 Open Access
Open Access
COMMENTARY
A commentary: harnessing vesicles power with new scenes of membrane-based devices for drug delivery
1 Departamento de Química, Universidad Nacional del Sur (UNS), Bahía Blanca, 8000, Argentina
2 INQUISUR-CONICET, Universidad Nacional del Sur (UNS), Bahía Blanca, 8000, Argentina
3 Departamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur (UNS), Bahía Blanca, 8000, Argentina
* Corresponding Author: LUCIANO A. BENEDINI. Email:
BIOCELL 2024, 48(10), 1401-1403. https://doi.org/10.32604/biocell.2024.055512
Received 28 June 2024; Accepted 03 September 2024; Issue published 02 October 2024
Abstract
This work shows relevant interactions between cells and drug-delivery systems based on vesicles crucial for therapeutic activity. This interplay drives strategies for the design of new drug-carry. Among the described systems are found liposomes, extracellular vesicles, and hybrid systems. The text details their properties, advantages, and constraints, and eventually, a perspective about the future of these formulations is proposed.Keywords
Cells and Vesicles: Understanding Interaction for Treatments
Cells contain complex membrane systems that compartmentalize internal structures and delineate external boundaries. Both frontiers show structural and dynamic features associated with the compositions, proportions of constitutive lipids, and either free or united proteins. The interrelationship between proteins and lipids in subcellular regions regulates biological processes occurring both in these regions and in the neighborhood of the peripheral membrane. These interactions also trigger cell signaling and modify the cytoskeletal dynamics that intervene in the modulation of lipid composition, shape, and dynamics of the membranes [1].
More than a thousand types of molecules and a combination of variable rates of glycerophospholipids and sphingolipids build the membranes of mammalian cells [2]. Active pharmaceutical ingredients (API) released from a drug delivery system (DDS) will only induce a therapeutic effect after interaction with specific receptors. These receptors can be either inserted in this membrane or inside the cell. Therefore, the interaction with cell membranes is a fundamental process for therapeutic response in those formulations based on vesicle systems. Different membrane-based models have been developed to enhance the understanding of specific membrane processes such as fusion, transport, and vesicle formation, as well as other events at a cellular and molecular level, including drug penetration in the membranes [2]. Considering the relevant models, liposomes are among the first systems to exhibit these features since the 1950s. Scientific advances in this field led to the wide application of liposomes, from biology to clinical use, resulting from a keen awareness of interaction mechanisms (endocytosis) between cell membranes and membrane systems such as adsorption, fusion, pinocytosis (non-specific or receptor-mediated), and lipid transfer (Fig. 1). However, these mechanisms rarely occur in isolation and are laborious to demonstrate separately in in-vivo biological systems. Recently, another type of nano-vesicles responsible for cell communications has emerged. Extracellular Vesicles (EVs) are implicated in many physiological processes, such as cellular homeostasis, and engaged in pathological ones such as cardiovascular diseases, infections, and cancer. Owing to their similarities with liposomes, EVs have been proposed as promising devices as DDSs [3].
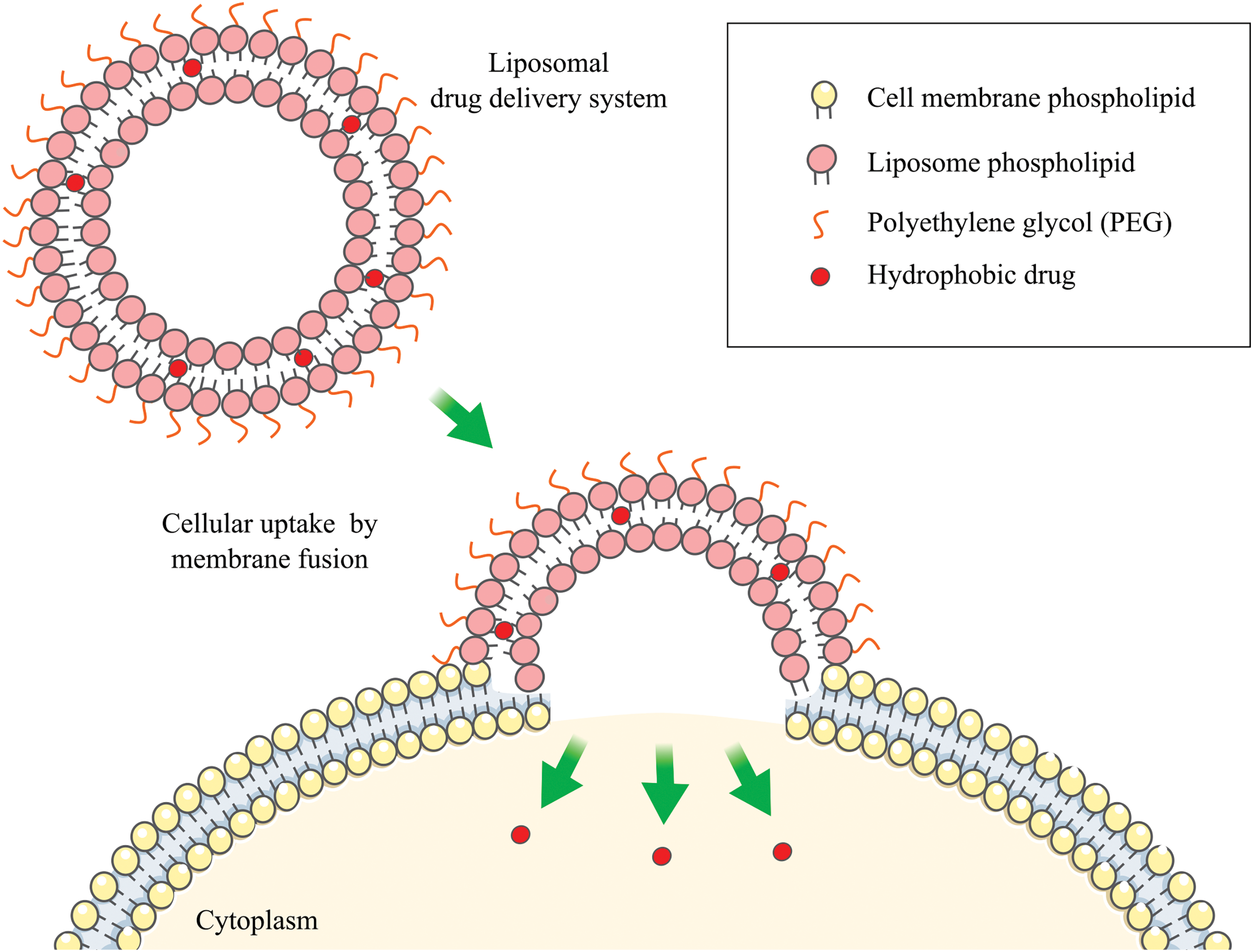
Figure 1: Fusion liposome-cell. The figure contains modified Images from Servier Medical Art (https://smart.servier.com, accessed 10 October 2024) and is licensed by a Creative Commons Attribution 4.0 Unported License.
The composition and state of the membrane strictly mediate interactions between cells and vesicles. For instance, the liquid crystal phase in liposomes and a particular content of phosphatidylcholine favor fusion. This fact engages distinct cellular uptake [4]. The complexity of signaling mechanisms associated with healthy biological systems, and those associated with drug resistance in specific diseases, such as cancer or insensitivity to antiepileptic drugs (Multi Drug Resistance), is also arduous to understand [5]. Due to these difficulties and the potential solutions that membrane-based systems offer for treatments; these systems have been studied to address increasingly complex problems. Today, the relationship between synthetic membrane-based systems (liposomes) and cell-based systems (EVs) has undergone significant evolution and will continue to do so as scientific advancements progress.
DDSs are developed to bring APIs to the sites of action, maximizing their concentration at the target site. This type of formulation plays a crucial role in absorption, distribution, metabolism, and excretion (ADME) processes. In particular, nano-systems may increase the distribution process of the non-free drug, avoiding the release in unintended sites and potentially prolonging release effects [5]. Liposomes first, and then EVs, are membrane-based drug delivery systems (MBDDSs) used for drug delivery (doxorubicin and paclitaxel) and other molecules such as RNAs (mi- and si-RNAs), DNAs, and enzymes [6].
Liposomes are vesicles mainly formed by lipophilic surfactants sizing 20–1000 nm. Originally conceived as multilamellar structures in the early 1960s, it was only about 15 years later that they were proposed for medical applications. The objective was to improve the pharmacokinetics profile of drugs, bearing in mind their versatility for delivering different solubility drugs. Extracellular Vesicles (EVs), in turn, are also nano-sized structures that can support hydrophobic drug loads, just like liposomes. Cells release EVs as exosomes (30–150 nm), ectosomes (100–1000 nm), microvesicles (100–1000 nm), and apoptotic bodies (1000–5000 nm) [7]. Therefore, these EVs form a polydisperse group.
Liposomes and EVs have differences and similarities, and the obtention method is notoriously different. On one hand, liposomes are designed and developed through a mixture of the components followed by evaporation and filtration processes. On the other hand, EVs are isolated from cells by density methods (ultracentrifugation) and size-based techniques (ultrafiltration) among others [8]. Drug-loading methods also show differences. For liposomes, drugs are loaded during the membrane-forming process [9]. EVs can be loaded into the donor cell or added into isolated EVs through electroporation, sonication, and other methods [7]. Compositionally, liposomes have high cholesterol levels to improve stability, while EVs show associations between cholesterol and glycosphingolipids in certain membrane zones. The lipids forming liposomes are diverse (different head groups, chain lengths, and saturation), whereas EVs show the same constituent membrane lipids as the origin cells but in distinct proportions [3,5]. As liposomes are performed artificially, their charge, laminarity, and size are defined based on the requirements. Contrarily, in EVs, these properties are strictly dependent on their cellular origin. For example, liposomes can show both negative or positive surface charge, while EVs mainly exhibit negative charge. Charge and size influence the stability of these systems. Additionally, proteins such as CD47 on the surface of EVs improve the circulation time just as polyethylene glycol (PEG) attachment transforms regular liposomes into stealthy carriers by suppressing the phagocytic activity of macrophages [5,10]. Each system is situated in the extremes of the design of MBDDSs; therefore, to merge their advantages, a combination of both forming hybrid systems will be desirable.
The comprehension of interaction mechanisms between cells and MBDDSs improves future strategies for their design and development. This work aims to show considerations of these interactions giving relevance to systems based on membranes that have lost importance, such as liposomes. Today liposomes have recovered territory thanks to EVs because both vesicles can be combined in hybrid systems harnessing their respective advantages. In the future, pure liposomes probably will be replaced by EVs or hybrid systems. Thus, this fact represents an encouraging alternative to promote MBDDSs as essential devices within pharmaceutical formulations.
Acknowledgement: The authors acknowledge CONICET for the financial support and Dr. Javier Sartuqui for his valuable insight in reading the manuscript.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Luciano A. Benedini, Noelia L. D´Elía and A. Noel Gravina; draft manuscript preparation: Luciano A. Benedini, Noelia L. D´Elía; review and editing: Luciano A. Benedini, Noelia L. D´Elía; visualization: A. Noel Gravina, Noelia L. D´Elía; supervision: Paula V. Messina, Luciano A. Benedini. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Zhao H, Lappalainen P. A simple guide to biochemical approaches for analyzing protein-lipid interactions. Mol Biol Cell. 2012;23(15):2823–30. doi:10.1091/mbc.e11-07-0645. [Google Scholar] [CrossRef]
2. Parchekani J, Allahverdi A, Taghdir M, Naderi-Manesh H. Design and simulation of the liposomal model by using a coarse-grained molecular dynamics approach towards drug delivery goals. Sci Rep. 2022;12(1):2371. doi:10.1038/s41598-022-06380-8. [Google Scholar] [PubMed] [CrossRef]
3. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–59. doi:10.1038/s41565-021-00931-2. [Google Scholar] [PubMed] [CrossRef]
4. Yaman S, Chintapula U, Rodriguez E, Ramachandramoorthy H, Nguyen KT. Cell-mediated and cell membrane-coated nanoparticles for drug delivery and cancer therapy. Cancer Drug Resist. 2020;3(4):879–911. doi:10.20517/cdr.2020.55. [Google Scholar] [PubMed] [CrossRef]
5. van der Koog L, Gandek TB, Nagelkerke A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv Healthc Mater. 2022;11(5):2100639. doi:10.1002/adhm.202100639. [Google Scholar] [PubMed] [CrossRef]
6. Nowak M, Górczyńska J, Kołodzińska K, Rubin J, Choromańska A. Extracellular vesicles as drug transporters. Int J Mol Sci. 2023;24(12):10267. doi:10.3390/ijms241210267. [Google Scholar] [PubMed] [CrossRef]
7. Han Y, Jones TW, Dutta S, Zhu Y, Wang X, Narayanan SP, et al. Overview and update on methods for cargo loading into extracellular vesicles. Processes. 2021;9(2):356. doi:10.3390/pr9020356. [Google Scholar] [PubMed] [CrossRef]
8. Du S, Guan Y, Xie A, Yan Z, Gao S, Li W, et al. Extracellular vesicles: a rising star for therapeutics and drug delivery. J Nanobiotechnol. 2023;21(1):231. doi:10.1186/s12951-023-01973-5. [Google Scholar] [PubMed] [CrossRef]
9. Benedini L, Antollini S, Fanani ML, Palma S, Messina P, Schulz P. Study of the influence of ascorbyl palmitate and amiodarone in the stability of unilamellar liposomes. Mol Membr Biol. 2014;31(2–3):85–94. doi:10.3109/09687688.2014.896956. [Google Scholar] [PubMed] [CrossRef]
10. Messina PV, Benedini LA, Placente D. Nano-targeted smart drug delivery systems and nanorobotics. In: Tomorrow’s healthcare by nano-sized approaches. Boca Raton: CRC Press; 2020. p. 202–29. doi:10.1201/9780429400360. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools