 Open Access
Open Access
ARTICLE
Bronchoalveolar lavage fluid metagenomic next-generation sequencing assay for identifying pathogens in lung cancer patients
1 Division of Pulmonary and Critical Care Medicine, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
2 Institute of Pulmonary Diseases, Sun Yat-sen University, Guangzhou, China
3 Department of Pharmacy, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
* Corresponding Author: KEJING TANG. Email:
(This article belongs to the Special Issue: Bioinformatics Study of Diseases)
BIOCELL 2024, 48(4), 623-637. https://doi.org/10.32604/biocell.2024.030420
Received 05 April 2023; Accepted 17 May 2023; Issue published 09 April 2024
Abstract
Background: For patients with lung cancer, timely identification of new lung lesions as infectious or non-infectious, and accurate identification of pathogens is very important in improving OS of patients. As a new auxiliary examination, metagenomic next-generation sequencing (mNGS) is believed to be more accurate in diagnosing infectious diseases in patients without underlying diseases, compared with conventional microbial tests (CMTs). We designed this study to find out whether mNGS has better performance in distinguishing infectious and non-infectious diseases in lung cancer patients using bronchoalveolar lavage fluid (BALF). Materials and Methods: This study was a real-world retrospective review based on electronic medical records of lung cancer patients with bronchoalveolar lavage (BAL) and BALF commercial mNGS testing as part of clinical care from 1 April 2019 through 30 April 2022 at The First Affiliated Hospital of Sun Yat-sen University. 164 patients were included in this study. Patients were categorized into the pulmonary non-infectious disease (PNID) group (n = 64) and the pulmonary infectious disease (PID) group (n = 100) groups based on final diagnoses. Results: BALF mNGS increased the sensitivity rate by 60% compared to CMTs (81% vs. 21%, p < 0.05), whereas there was no significant difference in specificity (75% vs. 98.4%, p > 0.1). Among the patients with PID, bacteria were the most common cause of infection. Fungal infections occurred in 32% of patients, and Pneumocystis Yersini was most common. Patients with Tyrosine kinase inhibitors (TKIs) therapy possess longer overall survival (OS) than other anti-cancer agents, the difference between TKIs and immuno-checkpoint inhibitors (ICIs) was insignificant (median OS TKIs vs. ICIs vs. Anti-angiogenic vs. Chemo vs. Radiotherapy = 76 vs. 84 vs. 61 vs. 58 vs. 60). Conclusions: our study indicates that BALF mNGS can add value by improving overall sensitivity in lung cancer patients with potential pulmonary infection, and was outstanding in identifying Pneumocystis infection. It could be able to help physicians adjust the follow-up treatment to avoid the abuse of antibiotics.Keywords
The non-specific symptoms, such as cough, fever, and dyspnea, can appear in both infectious and non-infectious diseases of pulmonary, including lung cancer. It is difficult to distinguish between pulmonary infectious disease (PID) and pulmonary non-infectious disease (PNID) according to these non-specific symptoms discovered by inquiry and physical examination alone [1]. Patients with lung cancer are often vulnerable to infection, and the risk is increased by tumor-associated immunosuppression and the effects of the treatments [2,3]. Infection negatively affects clinical outcomes and overall survival (OS) [4]; timely identification of PID/PNID, pathogens, and follow-up adjustment of antibiotics are very important for the management of lung cancer patients with new-onset infection-like symptoms and findings on computed tomography (CT) image [5,6].
Anti-cancer treatments can cause PNID, including radiation and interstitial pneumonitis [7], which calls for totally different management compared to pneumonia and other PIDs. When infectious diseases are mistaken for non-infectious diseases, the interruption of antibiotics treatment and the administration of immunosuppressive agents such as steroids can lead to disastrous results. Finding an auxiliary examination that can quickly and accurately distinguish infection from non-infection is particularly important.
Bronchoscopy has a unique advantage in the diagnosis of PID, such as pneumonia [8]. Bronchoalveolar lavage fluid (BALF) acquired via bronchoscopy can be used for conventional microbial test (CMT) methods and metagenomic next-generation sequencing (NGS) to identify pathogens. Compared with CMTs, mNGS is more superior in efficiency [9]. In non-tumor immunocompetent patients with suspected pulmonary infection, BALF mNGS was able to effectively distinguish PNID from PID [10,11]. However, many hospitals do not have the conditions for in-house mNGS tests, they have to rely on commercial organizations for NGS testing. So far it is unclear how the sensitivity and specificity of commercial BALF mNGS change in lung cancer patients.
This study aimed to determine whether commercial mNGS of BALF could differentiate between infectious and Non-infectious pulmonary lesions in patients with lung cancer; and accurately identify pathogens responsible for the infection, therefore enhancing the accuracy of clinical diagnosis.
Study design and eligibility criteria
This study was a real-world retrospective review based on electronic medical records of lung cancer patients with bronchoalveolar lavage (BAL) and BALF commercial mNGS testing as part of clinical care from 1 April 2019 through 30 April 2022 at the First Affiliated Hospital of Sun Yat-sen University. BAL was performed using a bronchoscope produced by OLYMPUS (BF-1T260). As the standard operating procedure (SOP) of BAL in our hospital, 10–15 mL of BALF is collected for testing during the BAL process. The BALF samples were sent to commercial mNGS labs within 6 h after collection. The order of commercial mNGS testing was not interfered with by researchers of this study. No treatment decisions were made or altered as a result of this study. The First Affiliated Hospital of Sun Yat-sen University is a class A tertiary hospital in Guangzhou, China. All patients reviewed had mNGS testing in the inpatient setting. All participants signed a written informed consent form before the procedures of bronchoscope and BAL.
The study included patients who met the following criteria: (1) having any type of lung cancer; (2) showing symptoms suggestive of a pulmonary infection such as fever, cough, or difficulty breathing, along with new findings on chest CT scans; (3) undergoing bronchoscopy and BAL procedures, with BAL fluid (BALF) being collected for mNGS during their hospital stay; (4) having BALF samples available for CMTs such as culture and BALF mNGS within 48 h of admission or the onset of new symptoms; (5) having complete electronic medical records documented.
The exclusion criteria were (1) suffering from any of the following: a) primary immune deficiency diseases; b) organ transplantation; c) hematopoietic stem cell transplantation; d) lung metastasis of other tumors; e) hematological malignancy, treatment naïve or not; (2) mugs and CMTs samples were not collected at the same site of bronchi; and (3) incomplete electronic medical records.
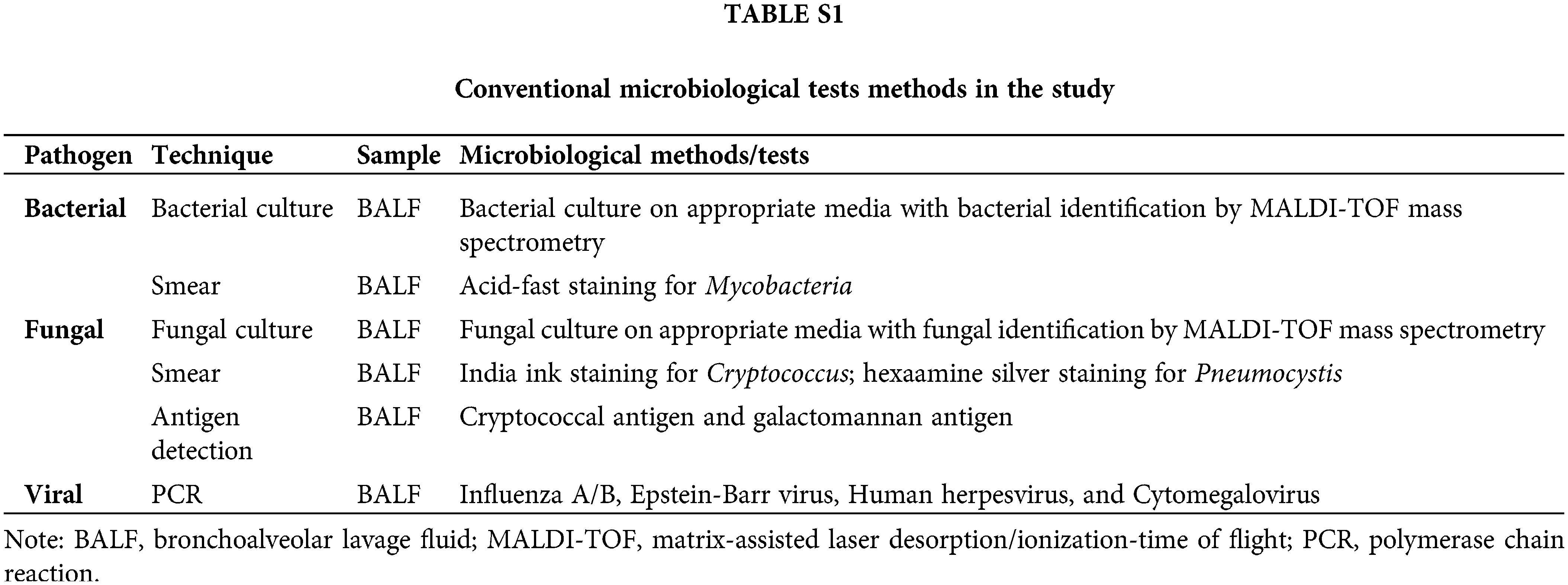
A total of 164 patients were included in the study, based on final diagnoses, these patients were categorized into two groups: PNID (n = 64) and PID (n = 100). Patient demographics, underlying medical conditions, CT images, and immune statuses were recorded. Commercial mNGS results were also recorded and evaluated. The data for all BALF CMTs (culture, smear, organism-specific PCR, antibody testing. Details were listed in Suppl. Table S1.) were ordered using the same batch of BALF as NGS. There are no institutional guidelines or limitations for mNGS ordering at The First Affiliated Hospital of Sun Yat-sen University.
Diagnosis of pulmonary infectious disease/pulmonary non-infectious disease
The diagnosis of pulmonary infectious and non-infectious diseases was determined by two specialized experts in the fields of pulmonary and critical care medicine. They thoroughly reviewed each patient’s electronic medical records, which included clinical symptoms, laboratory tests (such as complete blood counts, bronchoalveolar lavage fluid next-generation sequencing results), CT scan images, and responses to treatment. In case of any disagreement between the two specialists, they resolved it through discussion. If a consensus could not be reached, they sought the opinion of another senior specialist.
Definitions and statistical analyses
The final diagnosis was regarded as the gold standard, and both the conventional method and BALF mNGS were examined and compared accordingly. Discordant results were defined as cases where additional organisms were detected by mNGS or when there was a discrepancy between the organisms identified by mNGS and CMTs. To ascertain the impact of these discordant mNGS results, electronic patient records were examined to determine if any antimicrobials were added or modified based on these findings. Descriptive statistical analyses were conducted on laboratory tests, including white blood cell count, neutrophils, lymphocytes, eosinophils, CRP, and PCT levels. If the data did not follow a normal distribution, the Mann-Whitney test was employed to determine the statistical significance of any observed differences. All statistical analyses were performed using GraphPad Prism software, version 9.0.
The data used to compare the test performance of mNGS to that of CMTs, and the clinical impact of unique organisms identified by mNGS, are included in Suppl. Table S2 in the supplemental material.
Demographic and clinical characteristics
A total of 164 patients with suspected pulmonary infections were included in the final analysis. There were 64 patients in the PNID group (39.0%) and 100 patients in the PID group (61%) (Table 1). The differences in age and sex were insignificantly in the two groups. More patients were Immunocompromised (Fig. 1A 38 vs. 3 p < 0.0001) and received empiric antibiotic therapy before bronchoscopy and BAL in the PID group (Fig. 1A 78 vs. 32, p = 0.0002). As for laboratory blood test results, including white blood cell count, CRP, and PCT, showed no significant differences in the two groups, while the cell count and ratio of neutrophils, lymphocytes, and eosinophils were significantly higher in the PID group. Concerning CT findings of the BAL site, no significant differences were found in the bilateral lesions, consolidation, ground-glass opacities, solid nodules, tree-in-bud infiltrates, atelectasis, or cavities. One patient in the PID group had a panic attack during BAL; therefore, the scope could not reach the site suspected of infection, and the BALF sample was extracted from a random site in the bronchus (Fig. 1B). The PID group had less stage III (26% vs. 42.19%, p = 0.0399) and more stage I (11% vs. 3.13%, p < 0.001) patients compared to PNID group (Fig. 1C).
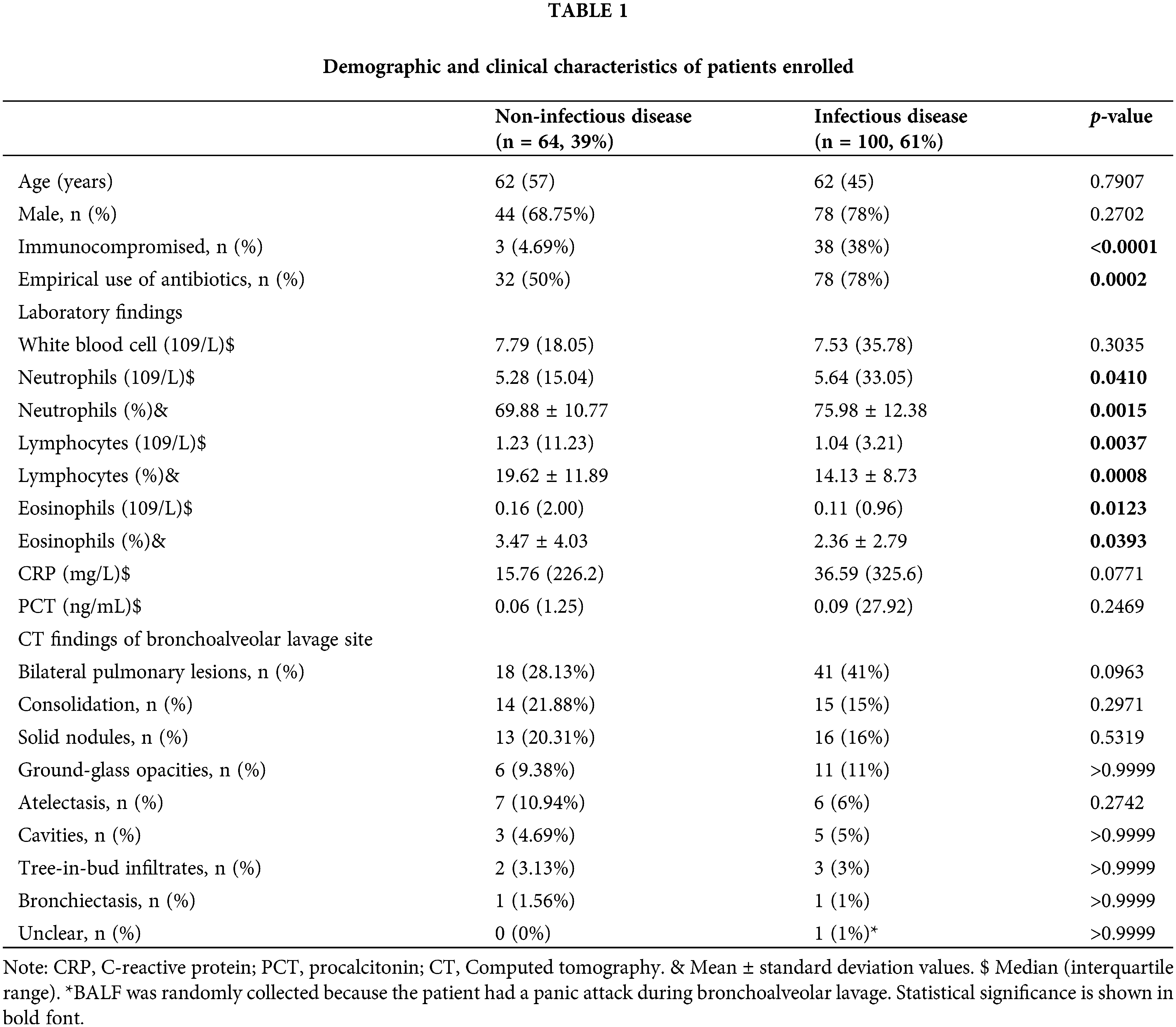

Figure 1: The ratio of clinical and cancer-related characteristics of patients enrolled. (A) Clinical characteristics of NID and ID groups. ID group is more likely to be immune-compromised (ID vs. NID = 38% vs. 4.69%, p < 0.0001) and empirically given antibiotics (ID vs. NID = 78% vs. 50%, p = 0.0002). (B) CT findings of bronchoalveolar lavage site of NID and ID groups. (C) Cancer staging of NID and ID groups. ID group has more stage I patients (ID vs. NID = 11% vs. 3.13%, p < 0.0001). (D) Pathological type of NID and ID groups. CT: computed tomography, NID: non-infectious disease, ID: infectious disease.
Distributions of pulmonary infectious pathogens and non-infectious diseases.
There was no significant difference in the pathological type of lung cancer (Fig. 1D & Table 2).
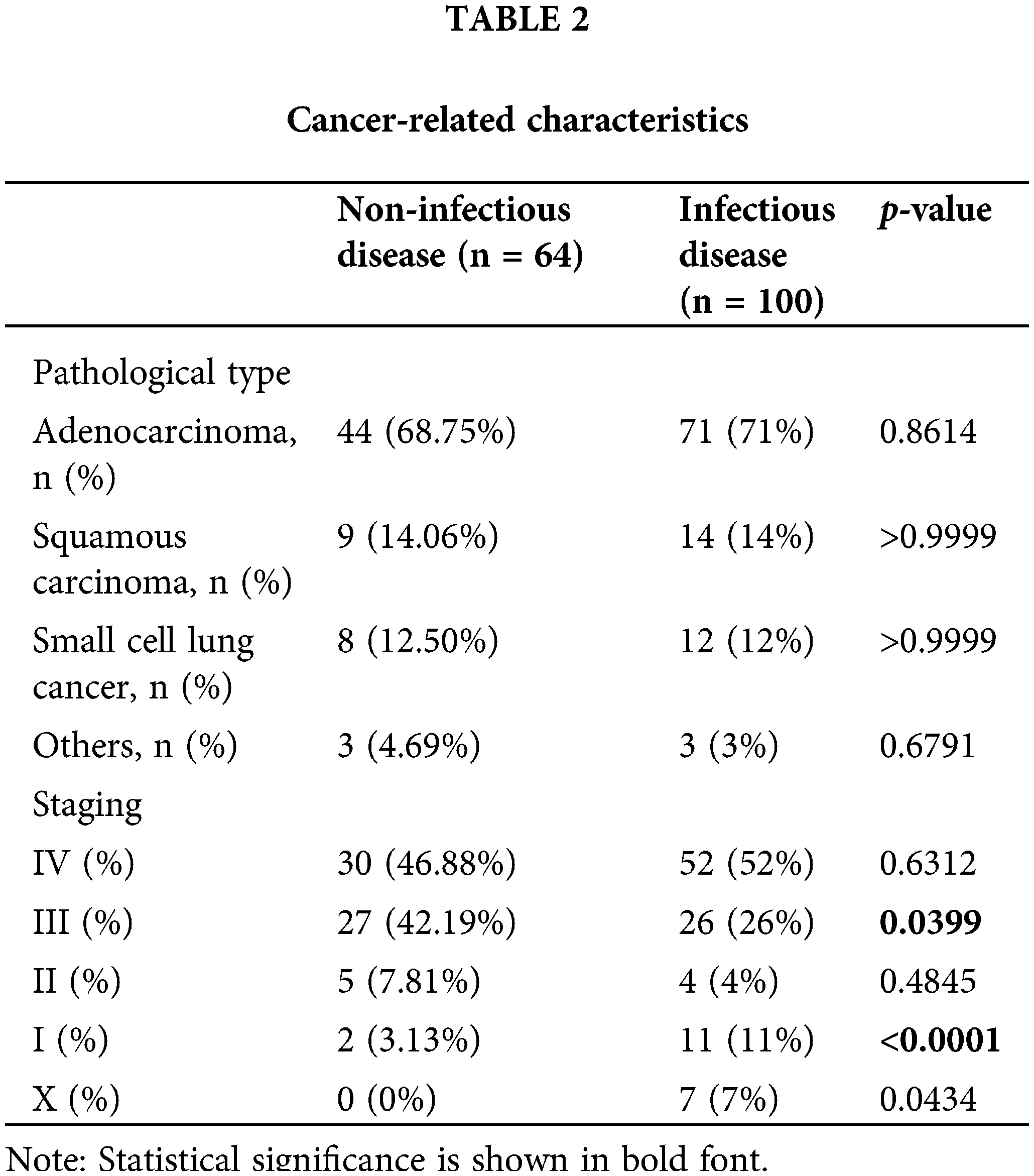
According to Table 3, among the 52 patients with PID, bacterial infections were the most common cause of infection. Staphylococcus aureus was detected in 8% of patients, making it the most commonly found pathogen. This was followed by Klebsiella pneumoniae, Nontuberculosis mycobacteria, Pseudomonas aeruginosa, and Streptococcus constellation, each accounting for 5% of the cases (as shown in Fig. 2A). Fungal infections were observed in 32% of patients, with the most common types being Pneumocystis Yersini (17%) and Aspergillus (6%). Additionally, one patient was found to be infected with Talaromyces marneffei (Fig. 2B).
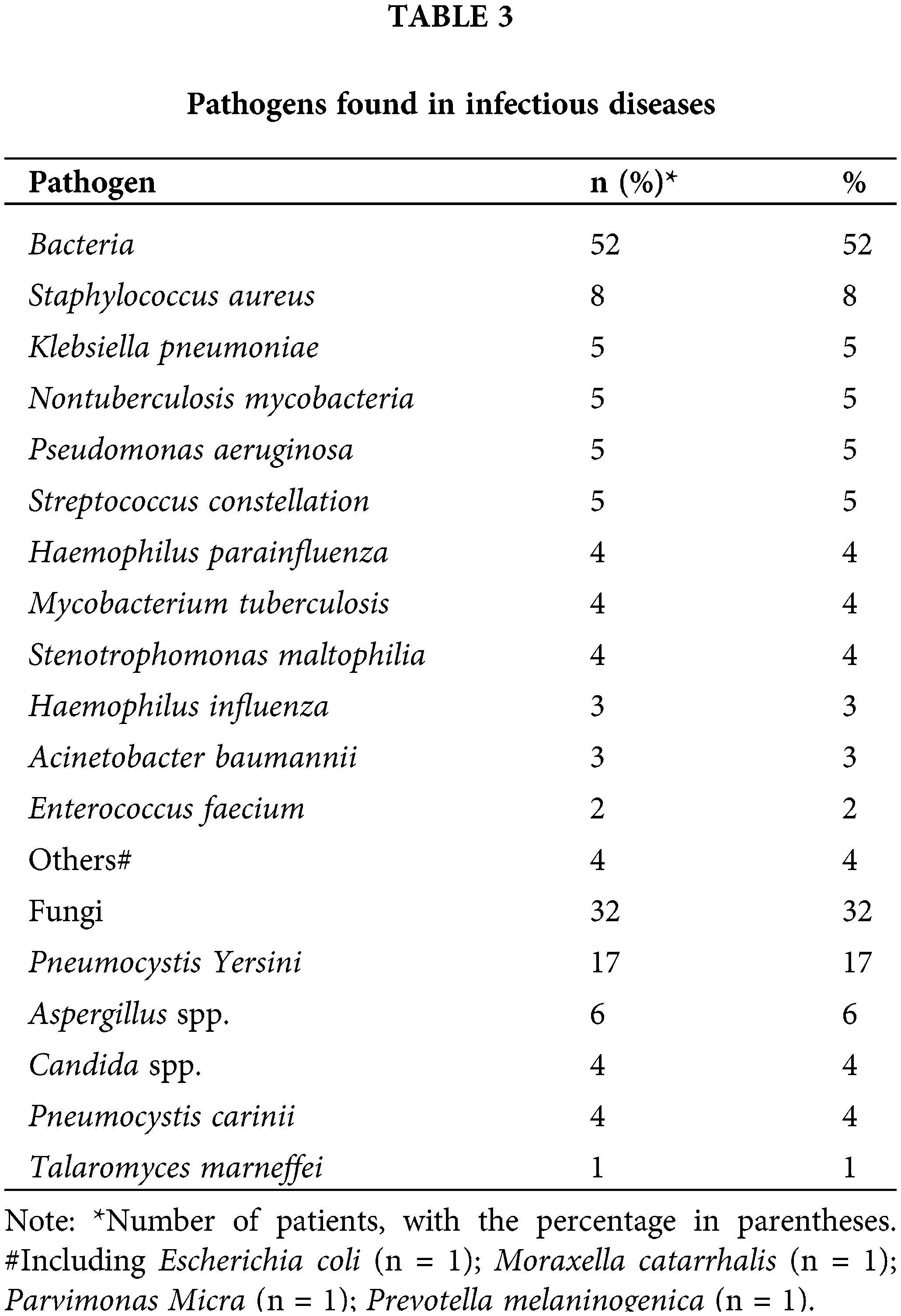

Figure 2: Pathogens found in infectious diseases. (A) Bacteria detected via BALF my test. Staphylococcus aureus (8%) was the most commonly detected pathogen, followed by Klebsiella pneumoniae (5%), nontuberculosis mycobacteria (5%), Pseudomonas aeruginosa (5%) and Streptococcus constellation (5%). (B) Fungi were found in the ID group. The most commonly detected fungi were Pneumocystis yersini (17%) and Aspergillus (6%). Aspergillus spp.: Aspergillus species, Candida spp.: Candida species. BALF: bronchoalveolar lavage fluid, ID: infectious disease, mNGS: metagenomic next-generation sequencing.
Comparison of diagnostic performance for differentiating PID from PNID with bronchoalveolar lavage fluid mNGS or conventional microbial tests
Fig. 3A displays the positivity rates of BALF mNGS and CMTs for PID and PNID. The positive predictive value for diagnosing PID using BALF mNGS was 83.5%, with a negative predictive value of 71.6%. The likelihood ratio was 3.24. Comparing BALF mNGS to CMTs, it was observed that BALF mNGS increased the sensitivity rate by approximately 60% (81% vs. 21%, p < 0.05), while there was no significant difference in specificity (75% vs. 98.4%, p > 0.1) (Fig. 1A). Out of the 100 patients, only two were confirmed by CMT, resulting in a low positive rate of 21% for CMT, whereas the positive rate for mNGS was 81% (Fig. 3B). For additional details, please refer to Suppl. Table S2.

Figure 3: Sensitivity and specificity of mNGS and CMTs. (A) Sensitivity and specificity of mNGS and CMTs. The BALF mNGS demonstrated a positive predictive value of 83.5% and a negative predictive value of 71.6% for diagnosing infectious diseases. Compared to CMTs, BALF mNGS increased the sensitivity rate by approximately 60% (81% vs. 21%; p < 0.01). (B) Moreover, despite comprehensive mNGS yielding negative results, two patients (2%) still tested positive for BALF CMT, indicating a non-infectious disease (NID) rather than an infectious disease (ID) scenario. mNGS: metagenomic next-generation sequencing, PPV: positive predictive value, NPV: negative predictive value, LR: likelihood ratio.
Diagnosis assisted by bronchoalveolar lavage fluid mNGS for pneumocystis
Among the 100 patients diagnosed with PID, 17 were confirmed Pneumocystis yersini infection by BALF mNGS, while one was confirmed by CMTs (Suppl. Table S2). Notably, all four cases of Pneumocystis carinii infection were BALF mNGS-positive despite comprehensive CMTs being negative, indicating mNGS is more sensitive in detecting Pneumocystis.
Immune status affects patients
As demonstrated in Fig. 4, PNID and PID made no significant difference in affecting OS of lung cancer patients (Fig. 4A, median OS NID vs. ID = 44 vs. 60 (months), p = 0.8904). On the other hand, patients with defective immunity had shorter OS than immunology intact patients (Fig. 4B median OS immunology defect vs. immunology intact = 60 vs. 77 (months), p = 0.0285). One main reason for this phenomenon is that patients with defection in immunity came with a heavier burden of tumor load and went through more cycles of treatments, impacting their immunological status.

Figure 4: OS of non-infectious disease, infectious disease, and different immune states. (A) The OS between NID and ID groups did not differ significantly (p = 0.8904). (B) The immuno-complete group possesses better OS compared to Immuno-defect (p = 0.0258). NID: non-infectious disease, ID: infectious disease, OS: overall survival.
Tyrosine kinase inhibitors (TKIs) cause longer overall survival in lung cancer patients
Patients with TKI therapy possess longer OS than other anti-cancer agents, except for immune checkpoint inhibitors (ICIs); the difference between TKIs and ICIs was insignificant (Fig. 5B, median OS TKIs vs. ICIs vs. anti-angiogenic vs. chemo vs. radiotherapy = 76 vs. 84 vs. 61 vs. 58 vs. 60 (months), p (TKIs vs. ICIs) = 0.0570, p (TKIs vs. anti-angiogenic) = 0.0297, p (TKIs vs. chemo) = 0.0028, p (TKIs vs. radiotherapy) = 0.0064). The differences among ICIs, anti-angiogenic, chemo-, and radiotherapy were insignificant. Moreover, the pathological of lung cancer made no significant differences to OS (Fig. 5A, p = 0.1023). However, when comparing the OS caused by TKI treatment brought in either PNID or PID patients, the difference was insignificant (Fig. 6A, median OS PNID vs. PID=NE vs. 77 (months), p = 0.1971). OS did not differ between ID and NID groups, irrespective of the treatment patients were given (Figs. 6B–6E).

Figure 5: OS of different cancer pathological types and anti-tumor therapy. (A) Pathological type does not affect patients’ OS (p = 0.1023). (B) TKI therapy entails longer OS than other anti-cancer agents, median OS TKIs vs. ICIs vs. Anti-angiogenic vs. Chemo vs. Radiotherapy = 76 vs. 84 vs. 61 vs. 58 vs. 60 (months), p (TKIs vs. ICIs) = 0.0570, p (TKIs vs. anti-angiogenic) = 0.0297, p (TKIs vs. Chemo) = 0.0028, p (TKIs vs. radiotherapy) = 0.0064. Adeno: adenocarcinoma, Squamous: squamous carcinoma, SCLC: small cell lung cancer, TKIs: tyrosine kinase inhibitors, ICIs: immuno-checkpoint inhibitors, Anti-angiogenic: antiangiogenic agents, Chemo: chemotherapy, OS: overall survival.

Figure 6: OS of different anti-tumor therapy in NID or ID patients. (A–E) No difference in OS was observed between ID and NID groups, regardless of anti-cancer treatments. TKIs: tyrosine kinase inhibitors, ICIs: immuno-checkpoint inhibitors, anti-angiogenic: antiangiogenic agents, Chemo: chemotherapy, NID: non-infectious disease, ID: infectious disease.
Our study showed that in patients with lung cancer, compared with CMTs, commercial mNGS using BALF had 60% higher diagnostic sensitivity and effectively filled the gap when CMT evidence was not sufficient, regardless of pathological types.
Infection and tumors are interrelated and influence each other on many levels [12–14]. Infection and cancer can both lead to continuous stimulation of T-cells, and exhausted T-cells gradually lose effector function and characteristics of memory T-cells [15,16]. Patients with lung cancer are at greater risk of dying from pneumonia [17]; therefore, accurate and quick identification of pathogens is the key to reducing mortality and prolonging the OS of lung cancer patients with ID. Regardless of the underlying mechanism, most of the pathogens negatively affect OS and the quality of life of patients with lung cancer, with or without anti-cancer treatments [18,19]. What’s more, antibiotic administration was significantly associated with worsened PFS in lung cancer patients treated with ICIs [12–14]; therefore, it is also important to reduce the unnecessary prescription of antibiotics. As anti-cancer therapies are being developed, PNID caused by ICIs and TKIs brings challenges to the management of lung cancer [20–22]. It urgently needs a reliable method to distinguish the nature of new lesions found in CT imaging of patients with lung cancer.
Generally speaking, BAL is a safe method for patients with possible lung diseases. The common complications of BAL are bleeding, fever, laryngeal edema, and hypoxia. With adequate preoperative preparation, most of the complications are preventable. In addition to collecting BALF for testing, the process of BAL can also flush the patient’s accumulated sputum in the bronchus, providing an unobstructed pulmonary drainage pathway. As an important means of diagnosis and treatment of lung diseases, bronchoscopes, and BAL have been widely used in clinical work.
CMTs, or complete blood counts, are widely utilized in medical diagnostics and can provide insight into the degree of infection [23]. Physicians in both primary care settings and specialized hospitals typically rely on routine blood tests to make accurate infection diagnoses [24]. To identify ID, cell count and the ratio of neutrophils was proved reliable in our study. However, in contrast to the present study and clinical practice experience, Zhu’s study found no difference in blood routine indexes between PNID and PID groups [25]. Therefore, blood routine cannot be used alone to diagnose PID. Moreover, routine blood tests cannot determine the type of pathogen. The liquid-based culture method is capable of sorting out the pathogen in blood, BALF, or other body fluids [26] but the limited number of pathogens could be detected at the same time causing a repetition of the tests [27].
Compared to CMTs, mNGS is broad-spectrum, and has a faster detection process than the liquid culture method, with a higher positive rate in a single test [28,29]. Although the specimens to be tested by NGS can be different kinds of body fluids, blood is still the most commonly used [30]. According to Sun’s study, BALF mNGS is more sensitive and exhibits better diagnostic accuracy in patients with suspected severe pneumonia [31], indicating that BALF is more suitable for identifying PID/PNID in patients with lung cancer.
As a new method of auxiliary examination, mNGS is expensive, and unaudited usage increases the financial burden of patients [32,33]. Primary hospitals usually cannot afford in-house mNGS stations; therefore, the accessibility of commercial mNGS tests could better help patients in primary hospitals when facing PID. In our study, compared to CMTs, commercial mNGS showed great potential in diagnosing PID, especially when smear or staining in CMTs cannot offer sufficient evidence.
However, certain limitations of this study must be stressed. First, the NGS tests evaluated in this study were offered by commercial test centers, unlike CMTs, which were carried out in the in-house laboratory. The transportation from the hospital to the commercial lab may affect the accuracy of the NGS results even though an SOP of the cold chain was applied. Second, the present study was single-center research, and the randomness of cases involved in the study was limited due to the nature of real-world retrospective studies. Further randomized control trial is needed to rule out the physician`s subjective influence in BAL implementation.
In conclusion, our study indicates that BALF mNGS can add value by improving overall sensitivity in lung cancer patients with suspected pulmonary infection and was outstanding in identifying Pneumocystis infection. It may help physicians to determine the follow-up treatment adjustment and avoid the abuse of antibiotics. In primary hospitals without access to complex equipment and in-house mNGS testing instruments, commercial BALF mNGS could be a safe, convenient, and economical method that can improve the accuracy of PNID and PID diagnoses in lung cancer patients. In identifying pathogens in patients with intact immune function, BALF mNGS is superior to the combination of CMTS, the combination of mNGS and CMTs may be a better diagnostic strategy.
Acknowledgement: None.
Funding Statement: This study was funded by Science and Technology Projects in Guangzhou (No. 202002030023).
Author Contributions: Study conception and design: Kejing Tang and Jiyu Wang; data collection: Deyuan Zhou and Yuxia Li; analysis and interpretation of results: Jiyu Wang, Lihong Bai, Kejing Tang; draft manuscript preparation: Jiyu Wang and Lihong Bai. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval: The research plan and electronic medical records of patients involved in this study were reviewed and approved by the Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University. ethical approval code: IIT-2023-145. Date of approval: 2nd March 2023. This study does not interfere with clinical decisions.
Conflicts of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Raghu G, Meyer KC. Cryptogenic organising pneumonia: current understanding of an enigmatic lung disease. Eur Respir Rev. 2021;30(161):210094. [Google Scholar] [PubMed]
2. Bertaglia V, Morelli AM, Solinas C, Aiello MM, Manunta S, Denaro N, et al. Infections in lung cancer patients undergoing immunotherapy and targeted therapy: an overview on the current scenario. Crit Rev Oncol Hematol. 2023;184:103954. [Google Scholar] [PubMed]
3. Osorio JC, Candia-Escobar F, Corvalán AH, Calaf GM, Aguayo F. High-risk human papillomavirus infection in lung cancer: mechanisms and perspectives. Biol. 2022;11(12):1691. [Google Scholar]
4. Burns EA, Gee K, Kieser RB, Xu J, Zhang Y, Crenshaw A, et al. Impact of infections in patients receiving pembrolizumab-based therapies for non-small cell lung cancer. Cancer. 2022;15(1):81. [Google Scholar]
5. Niu J, Wang J, Jia P, Zhang M, Wei E. Clinical features and diagnostic value of metagenomic next-generation sequencing in five cases of non-HIV related Pneumocystis jirovecii pneumonia in children. Front Cell Infect Microbiol. 2023;13:1132472. [Google Scholar] [PubMed]
6. Reid NK, Joyner KR, Lewis-Wolfson TD. Baricitinib versus Tocilizumab for the treatment of moderate to severe COVID-19. Ann Pharmacother. 2023;57(7):769–75. [Google Scholar] [PubMed]
7. Bi J, Qian J, Yang D, Sun L, Lin S, Li Y, et al. Dosimetric risk factors for acute radiation pneumonitis in patients with prior receipt of immune checkpoint inhibitors. Front Immunol. 2021;12:828858. [Google Scholar] [PubMed]
8. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67. [Google Scholar] [PubMed]
9. Huang Z, Li W, Lee GC, Fang X, Xing L, Yang B, et al. Metagenomic next-generation sequencing of synovial fluid demonstrates high accuracy in prosthetic joint infection diagnostics: mNGS for diagnosing PJI. Bone Joint Res. 2020;9(7):440–9. [Google Scholar] [PubMed]
10. Pan Y, Zhang X, Sun Y, Zhang Y, Bao W, Yin D, et al. Cellular analysis and metagenomic next-generation sequencing of bronchoalveolar lavage fluid in the distinction between pulmonary non-infectious and infectious disease. Front Cell Infect Microbiol. 2022;12:1023978. [Google Scholar] [PubMed]
11. Zhu L, Hao Y, Li W, Shi B, Dong H, Gao P. Significance of pleural effusion detected by metagenomic next-generation sequencing in the diagnosis of aspiration pneumonia. Front Cell Infect Microbiol. 2022;12:992352. [Google Scholar] [PubMed]
12. Hassan M, Flanagan TW, Kharouf N, Bertsch C, Mancino D, Haikel Y. Antimicrobial proteins: structure, molecular action, and therapeutic potential. Pharm. 2022;15(1):72. [Google Scholar]
13. Moreira H, Dobosz A, Cwynar-Zając Ł., Nowak P, Czyżewski M, Barg M, et al. Unraveling the role of Breg cells in digestive tract cancer and infectious immunity. Front Immunol. 2022;13:981847. [Google Scholar] [PubMed]
14. Luo Q, Yang H, Hu B. Application of artificial intelligence in the endoscopic diagnosis of early gastric cancer, atrophic gastritis, and Helicobacter pylori infection. J Dig Dis. 2022;23(12):666–74. [Google Scholar] [PubMed]
15. Gao Z, Feng Y, Xu J, Liang J. T-cell exhaustion in immune-mediated inflammatory diseases: new implications for immunotherapy. Front Immunol. 2022;13:977394. [Google Scholar] [PubMed]
16. Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory receptor expression depends more dominantly on differentiation and activation than exhaustion of human CD8 T cells. Front Immunol. 2013;4:455. [Google Scholar] [PubMed]
17. Hespanhol V, Bárbara C. Pneumonia mortality, comorbidities matter? Pulmonol. 2020;26(3):123–9. [Google Scholar]
18. Zhou Y, Xu M. Analysis of the effect of quality nursing on recovery after thoracic surgery. Emerg Med Int. 2022; 2022;2022:6204832. [Google Scholar] [PubMed]
19. Zhang T, Lu J, Fan Y, Wang L. Evidence-based nursing intervention can improve the treatment compliance, quality of life and self-efficacy of patients with lung cancer undergoing radiotherapy and chemotherapy. Am J Transl Res. 2022;14(1):396–405. [Google Scholar] [PubMed]
20. Rudin CM, Cervantes A, Dowlati A, Besse B, Ma B, Costa DB, et al. Safety and clinical activity of atezolizumab plus erlotinib in patients with non-small-cell lung cancer. ESMO Open. 2023;8(2):101160. doi:https://doi.org/10.1016/j.esmoop.2023.101160. [Google Scholar] [PubMed] [CrossRef]
21. Chihara Y, Takeda T, Goto Y, Nakamura Y, Tsuchiya-Kawano Y, Nakao A, et al. A phase II trial on osimertinib as a first-line treatment for egfr mutation-positive advanced NSCLC in elderly patients: the SPIRAL-0 study. Oncol. 2022;27(11):903–e834. [Google Scholar]
22. Romão R, Mendes AS, Ranchor R, Ramos MJ, Coelho J, Pichel RC, et al. Impact of immune-related adverse events on immune checkpoint inhibitors treated cancer patients’ survival: single center experience and literature review. Cancer. 2023;15(3):888. [Google Scholar]
23. Munsell MK, Fadelu T, Stuver SO, Baker KP, Glotzbecker B, Simmons JL, et al. The utility of procalcitonin for diagnosing bacteremia and bacterial pneumonia in hospitalized oncology patients. J Cancer Res Clin Oncol. 2023;149(8):5193–204. [Google Scholar] [PubMed]
24. Karuniawati A, Ambarwulan M, Shahab SN, Moenadjat Y, Lalisang TJM, Kurniati ND, et al. Ceftolozane/tazobactam in-vitro activity against clinical isolates from complicated intra-abdominal infection patients in three indonesian referral hospitals. Antibiot. 2022;12(1):52. [Google Scholar]
25. Zhu G, Zhu J, Song L, Cai W, Wang J. Combined use of biomarkers for distinguishing between bacterial and viral etiologies in pediatric lower respiratory tract infections. Infect Dis. 2015;47(5):289–93. [Google Scholar]
26. Lecerf P, De Paepe R, Jazaeri Y, Normand AC, Martiny D, Packeu A. Evaluation of a liquid media MALDI-TOF MS protocol for the identification of dermatophytes isolated from tinea capitis infections. J Fungi. 2022;8(12):1248. [Google Scholar]
27. Kite KA, Loomba S, Elliott TJ, Yongblah F, Lightbown SL, Doyle TJ, et al. FcMBL magnetic bead-based MALDI-TOF MS rapidly identifies paediatric blood stream infections from positive blood cultures. PLoS One. 2022;17(11):e0276777. [Google Scholar] [PubMed]
28. He D, Liu M, Chen Q, Liu Y, Tang Y, Shen F, et al. Clinical characteristics and the effect of timing for metagenomic next-generation sequencing in critically ill patients with sepsis. Infect Drug Resist. 2022;15:7377–87. [Google Scholar] [PubMed]
29. Bassi C, Guerriero P, Pierantoni M, Callegari E, Sabbioni S. Novel virus identification through metagenomics: a systematic review. Life. 2022;12(12):2048. [Google Scholar] [PubMed]
30. Gökdemir F, İşeri Ö. D, Sharma A, Achar PN, Eyidoğan F. Metagenomics next generation sequencing (mNGSan exciting tool for early and accurate diagnostic of fungal pathogens in plants. J Fungi. 2022;8(11):1195. [Google Scholar]
31. Sun T, Liu Y, Cai Y, Zhai T, Zhou Y, Yang B, et al. A paired comparison of plasma and bronchoalveolar lavage fluid for metagenomic next-generation sequencing in critically ill patients with suspected severe pneumonia. Infect Drug Resist. 2022;15:4369–79. [Google Scholar] [PubMed]
32. Fan C, Gong L, An M, Li Z, Li X, Fang J. Diagnosis and treatment to a post-craniotomy intracranial infection caused by corynebacterium. Infect Drug Resist. 2022;15:6681–7. [Google Scholar] [PubMed]
33. Zhou C, Wang K, Li H, Zhang X. Idiopathic thrombocytopenic purpura with brain abscess caused by Nocardia farcinica diagnosed using metagenomics next-generation sequencing of the cerebrospinal fluid: a case report. BMC Infect Dis. 2021;21(1):380. [Google Scholar] [PubMed]
Supplementary Materials
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools