 Open Access
Open Access
ARTICLE
Knockdown of RCN1 contributes to the apoptosis of colorectal cancer via regulating IP3R1
1 Department of Gastroenterology, The First Affiliated Hospital of Dalian Medical University, Dalian, China
2 Department of Gastroenterology, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, China
3 The First Clinical College, Dalian Medical University, Dalian, China
* Corresponding Author: AIXIA GONG. Email:
(This article belongs to the Special Issue: Navigating the Interplay of Cancer, Autophagy, ER Stress, Cell Cycle and Apoptosis: Mechanisms, Therapies, and Future Directions)
BIOCELL 2024, 48(5), 835-845. https://doi.org/10.32604/biocell.2024.048076
Received 27 November 2023; Accepted 06 February 2024; Issue published 06 May 2024
Abstract
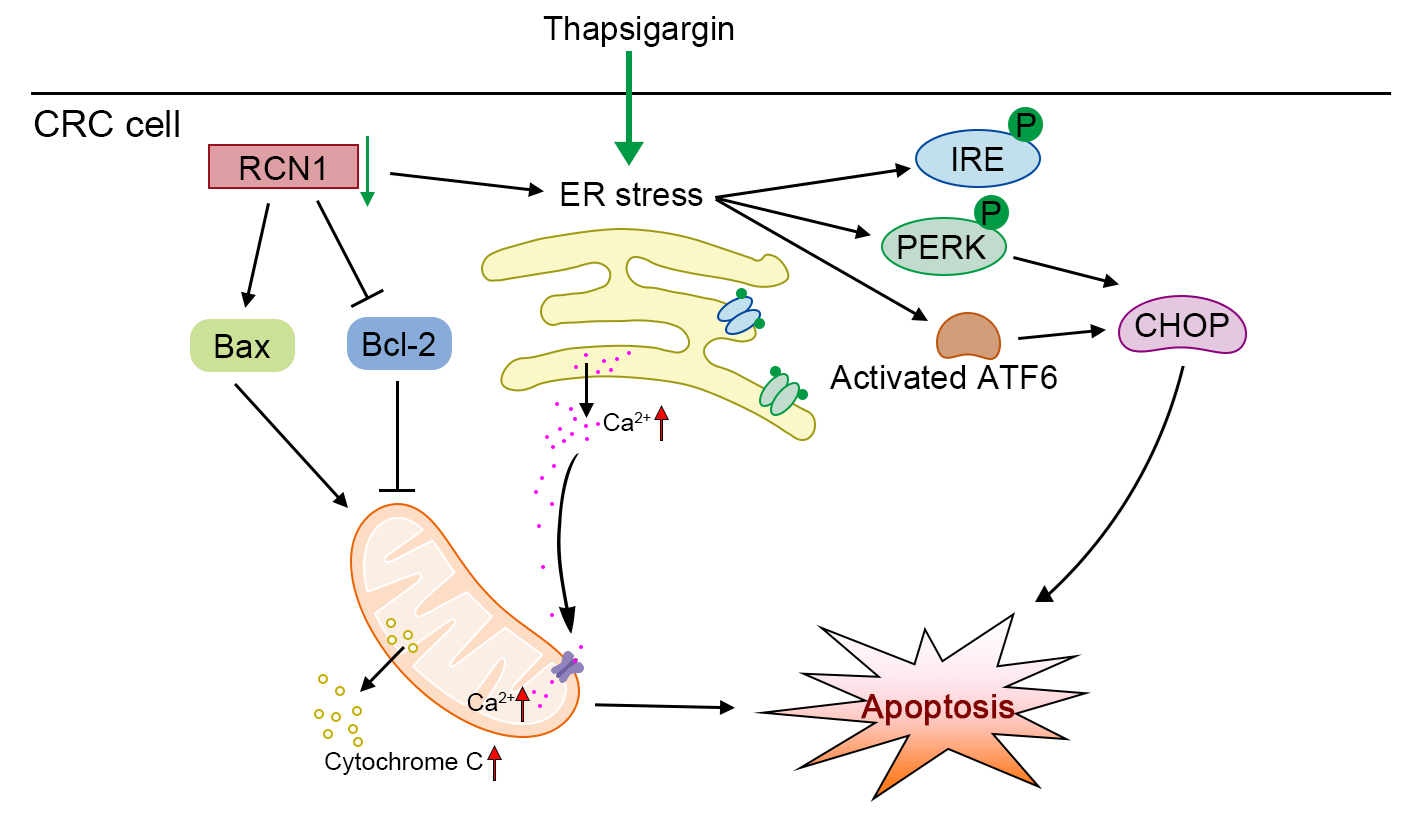
Background: The incidence of colorectal cancer (CRC) has been increasing in recent years. Thus, the discovery of factors that can assist in alleviating CRC is urgently warranted. Methods: To identify a potential factor involved in the development of CRC, we screened the upregulated genes in tumor tissues through four datasets from an online database. The expression of reticulocalbin 1 (RCN1), a Ca-binding protein, was upregulated in the four datasets. Based on loss-of-function experiments, the effect of RCN1 on cell viability was assessed by Cell Counting Kit-8 (CCK-8) assay. The regulatory effect of RCN1 on apoptosis was evaluated through Annexin V-fluorescein 5-isothiocyanate (FITC)/propidium iodide (PI) staining assay and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay in RKO and SW480 cells. Activation of endoplasmic reticulum (ER) stress signaling pathways was confirmed by estimating the phosphorylation and expression of PRKR-like ER kinase (PERK), inositol-requiring kinase-1 (IRE1), transcription factor 6 (ACT6), and CCAAT/enhancer-binding protein-homologous protein (CHOP). The intracellular Ca homeostasis regulated by RCN1 was determined through the detection of Ca concentration and mitochondrial membrane potential (MMP) measurement. Moreover, whether inositol 1,4,5-trisphosphate receptor type 1 (IP3R1) was involved in the regulation of RCN1 in CRC was verified through the depletion of IP3R1 in RKO cells. Results: Knockdown of RCN1 reduced cell viability and facilitated apoptosis in RKO and SW480 cells. Phosphorylation of PERK and IRE1, activation of ATF6, and upregulation of CHOP were induced by the absence of RCN1, suggesting that the unfolded protein response (UPR) was activated in CRC cells. The concentration of Ca in mitochondria was increased after RCN1 depletion, followed by reduction in the MMP and release of cytochrome c from mitochondria to the cytoplasm in RKO and SW480 cells. Moreover, it was demonstrated that IP3R1 mediates the effect of RCN1 on apoptosis induced by ER stress in CRC cells. The downregulation of IP3R1 restored the RCN1 loss-induced apoptosis and the increased Ca concentration. Conclusion: Taken together, our results confirmed that silencing of RCN1 disrupted intracellular Ca homeostasis and promoted cell apoptosis caused by TG-induced ER stress by regulating IP3R1 and activating the UPR signaling pathways.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools