

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011515
REVIEW
Transcatheter Closure of Coronary Artery Fistulae: A Literature Review
1Cardiovascular Intervention Research Center, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran
2Department of Cardiovascular Medicine, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran
3Cardio-Oncology Research Center, Rajaie Cardiovascular Medical and Research Center, Tehran, Iran
*Corresponding Author: Mohammad Javad Alemzadeh-Ansari. Email: mj.aansari@gmail.com
Received: 13 May 2020; Accepted: 10 June 2020
Abstract: Coronary artery fistulae (CAFs) are anomalous connections that bypass the myocardial capillary bed between 1 or more coronary arteries and other cardiac chambers or other vessels. These fistulae are usually asymptomatic and are, thus, diagnosed incidentally. However, larger CAFs can cause various symptoms such as angina, exertional dyspnea, syncope, palpitation, and even sudden cardiac death. Treatment options include surgical closure and percutaneous transcatheter closure (TCC) with comparable safety and efficacy. The choice of device in TCC depends on the anatomic characteristics of the CAF, the age and size of the patient, the size of the occluded vessel, the appropriate size of the catheter to be used, and the tortuosity of the catheter course to reach the intended point. Herein, we present 4 cases treated via TCC and then offer an in-depth discussion regarding this coronary artery anomaly.
Keywords: Coronary artery fistulae; percutaneous transcatheter closure; surgical closure
Coronary artery fistulae (CAFs) are major coronary artery anomalies defined as anomalous connections bypassing the myocardial capillary bed between 1 or more coronary arteries and other cardiac chambers (coronary-cameral fistulae) or other vessels. Such fistulae are present in 0.002% of the general population, are mainly congenital in origin, and comprise between 0.2% and 0.4% of all congenital cardiac anomalies [1]. Although CAFs are often secondary to congenital cardiac anomalies, they may be acquired from invasive surgical procedures, trauma, infective endocarditis, aortic dissection, and Kawasaki disease [2–4]. Fistula development may also be a consequence of surgical or percutaneous interventions such as coronary artery bypass grafting, valve replacement, cardiac transplantation, endomyocardial biopsy, coronary angioplasty, permanent pacemaker placement, and the closed-chest ablation of accessory pathways [1].
Herein, we describe 4 patients with CAFs managed via different percutaneous transcatheter closure (TCC) techniques and thereafter present an in-depth discussion concerning this anomaly of coronary arteries.
A 26-year-old woman with dyspnea and palpitation in the postpartum period referred to our center (2 months after delivery) for further evaluation (Tab. 1). In physical examination, she had normal S1 and S2, lateral deviation of LV apex with loud continuous murmur in both low LSB and RSB without RV heave. Chest radiography showed cardiomegaly, left ventricular (LV) enlargement, and shunt vascularity.
Transthoracic echocardiography (TTE) revealed severe LV enlargement (LV end-diastolic volume index = 95 cc/m2), mild LV systolic dysfunction (left ventricular ejection fraction [LVEF] = 50%), severe biatrial enlargement (left atrial volume index = 51 cm/m2 and right atrial [RA] volume index = 48 cc/m2), moderate right ventricular (RV) enlargement with mild systolic dysfunction, bileaflet mitral valve prolapse with a posteriorly directed mitral regurgitation jet, a dilated aortic root (4.3 cm) with the aneurysmal formation of the left coronary cusp, and moderate aortic regurgitation. TTE also illustrated a tubular flow originating from the aneurysmal left coronary cusp of the aorta with a continuous flow terminating in the RA, indicative of a cameral fistula between the left circumflex artery (LCx) and the RA.
Computed tomography angiography (CTA) showed severely dilated left main artery (diameter = 23 mm) and LCx (diameter = 19 mm). It also visualized a fistulous tract originating from the LCx and extending to the distalmost end of the coronary sinus near its entrance into the RA.
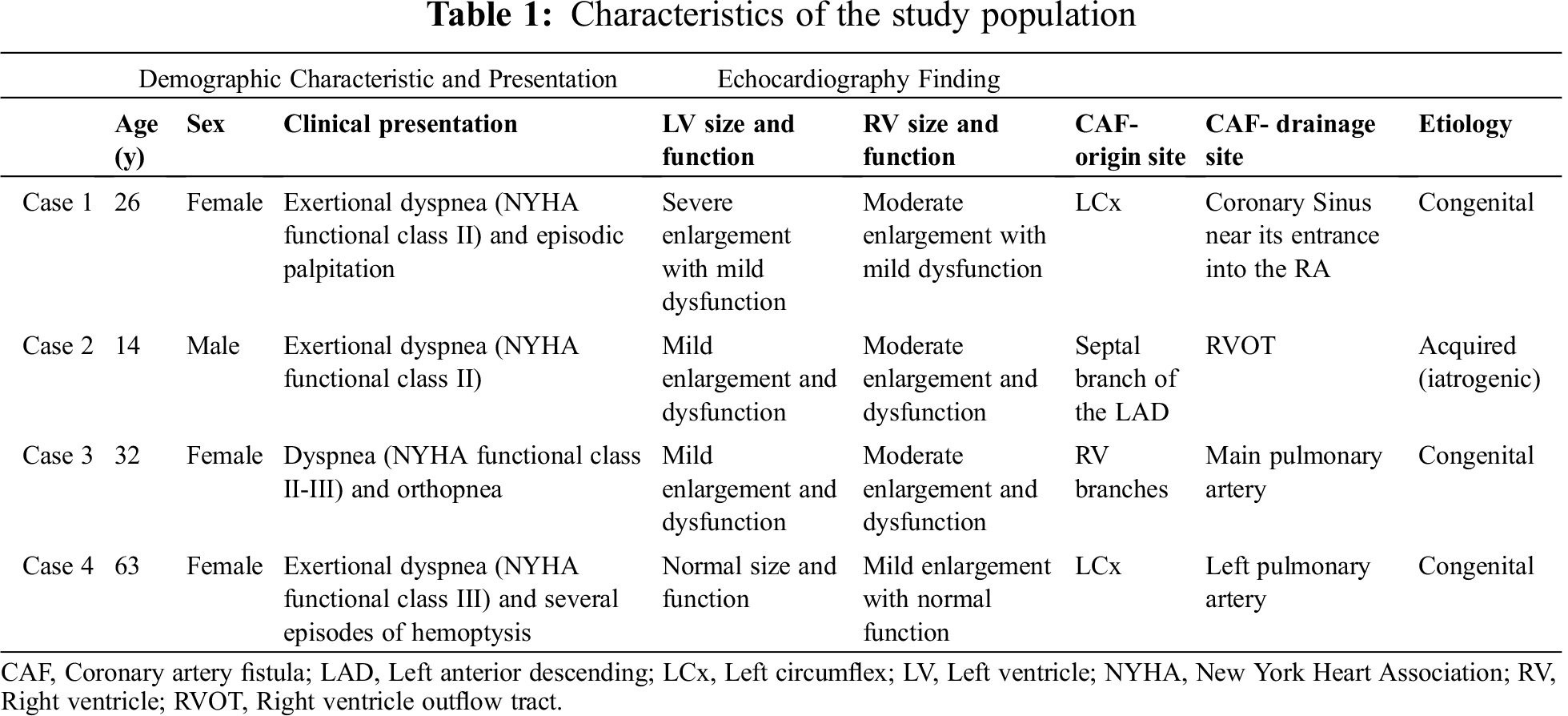
The coronary angiography revealed a large CAF from the LCx to the RA. Moreover, both left main and LCx were significantly dilated with no evidence of atherosclerotic coronary artery disease. Via the venous access through the femoral vein, a 14/18 Occlutech patent ductus arteriosus (PDA) occluder device (Occlutech international AB, Sweden) was deployed at the distal part of the fistula, resulting in a complete CAF closure with no residual shunt (Fig. 1). At 1 month’s follow-up, the patient was asymptomatic, and TTE and CTA confirmed the complete closure of the CAF with no residual shunt.
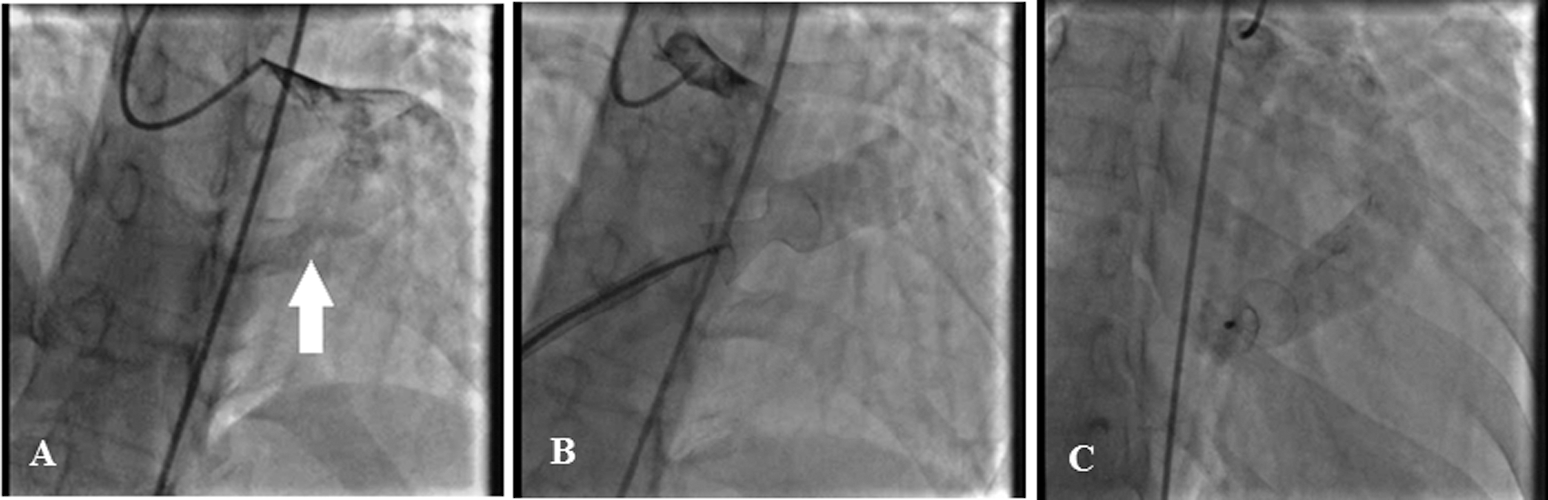
Figure 1: The coronary angiogram reveals a large coronary artery fistula, from the left circumflex artery to the right atrium (white arrow). Both left main and left circumflex coronary arteries are significantly dilated (Fig. A). Via the venous access through the femoral vein, a 14/18 Occlutech patent ductus arteriosus occluder device (Occlutech international AB, Sweden) was deployed successfully with no residual shunt (Figs. B and C)
A 14-year-old boy presented to our hospital with complaints of exertional dyspnea (New York Heart Association [NYHA] functional class II) and palpitations of 6 months’ duration (Tab. 1). He had a history of the tetralogy of Fallot which was totally surgical corrected at the age of 2.5 years. The patient’s recent echocardiogram revealed mild LV enlargement, mild LV systolic dysfunction (LVEF = 50%), moderate RA and RV enlargement, no residual ventricular septal defect (VSD), residual pulmonary stenosis with a peak gradient of 50 mm Hg, and mild tricuspid regurgitation. Additionally, there was a dilated left coronary artery proximal to the left anterior descending (LAD), together with a tubular structure originating from the proximal portion of the LAD with a continuous flow terminating in the right ventricular outflow tract (RVOT), suggestive of a cameral fistula between the LAD and the RVOT.
Coronary angiography revealed a dilated left coronary artery proximal to the LAD and also a large CAF from the septal branch of the LAD to the RVOT. No detailed follow-up data during postoperative years were available, but iatrogenic fistula formation following surgery is a possibility. During cardiac catheterization, there was a significant O2 step up in the RVOT, prompting the medical team to proceed with fistula occlusion.
First, the LAD was wired to the RVOT with a 0.014 guidewire, and snaring was done via the right femoral vein access. Then an 8-F right Judkins guiding catheter was introduced via the femoral vein right access toward the distal part of the CAF. The 0.014 guidewire helped the operator to negotiate the 8-F right Judkins guiding catheter to the distal part of the CAF and also to stabilize the catheter during the release of the occluder device. Thereafter, a detachable coil device (5 × 4 mm Duct Occluder pfm, Nit-Occlud PDA, pfm medical ag, Germany) was inserted in the distal part of the CAF, with a mild residual shunt (Fig. 2). Fortunately, at 6 months’ follow-up, the patient was asymptomatic. The echocardiogram showed no residual shunt flow and a Qp/Qs ratio of 1.1:1.
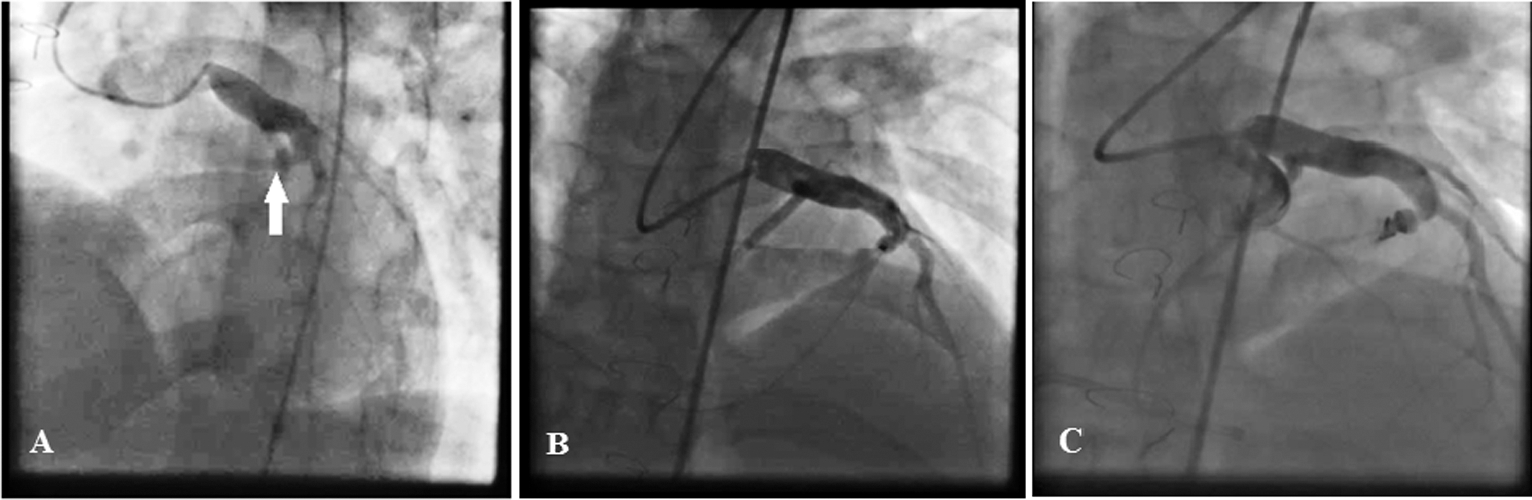
Figure 2: The coronary angiogram reveals a dilated left coronary artery proximal to the left anterior descending and also a large coronary artery fistula from the septal branch of the left anterior descending (white arrow) to the right ventricular outflow tract (Fig. A). The left anterior descending artery was wired to the right ventricular outflow tract with a 0.014 guidewire, and snaring was done via the right femoral vein access (Fig. B). Subsequently, an 8-F right Judkins guiding catheter was introduced through the right femoral vein access toward the distal part of the coronary artery fistula. Finally, a detachable coil device (5 × 4 mm Duct Occluder pfm, Nit-Occlud, pfm medical ag, Germany) was inserted in the distal part of the coronary artery fistula, with a mild residual shunt (Fig. C)
A 32-year-old woman, a known case of congenital heart disease, referred to our hospital with a complaint of dyspnea (Tab. 1). The patient had a history of the surgical closure of a VSD and a patent ductus arteriosus (PDA) during infancy, in conjunction with permanent pacemaker implantation due to postoperative atrioventricular nodal block. The first pacemaker was epicardial, which was substituted with an endocardial pacemaker after 10 years. She remained in a relatively good condition but developed progressive dyspnea (NYHA functional class II–III) and then orthopnea over the year leading to her referral to us.
Physical examination showed normal S1 and S2 and audible loud continuous murmurs at the upper left sternal border. Chest radiography showed mild cardiomegaly, RV enlargement, and shunt vascularity. Left coronary injection helped visualize a small fistula from the septal branch of the LAD to the main pulmonary artery. Right coronary artery injection showed a developed fistula with multiple feeding branches from the RV branches and also the distal portion of the right coronary artery (the posterior descending artery and the posterolateral branch) to the main pulmonary artery with a single drainage site. The fistula appeared to be congenital in origin with hemodynamic effects having become more prominent in adulthood. For CAF closure, a microcatheter (Caravel, ASAHI INTECC, Japan) was introduced from the larger RV branch to the distal part of the CAF in close proximity to the pulmonary artery. Additionally, a right Judkins guiding catheter was placed at the exit of the CAF via the venous access. Two coils (7 mm × 30 cm MicroPlex-10, MicroVention-Terumo, Japan, and 7 mm × 40 cm Cook UK Ltd.) were released successfully with the aid of the microcatheter. The final injection showed no residual shunt (Fig. 3). At 1 month’s follow-up, the patient was completely asymptomatic. CTA confirmed no residual shunt and showed proper coil position.
Figure 3: Right coronary artery injection illustrates a developed fistula with frequent feeding branches from the right ventricular branches and also the distal branches of the right coronary artery to the main pulmonary artery with a single drainage site (Fig. A). For the closure of the coronary artery fistula, a microcatheter was introduced from the larger right ventricular branch to the distal part of the fistula near the pulmonary artery. In addition, a right Judkins guiding catheter was placed at the exit site of the coronary artery fistula through the vein access. Two coils were released successfully by using the microcatheter. The final injection showed no residual shunt (Fig. B)
A 63-year-old woman with a history of exertional dyspnea and several episodes of hemoptysis of 3 months’ duration referred to our hospital (Tab. 1). Chest radiography and lung spiral CT scan showed left lower lobe bronchiectasis (Fig. 4). TTE revealed normal LV size with a good systolic function (LVEF = 55%), mild RV enlargement, and no valvular abnormality. Coronary angiography showed an anomalous vessel from the LCx to the left pulmonary artery, which was subsequently closed successfully via the arterial access (the femoral artery) with 3 coils (8 mm × 30 cm Axium Helix [Covidien, USA], 8 mm × 30 cm Stryker Neurovascular [Target, 360 Ultra, GB Tech USA Inc.], and 6 mm × 10 cm [Target, 360 Ultra, GB Tech USA Inc.] with no residual shunt (Fig. 4). At 1 month’s follow-up, TTE and CTA showed complete closure with no residual shunt.
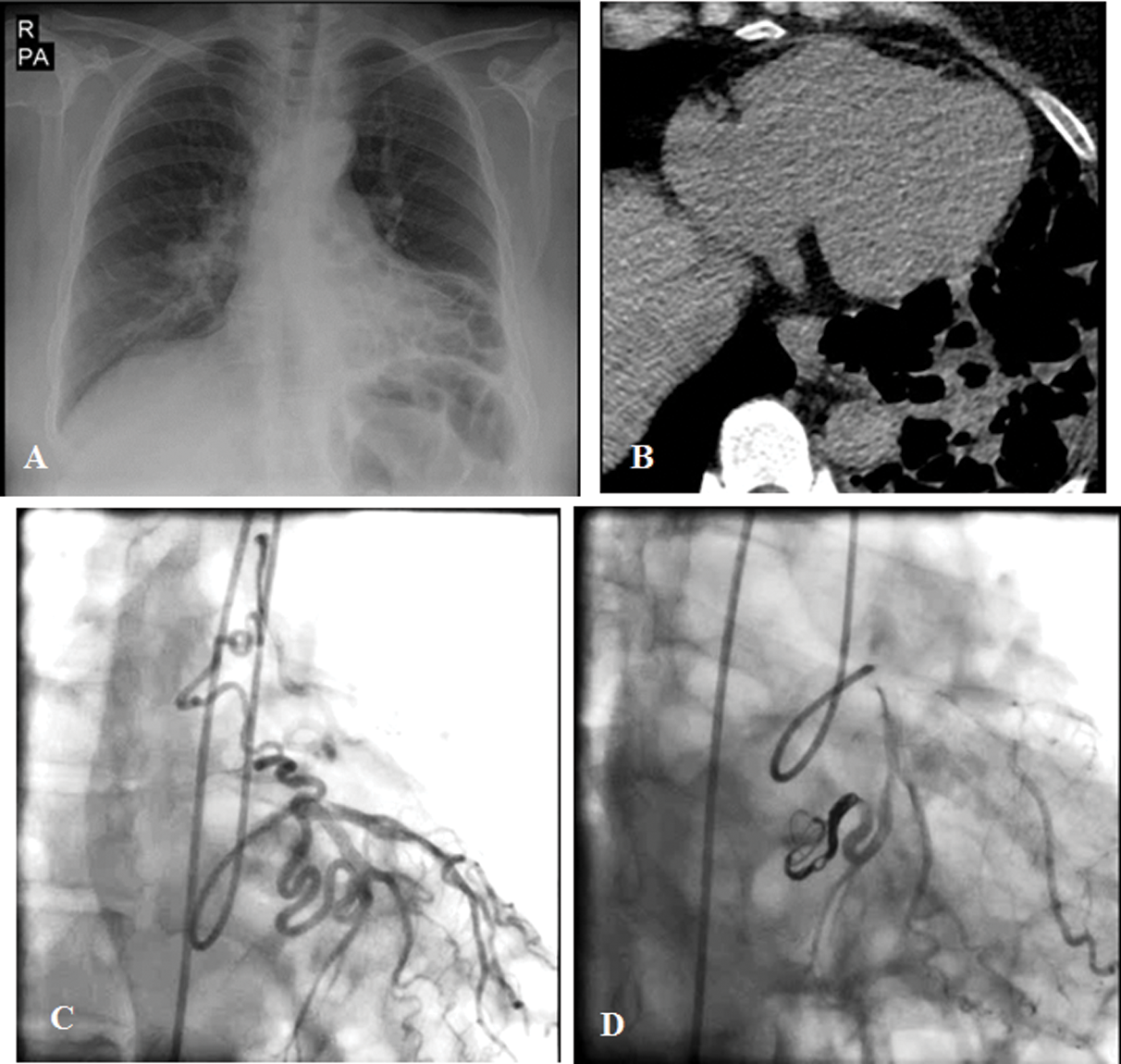
Figure 4: The chest radiograph and lung spiral computed tomography scan show left lower lobe bronchiectasis (Figs. A and B). The coronary angiogram illustrates an anomalous vessel from the left circumflex artery to the left pulmonary artery (Fig. C), which was successfully closed via the femoral artery access with 3 coils, with no residual shunt (Fig. D)
CAFs are visualized in nearly 0.25% of the patients undergoing catheterization, and there is no race or sex predilection [5]. If there is the involvement of 1 or more coronary arteries communicating with any cardiac chambers, the CAF is referred to as “the coronary cameral fistula”, and if there is the involvement of any great vessels such as the vena cava, the coronary sinus, the pulmonary artery, the pulmonary veins, and the bronchial vessels, the CAF is referred to as “ the coronary arteriovenous fistula” [1]. CAFs may be isolated in 55% to 80% of cases or associated with other congenital heart diseases in 20% to 45%. The associated anomalies may be seen in about 35% of cases; they include the tetralogy of Fallot, atrial septal defects (ASDs), PDAs, VSDs, pulmonary atresia/intact ventricular septa, and superimposed coronary artery disease. Single CAFs are more common, ranging from 74% to 90%, while multiple fistulae are present in 10.7% to 16%. Moreover, there are fistulae originating from both coronary arteries in 4% to 18% of cases.
The nomenclature is based on a descriptive analysis of the vessel of origin and its termination. Although CAFs may arise from any branch of the coronary artery system, the right coronary artery is the most common vessel of origin (50–60%). The right heart (i.e., RA, RV, and pulmonary artery) is the most common site of drainage (about 80%) [6]. Sakakibara et al. [7] introduced an angiographic classification: the proximal type, in which the CAF originates from the proximal third of the native vessel while the distal end is normal, and the distal type, in which the CAF originates beyond the proximal third of the native vessel. In the former type, the proximal coronary segment is dilated at the origin of the fistula and the distal end is normal, whereas in the latter type, the coronary artery is dilated through its entire length and terminates as a fistula mainly to the right side of the heart and the proximal coronary segment has regular branches.
3.1 Pathophysiology and Complications
The most widely accepted concept of the coronary steal phenomenon is the primary pathophysiological basis of ischemic symptoms in CAFs with large calibers. The resistance is determined by the size, tortuosity, and length of the pathway. The diversion of the blood flow from the high-pressure coronary artery vasculature to the low-resistance right-sided or venous structure (left-to-right shunting) due to the diastolic pressure gradient causes ischemia in the distal myocardial segments and the compensatory dilation of the diseased coronary artery. When the drainage site of the CAF is the left atrium or the pulmonary vein, there is an effective left-to-left shunt that volume-overloads the left heart only. A CAF flow into the LV causes volume overload in the LV with hemodynamics mimicking aortic valve regurgitation [8,9].
Other possible complications of CAFs include aneurysmal formation, intimal ulceration, medial degeneration, calcification and accelerated atherosclerosis, side-branch obstruction, mural thrombosis, and rupture [5].
Most CAFs are small and asymptomatic and are usually detected incidentally. Nonetheless, such small fistulae may slowly increase in size with advancing age and changes in the systemic blood pressure and aortic compliance. Small CAFs, especially those that drain into the main pulmonary artery and the LV, are much more common than are large ones and are usually considered benign [10]. In untreated CAFs, the symptoms may develop in 19% of patients under 20 years of age and in 63% of older patients [11].
Symptoms depend on the amount of the shunted blood or the presence of the coronary steal phenomenon. Small-to-medium-sized CAFs are usually asymptomatic, while medium-to-large-sized ones with pulmonary-systemic flow ratios of greater than 1.5:1 can lead to different signs and symptoms and need treatment [10]. The symptoms in young adults with large CAFs are exertional dyspnea (60%), endocarditis in the fistula (20%), angina (3–7%), syncope, palpitations, myocardial ischemia, and infarction. In older adults, the symptoms include congestive heart failure (19%), atherosclerosis, and cardiac arrhythmias. The drainage of CAFs into the right-sided structures results in congestion and pulmonary hypertension [5]. In rare cases, the fistula may rupture spontaneously, causing hemopericardium and cardiac tamponade [12]. In neonates and infants with a large CAF, congestive heart failure secondary to a large-volume shunt is the common presentation [13]. An important complication of CAFs in pediatric patients is late stenosis secondary to intimal hyperplasia, which could increase the risk of myocardial infarction later in life [1]. In children, associated congenital cardiovascular anomalies such as ASDs, VSDs, PDAs, and the tetralogy of Fallot are more common [5], and the signs and symptoms of these anomalies may further complicate the detection of the CAF.
The most prominent finding in physical examinations in large fistulae is an II/VI-IV/VI continuous murmur over the left upper sternal border, which is louder in diastole. There is a correlation between the site of the drainage of the fistula and the site of the loudest intensity of the murmur. By way of example, if the CAF drains into the RA, the loudest murmur is along the sternal border; if the CAF drains into the pulmonary artery, the loudest murmur is at the second intercostal space to the left of the sternum; and if the CAF drains into the LV, the loudest murmur is near the apex. CAFs in asymptomatic patients are usually suspected when a murmur is detected [1,5].
Electrocardiography and chest radiography are usually unhelpful, while echocardiography may show the effects of LV or RV volume overload and sometimes ischemic changes. In adult patients with normal electrocardiograms, stress imaging with the treadmill, echocardiography, or perfusion scan could be helpful in the detection of myocardial ischemia related to CAFs. Generally, chest radiographs are normal; still, in patients with large left-to-right shunts, moderate cardiomegaly and shunt vascularity may be present [14].
In patients with suspected CAFs, color Doppler echocardiography may be useful in the detection of coronary artery dilatation, termination chamber, turbulent flow, chamber volume overload, and alterations in the kinesis of the LV wall segments. The modality is also useful after CAF treatment in order to monitor the status of the fistula and the LV walls [1]. Although TTE in children and transesophageal echocardiography in adults may assist in the evaluation of CAFs [15], cardiac catheterization and coronary angiography remain the gold-standard methods to obtain hemodynamic data and to assess the anatomy of the coronary artery. Cardiac catheterization and coronary angiography provide substantial details about CAFs concerning the size, the course, the origin, the presence of any stenosis, and the drainage site; additionally, they help to exclude various anomalies and defects such as PDAs, VSDs, ASDs, aortic regurgitation, and arteriovenous fistulae in the lungs or chest walls [5]. In addition, intravascular ultrasound can be drawn upon for the assessment of intimal integrity, mural clots, vessel size, and localized aneurysms, and pressure-wire studies can be utilized to evaluate pressure loss along fistulous arteries [10].
Magnetic resonance imaging and multidetector CTA are both noninvasive and helpful in the evaluation of CAFs. Nevertheless, the latter is much faster than is the former and can be performed in a single breath-hold. Moreover, the ability to provide submillimeter reconstruction confers multidetector CTA high temporal and spatial resolution by comparison with magnetic resonance imaging. In overweight patients, CTA is also superior to echocardiography in that it allows an excellent anatomical delineation. Vis-à-vis preoperative evaluations, not only can CTA illustrate anomalous origins of the coronary arteries and their courses but also it is capable of assessing the complexity of fistulae and the presence or absence of obstruction [16].
In addition to other imaging modalities for the evaluation of CAFs, 3D-printed models can provide a better guide to surgeons or interventionists for preprocedural planning, not least in complex or recurrent CAFs [17–19].
There is a consensus regarding the notion that symptomatic patients with CAFs should be treated [5]. In adult patients, according to the guidelines of the American College of Cardiology and the American Heart Association, a large CAF, regardless of symptomatology, should be closed via either the transcatheter route or the surgical route after the delineation of its course and the possibility to fully obliterate the fistula (Class I, Level of Evidence: C). Further, a small-to-moderate-sized CAF accompanied by documented myocardial ischemia, arrhythmia, otherwise unexplained ventricular systolic or diastolic dysfunction or enlargement, or endarteritis should be closed after the delineation of its course and its potential to fully obliterate the fistula (Class I, Level of Evidence: C). On the other hand, CAF closure is not recommended for those with small and/or asymptomatic CAFs (Class III, Level of Evidence: C) [20]. There have, however, been recommendations that CAFs be closed irrespective of symptoms or size in patients undergoing an invasive cardiac procedure for other reasons [1].
In children, particularly those that are older than 5 years, elective CAF closure is recommended, even if the patient is asymptomatic. The closure of the proximal type of CAF, which originates from the proximal third of the native coronary, is highly recommended because the risk of aneurysmal formation and rupture increases in the future [1,5].
Therapeutic interventions consist of the surgical ligation and percutaneous TCC of CAFs. The best approach to CAF closure is based on its morphology, course, and tortuosity, as well as the presence of aneurysmal dilatation. Surgical CAF ligation is a safe and effective treatment method; it is reserved for large and/or symptomatic CAFs with very tortuous pathways, multiple terminations, significant aneurysmal formations, and a need for concurrent bypass grafting [5].
3.4.2 Percutaneous Trans-Catheter Closure
Percutaneous TCC is an invasive strategy associated with fewer complications than the surgical approach. Aside from better cosmetic results, TCC is allied to lower incidence rates of bleeding, infection, arrhythmias, inflammation, and myocardial ischemia, as well as lower costs, recovery time, and morbidity [1]. Interestingly, after successful TCC, 9% to 30% of patients have residual fistulae at follow-up angiography [21,22]. The aim of TCC is to occlude the CAF as distally and as closely to its termination point as possible so as to avoid any possibility of occlusion in the branches to the normal myocardium. However, if the occluder device is released very distally in the CAF without any significant stenosis at the distal part of the CAF, the migration of the device to the distal cavity or the large vessel can occur [14].
The success rate of TCC for the closure of CAFs depends on some factors, including the ability to safely cannulate the branch of the coronary artery that supplies the fistula; the absence of large branch vessels that can be inadvertently embolized; the presence of a single, narrow restrictive drainage site into the cardiac chamber or vessel; and the absence of multiple fistulous communications [23]. Thus, TCC is a suitable strategy with a high success rate in patients with a proximal CAF, not least when there are single drainage sites, non-tortuous vessels, distal CAF narrowing, accessible routes for the closure device, the absence of important branches that could accidentally be embolized, the absence of other cardiac disorders, or high perioperative surgical risks [1].
Two approaches are considered toward TCC: arterial and venous. The arterial approach is suitable for smaller CAFs that can be closed with coils or covered stents, whereas the venous approach is appropriate when the fistula is large and requires a device or a plug. Moreover, in cases with tortuous and long CAFs that can be approached easily through the venous side, the venous approach is preferable [23]. Be that as it may, in some cases, both accesses may be needed for arteriovenous loop formation [24]. The techniques for the TCC of CAFs include the use of various types of occlusion coils and microcoils, umbrella devices, detachable balloons, vascular plugs, covered stents, polyvinyl alcohol foam, glue, and histoacryl resin or other occlusive oils that are mainly used in smaller fistulae [1]. Based on our experience, residual shunt rates are low with Amplatzer or duct occluders compared with coils. The Amplatzer and Occlutech duct occluders are ideal devices for CAF closure provided that the drainage is large enough to allow the passage of the long sheath [1]. For example, in CASE 1, we used an Occlutech duct occluder device for the closure of a large CAF via the venous access.
In some patients with cameral fistulae, because of the high blood flow from the coronary system toward the cardiac chambers, the insertion of the device via the arterial access may be challenging. We have previously presented a case of an iatrogenic CAF following mitral valve surgery. In that patient, the CAF was placed between the proximal LCx and the LV. We opted for the arterial access and at the first attempt dropped the coil into the LV. After removing the coil, we deployed a Scepter C balloon (MicroVention-Terumo, USA) in the ostioproximal portion of the LCx to decrease the blood flow toward the LCx. We also managed to insert other coils [25].
Although TCC is a safe technique in selected patients, some periprocedural complications may occur; these complications include device migration, myocardial ischemia and infarction, bundle branch block, minor or major bleeding due to anticoagulation during the procedure, arrhythmias, coronary artery spasm or perforation, and guidewire entrapment [23]. Patients with the distal type CAF, large CAFs, and older age at presentation may be at higher risk for myocardial infarction. Patients with large distal CAFs arising from ectatic parent vessels with the subsequent stagnation of the flow after CAF closure are more prone to myocardial infarction [24,26,27]. What is more, incomplete occlusion with coils and the presence of a foreign body in the vessel might increase the risk of bacterial endocarditis in the future [4].
According to the guidelines of the American College of Cardiology and the American Heart Association, echocardiography with clinical follow-ups every 3 to 5 years can be useful for patients with small, asymptomatic CAFs to exclude the development of symptoms or arrhythmias or the progression of size or chamber enlargement, which might alter management (Class IIa, Level of Evidence: C) [20]. Long-term follow-ups after successful percutaneous or surgical CAF closure are essential due to the possibility of recanalization, persistent dilation of the coronary artery and ostium, thrombus formation, calcification, and myocardial ischemia [28].
Although the rates for residual shunts following procedures are variable among the published reports, based on an investigation by Kiefer et al. [23] into previous reports, the rates are similar in terms of the residual fistula flow (20–30%) during follow-ups after TCC and surgical techniques. High-risk features for subsequent adverse events were found to be more likely in patients with a fistula draining into the coronary sinus regardless of whether the patients had undergone TCC or surgical correction. In cases with recurrent/residual CAFs, attention to clinical symptoms and hemodynamic findings is mandatory. Recurrent/residual CAFs may have a relatively high incidence, but they are usually small and are of no hemodynamic significance [30]. In symptomatic or large recurrent/residual CAFs, the use of 3D-printed models can provide a better guide for surgeons or interventionists for preprocedural planning concerning patients with recurrent CAFs [17–19]. Given the high risk of redo-surgery, our experience indicates that TCC is a more appropriate technique in these patients provided that the anatomy is suitable for device closure.
Echocardiography with clinical follow-ups 1 month after the procedure is recommended [1]. Although most of the adult patients who are asymptomatic after successful CAF closure remain free of symptoms for long periods [28], a close clinical follow-up with echocardiographic evaluations every 6 to 12 months is recommended initially and then every 2 or 3 years [1].
We herein reported 4 cases of CAFs treated successfully via different percutaneous TCC techniques. With the advent of newer techniques and devices, a wide range of equipment will be available for further interventions on various types of CAFs. Awareness of possible complications and readiness for their management can confer excellent outcomes. We would recommend a close follow-up for all patients with CAFs regardless of size, symptoms, and treatment course.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Buccheri, D., Chirco, P. R., Geraci, S., Caramanno, G., Cortese, B. (2018). Coronary artery fistulae: anatomy, diagnosis and management strategies. Heart, Lung and Circulation, 27(8), 940–951. DOI 10.1016/j.hlc.2017.07.014. [Google Scholar] [CrossRef]
2. Said, S. A., El Gamal, M. I. H., Van Werf, T. D. (1997). Coronary arteriovenous fistulas: collective review and management of six new cases—changing etiology, presentation, and treatment strategy. Clinical cardiology, 20(9), 748–752. DOI 10.1002/clc.4960200907. [Google Scholar] [CrossRef]
3. Koenig, P. R., Kimball, T. R., Schwartz, D. C. (1993). Coronary artery fistula complicating the evaluation of Kawasaki disease. Pediatric Cardiology, 14(3), 179–180. DOI 10.1007/BF00795651. [Google Scholar] [CrossRef]
4. Mangukia, C. V. (2012). Coronary artery fistula. The Annals of Thoracic Surgery, 93(6), 2084–2092. DOI 10.1016/j.athoracsur.2012.01.114. [Google Scholar] [CrossRef]
5. Challoumas, D., Pericleous, A., Dimitrakaki, I. A., Danelatos, C., Dimitrakakis, G. (2014). Coronary arteriovenous fistulae: a review. International Journal of Angiology, 23(1), 1–10. DOI 10.1055/s-0033-1349162. [Google Scholar] [CrossRef]
6. Dodge-Khatami, A., Mavroudis, C., Backer, C. L. (2000). Congenital heart surgery nomenclature and database project: anomalies of the coronary arteries. The Annals of Thoracic Surgery, 69(3), 270–297. DOI 10.1016/S0003-4975(99)01248-5. [Google Scholar] [CrossRef]
7. Sakakibara, S., Yokoyama, M., Takao, A., Nogi, M., Gomi, H. (1966). Coronary arteriovenous fistula: nine operated cases. American Heart Journal, 72(3), 307–314. DOI 10.1016/S0002-8703(66)80004-2. [Google Scholar] [CrossRef]
8. Sommer, R. J., Hijazi, Z. M., Rhodes Jr, J. F. (2008). Pathophysiology of congenital heart disease in the adult: part I: shunt lesions. Circulation, 117(8), 1090–1099. DOI 10.1161/CIRCULATIONAHA.107.714402. [Google Scholar] [CrossRef]
9. Liberthson, R. R., Sagar, K. A. R. E. N., Berkoben, J. P., Weintraub, R. M., Levine, F. H. (1979). Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation, 59(5), 849–854. DOI 10.1161/01.CIR.59.5.849. [Google Scholar] [CrossRef]
10. Angilini, P. (2002). Coronary artery anomalies-current clinical issues. Texas Heart Institute Journal, 29, 271–278. [Google Scholar]
11. Mavroudis, C., Backer, C. L., Rocchini, A. P., Muster, A. J., Gevitz, M. (1997). Coronary artery fistulas in infants and children: a surgical review and discussion of coil embolization. The Annals of Thoracic Surgery, 63(5), 1235–1242. DOI 10.1016/S0003-4975(97)00251-8. [Google Scholar] [CrossRef]
12. Olearchyk, A. S., Runk, D. M., Alavi, M., Grosso, M. A. (1997). Congenital bilateral coronary-to-pulmonary artery fistulas. The Annals of Thoracic Surgery, 64(1), 233–235. DOI 10.1016/S0003-4975(97)00347-0. [Google Scholar] [CrossRef]
13. Latson, L. A. (2007). Coronary artery fistulas: how to manage them. Catheterization and Cardiovascular Interventions, 70(1), 111–118. DOI 10.1002/ccd.21125. [Google Scholar] [CrossRef]
14. Qureshi, S. A. (2006). Coronary arterial fistulas. Orphanet Journal of Rare Diseases, 1(1), 51. DOI 10.1186/1750-1172-1-51. [Google Scholar] [CrossRef]
15. Krishnamoorthy, K. M., Rao, S. (2004). Transesophageal echocardiography for the diagnosis of coronary arteriovenous fistula. International Journal of Cardiology, 96(2), 281–283. DOI 10.1016/j.ijcard.2003.03.031. [Google Scholar] [CrossRef]
16. Zenooz, N. A., Habibi, R., Mammen, L., Finn, J. P., Gilkeson, R. C. (2009). Coronary artery fistulas: CT findings. Radiographics, 29(3), 781–789. DOI 10.1148/rg.293085120. [Google Scholar] [CrossRef]
17. Zhang, J., Ma, W., Zhang, W., Kong, Y. (2019). Three-dimensional printed models-guided surgical repair for recurrent coronary artery fistula. The Annals of Thoracic Surgery, 107(3), e161–e163. DOI 10.1016/j.athoracsur.2018.07.085. [Google Scholar] [CrossRef]
18. Tari, C. K., Erdol, M. A., Ilkay, E. (2020). Printing the procedure: successful closure of a coronary cameral fistula with 3-dimensional model. JACC: Case Reports, 2(3), 488–492. DOI 10.1016/j.jaccas.2019.11.047. [Google Scholar] [CrossRef]
19. Aroney, N., Lau, K., Daniele, L., Burstow, D., Walters, D. (2018). Three-dimensional printing: to guide management of a right coronary artery to left ventricular fistula. European Heart Journal-Cardiovascular Imaging, 19(3), 268–268. DOI 10.1093/ehjci/jex317. [Google Scholar] [CrossRef]
20. Warnes, C. A., Williams, R. G., Bashore, T. M., Child, J. S., Connolly, H. M. et al. (2008). ACC/AHA, 2008 guidelines for the management of adults with congenital heart disease: executive summary: a report of the american college of cardiology/American heart association task force on practice guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation, 118(23), 2395–23451. DOI 10.1161/circulationaha.108.190811. [Google Scholar] [CrossRef]
21. Armsby, L. R., Keane, J. F., Sherwood, M. C., Forbess, J. M., Perry, S. B. et al. (2002). Management of coronary artery fistulae: patient selection and results of transcatheter closure. Journal of the American College of Cardiology, 39(6), 1026–1032. DOI 10.1016/S0735-1097(02)01742-4. [Google Scholar] [CrossRef]
22. Jama, A., Barsoum, M., Bjarnason, H., Holmes, D. R., Rihal, C. S. (2011). Percutaneous closure of congenital coronary artery fistulae: results and angiographic follow-up. JACC: Cardiovascular Interventions, 4(7), 814–821. DOI 10.1016/j.jcin.2011.03.014. [Google Scholar] [CrossRef]
23. Kiefer, T. L., Crowley, A. L., Jaggers, J., Harrison, J. K. (2012). Coronary arteriove- nous fistulae: the complexity of coronary artery-to-coronary sinus connections. Texas Heart Institute Journal, 39(2), 218–222. [Google Scholar]
24. Harikrishnan, S., Bimal, F., Ajithkumar, V., Bhat, A., Krishnamoorthy, K. M. et al. (2011). Percutaneous treatment of congenital coronary arteriovenous fistulas. Journal of Interventional Cardiology, 24(3), 208–215. DOI 10.1111/j.1540-8183.2010.00621.x. [Google Scholar] [CrossRef]
25. El-Sabawi, B., Al-Hijji, M. A., Eleid, M. F., Cabalka, A. K., Ammash, N. M. et al. (2020). Transcatheter closure of coronary artery fistula: A 21-year experience. Catheterization and Cardiovascular Interventions, DOI 10.1002/ccd.28721. [Google Scholar]
26. Firuzi, A., Alemzadeh-Ansari, M. J., Pouraliakbar, H. R. (2017). Transcatheter coil embolization of iatrogenic coronary artery-left ventricle fistula after mitral valve replacement. Journal of the Saudi Heart Association, 29(2), 148–152. DOI 10.1016/j.jsha.2016.10.009. [Google Scholar] [CrossRef]
27. Mottin, B., Baruteau, A., Boudjemline, Y., Piechaud, F. J., Godart, F. et al. (2016). Transcatheter closure of coronary artery fistulas in infants and children: A French multicenter study. Catheterization and Cardiovascular Interventions, 87(3), 411–418. DOI 10.1002/ccd.26320. [Google Scholar] [CrossRef]
28. Gowda, S. T., Latson, L. A., Kutty, S., Prieto, L. R. (2011). Intermediate to long-term outcome following congenital coronary artery fistulae closure with focus on thrombus formation. The American Journal of Cardiology, 107(2), 302–308. DOI 10.1016/j.amjcard.2010.09.018. [Google Scholar] [CrossRef]
29. Gowda, R. M., Vasavada, B. C., Khan, I. A. (2006). Coronary artery fistulas: clinical and therapeutic considerations. International Journal of Cardiology, 107(1), 7–10. DOI 10.1016/j.ijcard.2005.01.067. [Google Scholar] [CrossRef]
30. Cheung, D. L., Au, W. K., Cheung, H. H., Chiu, C. S., Lee, W. T. (2001). Coronary artery fistulas: long-term results of surgical correction. The Annals of Thoracic Surgery, 71(1), 190–195. DOI 10.1016/S0003-4975(00)01862-2. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |