

 | Congenital Heart Disease |  |
DOI: 10.32604/CHD.2020.011579
ARTICLE
Outcomes of Patients with Pulmonary Atresia with Intact Ventricular Septum Reaching Adulthood
1Department of Cardiovascular Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, 700-8558, Japan
2Department of Cardiovascular Surgery, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, 700-8558, Japan
3Department of Pediatric Cardiology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, 700-8558, Japan
*Corresponding Author: Norihisa Toh. Email: Norihisa.Toh@okayama-u.ac.jp
Received: 19 May 2020; Accepted: 08 June 2020
Abstract: Background: There is limited information on outcomes of adult patients with pulmonary atresia with intact ventricular septum (PA-IVS) due to the low incidence of disease and the large variation of surgical histories. Methods: Among 58 patients with repaired PA-IVS, a total of 32 patients aged ≥16 years and who were followed at our institution between January 2003 and December 2018 were reviewed. Surgical history, clinical outcomes, and laboratory, echocardiographic and electrocardiographic data were obtained by chart review. Results: Follow-up was from the age of 16 years and the median age at the latest follow-up was 23.7 years. Twenty-four patients had undergone biventricular repair (BVR), 3 had undergone one-and-a half ventricular repair (1.5VR), and 5 had undergone univentricular repair. Over a median follow-up period of 7.7 years (interquartile range: 4.1–11.0 years), 1 BVR patient died suddenly and 7 patients had heart failure. Arrhythmias were present in 5 patients. Ten patients underwent surgical re-interventions, including 4 BVR take-downs with conversion to 1.5VR and 3 Fontan conversions. Overall survival, heart failure-free, arrhythmia-free, and surgical re-intervention-free rates at 5 years and 10 years from the age of 16 years were 96.2% (95% confidence interval [CI], 77.2–99.4) and 96.2% (95% CI, 77.2–99.4), 81.4% (95% CI, 62.1–92.1) and 74.6% (95%CI, 52.3–88.7), 88.7% (95% CI, 70.1–96.3) and 75.9% (95% CI, 51.7–90.2), and 80.7% (95% CI, 60.8–91.8) and 70.8% (95% CI, 49.7–85.7), respectively. Conclusion: Adults with PA-IVS have preserved long-term survival regardless of the early operative strategy, while they are at risk for heart failure, arrhythmia, and surgical re-intervention. Thus, detailed and continued follow-up is mandatory for all PA-IVS patients from childhood to adulthood.
Keywords: Pulmonary atresia with intact ventricular septum; adult congenital heart disease; outcome
Pulmonary atresia with intact ventricular septum (PA-IVS) is a rare congenital heart defect characterized by an imperforate right ventricular outflow tract, intact ventricular septum, and varying degrees of right ventricular and tricuspid valve hypoplasia [1]. In addition to its wide spectrum of right ventricle (RV) morphologic features, RV-dependent coronary circulation (RVDCC) makes treatment strategies for this unique congenital heart disease more complicated. Various surgical procedures have been performed for patients with PA-IVS in early childhood according to their anatomic characteristics [2–8]. To avert abnormal hemodynamics and late complications in univentricular repair (UVR), our institutional strategy is to aim for biventricular repair (BVR) based on evidence of potential RV growth after adequate creation of RV and pulmonary artery continuity [2,9,10]. On the other hand, one-and-a half ventricular repair (1.5VR) was proposed as an alternative surgical procedure to avert Fontan circulation.
Due to a better understanding of the anatomic and physiologic characteristics and appropriate surgical strategy, an increasing number of children with PA-IVS reach adulthood [3–8]. Although some previous reports have shown clinical outcomes of adult survivors of PA-IVS [11,12], details of clinical courses and patients’ characteristics according to the type of surgical repair remain unknown. In the present study, we reviewed clinical outcomes and features of adults with PA-IVS at our institution.
This study was a retrospective cohort study. We identified 58 patients with PA-IVS who were followed at Okayama University Hospital. From this group of patients, we focused on the subset of adult patients who fulfilled the following criteria: 1) age of 16 years or older, 2) clinical visit to our institution between January 2003 and December 2018, and 3) having been diagnosed with PA-IVS by echocardiography and having undergone definitive repair during childhood. Patients with additional cyanotic congenital heart disease were excluded from this study. The final study population included 32 patients. Dropout was due to patient death before the age of 16 years (6 patients), loss of follow-up (3 patients), and age of less than 16 years at the time of data collection (17 patients). The Okayama University Hospital Institutional Review Board approved the study protocol and waived the need to obtain patient consent.
Clinical, laboratory, echocardiographic, and electrocardiographic data at the initial clinical visit after the age of 16 years were obtained and invasive hemodynamic data were obtained for BVR patients with 1.5VR conversion from our electronic database and from hospital case notes. Demographic and clinical data included age at the latest clinical follow-up, follow-up duration since the age of 16 years, gender, associated lesions, New York Heart Association (NYHA) functional classification, and detailed surgical records (number of operations and initial type of definitive repair). Laboratory data included data for serum sodium (mEq/L), serum creatinine (mg/dL), and B-type natriuretic peptide (BNP) (pg/mL). Echocardiography data were reviewed for left ventricular ejection fraction (LVEF) and severity of mitral regurgitation, tricuspid regurgitation (TR), and pulmonary regurgitation (PR). Data for cardiac rhythm and QRS duration were obtained from electrocardiograms (ECGs).
Patient-specific timelines allowed calculation of Kaplan-Meier plots for mortality, heart failure, arrhythmia, and surgical re-intervention. Patients were categorized as having heart failure if at least one of the following signs was present: orthopnea, nocturnal dyspnea, pulmonary edema, increasing peripheral edema, and radiological signs. Arrhythmias were identified on ECGs or Holter monitors or at the time of an electrophysiology study. Follow-up was from the age of 16 years. Patients were censored at the time of their last clinic review or date of death.
All of the patients were divided into groups according to the type of surgical repair at the age of 16 years.
Our institutional strategy is to aim for BVR to avoid abnormal hemodynamics as much as possible. Our staged strategy for PA-IVS was described in previous reports [2,9,10]. In brief, to achieve growth of right-sided heart structures, our choice of the initial palliation includes a modified Blalock-Taussig shunt with pulmonary valvotomy. After the initial surgical palliation, an RV overhaul procedure is performed for patients with RV end-diastolic volume of less than 50% of the predicted normal value [2,9]. Finally, we generally consider a tricuspid valve (TV) z-score of larger than –3 as the indication for BVR. If the TV z-score is less than –8, we consider UVR is favorable. Patients having a TV z-score of between –8 and –3 are considered as candidates for 1.5VR. The TV z-score is based on published data [13].
The BVR included excision of the hypertrophied RV muscle, cavity enlargement, and pulmonary valve repair to create a normal circulation from both ventricle pumps to each respective great artery after several surgical palliations and catheter interventions. A UVR meant completed Fontan physiology obtained by atriopulmonary, lateral tunnel, or extracardiac type Fontan. A 1.5VR consisted of bidirectional superior cavopulmonary anastomosis without Fontan circulation.
2.4 Strategy for Surgical Re-Interventions
In our institution, conversion to 1.5VR was performed in patients with BVR who had heart failure symptoms, significant PR indicated by echocardiography, elevated RAP (>10 mmHg), and liver damage assessed by ultrasound [2]. Fontan conversion was performed for patients who had a dilated right atrium, large thrombus in the right atrium and atrial tachyarrhythmia after an atriopulmonary Fontan operation.
Data are expressed as number (%) for categorical variables and mean ± standard deviation or median (interquartile range [IQR]) for continuous variables, as appropriate. Rates for actuarial survival and freedom from heart failure, arrhythmia, and surgical re-intervention were determined using the Kaplan-Meier method. Cox proportional hazards models were used to identify predictors for death or heart failure. For all analyses, a p-value less than 0.05 was considered to be statistically significant. All analyses were performed with JMP 14.0 (SAS Institute, Cary, NC).
We first identified 58 patients in the pediatric population (Fig. 1). Patients were censored at the time of their last clinical follow-up or when they died. Of the 58 patients, 49 patients had ongoing follow-up, 1 UVR patient died at the age the of 4 years and 5 patients died after palliative surgeries, and 3 patients were lost to follow-up. Of the 49 ongoing follow-up patients, 17 were less than 16 years of age at the time of data collection. Therefore, there were 32 PA-IVS patients aged ≥16 years and all of them were reviewed in the present study. The dotted line in Fig. 1 indicates the period from 16 years of age.
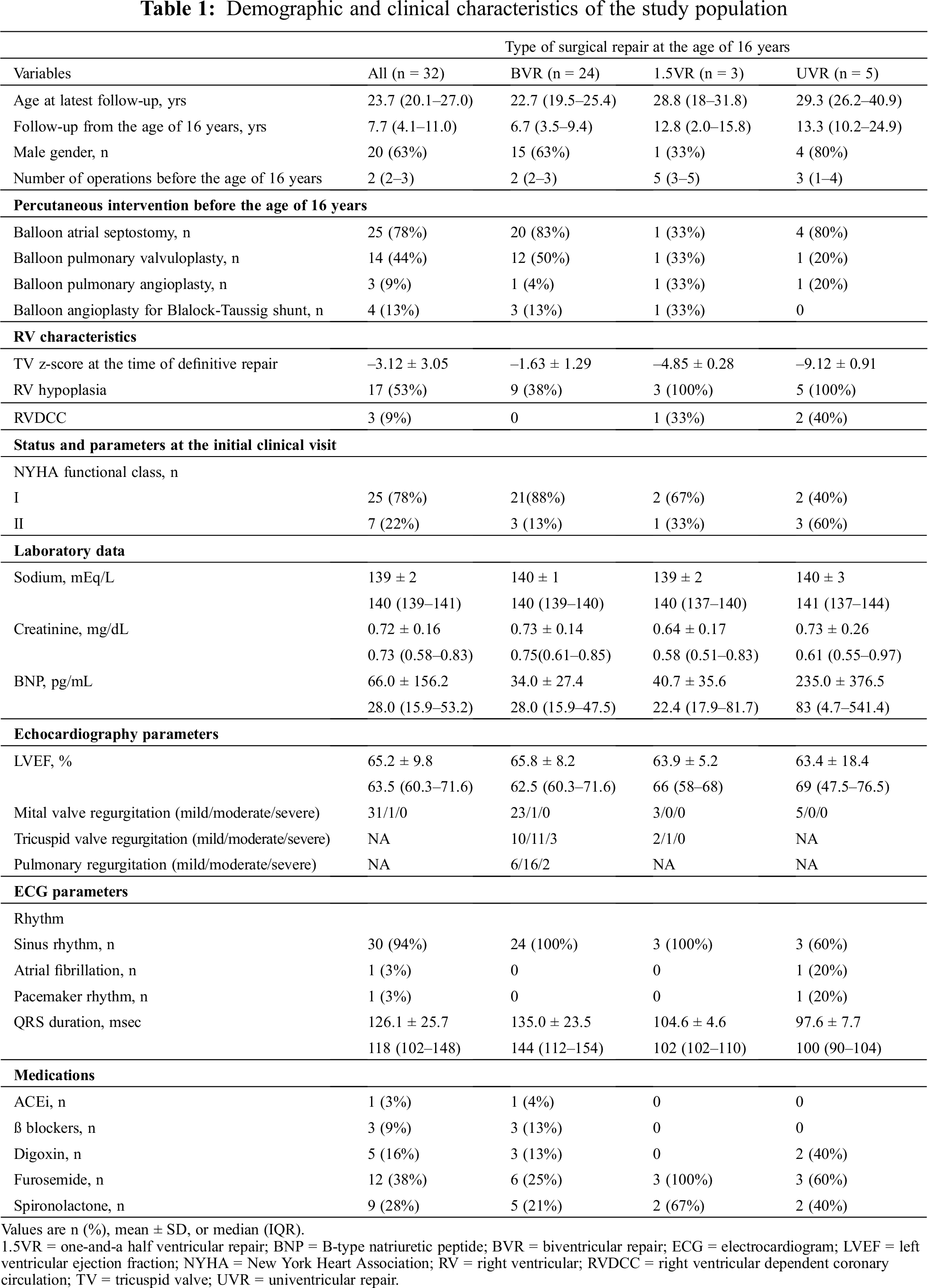
Patients’ demographics and characteristics are presented in Tab. 1. Their median age was 23.7 (IQR: 20.1–27.0) years at the latest follow-up. The median follow-up duration after the age of 16 years was 7.7 years (IQR: 4.1–11.0) and 20 patients were males. At the age of 16 years, 3 out of 5 UVR patients had atriopulmonary Fontan and the other two UVR patients had extracardiac Fontan. Before the age of 16 years, the median number of surgical interventions was 2 (IQR: 2–3). Details of percutaneous interventions are described in Tab. 1. TV z-scores at the time of definitive repair were –1.63 ± 1.29 in the BVR group, –4.85 ± 0.28 in the 1.5VR group, and –9.12 ± 0.91 in the UVR group. RV hypoplasia was found in all patients who underwent UVR and 1.5VR and in 9 of the 24 patients who underwent BVR. Two patients who underwent UVR and 1 patient who underwent 1.5VR had RVDCC. Two UVR patients underwent TV closure and no UVR patients experienced ischemic events. Before the age of 16 years, 3 UVR patients experienced heart failure and 2 UVR patients underwent epicardial pacemaker implantation due to sick sinus syndrome.
At the initial clinical visit after the age of 16 years, 25 patients were NYHA functional I and 7 patients were NYHA functional class II. Baseline serum BNP levels were 34.0 ± 27.4 pg/mL in the BVR group, 40.7 ± 35.6 pg/mL in the 1.5VR group, and 235.0 ± 376.5 pg/mL in the UVR group. Echocardiography revealed relatively preserved LVEF in the three groups, and 18 of the 24 BVR patients had moderate or severe PR. In the initial ECG, 30 patients had sinus rhythm, 1 UVR patient had atrial fibrillation, and 1 UVR patient had pacemaker rhythm. Only 1 BVR patient was prescribed an angiotensin-converting enzyme inhibitor.
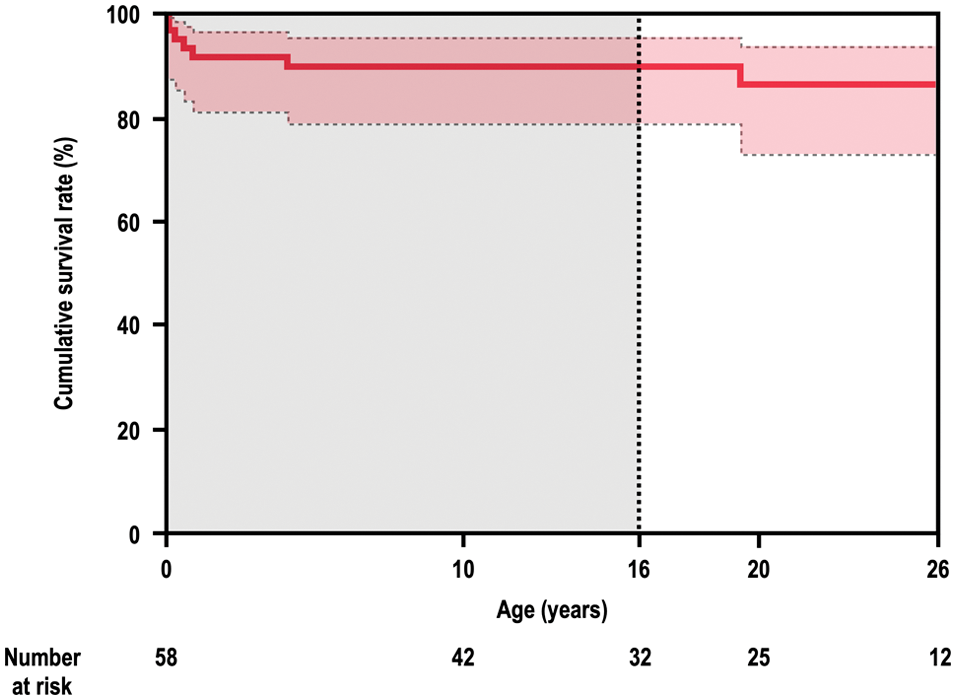
Figure 1: Long-term survival in 58 patients with PA-IVS
Firstly, we identified 58 patients in the pediatric population. Of the 58 patients, 49 had ongoing follow-up, 6 died, and 3 were lost to follow-up. Of the 49 ongoing follow-up patients, 17 were less than 16 years of age at the time of data collection. Thus, there were 32 adult survivors with PA-IVS and all of them were included in the present study. In the present study, we could not avoid immortal time bias because of the unique study population. The dotted line indicates the period from the age of 16 years.
PA-IVS = pulmonary atresia with intact ventricular septum.
The clinical outcomes of the study population are summarized in Tab. 2. During the median follow-up period of 7.7 years, one patient who underwent BVR died suddenly at the age of 19 years. He had undergone a Blalock-Taussig shunt procedure and pulmonary valvotomy at one month of age followed by right ventricular outflow tract reconstruction and atrial septal defect closure at the age of 2 years. In adult survivors with PA-IVS, the overall survival rates at 5 years and 10 years were 96.2% (95% confidence interval [CI], 77.2–99.4) and 96.2% (95% CI, 77.2–99.4), respectively (Fig. 2A).
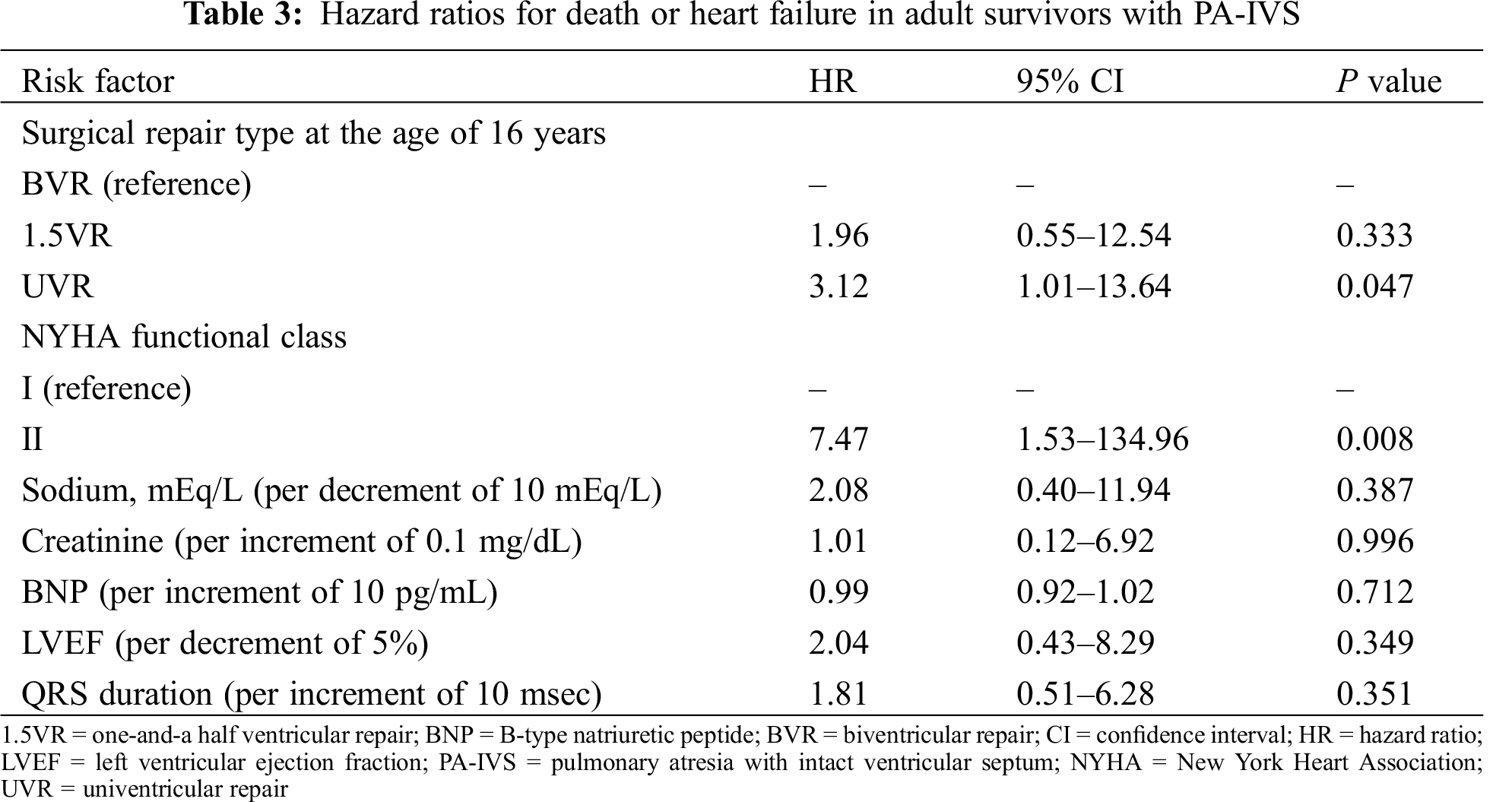
Heart failure occurred in 4 patients who underwent BVR, in 1 patient who underwent 1.5VR, and in 2 patient who underwent UVR. Protein losing enteropathy (PLE) and subsequent heart failure was observed in 1 UVR patient with atriopulmonary connection at the age of 16 years. The cumulative heart failure-free survival rates at 5 years and 10 years were 81.4% (95% CI, 62.1–92.1) and 74.6% (95%CI, 52.3–88.7), respectively (Fig. 2B). A univariate analysis with a Cox proportional hazards model was performed to establish risk factors for death or heart failure (Tab. 3). NYHA functional status at baseline and UVR were associated with risk of death or heart failure, albeit with wide confidence intervals.
Five patients had atrial arrhythmias including atrial re-entrant tachycardia (n = 3) and atrial fibrillation (n = 2) (Tab. 2), whereas none of the patients had ventricular arrhythmia. Atrial tachycardia occurred in 2 patients who underwent BVR and in 1 patient who underwent 1.5VR. Although 1 BVR patient underwent three radiofrequency catheter ablations for atrial tachycardia, atrial arrhythmia was not completely eliminated, and a class III antiarrhythmic drug was administered. Atrial fibrillation was observed in 1 BVR patient and 1 UVR patient. The cumulative arrhythmia-free survival rates at 5 years and 10 years were 88.7% (95% CI, 70.1–96.3) and 75.9% (95% CI, 51.7–90.2), respectively (Fig. 2C).
Three patients underwent percutaneous intervention after the age of 16 years. One BVR patient underwent coil embolization to occlude aortopulmonary collateral vessels, one 1.5VR patient underwent catheter closure of fenestration, and one UVR patient underwent pulmonary artery stenting.
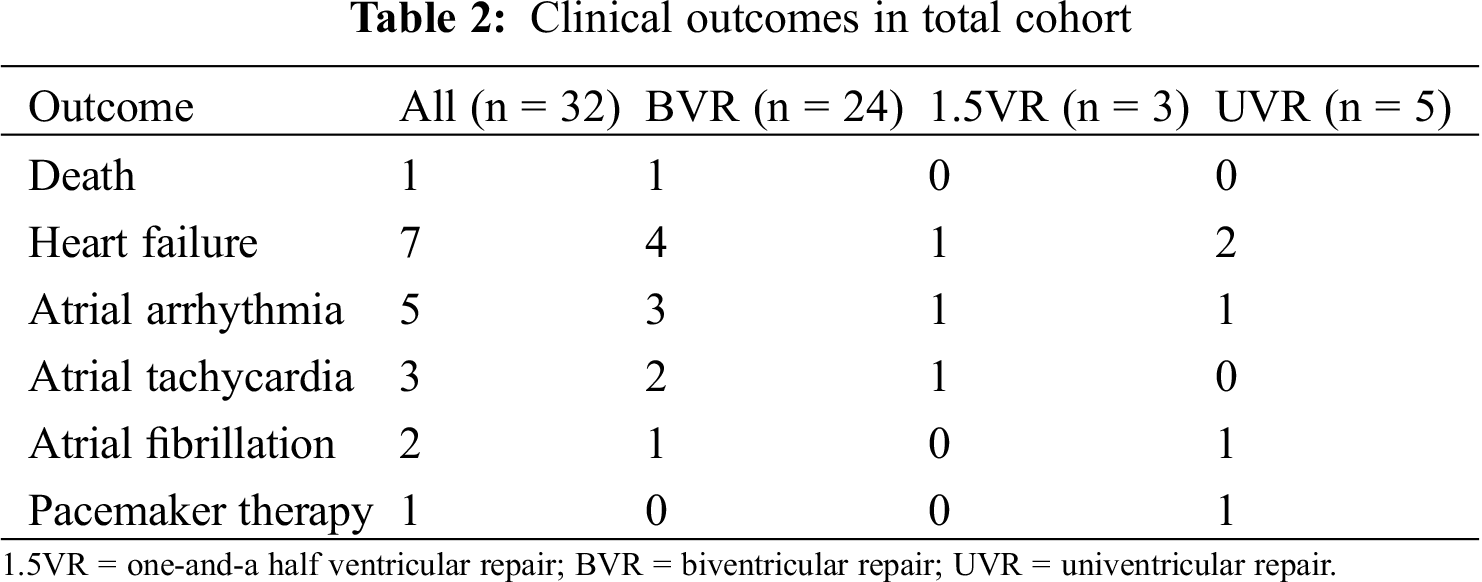
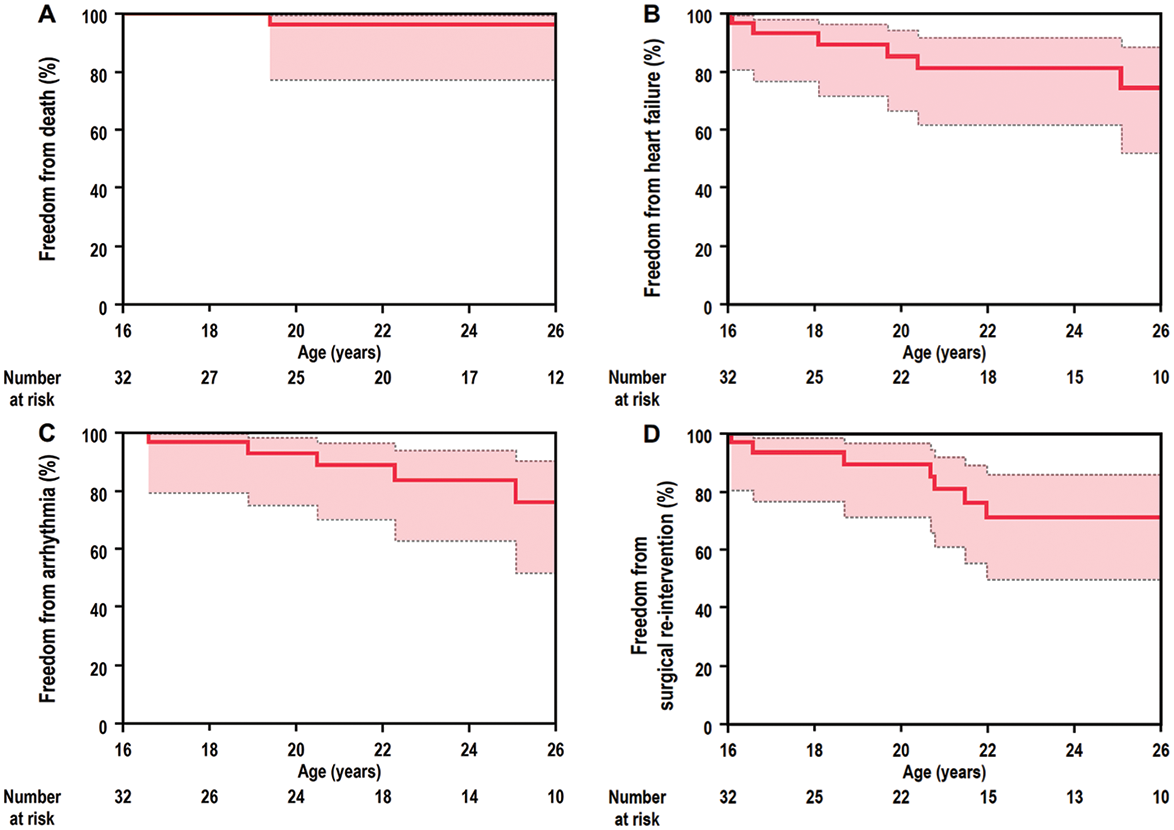
Figure 2: Rates for (A) cumulative survival, (B) heart failure-free survival, (C) arrhythmia-free survival, and (D) surgical re-intervention-free survival in 32 adult survivors of PA-IVS
In adult survivors with PA-IVS, overall survival, heart failure-free, arrhythmia-free, and surgical re-intervention-free rates at 5 years and 10 years were 96.2% (95% CI, 77.2–99.4) and 96.2% (95% CI, 77.2–99.4), 81.4% (95% CI, 62.1–92.1) and 74.6% (95%CI, 52.3–88.7), 88.7% (95% CI, 70.1–96.3) and 75.9% (95% CI, 51.7–90.2), and 80.7% (95% CI, 60.8–91.8) and 70.8% (95% CI, 49.7–85.7), respectively.
CI = confidence interval; PA-IVS = pulmonary atresia with intact ventricular septum.
Details of surgical re-interventions after the age of 16 years are shown in Fig. 3. Right atrial pressures (RAPs) in 4 BVR patients with heart failure were 10, 12, 11, and 10 mmHg, respectively. Liver ultrasound revealed an irregular and nodular liver surface and a coarse echo pattern in all of those 4 patients. Therefore, all of the BVR patients with heart failure fulfilled our institutional criteria for conversion (heart failure symptoms, significant PR, elevated RAP ≥ 10 mmHg, and ultrasound-based liver damage) and they subsequently underwent take-down of BVR and 1.5VR conversion. The median age of patients with 1.5 VR conversion was 19.7 years (IQR: 18.2–20.7 years) and the median duration between initial BVR and 1.5VR conversion was 18.6 years (IQR: 17.7–18.8 years). 1.5VR conversions were performed concomitant with right ventricular outflow tract reconstruction using valved conduit in the first and second patients, with pulmonary valve replacement using bioprosthetic valve in the third patient, and with pulmonary valve plasty in the fourth patient. After 1.5VR conversion, RAP decreased from 10, 12, 11, and 10 mmHg to 5, 8, 5, and 6 mmHg, respectively. Serum BNP levels also decreased from 55.9, 91.5, 51.0, and 125.4 pg/mL to 40.7, 58.7, 14.8, and 72.1 pg/mL, respectively. Four BVR patients with heart failure and subsequent 1.5VR conversion tended to have smaller TV z-scores than those in the other BVR patients, but the difference was not statistically significant (–2.68 ± 0.43 vs. –1.42 ± 1.30, P = 0.074).
Two BVR patients underwent tricuspid valve and pulmonary valve surgery because of significant TR and PR without elevated RAP. One patient underwent tricuspid and pulmonary valve plasty at the age of 22 years and the other patient underwent pulmonary valve replacement and tricuspid valve plasty at the age of 22 years. None of the BVR patients had heart failure or surgical re-interventions before the age of 16 years.
One 1.5VR patient had right ventricular outflow tract reconstruction using a valved conduit and tricuspid valve plasty at the age of 29 years due to frequent atrial tachycardia, significant TR and PR, and subsequent heart failure. Fontan conversion was performed in 3 of the 5 patients. The reasons for Fontan conversion were heart failure, PLE and heart failure, and right atrial thrombus, respectively. The ages at the time of the initial Fontan procedure were 7, 5, and 4 years and the ages at the time of Fontan conversion were 29, 16, and 19 years. After Fontan conversion, significant improvement in heart failure symptom was observed in the first patient, whereas PLE and heart failure symptom did not improve in the second patient.
The cumulative surgical re-intervention-free survival rates at 5 years and 10 years were 80.7% (95% CI, 60.8–91.8) and 70.8% (95% CI, 49.7–85.7), respectively (Fig. 2D).
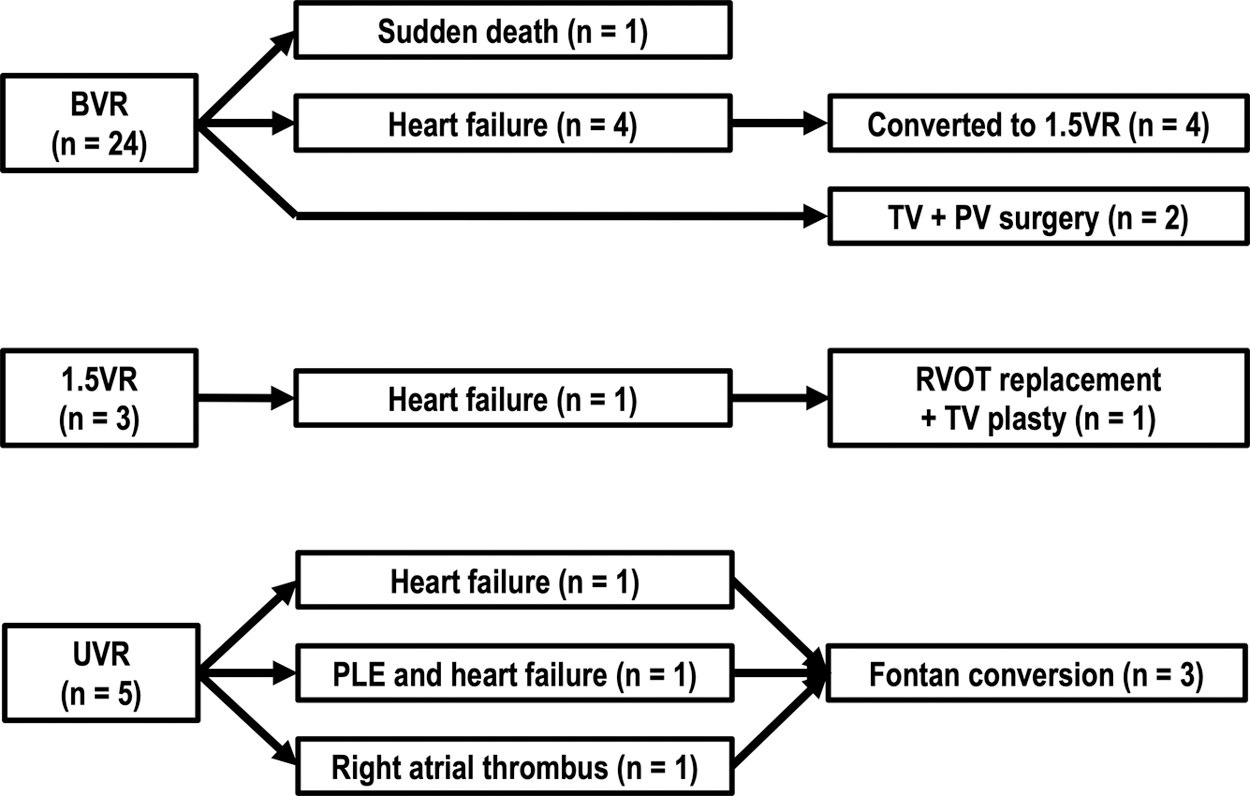
Figure 3: Summary of clinical courses in adult survivors with definitive repair for PA-IVS
Of 24 BVR patients, 4 patients underwent take-down of BVR and 1.5VR conversion. Of 3 1.5VR patients, 1 patient underwent RVOT replacement using a valved conduit and TV plasty. Of 5 UVR patients, 3 patients underwent Fontan conversion.
1.5VR = one-and-a half ventricular repair; BVR = biventricular repair; PA-IVS = pulmonary atresia with intact ventricular septum; PLE = protein losing enteropathy; PV = pulmonary valve; RVOT = right ventricular outflow tract; TV = tricuspid valve; UVR = univentricular repair.
The clinical courses and outcomes in adult survivors with PA-IVS were shown in this study. Since PA-IVS is an extremely rare congenital heart defect and has great morphological heterogeneity, various surgical repairs have been performed and thus there are limited data on long-term outcome of PA-IVS according to the type of surgical approach. In our patient population, BVR was the most common initial definitive repair, accounting for 75% of the study population, because of our institutional bias towards BVR. Among the 32 late survivors, 1 BVR patient died suddenly and 7 patients had admission for heart failure during the study period. Surgical re-interventions were performed in 10 patients including all of the 7 patients with heart failure.
4.1 Type of Repair and Clinical Outcome
Although the surgical outcomes of children with PA-IVS have been improved over the past decade with 10-year survival ranging from 80% to 94% [3–8], there is insufficient information on the prognosis of adult survivors with PA-IVS. Additionally, differences in long-term outcomes depending on the type of surgery are still under debate. The mortality rate in our cohort was 3.1%, which is lower than that in a previous study of adults with PA-IVS [11]. In that previous study, 5 (25%) of 20 adults with PA-IVS died during the study period and the mean age at the time of death was 32 years [11]. Unlike our data, more than half of the patients underwent UVR or univentricular palliation, which was reported to be associated with high mortality. On the other hand, another study that included fewer UVR patients than those in the aforementioned study showed a mortality rate (4.1%) [12] similar to that in our study. Regarding the mortality in BVR patients, in a study that included children who underwent BVR between 1965 to 1998, 20 (34%) of 58 BVR patients died, but the mortality rate was significantly higher in patients who underwent operations during the early era than that in patients who underwent operations during the late era [7]. In addition, in a study in which clinical features of PA-IVS patients after BVR were investigated, 2 (9%) of 23 patients died within 5 years after BVR, but no patients died after the age of 15 years [5]. Similarly, in a study on outcomes in PA-IVS, 1 (3.7%) of 27 BVR patients died at the age of 10 months [8]. Therefore, the mortality rate for adult survivors who have undergone BVR is considered to be acceptable.
The mortality rate for adults with PA-IVS after UVR is also uncertain. In a previous study, 2 out of 5 UVR patients died due to sepsis at the age of 31 years and due to sudden cardiac arrest after Fontan revision at the age of 32 years [11]. In contrast to that study, in our cohort, one UVR patient died at the age of 4 years, but no UVR patients died after the age of 16 years. Similarly, in several prior studies, most of the deaths in PA-IVS patients after UVR occurred during childhood [14–17].
The prevalence of heart failure in adult survivors with repaired PA-IVS is unknown. In our population, heart failure occurred in 7 (22%) of the 32 patients. In a previous study, the proportion of patients with admission for heart failure was 4.4% in adults with repaired pulmonary atresia during a median follow-up period of 8.8 years [12]. However, patients with various types of pulmonary atresia including patients with and those without an intact ventricular septum were enrolled in that study. In addition, although the most frequent reason for hospital admission in that study was invasive interventions, which were performed in 26% of the study population, the reason for surgical or percutaneous intervention was not stated in the report [12]. Therefore, heart failure might be more prevalent than expected. In adult patients with heart failure with or without congenital heart disease, baseline NYHA functional class, serum sodium, creatinine, and BNP levels, LVEF by echocardiography, and QRS duration on an ECG are well-established risk factors for worsening heart failure [18–20]. Among these risk factors, NYHA functional class at the initial visit after the age of 16 years was shown to be associated with risk of death or heart failure in the present study. UVR patients also had >3-fold risk (HR, 3.12) of death or heart failure compared with BVR patients. However, the number of UVR patients was small in the current study and thus further studies are required to draw a definitive conclusion. In our institution, although BVR was selected for patients with a TV z-score of larger than –3, BVR patients with heart failure and subsequent 1.5VR conversion had relatively small TV z-scores. The present study has proved that our early surgical strategy resulted in preserved long-term survival regardless of the early surgical repair, but further investigations are required to determine the optimal surgical strategy in patients with PA-IVS.
Atrial tachyarrhythmias were observed in 5 (16%) of the 32 patients in our study. Although atrial tachyarrhythmia is the most common arrhythmia in adults with repaired PA-IVS [5,11], the incidence in the present study was low compared with that in a previous study [11], probably due to the large percentage of patients who underwent BVR in our cohort.
4.2 Strategy for Surgical Re-Intervention after BVR
Based on published evidence from our institution [2,9,10], our institutional policy for PA-IVS management has aimed for BVR. On the other hand, restrictive RV physiology after BVR is not uncommon [21,22] and it is associated with elevated end-diastolic RV pressure, right atrial dysfunction [23], and increased central venous pressure followed by end organ damage, including liver congestion. The duration between the BVR procedure and manifestation of restrictive RV physiology is uncertain. In a previous study [24], conversion of BVR to 1.5VR was performed at 3.6 to 7.1 years of age, which was much younger than that in our cohort. Although there are no definitive criteria for take-down to 1.5VR from BVR, our institutional criteria for conversion include heart failure symptoms, significant PR, elevated RAP, and liver damage confirmed by ultrasound, and these criteria are consistent with previous reports [2,24,25]. However, reversed hepatic flow during atrial contraction and diastolic liver expansion was observed in a patient with PA-IVS after 1.5VR in a previous case report [26], and therefore long-term outcomes after 1.5VR conversion should be investigated.
There are some limitations in this study. First, since this study was a retrospective analysis of a single center’s experience of adult patients with repaired PA-IVS, which is an extremely rare congenital heart defect, the number of patients in each surgical group was small. Therefore, the number of patients in our cohort was not sufficient to represent all adult PA-IVS survivors of various ages, anatomies, and surgical histories. Second, since we aimed to focus on adult PA-IVS patients and only patients aged ≥16 years were reviewed in the present study, we could not avoid potential immortal time bias. Third, not all of the data for arrhythmic events were obtained because arrhythmic data were reviewed from medical records and thus unrecorded or asymptomatic arrhythmias were not included. Fourth, the decision for surgical re-intervention was made on the basis of a single-center policy. There are limited reports on detailed indication for conversion from BVR to 1.5VR in patients with PA-IVS, and our institutional criteria are thus based on previous reports [24,25] and on our accumulated experiences [2]. Fifth, we did not assess cardiopulmonary exercise test results, RV volume, or RV function because sufficient data for accurate analysis were not available at the time of data collection. In previous studies [27,28], decreased exercise capacity and RV function were observed late after definitive surgery regardless of the type of repair, and these parameters would thus be helpful for risk stratification in adults with PA-IVS. Sixth, although echocardiographic data and reports were reviewed to assess LVEF and valvular heart disease, sufficient data for accurate evaluation of RV function were not available due to the lack of RV-focused images, tricuspid annular plane systolic excursion, systolic velocity of tricuspid annulus from pulsed tissue Doppler, and other echocardiographic parameters for the assessment of RV function [29].
In our cohort, the mortality rate for adult survivors with PA-IVS was low compared with the mortality rates in previous reports, though our surgical strategy has bias towards BVR and the study sample size and study duration were limited. On the other hand, adult survivors with PA-IVS are at risk for heart failure, arrhythmic events, and surgical re-intervention. Therefore, detailed and continued follow-up at a specialized center is essential for all PA-IVS patients from childhood to adulthood.
Data Availability Statement: Data are available on reasonable request.
Author Contributions: NT and YK contributed to the research conception and design, data analysis and interpretation, manuscript drafting, and critical revision of the manuscript. TA is the guarantor of the study. YK and KB contributed to data acquisition, data interpretation, manuscript drafting and critical revision of the manuscript. SO, SK, and HI contributed to critical revision of the manuscript. All authors had full access to all of the statistical analyses, graphs and tables in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement: The author(s) received no specific funding for this study.
Conflict of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Nykamen, D. G. (2016). Pulmonary atresia and intact ventricular septum. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents. 9th ed. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
2. Kotani, Y., Kasahara, S., Fujii, Y., Eitoku, T., Sano, S. (2016). A staged decompression of right ventricle allows growth of right ventricle and subsequent biventricular repair in patients with pulmonary atresia and intact ventricular septum. European Journal of Cardio-Thoracic Surgery, 50(2), 298–303. DOI 10.1093/ejcts/ezw124. [Google Scholar] [CrossRef]
3. Odim, J., Laks, H., Plunkett, M. D., Tung, T. C. (2006). Successful management of patients with pulmonary atresia with intact ventricular septum using a three tier grading system for right ventricular hypoplasia. Annals of Thoracic Surgery, 81(2), 678–684. DOI 10.1016/j.athoracsur.2005.07.060. [Google Scholar] [CrossRef]
4. Liava’a, M., Brooks, P., Konstantinov, I., Brizard, C., d’Udekem, Y. (2011). Changing trends in the management of pulmonary atresia with intact ventricular septum: the Melbourne experience. European Journal of Cardio-Thoracic Surgery, 40(6), 1406–1411. DOI 10.1016/j.ejcts.2011.02.036. [Google Scholar] [CrossRef]
5. Hoashi, T., Kagisaki, K., Kitano, M., Kurosaki, K., Shiraishi, I. et al. (2012). Late clinical features of patients with pulmonary atresia or critical pulmonary stenosis with intact ventricular septum after biventricular repair. Annals of Thoracic Surgery, 94(3), 833–841. DOI 10.1016/j.athoracsur.2012.04.071. [Google Scholar] [CrossRef]
6. Ashburn, D. A., Blackstone, E. H., Wells, W. J., Jonas, R. A., Pigula, F. A. et al. (2004). Determinants of mortality and type of repair in neonates with pulmonary atresia and intact ventricular septum. Journal of Thoracic and Cardiovascular Surgery, 127(4), 1000–1007. DOI 10.1016/j.jtcvs.2003.11.057. [Google Scholar] [CrossRef]
7. Dyamenahalli, U., Mccrindle, B. W., Mcdonald, C., Trivedi, K. R., Freedom, R. M. (2004). Pulmonary atresia with intact ventricular septum: management of, and outcomes for, a cohort of 210 consecutive patients. Cardiology in the Young, 14(3), 299–308. DOI 10.1017/s1047951104003087. [Google Scholar] [CrossRef]
8. Schneider, A. W., Blom, N. A., Bruggemans, E. F., Hazekamp, M. G. (2014). More than 25 years of experience in managing pulmonary atresia with intact ventricular septum. Annals of Thoracic Surgery, 98(5), 1680–1686. DOI 10.1016/j.athoracsur.2014.05.085. [Google Scholar] [CrossRef]
9. Sano, S., Ishino, K., Kawada, M., Fujisawa, E., Kamada, M. et al. (2000). Staged biventricular repair of pulmonary atresia or stenosis with intact ventricular septum. Annals of Thoracic Surgery, 70(5), 1501–1506. DOI 10.1016/s0003-4975(00)01974-3. [Google Scholar] [CrossRef]
10. Huang, S. C., Ishino, K., Kasahara, S., Yoshizumi, K., Kotani, Y. et al. (2009). The potential of disproportionate growth of tricuspid valve after decompression of the right ventricle in patients with pulmonary atresia and intact ventricular septa. Journal of Thoracic and Cardiovascular Surgery, 138(5), 1160–1166. DOI 10.1016/j.jtcvs.2009.05.015. [Google Scholar] [CrossRef]
11. John, A. S., Warnes, C. A. (2012). Clinical outcomes of adult survivors of pulmonary atresia with intact ventricular septum. International Journal of Cardiology, 161(1), 13–17. DOI 10.1016/j.ijcard.2011.04.026. [Google Scholar] [CrossRef]
12. Montanaro, C., Merola, A., Kempny, A., Alvarez-Alvarez, B., Alonso-Gonzalez, R. (2019). The outcome of adults born with pulmonary atresia: high morbidity and mortality irrespective of repair. International Journal of Cardiology, 280, 61–66. DOI 10.1016/j.ijcard.2018.11.011. [Google Scholar] [CrossRef]
13. Zilberman, M. V., Khoury, P. R., Kimball, R. T. (2005). Two-dimensional echocardiographic valve measurements in healthy children: gender-specific differences. Pediatric Cardiology, 26(4), 356–360. DOI 10.1007/s00246-004-0736-z. [Google Scholar] [CrossRef]
14. Mair, D. D., Julsrud, P. R., Puga, F. J., Danielson, G. K. (1997). The Fontan procedure for pulmonary atresia with intact ventricular septum: operative and late results. Journal of the American College of Cardiology, 29(6), 1359–1364. DOI 10.1016/s0735-1097(97)00051-x. [Google Scholar] [CrossRef]
15. Zheng, J., Gao, B., Zhu, Z., Shi, G., Xu, Z. et al. (2016). Surgical results for pulmonary atresia with intact ventricular septum: a single-centre 15-year experience and medium-term follow-up. European Journal of Cardio-Thoracic Surgery, 50(6), 1083–1088. DOI 10.1093/ejcts/ezw226. [Google Scholar] [CrossRef]
16. Elias, P., Poh, C. L., Du Plessis, K., Zannino, D., Rice, K. et al. (2018). Long-term outcomes of single-ventricle palliation for pulmonary atresia with intact ventricular septum: fontan survivors remain at risk of late myocardial ischaemia and death. European Journal of Cardio-Thoracic Surgery, 53(6), 1230–1236. DOI 10.1093/ejcts/ezy038. [Google Scholar] [CrossRef]
17. Rychik, J., Levy, H., Gaynor, J. W., De Campli, W. M., Spray, T. L. (1998). Outcome after operations for pulmonary atresia with intact ventricular septum. Journal of Thoracic and Cardiovascular Surgery, 116(6), 924–931. DOI 10.1016/s0022-5223(98)70042-x. [Google Scholar] [CrossRef]
18. Alexander, V. D. B., Hickey, E. J., Kovacs, A. H., Crean, A. M., Wald, R. M. et al. (2017). Phenotype, management and preditors of outcome in a large cohort of adult congenital heart disease patients with heart failure. International Journal of Cardiology, 252, 80–87. DOI 10.1016/j.ijcard.2017.10.086. [Google Scholar] [CrossRef]
19. Butler, J., Yang, M., Manzi, M. A., Hess, G. P., Patel, M. J. et al. (2019). Clinical course of patients with worsening heart failure with reduced ejection fraction. Journal of the American College of Cardiology, 73(8), 935–944. DOI 10.1016/j.jacc.2018.11.049. [Google Scholar] [CrossRef]
20. Wang, N. C., Maggioni, A. P., Konstam, M. A., Krasa, H. B., Zannad, F. et al. (2008). Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA, 299(22), 2656–2666. DOI 10.1001/jama.299.22.2656. [Google Scholar] [CrossRef]
21. Redington, A. N., Penny, D., Rigby, M. L., Hayes, A. (1992). Antegrade diastolic pulmonary arterial flow as a marker of right ventricular restriction after complete repair of pulmonary atresia with intact septum and critical pulmonary valvar stenosis. Cardiology in the Young, 2(4), 382–386. DOI 10.1017/S1047951100007988. [Google Scholar] [CrossRef]
22. Liang, X. C., Lam, W. W., Cheung, E. W., Wu, A. K., Wong, S. J. et al. (2010). Restrictive right ventricular physiology and right ventricular fibrosis as assessed by cardiac magnetic resonance and exercise capacity after biventricular repair of pulmonary atresia and intact ventricular septum. Clinical Cardiology, 33(2), 104–110. DOI 10.1002/clc.20711. [Google Scholar] [CrossRef]
23. To, A. H., Lai, C. T., Wong, S. J., Cheung, Y. F. (2016). Right atrial mechanics long-term after biventricular repair of pulmonary atresia or stenosis with intact ventricular septum. Echocardiography: A Journal of Cardiovascular Ultrasound and Allied Techniques, 33(4), 586–595. DOI 10.1111/echo.13121. [Google Scholar] [CrossRef]
24. Shi, J. Z., Chow, P. C., Li, W., Kwok, S. Y., Wong, W. H. et al. (2019). Fifty-five years follow-up of 111 adult survivors after biventricular repair of PAIVS and PS. Pediatric Cardiology, 40(2), 374–383. DOI 10.1007/s00246-018-2041-2. [Google Scholar] [CrossRef]
25. He, X. M., Gao, B., Shi, G. C., Chen, H. W., Du, X. W. et al. (2018). Surgical strategy and outcomes for the delayed diagnosis of pulmonary atresia with intact ventricular septum. Journal of Cardiology, 72(1), 50–55. DOI 10.1016/j.jjcc.2017.12.009. [Google Scholar] [CrossRef]
26. Radojevic, J., Redheuil, A., Iserin, L. (2011). Pulmonary atresia with intact ventricular septum and diastolic liver expansion. Heart, 97(21), 1813–1814. DOI 10.1136/heartjnl-2011-300746. [Google Scholar] [CrossRef]
27. Karamlou, T., Poynter, J. A., Walters, H. L. 3rd, Rhodes, J., Bondarenko, I. et al. (2013). Long-term functional health status and exercise test variables for patients with pulmonary atresia with intact ventricular septum: A congenital heart surgeons society study. Journal of Thoracic and Cardiovascular Surgery, 145(4), 1018–1027. DOI 10.1016/j.jtcvs.2012.11.092. [Google Scholar] [CrossRef]
28. Romeih, S., Groenink, M., van der Plas, M. N., Blom, N. A., Mulder, B. J. et al. (2012). Effect of age on exercise capacity and cardiac reserve in patients with pulmonary atresia with intact ventricular septum after biventricular repair. European Journal of Cardio-Thoracic Surgery, 42(1), 50–55. DOI 10.1093/ejcts/ezr267. [Google Scholar] [CrossRef]
29. Harjola, V. P., Mebazaa, A., Elutkien, J., Bettex, D., Bueno, H. et al. (2016). Contemporary management of acute right ventricular failure: A statement from the heart failure association and the working group on pulmonary circulation and right ventricular function of the European society of cardiology. European Journal of Heart Failure, 18(3), 226–241. DOI 10.1002/ejhf.478. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |