 Open Access
Open Access
REVIEW
Research progress on natural products against hepatocellular carcinoma
1 Chongqing Key Laboratory of High Active Traditional Chinese Drug Delivery System, Chongqing Medical and Pharmaceutical College, Chongqing, 400030, China
2 Provincial Key Laboratory of Pharmaceutical Bioactive Substances, School of Life Sciences and Biopharmaceuticals, Guangdong Pharmaceutical University, Guangzhou, 510006, China
* Corresponding Author: JINGXIN MAO. Email:
BIOCELL 2024, 48(6), 905-922. https://doi.org/10.32604/biocell.2024.050396
Received 05 February 2024; Accepted 24 April 2024; Issue published 10 June 2024
Abstract
Hepatocellular carcinoma (HCC) remains a prevalent and challenging malignancy globally, characterized by its numerous causal factors and generally unfavorable prognosis. In the relentless pursuit of effective treatment modalities, natural products have emerged as a promising and relatively non-toxic alternative, garnering significant interest. The integration of natural products with contemporary medical research has yielded encouraging therapeutic outcomes in the management of HCC. This review offers a comprehensive overview of the causal factors underlying HCC, and the diverse treatment options available, and highlights the advancements made by natural products in anti-HCC research. Particularly, we provide an outline of the various types of natural products, their corresponding nomenclature, target molecules, and mechanisms of action that exhibit anti-HCC activities. Natural products are anticipated to play a pivotal role in future comprehensive treatment plans for liver cancer, potentially offering patients improved survival rates and an enhanced quality of life.Keywords
Hepatocellular carcinoma (HCC) is a widely prevalent malignant tumor with a worldwide increasing incidence [1]. In 2020, the number of new cases of primary liver cancer exceeded 900,000, resulting in approximately 830,000 deaths [2]. Currently, the incidence rate of HCC in China has begun to stabilize, representing 4.7% of the global incidence, with a mortality rate of 8.3% globally [3]. It is estimated that the number of liver cancer patients worldwide will exceed one million annually by 2025 [4]. HCC is the most common pathological type of primary liver cancer, making its treatment a significant challenge in global healthcare. Due to late-stage diagnosis, the optimal treatment window often passes by the time of diagnosis, and HCC is a highly invasive cancer often accompanied by liver function damage. Advanced HCC patients exhibit a poor prognosis, marked by a significant decrease in survival rates and a high risk of recurrence, often reaching 70% or higher [5]. Therefore, current treatment outcomes for HCC are inadequate, and new treatment strategies are warranted [6].
As early as thousands of years ago, the medicinal uses of natural products such as fungi, plants, and animals have been recorded in ancient prescriptions of human history [7]. Therefore, natural products exhibit great potential in searching for new therapeutic candidate drugs [8]. By combining modern tools such as molecular biology, biochemistry, and chemistry, compounds extracted from natural products can not only be used to treat diseases but also play an important role in drug development. This enables us to better analyze the biological effects and potential interactions of these compounds on the human body [9]. These research advances have made compounds extracted from natural products potential new therapies for many complex diseases, including inflammation, infectious and neurodegenerative diseases, and cancer [10]. Therefore, people began to study various components extracted from natural products and applied them in clinical trials [11].
After achieving success [12], people began to actively explore different plants to find drugs with potential anti-cancer potential [13]. Previous research reports indicate that natural products and compounds extracted from natural products can inhibit cell proliferation [14], inhibit cancer transformation, and effectively prevent tumor metastasis [15]. Through the complex mechanism of traditional Chinese medicine (TCM) preparations with multi-target and multi-component characteristics in network pharmacology [16], new strategies for the treatment of HCC with natural products are provided (Fig. 1).
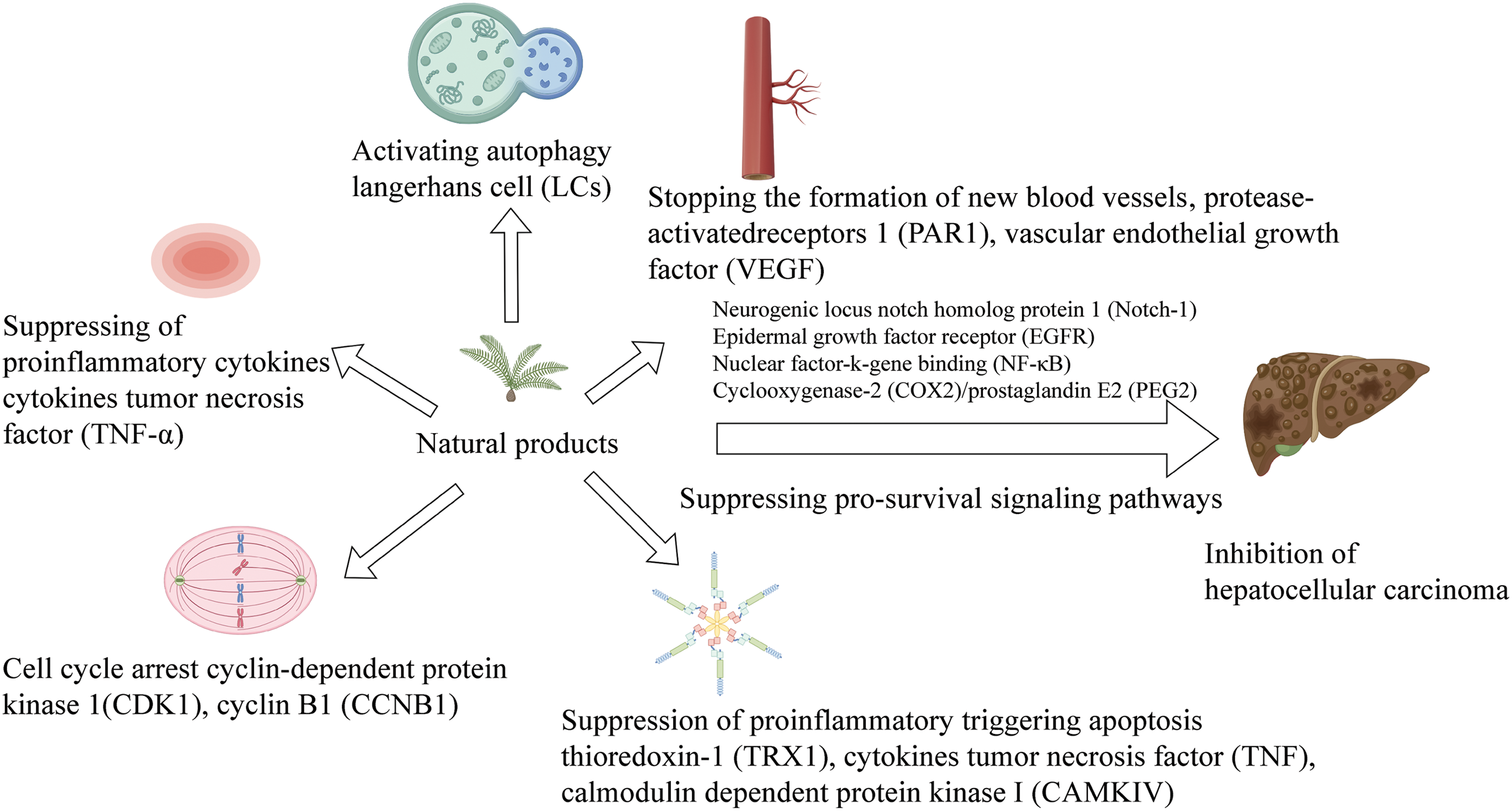
Figure 1: Natural product therapy for HCC from different pathways (By Figdraw).
The comprehensiveness, systematicity, and wholeness of network pharmacology are consistent with the multi-compound, multi-target, and multi-pathway characteristics of natural products [17]. Network pharmacology is to explore the mechanism of TCM in the treatment of HCC by integrating bioinformatics and molecular docking technology. Previous studies revealed that many decoctions may play an anti-HCC role by regulating specific signaling pathways and biological processes [18], which provides a new idea for the treatment of HCC, making traditional Chinese medicine play an important role in anti-cancer research. However, further research is still needed in this field to further understand the potential mechanism of TCM in the treatment of HCC and lay the foundation for individualized treatment and more effective clinical application. To explore the therapeutic potential of natural products, researchers have chosen different protein targets, including apoptotic proteins and transcription factors. This study encompasses the factors that initiate HCC, an examination of its etiology, the present-day treatment modalities available for HCC, and a subsequent assessment of the varying natural product components that can serve as potential therapeutic targets. From the perspective of natural products, we searched for relevant literature generated from various databases for the treatment of HCC. After searching the keywords “natural products” and “hepatocellular carcinoma” OR “HCC”, a total of 5,603 articles were found, of which 2,399 were retrieved from Web of Science, 604 from PubMed, and 2,600 records were retrieved from Scopus. After removing the repeated literature in the database, there were 980 references. After the screening of titles and abstracts, the remaining 612 articles were evaluated, and finally, 59 articles were included in the literature review for analysis. These literature studies finally highlighted the association of HCC and its treatment with natural products, providing a rich literature reference value for the treatment of HCC (Fig. 2).
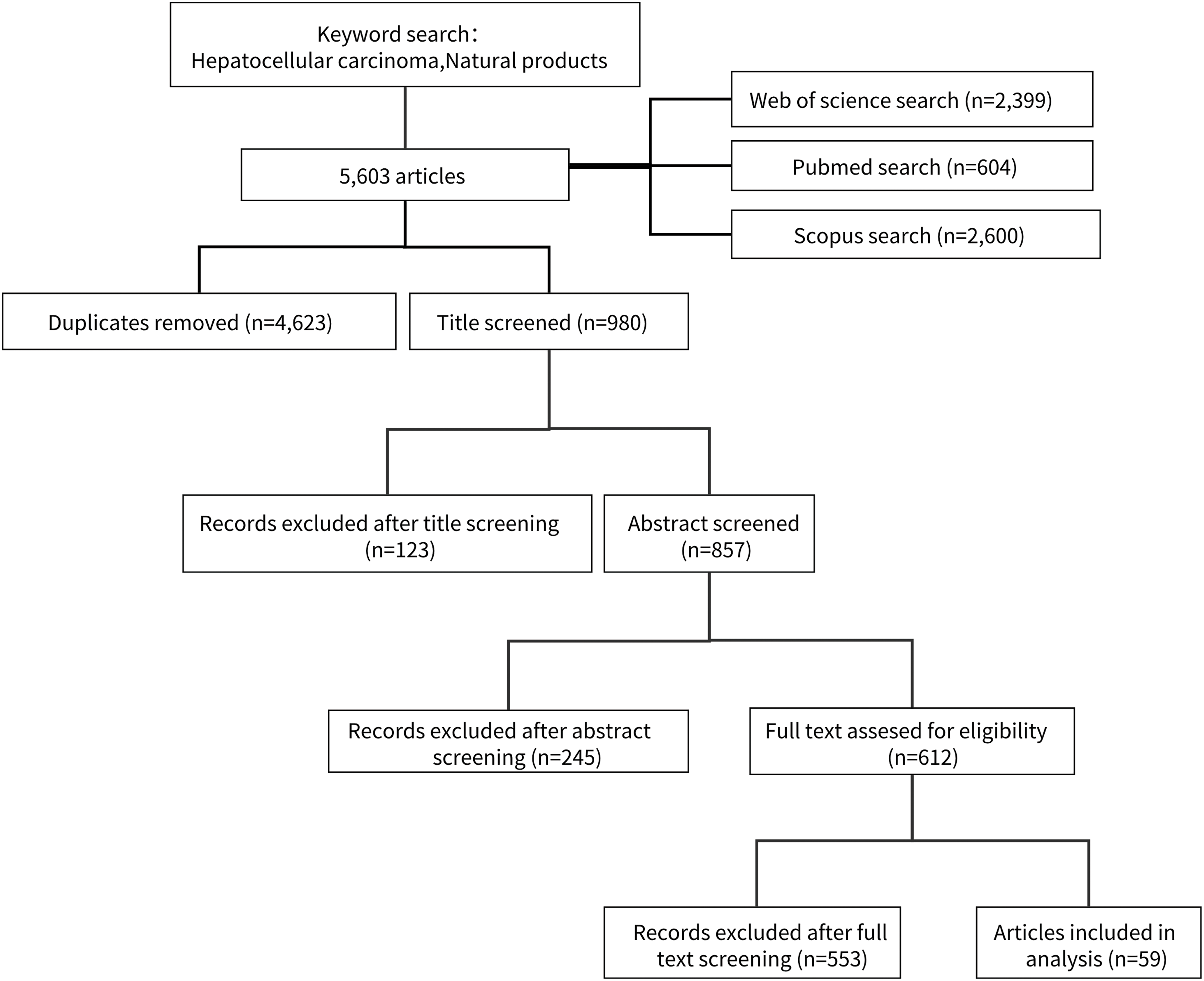
Figure 2: The flow chart of the study selection process.
The occurrence of HCC is the result of a combination of multiple factors. Liver fibrosis is a pathological process of fibrosis and scarring in liver tissue, usually caused by liver diseases such as chronic hepatitis, alcohol abuse, and fatty liver, which increases the risk of developing HCC. Hepatitis B virus (HBV) and hepatitis C virus (HCV) are the main viral causes, and their long-term infection can lead to chronic hepatitis and cirrhosis, exacerbating the development of HCC. The co-infection of hepatitis D virus (HDV) and HBV also increases the risk of developing HCC. Age and sex are also influencing factors, and males and older individuals are more likely to develop HCC. In addition, liver lesions, genetic factors, environmental factors, and exposure to certain drugs and chemicals may also increase the risk of developing HCC. These factors are often interrelated and together promote the development of HCC [19].
Liver fibrosis is one of the factors that may trigger HCC, and it is also a key factor leading to an increase in mortality in liver disease patients [20]. Individuals with progressive liver fibrosis will eventually develop into liver failure or HCC [21]. When our body develops fatty liver, if not actively controlled or treated, it may lead to liver fibrosis. Because of the fatty liver can lead to excessive accumulation of fat in the liver under different conditions, leading to sustained damage, hepatocyte steatosis, and ultimately evolving into liver fibrosis. In addition, patients with chronic viral hepatitis may also experience varying degrees of liver fibrosis. This is because the virus persists in the body and can cause recurrent or persistent inflammation in the liver, which in the long run will inevitably lead to liver fibrosis. Liver fibrosis can lead to liver failure or HCC, and mesenchymal stromal cells may play a crucial role.
HBV is a DNA virus that can integrate into the host genome, induce insertion mutagenesis, and activate oncogenes [22]. Moreover, HBV infection can inhibit the function of the immune system, which helps to promote the growth and spread of cancer cells, making it more difficult for the body to clear viruses and resist other pathogens. There is a close correlation between HBV infection and HCC. Firstly, HBV infection often leads to chronic hepatitis, which is a long-term and progressive inflammatory process that can damage liver tissue. Persistent hepatitis leads to gradual liver damage, increasing the risk of developing HCC. In addition, inflammation caused by HBV leads to oxidative stress, releasing free radicals, which can damage DNA and other cellular structures. Oxidative stress is related to the development of cancer, as it may lead to DNA mutations, thereby promoting tumor formation. Finally, HBV infection can also trigger secondary infections, which can increase the risk of HCC. According to a survey, 69.9% of Chinese HCC patients have a background of HBV infection, 5.2% have HCV infection, and 5.8% have both HBV and HCV infections [23]. The occurrence of HBV-induced HCC is a complex, multifactorial, and progressive process that involves the interaction between the virus and endogenous mutagens, as well as the host’s immune response to the virus [24].
HCV-induced HCC is mainly related to the host’s sustained immune response and chronic inflammation caused by acute infection clearance failure, leading to liver fibrosis and cirrhosis, ultimately developing into HCC [25,26]. There is also a close correlation between HCV infection and HCC. Firstly, HCV infection often leads to chronic hepatitis, causing fibrosis of liver tissue and transforming into cirrhosis. At this time, liver tissue becomes scarred, which increases the risk of developing HCC. Unlike HBV, HCV is an RNA virus that is not integrated into the host genome, and its treatment has also been quite successful. Due to the ongoing risk of developing HCC in patients with HCV-induced cirrhosis, we need to constantly monitor [27]. Secondly, HCV may also trigger carcinogenic changes in liver cells, directly promoting the development of HCC. Finally, the combination of alcohol abuse and HCV infection increases the risk of developing HCC.
HDV is an RNA virus that encodes only one type δ Protein or δ Antigen (HDAg) is a virus that requires the presence of HBV for replication and infection [28]. Large hepatitis D antigen (L-HDAg) may activate signal transduction and transcription activating factor 3 (STAT3) or oxidative stress-induced nuclear factor kappa-B (NF-κB) activation to promote inflammation. The effects of this inflammatory response include endoplasmic reticulum stress and necrotizing inflammation, as well as an increase in reactive oxygen species generation, which may ultimately lead to the occurrence of HCC [29]. Therefore, HDV cannot integrate viral genes into the host genome and must rely on HBV for replication. HDV indirectly causes HCC [30].
HDV is usually co-infected with HBV because it requires HBV to provide coating and other key factors for replication and infection. It means that the patient is carrying both viruses, namely HBV and HDV. This co-infection increases the severity of hepatitis and increases the risk of developing HCC. HDV infection can lead to a more severe course of hepatitis, and patients who are infected with HBV alone are more prone to liver inflammation, liver cell damage, and fibrosis. Persistent liver inflammation occurs, which stimulates liver cell proliferation and increases the risk of cancer. HDV infection is often associated with liver fibrosis and the progression of cirrhosis. Cirrhosis causes significant changes in liver tissue, causing serious damage to liver function, and is also a prerequisite for leading to HCC. According to investigations, the risk of HCC in patients with acute HDV infection (relative risk (RR) 6.1, 95% (confidence interval (CI) 2.8–11.7) or chronic HDV infection (RR 3.9, 95% CI 1.6–7.2) is significantly higher than that in individuals infected with HBV alone [31]. Overall, HDV infection increases the risk of multiple viruses in patients, increases the severity of hepatitis, and leads to persistent inflammation and liver fibrosis, all of which together increase the risk of HCC.
Age is an important risk factor for HCC. HCC is more common in middle-aged and elderly people, especially in the 70-year-old population, the age-specific incidence rate reported is the highest [32]. This may be related to long-term exposure to potential carcinogenic factors, such as viral infections or alcohol abuse. In addition, age is also associated with gradual damage and mutations in liver cells, increasing the risk of cancer development. Sex also plays an important role in the pathogenesis of HCC. Generally speaking, men are more likely to suffer from HCC, and its incidence rate is significantly higher than that of women. This may be partly due to men being more exposed to risk factors for liver cancer, such as alcohol abuse and viral infections [33]. Hormonal factors may also play a role in gender differences, although the specific mechanisms are not yet clear. According to the survey, the incidence rate varies with different races. This difference in incidence rate may be partly due to the high incidence rate of single nucleotide variation in patatin-like phospholipase domain containing 3 (PNPLA3), which is related to non-alcoholic steatohepatitis (NASH) related HCC [34]. Smoking can also increase the risk of HCC [35]. Genetic factors may also affect the risk of HCC. If there is a history of HCC in the family, the individual’s risk may increase. Certain genetic mutations may increase the genetic predisposition of HCC. The health of the immune system is crucial for preventing the development and spread of cancer cells. Damage or inhibition of the immune system may make cancer more likely to develop. However, the role of diet in regulating the risk of HCC is not yet clear, but studies have shown that coffee and aspirin have preventive effects [36].
With the progress of science and technology, in addition to surgical treatment, immunotherapy, targeted therapy, and interventional therapy, nanotechnology has emerged in recent years to treat hepatocellular carcinoma [37]. With the development of nanotechnology, more and more nanomedicines have been developed and applied in the biomedical field [38]. Through rational design, nanomedicines can be prepared into therapeutic agents with appropriate size, and surface modification specific liver cancer targeting ligands and simultaneously loaded with a variety of different mechanisms of action, to improve the bioavailability of drugs, enhance the targeting of liver cancer, reduce the toxic and side effects on normal tissues, and provide new hope for the treatment of liver cancer. Some receptors are often specifically expressed or overexpressed on the surface of hepatocellular carcinoma. Therefore, when designing nanomedicines, ligand molecules with high affinity for these receptors are coupled on the surface of nanomedicines to connect them with “missile molecules”, which can guide the drug delivery to the target site and realize the accumulation of drugs in liver tissues or liver cancer cells [39]. These active targeting ligand molecules include some small molecules, sugars, peptides, antibodies, etc. Under receptor ligand-mediated active targeting, nanomedicines can enter cells through the endocytic pathway, increasing the intracellular drug concentration. To achieve the purpose of treating hepatocellular carcinoma.
Surgical treatment is the main method for early HCC. When performing surgical treatment, it is necessary to conduct preoperative evaluation first, including checking the type, size, and location of the tumor, and whether there are signs of metastasis to other organs [40]. The overall health status of patients should also be evaluated to ensure they can withstand surgery. Surgical treatment will determine the type of surgery based on the nature and location of the tumor (Fig. 3A).
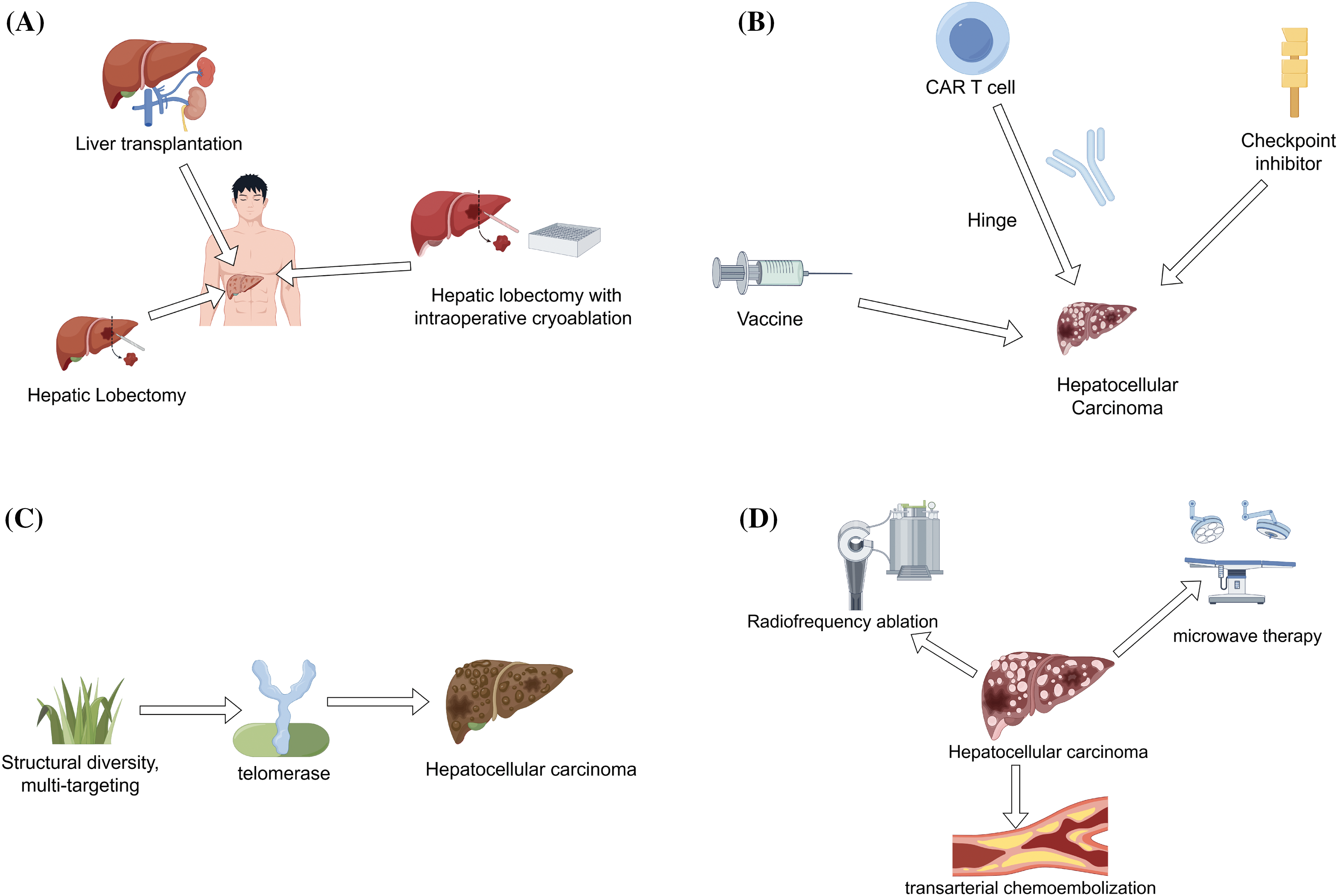
Figure 3: Treatment methods of HCC (By Figdraw). (A). Surgical treatment-lobectomy, intraoperative frozen resection, and liver transplantation, (B). Immunotherapy-checkpoint inhibitors, CAR-T cell therapy, and cancer vaccines, (C). Targeted therapy-inhibition of telomerase activity, (D). Interventional therapy-radiofrequency ablation, microwave therapy, and transarterial chemoembolization.
For example ① Hepatolobectomy. In this surgery, the doctor will remove a portion of the liver containing the tumor. ② Hepatolobectomy combined with intraoperative cryoresection. For some larger tumors, doctors may use intraoperative cryoresection to preserve healthy liver tissue to the greatest extent possible. ③ Liver transplantation. For some severe cases of HCC, liver transplantation surgery may be necessary, where the entire liver is replaced with a healthy donor liver.
Before surgery, patients need to follow the doctor’s advice, including stopping the use of specific medications, fasting, etc. Surgery is usually performed under general anesthesia, where the doctor enters the abdominal cavity through an incision and then removes the tumor or the entire liver according to the condition. After surgery, patients need to receive monitoring and treatment in the hospital to ensure no complications. Postoperative patients need to undergo regular follow-up and receive chemotherapy, radiation therapy, or other treatments to prevent the recurrence of cancer cells.
Immunotherapy is currently a promising method for treating HCC [41]. For example, monocarboxylate transporter (MCT) 4 in HCC is overexpressed, and VB124 (a high-potential MCT4 inhibitor) is used to inhibit MCT. This immunotherapy response targeting MCT4 has been shown to have anti-HCC effects and can produce an immunosuppressive liver cancer environment [42].
Immunotherapy is the process of enhancing or adjusting a patient’s immune system to better recognize and attack cancer cells. It includes various methods, such as checkpoint inhibitors, chimeric antigen receptor T cell immunotherapy (CAR-T) cell therapy, cancer vaccines, etc. (Fig. 3B). The common goal of these methods is to combat cancer by activating the immune system, stimulating immune cells and enhancing anti-cancer effects by infusing cytokines, vaccines, and T cells [43]. Immunotherapy is successful and safe in the treatment of HCC, with long-term survival and controllable toxicity [44,45]. One of the earliest immunotherapy methods used for HCC was vaccination. Due to the risk-free nature of vaccination, HepG2 cell lines and autologous tumor lysates have been used in clinical trials [46].
Targeted therapy is a new treatment method that can more accurately attack cancer cells, treating cancer by interfering with or blocking specific molecules or signaling pathways associated with cancer. According to the investigation, molecular targeted drugs currently have good effects in Phase III clinical practice and can significantly improve survival rates [47]. Due to the high expression of the angiogenesis promoter vascular endothelial growth factor (VEGF), it leads to vascular proliferation and causes HCC. Therefore, the current molecular-targeted therapy strategies for HCC mainly target VEGF and other angiogenic pathways [48].
Natural products have a wide range of pharmacological effects, with multiple components and targets, playing a crucial role in the treatment of HCC [49]. In recent years, natural products have been a hot topic and research direction in targeted therapies due to their structural diversity, multi-target effects, and minimal toxic side effects [50]. We are actively developing more molecular targeted drugs targeting the important targets that cause HCC. Telomerase is an attractive target for selective cancer treatment as it plays a crucial role in cell immortality. Previous research has shown that Imetelstat (GRN163L) [51], Periposine [52], KML001 [53], and BIBR1532 [54] can inhibit telomerase activity, cause telomere loss, and control cell growth rate to treat HCC (Fig. 3C).
Interventional therapy treatment
Radiofrequency ablation and microwave therapy are two commonly used interventional treatment methods for HCC, both aimed at destroying tumor tissue, but using different energy sources and principles (Fig. 3D). Both of these treatment methods can be used to treat early HCC or as adjuvant therapy to alleviate symptoms in patients. Radiofrequency ablation is a method of destroying liver cancer cells using the thermal energy generated by high-frequency currents. This process is usually completed by introducing radiofrequency electrodes directly into the interior of the tumor. Electrodes generate high temperatures, leading to thermal necrosis of surrounding tissues, which has a killing effect on tumor tissue. Radiofrequency ablation is commonly used for small, localized liver cancer lesions, especially in patients who are not suitable for surgical resection, such as due to liver dysfunction or other health reasons. This method is usually minimally invasive and recovers quickly but may require multiple treatments to ensure complete removal of the tumor.
Microwave therapy is also a method of destroying HCC by generating heat energy through high-frequency microwaves, but unlike radiofrequency ablation, it uses microwave energy instead of electric current. Microwave energy is directly directed into the interior of the tumor by introducing microwave antennas, generating high temperatures to destroy tumor tissue. Microwave therapy may be faster and more effective in certain situations, especially for large tumors or tumors at risk. Like radiofrequency ablation, microwave therapy is usually minimally invasive and helps reduce postoperative recovery time for patients.
Transcatheter arterial chemoembolization (TACE) is also an interventional treatment method for HCC. HCC is usually primary liver cancer, and the main goal of TACE is to slow down or inhibit the growth of HCC and improve the survival rate of patients. The principle of TACE combines two treatment methods, namely chemotherapy and embolization therapy. Chemotherapy: during TACE, anticancer drugs are delivered to the hepatic artery and directly act on the tumor, slowing down or inhibiting cancer growth [55]. Embolism therapy: in addition to drugs, TACE also includes embolic agents, which block the blood supply arteries of tumors, restrict blood flow supply, and thus allow drugs to stay inside the tumor and enhance its therapeutic effect [56]. During the treatment process, TACE is usually performed in the operating room or radiation therapy room by experienced interventional radiologists. Patients may require local anesthesia or sedatives but generally do not require general anesthesia. The doctor introduces a catheter into the hepatic artery system through arterial catheterization to accurately deliver drugs and embolic agents to the area where the tumor is located. The combination of drugs and embolic agents can lead to insufficient blood supply to tumors and drug effects, thereby achieving therapeutic effects. After surgery, the catheter will be removed, and patients need to be observed in the hospital for some time to ensure there are no serious complications.
The advantage of TACE treatment is that it can effectively slow down the growth of HCC, alleviate symptoms, and improve the survival rate. However, TACE typically requires multiple treatments as HCC is a recurrent cancer and may not be a permanent solution for some patients. The frequency and duration of treatment will vary depending on the nature of the tumor and the patient’s response.
Research on Natural Products against HCC
Although some progress has been made in the treatment and prevention of HCC, the overall survival rate of HCC patients remains low due to their susceptibility to recurrence and metastasis. In terms of surgical treatment, although the tumor can be completely removed to achieve a curative effect, and for early liver cancer, the cure rate after surgical resection is relatively high. However, for mid to late-stage liver cancer, the surgical risk is high, and the postoperative recurrence rate is high. Some patients cannot afford surgery due to poor physical condition. Immunotherapy can activate the patient’s immune system, improve their resistance to tumors, and have a broad-spectrum killing effect, which can reduce tumor recurrence and metastasis. Compared with other treatment methods, the side effects of immunotherapy are relatively small. However, the efficacy of immunotherapy varies from person to person, and some patients may not benefit from it. And immunotherapy needs to be combined with other treatment methods, such as surgery, radiotherapy, etc., to improve the treatment effect. In terms of targeted therapy, it inhibits or disrupts specific receptors or signaling pathways on the surface of tumor cells, with high specificity. Targeted therapy has a relatively small impact on normal cells, reducing the toxic side effects of traditional chemotherapy drugs. It can also target multiple targets and achieve therapeutic effects through multiple pathways and stages. However, targeted therapy requires genetic testing of the patient’s tumor to determine the presence of relevant mutated genes, which requires a certain amount of time and cost. Moreover, the long-term efficacy of targeted therapy still needs further observation and research, and some patients may experience drug resistance. Interventional therapy, which is a common approach for treating HCC, mainly treats local tumor lesions without surgery. It is suitable for patients with advanced liver cancer who cannot be operated on, have minimal side effects, and can be treated with embolization. However, the operation is difficult and prone to recurrence after treatment [57]. Both chemical and surgical treatment of interventional therapy could not provide a good prognosis. Therefore, there is an urgency to find an effective way (natural products) to treat HCC [58].
Due to the presence of multiple active ingredients and minimal side effects in natural products, TCM has been found to have good therapeutic effects in the treatment of cancer, such as choriocarcinoma, esophageal cancer, leukemia, and nasopharyngeal carcinoma, according to traditional folk therapies [59]. The evidence-based function of Chinese herbal medicine in anti-cancer is to prevent tumor growth and metastasis, as well as to alleviate the side effects or complications of treatment strategies such as chemotherapy, radiotherapy, and resection [60]. A large number of studies have adopted molecular docking techniques to explore the binding ability of key active ingredients and targets in natural products, to improve the prognosis of HCC patients after resection surgery [61]. An increasing number of studies indicate that natural products have enormous potential in developing alternative anti-cancer drugs [62]. Natural products have the characteristics of multiple compounds, targets, and pathways when applied in disease prevention and treatment. Molecular docking technology can simulate the results of drug-target interactions [63], making it easier to screen for anticancer drugs. At present, flavonoids [64–66], alkaloids, phenolic alcohols [67], and other TCM extracts in natural products have been found to exhibit strong anti-cancer activity in HCC treatment.
It was revealed that flavonoids have benefits in inhibiting the occurrence and development of cancer [68,69], and play a crucial role in the chemoprevention of HCC [70]. Flavonoids have anti-thrombotic, antioxidant, hepatoprotective, and anti-inflammatory properties [71], and have a wide range of pharmacological effects in TCM [72]. Flavonoids extracted from natural products have been proven to have neuroprotective effects, myocardial injury protective effects, alleviating liver steatosis, and inducing apoptosis in human epithelial ovarian cancer cell SK-OV-3 [73–76], exhibiting anticancer activity. Moreover, flavonoids can also treat chronic venous insufficiency (CVI) and improve capillary function and have been successfully proven to be safe and effective in elderly patients and pregnant women, with no reported side effects [77]. Flavonoids in some natural products can be applied to daily beverages, and daily consumption can better prevent anti-inflammatory, antioxidant, antimicrobial, antiviral, and hypoglycemic effects [78]. Flavonoids not only exhibit partial anti-cancer and cancer prevention effects, reducing liver damage [79–81] but are also used as liver detoxifiers, possibly inhibiting the survival and angiogenesis of liver cancer cells [82]. We have summarized different kinds of flavonoids that may be used for the treatment of HCC, including their names, sources, targets, and mechanisms of action (Table 1).
In folk therapies, alkaloids extracted from natural products exhibit an excellent effect on inhibiting cancer by inhibiting cell division, inducing apoptosis, and inhibiting angiogenesis, thereby inhibiting the activity of cancer cells [98,99]. Alkaloid compounds can inhibit the growth of HCC by inducing cell aging and cell cycle arrest, as cell aging is an irreversible state of cell cycle arrest, in which senescent cells permanently lose their proliferative ability [100]. Currently, inducing cell aging to slow down cancer cell proliferation is also a powerful defense against cancer (Table 2).
It was reported that phenolic compounds have benefits in inhibiting the occurrence and development of cancer, exhibiting not only significant anti-tumor, antioxidant, and anti-inflammatory effects [102–104] but also anti-cancer and anti-inflammatory effects [105]. Previous studies have confirmed that phenolic compounds can induce tumor cell apoptosis, arrest cell cycle, and autophagy, and therefore may also apply to liver cancer cells [106–108]. In TCM prescriptions, according to surveys, phenolic compounds are the basic components for treating various diseases (such as blood stasis, pain relief, etc.) [109], and have far-reaching therapeutic effects on tumors [110], which can reduce inflammation [111] and protect the liver [112–114]. At present, bioactive extracts have been extracted from natural products of TCM to combat HCC [115], mainly by inducing cell apoptosis, regulating inflammatory response, and increasing the expression of HCC-specific proteins (Table 3).
Apart from the compounds enumerated in the aforementioned categories, there exist extracts derived from certain natural products, which possess the capability of addressing HCC. The derivative compounds isolated from natural product extracts have been proven to inhibit the growth of HCC and induce cell apoptosis [120]. In TCM formulas, there are medicinal herbs specifically used to treat diseases related to the liver meridian and related organs, which have bioactive ingredients against HCC [121] and can effectively eliminate ‘liver fire’. They have been confirmed in liver diseases such as HCC [122], non-alcoholic fatty liver [123], and liver failure [124]. Extracts of other natural products can also reduce the activity of HCC cells [125], inhibit proliferation [126], prevent migration [127] and invasion [128], and induce cell apoptosis [129]. They have physiological activities such as anti-tumor [130], liver protection [131], immune regulation [132], inhibition of neointimal formation [133], lowering blood pressure and cholesterol [134], and inhibition of platelet aggregation [135]. They can also promote angiogenesis and endothelial cell proliferation or growth, which exhibits growth inhibition and induces cell apoptosis [136] (Table 4).
The liver is a critical organ in the human body. Primarily, it serves as a metabolic hub, participating in the breakdown and synthesis of essential nutrients such as carbohydrates, lipids, and proteins. This helps to maintain blood sugar levels and stores energy for later use. Additionally, the liver has a robust detoxification capacity. It efficiently purifies the blood, ridding the body of harmful substances, including those produced during metabolism and environmental toxins [141]. By converting these substances into harmless metabolites, the liver effectively removes them from the body [142]. Furthermore, the liver plays a crucial role in lipid metabolism. It synthesizes and regulates cholesterol levels, ensuring a balanced lipid profile in the body. A key function of the liver is secreting bile, which assists in the digestion of fats. The liver is also responsible for producing various proteins essential for bodily functions. These include plasma proteins, coagulation factors, and hormones. Importantly, the liver serves as a nutrient storage depot. It stores glycogen, the stored form of glucose, and vitamins to meet bodily demands. Additionally, it synthesizes coagulation factors, which control blood clotting and hemostasis [143]. Dysfunction of the liver can lead to bleeding issues and obesity, while obesity increases the risk of developing HCC [144]. Lastly, the liver boosts immune function, assisting the body in defending against infections and diseases. Therefore, it is imperative to prioritize liver health, particularly regarding HCC, and pursue early detection and treatment methods [145].
At present, HCC is of great concern in the medical field, mainly because it is one of the most common cancers worldwide, especially in Asia and Africa [146]. Its high incidence and mortality rate drive the medical community to seek more effective treatment methods [147]. Although the incidence rate and mortality of HCC in China are stable, the limitations of traditional therapies have prompted the medical community to constantly explore new therapeutic strategies such as immunotherapy, targeted therapy, and natural products [148]. Technological progress has also provided more accurate diagnostic and treatment methods, bringing hope for early detection and personalized treatment of HCC. Therefore, HCC, as a difficult cancer to treat, has always been one of the focuses of research and attention in the medical community.
The treatment options for HCC are still relatively limited. Although liver transplantation [149] and surgical resection [150] are considered one of the best treatment options and can improve patient survival [151], there are still many patients who have not adapted to these treatment methods or have drug resistance issues. Due to the significant drawback of high drug resistance in HCC, there is currently no small molecule or antibody therapy that can completely cure HCC. Methods such as liver transplantation and surgical resection only lead to a moderate overall increase in patient survival [152], which is in stark contrast to many other cancers. Even with some approved systemic therapies and surgical options, there is no universal treatment plan applicable to all patients with HCC [153]. The differences in disease conditions, tumor characteristics, and individual reactions among different patients still pose challenges to personalized and precise treatment. Some therapies may be effective in the early stages, but there may be issues with tolerance or reduced efficacy in long-term treatment, which limits the sustainability and effectiveness of the treatment. Although there are many exciting emerging treatment strategies, such as molecular targeted drugs and immunotherapy, many are still in the clinical trial stage and require more research to prove their safety and effectiveness, gradually applied to clinical treatment of HCC patients, and then widely promoted.
At present, the forefront of treating HCC lies in the emergence of immunotherapy and new drugs. Systemic drug treatments such as cabozantinib [154], ramucirumab [155], and immune checkpoint inhibitors [156] significantly improve patient prognosis. The progress of systemic therapy has also attracted attention, and new drugs have shown potential in the treatment of advanced HCC. Although systematic treatment plans have been promoted, their efficacy and survival time are still limited [157]. Natural products possess various beneficial characteristics, including multi-target, multi-level treatment, safety, and bioavailability advantages [158]. The ancient classic work “Compendium of Materia Medica” records the use of various natural products for the treatment of liver cancer. Some of these herbs, such as Astragalus membranaceus, Radix liquidities, Angelica Sinensis, etc., are described as exhibiting the effects of regulating ‘qi’ and blood, tonifying the spleen and kidneys, and enhancing the human immune system, which is helpful in the adjuvant treatment of HCC [159]. ‘Qi’ is an invisible energy phenomenon that exists in every living or inanimate object in the universe, constituting the human body and maintaining its life activities. It is an extremely delicate material, which embodies the unity of important substances and physiological functions in life [160]. In addition, Mulberry leaves, Gardenia, Hawthorn, etc., are also considered to exhibit the effects of clearing heat and detoxifying, promoting blood circulation and removing blood stasis, and can be used to assist in regulating liver function [161]. These ancient works record various herbs and their effects, providing a traditional foundation and direction for the treatment of HCC with TCM. However, further optimization is needed to improve the bioavailability of natural products, reduce nontargeted effects, and maintain therapeutic efficacy. The current challenge is to fully explore the efficacy of natural products, enhance their efficacy against HCC, and reduce nontargeted side effects. Although natural products provide new possibilities for the treatment of HCC, more research and technological optimization are still needed in clinical applications.
In the past decade of research, significant therapeutic effects have been associated with the use of natural products, indicating that innovative development of natural products poses challenges for the treatment of HCC. Starting from the direction of natural products, searching for targets for HCC can better cure the survival rate and reduce the recurrence rate of HCC patients. Compared with chemically synthesized drugs, these natural products have higher selectivity, can kill liver cancer, and have lower toxicity to healthy cells, making them attractive alternatives for cancer treatment [120]. Therefore, researchers are exploring the components extracted from natural products and these natural products themselves to block specific pathways of cancer formation, which is one of the common strategies for developing anti-cancer drugs [162].
Natural plant products have shown extensive potential for application in the treatment of HCC [163]. Researchers have conducted in-depth research on the mechanisms of action of various natural products in the treatment of HCC. Previous studies have shown that natural plant products, such as flavonoids [68,69], have significant inhibitory effects on liver cancer cells. Flavonoids exhibit multiple mechanisms in the treatment of HCC. They may interfere with the growth and spread of HCC through a variety of pathways, including the regulation of cyclins and apoptosis-related pathways. Especially the intervention of PI3K/Akt and Wnt/β-catenin transduction pathways of factors such as catenin, and inhibition of tumor angiogenesis. This multiple effect makes flavonoids a potential anti-liver cancer drug. Similarly, alkaloid compounds also exhibit multiple mechanisms of action in the treatment of HCC [98]. They play a crucial role in regulating cancer cell proliferation and growth, including inducing apoptosis, blocking the cell cycle, inhibiting angiogenesis, and intervening in tumor metastasis. These compounds may regulate key signaling pathways such as PI3K/Akt, MAPK, NF-κB, etc. [164,165], which affect the survival and proliferation of tumor cells, and inhibit their invasiveness, thereby limiting the growth and spread of tumors. Phenolic compounds also exhibit multiple mechanisms of action in the treatment of HCC. They have strong antioxidant properties, which can inhibit the proliferation of tumor cells, promote apoptosis, and block the invasion and metastasis of tumor cells. These compounds exert their effects by regulating key pathways such as cell cycle, apoptotic signaling pathways, and tumor angiogenesis [166]. In addition, they also affect the tumor microenvironment, reduce the invasiveness and angiogenesis of cancer cells, and limit the growth and spread of tumors. Phenolic alcohol compounds have become potential anti-HCC drugs due to their multiple mechanisms of action [102–104]. These products may exert therapeutic effects by regulating the cell cycle, inducing apoptosis, inhibiting metastasis, and angiogenesis. However, the clinical application of flavonoids in the treatment of HCC is more, the curative effect is better, and the side effects are less [167]. Previous studies have shown that flavonoids have the effects of antioxidation, improving blood circulation, protecting liver activity and multiple mechanisms of action, and helping to reduce the risk of hepatocellular carcinoma [79–81]. With the progress of science and technology, the development of the times, and the update of research methods, flavonoids will have breakthroughs and new understanding in the treatment of human diseases, especially HCC, and will have great practical significance in promoting the development of human health.
Natural products play a crucial role in preventing the development of HCC, partly because they can target specific receptors or regulate immune responses. Some natural products can interact with specific receptors to block the growth and spread of HCC. These products may bind to receptors on the surface of tumor cells, interfere with signaling pathways, and thus limit the proliferation and survival of tumor cells [165]. On the other hand, natural products may also combat HCC by regulating the immune response. They may activate or enhance the immune system, making it more effective in identifying and clearing tumor cells. This immune regulatory effect may include multiple mechanisms, such as enhancing T cell-mediated toxicity, promoting natural killer cell activity, and regulating immunosuppressive factors in the tumor microenvironment [168]. These mechanisms of action indicate that natural products have potential therapeutic value in inhibiting the development of HCC and have become important candidate drugs for studying immunotherapy and targeted therapy.
The application of natural products in inhibiting the development of HCC is not limited to drug therapy but also extends to surgical and interventional treatments. Natural products are widely used in the treatment process before and after surgery, helping to reduce surgical risk, reduce postoperative complications [169], and promote tissue repair [40]. In addition, natural products exhibit unique value in interventional therapy, such as serving as sensitizers or adjunctive therapy drugs during interventional radiation therapy, which helps to improve treatment efficacy and reduce side effects [170]. This comprehensive treatment model uses natural products as supplements or adjuncts to comprehensively apply in the overall treatment strategy of HCC, to achieve better treatment effects and improve patient survival rate.
In searching for treatment options for patients with HCC, we utilize targeted therapy to identify potential natural product drug candidates. The goal is to precisely target the genes associated with HCC, characterize ligand-target protein interactions, and validate the selected ligand as a potential drug candidate [170]. This approach offers an efficient means of screening target genes in HCC research, leveraging the benefits of time-savings and selective treatment with precise targeting of HCC. Although natural products have shown potential value in the treatment of HCC [171], more clinical trials are still needed to verify their safety and efficacy. These substances are expected to be used as independent therapies or in combination with traditional treatment methods, opening up new avenues for the future treatment of HCC. Looking ahead to the future, natural products may complement existing treatment models by combining chemotherapy, radiation therapy, and surgical treatment to improve treatment efficacy, reduce adverse reactions, and slow down tumor resistance [172]. With this comprehensive treatment strategy, natural products are expected to become an indispensable component of future comprehensive treatment plans for HCC, providing patients with higher survival rates and quality of life.
HCC is not only one of the most common malignant tumors in the world but also has a high mortality and recurrence rate. Due to the complex pathogenesis of hepatocellular carcinoma, the lack of predictable biomarkers, and the problem of persistent drug resistance, these factors have brought limitations to the treatment and continuous treatment. The clinical symptoms of early HCC are not obvious, and 50% of HCC patients are in the advanced stage at the time of diagnosis, and surgical treatment is recommended for advanced HCC. With the development of targeted therapy, the surgical treatment of advanced HCC has made some progress, but the survival benefit of HCC patients is still small, and there are still many limitations and challenges, for example, more than 2/3 of patients still have poor response to immunotherapy [173]. In the clinical practice of immunotherapy, due to the lack of T cells recognizing tumor-specific antigens in patients, some patients are still unable to eliminate tumors by relying on their T cells after the application of Immune checkpoint inhibitors (ICI) based immunotherapy [174]. At present, the pathogenesis of HCC is complex, and a single biomarker cannot accurately predict the prognosis of immunotherapy in HCC patients. Therefore, it is necessary to further explore the research of natural products in hepatocellular carcinoma, and then combine them with nanotechnology to deliver nanodrugs to the tumor site to achieve the accumulation in the tumor site, to achieve the purpose of anti-hepatocellular carcinoma.
Because of the difficulties in the diagnosis and treatment of HCC, the prospect of using natural products and molecular docking technology to develop therapeutic methods is promising and compelling. Compared with synthetic drugs, natural products have a wide variety and low toxicity, and are one of the reliable drug sources [175]. Natural products and their extracts, as anticancer agents, have been reported as natural triggers of apoptosis signaling in different cancers [176] and are effective against a variety of cancer characteristics. We are studying the combination of new technologies such as molecular docking with anticancer drugs. Molecular targeted drugs can regulate autophagy and cell cycle arrest, induce cell senescence, inhibit the occurrence and development of tumors, and play an anti-HCC role. After investigation, it was found that there are a large number of natural products with anticancer properties in nature. Although some of these drugs have been developed and achieved some effects in the treatment of HCC, there are still many unknown natural products that need further research and development. This exploration aims to find a new direction for the treatment of HCC.
Acknowledgement: None.
Funding Statement: This work was supported by Chongqing Natural Science Foundation General Project (2023NSCQ-MSX1633, CSTB2023NSCQ-MSX0393), Key Scientific and Technological Research Project of Chongqing Municipal Education Commission (KJ202302884457913, KJZD-K202302801), 2022 Scientific Research Project of Chongqing Medical and Pharmaceutical College (ygz2022104), Scientific Research and Seedling Breeding Project of Chongqing Medical Biotechnology Association (cmba2022kyym-zkxmQ0003), and Chongqing Natural Science Foundation (cstc2021jcyj-msxm3191, cstc2021jcyj-msxm0452), respectively.
Author Contributions: Jingxin Mao and Yan Li conceived and designed the research. Yan Li, Jingxin Mao, and Lingli Zhang carried out the data analysis and wrote the paper in the present study. Lingli Zhang, Jingxin Mao, and Yan Li finished the drawing and manuscript revision work. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare they have no conflicts of interest to report regarding the present study.
References
1. Younossi ZM, Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021;3(4):100305. doi:10.1016/j.jhepr.2021.100305 [Google Scholar] [PubMed] [CrossRef]
2. Bzeizi KI, Abdullah M, Vidyasagar K, Alqahthani SA, Broering D. Hepatocellular carcinoma recurrence and mortality rate post liver transplantation: meta-analysis and systematic review of real-world evidence. Cancers. 2022;14(20):5114. doi:10.3390/cancers14205114 [Google Scholar] [PubMed] [CrossRef]
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin. 2021;71(3):209–49. doi:10.3322/caac.v71.3. [Google Scholar] [CrossRef]
4. Llovet J, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi:10.1038/nrdp.2016.18 [Google Scholar] [PubMed] [CrossRef]
5. Nevola R, Ruocco R, Criscuolo L, Villani A, Alfano M, Beccia D, et al. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroentero. 2023;29(8):1243. doi:10.3748/wjg.v29.i8.1243 [Google Scholar] [PubMed] [CrossRef]
6. Dahlgren D, Lennernäs H. Antibody-drug conjugates and targeted treatment strategies for hepatocellular carcinoma: a drug-delivery perspective. Molecules. 2020;25(12):2861. doi:10.3390/molecules25122861 [Google Scholar] [PubMed] [CrossRef]
7. Shrivastava SS, Kharabe PM. Ancient roots of modern medicines; their prospects and promises. Pharmacogn Rev. 2021;15(29):20–31. doi:10.5530/phrev. [Google Scholar] [CrossRef]
8. Da Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol. 2001;1(4):364–9. doi:10.1016/S1471-4892(01)00063-7 [Google Scholar] [PubMed] [CrossRef]
9. Najmi A, Javed SA, Al Bratty M, Alhazmi HA. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules. 2022;27(2):349. doi:10.3390/molecules27020349 [Google Scholar] [PubMed] [CrossRef]
10. Muhammad N, Usmani D, Tarique M, Naz H, Ashraf M, Raliya R, et al. The role of natural products and their multitargeted approach to treat solid cancer. Cells. 2022;11(14):2209. doi:10.3390/cells11142209 [Google Scholar] [PubMed] [CrossRef]
11. Banerjee A, Sriramulu S, Catanzaro R, He F, Chabria Y, Balakrishnan B, et al. Natural compounds as integrative therapy for liver protection against inflammatory and carcinogenic mechanisms: from induction to molecular biology advancement. Curr Mol Med. 2023;23(3):216–31. doi:10.2174/1566524022666220316102310 [Google Scholar] [PubMed] [CrossRef]
12. Abdu S, Juaid N, Amin A, Moulay M, Miled N. Effects of sorafenib and quercetin alone or in combination in treating hepatocellular carcinoma: in vitro and in vivo approaches. Molecules. 2022;27(2):1–20. [Google Scholar]
13. Rodriguez S, Skeet K, Mehmetoglu-Gurbuz T, Goldfarb M, Karri S, Rocha J, et al. Phytochemicals as an alternative or integrative option, in conjunction with conventional treatments for hepatocellular carcinoma. Cancers. 2021;13(22):5753. doi:10.3390/cancers13225753 [Google Scholar] [PubMed] [CrossRef]
14. Li JJ, Liang Q, Sun GC. Traditional Chinese medicine for prevention and treatment of hepatocellular carcinoma: a focus on epithelial-mesenchymal transition. J Integr Med. 2021;19(6):469–77. doi:10.1016/j.joim.2021.08.004 [Google Scholar] [PubMed] [CrossRef]
15. Zhang S, Mo Z, Zhang S, Li X. A network pharmacology approach to reveal the underlying mechanisms of Artemisia annua on the treatment of hepatocellular carcinoma. Evid-Based Compl Alt. 2021;2021:1–9. [Google Scholar]
16. Luo TT, Lu Y, Yan SK, Xiao X, Rong XI, Guo J. Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chin J Integr Med. 2020;26(1):72–80. doi:10.1007/s11655-019-3064-0 [Google Scholar] [PubMed] [CrossRef]
17. Khan SA, Lee TKW. Network-pharmacology-based study on active phytochemicals and molecular mechanism of Cnidium monnieri in treating hepatocellular carcinoma. Int J Mol Sci. 2022;23(10):5400. doi:10.3390/ijms23105400 [Google Scholar] [PubMed] [CrossRef]
18. Guo W, Tan HY, Wang N, Feng Y. Chinese medicines for cancer treatment from the metabolomics perspective. In: Metabolomics-new insights into biology and medicine. London, UK: IntechOpen; 2019. p. 73–99. [Google Scholar]
19. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–62. doi:10.1016/S0140-6736(22)01200-4 [Google Scholar] [PubMed] [CrossRef]
20. O’Rourke JM, Sagar VM, Shah T, Shetty S. Carcinogenesis on the background of liver fibrosis: implications for the management of hepatocellular cancer. World J Gastroentero. 2018;24(39):4436. doi:10.3748/wjg.v24.i39.4436 [Google Scholar] [PubMed] [CrossRef]
21. Dhar D, Baglieri J, Kisseleva T, Brenner DA. Mechanisms of liver fibrosis and its role in liver cancer. Exp Biol Med. 2020;245(2):96–108. doi:10.1177/1535370219898141 [Google Scholar] [PubMed] [CrossRef]
22. Wang J, Chenivesse X, Henglein B, Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343(6258):555–7. doi:10.1038/343555a0 [Google Scholar] [PubMed] [CrossRef]
23. Rosa AS, Araujo OC, Savassi-Ribas F, Fernandes CA, Coelho HS, Niel C, et al. Prevalence of occult hepatitis B virus infection and Torque teno virus infection and their association with hepatocellular carcinoma in chronic hepatitis C patients. Virus Res. 2017;242:166–72. doi:10.1016/j.virusres.2017.09.022 [Google Scholar] [PubMed] [CrossRef]
24. Shen C, Jiang X, Li M, Luo Y. Hepatitis virus and hepatocellular carcinoma: recent advances. Cancers. 2023;15(2):533. doi:10.3390/cancers15020533 [Google Scholar] [PubMed] [CrossRef]
25. Ninio L, Nissani A, Meirson T, Domovitz T, Genna A, Twafra S, et al. Hepatitis C virus enhances the invasiveness of hepatocellular carcinoma via EGFR-mediated invadopodia formation and activation. Cells. 2019;8(11):1395. doi:10.3390/cells8111395 [Google Scholar] [PubMed] [CrossRef]
26. Koshiol J, Argirion I, Liu Z, Kim Lam T, O’Brien TR, Yu K, et al. Immunologic markers and risk of hepatocellular carcinoma in hepatitis B virus-and hepatitis C virus-infected individuals. Aliment Pharm Ther. 2021;54(6):833–42. doi:10.1111/apt.v54.6. [Google Scholar] [CrossRef]
27. Datfar T, Doulberis M, Papaefthymiou A, Hines IN, Manzini G. Viral hepatitis and hepatocellular carcinoma: state of the art. Pathogens. 2021;10(11):1366. doi:10.3390/pathogens10111366 [Google Scholar] [PubMed] [CrossRef]
28. Caviglia GP, Ciancio A, Rizzetto M. A review of HDV infection. Viruses. 2022;14(8):1749. doi:10.3390/v14081749 [Google Scholar] [PubMed] [CrossRef]
29. Conde de la Rosa L, Goicoechea L, Torres S, Garcia-Ruiz C, Fernandez-Checa JC. Role of oxidative stress in liver disorders. Livers. 2022;2(4):283–314. doi:10.3390/livers2040023. [Google Scholar] [CrossRef]
30. D’souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroentero. 2020;26(38):5759. doi:10.3748/wjg.v26.i38.5759 [Google Scholar] [PubMed] [CrossRef]
31. Baskiran A, Atay A, Baskiran D, Akbulut S. Hepatitis B/D-related hepatocellular carcinoma. A clinical literature review. J Gastrointest Canc. 2021;52(4):1–6. [Google Scholar]
32. He Y, Su Y, Duan C, Wang S, He W, Zhang Y, et al. Emerging role of aging in the progression of NAFLD to HCC. Ageing Res Rev. 2023;84:101833. doi:10.1016/j.arr.2022.101833 [Google Scholar] [PubMed] [CrossRef]
33. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73:4–13. doi:10.1002/hep.31288 [Google Scholar] [PubMed] [CrossRef]
34. Gellert-Kristensen H, Richardson TG, Davey Smith G, Nordestgaard BG, Tybjærg-Hansen A, Stender S. Combined effect of PNPLA3, TM6SF2, and HSD17B13 variants on risk of cirrhosis and hepatocellular carcinoma in the general population. Hepatology. 2020;72(3):845–56. doi:10.1002/hep.31238 [Google Scholar] [PubMed] [CrossRef]
35. Marti-Aguado D, Clemente-Sanchez A, Bataller R. Cigarette smoking and liver diseases. J Hepatol. 2022;77(1):191–205. doi:10.1016/j.jhep.2022.01.016 [Google Scholar] [PubMed] [CrossRef]
36. Kusnik A, Hunter N, Rasbach E, Miethke T, Reissfelder C, Ebert MP, et al. Co-medication and nutrition in hepatocellular carcinoma: potentially preventative strategies in hepatocellular carcinoma. Digest Dis. 2021;39(5):526–33. doi:10.1159/000514277 [Google Scholar] [PubMed] [CrossRef]
37. Wu H, Wang MD, Liang L, Xing H, Zhang CW, Shen F, et al. Nanotechnology for hepatocellular carcinoma: from surveillance, diagnosis to management. Small. 2021;17(6):2005236. doi:10.1002/smll.v17.6. [Google Scholar] [CrossRef]
38. Khan Y, Sadia H, Ali Shah SZ, Khan MN, Shah AA, Ullah N, et al. Classification, synthetic, and characterization approaches to nanoparticles, and their applications in various fields of nanotechnology: a review. Catalysts. 2022;12(11):1386. doi:10.3390/catal12111386. [Google Scholar] [CrossRef]
39. Alhalmi A, Beg S, Kohli K, Waris M, Singh T. Nanotechnology based approach for hepatocellular carcinoma targeting. Curr Drug Targets. 2021;22(7):779–92 [Google Scholar] [PubMed]
40. Zhu P, Liao W, Zhang WG, Chen L, Shu C, Zhang ZW, et al. A prospective study using propensity score matching to compare long-term survival outcomes after robotic-assisted, laparoscopic or open liver resection for patients with BCLC stage 0-A hepatocellular carcinoma. Ann Surg. 2022;277:103–11 [Google Scholar]
41. Yu SJ. Immunotherapy for hepatocellular carcinoma: recent advances and future targets. Pharmacol Therapeut. 2023;244:108387. doi:10.1016/j.pharmthera.2023.108387 [Google Scholar] [PubMed] [CrossRef]
42. Fang Y, Liu W, Tang Z, Ji X, Zhou Y, Song S, et al. Monocarboxylate transporter 4 inhibition potentiates hepatocellular carcinoma immunotherapy through enhancing T cell infiltration and immune attack. Hepatology. 2023;77(1):109–23. doi:10.1002/hep.32348 [Google Scholar] [PubMed] [CrossRef]
43. Colli LM, Machiela MJ, Zhang H, Myers TA, Jessop L, Delattre O, et al. Landscape of combination immunotherapy and targeted therapy to improve cancer management. Cancer Res. 2017;77(13):3666–71. doi:10.1158/0008-5472.CAN-16-3338 [Google Scholar] [PubMed] [CrossRef]
44. Mandlik DS, Mandlik SK, Choudhary HB. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. World J Gastroentero. 2023;29(6):1054. doi:10.3748/wjg.v29.i6.1054 [Google Scholar] [PubMed] [CrossRef]
45. Keilson JM, Knochelmann HM, Paulos CM, Kudchadkar RR, Lowe MC. The evolving landscape of immunotherapy in solid tumors. J Surg Oncol. 2021;123(3):798–806. doi:10.1002/jso.v123.3. [Google Scholar] [CrossRef]
46. Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2005;28(5):496–504. doi:10.1097/01.cji.0000171291.72039.e2 [Google Scholar] [PubMed] [CrossRef]
47. Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metast Rev. 2023;42:1–24. [Google Scholar]
48. Rinaldi L, Vetrano E, Rinaldi B, Galiero R, Caturano A, Salvatore T, et al. HCC and molecular targeting therapies: back to the future. Biomedicines. 2021;9(10):1345. doi:10.3390/biomedicines9101345 [Google Scholar] [PubMed] [CrossRef]
49. Zheng Y, Zhang W, Xu L, Zhou H, Yuan M, Xu H. Recent progress in understanding the action of natural compounds at novel therapeutic drug targets for the treatment of liver cancer. Front Oncol. 2022;11:795548. doi:10.3389/fonc.2021.795548 [Google Scholar] [PubMed] [CrossRef]
50. Xu J, Lin H, Wu G, Zhu M, Li M. IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma. Front Oncol. 2021;11:760971. doi:10.3389/fonc.2021.760971 [Google Scholar] [PubMed] [CrossRef]
51. Shen Z, Wang Y, Wang G, Gu W, Zhao S, Hu X, et al. Research progress of small-molecule drugs in targeting telomerase in human cancer and aging. Chem-Biol Interact. 2023;382:110631. doi:10.1016/j.cbi.2023.110631 [Google Scholar] [PubMed] [CrossRef]
52. Vishwakarma K, Dey R, Bhatt H. Telomerase: a prominent oncological target for development of chemotherapeutic agents. Eur J Med Chem. 2023;249:115121. doi:10.1016/j.ejmech.2023.115121 [Google Scholar] [PubMed] [CrossRef]
53. Relitti N, Saraswati AP, Federico S, Khan T, Brindisi M, Zisterer D, et al. Telomerase-based cancer therapeutics: a review on their clinical trials. Curr Top Med Chem. 2020;20(6):433–57. doi:10.2174/1568026620666200102104930 [Google Scholar] [PubMed] [CrossRef]
54. Altamura G, Degli Uberti B, Galiero G, De Luca G, Power K, Licenziato L, et al. The small molecule BIBR1532 exerts potential anti-cancer activities in preclinical models of feline oral squamous cell carcinoma through inhibition of telomerase activity and down-regulation of TERT. Front Vet Sci. 2021;7:620776. doi:10.3389/fvets.2020.620776 [Google Scholar] [PubMed] [CrossRef]
55. Larijani RS, Ravari NS, Goodarzi N, Akhlaghpour S, Larijani SS, Rouini MR, et al. Current status of transarterial chemoembolization (TACE) agents in hepatocellular carcinoma treatment. J Drug Deliv Sci Tec. 2022;77:103905. doi:10.1016/j.jddst.2022.103905. [Google Scholar] [CrossRef]
56. Liang B, Makamure J, Shu S, Zhang L, Sun T, Zheng C. Treatment response, survival, and safety of transarterial chemoembolization with CalliSpheres® microspheres versus conventional transarterial chemoembolization in hepatocellular carcinoma: a meta-analysis. Front Oncol. 2021;11:576232. doi:10.3389/fonc.2021.576232 [Google Scholar] [PubMed] [CrossRef]
57. Bajwa R, Madoff DC, Kishore SA. Embolotherapy for hepatic oncology: current perspectives and future directions. Dig Dis Int. 2020;4(2):134–47. doi:10.1055/s-0040-1712146 [Google Scholar] [PubMed] [CrossRef]
58. Liu W, Chen D, Su J, Zheng R, Kong R, Zhu B, et al. Quercetin induced HepG2 cells apoptosis through ATM/JNK/STAT3 signaling pathways. BIOCELL. 2023;47(1):187–94. doi:10.32604/biocell.2022.023030. [Google Scholar] [CrossRef]
59. Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15(5):283–98. doi:10.5582/bst.2021.01318 [Google Scholar] [PubMed] [CrossRef]
60. Chen F, Zhong Z, Tan HY, Guo W, Zhang C, Tan CW, et al. Uncovering the anticancer mechanisms of Chinese herbal medicine formulas: therapeutic alternatives for liver cancer. Front Pharmacol. 2020;11:293. doi:10.3389/fphar.2020.00293 [Google Scholar] [PubMed] [CrossRef]
61. Eissa IH, Ibrahim MK, Metwaly AM, Belal A, Mehany AB, Abdelhady AA, et al. Design, molecular docking, in vitro, and in vivo studies of new quinazolin-4 (3H)-ones as VEGFR-2 inhibitors with potential activity against hepatocellular carcinoma. Bioorg Chem. 2021;107:104532. doi:10.1016/j.bioorg.2020.104532 [Google Scholar] [PubMed] [CrossRef]
62. Man S, Luo C, Yan M, Zhao G, Ma L, Gao W. Treatment for liver cancer: from sorafenib to natural products. Eur J Med Chem. 2021;224:113690. doi:10.1016/j.ejmech.2021.113690 [Google Scholar] [PubMed] [CrossRef]
63. Zhang Q, Feng Z, Gao M, Guo L. Determining novel candidate anti-hepatocellular carcinoma drugs using interaction networks and molecular docking between drug targets and natural compounds of SiNiSan. PeerJ. 2021;9:e10745. doi:10.7717/peerj.10745 [Google Scholar] [PubMed] [CrossRef]
64. Liu CY, Sun YY, Wang SQ, Jia YQ, Wang HX, Pan LC, et al. Dihydromyricetin from Ampelopsis grossedentata and its derivatives: structural characterization and anti-hepatocellular carcinoma activity. J Mol Struct. 2022;1258:132677. doi:10.1016/j.molstruc.2022.132677. [Google Scholar] [CrossRef]
65. Vo TK, Ta QTH, Chu QT, Nguyen TT, Vo VG. Anti-hepatocellular-cancer activity exerted by β-sitosterol and β-sitosterol-glucoside from Indigofera zollingeriana Miq. Molecules. 2020;25(13):3021. doi:10.3390/molecules25133021 [Google Scholar] [PubMed] [CrossRef]
66. Zou Y, Lu N, Yang X, Xie Z, Lei X, Liu X, et al. Synthesis and anti-hepatocellular carcinoma evaluation of salicylic acid-modified indole trimethoxy flavonoid derivatives. RSC Med Chem. 2023;14(6):1172–85. doi:10.1039/D3MD00128H [Google Scholar] [PubMed] [CrossRef]
67. Cheng X, Zhong F, He K, Sun S, Chen H, Zhou J. EHHM, a novel phenolic natural product from Livistona chinensis, induces autophagy-related apoptosis in hepatocellular carcinoma cells. Oncol Lett. 2016;12(5):3739–48. doi:10.3892/ol.2016.5178 [Google Scholar] [PubMed] [CrossRef]
68. Ma H, Li X, Che J, Fan H, Liu Q, Xia H. The inhibitory effect of Periplaneta americana L. on hepatocellular carcinoma: explore the anti-hepatocellular carcinoma active site and its mechanism of action. J Ethnopharmacol. 2022;291:114884. doi:10.1016/j.jep.2021.114884 [Google Scholar] [PubMed] [CrossRef]
69. Al-Ghamdi MA, Alsulami RR, Bakkar A, Kumosani TA, Barrbour EK, Abulnaja KO, et al. Khalas date flavonoids inhibited cell viability, induced apoptosis and expression of the pro-autophagy LC3-B gene in human hepatocellular carcinoma cells (HepG2). Nat Prod Res. 2023;37(18):3109–13. doi:10.1080/14786419.2022.2140803 [Google Scholar] [PubMed] [CrossRef]
70. Baby J, Devan AR, Kumar AR, Gorantla JN, Nair B, Aishwarya TS, et al. Cogent role of flavonoids as key orchestrators of chemoprevention of hepatocellular carcinoma: a review. J Food Biochem. 2021;45(7):e13761 [Google Scholar] [PubMed]
71. Corrêa WR, Serain AF, Netto LA, Marinho JV, Arena AC, de Santana Aquino DF, et al. Anti-inflammatory and antioxidant properties of the extract, tiliroside, and patuletin 3-O-β-D-glucopyranoside from Pfaffia townsendii (Amaranthaceae). Evid-Based Compl Alt. 2018; 2018:1–9. [Google Scholar]
72. Guo Z, Lou Y, Kong M, Luo Q, Liu Z, Wu J. A systematic review of phytochemistry, pharmacology and pharmacokinetics on astragali radix: implications for astragali radix as a personalized medicine. Int J Mol Sci. 2019;20(6):1–44. [Google Scholar]
73. Yan X, Yu A, Zheng H, Wang S, He Y, Wang L. Calycosin-7-O-β-D-glucoside attenuates OGD/R-induced damage by preventing oxidative stress and neuronal apoptosis via the SIRT1/FOXO1/PGC-1α pathway in HT22 Cells. Neural Plast. 2019;2019:8798069 [Google Scholar] [PubMed]
74. Tsai CC, Wu HH, Chang CP, Lin CH, Yang HH. Calycosin-7-O-β-D-glucoside reduces myocardial injury in heat stroke rats. J Formos Med Assoc. 2019;118(3):730–8. doi:10.1016/j.jfma.2018.08.024 [Google Scholar] [PubMed] [CrossRef]
75. Xu W, Zhou F, Zhu Q, Bai M, Luo T, Zhou L, et al. Calycosin-7-O-β-D-glucoside attenuates palmitate-induced lipid accumulation in hepatocytes through AMPK activation. Eur J Pharmacol. 2022;925:174988. doi:10.1016/j.ejphar.2022.174988 [Google Scholar] [PubMed] [CrossRef]
76. Huang JZ, Li LL, Tan XY, Wu ZY, Chen DW, Luo X. The effect of calycosin-7-O-β-D-glucoside and its synergistic augmentation of cisplatin-induced apoptosis in SK-OV-3 Cells. Curr Pharm Des. 2022;28(26):2161–6. doi:10.2174/1381612828666220610164100 [Google Scholar] [PubMed] [CrossRef]
77. Casili G, Lanza M, Campolo M, Messina S, Scuderi S, Ardizzone A, et al. Therapeutic potential of flavonoids in the treatment of chronic venous insufficiency. Vasc Pharmacol. 2021;137:106825. doi:10.1016/j.vph.2020.106825 [Google Scholar] [PubMed] [CrossRef]
78. Wang Z, Clifford MN, Sharp P. Analysis of chlorogenic acids in beverages prepared from Chinese health foods and investigation, in vitro, of effects on glucose absorption in cultured Caco-2 cells. Food Chem. 2008;108(1):369–73. doi:10.1016/j.foodchem.2007.10.083. [Google Scholar] [CrossRef]
79. Gürler SB, Kiraz Y, Baran Y. Flavonoids in cancer therapy: current and future trends. Bio Biomed. 2020:403–40. [Google Scholar]
80. Liskova A, Koklesova L, Samec M, Smejkal K, Samuel SM, Varghese E, et al. Flavonoids in cancer metastasis. Cancers. 2020;12(6):1498. doi:10.3390/cancers12061498 [Google Scholar] [PubMed] [CrossRef]
81. Wu J, Huang G, Li Y, Li X. Flavonoids from Aurantii Fructus Immaturus and Aurantii Fructus: promising phytomedicines for the treatment of liver diseases. Chin Med. 2020;15:1–18. [Google Scholar]
82. Butt SS, Khan K, Badshah Y, Rafiq M, Shabbir M. Evaluation of pro-apoptotic potential of taxifolin against liver cancer. PeerJ. 2021;9:e11276. doi:10.7717/peerj.11276 [Google Scholar] [PubMed] [CrossRef]
83. O’Brien K, Matlin AJ, Lowell AM, Moore MJ. The biflavonoid isoginkgetin is a general inhibitor of pre-mRNA splicing. J Biol Chem. 2008;283(48):33147–54. doi:10.1074/jbc.M805556200 [Google Scholar] [PubMed] [CrossRef]
84. Sivaramakrishnan M, McCarthy KD, Campagne S, Huber S, Meier S, Augustin A, et al. Binding to SMN2 pre-mRNA-protein complex elicits specificity for small molecule splicing modifiers. Nat Commun. 2017;8(1):1476. doi:10.1038/s41467-017-01559-4 [Google Scholar] [PubMed] [CrossRef]
85. Li M, Li B, Hou Y, Tian Y, Chen L, Liu S, et al. Anti-inflammatory effects of chemical components from Ginkgo biloba L. male flowers on lipopolysaccharide-stimulated RAW264.7 macrophages. Phytother Res. 2019;33(4):989–97. doi:10.1002/ptr.v33.4. [Google Scholar] [CrossRef]
86. Li M, Li B, Xia ZM, Tian Y, Zhang D, Rui WJ, et al. Anticancer Effects of five biflavonoids from Ginkgo biloba L. male flowers in vitro. Molecules. 2019;24(8):1–13. [Google Scholar]
87. Yao J, Tang S, Shi C, Lin Y, Ge L, Chen Q, et al. Isoginkgetin, a potential CDK6 inhibitor, suppresses SLC2A1/GLUT1 enhancer activity to induce AMPK-ULK1-mediated cytotoxic autophagy in hepatocellular carcinoma. Autophagy. 2023;19(4):1221–38. doi:10.1080/15548627.2022.2119353 [Google Scholar] [PubMed] [CrossRef]
88. Yang C, Lu T, Liu M, Yuan X, Li D, Zhang J, et al. Tiliroside targets TBK1 to induce ferroptosis and sensitize hepatocellular carcinoma to sorafenib. Phytomedicine. 2023;111:154668. doi:10.1016/j.phymed.2023.154668 [Google Scholar] [PubMed] [CrossRef]
89. Wei X, Zeng Y, Meng F, Wang T, Wang H, Yuan Y, et al. Calycosin-7-glucoside promotes mitochondria-mediated apoptosis in hepatocellular carcinoma by targeting thioredoxin 1 to regulate oxidative stress. Chem-Biol Interact. 2023;374:110411. doi:10.1016/j.cbi.2023.110411 [Google Scholar] [PubMed] [CrossRef]
90. Sun J, Yang X, Sun H, Huang S, An H, Xu W, et al. Baicalin inhibits hepatocellular carcinoma cell growth and metastasis by suppressing ROCK1 signaling. Phytother Res. 2023;37(9):4117–32. doi:10.1002/ptr.v37.9. [Google Scholar] [CrossRef]
91. Yang H, Khan S, Sun A, Bai Q, Cheng H, Akhtari K. Enhancement of interferon gamma stability as an anticancer therapeutic protein against hepatocellular carcinoma upon interaction with calycosin. Int J Biol Macromol. 2021;185:813–20. doi:10.1016/j.ijbiomac.2021.06.159 [Google Scholar] [PubMed] [CrossRef]
92. Wang M, Ren S, Bi Z, Zhang L, Cui M, Sun R, et al. Myricetin reverses epithelial-endothelial transition and inhibits vasculogenic mimicry and angiogenesis of hepatocellular carcinoma by directly targeting PAR1. Phytother Res. 2022;36(4):1807–21. doi:10.1002/ptr.v36.4. [Google Scholar] [CrossRef]
93. Wan H, Ge L, Li J, Zhang K, Wu W, Peng S, et al. Effects of a novel biflavonoid of Lonicera japonica flower buds on modulating apoptosis under different oxidative conditions in hepatoma cells. Phytomedicine. 2019;57:282–91. doi:10.1016/j.phymed.2018.12.044 [Google Scholar] [PubMed] [CrossRef]
94. Thomas NS, George K, Selvam AAA. Anticancer mechanism of troxerutin via targeting Nrf2 and NF-κB signalling pathways in hepatocarcinoma cell line. Toxicol in Vitro. 2019;54:317–29. doi:10.1016/j.tiv.2018.10.018 [Google Scholar] [PubMed] [CrossRef]
95. Khalid HR, Aamir M, Tabassum S, Alghamdi YS, Alzamami A, Ashfaq UA. Integrated system pharmacology approaches to elucidate multi-target mechanism of solanum surattense against hepatocellular carcinoma. Molecules. 2022;27(19):6220. doi:10.3390/molecules27196220 [Google Scholar] [PubMed] [CrossRef]
96. Hong M, Almutairi MM, Li S, Li J. Wogonin inhibits cell cycle progression by activating the glycogen synthase kinase-3 beta in hepatocellular carcinoma. Phytomedicine. 2020;68:153174. doi:10.1016/j.phymed.2020.153174 [Google Scholar] [PubMed] [CrossRef]
97. Liu G, Shi A, Wang N, Li M, He X, Yin C, et al. Polyphenolic Proanthocyanidin-B2 suppresses proliferation of liver cancer cells and hepatocellular carcinogenesis through directly binding and inhibiting AKT activity. Redox Biol. 2020;37:101701. doi:10.1016/j.redox.2020.101701 [Google Scholar] [PubMed] [CrossRef]
98. Bao X, Liu Y, Huang J, Yin S, Sheng H, Han X, et al. Stachydrine hydrochloride inhibits hepatocellular carcinoma progression via LIF/AMPK axis. Phytomedicine. 2022;100:154066. doi:10.1016/j.phymed.2022.154066 [Google Scholar] [PubMed] [CrossRef]
99. Li Y, Li Y, Zhang J, Ji L, Li M, Sun X, et al. Current perspective of traditional Chinese medicines and active ingredients in the therapy of hepatocellular carcinoma. J Hepatocell Carcino. 2022;9:41–56. doi:10.2147/JHC.S346047 [Google Scholar] [PubMed] [CrossRef]
100. Rodenak-Kladniew B, Castro A, Stärkel P, Galle M, Crespo R. 1, 8-Cineole promotes G0/G1 cell cycle arrest and oxidative stress-induced senescence in HepG2 cells and sensitizes cells to anti-senescence drugs. Life Sci. 2020;243:117271. doi:10.1016/j.lfs.2020.117271 [Google Scholar] [PubMed] [CrossRef]
101. Li S, Hao L, Hu X, Li L. A systematic study on the treatment of hepatitis B-related hepatocellular carcinoma with drugs based on bioinformatics and key target reverse network pharmacology and experimental verification. Infect Agents Cancer. 2023;18(1):41. doi:10.1186/s13027-023-00520-z [Google Scholar] [PubMed] [CrossRef]
102. Liu Y, Zhang Y, Muema FW, Kimutai F, Chen G, Guo M. Phenolic compounds from Carissa spinarum are characterized by their antioxidant, anti-inflammatory and hepatoprotective activities. Antioxidants. 2021;10(5):652. doi:10.3390/antiox10050652 [Google Scholar] [PubMed] [CrossRef]
103. Ahmed OM, Fahim HI, Mohamed EE, Abdel-Moneim A. Protective effects of Persea americana fruit and seed extracts against chemically induced liver cancer in rats by enhancing their antioxidant, anti-inflammatory, and apoptotic activities. Environ Sci Pollut Res. 2022;29(29):43858–73. doi:10.1007/s11356-022-18902-y [Google Scholar] [PubMed] [CrossRef]
104. Morsi EA, Ahmed HO, Abdel-Hady H, El-Sayed M, Shemis MA. GC-analysis, and antioxidant, anti-inflammatory, and anticancer activities of some extracts and fractions of linum usitatissimum. Curr Bioinf C. 2020;16(9):1306–18. [Google Scholar]
105. Zafar S, Sarfraz I, Rasul A, Shah MA, Hussain G, Zahoor MK, et al. Osthole: a multifunctional natural compound with potential anticancer, antioxidant and anti-inflammatory activities. Mini Rev Med Chem. 2021;21(18):2747–63. doi:10.2174/1389557520666200709175948 [Google Scholar] [PubMed] [CrossRef]
106. Wu P, Meng X, Zheng H, Zeng Q, Chen T, Wang W, et al. Kaempferol attenuates ROS-induced hemolysis and the molecular mechanism of its induction of apoptosis on bladder cancer. Molecules. 2018;23(10):2592. doi:10.3390/molecules23102592 [Google Scholar] [PubMed] [CrossRef]
107. Zhu L, Xue L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol Res. 2019;27(6):629–34. doi:10.3727/096504018X15228018559434 [Google Scholar] [PubMed] [CrossRef]
108. Kim TW, Lee SY, Kim M, Cheon C, Ko SG. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018;9(9):875. doi:10.1038/s41419-018-0930-1 [Google Scholar] [PubMed] [CrossRef]
109. Xia Q, Zhao KJ, Huang ZG, Zhang P, Dong TT, Li SP, et al. Molecular genetic and chemical assessment of Rhizoma Curcumae in China. J Agric Food Chem. 2005;53(15):6019–26. doi:10.1021/jf0508495 [Google Scholar] [PubMed] [CrossRef]
110. Liu W, Cui X, Zhong Y, Ma R, Liu B, Xia Y. Phenolic metabolites as therapeutic in inflammation and neoplasms: molecular pathways explaining their efficacy. Pharmacol Res. 2023;193:106812. doi:10.1016/j.phrs.2023.106812 [Google Scholar] [PubMed] [CrossRef]
111. Rahman MM, Rahaman MS, Islam MR, Rahman F, Mithi FM, Alqahtani T, et al. Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules. 2021;27(1):233. doi:10.3390/molecules27010233 [Google Scholar] [PubMed] [CrossRef]
112. Li Y, Qin C, Dong L, Zhang X, Wu Z, Liu L, et al. Whole grain benefit: synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022;13(24):12686–96. doi:10.1039/D2FO01746F [Google Scholar] [PubMed] [CrossRef]
113. Simón J, Casado-Andrés M, Goikoetxea-Usandizaga N, Serrano-Maciá M, Martínez-Chantar ML. Nutraceutical properties of polyphenols against liver diseases. Nutrients. 2020;12(11):3517. doi:10.3390/nu12113517 [Google Scholar] [PubMed] [CrossRef]
114. Cheng C, Li Z, Zhao X, Liao C, Quan J, Bode AM, et al. Natural alkaloid and polyphenol compounds targeting lipid metabolism: treatment implications in metabolic diseases. Eur J Pharmacol. 2020;870:172922. doi:10.1016/j.ejphar.2020.172922 [Google Scholar] [PubMed] [CrossRef]
115. Kiruthiga C, Devi KP, Nabavi SM, Bishayee A. Autophagy: a potential therapeutic target of polyphenols in hepatocellular carcinoma. Cancers. 2020;12(3):562. doi:10.3390/cancers12030562 [Google Scholar] [PubMed] [CrossRef]
116. Zhang Q, Chen L, Gao M, Wang S, Meng L, Guo L. Molecular docking and in vitro experiments verified that kaempferol induced apoptosis and inhibited human HepG2 cell proliferation by targeting BAX, CDK1, and JUN. Mol Cell Biochem. 2023;478(4):767–80. doi:10.1007/s11010-022-04546-6 [Google Scholar] [PubMed] [CrossRef]
117. Siddiqui S, Upadhyay S, Ahmad I, Hussain A, Ahamed M. Cytotoxicity of Moringa oleifera fruits on human liver cancer and molecular docking analysis of bioactive constituents against caspase-3 enzyme. J Food Biochem. 2021;45(5):e13720 [Google Scholar] [PubMed]
118. Naz H, Tarique M, Ahamad S, Alajmi MF, Hussain A, Rehman MT, et al. Hesperidin-CAMKIV interaction and its impact on cell proliferation and apoptosis in the human hepatic carcinoma and neuroblastoma cells. J Cell Biochem. 2019;120(9):15119–30. doi:10.1002/jcb.v120.9. [Google Scholar] [CrossRef]
119. Huang X, Rehman HM, Szöllősi AG, Zhou S. Network pharmacology-based approach combined with bioinformatic analytics to elucidate the potential of curcumol against hepatocellular carcinoma. Genes. 2022;13(4):653. doi:10.3390/genes13040653 [Google Scholar] [PubMed] [CrossRef]
120. Shindoh J, Kawamura Y, Kobayashi Y, Kobayashi M, Akuta N, Okubo S, et al. Prognostic impact of surgical intervention after lenvatinib treatment for advanced hepatocellular carcinoma. Ann Surg Oncol. 2021;28(12):7663–72. doi:10.1245/s10434-021-09974-0 [Google Scholar] [PubMed] [CrossRef]
121. Zhao Q, Bai J, Chen Y, Liu X, Zhao S, Ling G, et al. An optimized herbal combination for the treatment of liver fibrosis: hub genes, bioactive ingredients, and molecular mechanisms. J Ethnopharmacol. 2022;297:115567. doi:10.1016/j.jep.2022.115567 [Google Scholar] [PubMed] [CrossRef]
122. Rehman G, Hamayun M, Iqbal A, Khan SA, Khan H, Shehzad A, et al. Effect of methanolic extract of dandelion roots on cancer cell lines and AMP-activated protein kinase pathway. Front Pharmacol. 2017;8:875. doi:10.3389/fphar.2017.00875 [Google Scholar] [PubMed] [CrossRef]
123. Davaatseren M, Hur HJ, Yang HJ, Hwang JT, Park JH, Kim HJ, et al. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem Toxicol. 2013;58:30–6. doi:10.1016/j.fct.2013.04.023 [Google Scholar] [PubMed] [CrossRef]
124. Pfingstgraf IO, Taulescu M, Pop RM, Orăsan R, Vlase L, Uifalean A, et al. Protective effects of Taraxacum officinale L.(Dandelion) root extract in experimental acute on chronic liver failure. Antioxidants. 2021;10(4):504. doi:10.3390/antiox10040504 [Google Scholar] [PubMed] [CrossRef]
125. Wu WD, Chen PS, Omar HA, Arafa ESA, Pan HW, Jeng J, et al. Antrodia cinnamomea boosts the anti-tumor activity of sorafenib in xenograft models of human hepatocellular carcinoma. Sci Rep. 2018;8(1):12914. doi:10.1038/s41598-018-31209-8 [Google Scholar] [PubMed] [CrossRef]
126. Zhu PL, Fu XQ, Li JK, Tse AKW, Guo H, Yin CL, et al. Antrodia camphorata mycelia exert anti-liver cancer effects and inhibit STAT3 signaling in vitro and in vivo. Front Pharmacol. 2018;9:1449. doi:10.3389/fphar.2018.01449 [Google Scholar] [PubMed] [CrossRef]
127. Chen YY, Liu FC, Wu TS, Sheu MJ. Antrodia cinnamomea inhibits migration in human hepatocellular carcinoma cells: the role of ERp57 and PGK-1. Am J Chin Med. 2015;43(8):1671–96. doi:10.1142/S0192415X15500950 [Google Scholar] [PubMed] [CrossRef]
128. Hsieh YC, Rao YK, Wu CC, Huang CYF, Geethangili M, Hsu SL, et al. Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin-and Bax-triggered mitochondrial pathway. Chem Res Toxicol. 2010;23(7):1256–67. doi:10.1021/tx100116a [Google Scholar] [PubMed] [CrossRef]
129. Song TY, Hsu SL, Yen GC. Induction of apoptosis in human hepatoma cells by mycelia of Antrodia camphorata in submerged culture. J Ethnopharmacol. 2005;100(1–2):158–67 [Google Scholar] [PubMed]
130. Sureda A, Martorell M, Capó X, Monserrat-Mesquida M, Quetglas-Llabrés MM, Rasekhian M, et al. Antitumor effects of triterpenes in hepatocellular carcinoma. Curr Med Chem. 2021;28(13):2465–84. doi:10.2174/0929867327666200602132000 [Google Scholar] [PubMed] [CrossRef]
131. Bachar SC, Bachar R, Jannat K, Jahan R, Rahmatullah M. Hepatoprotective natural products. Annu Rep Med Chem. 2020;55:207–49. [Google Scholar]
132. Nuzzo G, Senese G, Gallo C, Albiani F, Romano L, d’Ippolito G, et al. Antitumor potential of immunomodulatory natural products. Mar Drugs. 2022;20(6):386. doi:10.3390/md20060386 [Google Scholar] [PubMed] [CrossRef]
133. Zhang Y, Ma A, Xi H, Chen N, Wang R, Yang C, et al. Antrodia cinnamomea ameliorates neointimal formation by inhibiting inflammatory cell infiltration through downregulation of adhesion molecule expression in vitro and in vivo. Food Sci Hum Well. 2021;10(4):421–30. doi:10.1016/j.fshw.2021.04.004. [Google Scholar] [CrossRef]
134. Gnocchi D, Del Coco L, Girelli CR, Castellaneta F, Cesari G, Sabbà C, et al. 1H-NMR metabolomics reveals a multitarget action of Crithmum maritimum ethyl acetate extract in inhibiting hepatocellular carcinoma cell growth. Sci Re. 2021;11(1):1259. [Google Scholar]
135. Huang W, Kong L, Cao Y, Yan L. Identification and quantification, metabolism and pharmacokinetics, pharmacological activities, and botanical preparations of protopine: a review. Molecules. 2021;27(1):215. doi:10.3390/molecules27010215 [Google Scholar] [PubMed] [CrossRef]
136. Ballmer-Hofer K. Vascular endothelial growth factor, from basic research to clinical applications. Int J Mol Sci. 2018;19(12):3750. doi:10.3390/ijms19123750 [Google Scholar] [PubMed] [CrossRef]
137. Zheng Y, Ji S, Li X, Feng Q. Active ingredients and molecular targets of Taraxacum mongolicum against hepatocellular carcinoma: network pharmacology, molecular docking, and molecular dynamics simulation analysis. PeerJ. 2022;10:e13737. doi:10.7717/peerj.13737 [Google Scholar] [PubMed] [CrossRef]
138. Zhang Y, Lv P, Ma J, Chen N, Guo H, Chen Y, et al. Antrodia cinnamomea exerts an anti-hepatoma effect by targeting PI3K/AKT-mediated cell cycle progression in vitro and in vivo. Acta Pharm Sin B. 2022;12(2):890–906. doi:10.1016/j.apsb.2021.07.010 [Google Scholar] [PubMed] [CrossRef]
139. Samreen S, Khan E, Ahmad IZ. Molecular docking and molecular dynamics simulation analysis of bioactive compounds of Cichorium intybus L. seed against hepatocellular carcinoma. J Biomol Struct Dyn. 2023;25:1–12. [Google Scholar]
140. Qin L, Huang D, Huang J, Qin F, Huang H. Integrated analysis and finding reveal anti-liver cancer targets and mechanisms of pachyman (Poria cocos polysaccharides). Front Pharmacol. 2021;12:742349. doi:10.3389/fphar.2021.742349 [Google Scholar] [PubMed] [CrossRef]
141. Batool S, Morton Cuthrell K, Tzenios N, Shehryar Z. Hepatocellular carcinoma in non-alcoholic fatty liver disease: emerging burden. Int J Oncol. 2022;6(4):93–104. [Google Scholar]
142. Panda AK. Ayurveda-an indigenous medicine in holistic liver care. Int J Aayush Trad Med. 2022;2(2):32–7. [Google Scholar]
143. Trivedi P, Wang S, Friedman SL. The power of plasticity—metabolic regulation of hepatic stellate cells. Cell Metab. 2021;33(2):242–57. doi:10.1016/j.cmet.2020.10.026 [Google Scholar] [PubMed] [CrossRef]
144. Sohn W, Lee HW, Lee S, Lim JH, Lee MW, Park CH, et al. Obesity and the risk of primary liver cancer: a systematic review and meta-analysis. Clin Mol Hepatol. 2021;27(1):157–74. doi:10.3350/cmh.2020.0176 [Google Scholar] [PubMed] [CrossRef]
145. Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;77(1):128–39. doi:10.1016/j.jhep.2022.01.023 [Google Scholar] [PubMed] [CrossRef]
146. Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol. 2021;15(11):1295–307. doi:10.1080/17474124.2021.1991792 [Google Scholar] [PubMed] [CrossRef]
147. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–61. doi:10.1016/j.jhep.2019.08.025 [Google Scholar] [PubMed] [CrossRef]
148. Ganesan P, Kulik LM. Hepatocellular carcinoma: new developments. Clin Liver Dis. 2023;27(1):85–102. doi:10.1016/j.cld.2022.08.004 [Google Scholar] [PubMed] [CrossRef]
149. Yang JD, Larson JJ, Watt KD, Allen AM, Wiesner RH, Gores GJ, et al. Hepatocellular carcinoma is the most common indication for liver transplantation and placement on the waitlist in the United States. Clin Gastroenterol H. 2017;15(5):767–75.e3. doi:10.1016/j.cgh.2016.11.034 [Google Scholar] [PubMed] [CrossRef]
150. Kim H, Ahn S, Hong S, Yoon K, Kim H, Choi Y, et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Brit J Surg. 2017;104(8):1045–52. doi:10.1002/bjs.10541 [Google Scholar] [PubMed] [CrossRef]
151. Mehta N, Guy J, Frenette CT, Dodge JL, Osorio RW, Minteer WB, et al. Excellent outcomes of liver transplantation following down-staging of hepatocellular carcinoma to within Milan criteria: a multicenter study. Clin Gastroenterol H. 2018;16(6):955–64. doi:10.1016/j.cgh.2017.11.037 [Google Scholar] [PubMed] [CrossRef]
152. Lee DD, Sapisochin G, Mehta N, Gorgen A, Musto KR, Hajda H, et al. Surveillance for HCC after liver transplantation: increased monitoring may yield aggressive treatment options and improved postrecurrence survival. Transplantation. 2020;104(10):2105–12. doi:10.1097/TP.0000000000003117 [Google Scholar] [PubMed] [CrossRef]
153. Javan H, Dayyani F, Abi-Jaoudeh N. Therapy in advanced hepatocellular carcinoma. Semin Intervent Radiol. 2020;37(5):466–74. doi:10.1055/s-0040-1719187 [Google Scholar] [PubMed] [CrossRef]
154. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002 [Google Scholar] [PubMed] [CrossRef]
155. Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121Ba fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28(5):780–7. doi:10.1200/JCO.2009.23.7537 [Google Scholar] [PubMed] [CrossRef]
156. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. The Lancet. 2017;389(10088):2492–502. doi:10.1016/S0140-6736(17)31046-2 [Google Scholar] [PubMed] [CrossRef]
157. Chan LK, Ng IOL. Joining the dots for better liver cancer treatment. Nat Rev Gastro Hepat. 2020;17(2):74–5. doi:10.1038/s41575-019-0238-3 [Google Scholar] [PubMed] [CrossRef]
158. Wang S, Bao YR, Meng XS. Analysis of the absorbed constituents and mechanism of liquidambaris fructus extract on hepatocellular carcinoma. Front Pharmacol. 2022;13:999935. doi:10.3389/fphar.2022.999935 [Google Scholar] [PubMed] [CrossRef]
159. Dai X, Feng J, Chen Y, Huang S, Shi X, Liu X, et al. Traditional Chinese medicine in nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Chin Med. 2021;16:1–17. [Google Scholar]
160. Li XT. Investigation on the mechanism of Qi-invigoration from a perspective of effects of Sijunzi decoction on mitochondrial energy metabolism. Altern Med. 2012;12:247–75. [Google Scholar]
161. Liao K, Gong L, Yang Y, He Y, Wang F, Huang Y, et al. A comprehensive review of research progress in Chinese medicines for primary liver cancer treatment. Tradit Med Res. 2022;7(2):10. doi:10.53388/TMR20220207263. [Google Scholar] [CrossRef]
162. Suganya V, Anuradha V. In silico molecular docking of astaxanthin and sorafenib with different apoptotic proteins involved in hepatocellular carcinoma. Biocatal Agric Biotech. 2019;19:101076. doi:10.1016/j.bcab.2019.101076. [Google Scholar] [CrossRef]
163. Al-Malki AL. In vitro cytotoxicity and pro-apoptotic activity of phycocyanin nanoparticles from Ulva lactuca (Chlorophyta) algae. Saudi J Biol Sci. 2020;27(3):894–8. doi:10.1016/j.sjbs.2019.12.037 [Google Scholar] [PubMed] [CrossRef]
164. Wang Y, Li J, Xia L. Plant-derived natural products and combination therapy in liver cancer. Front Oncol. 2023;13:1116532. doi:10.3389/fonc.2023.1116532 [Google Scholar] [PubMed] [CrossRef]
165. Narayanankutty A. Natural products as PI3K/Akt inhibitors: implications in preventing hepatocellular carcinoma. CCurr Mol Pharmacol. 2021;14(5):760–9. doi:10.2174/1874467214666210120152657 [Google Scholar] [PubMed] [CrossRef]
166. Hemalatha G, Sivakumari K, Rajesh S, Shyamala Devi K. Phytochemical profiling, anticancer and apoptotic activity of graviola (Annona muricata) fruit extract against human hepatocellular carcinoma (HepG-2) cells. Int J Zool Appl Biosci. 2020;5(1):32–47. [Google Scholar]
167. Khan H, Ullah H, Martorell M, Valdes SE, Belwal T, Tejada S, et al. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. In: Semin cancer biology. Amsterdam: Elsevier; 2021. [Google Scholar]
168. Hsu CY, Mustafa MA, Kumar A, Pramanik A, Sharma R, Mohammed F, et al. Exploiting the immune system in hepatic tumor targeting: unleashing the potential of drugs, natural products, and nanoparticles. Pathol Res Pract. 2024;256:155266. doi:10.1016/j.prp.2024.155266 [Google Scholar] [PubMed] [CrossRef]
169. Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GAM, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229–35. doi:10.1097/01.sla.0000197706.21803.a1 [Google Scholar] [PubMed] [CrossRef]
170. Mustafa G, Mahrosh HS, Attique SA, Arif R, Farah MA, Al-Anazi KM, et al. Identification of plant peptides as novel inhibitors of orthohepevirus A (HEV) capsid protein by virtual screening. Molecules. 2023;28(6):2675. doi:10.3390/molecules28062675 [Google Scholar] [PubMed] [CrossRef]
171. Singh AK, Singh SV, Kumar R, Kumar S, Senapati S, Pandey AK. Current therapeutic modalities and chemopreventive role of natural products in liver cancer: progress and promise. World J Hepatol. 2023;15(1):1–18. doi:10.4254/wjh.v15.i1.1 [Google Scholar] [PubMed] [CrossRef]
172. Wei L, Wang Z, Jing N, Lu Y, Yang J, Xiao H, et al. Frontier progress of the combination of modern medicine and traditional Chinese medicine in the treatment of hepatocellular carcinoma. Chin Med. 2022;17(1):90. doi:10.1186/s13020-022-00645-0 [Google Scholar] [PubMed] [CrossRef]
173. Pinter M, Jain RK, Duda DG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: a review. JAMA Oncol. 2021;7(1):113–23. doi:10.1001/jamaoncol.2020.3381 [Google Scholar] [PubMed] [CrossRef]
174. Chaoul N, Mancarella S, Lupo L, Giannelli G, Dituri F. Impaired anti-tumor T cell response in hepatocellular carcinoma. Cancers. 2020;12(3):627. doi:10.3390/cancers12030627 [Google Scholar] [PubMed] [CrossRef]
175. Rawat D, Shrivastava S, Naik RA, Chhonker SK, Mehrotra A, Koiri RK. An overview of natural plant products in the treatment of hepatocellular carcinoma. Anti-Cancer Agent Me. 2018;18(13):1838–59. [Google Scholar]
176. Kim C, Kim B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: a review. Nutrients. 2018;10(8):1021. doi:10.3390/nu10081021 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF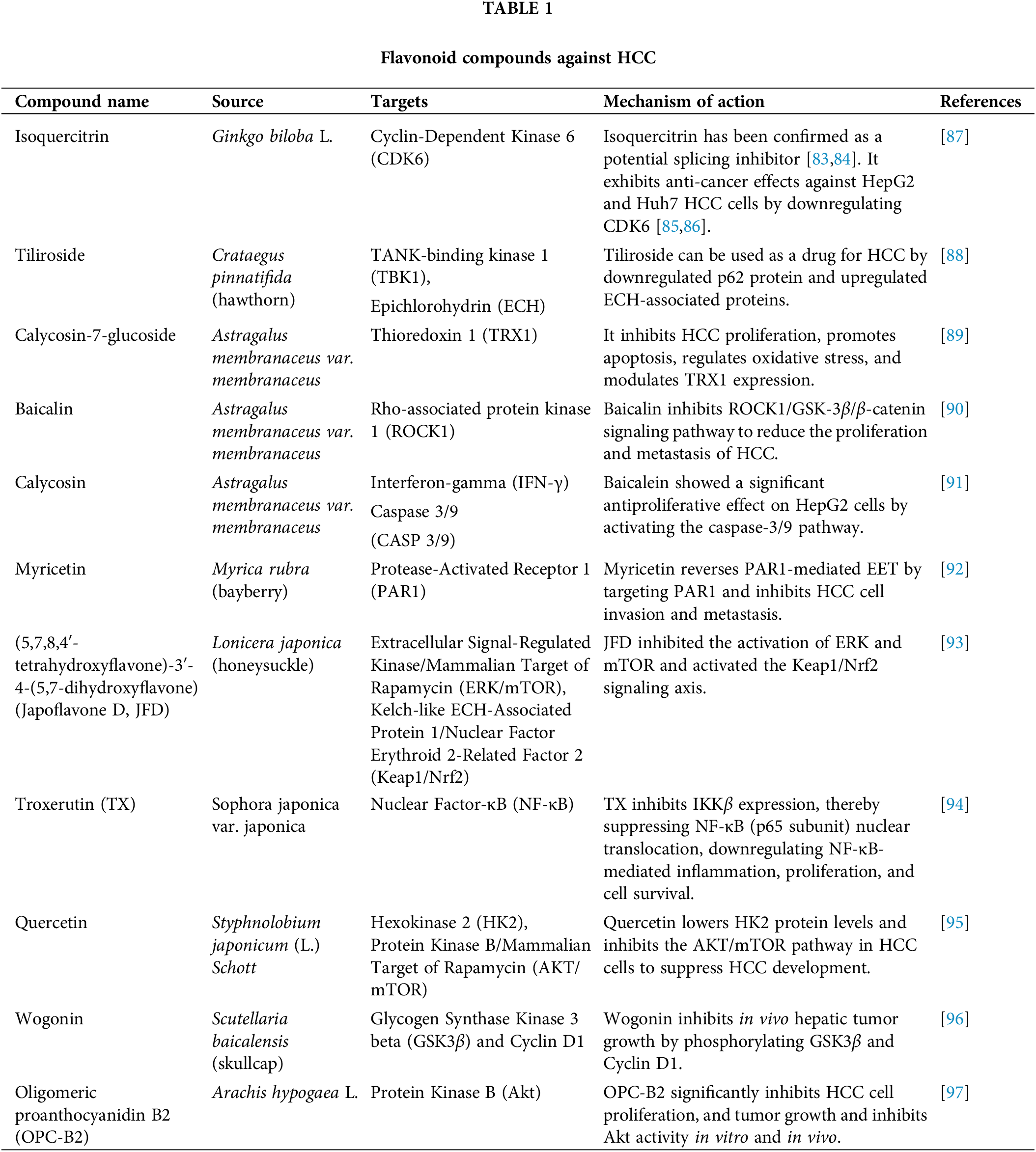
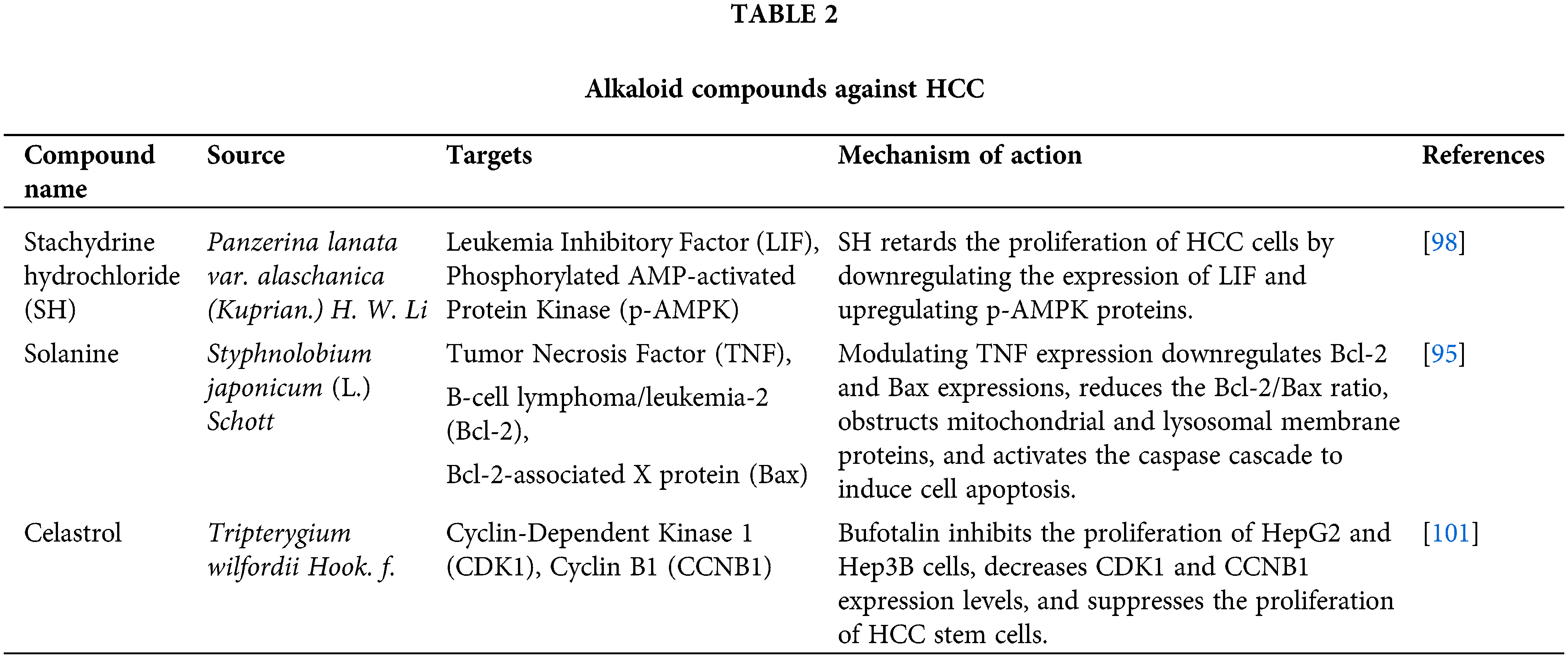
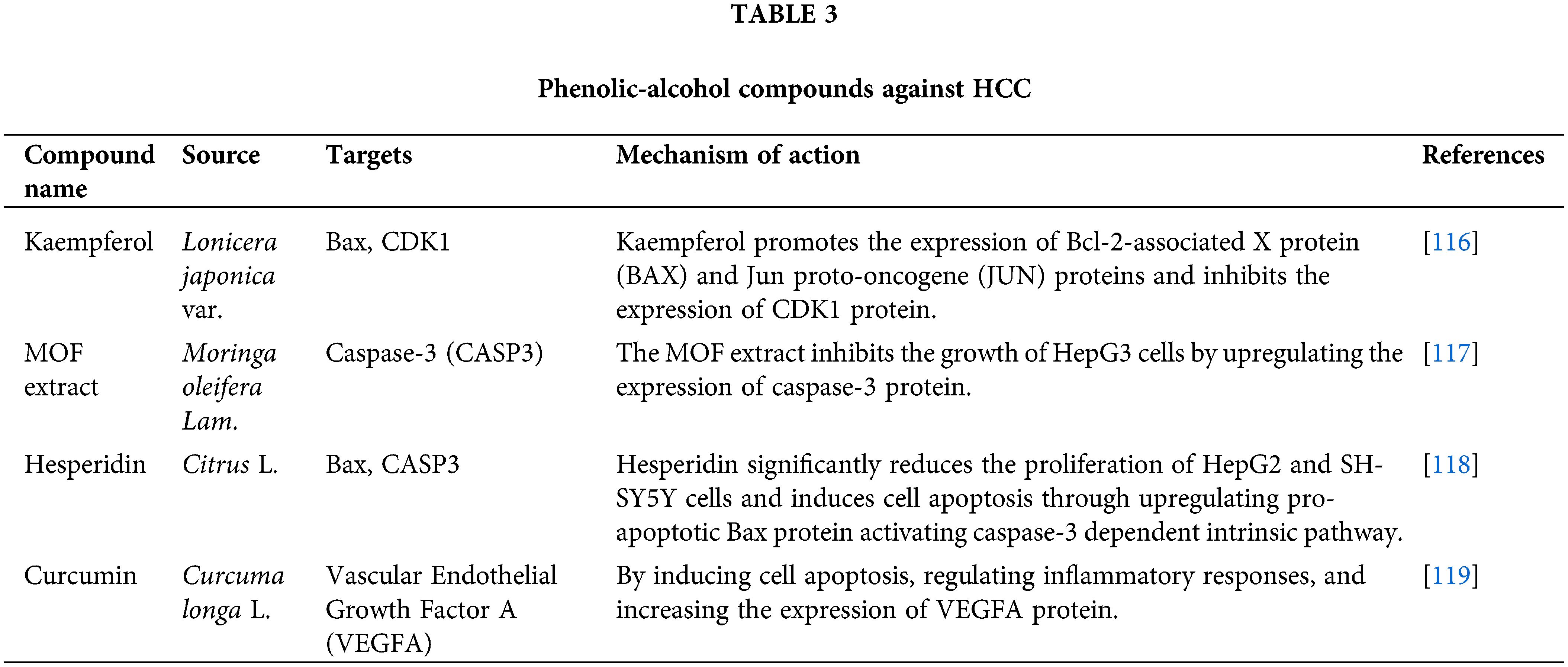
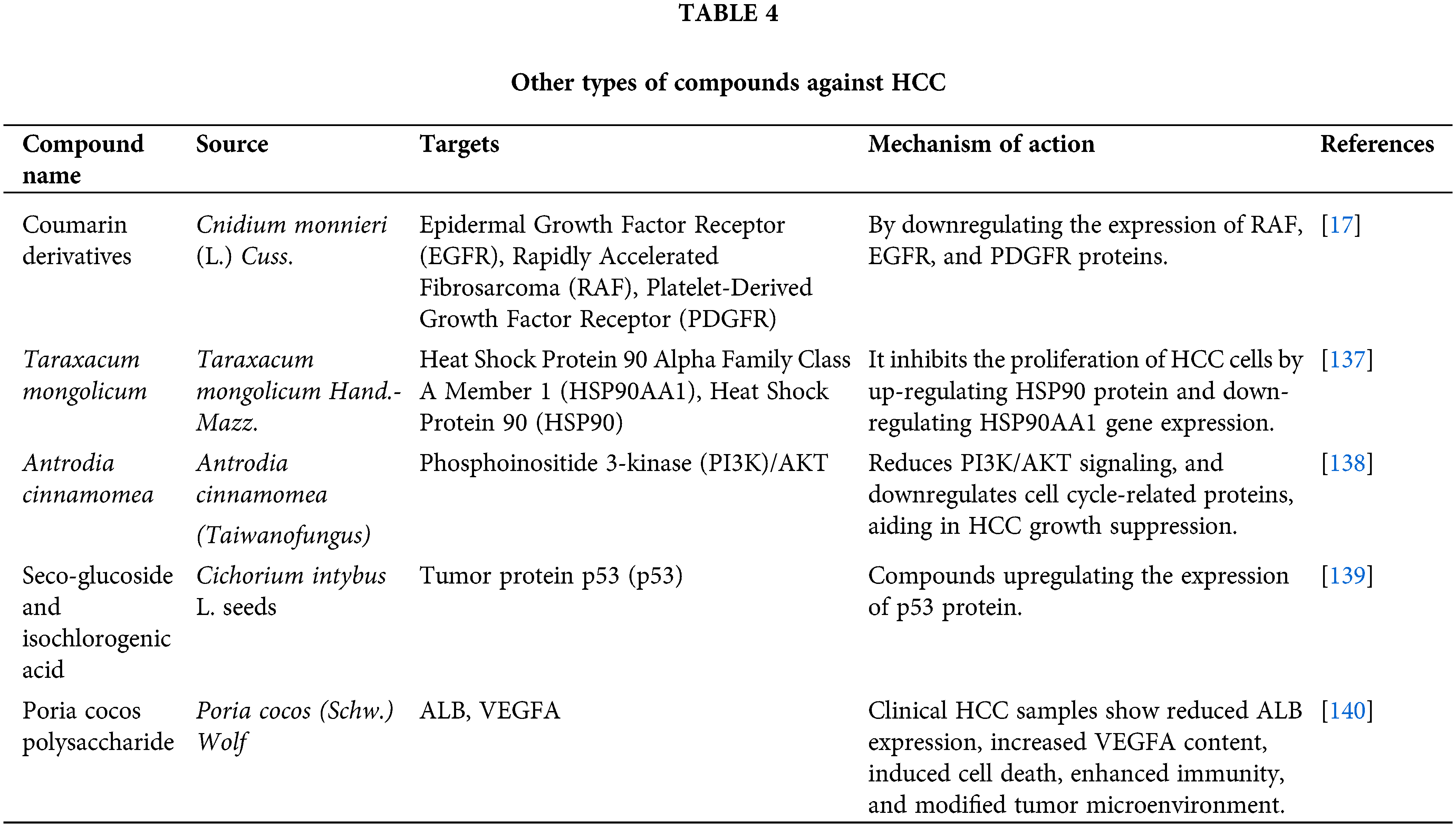
 Downloads
Downloads
 Citation Tools
Citation Tools