 Open Access
Open Access
REVIEW
Does young feces make the elderly live better? Application of fecal microbiota transplantation in healthy aging
1 Department of Endodontics, Stomatological Hospital of Chongqing Medical University, Chongqing, China
2 Chongqing Key Laboratory of Oral Diseases and Biomedical Sciences, Chongqing, China
3 Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing, China
4 Department of Research & Development, Zhejiang Charioteer Pharmaceutical Co., Ltd., Taizhou, China
5 Department of Implantology, Stomatological Hospital of Chongqing Medical University, Chongqing, China
* Corresponding Author: TING GONG. Email:
(This article belongs to the Special Issue: Gut Microbiota in Human Health: Exploring the Complex Interplay)
BIOCELL 2024, 48(6), 873-887. https://doi.org/10.32604/biocell.2024.050324
Received 02 February 2024; Accepted 26 March 2024; Issue published 10 June 2024
Abstract
As we are facing an aging society, anti-aging strategies have been pursued to reduce the negative impacts of aging and increase the health span of human beings. Gut microbiota has become a key factor in the anti-aging process. Modulation of gut microbiota by fecal microbiota transplantation (FMT) to prevent frailty and unhealthy aging has been a hot topic of research. This narrative review summarizes the benefits of FMT for health span and lifespan, brains, eyes, productive systems, bones, and others. The mechanisms of FMT in improving healthy aging are discussed. The increased beneficial bacteria and decreased pathological bacteria decreased gut permeability and systemic inflammation, increased short-chain fatty acid (SCFA) and SCFA-producing bacteria, and other factors are listed as mechanisms of FMT to improve healthy aging. The points that need to be considered to ensure the optimal outcomes of FMT are also discussed, such as recipients’ age, sex, genetic background, and gut microbiota after FMT. Although this field is still in its infancy, it has shown that FMT has great potential to improve healthy aging.Keywords
The aging population is a social and economic problem worldwide. In the next 30 years, the number of people aged ≥65 years is estimated to more than double, reaching 1.5 billion globally, with the majority in less-developed countries. As a result, the costs of health care will escalate enormously [1]. Reducing the negative impacts of advanced age and increasing health span has therefore been an important goal of anti-aging research. The human gut microbiota which is composed of approximately 10 to 100 trillion microorganisms is estimated to be almost equal to the number of human body cells [2]. Gut microbiota has been proposed as an additional organ of the human body [3–6], shifting with age [1,7] and influencing not only the digestive systems [8] but also other organs such as the brain [9], muscles [10], eye [11], bones [12], reproductive systems [13], etc. Microbiome disturbance has been suggested as a new hallmark of aging [14] in addition to the original nine hallmarks of aging proposed by López-Otín and colleagues in 2013 [15]. The idea of modulating gut microbiota to improve healthy aging is captivating. Methods of gut microbiota modulation include dietary intervention, prebiotics, probiotics, postbiotics, synbiotics, and fecal microbiota transplantation (FMT) [16,17]. FMT is the process of transplanting fecal bacteria from healthy donors to those with gut dysbiosis to restore the community and function of gut microbiota [18]. The main indication for FMT is antibiotic-refractory Clostridium difficile infection (CDI), with a satisfactory cure rate of 87%–90% [19]. FMT has also been applied in treating other digestive diseases such as inflammatory bowel diseases, irritable bowel syndrome, constipation, colon cancer, etc. Beyond the digestive tract, FMT has been proven beneficial for problems with metabolism, autoimmunity, and nervous system development [20,21]. In 2017, researchers found that feeding middle-aged African turquoise killifish with gut contents from young donors resulted in lifespan extension and delayed behavioral decline [22,23], which has encouraged enthusiasm for application of FMT to improve healthy aging. There was a surge of research in applying FMT to reverse aged-related pathology, such as cognitive impairment, inflammation, decreased reproductive functions, age-related macular degeneration, osteoporosis, etc. In this review, we define transplanting gut microbiota from young donors to aged ones as yFMT, and the reverse one as aFMT. This review highlights the beneficial effects of young feces and the detrimental effects of old feces, the mechanisms of yFMT to improve healthy aging, and key points to achieve optimal FMT outcomes. Considering the progress in simplified FMT procedures and deep understanding of the mechanisms of FMT, the promising results of applying FMT in improving healthy aging may show an alternative way of anti-aging strategies.
Beneficial Effects of Young Feces
African turquoise killifish is a naturally short-lived vertebrate, transplanting the gut microbiota from young donors to middle-aged individuals resulted in a 41% increase in the median lifespan, compared to transplanting the gut microbiota from the same age [22]. FMT from wild-type mice to progeroid mice enhanced the health span and lifespan of progeroid mice [24].
Stroke is a disease predominately associated with aging, yFMT to aged mice before a stroke can decrease the mortality rate after experimental stroke, with decreased neurological deficits, and increased hang times and activities. The inflammatory cytokines in plasma were decreased [25]. Another study validated that yFMT to aged mice before stroke can decrease neurological deficits and infarct volume in the brain after stroke, the decreased interleukin-17 (IL-17) in serum, colon, and brain might be the mediated factor of neuroprotective effects of yFMT [26]. yFMT to aged mice after stroke can rescue behavioral impairment, and reduce brain and gut inflammation, suggesting yFMT may be a “post-stroke bacteriotherapy”. Transplanting short-chain fatty acids (SCFAs)-producing bacteria to aged mice after stroke can also alleviate post-stroke neurological deficits and inflammation, which suggests the effect of yFMT was mediated by SCFAs [27]. yFMT to aged mice can decrease the portion of activated microglia in the hippocampus, and restore the aged hippocampal metabolome, with glutamine as a potential driver. For aging-associated behavioral deficits, yFMT can improve aging-associated impairments in long-term spatial memory and learning and also show a potential anxiety-alleviating therapeutic effect [28]. yFMT to middle-aged killifish can delay motor decline. This effect can last for 16 weeks after FMT, which shows the long-lasting effects of FMT [22]. To sum up, yFMT to aged mice can decrease stroke symptoms and mortalities when used before stroke or after stroke, and yFMT to aged mice can improve memory impairments and alleviate anxiety.
Benefits for reproductive organs
yFMT to aged zebrafish promoted oocyte growth, decreased the blood concentration of estradiol, and returned the balance of sex hormones in the elderly [29]. This effect was associated with reestablishing youth-like transcriptomic phenotype in the elderly gonads, thus attenuating the functional decline [30]. yFMT to aged female mice can decrease follicle atresia and apoptosis in ovaries, and increase cellular proliferation in the ovaries. Besides, the first litter size of FMT mice after pregnancy was much bigger than non-FMT mice [31]. Vulvovaginal atrophy is a common menopause-related symptom in middle-aged women, transplanting feces of ovary-intact fecund mice to ovariectomized mice can alleviate vaginal epithelial atrophy and enhance vaginal regeneration [32].
Numerous studies have shown that yFMT can restore the aged gut microbiota to young-like gut microbiota [27,33,34]. yFMT to aged mice can enhance intestinal barrier function, with increasing occludin, claudin, and zonula occludens-1 (ZO-1) expression in the intestine 24 weeks after FMT, the intestinal structure showed less swelling mucosa, sub-epithelial space expansion, and well-arranged villi compared to non-FMT mice [35]. Regulatory T (Treg) cells in the intestinal lamina propria are anti-inflammatory and suppress immune responses [36]. yFMT to aged mice increased CD4+Foxp3+ Treg cells in the small intestine. The mucins are produced by goblet cells in the intestine and are protective of the host. yFMT to aged mice can enhance the number of mature goblet cells and increase mucin gene expression in large intestines. The intestinal integrity was increased by yFMT [27]. Germinal center reaction in Peyer’s patches of the small intestine is diminished in aged mice, yFMT or co-housing with young mice can rescue the reduced germinal center reaction in aged mice [37]. M cells are specialized enterocytes in Peyer’s patches of the small intestine, which can transport antigens and show immunosurveillance function. Aging causes a decline in functional M cell maturation. However, housing aged mice on used bedding from young mice was sufficient to enhance the functional maturation of M cells in Peyer’s patches of aged mice [38]. Aging is characterized by disordered bile acid homeostasis. Intermittent co-housing with young mice for ten weeks can decrease the ratios of primary to secondary bile acids in the liver, serum, and intestinal segments in aged mice, thus rescuing the disordered bile acids homeostasis [39]. Housing aged mice in dirty cages of young mice for one week can protect aged mice from subsequent CDI infection, with decreased mortality rate and increased early innate immune responses [40,41]. To conclude, these findings suggest that yFMT can restore the aged gut microbiota to young-like gut microbiota, increase intestinal barrier function, and rescue the disordered bile acids homeostasis.
Eyes are sensitive and vulnerable to age-related functional decline and inflammatory damage. As age advances, the complement protein C3 and pro-inflammatory cytokines increase in the retina which causes retina degeneration. yFMT to aged mice significantly reduced C3 and pro-inflammatory cytokines in the eyes of aged mice. Retinal pigment epithelial protein RPE65, which is critical for retinal visual pigment regeneration in photoreceptors, was increased after yFMT in aged mice [34]. The lacrimal glands are highly susceptible to aging, with increased inflammation, lipid deposition, and disturbed rhythmic transcriptomic profiling. yFMT to aged mice can reduce chronic inflammation and lipid deposition, improve rhythmic transcriptomic profiling, and change the aberrant neural response of the aging lacrimal glands in aged mice [33]. yFMT to aged zebrafish can diminish the areas of all retinal layers and re-organize the histological assembly of aged eyes. Proteome perturbation of aged eyes caused by perfluorobutane sulfonate can be effectively shifted by the yFMT towards the control phenotype, suggesting the high ameliorative potential of yFMT along the gut-retina axis [42]. In sum, these findings indicate that yFMT can reduce inflammation of the aged eyes, and improve rhythmic transcriptomic profiling of the aging lacrimal glands.
yFMT can alleviate bone loss in aged rats with senile osteoporosis [35]. Transplanting the gut microbiota of young children to osteoporotic mice can prevent bone loss, enhance bone growth, and maintain bone strength, while the gut microbiota of aged people could not induce bone protective effects in osteoporotic mice [43].
yFMT to aged zebrafish can alleviate the hyperglycemia symptom in the elderly by stimulating the secretion of insulin [44]. yFMT to aged zebrafish can increase body weights, and decrease vacuolization structure defects in aged livers. The digestive activity of lipids was improved, which was associated with activating mitochondrial β-oxidation of fatty acids in the liver of aged zebrafish [45]. Older BALB/c mice receiving FMT from younger BALB/c mice showed increased tight junction-related genes in the colon, and increased Salmonella infection survival compared to control mice [46]. Co-housing with young mice can reduce hepatic inflammation and splenomegaly in old mice (Fig. 1) [39].
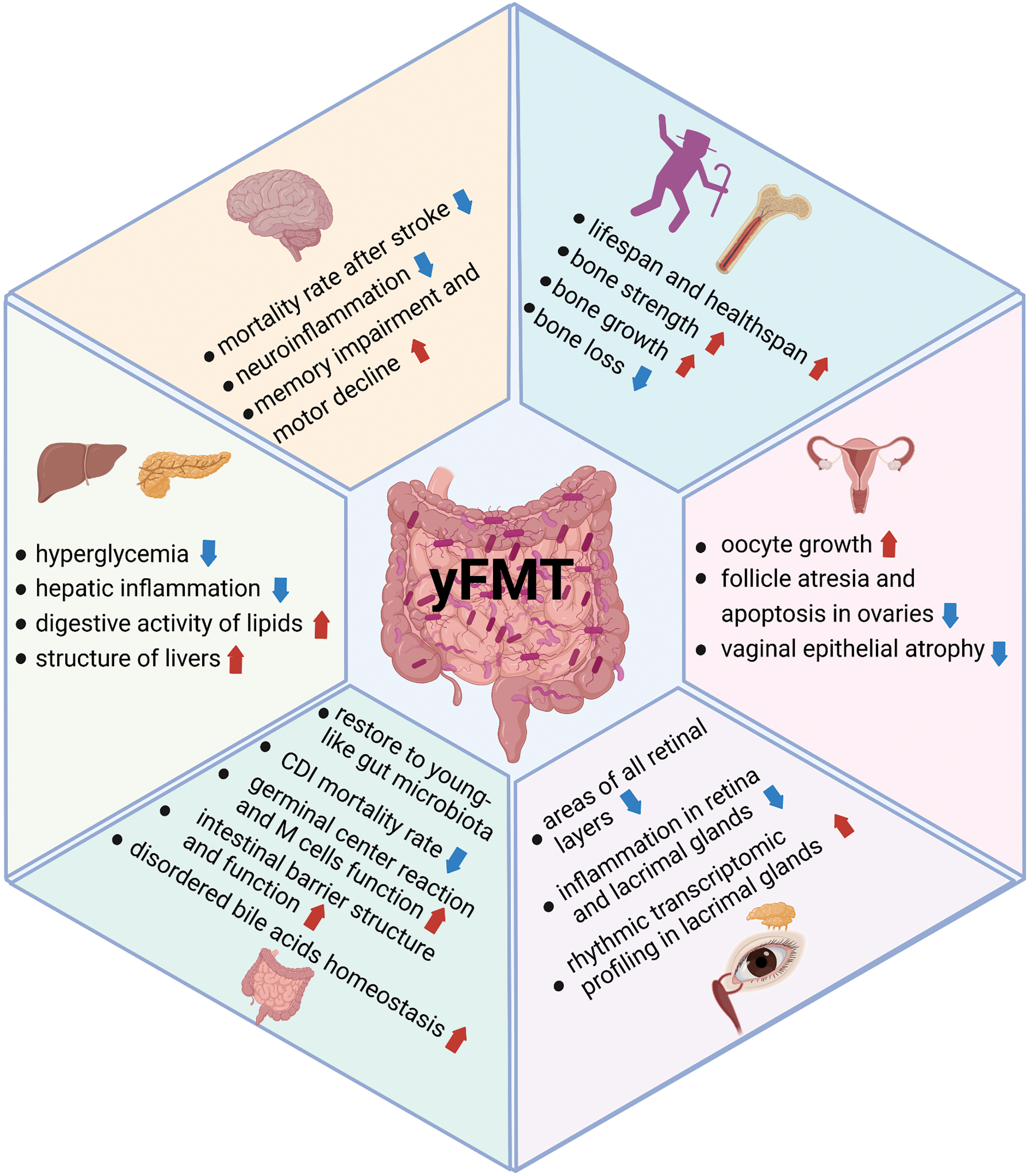
Figure 1: Benefits of yFMT for healthy aging. The benefits of yFMT are summarized, including extending lifespan, improving brain functions and bone health, rejuvenating reproductive organs, and benefits for the eyes, gut, liver, and pancreas. Created with BioRender.Com.
Clinical studies of yFMT for healthy aging
Four frail older patients with CDI were treated at home using nasojejunal tube-delivered or encapsulated donor feces. All patients aged more than 80 years and improved clinically with one FMT. No adverse events related to FMT were observed. This case report shows that frail older people may benefit from FMT both for clinical cure and for palliation [41]. Another study demonstrated that FMT can palliate CDI symptoms and improve cognitive functions for older patients with both CDI and dementia when compared to those who did not receive FMT. FMT led to changes in the gut microbiota composition, alanine, aspartate, and glutamate metabolism pathways were also changed by FMT. The authors suggest that FMT may effectively delay cognitive decline in patients with dementia [47].
Detrimental Effects of Old Feces
FMT from older progeroid mice to younger progeroid mice caused reduced survival with a reduction in median lifespan [24]. Flies that were fed with aged-fly homogenate showed significantly decreased lifespan, compared to flies that were fed with young-fly homogenate [48].
Detrimental for brain function
Five studies have shown that aFMT in young ones causes cognitive impairment [49–53]. aFMT to young mice causes decreased spatial learning and memory but does not affect anxiety-like behavior or motor activity [49]. FMT from aged people or aged mice to young mice can induce cognitive impairment [50,52]. aFMT to young rats also caused cognitive impairment [51]. aFMT to young germ-free (GF) mice induced depressive-like behavior, and impaired short-term memory and spatial memory [53]. The mechanisms for this cognitive impairment include changed brain structure [49,51,52], increased pro-inflammatory cytokines and oxidative stress [51], decreased SCFA-producing bacteria [49,53], and decreased vagus ascending activity [52]. aFMT to young mice decreased neurogenesis and novelty-induced neuronal activation [52], altered expression of proteins involved in synaptic plasticity and neurotransmission in the hippocampus, and changed microglia to an aging-like phenotype [49]. aFMT to young rats decreased the regional homogeneity in the medial prefrontal cortex and hippocampus, changed synaptic structures and decreased dendritic spines, and reduced protein expression of synaptic plasticity [51]. However, one study indicated healthy old gut microbiota transplanted to young GF mice have beneficial effects with increased adult neurogenesis, which may be associated with increased butyrate-producing microbes and pro-longevity hormone fibroblast growth factor 21 (FGF21) [54]. Another study showed that transplanting gut microbiota from centenarians to mice can reduce age-related indices and increase probiotic bacteria and SCFA-producing bacteria [55]. This difference shows that aged gut microbiota may be a double-edged sword. During healthy aging, the gut microbiota may support the health of the host, but when in gut dysbiosis, the microbiome may elicit typical aging characteristics.
aFMT to young GF mice can promote inflammation, with an increased translocation of inflammatory bacterial products into the circulation, and enhanced CD4+ T cell differentiation in the spleen. This effect was associated with lower levels of Akkermansia and higher levels of TM7 bacteria and Proteobacteria [56]. Cohousing aged specific-pathogen-free (SPF) mice with young GF mice can increase levels of plasma tumor necrosis factor (TNF) in young GF mice more than cohousing with young SPF mice [57].
Cohousing aged SPF mice with young GF mice can increase gut paracellular permeability of GF mice, compared to cohousing young SPF mice with young GF mice, which suggests that aFMT increases gut permeability [57].
aFMT to young GF mice can transfer the obese phenotypes of aged mice to GF mice, which shows that gut microbiota alone is sufficient to induce some of the manifestations of obesity [58]. Transplanting the gut microbiota of senile osteoporotic rats to young female rats can cause osteoporosis. The changed gut microbiota and the impaired intestinal barrier contributed to the pathogenesis of osteoporosis [59]. Transplanting gut microbiota of aged people to mice can exacerbate acute pancreatitis during both the early and recovery stages, which may be caused by the absence of multiple types of non-dominant species in aged gut microbiota compared to the young gut microbiota, thus decreasing antimicrobial peptides in the pancreas and ileum of recipient mice (Table 1 and Fig. 2) [60].
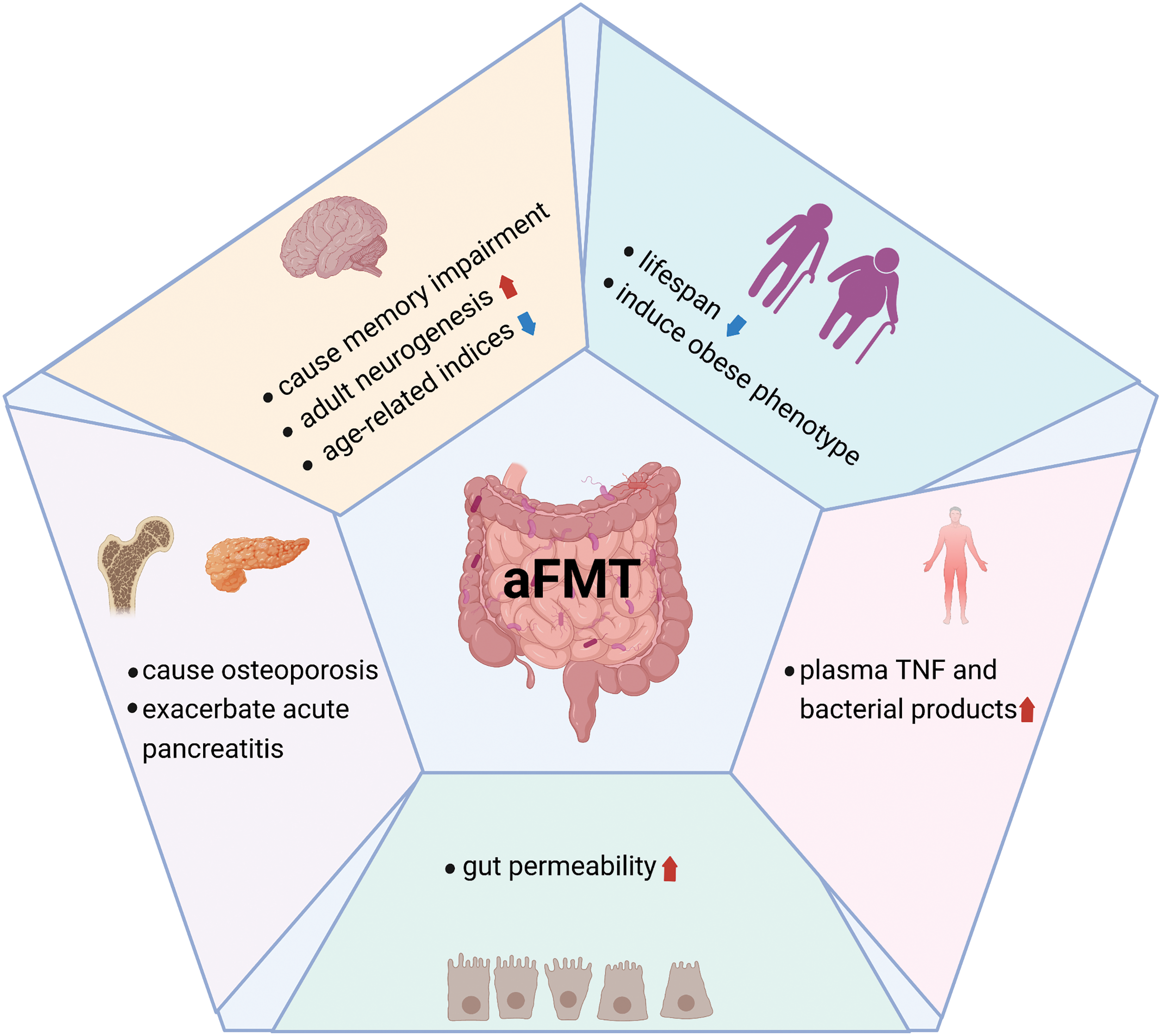
Figure 2: Detrimental effects of aFMT. Detrimental effects of aFMT are summarized, including decreasing lifespan, inducing obese phenotype, causing memory impairment, systemic inflammation, osteoporosis, and increasing gut permeability. Created with BioRender.Com.
Increased pathogenic bacteria in old feces and increased beneficial bacteria in young feces
Paenalcaligenes hominis is frequently detected in the elderly, but not in children and young adults. Paenalcaligenes hominis and its extracellular vesicles (EVs) can induce cognitive impairment in SPF mice and GF mice. The EVs may penetrate the brain through the blood as well as the vagus nerve [50]. Akkermansia muciniphila (Akk), is a Gram-negative, anaerobic mucin-degrading bacterium that belongs to the phylum Verrucomicrobia and resides in the gastrointestinal tract of humans and animals. The abundance of Akk is reduced in aged humans and mice [62,63]. A higher abundance of Akk is associated with decreased systemic inflammation, increased gut integrity, and a healthier metabolic status [64,65]. Transplanting gut microbiota from children (CGM) but not from the elderly (EGM) prevents decreases in bone mass and bone strength in osteoporotic mice. The higher abundance of Akk in CGM than that in EGM, which can rescue the reduction of Akk in osteoporotic mice, can explain the beneficial effect of CGM. Direct replenishment of Akk or Akk EVs is sufficient to increase bone mass and bone strength in osteoporotic mice. Inhibiting EVs’ secretion of CGM or Akk abolishes the beneficial effect of CGM [43]. Transplanting gut microbiota of wild-type mice to progeroid mice can extend lifespan, while Akk supplementation to progeroid mice can also extend lifespan [24]. Cage switching with young mice can improve CDI outcomes for aged mice, as three signature bacteria, Bacteroides, Alistipes, and rc4-4 genera, were replenished in aged mice by cage switching [40]. Bacteroides and Alistipes have been associated with protection against CDI in human studies [66–68]. yFMT to osteoporosis rats can alleviate bone loss in aged rats, with increased Blautia and decreased Helicobacter and Prevotella in the recipient gut of aged rats [35]. Blautia is a SCFA-producing bacterium, which is beneficial for bone health [69], while Prevotella is associated with inflammatory bone loss [70].
SCFAs and SCFA-producing bacteria
SCFAs, primarily butyrate, propionate, and acetate, are almost exclusively derived from bacterial metabolism in the gut, and play a significant role in stabilizing the gut epithelial barrier. SCFAs can promote the secretion of mucus and immunoglobulin A, enhance Treg cell responses, and have anti-inflammatory effects in the gut [71]. Additionally, SCFAs can mediate the maturation of brain microglia and influence the blood-brain barrier [72,73]. SCFAs were lower in aged mice compared to young. yFMT to aged mice can increase SCFAs, which is associated with a better outcome of experimental stroke in aged mice [25]. The replenishment with SCFA-producing bacteria and prebiotic inulin reproduces the beneficial effects of yFMT on stroke recovery in aged mice, which suggests SCFAs played a significant role in yFMT [27]. aFMT to young GF mice can increase butyrate-producing bacteria. Fecal butyrate, neurogenesis, and intestinal growth were also increased. This phenotype can be replicated by treating young GF mice with sodium butyrate, which suggests the significant role played by butyrate in the mechanism of FMT [54]. Other studies showed that aFMT to young GF mice [27] or young SPF mice [49] decreased SCFA-producing bacteria and SCFA production, and impaired spatial memory. aFMT to young SPF mice also decreased SCFA-producing bacteria, accelerated central nervous system (CNS) inflammation and retinal inflammation, and increased intestinal barrier permeability [34]. Transplanting gut microbiota from centenarians to mice can increase SCFA-producing bacteria and intestinal vill length, and decrease age-related indices [55]. However, these studies only show the association between SCFAs after FMT and age-related phenotype, the casual relationship needs further investigation.
Gut barrier function and systemic inflammation
Aging is associated with declined gut barrier function and increased gut permeability [74], which is manifested in decreased thickness of the gut mucus layer [75] and decreased tight junction protein expression in colons [76]. The increased gut permeability allowing translocation of commensal microbes, microbial debris, and/or luminal metabolites to systemic dissemination may explain the chronic inflammation with advancing age [77]. The gut microbiota plays a significant role in gut barrier function. It is intricate that increased antimicrobial peptide expression can identify gut barrier dysfunction in individual flies [78], and gut dysbiosis precedes and predicts the onset of intestinal barrier dysfunction in aged flies [48]. The aFMT to young GF mice [56,57] or SPF rats [59] can increase gut permeability and serum inflammatory cytokines. aFMT to young SPF mice can also increase serum inflammatory cytokines in young SPF mice [25,51], while the yFMT to aged SPF mice improved intestinal structure and barrier function [34,35], decreased serum inflammatory cytokines [27,31] and translocation of bacterial products [25,34]. Together, these results show that aFMT promotes the breakdown of the gut barrier, the translocation of bacterial products, and the elevated serum levels of pro-inflammatory cytokines, while yFMT has the opposite function. This may explain the beneficial effect of yFMT on stroke outcome [25,27], osteoporosis [35], ovary functions [31], and impaired memory [51] in aged ones.
aFMT to young mice reduced neuronal activity in the ascending-vagus nerve output brain structure, compared to yFMT to young mice, thus impairing hippocampus-dependent memory. Increasing vagal ascending activity alleviated the adverse effects of aFMT on hippocampal functions [52]. Secondary bile acids which are produced by the gut microbiota can regulate metabolism and anti-inflammatory signals. The restoration of secondary bile acids by FMT might contribute to extending lifespan in progeroid mice [24]. yFMT to middle-aged zebrafish increased a distinct signature of defense response to bacteria in the recipient gut, which may explain the lifespan extension effect of yFMT. While aFMT to middle-aged zebrafish increased hyaluronic acid metabolism, which has been associated with increased inflammation and deregulated immune response [22]. Aging is associated with metabolic and immune alterations that lead to perturbation of brain function and behavior, yFMT to aged mice can modulate age-induced peripheral and brain immunity, as well as the hippocampal metabolome and transcriptome, which may explain the attenuation of impaired learning in aging (Table 2 and Fig. 3) [28].
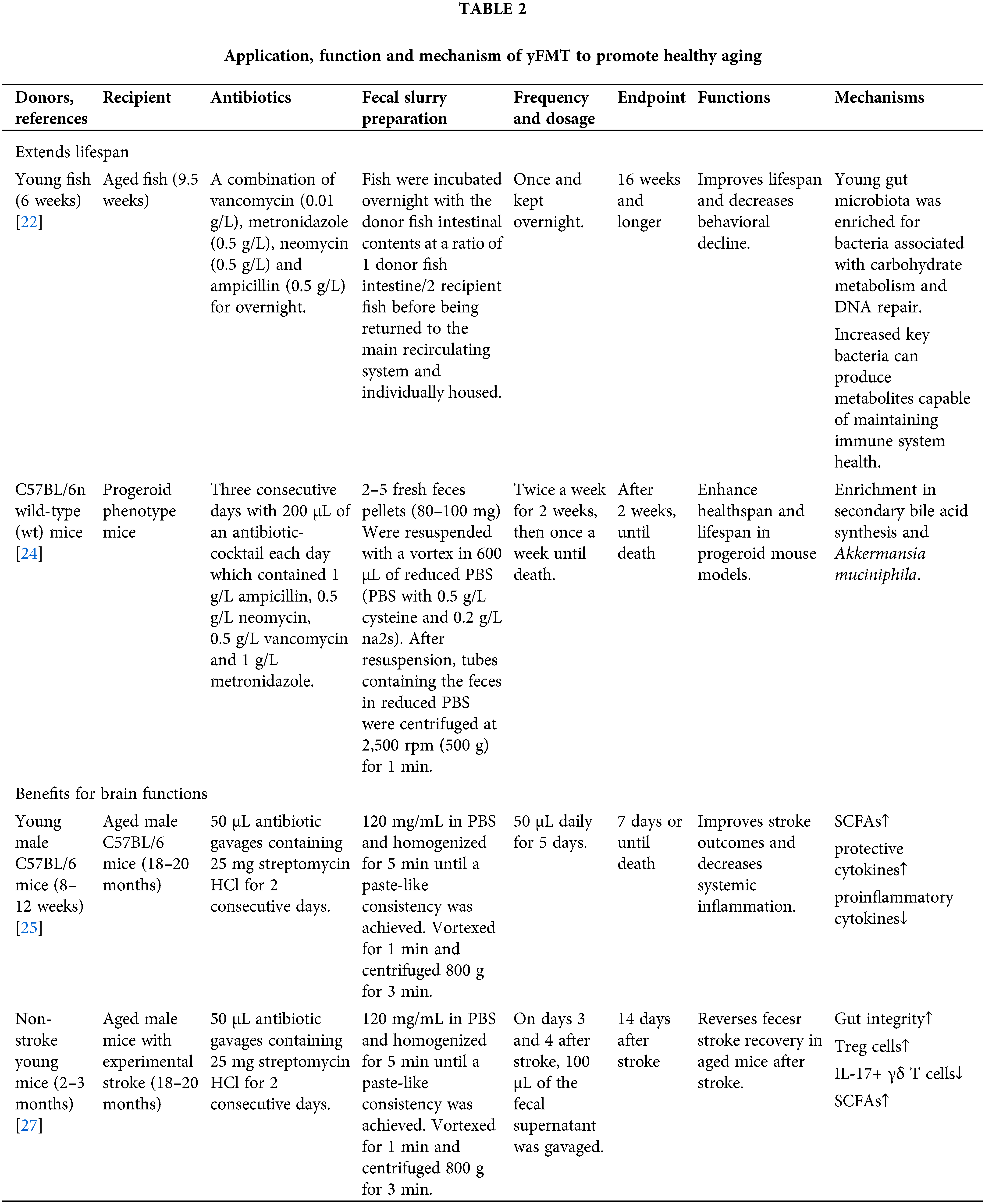
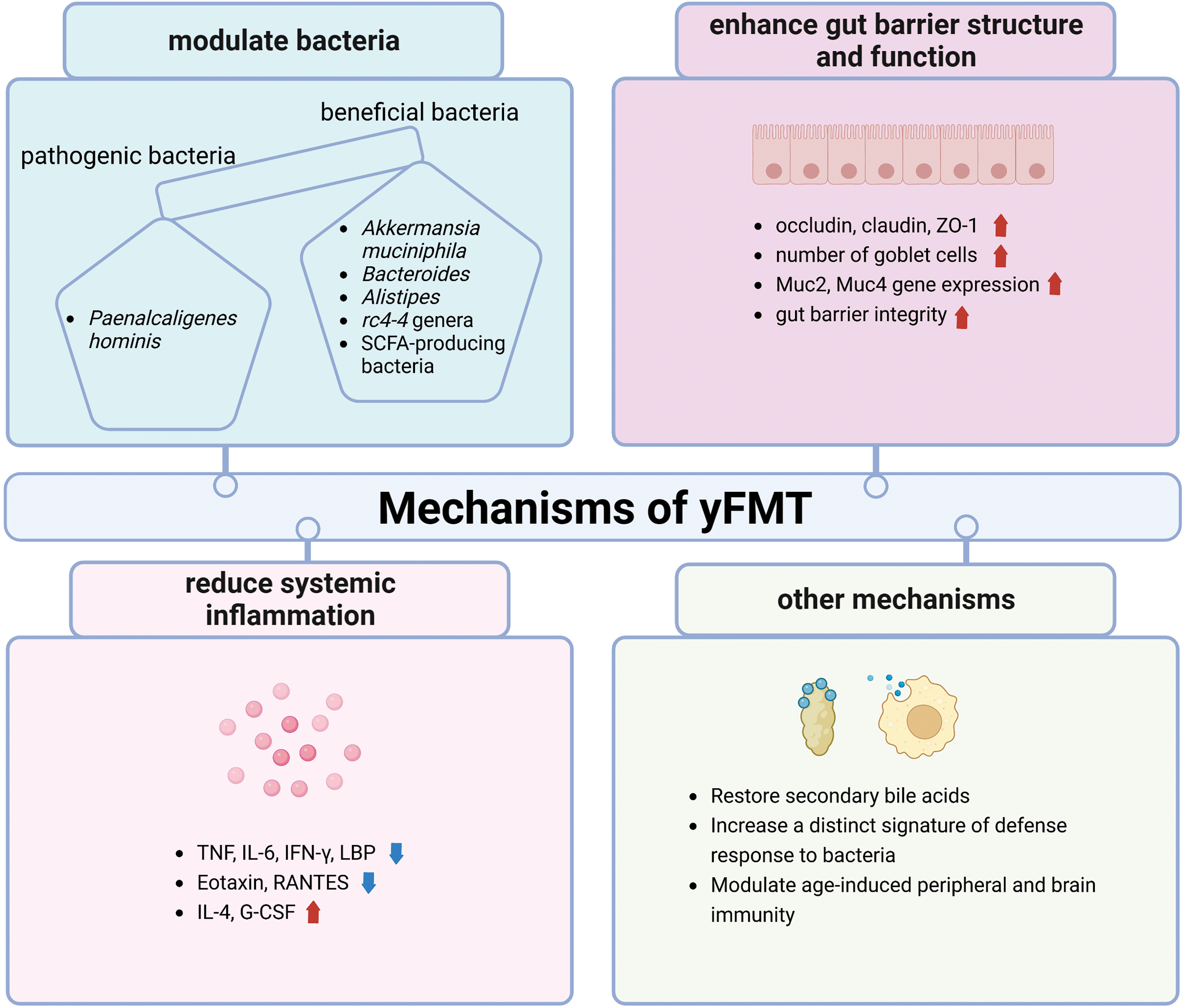
Figure 3: Mechanisms of yFMT for healthy aging is summarized, including modulating bacteria, enhancing gut barrier structure and function, reducing systemic inflammation, restoring secondary bile acids, increasing a distinct signature of defense response to bacteria, and modulating periphery and brain immunity. tumor necrosis factor (TNF); interleukin 6 (IL-6); interferon-Gamma (IFN-γ); lipopolysaccharide binding protein (LBP); regulate upon activation normal T cell expressed and secreted (RANTES); mucin 2 (muc2); mucin 4 (muc4); granulocyte colony-stimulating factor (G-CSF). Created with BioRender.Com.
It seems that the recipients’ age is critical for the effect of FMT. For instance, middle-aged fish receiving gut microbiota from young donors showed increased lifespan compared to the same age without FMT, but young-aged fish receiving gut microbiota from old donors did not have a different lifespan compared to the same age without FMT. The effect of recipients’ age may lie in the composition of recipients’ gut microbiota and immune function. The middle-aged gut microbiota composition might be primed to induce damage in the host and its removal is therefore beneficial. While for young recipients, the strong immune function can maintain gut homeostasis even when colonized with old gut microbiota [22]. Young GF mice receiving gut microbiota from healthy old mice showed increased neurogenesis in the hippocampus of the brain and increased intestinal growth, but this effect was lost in old GF recipient mice. This recipients’ age-related difference suggested that the early life response to microbial cues may differ considerably from that later in life and that both recipients’ age and donor microbiota signatures play a role in the effect of FMT [54]. Cage switching between aged mice and young mice can improve the clinical outcome of subsequent CDI infection in aged mice, but did not worsen clinical outcome in young mice, suggesting that the gut microbiota of young mice has colonization resistance towards aged gut microbiota, or that aged gut microbiota lost the capacity for colonization in young mice [79].
The recipients’ sex also determines the effect of FMT. Co-housing with young mice of the same sex increased hepatic genes related to bile acids homeostasis in old male mice, but there was no significant change in old female mice. Moreover, the intestinal concentrations of bile acids were divergently altered upon co-housing in the jejunum, ileum, cecum, and colon in a sex-dependent manner [39]. This recipients’ sex-specific difference was also shown in zebrafish. yFMT to aged zebrafish caused more metabolic pathways activation in aged testes than in aged ovaries [30]. This recipients’ sex difference highlights the potential of sex-specific strategies to prevent or treat aging-related disorders.
The recipients’ genetic background
The choice of recipients’ strains seems important for the effect of FMT. In the same strains, yFMT to aged mice in both C57BL/6 background and BALB/c background can rescue the defective germinal center reaction in Peyer’s patches of aged mice. However, cross-strain FMT, FMT from 3-month-old BALB/c mice into 3-month-old C57BL/6 mice could not enhance germinal center reaction, which shows that host genetics might impact the cross-talk between the gut microbiota and the germinal center reaction [37]. yFMT to aged mice can improve more survival rate of Salmonella infection in BALB/c background than in C56BL/6 background [46], which shows that the recipients’ strains play a role in FMT.
The recipients’ gut microbiota after FMT
It is interesting that among the aFMT studies on the effect of the brain [49–52,54], only one study by Kundu et al. showed increased neurogenesis and pro-longevity signatures [54], other studies showed decreased neurogenesis [52], decreased hippocampal synaptic plasticity [49,51], increased astrogliosis [52] and microgliosis [49], and deterioration of memory [49–52]. This contradictory result may be explained by the recipient’s gut microbiota after FMT. In Kundu’s study, although the aged donors’ gut microbiota showed decreased Akkermansia and Alistipes genera which can produce SCFAs compared to young donors’ gut microbiota, the recipients’ gut microbiota with aFMT showed increased butyrate-producing bacteria compared to yFMT [54]. But in D’Amato’s study, the aged donors’ gut microbiota showed decreased Lachnospiraceae and Ruminococcaceae which can produce SCFAs compared to young donors’ gut microbiota, and recipients’ gut microbiota with aFMT showed decreased SCFA-producing bacteria compared to yFMT [49]. Together, it seems that both the recipient and the donor microbiota signatures play a critical role in the effect of FMT.
Conclusions and Future Perspectives
The idea of a microbiota-based modulation to influence human health and longevity is captivating, even if improbable at present. This review summarized the recent findings of FMT to improve healthy aging, including benefits for lifespan and health span, brain functions, reproductive organs, gut, eyes, bones, and others. The possible mechanisms of this beneficial effect were highlighted, including the increased beneficial bacteria and decreased pathological bacteria, decreased gut permeability and systemic inflammation, increased short-chain fatty acid (SCFA) and SCFA-producing bacteria, increased vagal ascending activity, restored bile acids homeostasis, etc. The recipients’ age, sex, genetic background, and gut microbiota after FMT, were all influencing factors of FMT. This review also has limitations. The potential hazards of FMT should be discussed in the future research. And mechanisms of FMT should be further explored.
Based on the above benefits of yFMT, an idea of rejuvenating the human gut microbiome was proposed. Similar to cord blood banking for an autologous transplant, autologous FMT, which means collecting the hosts’ stool samples at a younger age when they are disease-free and cryopreserving the samples in a stool bank, then transplanting the stool samples for the hosts’ future use, may be an alternative solution. The potential applications may include recurrent CDI, obesity, inflammatory bowel disease, allogeneic hematopoietic stem-cell transplantation, and aging [80].
Acknowledgement: None.
Funding Statement: This work was sponsored by Natural Science Foundation of Chongqing, China (cstc2021jcyjbshX0176 to Ting Gong) and National Natural Science Foundation of China (81900980 to Huifen Ding).
Author Contributions: Study conception and design: Ting Gong; data collection: Xinsi Li and Qian Li; analysis and interpretation of results: Xiujun Tan, Yizhong Wang; draft manuscript preparation: Yuanyuan Liao. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Ghosh TS, Shanahan F, O’Toole PW. The gut microbiome as a modulator of healthy aging. Nat Rev Gastroenterol Hepatol. 2022;19(9):565–84. doi:10.1038/s41575-022-00605-x [Google Scholar] [PubMed] [CrossRef]
2. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–40. doi:10.1016/j.cell.2016.01.013 [Google Scholar] [PubMed] [CrossRef]
3. Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28(8):1221–38. doi:10.1210/me.2014-1108 [Google Scholar] [PubMed] [CrossRef]
4. Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6(4):295–308. doi:10.1177/1756283X13482996 [Google Scholar] [PubMed] [CrossRef]
5. Seo DO, Holtzman DM, Masternak M. Gut microbiota: from the forgotten organ to a potential key player in the pathology of Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2020;75(7):1232–41. doi:10.1093/gerona/glz262 [Google Scholar] [PubMed] [CrossRef]
6. Stephens RW, Arhire L, Covasa M. Gut microbiota: from microorganisms to metabolic organ influencing obesity. Obesity. 2018;26(5):801–9. doi:10.1002/oby.v26.5. [Google Scholar] [CrossRef]
7. Haran JP, McCormick BA. Aging, frailty, and the microbiome-how dysbiosis influences human aging and disease. Gastroenterol. 2021;160(2):507–23. doi:10.1053/j.gastro.2020.09.060 [Google Scholar] [PubMed] [CrossRef]
8. Yu J, Marsh S, Hu J, Feng W, Wu C. Gut microbiota and metagenomic advancement in digestive disease. Gastroenterol Res Pract. 2016;2016:4703406 [Google Scholar] [PubMed]
9. Agirman G, Hsiao EY. SnapShot: the microbiota-gut-brain axis. Cell. 2021;184(9):2524.e1. [Google Scholar]
10. Zhang T, Cheng JK, Hu YM. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res Rev. 2022;81:101739. doi:10.1016/j.arr.2022.101739 [Google Scholar] [PubMed] [CrossRef]
11. Lin P. Importance of the intestinal microbiota in ocular inflammatory diseases: a review. Clin Exp Ophthalmol. 2019;47(3):418–22. doi:10.1111/ceo.2019.47.issue-3. [Google Scholar] [CrossRef]
12. Jones RM, Mulle JG, Pacifici R. Osteomicrobiology: the influence of gut microbiota on bone in health and disease. Bone. 2018;115:59–67. doi:10.1016/j.bone.2017.04.009 [Google Scholar] [PubMed] [CrossRef]
13. Chadchan SB, Singh V, Kommagani R. Female reproductive dysfunctions and the gut microbiota. J Mol Endocrinol. 2022;69(3):R81–94. doi:10.1530/JME-21-0238 [Google Scholar] [PubMed] [CrossRef]
14. Schmauck-Medina T, Moliere A, Lautrup S, Zhang J, Chlopicki S, Madsen HB, et al. New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging. 2022;14(16):6829–39. doi:10.18632/aging.v14i16. [Google Scholar] [CrossRef]
15. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. doi:10.1016/j.cell.2013.05.039 [Google Scholar] [PubMed] [CrossRef]
16. Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi:10.7717/peerj.7502 [Google Scholar] [PubMed] [CrossRef]
17. Holmes A, Finger C, Morales-Scheihing D, Lee J, McCullough LD. Gut dysbiosis and age-related neurological diseases; an innovative approach for therapeutic interventions. Transl Res. 2020;226:39–56. doi:10.1016/j.trsl.2020.07.012 [Google Scholar] [PubMed] [CrossRef]
18. Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13(9):508–16. doi:10.1038/nrgastro.2016.98 [Google Scholar] [PubMed] [CrossRef]
19. Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500–8. doi:10.1038/ajg.2013.59 [Google Scholar] [PubMed] [CrossRef]
20. Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29(1):79–84. doi:10.1097/MOG.0b013e32835a4b3e [Google Scholar] [PubMed] [CrossRef]
21. Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145(5):946–53. doi:10.1053/j.gastro.2013.08.058 [Google Scholar] [PubMed] [CrossRef]
22. Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, et al. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. Elife. 2017;6:e27014. doi:10.7554/eLife.27014 [Google Scholar] [PubMed] [CrossRef]
23. Callaway E. Young poo’ makes aged fish live longer. Nature. 2017;544(7649):147. doi:10.1038/nature.2017.21770 [Google Scholar] [PubMed] [CrossRef]
24. Barcena C, Valdes-Mas R, Mayoral P, Garabaya C, Durand S, Rodriguez F, et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med. 2019;25(8):1234–42. doi:10.1038/s41591-019-0504-5 [Google Scholar] [PubMed] [CrossRef]
25. Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84(1):23–36. doi:10.1002/ana.v84.1. [Google Scholar] [CrossRef]
26. Feng Y, Zhang D, Zhao Y, Duan T, Sun H, Ren L, et al. Effect of intestinal microbiota transplantation on cerebral ischemia reperfusion injury in aged mice via inhibition of IL-17. Neurogastroenterol Motil. 2022;34(7):e14313. doi:10.1111/nmo.v34.7. [Google Scholar] [CrossRef]
27. Lee J, d’Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127(4):453–65. doi:10.1161/CIRCRESAHA.119.316448 [Google Scholar] [PubMed] [CrossRef]
28. Boehme M, Guzzetta KE, Bastiaanssen TFS, van de Wouw M, Moloney GM, Gual-Grau A, et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat Aging. 2021;1(8):666–76. doi:10.1038/s43587-021-00093-9 [Google Scholar] [PubMed] [CrossRef]
29. Hu C, Liu M, Sun B, Tang L, Zhou X, Chen L. Young fecal transplantation mitigates the toxicity of perfluorobutanesulfonate and potently refreshes the reproductive endocrine system in aged recipients. Environ Int. 2022;167:107418. doi:10.1016/j.envint.2022.107418 [Google Scholar] [PubMed] [CrossRef]
30. Tang L, Li J, Sun B, Bai Y, Zhou X, Chen L. Transcriptomic interaction between young fecal transplantation and perfluorobutanesulfonate in aged zebrafish gonads. Toxics. 2022;10(11):631. doi:10.3390/toxics10110631 [Google Scholar] [PubMed] [CrossRef]
31. Xu L, Zhang Q, Dou X, Wang Y, Wang J, Zhou Y, et al. Fecal microbiota transplantation from young donor mice improves ovarian function in aged mice. J Genet Genomics. 2022;49(11):1042–52. doi:10.1016/j.jgg.2022.05.006 [Google Scholar] [PubMed] [CrossRef]
32. Huang J, Shan W, Li F, Wang Z, Cheng J, Lu F, et al. Fecal microbiota transplantation mitigates vaginal atrophy in ovariectomized mice. Aging. 2021;13(5):7589–607. doi:10.18632/aging.v13i5. [Google Scholar] [CrossRef]
33. Jiao X, Pei X, Lu D, Qi D, Huang S, He S, et al. Microbial reconstitution improves aging-driven lacrimal gland circadian dysfunction. Am J Pathol. 2021;191(12):2091–116. doi:10.1016/j.ajpath.2021.08.006 [Google Scholar] [PubMed] [CrossRef]
34. Parker A, Romano S, Ansorge R, Aboelnour A, Le Gall G, Savva GM, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. 2022;10(1):68. doi:10.1186/s40168-022-01243-w [Google Scholar] [PubMed] [CrossRef]
35. Ma S, Wang N, Zhang P, Wu W, Fu L. Fecal microbiota transplantation mitigates bone loss by improving gut microbiome composition and gut barrier function in aged rats. PeerJ. 2021;9:e12293. doi:10.7717/peerj.12293 [Google Scholar] [PubMed] [CrossRef]
36. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–41. doi:10.1126/science.1198469 [Google Scholar] [PubMed] [CrossRef]
37. Stebegg M, Silva-Cayetano A, Innocentin S, Jenkins TP, Cantacessi C, Gilbert C, et al. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat Commun. 2019;10(1):2443. doi:10.1038/s41467-019-10430-7 [Google Scholar] [PubMed] [CrossRef]
38. Donaldson DS, Pollock J, Vohra P, Stevens MP, Mabbott NA. Microbial stimulation reverses the age-related decline in M cells in aged mice. iScience. 2020;23(6):101147. doi:10.1016/j.isci.2020.101147 [Google Scholar] [PubMed] [CrossRef]
39. Ma J, Hong Y, Zheng N, Xie G, Lyu Y, Gu Y, et al. Gut microbiota remodeling reverses aging-associated inflammation and dysregulation of systemic bile acid homeostasis in mice sex-specifically. Gut Microbes. 2020;11(5):1450–74. doi:10.1080/19490976.2020.1763770 [Google Scholar] [PubMed] [CrossRef]
40. Shin JH, Gao Y, Moore JH II, Bolick DT, Kolling GL, Wu M, et al. Innate immune response and outcome of clostridium difficile infection are dependent on fecal bacterial composition in the aged host. J Infect Dis. 2018;217(2):188–97. doi:10.1093/infdis/jix414 [Google Scholar] [PubMed] [CrossRef]
41. Jorgensen SMD, Rubak TMM, Damsgaard EM, Dahlerup JF, Hvas CL. Faecal microbiota transplantation as a home therapy to frail older people. Age and Ageing. 2020;49(6):1093–6. doi:10.1093/ageing/afaa073 [Google Scholar] [PubMed] [CrossRef]
42. Hu C, Li J, Liu M, Lam PKS, Chen L. Young fecal transplantation modulates the visual toxicity of perfluorobutanesulfonate in aged zebrafish recipients. Aquat Toxicol. 2022;251:106295. doi:10.1016/j.aquatox.2022.106295 [Google Scholar] [PubMed] [CrossRef]
43. Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, Jin L, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci. 2021;8(9):2004831. doi:10.1002/advs.v8.9. [Google Scholar] [CrossRef]
44. Liu M, Sun B, Zhou X, Chen L. Disturbed glucose metabolism by perfluorobutanesulfonate pollutant and benefit of young fecal transplantation in aged zebrafish. Ecotoxicol Environ Saf. 2022;241:113721. doi:10.1016/j.ecoenv.2022.113721 [Google Scholar] [PubMed] [CrossRef]
45. Hu C, Sun B, Liu M, Yu J, Zhou X, Chen L. Fecal transplantation from young zebrafish donors efficiently ameliorates the lipid metabolism disorder of aged recipients exposed to perfluorobutanesulfonate. Sci Total Environ. 2022;823:153758. doi:10.1016/j.scitotenv.2022.153758 [Google Scholar] [PubMed] [CrossRef]
46. Pradhan S, Ray P, Aich P. Microbiota transplantation from younger to older mice could restore lost immunity to effectively clear salmonella infection in Th2-biased BALB/c mice. Life Sci. 2022;288:120201. doi:10.1016/j.lfs.2021.120201 [Google Scholar] [PubMed] [CrossRef]
47. Park SH, Lee JH, Kim JS, Kim TJ, Shin J, Im JH, et al. Fecal microbiota transplantation can improve cognition in patients with cognitive decline and Clostridioides difficile infection. Aging. 2022;14(16):6449–66. doi:10.18632/aging.v14i16. [Google Scholar] [CrossRef]
48. Clark RI, Salazar A, Yamada R, Fitz-Gibbon S, Morselli M, Alcaraz J, et al. Distinct shifts in microbiota composition during drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015;12(10):1656–67. doi:10.1016/j.celrep.2015.08.004 [Google Scholar] [PubMed] [CrossRef]
49. D’Amato A, di Cesare Mannelli L, Lucarini E, Man AL, Le Gall G, Branca JJV, et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome. 2020;8(1):140. doi:10.1186/s40168-020-00914-w [Google Scholar] [PubMed] [CrossRef]
50. Lee KE, Kim JK, Han SK, Lee DY, Lee HJ, Yim SV, et al. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8(1):107. doi:10.1186/s40168-020-00881-2 [Google Scholar] [PubMed] [CrossRef]
51. Li Y, Ning L, Yin Y, Wang R, Zhang Z, Hao L, et al. Age-related shifts in gut microbiota contribute to cognitive decline in aged rats. Aging. 2020;12(9):7801–17. doi:10.18632/aging.v12i9. [Google Scholar] [CrossRef]
52. Rei D, Saha S, Haddad M, Rubio AH, Perlaza BL, Berard M, et al. Age-associated gut microbiota impair hippocampus-dependent memory in a vagus-dependent manner. JCI Insight. 2022;7(15):e147700. doi:10.1172/jci.insight.147700 [Google Scholar] [PubMed] [CrossRef]
53. Lee J, Venna VR, Durgan DJ, Shi H, Hudobenko J, Putluri N, et al. Young versus aged microbiota transplants to germ-free mice: increased short-chain fatty acids and improved cognitive performance. Gut Microbes. 2020;12(1):1–14. [Google Scholar]
54. Kundu P, Lee HU, Garcia-Perez I, Tay EXY, Kim H, Faylon LE, et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci Transl Med. 2019;11(518):eaau4760. doi:10.1126/scitranslmed.aau4760 [Google Scholar] [PubMed] [CrossRef]
55. Chen Y, Zhang S, Zeng B, Zhao J, Yang M, Zhang M, et al. Transplant of microbiota from long-living people to mice reduces aging-related indices and transfers beneficial bacteria. Aging. 2020;12(6):4778–93. doi:10.18632/aging.v12i6. [Google Scholar] [CrossRef]
56. Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de Jongh C, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. doi:10.3389/fimmu.2017.01385 [Google Scholar] [PubMed] [CrossRef]
57. Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455–66 e4. doi:10.1016/j.chom.2017.03.002 [Google Scholar] [PubMed] [CrossRef]
58. Binyamin D, Werbner N, Nuriel-Ohayon M, Uzan A, Mor H, Abbas A, et al. The aging mouse microbiome has obesogenic characteristics. Genome Med. 2020;12(1):87. doi:10.1186/s13073-020-00784-9 [Google Scholar] [PubMed] [CrossRef]
59. Wang N, Ma S, Fu L. Gut microbiota dysbiosis as one cause of osteoporosis by impairing intestinal barrier function. Calcif Tissue Int. 2022;110(2):225–35. doi:10.1007/s00223-021-00911-7 [Google Scholar] [PubMed] [CrossRef]
60. Jing H, Chang Q, Xu Y, Wang J, Wu X, Huang J, et al. Effect of aging on acute pancreatitis through gut microbiota. Front Microbiol. 2022;13:897992. doi:10.3389/fmicb.2022.897992 [Google Scholar] [PubMed] [CrossRef]
61. Albouery M, Buteau B, Grégoire S, Cherbuy C, Pais de Barros JP, Martine L, et al. Age-related changes in the gut microbiota modify brain lipid composition. Front Cell Infect Microbiol. 2019;9:444 [Google Scholar] [PubMed]
62. Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73(23):7767–70. doi:10.1128/AEM.01477-07 [Google Scholar] [PubMed] [CrossRef]
63. van der Lugt B, Rusli F, Lute C, Lamprakis A, Salazar E, Boekschoten MV, et al. Integrative analysis of gut microbiota composition, host colonic gene expression and intraluminal metabolites in aging C57BL/6J mice. Aging. 2018;10(5):930–50. doi:10.18632/aging.v10i5. [Google Scholar] [CrossRef]
64. Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–36. doi:10.1136/gutjnl-2014-308778 [Google Scholar] [PubMed] [CrossRef]
65. Bodogai M, O’Connell J, Kim K, Kim Y, Moritoh K, Chen C, et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci Transl Med. 2018;10(467):eaat4271. doi:10.1126/scitranslmed.aat4271 [Google Scholar] [PubMed] [CrossRef]
66. Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4(2):125–35. doi:10.4161/gmic.23571 [Google Scholar] [PubMed] [CrossRef]
67. Manges AR, Labbe A, Loo VG, Atherton JK, Behr MA, Masson L, et al. Comparative metagenomic study of alterations to the intestinal microbiota and risk of nosocomial Clostridum difficile-associated disease. J Infect Dis. 2010;202(12):1877–84. doi:10.1086/653024. [Google Scholar] [CrossRef]
68. Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51(5):448–54. doi:10.1099/0022-1317-51-5-448 [Google Scholar] [PubMed] [CrossRef]
69. Zhang C, He X, Sheng Y, Yang C, Xu J, Zheng S, et al. Allicin-induced host-gut microbe interactions improves energy homeostasis. FASEB J. 2020;34(8):10682–98. doi:10.1096/fsb2.v34.8. [Google Scholar] [CrossRef]
70. Choi EY, Bae SH, Ha MH, Choe SH, Hyeon JY, Choi JI, et al. Genistein suppresses Prevotella intermedia lipopolysaccharide-induced inflammatory response in macrophages and attenuates alveolar bone loss in ligature-induced periodontitis. Arch Oral Biol. 2016;62:70–9. doi:10.1016/j.archoralbio.2015.11.019 [Google Scholar] [PubMed] [CrossRef]
71. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and western-lifestyle inflammatory diseases. Immunity. 2014;40(6):833–42. doi:10.1016/j.immuni.2014.05.014 [Google Scholar] [PubMed] [CrossRef]
72. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158 [Google Scholar] [PubMed]
73. Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–77. doi:10.1038/nn.4030 [Google Scholar] [PubMed] [CrossRef]
74. Hohman LS, Osborne LC. A gut-centric view of aging: do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence? Aging Cell. 2022;21(9):e13700. doi:10.1111/acel.v21.9. [Google Scholar] [CrossRef]
75. Sovran B, Hugenholtz F, Elderman M, van Beek AA, Graversen K, Huijskes M, et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci Rep. 2019;9(1):1437. doi:10.1038/s41598-018-35228-3 [Google Scholar] [PubMed] [CrossRef]
76. Tran L, Greenwood-van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci. 2013;68(9):1045–56. doi:10.1093/gerona/glt106 [Google Scholar] [PubMed] [CrossRef]
77. Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739–51. doi:10.1038/s41577-021-00538-7 [Google Scholar] [PubMed] [CrossRef]
78. Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A. 2012;109(52):21528–33. doi:10.1073/pnas.1215849110 [Google Scholar] [PubMed] [CrossRef]
79. Fischer N, Relman DA. Clostridium difficile, aging, and the gut: ccan microbiome rejuvenation keep us young and healthy? J Infect Dis. 2018;217(2):174–6. doi:10.1093/infdis/jix417 [Google Scholar] [PubMed] [CrossRef]
80. Ke S, Weiss ST, Liu YY. Rejuvenating the human gut microbiome. Trends Mol Med. 2022;28(8):619–30. doi:10.1016/j.molmed.2022.05.005 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF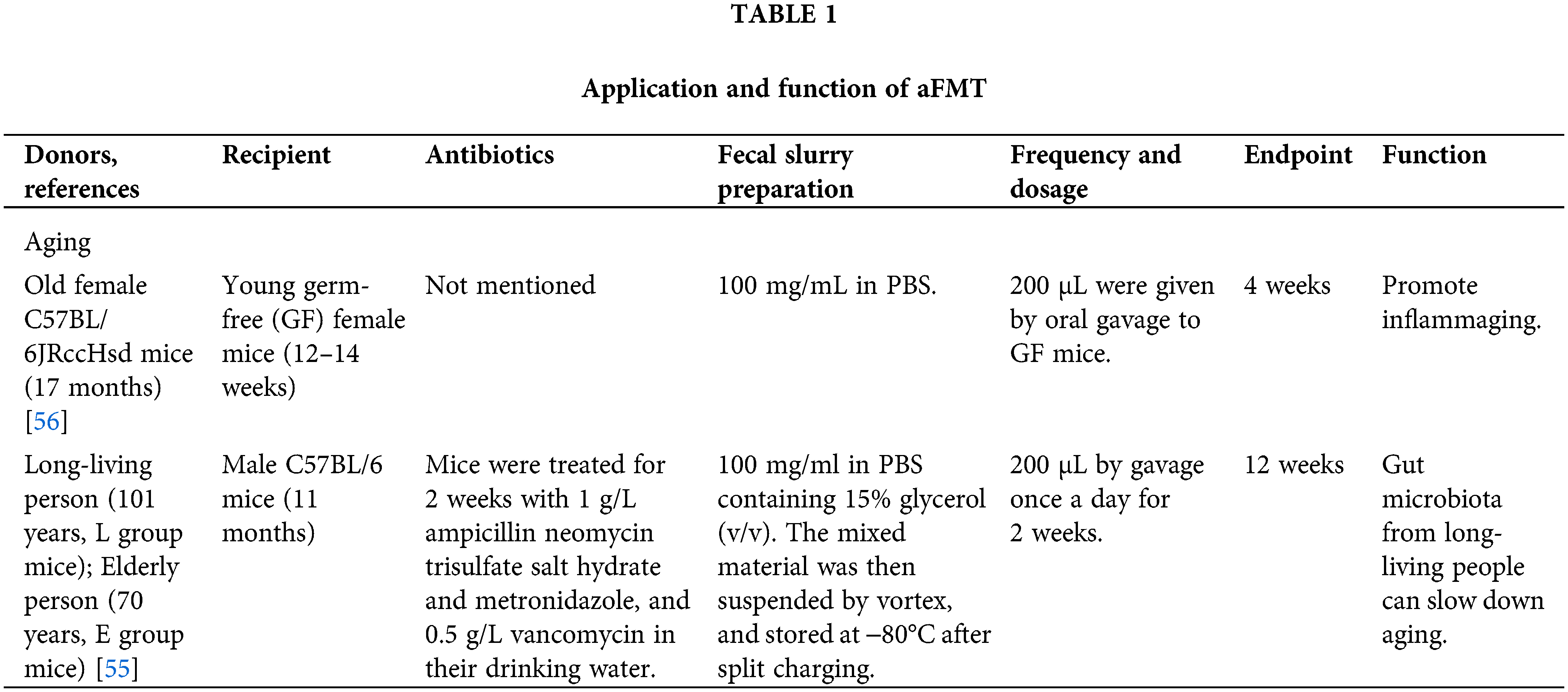
 Downloads
Downloads
 Citation Tools
Citation Tools