 Open Access
Open Access
ARTICLE
The Anti-Senescence Effect and Mechanism of 17β-Estradiol on Pelvic Organ Prolapse Derived Fibroblasts
1 Department of Otolaryngology Head and Neck Surgery/Deep Underground Space Medical Center, West China Hospital, Sichuan University, Chengdu, 610064, China
2 West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu, 610064, China
3 Department of Obstetrics and Gynecology, Key Laboratory of Birth Defects and Related Diseases of Women and Children of MOE, West China Second University Hospital, Sichuan University, Chengdu, 610064, China
* Corresponding Authors: Yali Miao. Email: ; Jiang Wu. Email:
# These two authors contributed equally to this work and shared the first authorship
BIOCELL 2025, 49(2), 335-348. https://doi.org/10.32604/biocell.2025.059573
Received 11 October 2024; Accepted 13 January 2025; Issue published 28 February 2025
Abstract
Objectives: Recently, pre-/post-operative Local Estrogen Therapy (LET) has shown effectiveness in alleviating Pelvic Organ Prolapse (POP) symptoms in clinical therapy. However, there is a lack of scientific evidence to support these claims. Therefore, we aimed to explore the anti-senescence effects and mechanisms of 17β-estradiol (E2) on POP-derived fibroblasts. Methods: The primary fibroblast cells were isolated and cultured from the surgical samples of postmenopausal women clinically diagnosed with pelvic organ prolapse (POP) at stages III-IV (quantified using the POP-Q system) and without any other treatment within 6 months. (n = 12, age 50–75). Colorimetric Cell Counting Kit (CCK-8) assay and Senescence-Associated-β-Galactosidase (SA-β-Gal) staining were used to test the cell proliferative capacity and the senescence rate. Western blotting (WB) was used to detect the expression of Collagen Type I (COL-I), Collagen Type III (COL-III), Cyclin-dependent kinase 4 inhibitor A (p16INK4a), Cyclin-dependent kinase inhibitor 1A (p21), Tumor Protein 53 (p53), Sirtuin 1 (SIRT-1) and Microtubule-associated protein 1A/1B-light chain 3-I/II (LC3-I/II) protein. A transmission Electron Microscope (TEM) was used to observe the ultrastructure of fibroblasts. Results: The results showed that E2 significantly promoted the proliferation of fibroblasts derived from POP and reduced the staining rate of SA-β-Gal. It markedly enhanced the extracellular matrix proteins COL-I and COL-III, accompanied by inhibition of the senescent maker p16INK4a. Additionally, our results improved the cells’ autophagy and metabolic activity. Additionally, our results indicate the anti-senescence mechanism of E2 through the mediated SIRT-1/p53/p21 axis pathway. Conclusion: We provide preliminary evidence for the anti-aging effects and mechanisms of E2 on POP, hoping to provide a theoretical basis for estrogen against POP senescence and guide the clinical application and local administration of estrogen in POP treatment.Keywords
Supplementary Material
Supplementary Material FilePelvic Organ Prolapse (POP) is one of the common diseases of middle-aged and elderly women, mainly manifested as uterine prolapse, anterior and posterior vaginal wall uterine prolapse, urinary retention, and sexual dysfunction, which seriously affected the life quality of women [1]. It is estimated that the prevalence of at least one symptom of pelvic floor dysfunction among women of reproductive age is 25% in the United States [2], 40% among women in Spanish [3], and more than 64% among nurses in Japan [4]. At present, the causes of POP are not entirely understood but may be multifactorial, including increased age, vaginal delivery, parity, decreased estrogen levels, high body mass index (BMI), increased intra-abdominal pressure, and genetic factors [5].
Currently, 17β-estradiol (E2) has been widely used in clinical local estrogen therapy (LET) before and after POP surgery. It is reported that pre-operatively vaginal estrogen application for 4–6 weeks improved the matrix restoration and the integrity maintenance of pelvic floor connective tissues through increased collagen synthesis and enhanced blood circulation [6]. Moreover, post-operative estrogen treatment could reduce the incidence and severity of urinary frequency and urgency without obvious adverse events [7]. Although LET is effective in alleviating POP symptoms after surgery, the duration, optimal dosage, long-term effects, and cost-effectiveness of LET are still unclearly [8].
Estrogen’s anti-senescence role is not only widely used in clinical, but also widely studied at the cell and animal level. Dermal aging accelerates immediately after menopause owing to the lack of estrogen [9]. E2 also regulates bone development and metabolism, promoting cell growth and differentiation to resist age-related bone resorption and stimulate bone formation [10]. Recently, evidence suggests that E2-mediated activation of Sirtuin 1 (SIRT-1), contributes to the anti-aging of the vascular system and the repair of neurodegenerative diseases by regulating endothelial nitric oxide synthase (eNOS) activation, autophagy, oxidative stress, inflammation, and DNA damage [11]. Animal studies have proved that SIRT-1 has a protective effect on vascular endothelial cells and smooth muscle cells. Besides, E2 promotes autophagy to inhibit apoptosis in osteoblast and chondrocytes through induced upregulation of SIRT-1 mediated by the AMP-activated protein kinase (AMPK)/mammalian target of the rapamycin (mTOR) pathway [12]. However, the relationship between estrogen and SIRT-1 in the background of menopause-induced POP development remains unclear, the relative anti-senescence mechanism of estrogen on POP has not been reported yet.
With the extension of human longevity, women live in a long stage of estrogen-deficient state after menopause, and the ovarian steroid hormone E2 deficiency results in a series of age-related diseases especially the pelvic floor organ prolapse (POP) [13]. Pelvic organs generally contain estradiol receptors α and β (ERα/β), which are very sensitive to estrogen levels. Decreased estrogen levels may have a significant effect on reproductive organs [14]. In addition, estrogen profoundly affects the synthesis and metabolism of the components of pelvic connective tissue. Atrophy of these tissues would weaken the capacity of the pelvic floor muscles, connective tissues, and ligaments, ultimately leading to POP [15].
Although estrogen is generally used in POP clinical treatment, however, the certain effects and mechanisms of estrogen against POP senescence have not been reported yet, and patients are still very cautious about estrogen therapy. Therefore, we aimed to provide preliminary evidence of the anti-aging effect and mechanism of estrogen on POP fibroblasts, hoping to provide a theoretical basis for the study of estrogen against POP senescence and guide the clinical application and local administration of estrogen treatment for POP.
All vaginal anterior wall prolapse samples (n = 12, age from 50–75) were obtained from the postmenopausal women who were clinically diagnosed with POP-Q III-IV stage of prolapse and without any other treatment within 6 months. All samples were obtained from the surgical patients at the department of obstetrics and gynecology department, West China Second University Hospital (Chengdu, China). This study was approved by the Ethics Committee of Sichuan University (approval number: 2024025), Patients consented to an informed consent process that was reviewed by the Ethics Committee of Sichuan University and certified that the study was performed by the ethical standards as laid down in the 1964 Declaration of Helsinki.
2.2 Pelvic Floor Fibroblast Isolation and Culture
The obtained surgical sample was immediately cut into small pieces, digested with type-I collagenase (Sigma, C0130, Shanghai, China) at a concentration of 1 mg/mL, placed in a 37°C water bath shaker (Crystal Technology & Industries, Inc., SY-2230, Dallas, TX, USA) at 150 rpm for 2 h, and then passed through 70 and 40 μm cell filters filtration in sequence, the obtained single cell suspension were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Hyclone, SH30022.01B, Shanghai, China) supplemented with 15% fetal bovine serum (FBS) (Gibco, A5256701, Shanghai, China) and 1% penicillin-streptomycin (P/S) (Hyclone, SV30010, Shanghai, China), and all cell cultured in a humidified incubator (Heraeus, BB15, Germany) with 5% CO2 saturation at 37°C, the medium was changed for every 3 days, the first passage cell confluence at 80% takes about 15 days and can be sub-cultured for further test, and the cells used in our experiment in the third or fourth passage. All primary cells were free from mycoplasma contamination during the culture process.
2.3 Grouping and Administration
To obtain the best administration concentration of 17β-estradiol (E2) (Solarbio, IE0210, Beijing, China), we tested the effect of different concentrations (range from 10−5–10−10 mol/L) of E2 on pelvic floor fibroblast proliferation at 12, 24 and 48 h by Colorimetric Cell Counting Kit (CCK-8) (Beyotime, C0037, Shanghai, China) assay, according to the results, the best administration concentration of E2 is 10−7 mol/L. For further study, cells treated with equal amounts of Dimethyl sulfoxide (DMSO) (Solarbio, D8371, Beijing, China) (Control group), 10−7 mol/L of E2 (Experiment group), and 10−8 mol/L of rapamycin (Rapa) (Solarbio, R8140, Beijing, China) (Positive control group), respectively [16]. Besides, for measuring “autophagy flux”, cells are treated with lysosomotropic reagents chloroquine (CQ) (Solarbio, IC4440, Beijing, China) at the concentration of 40 μmol/L [17].
Colorimetric Cell Counting Kit was used to test the cell proliferative capacity. The cells were seeded at a density of 1 × 104 cells/well into the 96-well plate, and 10 μL CCK-8 reaction solution was added to each well after administration for 12, 24, and 48 h and incubated for 2 h. The microplate reader (S/N 601-1034, Ortenberg, Germany) was used to measure the absorbance at 450 nm. The final absorbance value is calculated from the absorbance value of the test well minus the absorbance value of the reagent background.
2.5 Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
The senescence of the pelvic floor fibroblasts was evaluated by an SA-β-Gal staining kit (Beyotime, G1580, Shanghai, China). The cells were seeded at a density of 1 × 105 cells/well on a coverslip, when the cell confluence at 80%–90%, fixed with 4% paraformaldehyde (Beyotime, P1110, Shanghai, China) for 15 min, and washed 3 times with phosphate buffered solution (PBS) (Beyotime, P1020, Shanghai, China), finally, incubated the cells with SA-β-Gal reaction solution at 37°C for 12 h according to the manufacturer’s protocol, then washed three times with double-steaming water, SA-β-Gal positive cells were counted under the optical microscope (Nikon, ECLIPSE TS100-F, DS-U3, Tokyo, Japan).
2.6 Western Blotting (WB) Analysis
RIPA (Beyotime, P0038, Shanghai, China) buffer was used to lyse the cells and extract total protein, and the BCA assay kit (Beyotime, P0010S, Shanghai, China) was used to determine the protein concentration. WB was used to measure protein expression. The total protein equivalent (30 μg) of each sample was separated by 10% sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE) (Invitrogen Life Technologies, Inc., NP0302, Carlsbad, CA, USA) and polyvinylidene fluoride (PVDF) (Invitrogen Life Technologies, Inc., IPVH00010, USA) membranes in the membrane transfer system. Then, the membrane was placed in 5% fat-free milk and sealed at room temperature for 2 h. After washing with Tris Buffer Saline-Tween 20 (TBST) (Sigma-Aldrich, T9039, Saint Louis, MO, USA), Followed by incubated with primary antibodies against p16INK4A, p21, p-53 and SIRT-1 (1:3000 dilution, Cell Signaling Technology, Inc., 18769T, 2947T, 9282T, 8469, Danvers, MA, USA), COL-I & COL-III (1:3000 dilution, Proteintech, Inc., 14695-1-AP, 22734-1-AP, Rosemont, IL, USA), LC3-I/II and GAPDH (1:3000 and 1:5000 dilution, Signalway Antibody, Inc., 29357, 37985-1, Shanghai, China) at 4°C overnight, and then incubated with anti-rabbit IgG (HRP) (1:3000 dilution, Cell Signaling Technology, 7074, USA) or anti-mouse IgG (HRP) (1:3000 dilution, Sigma-Aldrich, A4416, USA) at room temperature for 1 h. The membranes were visualized in Molecular Image® ChemiDocTM XRS+ system (Bio-Rad Inc., ChemiDoc, 1708265, Boulder, CO, USA) with Image LabTM Software and analyzed by Image J 1.44p software (National Institutes of Health, Version 1.44p, Bethesda, MD, USA).
2.7 Ultrastructure of Fibroblasts Observed under Transmission Electron Microscope (TEM)
Take the logarithmic growth phase fibroblasts, expand the culture, and collect cells, so that the total number of cells reaches 2 × 107, washing with PBS and centrifuge (Anhui USTC Zonkia Scientific Instruments Co., Ltd., SC-3614, Hefei, China) at 1000 rpm for 5 min, then resuspend the cells with 1:6 diluted fixating solution (3% glutaraldehyde: PBS) and stand at 4°C for 5 min, after that, centrifuge at 10000 rpm for 15 min, discard the supernatant, and then fix with 3% glutaraldehyde at 4°C for 2 h. after dehydration, immersion, embedding, slicing and staining, the cells are observed under a transmission electron microscope (TEM) (Hitachi, HT7800, Tokyo, Japan).
Results were analyzed by GraphPad Software (GraphPad Prism 9.0, San Diego, CA, USA). The values are expressed as mean ± SEM in at least three independent experiments performed. The data were analyzed using one-way analysis of variance (ANOVA) and post-hoc analysis, A p-value of less than 0.05 was considered statistically significant (*p < 0.05, **p < 0.01), and the significance was calculated by comparing the controls with experimental groups.
3.1 The Effect of E2 on Human Pelvic Floor Fibroblast Proliferation
The isolated primary cultured fibroblasts of the human vaginal anterior wall generally need about 15 days to reach 80%–90% confluence, the growth process of these primary fibroblasts is shown in Fig. S1. We performed cellular immunocytochemistry to identify the isolated and cultured fibroblasts. Fig. S2 showed that the specific structural proteins of fibroblasts, including vimentin and α-smooth muscle protein (α-SMA), are stained as brown granular substances in the cytoplasm, and epithelial cell markers Cytokeratin staining was negative, Indicating the isolated cells were verified as fibroblasts.
As shown in Fig. 1A, the absorbance of primary fibroblasts after being treated with E2 for 48 h was higher than that of 12 and 24 h, indicating that E2 treatment for 48 h has a stronger ability to promote the proliferation of fibroblasts. In addition, the absorbance value of fibroblasts increased with the E2 concentration until 10−7 mol/L in each treated time point, after that, gradually decreased, and the absorbance value had a significant difference between the concentration of 10−7 mol/L and control (*p < 0.05). Therefore, we choose E2 treatment for 48 h and a concentration of 10−7 mol/L for further research. We further illustrated the proliferation ability of E2 and found that E2 can significantly enhance the proliferation of fibroblasts (*p < 0.05), however, rapamycin as a positive control seems to not affect cell proliferation, as shown in Fig. 1B.
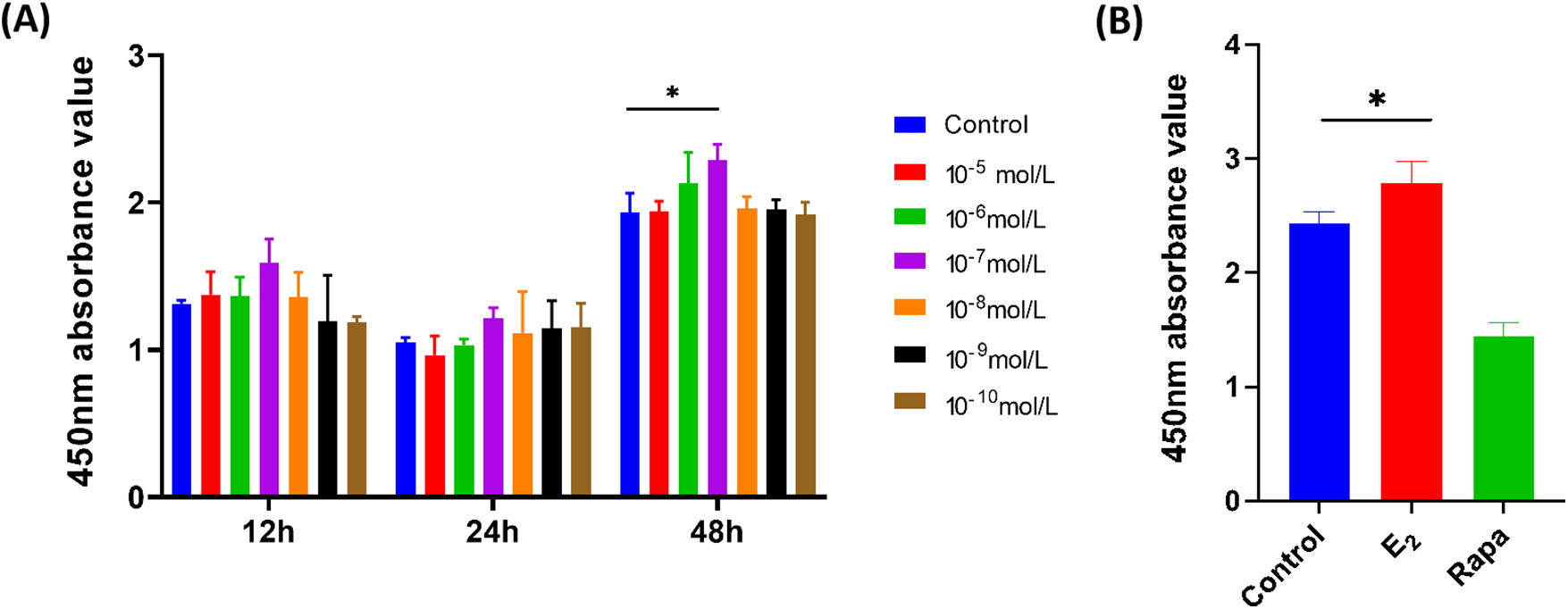
Figure 1: The effect of E2 on human pelvic floor fibroblast proliferation. (A) Selection of estrogen treatment time and concentration (x ± s, n = 3); *p < 0.05, E2 vs. control group. (B) The effect of E2 on fibroblast proliferation (x ± s, n = 3); *p < 0.05, E2 vs. control group. Note: E2: 17β-estradiol, Rapa: Rapamycin
3.2 SA-β-Gal of Human Pelvic Floor Fibroblasts
The SA-β-gal staining was tested to detect the anti-aging effect of E2. As shown in Fig. 2A, the cells stained blue are SA-β-gal staining positive cells. Compared with the control, estrogen administration significantly decreased the number of positive cells, and there is no significant difference between the E2 and rapamycin groups. Fig. 2B shows the statistical results of the staining positive rate, the staining rate of the E2 group was significantly reduced by 3.9% compared with the control (**p < 0.01), the staining results of all samples are shown in Fig. S3, indicating that E2 had a significant anti-aging effect.
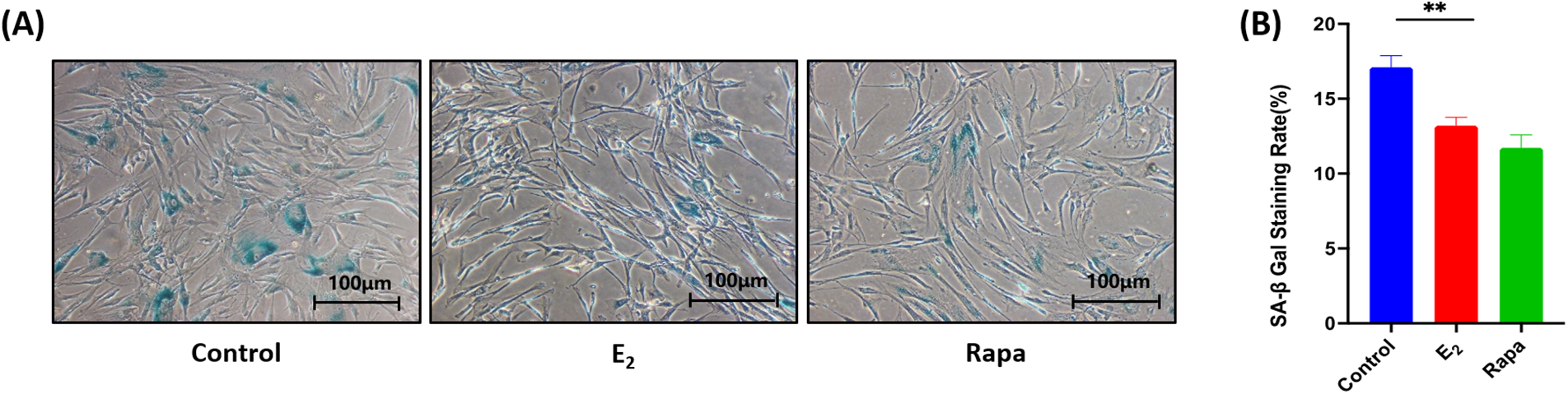
Figure 2: SA-β-gal of human pelvic floor fibroblasts ×100. (A) SA-β-Gal staining of fibroblasts (B) Statistical plots of the rate of positive SA-β-Gal staining cells (x ± s, n = 6); **p < 0.01, E2 vs. control group. Note: E2: 17β-estradiol, Rapa: Rapamycin, SA-β-Gal: Senescence-Associated-β-Galactosidase
3.3 The Expression of Senescence-Related Function and Marker Proteins of Human Pelvic Floor Fibroblasts
Collagen fibers as the main component of the ligament tissue, mainly composed of type-I and III collagen. The decrease in the quantity and quality of collagen fibers and the change in the ratio of collagen subtypes would result in the flabby of the pelvic floor tissue, ultimately leading to pelvic floor organ prolapse. The senescent cells usually highly expressed the senescence marker protein p16INK4a, indicating that p16INK4a plays a crucial role in regulating cell aging. As shown in Fig. 3A,B, our results showed that the expression level of COL-I and COL-III in the E2 treated fibroblasts are remarkedly increased by 1.88 (**p < 0.01) and 1.84 (**p < 0.01) times respectively, and the senescence marker protein p16INK4a significantly decreased by 0.66 (**p < 0.01) times compared to control, and a similar p16INK4a expression trend was also observed in the rapamycin group, indicating that E2 may improve the synthesis of functional proteins of senescent cells to remodel the Extracellular Matrix (ECM), and remit the cells senescence by reduce the aging protein expression, thereby exerting anti-senescence effects.
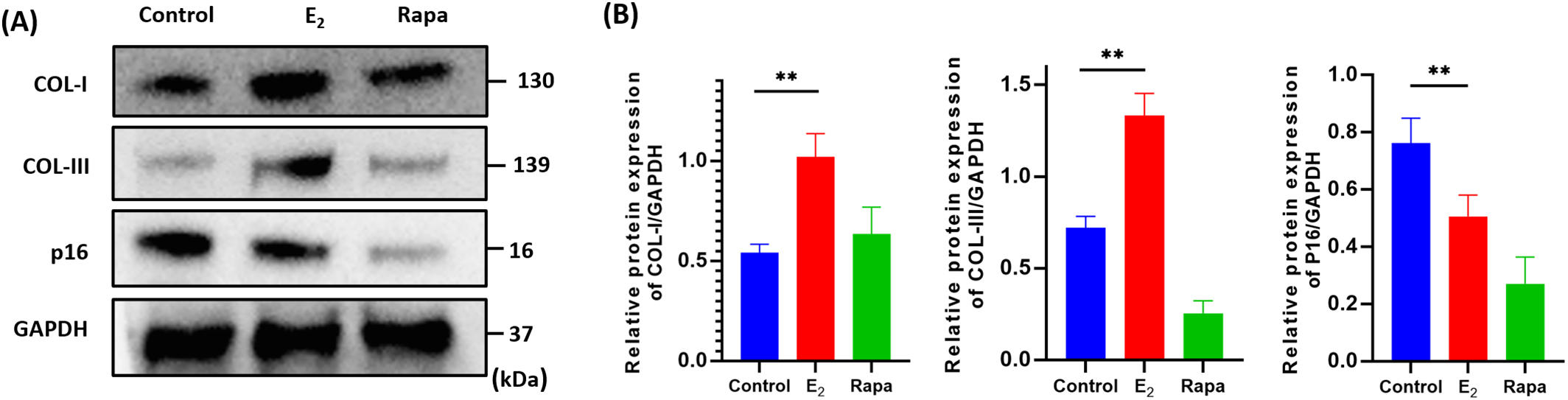
Figure 3: The expression of functional and senescence-related proteins of human pelvic floor fibroblasts. (A) The expression levels of COL-I, COL-III, p16INK4a proteins using western blots to test (x ± s, n = 3); (B) The statistical plots of detected protein (x ± s, n = 3); **p < 0.01, E2 vs. control group. Note: COL-I: Collagen Type I, COL-III: Collagen Type III, p16INK4a: Cyclin-dependent kinase 4 inhibitor A, E2: 17β-estradiol, Rapa: Rapamycin
3.4 Changes of Autophagy Flux and Ultrastructure of Pelvic Floor Fibroblasts
To obtain further insights into the anti-senescence capability of E2 on fibroblasts, we measured the cell autophagy flux-LC3 turnover rate. The difference in the ratio of LC3-II/I with and without chloroquine between the different treatment groups represents the degradation amount of LC3 delivered to the lysosome. As shown in western blot analysis (Fig. 4A), the ratio of LC3-II/I of the control and control + CQ group were 0.925 ± 0.07 and 1.293 ± 0.02 respectively, while the ratio of LC3-II/I of E2 and E2 + CQ group was increased to 1.152 ± 0.08 and 1.826 ± 0.09, respectively (Fig. 4B). The ratio of LC3-II/I in the E2 + CQ group were significantly increased compared to the control + CQ (#p < 0.05) and E2 group (*p < 0.05), the increase of the ratio which indicated an increase of autophagic flux. Indicating that E2 regulated the formation and degradation of autophagy and increased phagocytic flux to play an anti-senescence role.
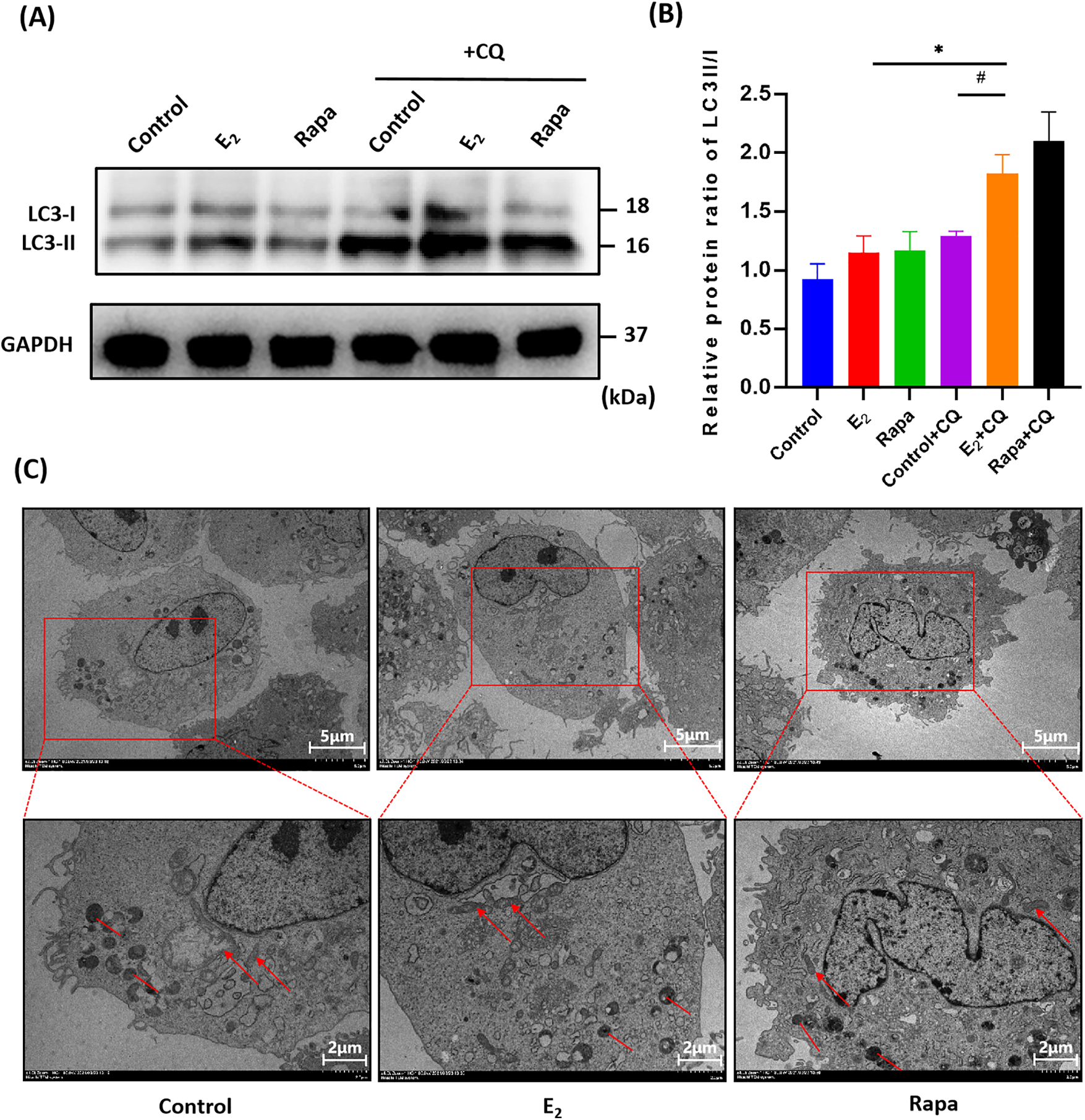
Figure 4: Changes of autophagy flux and ultrastructure of pelvic floor fibroblasts after E2 treatment. (A) the protein levels of LC3-I and LC3-II with or without chloroquine (CQ) using western blots to detect (x ± s, n = 3); (B) The statistical plots of detected proteins (x ± s, n = 3); *p < 0.05, E2 + CQ vs. E2 group; #p < 0.05, E2 + CQ vs. Control + CQ group. (C) Ultrastructure of fibroblasts observed under transmission electron microscopy at ×2000/4000 magnification. The red arrows indicate mitochondria, while the red lines represent autophagosomes. In the control group, mitochondria exhibited swelling and vacuolation, with ruptured and absent cristae (red arrows), and a limited formation of autophagosomes was observed (red lines). In the E2 treatment group, there was an increase in the number of mitochondria, a reduction in mitochondrial swelling, and a clearer appearance of cristae (red arrows), alongside an increase in the number of autophagosomes and autolysosomes (red lines). The rapamycin group presented results that were similar to those of the E2 group (x ± s, n = 3). Note: LC3-I: Microtubule-associated protein 1A/1B-light chain 3-I, LC3-II: Microtubule-associated protein 1A/1B-light chain 3-II, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, CQ: Chloroquine, E2: 17β-estradiol, Rapa: Rapamycin
The ultrastructure of fibroblasts was examined by transmission electron microscopy (TEM). As shown in Fig. 4C, in the control group, mitochondria were swollen, vacuolated, and enlarged, mitochondria cristae ruptured and disappeared, the endoplasmic reticulum expanded and a small number of autophagosomes and autolysosomes were observed. In the E2 treatment group, mitochondria quantity increased accompanied by swelling decreased, mitochondria cristae were distinct, autophagosomes and autolysosomes increased, and similar results were also observed in the rapamycin-treated group. Implying that E2 inhibits senescence by promoting the cell metabolic activity.
3.5 Changes in the SIRT-1/p53/p21 Axis in Fibroblasts after E2 Treatment
Sirtuin 1 (SIRT-1) is a histone deacetylase, which regulates cell proliferation, differentiation, metabolism, aging, and apoptosis to play a crucial role in the anti-senescence process. In our study, SIRT-1 was markedly down-regulated in senescent fibroblasts, accompanied by markedly up-regulation of p53 and p21. However, after E2 administration, The SIRT-1 was significantly up-regulated by 1.50 times (**p < 0.01), and p53 and p21 were significantly down-regulated by 2.40 and 1.59 times (**p < 0.01) compared to the control, a similar trend of the level of p21 and p53 were also observed in the rapamycin group (Fig. 5A, B). The SIRT-1/p53/p21 axis is a typical aging regulation pathway, these results indicated that the SIRT-1/p53/p21 pathway was involved in regulating the E2-mediated anti-aging process of fibroblasts.
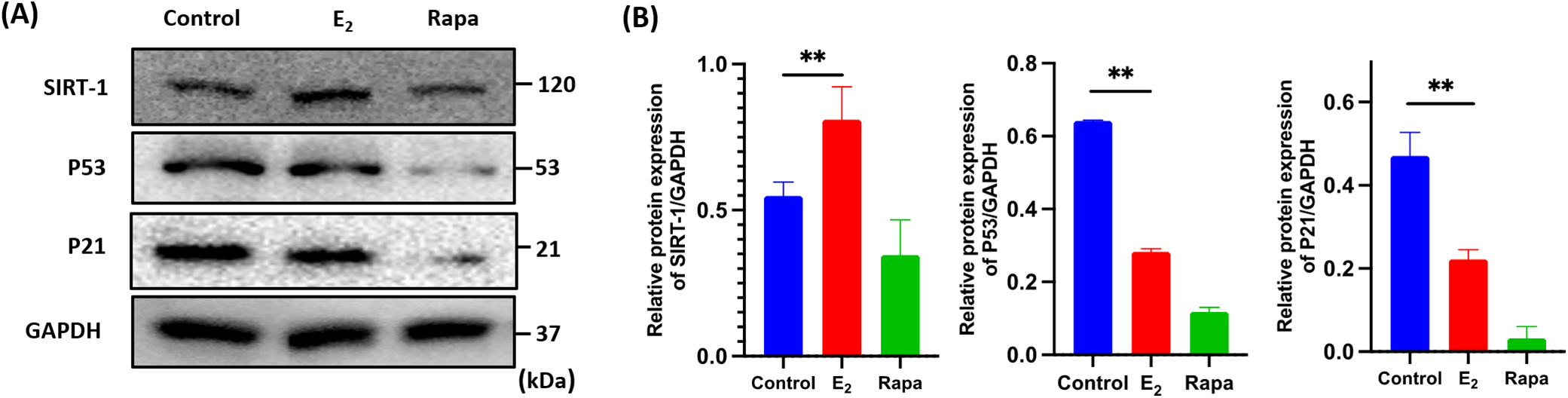
Figure 5: Changes in the SIRT-1/p53/p21 axis in fibroblasts after E2 treatment. (A): Fibroblasts were harvested to detect the protein levels of SIRT-1, p53 and p21 using western blots (x ± s, n = 3); (B) The statistical plots of SIRT-1, p53 and p21 protein (x ± s, n = 3); **p < 0.01, E2 vs. control group. Note: SIRT-1: Sirtuin 1, p53: Tumor Protein 53, p21: Cyclin-dependent kinase inhibitor 1A, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, E2: 17β-estradiol, Rapa: Rapamycin
With the increase of age, women experience prolonged estrogen deficiency post-menopause. The increase in senescent cells is believed to have a profound negative impact on tissue function in elderly animals [18]. Thus, the basic biological mechanisms associated with cellular senescence may partly be responsible for age-related tissue dysfunction, degeneration, and pathological alterations [19]. The main functional cells in the connective tissue of the pelvic floor are fibroblasts, altered in their quantity, and activity, and extracellular matrix (ECM) secretion capabilities may contribute to the damage to the tissue elasticity and strength. To verify the anti-aging effect and mechanism of estrogen, we cultured primary fibroblasts and identified the estrogen-mediated improvement of the aging phenotype. According to our results, after being treated with estrogen, the senescent fibroblast proliferation activity, autophagy flux, and metabolic activity were enhanced, while SA-β-Gal activity was decreased. Additionally, the expression of functional proteins including type-I and type-III collagen was significantly increased, and senescence-related marker p16INK4a was significantly reduced. These results were consistent with several key anti-senescence phenotypes displayed in neurons, liver cells, and adipocytes [20]. Furthermore, we illustrated that estrogen exerts anti-senescence effects by mediating the SIRT-1 axis.
According to the results, E2 has the most significant ability to promote proliferation at the concentration of 10−7 mol/L, which was consistent with other research of E2 decreased senescence and improved the osteogenic ability of Bone marrow-derived mesenchymal stem cells (BM-MSCs) [21]. Also, E2 (10−7 and 10−9 mol/L) prevented telomere shortening by reducing oxidative stress and decreasing h-MSC senescence [22].
Since cell senescence is accompanied by the inhibition of cell proliferation, our results showed that E2 promoted the proliferation of senescent fibroblasts. Savoia et al. [23] also found that E2 improved the proliferation activity of human skin keratinocytes/fibroblast to prevent the cells from UV damage. SA-β-Gal is a method used by other studies to verify the reversal of aging, in our study, we also found that E2 was able to inhibit the senescence of fibroblasts.
Changes in the collagen content of the pelvic floor connective tissue, such as collagen degradation, structural alterations, and proportions imbalances, also play an important role in POP. The immature collagen and elastic fibers would weaken the mechanism support effect of the pelvic floor [24]. Moalli et al. [25] found that the decrease in the type-I/-III ratio of POP patients’ fascia caused a significant weakening of the pelvic floor tissue. In estrogen-deficient women, Type-I and type-III collagens are also considered to reduce. The clinical results of pre-operatively vaginal estrogen application for 6 weeks increased the synthesis and maturity of collagen, and enhanced the thickness of the vaginal wall, thereby improving the postoperative matrix repair and maintaining the organizational integrity of pelvic floor connection [6]. Our results found that E2 may promoted the expression of type-I/-III collage, as Fig. 3A,B shown. In addition, researchers have illustrated that E2 increased collagen by induced vascular endothelial growth factor (VEGF) improved the transforming growth factor-β (TGF-β), and reduced collagen degradation by inhibited matrix metalloproteinases (MMPs) and increased tissue inhibitor of metalloproteinase (TIMP) in the dermal fibroblasts [26].
It is generally believed that E2 plays a crucial role in mitochondrial biogenesis and macrophage/autophagy function through estrogen receptors. The LC3 turnover rate is a parameter for measuring autophagy flux. As shown in Fig. 4A–C, we tested the changes in cell autophagy flux, mitochondria, and autophagosomes after E2 treatment, revealing that E2 played an anti-aging effect by mediating the increase of autophagy flux and cell metabolic activity. Singh et al. [27] found that hormone-induced activation of ERα regulated cell autophagy and mitochondrial division and biogenesis, while ERβ also induced autophagy to inhibit the migration and invasion of breast cancer cells. Additionally, Gavali et al. [28] found demonstrated that E2 promoted autophagy during osteoblast differentiation by up-regulating rab3gap1, and increased the survival and mineralization capacity of osteoblasts. Furthermore, mitochondria and autophagy play a central role in cell energy metabolism, its dysfunction leads to metabolic disorders and pathological features of aging [29].
SIRT-1 is a mammalian NAD+-dependent histone deacetylase responsible for deacetylating p53, thereby regulating apoptosis, stress response, cell metabolism, DNA repair, and cell aging. Consistent with our research, Sasaki’s data showed that E2 up-regulated SIRT-1 in ovariectomy (OVX) models, which induced senescent vascular endothelial cells and deacetylated p53, thereby, remitting arterial senescence and atherosclerosis which caused by menopause [30]. Estrogen usually mediated downstream reactions through Estrogen Relative Receptor (ERR) activation, and clinical local estrogen therapy has been shown to increase ERα expression, facilitating the proliferation of posterior vaginal tissues in postmenopausal women [31]. Studies have also found that E2 can eliminate oxidative stress in an ERα/SIRT-1-dependent manner to improve memory impairment, neuroinflammation, and neurodegeneration in adult mice [11]. In addition, both estrogen and hypothalamic ERα are related to aging, and long-term estrogen therapy may prolong the healthy lifespan of postmenopausal women [32]. In our study, the expression level of SIRT-1 was significantly up-regulated after estrogen treatment. More importantly, estrogen not only up-regulated SIRT-1 but also inhibited p53 acetylate and further inhibited the p21 expression to inhibit the senescence of pelvic floor fibroblasts, this supported the hypothesis that estrogen inhibits fibroblasts senescence through the SIRT-1/p53/p21 axis. Similar findings were reported by Wen et al., who demonstrated that the SIRT-1/p53/p21 signaling pathway played an important role in inhibiting the senescence of osteoblasts in aged rats [33]. As shown in Fig. 6, in conclusion, our study illustrated that estrogen mediated the signal pathway of the SIRT-1/p53/p21 axis to decrease the number of senescent cells and promote fibroblast proliferation and metabolic function. These effects contribute to alleviating pelvic floor muscle atrophy and degeneration, thereby inhibiting the progression of POP.
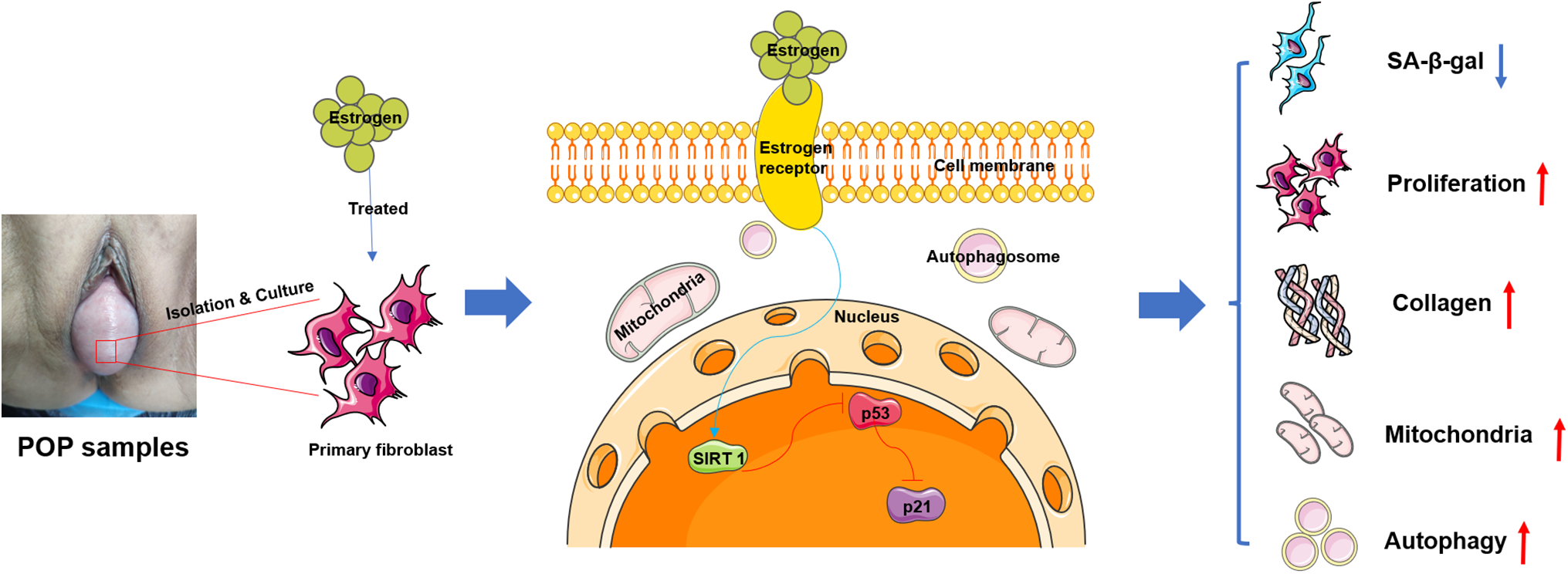
Figure 6: The effect and mechanism of estrogen anti-senescence. The primary fibroblasts derived from pelvic floor vaginal anterior wall prolapse samples were isolated, cultured, and identified, then treated with estrogen and found that estrogen decreased the number of senescent cells and enhanced the synthesis, secretion, and maturation of ECM, promoted the proliferation activity and metabolic function of fibroblasts through mediated the SIRT-1/p53/p21 axis signal pathway to inhibit the POP progress. Note: ECM: Extracellular Matrix, POP: Pelvic Organ Prolapse, SIRT-1: Sirtuin 1, p53: Tumor Protein 53, p21: Cyclin-dependent kinase inhibitor 1A, SA-β-Gal: Senescence-Associated-β-Galactosidase
At present, our results are limited and focused on the role of estrogen in regulating the SIRT-1/p53/p21 axis, and the system anti-aging mechanism still needs to be supplemented. In addition, E2 exerts anti-aging effects by regulating other signaling pathways such as phosphatidylinositide 3-kinases-Akt kinase/mammalian target of rapamycin (PI3K-Akt/mTOR) and mitogen-activated protein kinase (MAPK) et al., which also need further in-depth study.
The present study indicated that estrogen promoted the proliferation and autophagy activity of fibroblasts, as well as enhanced the synthesis of extracellular matrix such as COL-I and COL-III. In addition, the current study observed that estrogen played an improvement role by regulating the SIRT-1/p53/p21 axis. These findings provide a theoretical basis for the anti-aging effect and mechanism of estrogen on POP fibroblasts, which indicates that the clinical application and local administration of estrogen on POP treatment may be helpful for long-term maintenance and rejuvenation of connective tissue of the pelvic floor.
Acknowledgement: We would like to express our sincere gratitude to the patients who participated in this study for providing samples and their invaluable support.
Funding Statement: This research work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21048), Foundation of Sichuan Provincial Science and Technology Program (2022JDR0091, 2023NSFSC0004, 2023NSFSC0639, 2023NSFSC1742), Cooperation Project for Academician & Expert Workstation (HXYS20001) and Sichuan University Education Foundation (0040206107011), National Natural Science Foundation of China (Nos. 82371883, 82402191), China Postdoctoral Science Foundation (2023M732456), Postdoctor Research Fund of West China Hospital (2024HXBH142), and Sichuan University “From 0 to 1” Innovation Research Project (2023SCUH0056).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Juan Cheng, Jiang Wu, Zhiwei Zhao, Yali Miao; data collection, analysis and interpretation of study: Juan Cheng, Zhiwei Zhao, Ling Wang; draft manuscript preparation: Juan Cheng, Zhiwei Zhao; revise the important intellectual content: Jiang Wu, Yali Miao, Ling Wang, Jirui Wen. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Ethics Approval: All Pelvic floor vaginal anterior wall prolapse samples (n = 12, age from 50–75) were obtained from surgical patients at the department of obstetrics and gynecology department, West China Second University Hospital (Chengdu, China). This study was approved by the Ethics Committee of Sichuan university (approval number: 2024025), Patients were consented by an informed consent process that was reviewed by the Ethics Committee of Sichuan university and certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/biocell.2025.059573.
Abbreviations
| COL-I | Collagen Type I |
| COL-III | Collagen Type III |
| p16INK4a | Cyclin-dependent kinase 4 inhibitor A |
| p21 | Cyclin-dependent kinase inhibitor 1A |
| p53 | Tumor Protein 53 |
| LC3-I/II | Microtubule-associated protein 1A/1B-light chain 3-I/II |
| LET | Local Estrogen Therapy |
| POP | Pelvic Organ Prolapse |
| E2 | 17β-estradiol |
| POP-Q | POP Quantification system |
| CCK-8 | Colorimetric Cell Counting Kit |
| SA-β-Gal | Senescence-Associated-β-Galactosidase |
| WB | Western Blotting |
| TEM | Transmission Electron Microscope |
| BMI | Body Mass Index |
| SIRT-1 | Sirtuin 1 |
| eNOS | Endothelial Nitric Oxide Synthase |
| AMPK | AMP-activated Protein Kinase |
| ERα/β | Estradiol Receptors α and β |
| DMSO | Dimethyl sulfoxide |
| Rapa | Rapamycin |
| CQ | Chloroquine |
| SDS-PAGE | Sodium Dodecyl Sulphate-polyacrylamide Gel |
| PVDF | Polyvinylidene Fluoride |
| TBST | Tris Buffer Saline-Tween 20 |
| p | p-value |
| α-SMA | α-Smooth Muscle Protein |
| ECM | Extracellular Matrix |
| OVX | Ovariectomy |
| ERR | Estrogen Relative Receptor |
| BM-MSCs | Bone Marrow Derived Mesenchymal Stem Cells |
| VEGF | Vascular Endothelial Growth Factor |
| TGF-β | Transforming Growth Factor-β |
| MMPs | Matrix Metalloproteinases |
| TIMP | Tissue Inhibitor of Metalloproteinase |
| PI3K-Akt | Phosphatidylinositide 3-kinases-Akt kinase |
| mTOR | Mammalian Target Of the Rapamycin |
| MAPK | Mitogen-Activated Protein Kinase |
References
1. Cheng J, Zhao ZW, Wen JR, Wang L, Huang LW, Yang YL, et al. Status, challenges, and future prospects of stem cell therapy in pelvic floor disorders. World J Clin Cases. 2020;8(8):1400–13. doi:10.12998/wjcc.v8.i8.1400. [Google Scholar] [PubMed] [CrossRef]
2. Wilczak M, Chmaj-Wierzchowska K, Wójtowicz M, Kądziołka P, Paul P, Gajdzicka A, et al. Safety and effectiveness of G-Mesh(®) gynecological meshes intended for surgical treatment of pelvic organ prolapse-a retrospective analysis. J Clin Med. 2024;13(23):7421. doi:10.3390/jcm13237421. [Google Scholar] [PubMed] [CrossRef]
3. Peinado-Molina RA, Hernández-Martínez A, Martínez-Vázquez S, Rodríguez-Almagro J, Martínez-Galiano JM. Pelvic floor dysfunction: prevalence and associated factors. BMC Public Health. 2023;23(1):2005. doi:10.1186/s12889-023-16901-3. [Google Scholar] [PubMed] [CrossRef]
4. Sawai M, Yuno C, Shogenji M, Nakada H, Takeishi Y, Kawajiri M, et al. Prevalence of symptoms of pelvic floor dysfunction and related factors among Japanese female healthcare workers. Lower Urinary Tract Sympt. 2022;14(5):380–6. doi:10.1111/luts.12455. [Google Scholar] [PubMed] [CrossRef]
5. Barber MD. Measuring pelvic organ prolapse: an evolution. Int Urogynecol J. 2024;35(5):967–76. doi:10.1007/s00192-024-05798-0. [Google Scholar] [PubMed] [CrossRef]
6. Rahn DD, Good MM, Roshanravan SM, Shi H, Schaffer JI, Singh RJ, et al. Effects of preoperative local estrogen in postmenopausal women with prolapse: a randomized trial. J Clin Endocrinol Metab. 2014;99(10):3728–36. doi:10.1210/jc.2014-1216. [Google Scholar] [PubMed] [CrossRef]
7. Freitas SVTD, Faria CA. Quality of life before and after the use of vaginal estriol in postmenopausal women with lower urinary tract symptoms. Open J Obstet Gynecol. 2020;10(4):452–62. doi:10.4236/ojog.2020.1040041. [Google Scholar] [CrossRef]
8. Bodner-Adler B, Alarab M, Ruiz-Zapata AM, Latthe P. Effectiveness of hormones in postmenopausal pelvic floor dysfunction-International Urogynecological Association research and development-committee opinion. Int Urogynecol J. 2020;31(8):1577–82. doi:10.1007/s00192-019-04070-0. [Google Scholar] [PubMed] [CrossRef]
9. Lephart ED. Skin aging and oxidative stress: equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res Rev. 2016;31(Suppl. 1):36–54. doi:10.1016/j.arr.2016.08.001. [Google Scholar] [PubMed] [CrossRef]
10. Zhou Y, Xue X, Guo Y, Liu H, Hou Z, Chen Z, et al. A quinoxaline-based compound ameliorates bone loss in ovariectomized mice. Exp Biol Med. 2021;246(23):2502–10. doi:10.1177/15353702211032133. [Google Scholar] [PubMed] [CrossRef]
11. Khan M, Ullah R, Rehman SU, Shah SA, Saeed K, Muhammad T, et al. 17beta-estradiol modulates SIRT1 and halts oxidative stress-mediated cognitive impairment in a male aging mouse model. Cells. 2019;8(8):928. doi:10.3390/cells8080928. [Google Scholar] [PubMed] [CrossRef]
12. Mei R, Lou P, You G, Jiang T, Yu X, Guo L. 17β-estradiol induces mitophagy upregulation to protect chondrocytes via the SIRT1-mediated AMPK/mTOR signaling pathway. Front Endocrinol. 2020;11:615250. doi:10.3389/fendo.2020.615250. [Google Scholar] [PubMed] [CrossRef]
13. Reddy RA, Cortessis V, Dancz C, Klutke J, Stanczyk FZ. Role of sex steroid hormones in pelvic organ prolapse. Menopause. 2020;27(8):941–51. doi:10.1097/GME.0000000000001546. [Google Scholar] [PubMed] [CrossRef]
14. Takuya M, Kazumi N, Toru I. Rat uterine oxytocin receptor and estrogen receptor α and β mRNA levels are regulated by estrogen through multiple estrogen receptors. J Reprod Develop. 2014;60(1):55–61. doi:10.1262/jrd.2012-139. [Google Scholar] [PubMed] [CrossRef]
15. Huang L, Zhao Z, Wen J, Ling W, Miao Y, Wu J. Cellular senescence: a pathogenic mechanism of pelvic organ prolapse (Review). Mol Med Rep. 2020;22(3):2155–62. doi:10.3892/mmr.2020.11339. [Google Scholar] [PubMed] [CrossRef]
16. Zhu W, Yuan Y, Liao G, Li L, Liu J, Chen Y, et al. Mesenchymal stem cells ameliorate hyperglycemia-induced endothelial injury through modulation of mitophagy. Cell Death Dis. 2018;9(8):837. doi:10.1038/s41419-018-0861-x. [Google Scholar] [PubMed] [CrossRef]
17. Xin P, Xu W, Zhu X, Li C, Zheng Y, Zheng T, et al. Protective autophagy or autophagic death: effects of BEZ235 on chronic myelogenous leukemia. Cancer Manag Res. 2019;11:7933–51. doi:10.2147/CMAR.S204472. [Google Scholar] [PubMed] [CrossRef]
18. Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75–95. doi:10.1038/s41580-020-00314-w. [Google Scholar] [PubMed] [CrossRef]
19. Secomandi L, Borghesan M, Velarde M, Demaria M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum Reprod Update. 2022;28(2):172–89. doi:10.1093/humupd/dmab038. [Google Scholar] [PubMed] [CrossRef]
20. Yin Q, Tang TT, Lu XY, Ni WJ, Yin D, Zhang YL, et al. Macrophage-derived exosomes promote telomere fragility and senescence in tubular epithelial cells by delivering miR-155. Cell Commun Signal. 2024;22(1):357. doi:10.1186/s12964-024-01708-5. [Google Scholar] [PubMed] [CrossRef]
21. Soltanyzadeh M, Ghollasi M, Halabian R, Shams M. A comparative study of hBM-MSCs’ differentiation toward osteogenic lineage in the presence of progesterone and estrogen hormones separately and concurrently in vitro. Cell Biol Int. 2020;44(8):1701–13. doi:10.1002/cbin.11364. [Google Scholar] [PubMed] [CrossRef]
22. Breu A, Sprinzing B, Merkl K, Bechmann V, Kujat R, Jenei-Lanzl Z, et al. Estrogen reduces cellular aging in human mesenchymal stem cells and chondrocytes. J Orthop Res. 2011;29(10):1563–71. doi:10.1002/jor.21424. [Google Scholar] [PubMed] [CrossRef]
23. Savoia P, Raina G, Camillo L, Farruggio S, Mary D, Veronese F, et al. Anti-oxidative effects of 17 beta-estradiol and genistein in human skin fibroblasts and keratinocytes. J Dermatol Sci. 2018;92(1):62–77. doi:10.1016/j.jdermsci.2018.07.007. [Google Scholar] [PubMed] [CrossRef]
24. Zhang D, Xu D, Huang X, Wei Y, Tang F, Qin X, et al. Puerarin-loaded electrospun patches with anti-inflammatory and pro-collagen synthesis properties for pelvic floor reconstruction. Adv Sci. 2024;11(21):e2308590. doi:10.1002/advs.202308590. [Google Scholar] [PubMed] [CrossRef]
25. Moalli PA, Talarico LC, Sung VW, Klingensmith WL, Shand SH, Meyn LA, et al. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol. 2004;190(3):620–7. doi:10.1016/j.ajog.2003.08.040. [Google Scholar] [PubMed] [CrossRef]
26. Giardina S, Michelotti A, Zavattini G, Finzi S, Ghisalberti C, Marzatico F. Efficacy study in vitro: assessment of the properties of resveratrol and resveratrol + N-acetyl-cysteine on proliferation and inhibition of collagen activity. Minerva Ginecol. 2010;62(3):195–201. [Google Scholar] [PubMed]
27. Singh BK, Sinha RA, Tripathi M, Mendoza A, Ohba K, Sy JAC, et al. Thyroid hormone receptor and ERRalpha coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci Signal. 2018;11(536):eaam5855. doi:10.1126/scisignal.aam5855. [Google Scholar] [PubMed] [CrossRef]
28. Gavali S, Gupta MK, Daswani B, Wani MR, Sirdeshmukh R, Khatkhatay MI. Estrogen enhances human osteoblast survival and function via promotion of autophagy. Biochim Biophys Acta Mol Cell Res. 2019;1866(9):1498–507. doi:10.1016/j.bbamcr.2019.06.014. [Google Scholar] [PubMed] [CrossRef]
29. Juszczak F, Caron N, Mathew AV, Declèves AE. Critical role for AMPK in metabolic disease-induced chronic kidney disease. Int J Mol Sci. 2020;21(21):7994. doi:10.3390/ijms21217994. [Google Scholar] [PubMed] [CrossRef]
30. Sasaki Y, Ikeda Y, Miyauchi T, Uchikado Y, Akasaki Y, Ohishi M. Estrogen-SIRT1 axis plays a pivotal role in protecting arteries against menopause-induced senescence and atherosclerosis. J Atheroscler Thromb. 2020;27(1):47–59. doi:10.5551/jat.47993. [Google Scholar] [PubMed] [CrossRef]
31. Fuermetz A, Schoenfeld M, Ennemoser S, Muetzel E, Jeschke U, Jundt K. Change of steroid receptor expression in the posterior vaginal wall after local estrogen therapy. Eur J Obstet Gynecol Reprod Biol. 2015;187(1):45–50. doi:10.1016/j.ejogrb.2015.02.021. [Google Scholar] [PubMed] [CrossRef]
32. Paganini-Hill A, Corrada MM, Kawas CH. Increased longevity in older users of postmenopausal estrogen therapy: the Leisure World Cohort study. Menopause. 2018;25(11):1256–61. doi:10.1097/GME.0000000000001227. [Google Scholar] [PubMed] [CrossRef]
33. Wen J, Bao M, Tang M, He X, Yao X, Li L. Low magnitude vibration alleviates age-related bone loss by inhibiting cell senescence of osteogenic cells in naturally senescent rats. Aging. 2021;13(8):12031–45. doi:10.18632/aging.202907. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools