 Open Access
Open Access
ARTICLE
Curcumin inhibits colorectal cancer development by blocking the YAP/TAZ signaling axis
1 Department of Pharmacy, Affiliated Cancer Hospital of Shantou University School of Medicine, Shantou, China
2 Department of Pharmacy, Shantou University Mental Health Center, Shantou, China
3 Static Distribution Center, Affiliated Cancer Hospital of Shantou University School of Medicine, Shantou, China
* Corresponding Authors: MINGHAO ZHENG. Email: ; JIAOLING CHEN. Email:
(This article belongs to the Special Issue: Herbal Active Ingredients: Potential for the Prevention and Treatment of Cancer)
BIOCELL 2024, 48(3), 443-451. https://doi.org/10.32604/biocell.2023.029188
Received 06 February 2023; Accepted 08 May 2023; Issue published 15 March 2024
Abstract
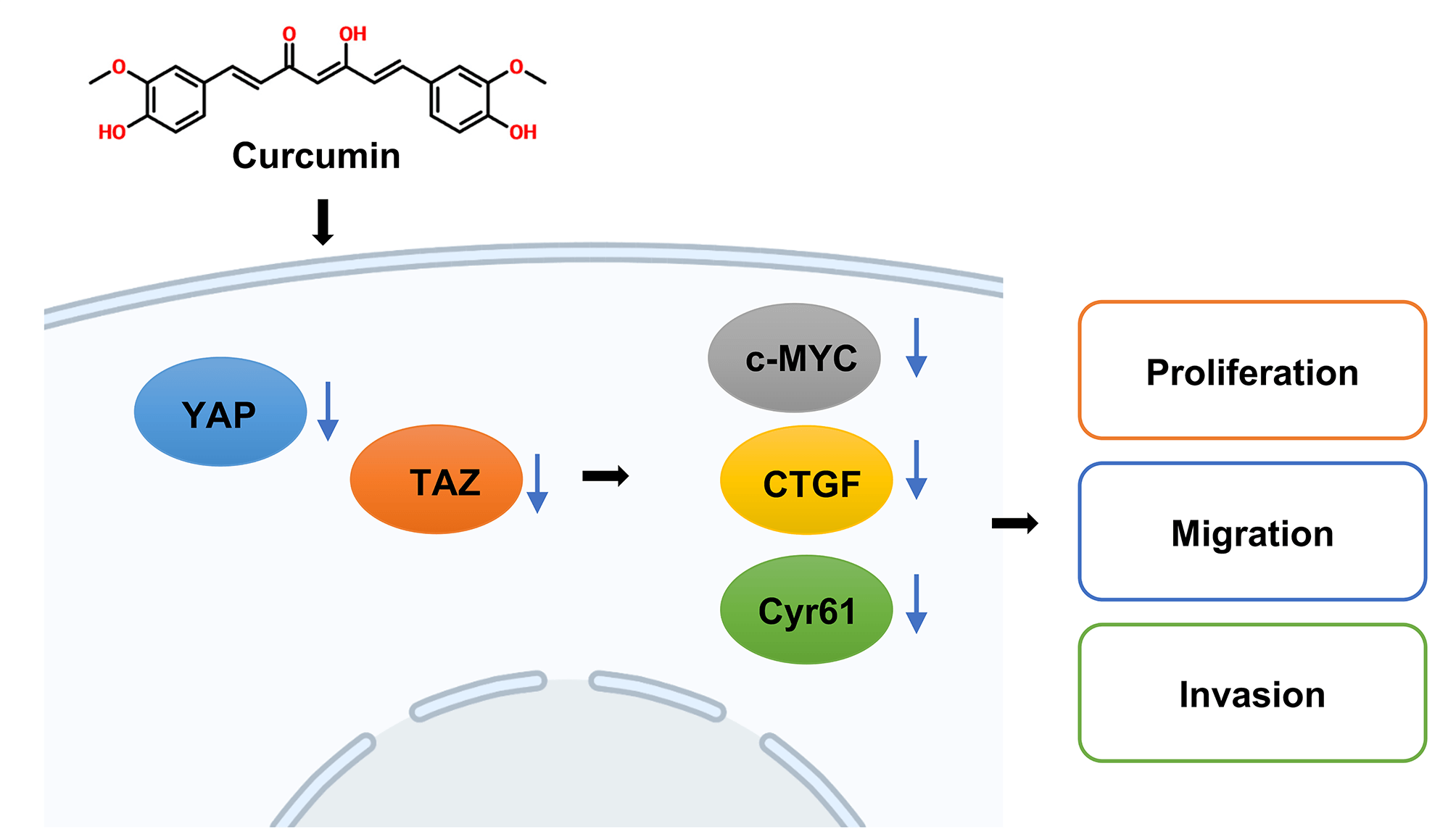
Background: Curcumin is a plant polyphenol with antitumor properties and inhibits the development of colorectal cancer (CRC). However, as the molecular mechanism associated is still unclear, our study aimed to explore the underlying molecular mechanisms by which curcumin inhibits CRC. Methods: HT29 and SW480 cells were treated with curcumin or/and Doxycycline (DOX), and cell viability, colony forming ability, migration and invasion were confirmed by cell counting kit-8 (CCK-8), colony forming, Transwell assays. And Yes-associated protein 1 (YAP) and PDZ-binding motif (TAZ) signaling-related genes or proteins were analyzed using reverse transcription quantitative real-time PCR (RT-qPCR), western blot, and immunofluorescence assays. Then nude mice xenograft tumor model was constructed, YAP and Ki67 expressions were tested by immunohistochemistry (IHC) staining. Results: In our study, we proved that curcumin significantly inhibited the CRC cell viability, cell migration, and cell invasion abilities. In addition, curcumin inhibited YAP and Transcriptional coactivator with TAZ or the YAP/TAZ signaling axis in CRC cells. Further, in the nude mice model, curcumin treatment significantly decreased the size and weight of xenotransplant tumors. Conclusion: Therefore, curcumin significantly inhibited CRC development and invasion by regulating the YAP/TAZ signaling axis.Graphic Abstract
Keywords
Abbreviations
| CRC | Colorectal cancer |
| YAP | Yes-associated protein 1 |
| TAZ | PDZ-binding motif |
| DOX | Doxycycline |
| CTGF | Connective tissue growth factor |
| CCK-8 | Cell counting kit-8 |
| IHC | Immunohistochemistry |
| RT-qPCR | Reverse transcription quantitative real-time PCR |
| SDS | Sodium dodecyl sulfate |
| PAGE | Polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene difluoride |
Colorectal cancer (CRC) is one of the most common digestive tract malignancies, with more than one million diagnoses worldwide each year, and more than half of such patients die from it [1,2]. Although the research on CRC has made great progress in recent years, there is still a lack of effective clinical treatments for CRC patients [3]. Therefore, developing new therapeutic drugs for CRC has very important clinical application values.
Curcumin is a plant polyphenol mainly derived from the rhizomes of the genus Curcuma [4]. Curcumin has documented various properties on tumor cells, including anti-proliferative, anti-inflammatory, and antioxidant activities [5,6]. Studies have shown that curcumin could effectively inhibit the invasion and proliferation of human cancers, such as Wilms tumor (WT), esophageal cancer, and CRC [7–10]. Further, curcumin inhibits the occurrence and development of CRC mainly through anti-inflammatory mechanisms and anti-tumor-related pathways [11,12].
Dysregulation of the Hippo signaling pathway can induce development in various organs, such as in CRC, lung cancer, liver cancer, gastric cancer, and breast cancer, to name a few. For example, a mutation or abnormality of the Hippo signaling pathway resulted in overexpression of Yes-associated protein 1 (YAP) and PDZ-binding motif (TAZ) and their accumulation in the nucleus promoted the development and development of disease [13,14]. Few latest studies found that elevated expression of YAP/TAZ or nuclear enrichment of YAP/TAZ was observed in various types of tumors [15–17]. In addition, the expression level of YAP/TAZ is considered to be a new tumor molecular marker and a potential therapeutic target. It can be also used as an independent prognostic indicator in some malignant tumors [18]. Previous studies also reported that curcumin interacts with YAP/TAZ in prostate cancer [19]. Furthermore, a recent analysis reported that curcumin inhibits the Hippo signaling pathway via miR-30a-5p in CRC [20]. However, it is not yet clear whether the inhibitory effect of curcumin on colorectal carcinogenesis is related to the YAP/TAZ axis. Based on the above studies, we hypothesize that curcumin inhibits CRC by inhibiting the YAP/TAZ axis. Our data showed that the inhibition of CRC by curcumin depended on the TAZ/YAP axis. In addition, curcumin inhibited the TAZ/YAP axis by phosphorylating YAP to prevent it from entering the nucleus.
Curcumin (SC0299), Trizol (R0016), Crystal Violet Staining Solution (C0121), Immunol Fluorescence Staining Kit with Alexa Fluor 555 (P0179), Cell Counting Kit-8 (C0039) and Doxycycline (ST039A) were purchased from Beyotime Biotechnology, Shanghai, China. Trans-well (Snapwell™, 3407) was purchased from Corning Incorporated, New York, USA. The following antibodies, anti-TAZ (ab110239), anti-YAP (ab205270), anti-GAPDH (ab76523), anti-cMYC (ab71676), anti-connective tissue growth factor (CTGF) (ab209780), anti-Cyr61 (ab252421) and anti-Ki67 (ab92742) we all purchased from Abcam Co., Ltd., Cambridge, UK. HRP-labeled Goat Anti-Rabbit IgG was purchased from Beyotime Biotechnology, Shanghai, China.
SW480, HT29, and HEK293 cells were purchased from American Type Culture Collection (ATCC). While HT29 cells were maintained in the HT-29 medium (SNLM-069, SUNNCELL Biotechnology Co., Ltd., Wuhan, China), SW480 cells were maintained in the SW480 cell-specific medium (CM-0223B, ProcellLife Science & Technology Co., Ltd., Wuhan, China) and HEK293 cells were maintained in the HEK293 cell-special medium (CM-0001, Procell Life Science & Technology Co., Ltd., Wuhan, China). All cells were cultured in a cell incubator at humidified atmosphere conditions of 37°C and 5% CO2.
After counting single-cell suspensions of HT29 and SW480 cells, 1000 cells per well were plated into 96-well plates. Subsequently, curcumin was added at final concentrations of 0, 5, 10 or 20 μM for treatment. The cell viability of each treatment group was measured using the CCK-8 kit at 24, 48, 72, and 96 h.
After HT29 and SW480 cells were digested into a single cell suspension, 200 cells per well were seeded in a 6-well plate. Curcumin was added at concentrations of 0, 5, 10 and 20 μM. After 2–3 weeks of cell culture, the cells were stained with crystal violet staining solution, and the number of colonies formed was subsequently counted.
After HT29 and SW480 cells were digested into single cell suspensions, 200 µl of the cell suspension was added to the Trans-well chamber with Matrigel (invasion) or Trans-well chamber without Matrigel (migration). The concentration was adjusted so that the number of cells in 200 µl of cell suspension was 20,000. Subsequently, 600 µl of medium containing 15% fetal bovine serum (FBS) was added to the lower chamber of the 24-well plate and curcumin was added at concentrations of 0, 5, 10 or 20 μM, respectively. After 24 h of cell culture, the cells were stained with crystal violet staining solution, and the number of cells was subsequently counted.
We used pre-cooled cell lysis buffer (P0013, Beyotime Biotechnology, Shanghai, China) to lyse cells in each curcumin treatment group and then centrifuged at 4°C at 12000 rpm for 15 min. After the protein concentration in each group was detected, a total of 30–40 μg protein was used to perform sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). After the electrophoretic separation, protein samples were transferred to polyvinylidene difluoride (PVDF) membranes by electro-transfer. These PVDF membranes were blocked with 5% skimmed milk for 1 h at room temperature and then incubated overnight at 4°C with the corresponding primary antibodies mentioned above (1:500).
Reverse transcription quantitative real-time PCR (RT-qPCR)
The total RNA of cells of each treatment group was extracted by the Trizol method. RT-qPCR was then performed using the HiScript II One Step qRT-PCR SYBR Green Kit (Q221-01, Vazyme, Nanjing, China) kit. The primer sequences for Homo sapiens genes are shown below:
cMYC-Forward: 5′-CCTGGTGCTCCATGAGGAGAC-3′
cMYC-Reverse: 5′-CAGACTCTGACCTTTTGCCAGG-3′
CTGF-Forward: 5′-CTTGCGAAGCTGACCTGGAAGA-3′
CTGF-Reverse: 5′-CCGTCGGTACATACTCCACAGA-3′
Cyr61-Forward: 5’-GGAAAAGGCAGCTCACTGAAGC-3′
Cyr61-Reverse: 5′-GGAGATACCAGTTCCACAGGTC-3′
GAPDH-Forward: 5′-GTCTCCTCTGACTTCAACAGCG-3′
GAPDH-Reverse: 5′-ACCACCCTGTTGCTGTAGCCAA-3′
Immunofluorescence staining assay
SW480 cells on coverslips of different treatment groups were fixed with fixative, and immunofluorescence experiments were then done according to the instructions of the Immunol Fluorescence Staining Kit with Alexa Fluor 555 (P0179, Beyotime Biotechnology, Shanghai, China).
Generation of the transfected engineered cell line
We transfected pSPAX2, pMD2.g, and pLVX-TetOne-YAP plasmids into HEK293 cells according to the instruction of the Lipo293F™ Transfection Reagent (C0518, Beyotime Biotechnology, Shanghai, China). After 48 h of transfection, the medium containing the recombinant virus was filtered through a 0.22 μM filter, and the filtered virus solution was used to infect SW480 cells with polyene (8 μg/mL). The infected SW480 cells were screened with puromycin (0.5 μg/mL) for 7 days before proceeding to subsequent corresponding experiments.
All animal experimental protocols in this study were approved by the Institutional Animal Care and Use Committee of Shantou University School of Medicine (No. 20200921021). All animal work was performed in strict accordance with approved protocols. A total of 15 healthy BALB/c nude mice (6–8 weeks old; Shanghai Model Organisms Center, Shanghai, China) were housed in a pathogen-free environment with a 12-h light/dark cycle. We subcutaneously injected 1 × 106 SW480-tet cells into each group of nude mice. The tumor volume was monitored on 7, 10, 13, 16, 19, 22, 25, and 28 days post-injection. After 28 days of injection, mice were euthanized with CO2, and tumors were collected for subsequent assays.
Immunohistochemistry (IHC) assay
The tumor tissues of each group were fixed with 4% paraformaldehyde, dehydrated with different concentrations of ethanol, transparent with xylene II, and then immersed in wax. After embedding in paraffin, the tissues were sectioned (4 μM thick). Subsequently, sections were deparaffinized with xylene and hydrated with gradient ethanol, and rinsed with distilled water and PBS. After 10 min of peroxidase blocking, sections were added with primary antibody (Abcam, 1:300) followed by secondary antibody (Abcam, 1:400). After DAB coloration, sections were counterstained by dropping hematoxylin nucleating and dehydrated with gradient ethanol. After sealing the slides, the samples were observed under a microscope.
Data presented in this study are the mean ± standard error (SE) of three independent measurements. Data were statistically analyzed by t-test, two-way analysis of variance (ANOVA), and one-way ANOVA. The data analysis software used in this research was GraphPad Prism 7.0. Statistical significance was considered when the p-value < 0.05 (Note that *p < 0.05; **p < 0.01; ***p < 0.001).
Curcumin suppresses the malignancy of CRC cells
We used curcumin at varying doses to treat human CRC cell lines HT29 and SW480 to validate its potential as a CRC treatment agent. We found that curcumin significantly inhibited the cell viability and colony-forming ability of HT29 and SW480 cells in a dose-dependent manner (Figs. 1A–1D).
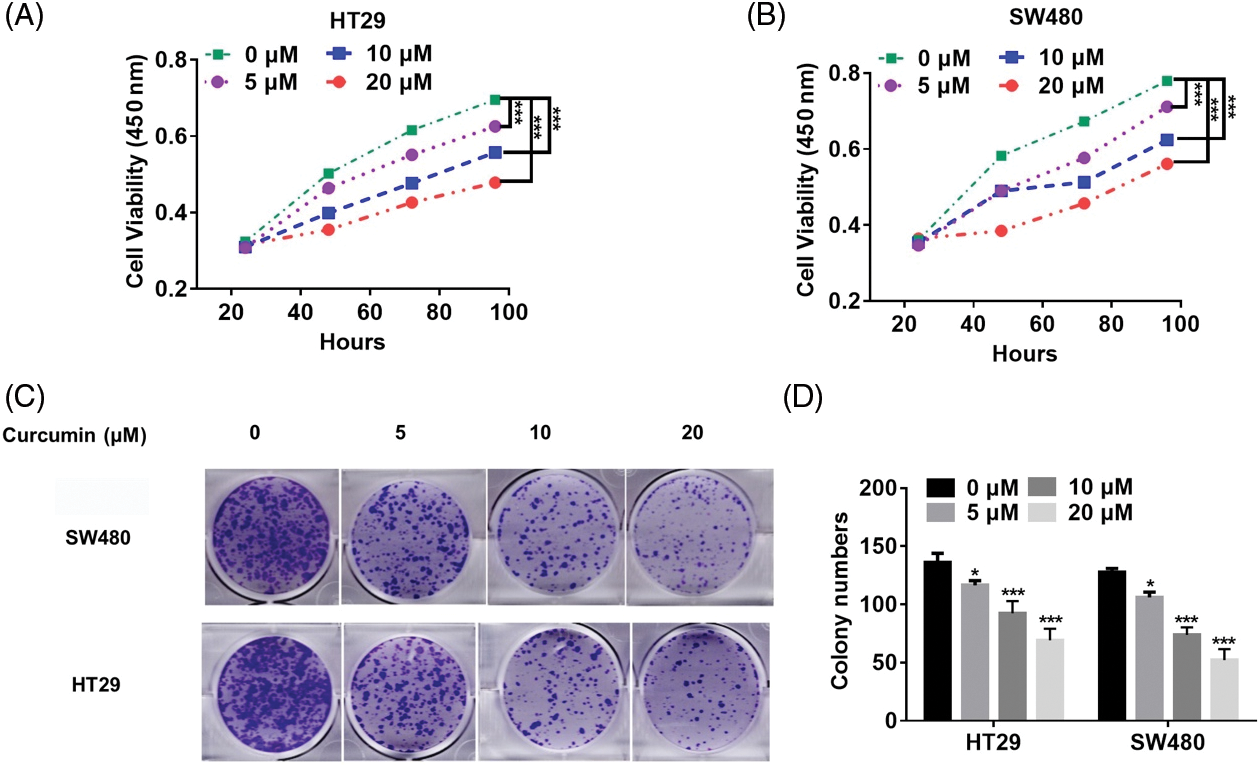
Figure 1: Curcumin inhibits CRC cell proliferation. (A) Curcumin inhibited HT29 cell viability. HT29 cells (1 × 103) were treated with 0, 5, 10 and 20 µM of curcumin, and the cell viability was detected by the cell counting kit-8 (CCK-8) assay kit for indicated time points. (B) Curcumin inhibited SW480 cell viability. SW480 cells (1 × 103) were treated with 0, 5, 10, and 20 µM curcumin, and the cell viability was detected by the CCK-8 assay kit for indicated time points. (C) Curcumin inhibited the 2-D colony forming ability of HT29 and SW480 cells. HT29 and SW480 cells were treated with 0, 5, 10, and 20 µM curcumin for 48 h before the colony forming assay. (D) Details of the clone formation assay of HT29 and SW480 cells. Each experiment was repeated three times; *p < 0.05; ***p < 0.001.
Then we also observed such a dose-dependent inhibitory effect of curcumin on cell migration of HT29 and SW480 (Figs. 2A and 2B). In addition, curcumin could significantly inhibit the invasion of HT29 and SW480 cells in a dose-dependent manner (Figs. 2C and 2D). Our overall results suggested that curcumin could suppress the malignancy of CRC cells in vitro.
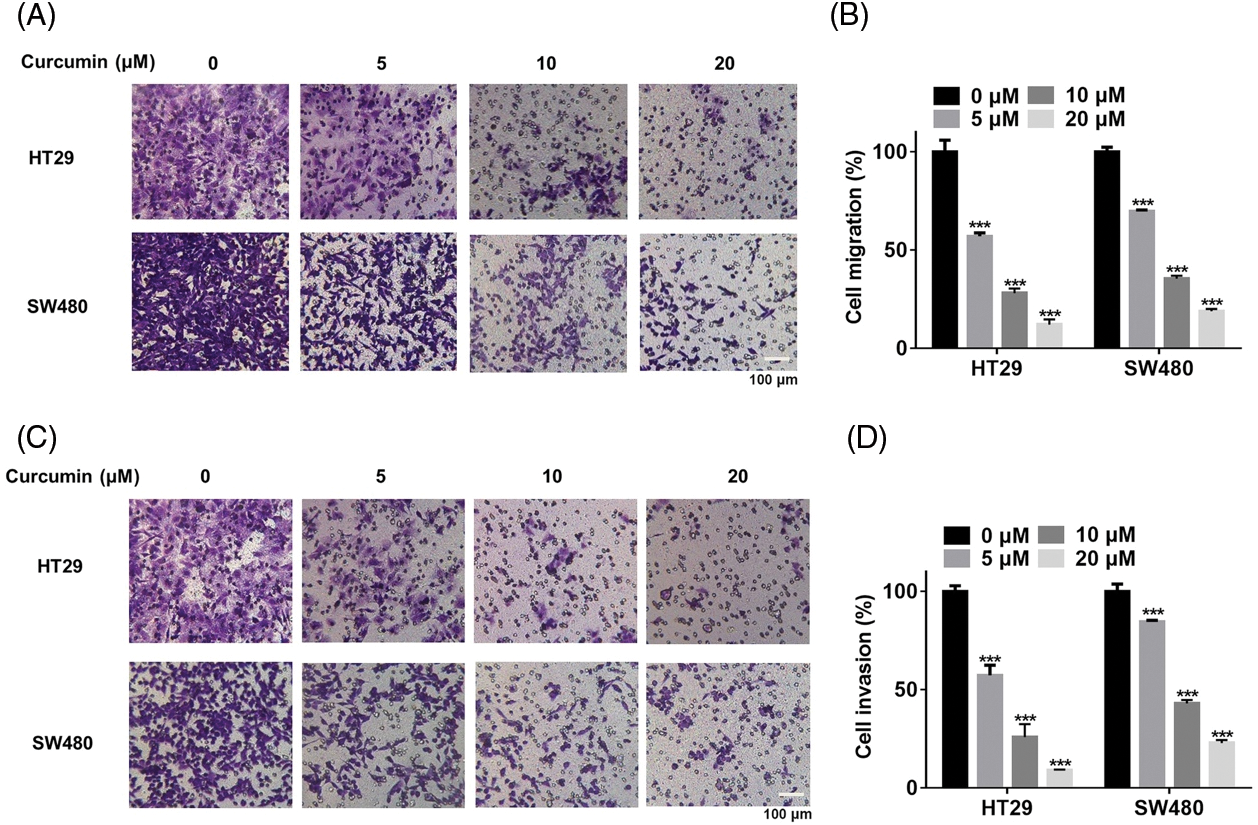
Figure 2: Curcumin inhibited the migration and invasion of CRC cells. (A) HT29 and SW480 cells were treated with 0, 5, 10, and 20 µM of curcumin for 48 h before cells migration analysis using the trans-well chamber assay, scale bar: 100 μM, magnification: 100×. (B) Details of cell migration detected in HT29 and SW480 cells. (C) HT29 and SW480 cells were treated with 0, 5, 10, and 20 µM of curcumin for 48 h before cells invasion analysis using the trans-well chamber assay, scale bar: 100 μM, magnification: 100×. (D) Statistical analysis of cell invasion analysis of HT29 and SW480 cells. Each experiment was repeated three times; ***p < 0.001.
Curcumin inhibits the YAP/TAZ signaling axis in CRC cells
Previous studies have reported that when the Hippo signaling pathway is mutated or abnormal, YAP/TAZ is overexpressed and is accumulated in the nucleus. Subsequently, its ability to promote cell proliferation is activated that promotes the occurrence and development of tumors [13,14]. In addition, some studies reported that curcumin can influence the YAP/TAZ signaling axis [19,21]. Hence, we hypothesized that curcumin may inhibit the viability, migration, and invasion of CRC cells by affecting the YAP/TAZ signaling axis. As shown in Figs. 3A and 3B, curcumin inhibited the expression of TAZ and YAP in HT29 and SW480 cells in a dose-dependent manner. In addition, curcumin also significantly inhibited the transcription and protein expression levels of genes downstream of the YAP/TAZ signaling axis namely that of c-myc, CTGF, and Cyr61 (Figs. 3C–3F). Given that the phosphorylation of YAP can cause YAP protein degradation and nuclear entry, we examined the effect of curcumin on YAP phosphorylation in HT29 and SW480 cells. Western blotting results showed that curcumin could significantly inhibit the phosphorylation of YAP (Figs. 3E and 3F). Further, as YAP needs to enter the nucleus to transcribe downstream genes, we examined the effect of curcumin on the entry of YAP into the nucleus. As shown in Figs. 3G and 3H, curcumin could significantly inhibit YAP nuclear entry in SW480 cells. Combining our observations, we found that curcumin can inhibit the activation of the YAP/TAZ signaling axis in CRC cells.
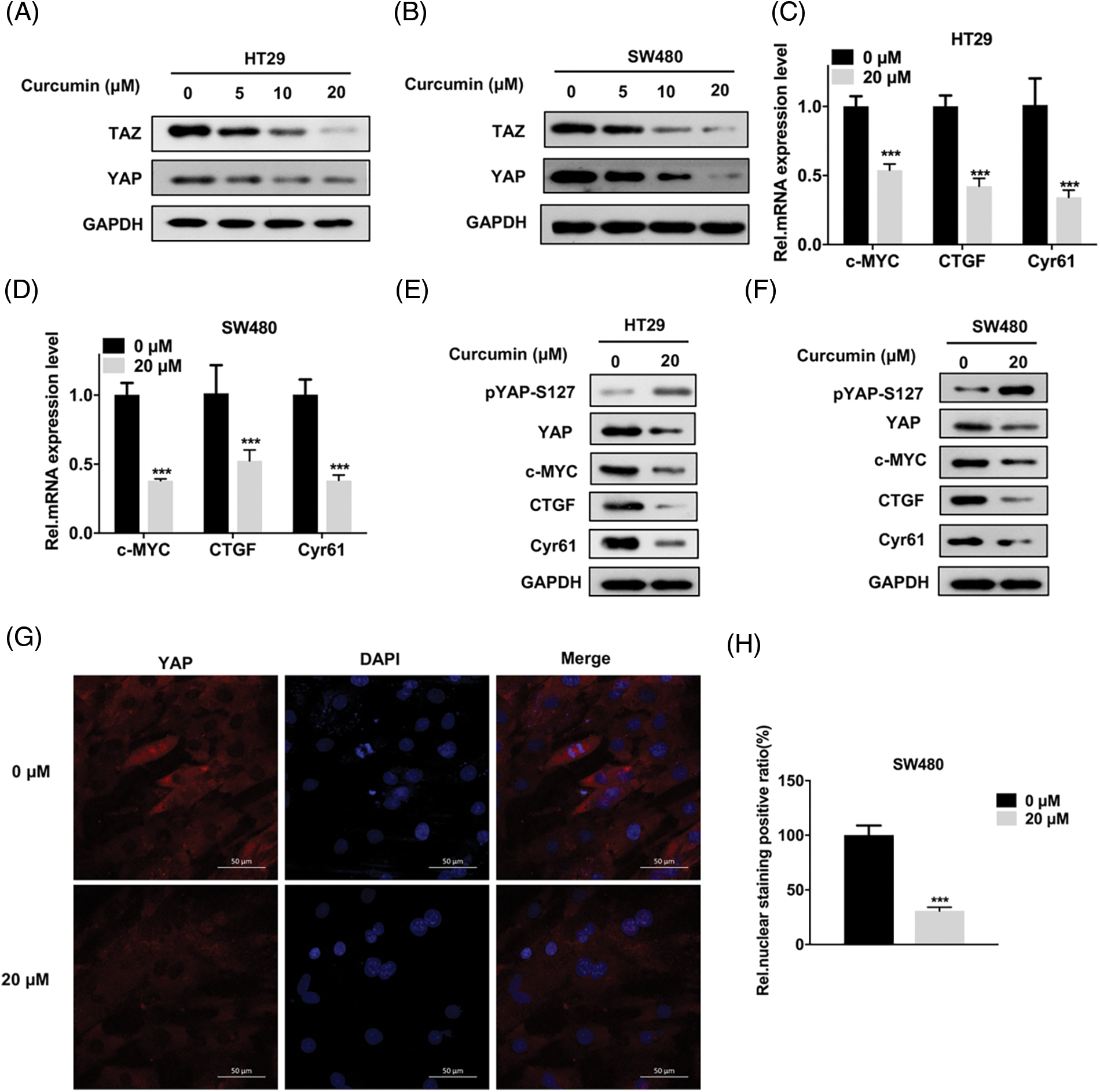
Figure 3: Curcumin inhibits the Yes-associated protein 1 (YAP) and PDZ-binding motif (TAZ) signaling axis in CRC cells. (A and B) HT29 and SW480 cells were treated with 0, 5, 10 and 20 µM curcumin for 48 h and the YAP/TAZ protein expression level was detected by Western Blot assay; the indicated antibodies were added during Western Blot assay; HT29 (A) and SW480 (B) cells (C and D) HT29 and SW480 cells were treated with 0, 5, 10 and 20 µM curcumin for 48 h and the YAP/TAZ signaling pathway downstream target gene mRNA expression levels were detected by reverse transcription quantitative real-time PCR (RT-qPCR). The indicated gene mRNA expression levels were detected during the qPCR assay in HT29 (C) and SW480 (D) cells; (E and F) HT29 and SW480 cells were treated with 0, 5, 10, and 20 µM curcumin for 48 h and western blotting was done for the downstream target genes protein expression analysis of the YAP/TAZ signaling pathway in HT29 (E) and SW480 (F) cells; the indicated antibodies were added during the western blotting assay; (G) SW480 cells were treated with 0 and 20 µM curcumin for 48 h before the immunofluorescence assay to detect YAP protein cellular sub-localization, the yellow arrow points to positive staining for YAP protein, scale bar: 50 μM, magnification: 200×; (H) Statistical analysis for the immunofluorescence assay of YAP protein cellular sub-localization. Each experiment was repeated three times; ***p < 0.001.
Malignant inhibition of CRC cells by curcumin depends on the YAP protein
We further explored whether these inhibitory effects of curcumin on CRC were caused by the inhibition of YAP entry into the nucleus. We constructed SW480-tet-YAP cells (hereinafter referred to as SW480-tet) with DOX (Doxycycline)-induced YAP expression through the Tet-on system for probing the same. As shown in Fig. 4A, DOX could significantly increase the expression of YAP in SW480 cells. Further, the results of the CCK-8 assay showed that the restoration of YAP expression could significantly rescue the inhibition of SW480-tet cell viability by curcumin (Fig. 4B). Colony forming assays and the trans-well assay results showed that YAP could also rescue the curcumin-induced diminishing of clone formation, cell migration and cell invasion ability of SW480-tet cells (Figs. 4C–4G). Furthermore, we also found that YAP could also rescue the curcumin-induced suppression of the YAP/TAZ signaling axis in SW480-tet cells (Figs. 4H and 4I). These observations suggested that the suppression of CRC cell line malignancy by curcumin is dependent on the YAP protein.
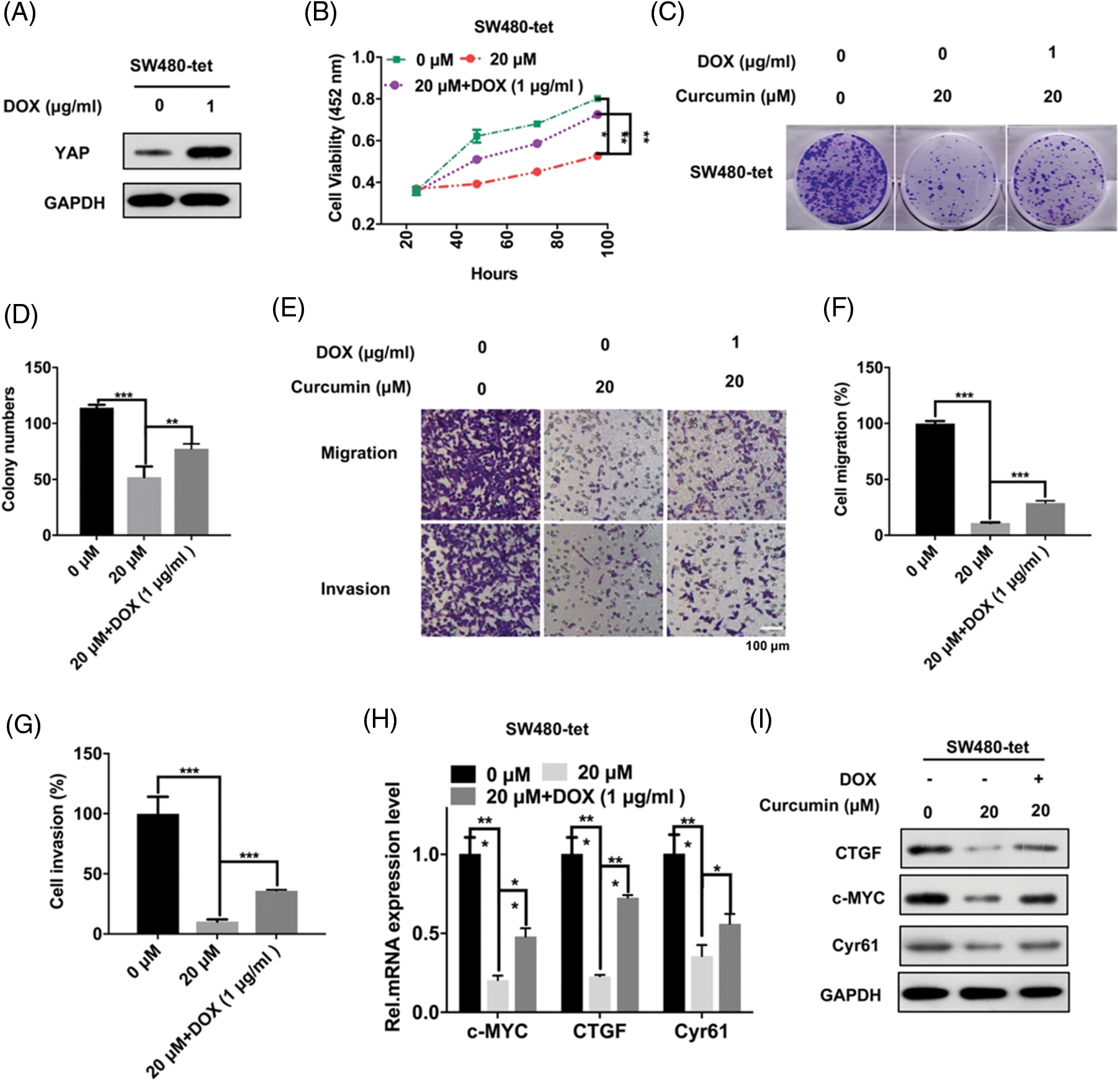
Figure 4: CRC cell malignancy inhibition by curcumin depends on YAP. (A) SW480-tet cells were treated with 0 and 1 µg/mL of doxycycline (DOX) for 48 h before YAP protein expression level detection by Western Blot assay in SW480-tet cells, indicated antibodies were added during western blotting. (B) SW480-tet cells (1 × 103) were treated with 0, 20, and 20 M curcumin + 1 µg/mL DOX, the cell viability was detected by the cell counting kit-8 (CCK-8) assay kit for indicated time points. (C) SW480-tet cells (1 × 103) were treated with 0, 20 and 20 µM curcumin + 1 µg/mL DOX for 48 h before the colony forming assay. (D) Analysis of the clone formation assays of SW480-tet cells treated with 0, 20 and 20 µM curcumin + 1 µg/mL DOX. (E) SW480-tet cells were treated with 0, 20 and 20 µM curcumin + 1 µg/mL DOX for 48 h before cells migration assays and cell invasion assays by the trans-well chamber assay, scale bar: 100 μM; magnification: 100×. (F and G) Analysis of the cell migration assay (F) and invasion assay (G) in SW480-tet cells. (H) SW480-tet cells were treated with 0, 20 and 20 µM curcumin + 1 µg/mL DOX for 48 h before detecting the mRNA expression level of the YAP/TAZ signaling pathway downstream target genes by RT-qPCR in SW480-tet cells, indicated genes mRNA expression level were detected during the qPCR assay. (I) SW480-tet cells were treated with 0, 20 and 20 µM curcumin + 1 µg/mL DOX for 48 h before detecting the protein expression level of the YAP/TAZ signaling pathway downstream target genes by western blotting in SW480-tet cells, indicated antibodies were added during the western blotting assay. Each experiment was repeated three times; *p < 0.05; **p < 0.01; ***p < 0.001.
Curcumin inhibits the in vivo development of CRC
We next studied whether the inhibition of CRC by curcumin was also seen in in vivo conditions. For this study, we subcutaneously injected 1 × 106 SW480-tet cells into 6–8 weeks-old healthy nude mice. On the seventh day after injection, mice were randomly divided into three groups (five per group): the control group fed normal food, the curcumin group fed normal food + curcumin gavage, and the curcumin + DOX group fed DOX food + curcumin gavage (Fig. 5A). We measured the tumor volume every three days in all these group animals. As shown in Fig. 5B curcumin treatment could significantly inhibit the tumor growth rate of CRC, and this inhibition was rescued by the expression of YAP. We then took out the xenografts from these three groups of mice 28 days after injection and found that curcumin treatment could significantly inhibit the volume and weight of CRC tumors, and this inhibition could be rescued by the expression of YAP (Figs. 5C and 5D). Further, we observed no significant changes in the body weight of mice during the experimental period, indicating that neither curcumin nor DOX food had toxic effects on the study animals (Fig. 5E). Furthermore, IHC results showed that curcumin treatment significantly inhibited the expression of the proliferative makers (YAP and Ki67) in CRC tumor samples, and this inhibition could be rescued by the expression of YAP (Fig. 5F). Our overall observations suggested that curcumin also inhibits the development and development of CRC in vivo.
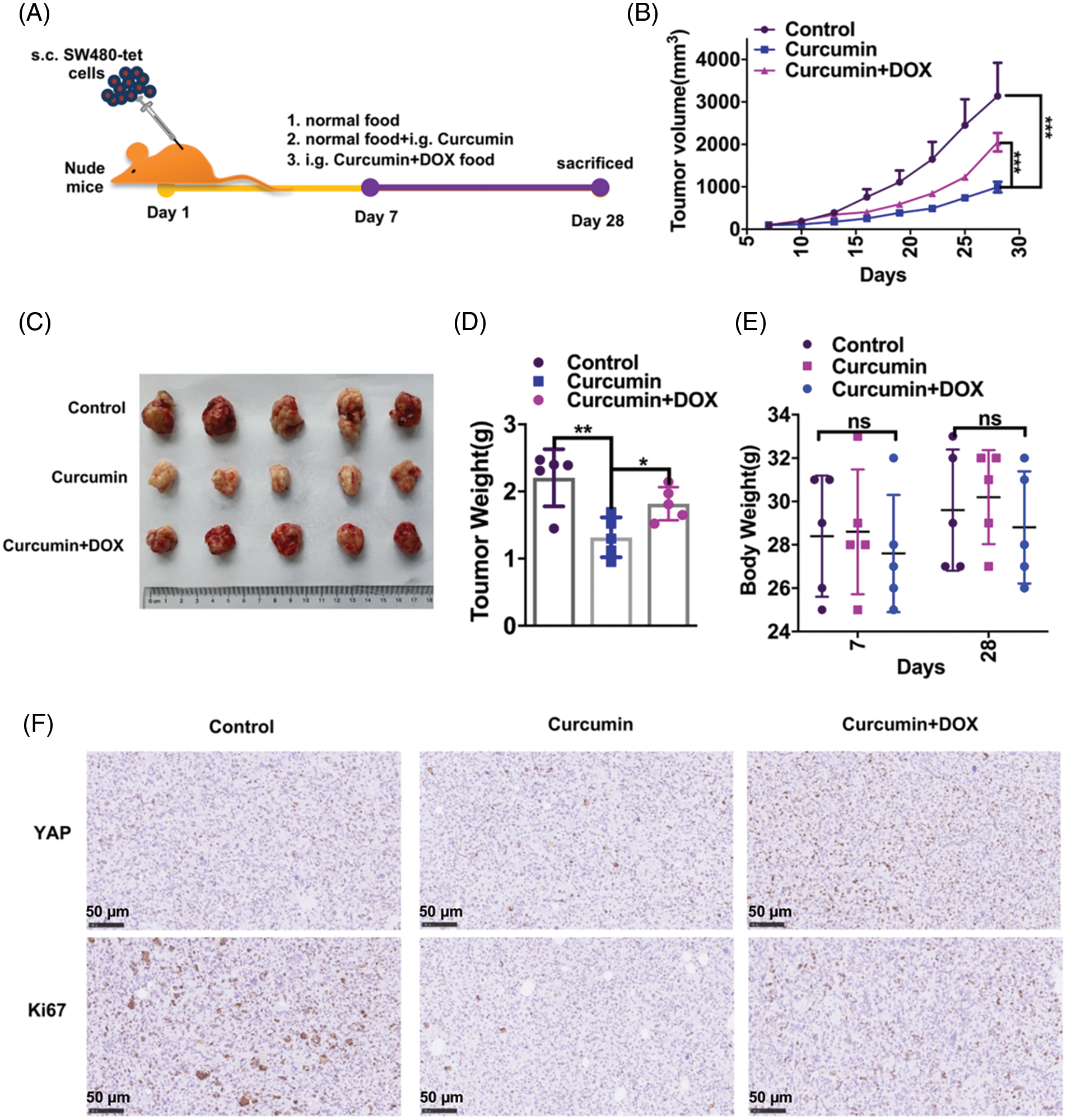
Figure 5: Curcumin inhibits the in vivo development and development of CRC in mice. (A) Schematic diagram of our experimental design of nude mice with xenograft tumors. (B) Tumor growth curves of mice fed with normal food (group control), mice fed with normal food + i.g. curcumin (group curcumin) and mice fed with DOX food i.g. curcumin (group curcumin + DOX). The tumor growth was measured at intervals of 3 days by measuring its diameter with a vernier caliper, n = 5. (C) Representative pictures of tumors from the control group, curcumin group, and curcumin + DOX group mice; (D) Tumor weights of the control group, curcumin group and curcumin + DOX group mice, n = 5. (E) The body weight of mice of the control group, curcumin group, and curcumin + DOX group mice, n = 5. (F) Immunohistochemistry (IHC) staining for YAP and Ki67 on paraffin sections from the control group, curcumin group, and curcumin + DOX group mice tumors, scale bar: 50 μM, magnification: 100×. Each experiment was repeated three times; *p < 0.05; **p < 0.01; ***p < 0.001.
As the third most commonly occurring cancer, CRC is a highly complex and heterogeneous disease on a molecular basis with frequent mutations that confer resistance to conventional treatments [22,23]. Despite recent progress in identifying therapeutic targets for CRC, there is a far unmet need in the clinical treatment of CRC. Current clinical treatments have shown that drug resistance is one of the major obstacles to treating CRC patients [24,25]. Therefore, the development of new therapeutic CRC targets remains of great importance to improve the clinical outcomes of CRC patients. Previous studies have shown that curcumin has an inhibitory effect on CRC. Our experimental results that curcumin inhibited the cell viability, migration, and invasion of CRC in a dose-dependent manner are also consistent with such reports.
The Hippo signaling pathway in cells is a highly conserved protein kinase cascade pathway that was discovered in recent years. YAP is the core effector molecule in this signaling pathway [26]. Recent studies have also confirmed that activation of this pathway results in YAP phosphorylation, which is retained in the cytoplasm and subsequently degraded [27,28]. Our experimental data suggest that curcumin promotes the phosphorylation of YAP and prevents its entry into the nucleus. This suggests that curcumin may activate the Hippo signaling pathway through a particular mechanism, which is our main research topic in future studies.
Nude mice are a crucial cancer research model widely applied for tumor immunology studies and drug screening. Therefore, constructing a CRC nude mouse transplantation tumor model could further explore the therapeutic effects of curcumin in vivo. Our study also confirmed that curcumin could inhibit the growth of CRC in xenograft tumors in nude mice.
In conclusion, this study demonstrates that curcumin inhibits the YAP/TAZ signaling pathway by promoting YAP phosphorylation and ultimately inhibits CRC tumorigenesis (Graphical abstract). Our study also further clarified the mechanism of curcumin in the treatment of CRC, laying an experimental and theoretical basis for further elucidation of the mechanism of curcumin in CRC treatment. This finding has promising significance for promoting the application of curcumin for treating CRC.
Acknowledgement: None.
Funding Statement: This work was financially supported by the Second Batch of Medical and Health Science and Technology Plan (self-financing) Projects in Shantou in 2020, Shantou Science and Technology Bureau Document Shantou ([2020] No. 58).
Author Contributions: The authors confirm their contribution to the paper as follows: SF and XD performed the experiments. XJ, ZZ and LW conducted literature research. CX and LF performed data processing. CJ designed and conceived the study. CJ and ZM wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: All animal experimental protocols in this study were approved by the Institutional Animal Care and Use Committee of Shantou University School of Medicine (No. 20200921021).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci. 2018;6:31. [Google Scholar]
2. Liang Z, Xie H, Shen W, Shao L, Zeng L, Huang X, et al. The synergism of natural compounds and conventional therapeutics against colorectal cancer progression and metastasis. Front Biosci. 2022;27:263. [Google Scholar]
3. Pericleous S, Bhogal RH, Mavroeidis VK. The role of circulating biomarkers in the early detection of recurrent colorectal cancer following resection of liver metastases. Front Biosci. 2022;27(6):189. doi:10.31083/j.fbl2706189. [Google Scholar] [PubMed] [CrossRef]
4. Xu X, Zhu Y. Curcumin inhibits human non-small cell lung cancer xenografts by targeting STAT3 pathway. Am J Transl Res. 2017;9:3633–41. [Google Scholar] [PubMed]
5. Kesharwani SS, Ahmad R, Bakkari MA, Rajput MKS, Dachineni R, Valiveti CK, et al. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBDformulation development, characterization and pharmacological evaluation. J Control Release. 2018;290:165–79. [Google Scholar] [PubMed]
6. Wang XP, Wang QX, Lin HP, Chang N. Anti-tumor bioactivities of curcumin on mice loaded with gastric carcinoma. Food Funct. 2017;8(9):3319–26. [Google Scholar] [PubMed]
7. Subramaniam D, Ponnurangam S, Ramamoorthy P, Standing D, Battafarano RJ, Anant S, et al. Curcumin induces cell death in esophageal cancer cells through modulating notch signaling. PLoS One. 2012;7(2):e30590. [Google Scholar] [PubMed]
8. Zheng BZ, Liu TD, Chen G, Zhang JX, Kang X. The effect of curcumin on cell adhesion of human esophageal cancer cell. Eur Rev Med Pharmacol Sci. 2018;22:551–60. [Google Scholar] [PubMed]
9. Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171(7):3863–71. [Google Scholar] [PubMed]
10. Glienke W, Maute L, Wicht J, Bergmann L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of survivin/BIRC5 gene expression. Cancer Invest. 2010;28(2):166–71. [Google Scholar] [PubMed]
11. Marjaneh RM, Rahmani F, Hassanian SM, Rezaei N, Hashemzehi M, Bahrami A, et al. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J Cell Physiol. 2018;233(10):6785–98. [Google Scholar] [PubMed]
12. Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS One. 2013;8(2):e57218. [Google Scholar] [PubMed]
13. Cai X, Wang KC, Meng Z. Mechanoregulation of YAP and TAZ in cellular homeostasis and disease progression. Front Cell Dev Biol. 2021;9:673599. [Google Scholar] [PubMed]
14. Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Dev. 2014;141(8):1614–26. [Google Scholar]
15. Astudillo P. An emergent Wnt5a/YAP/TAZ regulatory circuit and its possible role in cancer. Semin Cell Dev Biol. 2022;125:45–54. [Google Scholar] [PubMed]
16. Huh HD, Kim DH, Jeong HS, Park HW. Regulation of TEAD transcription factors in cancer biology. Cells. 2019;8(6):600. [Google Scholar] [PubMed]
17. Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15(2):73–9. [Google Scholar] [PubMed]
18. Bouvier C, Macagno N, Nguyen Q, Loundou A, Jiguet-Jiglaire C, Gentet JC, et al. Prognostic value of the Hippo pathway transcriptional coactivators YAP/TAZ and β1-integrin in conventional osteosarcoma. Oncotarget. 2016;7(40):64702–10. [Google Scholar] [PubMed]
19. Zhou X, Su J, Feng S, Wang L, Yin X, Yan J, et al. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget. 2016;7(48):79076–88. [Google Scholar] [PubMed]
20. Yu D, Liu H, Qin J, Huangfu M, Guan X, Li X, et al. Curcumol inhibits the viability and invasion of colorectal cancer cells via miR-30a-5p and hippo signaling pathway. Oncol Lett. 2021;21(4):299. [Google Scholar] [PubMed]
21. Shen Y, Han Z, Liu S, Jiao Y, Li Y, Yuan H. Curcumin inhibits the tumorigenesis of breast cancer by blocking tafazzin/yes- associated protein axis. Cancer Manag Res. 2020;12:1493–502. [Google Scholar] [PubMed]
22. Hahn MM, de Voer RM, Hoogerbrugge N, Ligtenberg MJ, Kuiper RP, van Kessel AG. The genetic heterogeneity of colorectal cancer predisposition-guidelines for gene discovery. Cell Oncol. 2016;39:491–510. [Google Scholar]
23. Liu W, Zhang X, Xu H, Li S, Lau HC, Chen Q, et al. Microbial community heterogeneity within colorectal neoplasia and its correlation with colorectal carcinogenesis. Gastroenterol. 2021;160:2395–408. [Google Scholar]
24. Luo M, Yang X, Chen HN, Nice EC, Huang C. Drug resistance in colorectal cancer: an epigenetic overview. Biochim Biophys Acta (BBA)-Rev Cancer. 2021;1876:188623. [Google Scholar]
25. Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834–48. [Google Scholar] [PubMed]
26. Yin F, Dong J, Kang LI, Liu X. Hippo-YAP signaling in digestive system tumors. Am J Cancer Res. 2021;11:2495–507. [Google Scholar] [PubMed]
27. Morice S, Danieau G, RØdini F, Brounais-Le-Royer B, Verrecchia F. Hippo/YAP signaling pathway: a promising therapeutic target in bone paediatric cancers? Cancers. 2020;12:645. [Google Scholar] [PubMed]
28. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–312. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools