 Open Access
Open Access
REVIEW
Pioneering a new era in Parkinson’s disease management through adipose-derived mesenchymal stem cell therapy
Novel Diagnostics and Therapeutics Research Group, Institute of Biotechnology, Ferdowsi University of Mashhad, Mashhad, 9177948974, Iran
* Corresponding Author: FATEMEH B. RASSOULI. Email:
(This article belongs to the Special Issue: Perspectives on Stem Cells and Regenerative Medicine)
BIOCELL 2024, 48(10), 1419-1428. https://doi.org/10.32604/biocell.2024.053597
Received 06 May 2024; Accepted 08 July 2024; Issue published 02 October 2024
Abstract
Parkinson’s disease (PD) is one of the fastest-growing neurodegenerative disorders worldwide. So far, PD treatments only offer little clinical relief and cannot reverse or stop the disease progression. Stem cell (SC) therapy is a rapidly evolving technology that holds significant promise for enhancing current therapeutic approaches. Adipose-derived mesenchymal SCs (AD-MSCs) have many features such as easy harvest with minimal invasive techniques, high plasticity, non-immunogenicity, and no ethical issues, which have made them suitable choices for clinical applications in regenerative research. AD-MSCs are ideal tools to treat PD, as they have the potential to differentiate into functional dopaminergic neurons, and also could produce and secrete useful paracrine factors and extracellular vesicles, such as cytokines and growth factors, and thus promote the repair and regeneration of damaged nerve tissue. Studies revealed that AD-MSCs induced angiogenesis, nerve regeneration, and memory and motor improvement in cellular and animal models of PD. Moreover, clinical studies demonstrated the safety of AD-MSC transplantation in PD patients. This review provides a comprehensive and current summary of the therapeutic potential of AD-MSC transplantation for the treatment of PD, by highlighting the ability of cells to differentiate into functional dopaminergic neurons.Keywords
Abbreviations
| AD-MSCs | Adipose-derived mesenchymal stem cells |
| ATG | Autophagy-related 1 |
| BDNF | Brain-derived neurotrophic factor |
| BMP-2 | Bone morphogenetic protein 2 |
| BM-MSC | Bone marrow mesenchymal stem cell |
| Cav-1 | Caveolin-1 |
| CM | Conditioned medium |
| ERK | Extracellular signal-regulated kinase |
| FADD | Fas-associated protein with death domain |
| FGF2 | Fibroblast growth factor 2 |
| GABA | Gamma-aminobutyric acid |
| GBA | Glucocerebrosidase |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| hAD-MSC | Human adipose-derived mesenchymal stem cell |
| HES1 | Hairy and enhancer of split-1 |
| 6-OHDA | 6-hydroxydopamine |
| LMX1A | LIM homeobox transcription factor 1, alpha |
| LRRK2 | Leucine-rich repeat kinase 2 |
| MAPK | Mitogen-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| NICD | Notch intracellular domain |
| NI-hADSC-CM | Neurogenic differentiation of human adipose-derived stem cell-conditioned medium |
| NTN | Neuturin |
| PD | Parkinson’s disease |
| PKB/AKT | Protein kinase B |
| PRKN | Parkin |
| PINK1 | PTEN-induced putative kinase 1 |
| PROTACs | Proteolysis-targeting chimeras |
| PTX 3 | Pentraxin3 |
| rhPTX3 | Human recombinant PTX3 |
| RORγt | Retinoic acid-related orphan receptor gamma t |
| SGZ | Subgranular zone |
| SNCA | α-synuclein |
| STAT | Signal transducers and activators of transcription |
| SVZ | Subventricular zone |
| TRADD | Tumor necrosis factor receptor type 1-associated DEATH domain protein |
| TH | Tyrosine hydroxylase |
| ULK1 | Unc-51 like autophagy activating kinase 1 |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless-related integration site |
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders that endangers human health and quality of life. PD is an age-related disorder that affects 3% of the population during older age and ranks second among neurodegenerative diseases in terms of prevalence [1]. As the world’s population is aging at an alarming rate, the incidence and mortality rates of neurodegenerative disorders are becoming serious health concerns worldwide. According to the latest global statistics, the incidence and prevalence of PD showed steadily increasing from 1990 to 2019. The majority of PD patients are aged more than 65 years, with the percentage of cases rapidly rising in the population aged 80 years and above [2].
PD phenotype is characterized by motor and non-motor abnormalities in the forms of muscle rigidity and tremor, impaired gait, hypokinesia, bradykinesia/akinesia, postural instability, disturbed cognition and sleep pattern, anxiety, and dementia [3,4]. Current treatment regimens for PD mainly involve the administration of levodopa, dopamine agonists, and monoamine oxidase B inhibitors or surgery [5–7]. Nevertheless, provided medications are only symptomatic and do not delay disease development. They are also associated with an array of side effects and lead to more difficulties with increasing age including dyskinesia, akinesia, nausea, hypotension, muscular rigidity, anxiety, and hallucinations [8].
PD is caused by an amalgamation of genetic and environmental risk factors. Around 15% of people with PD have a family history of the condition, which is linked to mutations in genes likeα-synuclein (SNCA), parkin (PRKN), glucocerebrosidase (GBA), leucine-rich repeat kinase 2 (LRRK2) and PTEN-induced putative kinase 1 (PINK1) [9,10]. Apart from aging, which is the most significant environmental risk component, exposure to pesticides (such as rotenone), consumption of dairy products, traumatic brain injury, hormone replacement therapy, and nicotine, caffeine, and aspirin intake are acknowledged as PD risk factors. In addition, diabetes, depression, and mood disorders increase the risk of PD [11–13]. Sex is also considered an important factor in the development and phenotypic expression of PD, as men are twice more at risk of PD than women, while women show a higher mortality rate [14].
The pathology of PD is linked with α-Synuclein, a natively unfolded protein that can form aggregated polymers upon misfolding. Notably, the interplay between a plethora of genetic and molecular factors as well as environmental conditions, contributes to the accumulation of α-Synuclein [15,16]. α-Synuclein is highly expressed in the brain and acts in the intracellular transport and release of neurotransmitters such as dopamine [17]. A proposed mechanism for PD progression is that α-Synuclein aggregates spread from one cell to another and trigger the conversion of soluble α-Synuclein into fibrillar-insoluble conformation, which finally leads to the loss of dopaminergic neurons in the substantia nigra and affects signaling in the circuits of the striatal projections [18,19]. Accordingly, targeting extracellular forms of α-Synuclein aggregates by monoclonal antibody, which attenuates the loss of striatal dopamine-transporter density, has been proposed as a disease-modifying treatment for PD [20–22]. Striatal dopamine-transporters are known as important modulators of dopamine release and uptake which act by clearing dopamine, influencing short-term plasticity, and interacting with α-Synuclein [23]. In addition, proteolysis-targeting chimeras (PROTACs), which operate through the ubiquitin-proteasome system, have been recently used for the degradation of α-Synuclein [24,25]. Gene therapy is another approach that aims at ailing PD symptoms by normalizing aberrant firing in the basal ganglia through the expression of either dopaminergic or GABAgenic (gamma-aminobutyric acid) enzymes. Besides the fact that this strategy is symptomatic and cannot modify the underlying pathophysiological process, technological developments are required to improve the specificity and transduction capacity and control transgene expression [26–29].
Cell transplantation offers a promising approach for restoring neurotransmission in neurodegenerative disorders. In this regard, clinical trials have demonstrated that fetal dopaminergic cell transplantation can effectively replace lost neurons in patients with PD [30,31]. Allografting of fetal ventral mesencephalic tissue is a dopaminergic replacement therapy for PD patients. However, the inconsistent clinical outcomes of this approach, which mainly arose from differences in the cell source, preparation, and transplantation paradigms, along with ethical concerns, have largely questioned the effectiveness of this method [32]. As a result, other cell-based platforms such as stem cell (SC) therapy have emerged.
SCs represent a population of undifferentiated, proliferative cells capable of undergoing differentiation into diverse cell lineages. Cellular turnover, which occurs throughout an organism’s lifespan, is essential for maintaining tissue homeostasis and overall health. As certain cellular populations, such as erythrocytes, possess a finite lifespan and necessitate replenishment, SCs offer the potential for the body to regenerate and replace damaged or lost cells with new ones [33,34]. There are various kinds of adult SCs including neural stem cells (NSCs), mesenchymal stem cells (MSCs), hematopoietic SCs, and induced pluripotent SCs [35]. NSCs possess the remarkable ability to generate various types of mature cells within the nervous system. However, they are primarily found in only two regions of the nervous system: the subventricular zone and the subgranular zone of the dentate gyrus in the hippocampus. Accordingly, other SC sources that are readily accessible and well-suited for transplantation have emerged as alternatives in the field of cell therapy for neurodegenerative disorders [36].
MSCs have a variety of characteristics such as plasticity, availability, the ability to strengthen in laboratory conditions, no risk of teratoma, lack of immunogenicity, few ethical issues, and the ability to cross the blood-brain barrier. In addition, the secretome of MSCs including various neurotrophic factors and exosomes containing detrimental molecules, such as miR-4639-5p, miR-137, miR-143, miR-21, and miR-133b, had shown beneficial effects for neurodegenerative disorders. These properties will explain why MSCs can be exploited as a suitable option for clinical applications [37–39]. MSCs can be isolated from adipose tissue (AD), bone marrow (BM), peripheral blood, placenta, amniotic fluid, umbilical cord, and dental pulp [40–42]. Among all these sources, AD and BM are the most abundant and readily available sources that require relatively simple methods for isolating MSCs [43]. Nevertheless, AD-MSCs have gained more attention as they exhibit superior performance compared to BM-MSCs in various aspects, including enhanced proliferative capacity, greater neural differentiation potential [44,45], increased expression of neurotrophic factors [46,47], and improved resilience under challenging circumstances [48]. Additionally, AD provides a more abundant source of adult SCs and necessitates less invasive procedures relative to BM extraction [49,50]. The present review aims to offer an up-to-date perspective on the application of AD-MSC transplantation for PD.
Information sources and search strategy
We conducted a comprehensive search across multiple databases, including PubMed, Google Scholar, and Web of Science, to identify studies on the therapeutic potential of AD-MSCs for PD. We utilized the following search terms: (“Human Adipose-Derived Mesenchymal Stem Cell” [Title/Abstract]) AND (“Parkinson’s disease” [Title/Abstract]), (“Murine Adipose-Derived Mesenchymal Stem Cell” [Title/Abstract]) AND (“Parkinson’s disease” [Title/Abstract]), ((“Mesenchymal Stem Cells” [Title/Abstract]) AND (“Neurodegenerative Disorders” [Title/Abstract])) AND (“Parkinson’s disease” [Title/Abstract]).
Therapeutic Potential of AD-MSCs in the Treatment of PD
So far, several in vitro and preclinical studies have indicated the great potential of AD-MSCs for the treatment of PD, which is mediated through increasing the level of dopamine secretion, nerve regeneration, angiogenesis, and anti-inflammatory effects (Fig. 1).
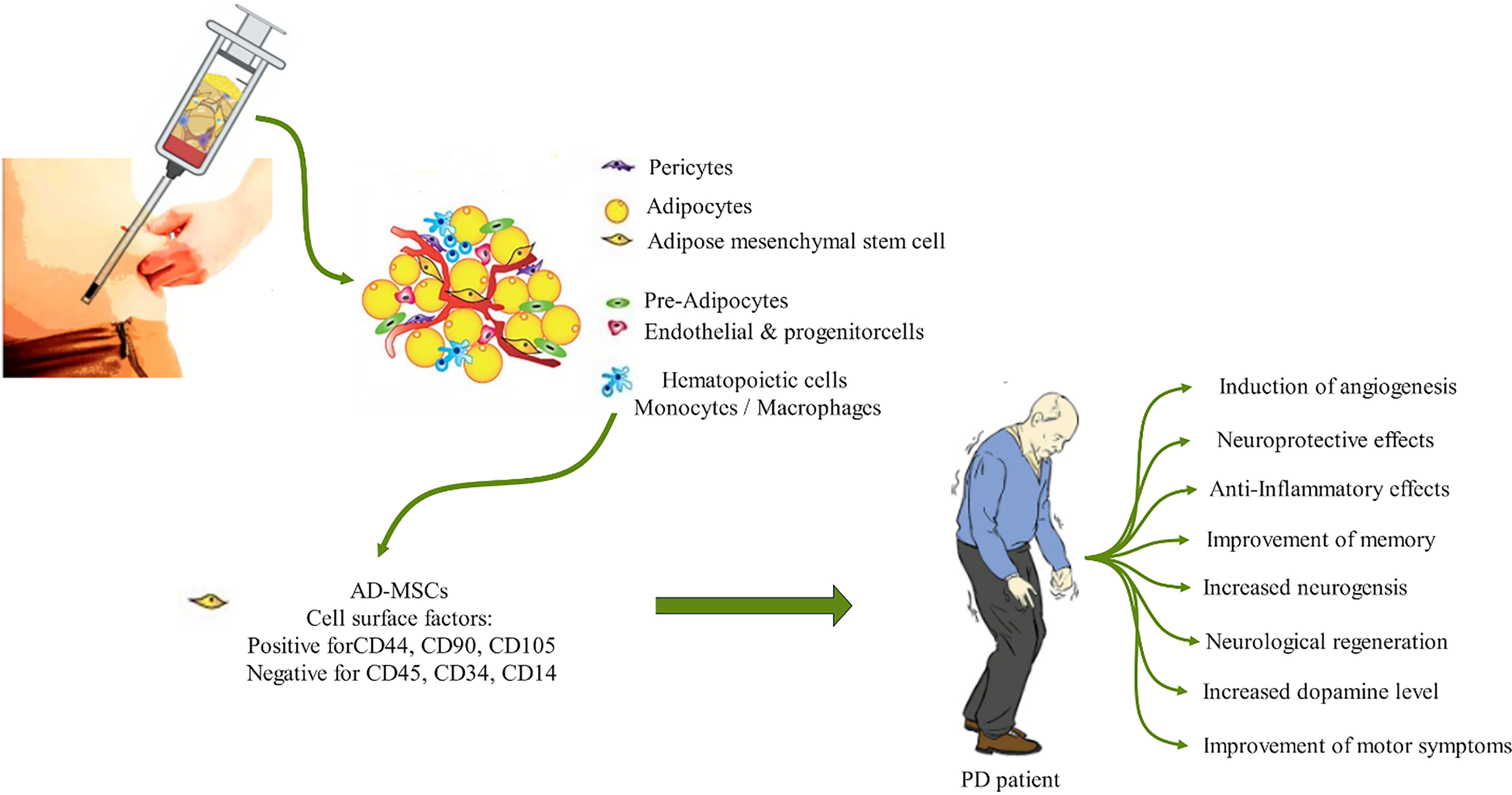
Figure 1: Therapeutic application of AD-MSCs for the treatment of PD. The procedure involves the extraction of a mixture of cells, including pericytes, adipocytes, endothelial cells, hematopoietic cells, and progenitor cells, along with stem cells (SCs) from the adipose tissue using relatively simple methods. AD-MSCs can then be isolated and purified using a cocktail of surface markers, including CD44, CD90, CD105, CD45, and CD34. Upon injection into PD patients, AD-MSCs are capable of inducing angiogenesis, neurogenesis, and anti-inflammatory effects, which ultimately improve memory and motor symptoms.
A summary of experimental and preclinical studies investigating the potential of AD-MSCs for PD treatment is provided in Table 1. MSCs not only have the potential to differentiate into dopamine-producing neurons, and thus restore neuronal function, but they can also release neurotrophic factors that promote the growth and viability of the remaining neurons in PD [13]. In the case of AD-MSCs, studies have also demonstrated the ability of human AD-MSCs to differentiate into dopaminergic neurons [51–53]. Furthermore, preliminary clinical studies have reported the safety of AD-MSC transplantation in individuals with neurological disorders, particularly PD [54–56].
AD-MSCs have demonstrated the ability to induce long-term neuroprotective and anti-inflammatory effects, as well as improve cognitive function in mouse models of PD. These cells were found to persist in the damaged substantia nigra for up to 6 months and provide protection to dopaminergic neurons. AD-MSCs also regulated anti-inflammatory cytokines, promoted neurogenesis, and enhanced memory in rat models of PD [57]. Furthermore, repeated and continuous intravenous transplantation of AD-MSCs into PD mouse models led to the restoration of dopaminergic neurons in the nigrostriatal pathway and significant improvements in motor performance [58]. The transplantation of AD-MSCs also resulted in increased expression of neurotrophic factors, including brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) [58]. Both factors are involved in the survival, axonal growth, and differentiation of dopamine-producing neurons in the brain, particularly those in the substantia nigra [59]. Notably, the nigrostriatal pathway, which originates in the substantia nigra and projects to the striatum, is critical for facilitating and regulating movements [60].
Several studies have focused on investigating the molecular mechanisms underlying the therapeutic effects of AD-MSCs in PD and provided valuable insights into the basic understanding of how these cells exert their therapeutic effects. One molecule of interest is Caveolin-1 (Cav-1), which is a component of caveolae found in the cell membrane. Cav-1 is involved in various signaling pathways and plays a role in cell migration, tumorigenesis, neurogenesis, and embryogenesis [61]. Studies have shown that Cav-1 negatively affects the vascular endothelial growth factor (VEGF), p44/42 MAPK, protein kinase B (PKB/AKT), signal transducer and activator of transcription 3 (STAT3), and Wnt/β-catenin signaling pathways, thereby inhibiting neural differentiation and modulating Notch1/Notch intracellular domain (NICD) and hairy and enhancer of split-1 (HES1) expression during astroglia differentiation in neural progenitor cells [62–64]. Notch1 is involved in neural differentiation, particularly in the development of the central nervous system. The NICD released by Notch1 cleavage translocates to the nucleus and regulates the expression of target genes including Hes-1, which ultimately regulates the proliferation, survival, and differentiation of neural progenitor cells [65–67].
Interestingly, during the differentiation process of AD-MSCs into dopaminergic neurons, the expression of Cav-1 protein gradually decreased [52]. This finding suggests that the downregulation of Cav-1 may play a role in facilitating the differentiation of AD-MSCs into dopaminergic neurons. Furthermore, as recently demonstrated for α-Synuclein degradation [24,25], PROTACs could be explored as a potential tool to modulate Cav-1 levels in AD-MSCs.
It has also been reported that human AD-MSCs not only secrete pentraxin 3 (PTX3) as a therapeutic protein, but also downregulate the expression of FAS-associated death domain (FADD), TNFR1-associated death domain protein (TRADD), caspase-8, and caspase-3 at both the gene and protein levels. PTX3, also known as TNF-inducible gene 14 protein, promotes neurogenesis by inducing neural differentiation, particularly in the final stages of neural stem cell differentiation [68]. Recombinant human PTX3 was shown to reduce the expression of apoptotic genes, thus the anti-apoptotic and neuroprotective effects of human AD-MSCs in PD models were partially attributed to the secretion of PTX3 [69]. More studies have also revealed that PTX3 can promote long-term neurovascular repair and protect neurons following ischemic stroke [70,71].
Studies have reported that AD-MSCs influence the differentiation of peripheral blood mononuclear cells due to their immune-regulatory effects. Specifically, AD-MSCs can suppress the production of T helper 17 cells by downregulating the expression of interleukin-6R (IL-6R), interleukin-23R (IL-23R), and retinoic acid-related orphan receptor gamma t (RORγt), thereby preventing the loss of nigral dopaminergic neurons [72].
Numerous studies have reported that GDNF promotes the survival and maintenance of dopaminergic neurons, leading to increased dopamine secretion and improved motor symptoms [73–76]. Moreover, GDNF has been shown to significantly enhance the survival and differentiation potential of AD-MSCs in vitro, leading to a substantial increase in the number of cells expressing glial fibrillary acid protein (GFAP), Nestin, and Tuj1, markers that are commonly used to identify and characterize neural stem/progenitor cells and their differentiation into various neural lineages [77–79]. Combining GDNF with AD-MSCs has demonstrated promising outcomes in enhancing the efficacy of cell therapy in PD mouse models induced by 6-hydroxydopamine [80]. In the study of Xu et al., lentivirus-mediated genetic engineering was employed to induce GDNF expression in AD-MSCs. These genetically modified cells displayed prolonged survival, lasting up to 90 days after transplantation, and successfully differentiated into dopaminergic cells. Moreover, they effectively alleviated clinical symptoms in PD rats [81]. These findings highlight the potential of genetically modified AD-MSCs as a promising approach for future clinical studies in PD treatment.
LIM homeobox transcription factor 1 alpha (LMX1A) is a transcription factor that regulates gene expression during neural differentiation, and neurturin (NTN) is a neurotrophic factor that promotes the survival and differentiation of dopaminergic [82]. In another gene and cell therapy approach, adenoviral vectors were employed to enhance the conversion of rhesus monkey-derived AD-MSCs into dopaminergic neurons. By introducing LMX1A and NTN using this vector, the neural lineage differentiation of AD-MSCs was significantly promoted [83]. However, it is essential to acknowledge certain limitations associated with adenoviral vectors, such as pro-inflammatory responses, immune reactions to the vector, and limited duration of transgene expression. These drawbacks may hinder long-term efficacy but could potentially be addressed or mitigated through further research and development efforts.
Reelin, a protein present in the extracellular matrix, plays an important role in brain development by regulating cell migration, influencing dendritogenesis, and modulating synaptic transmission. Notably, reelin suppresses α-Synuclein accumulation, promotes the survival and proliferation of AD-MSCs, and prevents premature cellular aging, an accelerated process characterized by the accumulation of senescent cells and the loss of cellular function and regenerative capacity [84]. Given these findings, reelin holds significant promise as both a factor for enhancing the functional capabilities of AD-MSCs, and as a therapeutic component for reducing α-Synuclein aggregation [85].
In the study conducted by Moriyama et al, it has been shown that oxidative stress induced by buthionine sulfoximine in human AD-multilineage progenitor cells (hAD-MPCs) resulted in p38 MAPK activation, which then led to bone morphogenetic protein (BMP2) and fibroblast growth factor 2 (FGF2) secretion and subsequently facilitated neural differentiation [86]. BMP2 and FGF play critical roles in neural differentiation, particularly in the differentiation of NSCs into dopaminergic neurons.
Conditioned medium (CM) from AD-MSCs is the culture supernatant containing a mixture of secreted chemokines, cytokines, growth factors, hormones, microvesicles, and exosomes with valuable therapeutic potentials. Studies have highlighted the significance of CM derived from AD-MSCs for neural induction, in comparison to CM from undifferentiated MSCs. CM obtained from neural-induced SCs can impact the regulation of the mTOR signaling pathway, which is known to play a crucial role in neurodegenerative diseases such as Alzheimer’s and Parkinson’s, as well as in neural development and autophagic functions [87]. CM derived from neutralized AD-MSCs, which were cells with eliminated or reduced ability for differentiation, has demonstrated the ability to enhance the phosphorylation of mTORC1 and mTORC2, contributing to the increased survival of dopaminergic neurons. Moreover, CM exerted effects on PKB/AKT, unc-51 like autophagy activating kinase 1 (ULK1), extracellular signal-regulated kinase (ERK), as well as autophagy-related genes (ATG), and lysosomal proteins, leading to the regulation of autophagy, a natural process that maintains cellular homeostasis [88]. In addition to its protective properties against cell death induced by oxidative stress and autophagy, CM has shown potential in reducing the phosphorylation and oligomerization of α-Synuclein during rotenone-induced toxicity [89].
Melatonin exhibits various pharmacological effects, including sedative, antioxidant, anti-anxiety, anti-depressant, anti-seizure, and pain-relieving properties. Moreover, melatonin has anti-apoptotic effects, as it inhibits the release of cytochrome c, which is a crucial step in the apoptotic process. Studies have revealed melatonin’s role in preventing apoptosis by neutralizing free radicals [90,91]. In the context of SC therapy, transplanted cells often face challenges such as oxidative stress, nutrient deprivation, and apoptosis, leading to limited survival at the transplant site. Enhancing cell survival is, therefore, of crucial importance for maximizing therapeutic benefits. Research on PD animal models demonstrated that combining the transplantation of dopaminergic neurons derived from AD-MSCs with melatonin treatment significantly improved the survival of transplanted neurons. This synergistic approach holds promise for enhancing the efficacy of cell-based therapies [92].
miR-188-3p, a key factor secreted by AD-MSCs, possesses therapeutic potential in PD by targeting NLRP3 and CDK5 pathways, both of which play important roles in the regulation of inflammation and neurodegeneration [93,94]. It has been reported that administration of exosomes enriched with miR-188-3p significantly enhanced cell proliferation in vitro. This highlights the promising therapeutic potential of miR-188-3p in PD treatment and underscores the potential of exosome-based therapies for the improvement of therapeutic outcomes [95].
Adipose-derived mesenchymal stem cells (AD-MSCs), Protein kinase B (PKB/AKT), Bone morphogenetic protein 2 (BMP-2), Extracellular signal-regulated kinase (ERK), Fibroblast growth factor 2 (FGF2), Forkhead box p3 (Foxp3), Glial cell line-derived neurotrophic factor (GDNF), Human adipose-derived mesenchymal stem cell (hAD-MSC), 6-hydroxydopamine (6-OHDA), Mitogen-activated protein kinase (MAPK), Mammalian target of rapamycin (mTOR), Neurogenic differentiation of human adipose-derived stem cell-conditioned medium (NI-hADSC-CM), Neuturin (NTN), Parkinson’s disease (PD), Tyrosine hydroxylase (TH), Pentraxin3 (PTX 3).
SC therapy encompasses two primary aspects in PD treatment: firstly, the ability to differentiate into diverse nerve cell types, including dopaminergic cells, and secondly, the secretion of bioactive molecules such as growth factors, cytokines, and non-coding RNAs like miRNA. AD-MSCs stand out among SC sources owing to their favorable attributes and widespread usage in nerve regeneration medicine. Researchers strive to optimize SC-based treatments for PD, addressing safety concerns such as unintended tumor formation, irreversible changes, hazardous factor transmission, unwarranted differentiation, impurity, and misidentification of SCs. Utilizing CM and biomaterial-based strategies can help manage these issues. On the other hand, enhanced therapeutic efficacy can be achieved through methods like gene therapy or employing natural compounds that boost growth factor secretion or improve the survival rate of transplanted SCs. Phytochemicals, such as phenolic acids, polyphenols, flavonoids, and terpenoids, display additional benefits for PD treatment by stimulating the production of neurotrophins, such as BDNF, and NGF, regulating signal transduction pathways involved in neural differentiation like Wnt/β-catenin and MAPK, and regulating the expression of neural-specific genes like Nestin, and GFAP [98–100]. Lastly, incorporating healthy lifestyle practices like regular physical activity alongside cell therapy offers promise for strengthening the treatment process. Such routines positively impact the nervous system and aid in managing PD.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and draft manuscript preparation and visualization: Mohammad-Sadegh Lotfi; review, editing and supervision: Fatemeh B. Rassouli. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available on request from the corresponding authors.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Kaur R, Mehan S, Singh S. Understanding multifactorial architecture of Parkinson’s disease: pathophysiology to management. Neurol Sci. 2019;40(1):13–23. doi:10.1007/s10072-018-3585-x. [Google Scholar] [PubMed] [CrossRef]
2. Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H, et al. Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front Public Health. 2021;9:776847. doi:10.3389/fpubh.2021.776847. [Google Scholar] [PubMed] [CrossRef]
3. Balestrino R, Schapira AH. Parkinson disease. Eur J Neurol. 2020;27(1):27–42. doi:10.1111/ene.v27.1. [Google Scholar] [CrossRef]
4. Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36(1):1–12. doi:10.1016/j.cger.2019.08.002. [Google Scholar] [PubMed] [CrossRef]
5. Fahn S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disord. 2015;30(1):4–18. doi:10.1002/mds.v30.1. [Google Scholar] [CrossRef]
6. Verhagen Metman L, Pal G, Slavin K. Surgical treatment of parkinson’s disease. Curr Treat Options Neurol. 2016;18(11):49. doi:10.1007/s11940-016-0432-3. [Google Scholar] [PubMed] [CrossRef]
7. Dumbhare O, Gaurkar SS. A review of genetic and gene therapy for Parkinson’s disease. Cureus. 2023;15(2):e34657. [Google Scholar] [PubMed]
8. Borovac JA. Focus: the aging brain: side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37. [Google Scholar] [PubMed]
9. Day JO, Mullin S. The genetics of Parkinson’s disease and implications for clinical practice. Genes. 2021;12(7):1006. [Google Scholar] [PubMed]
10. Zhang PL, Chen Y, Zhang CH, Wang YX, Fernandez-Funez P. Genetics of Parkinson’s disease and related disorders. J Med Genet. 2018;55(2):73–80. doi:10.1136/jmedgenet-2017-105047. [Google Scholar] [PubMed] [CrossRef]
11. Chen Y, Sun X, Lin Y, Zhang Z, Gao Y, Wu I. Non-genetic risk factors for Parkinson’s disease: an overview of 46 systematic reviews. J Parkinsons Dis. 2021;11(3):919–35. doi:10.3233/JPD-202521. [Google Scholar] [PubMed] [CrossRef]
12. Ascherio A. Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–72. doi:10.1016/S1474-4422(16)30230-7. [Google Scholar] [PubMed] [CrossRef]
13. Mattei V, Monache SD. Mesenchymal stem cells and their role in neurodegenerative diseases. Cells. 2024;13(9):779. doi:10.3390/cells13090779. [Google Scholar] [PubMed] [CrossRef]
14. Cerri S, Mus L, Blandini F. Parkinson’s disease in women and men: what’s the difference? J Parkinsons Dis. 2019;9(3):501–15. doi:10.3233/JPD-191683. [Google Scholar] [PubMed] [CrossRef]
15. Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364(9440):1167–9. doi:10.1016/S0140-6736(04)17103-1. [Google Scholar] [PubMed] [CrossRef]
16. Cannon JR, Greenamyre JT. Gene-environment interactions in Parkinson’s disease: specific evidence in humans and mammalian models. Neurobiol Dis. 2013;57:38–46. doi:10.1016/j.nbd.2012.06.025. [Google Scholar] [PubMed] [CrossRef]
17. Calabresi P, Mechelli A, Natale G, Volpicelli-Daley L, Di Lazzaro G, Ghiglieri V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023;14(3):176. doi:10.1038/s41419-023-05672-9. [Google Scholar] [PubMed] [CrossRef]
18. Volpicelli-Daley L, Brundin P. Prion-like propagation of pathology in Parkinson disease. Handb Clin Neurol. 2018;153:321–35. doi:10.1016/B978-0-444-63945-5.00017-9. [Google Scholar] [PubMed] [CrossRef]
19. Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. doi:10.1136/jnnp.2007.131045. [Google Scholar] [PubMed] [CrossRef]
20. Sardi SP, Cedarbaum JM, Brundin P. Targeted therapies for Parkinson’s disease: from genetics to the clinic. Mov Disord. 2018;33(5):684–96. doi:10.1002/mds.v33.5. [Google Scholar] [CrossRef]
21. Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front Mol Neurosci. 2019;12:299. doi:10.3389/fnmol.2019.00299. [Google Scholar] [PubMed] [CrossRef]
22. Weihofen A, Liu Y, Arndt JW, Huy C, Quan C, Smith BA, et al. Development of an aggregate-selective, human-derived α-synuclein antibody BIIB054 that ameliorates disease phenotypes in Parkinson’s disease models. Neurobiol Dis. 2019;124(6):276–88. [Google Scholar] [PubMed]
23. Threlfell S, Mohammadi AS, Ryan B, Connor-Robson N, Platt NJ, Anand R, et al. Striatal dopamine transporter function is facilitated by converging biology of α-Synuclein and cholesterol. Front Cell Neurosci. 2021;15:658244. doi:10.3389/fncel.2021.658244. [Google Scholar] [PubMed] [CrossRef]
24. Tong Y, Zhu W, Chen J, Wen T, Xu F, Pang J. Discovery of small-molecule degraders for alpha-synuclein aggregates. J Med Chem. 2023;66(12):7926–42. doi:10.1021/acs.jmedchem.3c00274. [Google Scholar] [PubMed] [CrossRef]
25. Wen T, Chen J, Zhang W, Pang J. Design, synthesis and biological evaluation of α-synuclein proteolysis-targeting chimeras. Molecules. 2023;28(11):4458. doi:10.3390/molecules28114458. [Google Scholar] [PubMed] [CrossRef]
26. Merola A, Van Laar A, Lonser R, Bankiewicz K. Gene therapy for Parkinson’s disease: contemporary practice and emerging concepts. Expert Rev Neurother. 2020;20(6):577–90. doi:10.1080/14737175.2020.1763794. [Google Scholar] [PubMed] [CrossRef]
27. Axelsen TM, Woldbye DPD. Gene therapy for Parkinson’s disease, an update. J Parkinsons Dis. 2018;8(2):195–215. doi:10.3233/JPD-181331. [Google Scholar] [PubMed] [CrossRef]
28. Gklinos P, Papadopoulou M, Stanulovic V, Mitsikostas DD, Papadopoulos D. Monoclonal antibodies as neurological therapeutics. Pharmaceuticals. 2021;14(2):92. doi:10.3390/ph14020092. [Google Scholar] [PubMed] [CrossRef]
29. Yasuhara T, Kameda M, Sasaki T, Tajiri N, Date I. Cell therapy for Parkinson’s disease. Cell Transplant. 2017;26(9):1551–9. doi:10.1177/0963689717735411. [Google Scholar] [PubMed] [CrossRef]
30. Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403–14. doi:10.1002/ana.v54:3. [Google Scholar] [CrossRef]
31. Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA neurol. 2014;71(1):83–7. doi:10.1001/jamaneurol.2013.4749. [Google Scholar] [PubMed] [CrossRef]
32. Park TY, Jeon J, Cha Y, Kim KS. Past, present, and future of cell replacement therapy for Parkinson’s disease: a novel emphasis on host immune responses. Cell Res. 2024;34:1–14. [Google Scholar]
33. Liao Y, Zhu H, Ivanova L, Cairo MS. Innovations in human stem cell research: a holy grail for regenerative medicine. In: Loewy Z, editor. Innovations in cell research and therapy. 2020. [Google Scholar]
34. Thiagarajan P, Parker CJ, Prchal JT. How do red blood cells die? Front Physiol. 2021;12:655393. doi:10.3389/fphys.2021.655393. [Google Scholar] [PubMed] [CrossRef]
35. Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10(6):678–84. doi:10.1016/j.stem.2012.05.005. [Google Scholar] [PubMed] [CrossRef]
36. Grochowski C, Radzikowska E, Maciejewski R. Neural stem cell therapy—brief review. Clin Neurol Neurosurg. 2018;173:8–14. doi:10.1016/j.clineuro.2018.07.013. [Google Scholar] [PubMed] [CrossRef]
37. Gorabi AM, KiNaie N, Pirro M, Bianconi V, Jamialahmadi T, Sahebkar AH. Effects of statins on the biological features of mesenchymal stem cells and therapeutic implications. Heart Fail Rev. 2021;26(5):1259–72. doi:10.1007/s10741-020-09929-9. [Google Scholar] [PubMed] [CrossRef]
38. Paccosi E, Proietti-De-Santis L. Parkinson’s disease: from genetics and epigenetics to treatment, a miRNA-based strategy. Int J Mol Sci. 2023;24(11):9547. [Google Scholar] [PubMed]
39. Vilaça-Faria H, Salgado AJ, Teixeira FG. Mesenchymal stem cells-derived exosomes: a new possible therapeutic strategy for Parkinson’s disease? Cells. 2019;8(2):118. [Google Scholar]
40. Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cell Int. 2016;2016:6901286. [Google Scholar] [PubMed]
41. Meirelles LDS, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(11):2204–13. doi:10.1242/jcs.02932. [Google Scholar] [PubMed] [CrossRef]
42. Minteer DM, Marra KG, Rubin JP. Adipose stem cells: biology, safety, regulation, and regenerative potential. Clin Plast Surg. 2015;42(2):169–79. doi:10.1016/j.cps.2014.12.007. [Google Scholar] [PubMed] [CrossRef]
43. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4(1):22. doi:10.1038/s41536-019-0083-6. [Google Scholar] [PubMed] [CrossRef]
44. Wu SH, Liao Y, Huang CH, Chen YC, Chiang ER, Wang JP. Comparison of the confluence-initiated neurogenic differentiation tendency of adipose-derived and bone marrow-derived mesenchymal stem cells. Biomedicines. 2021;9(11):1503. doi:10.3390/biomedicines9111503. [Google Scholar] [PubMed] [CrossRef]
45. Zheng Y, Huang C, Liu F, Lin H, Yang X, Zhang Z. Comparison of the neuronal differentiation abilities of bone marrow‐derived and adipose tissue‐derived mesenchymal stem cells. Mol Med Rep. 2017;16(4):3877–86. doi:10.3892/mmr.2017.7069. [Google Scholar] [PubMed] [CrossRef]
46. Zhou LN, Wang JC, Zilundu PL, Wang YQ, Guo WP, Zhang SX, et al. A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Res Ther. 2020;11(1):153. doi:10.1186/s13287-020-01661-3. [Google Scholar] [PubMed] [CrossRef]
47. Takahashi A, Nakajima H, Uchida K, Takeura N, Honjoh K, Watanabe S, et al. Comparison of mesenchymal stromal cells isolated from murine adipose tissue and bone marrow in the treatment of spinal cord injury. Cell Transplant. 2018;27(7):1126–39. doi:10.1177/0963689718780309. [Google Scholar] [PubMed] [CrossRef]
48. Balasubramanian S, Thej C, Venugopal P, Priya N, Zakaria Z, Sundarraj S, et al. Higher propensity of Wharton’s jelly derived mesenchymal stromal cells towards neuronal lineage in comparison to those derived from adipose and bone marrow. Cell Biol Int. 2013;37(5):507–15. doi:10.1002/cbin.v37.5. [Google Scholar] [CrossRef]
49. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150–4. doi:10.1016/j.tibtech.2006.01.010. [Google Scholar] [PubMed] [CrossRef]
50. Chu DT, Phuong TNT, Tien NLB, Tran DK, Minh LB, Thanh VV, et al. Adipose tissue stem cells for therapy: an update on the progress of isolation, culture, storage, and clinical application. J Clin Med. 2019;8(7):917. doi:10.3390/jcm8070917. [Google Scholar] [PubMed] [CrossRef]
51. Absalan F, Sharifi Pasandi M, Ghasemi Hamidabadi H, Saeednia S, Nazm Bojnordi M, Zahiri M, et al. Matrigel enhances differentiation of human adipose tissue-derived stem cells into dopaminergic neuron. Neurosci Lett. 2021;760:136070. doi:10.1016/j.neulet.2021.136070. [Google Scholar] [PubMed] [CrossRef]
52. Han C, Wang YJ, Wang YC, Guan X, Wang L, Shen LM, et al. Caveolin-1 downregulation promotes the dopaminergic neuron-like differentiation of human adipose-derived mesenchymal stem cells. Neural Regen Res. 2021;16(4):714–20. doi:10.4103/1673-5374.295342. [Google Scholar] [PubMed] [CrossRef]
53. Khademizadeh M, Messripour M, Ghasemi N, Momen Beik F, Movahedian Attar A. Differentiation of adult human mesenchymal stem cells into dopaminergic neurons. Res Pharm Sci. 2019;14(3):209–15. doi:10.4103/1735-5362.258487. [Google Scholar] [PubMed] [CrossRef]
54. Shigematsu K, Komori N, Tahara K, Yamagishi H. Repeated infusion of autologous adipose tissue-derived stem cells for Parkinson’s disease. Acta Neurol Scand. 2022;145(1):119–22. doi:10.1111/ane.v145.1. [Google Scholar] [CrossRef]
55. Choi SW, Park KB, Woo SK, Kang SK, Ra JC. Treatment of progressive supranuclear palsy with autologous adipose tissue-derived mesenchymal stem cells: a case report. J Med Case Rep. 2014;8:87. doi:10.1186/1752-1947-8-87. [Google Scholar] [PubMed] [CrossRef]
56. Nguyen NT, Phan HT, Le PM, Thi Nguyen LH, Do TT, Phan TP, et al. Safety and efficacy of autologous adipose tissue-derived stem cell transplantation in aging-related low-grade inflammation patients: a single-group, open-label, phase I clinical trial. Trials. 2024;25(1):309. doi:10.1186/s13063-024-08128-3. [Google Scholar] [PubMed] [CrossRef]
57. Schwerk A, Altschüler J, Roch M, Gossen M, Winter C, Berg J, et al. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson’s disease. Regen Med. 2015;10(4):431–46. doi:10.2217/rme.15.17. [Google Scholar] [PubMed] [CrossRef]
58. Park H, Chang KA. Therapeutic potential of repeated intravenous transplantation of human adipose-derived stem cells in subchronic MPTP-induced Parkinson’s disease mouse model. Int J Mol Sci. 2020;21(21):8129. doi:10.3390/ijms21218129. [Google Scholar] [PubMed] [CrossRef]
59. Popova N, Ilchibaeva T, Naumenko V. Neurotrophic factors (BDNF and GDNF) and the serotonergic system of the brain. Biochem. 2017;82:308–17. [Google Scholar]
60. Seo JP, Koo DK. Degeneration of nigrostriatal pathway in patients with middle cerebral infarct: a diffusion tensor imaging study. Medicine. 2023;102(14):e33370. doi:10.1097/MD.0000000000033370. [Google Scholar] [PubMed] [CrossRef]
61. Bhattachan P, Rae J, Yu H, Jung WR, Wei J, Parton RG, et al. Ascidian caveolin induces membrane curvature and protects tissue integrity and morphology during embryogenesis. Faseb J. 2020;34(1):1345–61. doi:10.1096/fsb2.v34.1. [Google Scholar] [CrossRef]
62. Bandara N, Gurusinghe S, Yong Lim S, Chen H, Chen S, Wang D, et al. Molecular control of nitric oxide synthesis through eNOS and caveolin-1 interaction regulates osteogenic differentiation of adipose-derived stem cells by modulation of Wnt/β-catenin signaling. Stem Cell Res Ther. 2016;7(1):182. doi:10.1186/s13287-016-0442-9. [Google Scholar] [PubMed] [CrossRef]
63. Li Y, Lau WM, So KF, Tong YT, Shen JS, et al. Caveolin-1 promote astroglial differentiation of neural stem/progenitor cells through modulating Notch1/NICD and Hes1 expressions. Biochem Biophys Res Commun. 2011;407(3):517–24. doi:10.1016/j.bbrc.2011.03.050. [Google Scholar] [PubMed] [CrossRef]
64. Peffer ME, Chandran UR, Luthra S, Volonte D, Galbiati F, Garabedian MJ, et al. Caveolin-1 regulates genomic action of the glucocorticoid receptor in neural stem cells. Mol Cell Biol. 2014;34(14):2611–23. doi:10.1128/MCB.01121-13. [Google Scholar] [PubMed] [CrossRef]
65. Jin YH, Kim H, Oh M, Ki H, Kim M. Regulation of Notch1/NICD and Hes1 expressions by GSK-3α/β. Mol Cells. 2009;27(1):15–9. doi:10.1007/s10059-009-0001-7. [Google Scholar] [PubMed] [CrossRef]
66. Cenciarelli C, Marei HE, Zonfrillo M, Casalbore P, Felsani A, Giannetti S, et al. The interference of Notch1 target Hes1 affects cell growth, differentiation and invasiveness of glioblastoma stem cells through modulation of multiple oncogenic targets. Oncotarget. 2017;8(11):17873–86. doi:10.18632/oncotarget.v8i11. [Google Scholar] [CrossRef]
67. Lasky JL, Wu H. Notch signaling, brain development, and human disease. Pediatr Res. 2005;57:104–9. doi:10.1203/01.PDR.0000159632.70510.3D. [Google Scholar] [PubMed] [CrossRef]
68. Rodriguez-Grande B, Varghese L, Molina-Holgado F, Rajkovic O, Garlanda C, Denes A, et al. Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J Neuroinflammation. 2015;12:1–11. [Google Scholar]
69. Lian C, Huang Q, Zhong X, He Z, Liu B, Zeng H, et al. Pentraxin 3 secreted by human adipose-derived stem cells promotes dopaminergic neuron repair in Parkinson’s disease via the inhibition of apoptosis. Faseb J. 2021;35(7):e21748. [Google Scholar] [PubMed]
70. Rajkovic I, Wong R, Lemarchand E, Rivers-Auty J, Rajkovic O, Garlanda C, et al. Pentraxin 3 promotes long-term cerebral blood flow recovery, angiogenesis, and neuronal survival after stroke. J Mol Med. 2018;96(12):1319–32. doi:10.1007/s00109-018-1698-6. [Google Scholar] [PubMed] [CrossRef]
71. Zhou C, Chen H, Zheng JF, Guo ZD, Huang ZJ, Wu Y, et al. Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice. Neural Regen Res. 2020;15(12):2318–26. doi:10.4103/1673-5374.285001. [Google Scholar] [PubMed] [CrossRef]
72. Bi Y, Lin X, Liang H, Yang D, Zhang X, Ke J, et al. Human adipose tissue-derived mesenchymal stem cells in Parkinson’s disease: inhibition of T helper 17 cell differentiation and regulation of immune balance towards a regulatory T cell phenotype. Clin Interv Aging. 2020;15:1383–91. doi:10.2147/CIA.S259762. [Google Scholar] [PubMed] [CrossRef]
73. Pascual Bravo A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R, López-Barneoal J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11(7):755–61. doi:10.1038/nn.2136. [Google Scholar] [PubMed] [CrossRef]
74. Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line—derived neurotrophic factor. J Neurosurg. 2005;102(2):216–22. doi:10.3171/jns.2005.102.2.0216. [Google Scholar] [PubMed] [CrossRef]
75. Wang F, Kameda M, Yasuhara T, Tajiri N, Kikuchi Y, Liang HB, et al. GDNF-pretreatment enhances the survival of neural stem cells following transplantation in a rat model of Parkinson’s disease. Neurosci Res. 2011;71(1):92–8. doi:10.1016/j.neures.2011.05.019. [Google Scholar] [PubMed] [CrossRef]
76. Hoban D, Howard L, Dowd E. GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson’s disease. Neurosci. 2015;303:402–11. doi:10.1016/j.neuroscience.2015.07.014. [Google Scholar] [PubMed] [CrossRef]
77. Lang H, Xing Y, Brown LSN, Samuvel DJ, Panganiban CH, Havens LT. Neural stem/progenitor cell properties of glial cells in the adult mouse auditory nerve. Sci Rep. 2015;26(5):13383. [Google Scholar]
78. Tavakol S, Mousavi SMM, Tavakol B, Hoveizi E, Ai J, Sorkhabadi SMR. Mechano-transduction signals derived from self-assembling peptide nanofibers containing long motif of laminin influence neurogenesis in in-vitro and in-vivo. Mol Neurobiol. 2017;54(4):2483–96. doi:10.1007/s12035-016-9836-z. [Google Scholar] [PubMed] [CrossRef]
79. Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One. 2011;6(2):e16816. doi:10.1371/journal.pone.0016816. [Google Scholar] [PubMed] [CrossRef]
80. Sun S, Zhang Q, Li M, Gao P, Huang K, Beejadhursing R, et al. GDNF promotes survival and therapeutic efficacy of human adipose-derived mesenchymal stem cells in a mouse model of Parkinson’s disease. Cell Transplant. 2020;29:0963689720908512. [Google Scholar] [PubMed]
81. Xu R, Wu J, Lang L, Hu J, Tang H, Xu J, et al. 1Implantation of glial cell line-derived neurotrophic factor-expressing adipose tissue-derived stromal cells in a rat Parkinson’s disease model. Neurol Res. 2020;42(8):712–20. doi:10.1080/01616412.2020.1783473. [Google Scholar] [PubMed] [CrossRef]
82. Zhang J, Yang B, Luo L, Li L, Yang X, Zhang J, et al. Effect of NTN and Lmx1α on the Notch signaling pathway during the differentiation of human bone marrow mesenchymal stem cells into dopaminergic neuron-like cells. Parkinson’s Dis. 2021;2021:1–11. [Google Scholar]
83. Zhou Y, Sun M, Li H, Yan M, He Z, Wang W, et al. Recovery of behavioral symptoms in hemi-parkinsonian rhesus monkeys through combined gene and stem cell therapy. Cytotherapy. 2013;15(4):467–80. doi:10.1016/j.jcyt.2013.01.007. [Google Scholar] [PubMed] [CrossRef]
84. Cho E, Park J, Kim K, Cho SR. Reelin alleviates mesenchymal stem cell senescence and reduces pathological α-synuclein expression in an in vitro model of Parkinson’s disease. Genes. 2021;12(7):1066. doi:10.3390/genes12071066. [Google Scholar] [PubMed] [CrossRef]
85. Kubben N, Misteli T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol. 2017;18(10):595–609. doi:10.1038/nrm.2017.68. [Google Scholar] [PubMed] [CrossRef]
86. Moriyama M, Moriyama H, Ueda A, Nishibata Y, Okura H, Ichinose A, et al. Human adipose tissue-derived multilineage progenitor cells exposed to oxidative stress induce neurite outgrowth in PC12 cells through p38 MAPK signaling. BMC Cell Biol. 2012;13:21. doi:10.1186/1471-2121-13-21. [Google Scholar] [PubMed] [CrossRef]
87. Ramalingam M, Jeong HS, Hwang J, Cho HH, Kim BC, Kim E, et al. Autophagy signaling by neural-induced human adipose tissue-derived stem cell-conditioned medium during rotenone-induced toxicity in SH-SY5Y cells. Int J Mol Sci. 2022;23(8):4193. doi:10.3390/ijms23084193. [Google Scholar] [PubMed] [CrossRef]
88. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi:10.1002/path.v221:1. [Google Scholar] [CrossRef]
89. Ramalingam M, Jang S, Jeong HS. Neural-induced human adipose tissue-derived stem cells conditioned medium ameliorates rotenone-induced toxicity in SH-SY5Y cells. Int J Mol Sci. 2021;22(5):2322. doi:10.3390/ijms22052322. [Google Scholar] [PubMed] [CrossRef]
90. Mayo JC, Sainz RM, Antolín I, Rodriguez C. Ultrastructural confirmation of neuronal protection by melatonin against the neurotoxin 6-hydroxydopamine cell damage. Brain Res. 1999;818(2):221–7. doi:10.1016/S0006-8993(98)01262-1. [Google Scholar] [PubMed] [CrossRef]
91. Rios ER, Venâncio ET, Rocha NF, Woods DJ, Vasconcelos S, Macedo D, et al. Melatonin: pharmacological aspects and clinical trends. Int J Neurosci. 2010;120(9):583–90. doi:10.3109/00207454.2010.492921. [Google Scholar] [PubMed] [CrossRef]
92. Asemi-Rad A, Moafi M, Aliaghaei A, Abbaszadeh HA, Abdollahifar MA, Ebrahimi MJ, et al. The effect of dopaminergic neuron transplantation and melatonin co-administration on oxidative stress-induced cell death in Parkinson’s disease. Metab Brain Dis. 2022;37(8):2677–85. doi:10.1007/s11011-022-01021-5. [Google Scholar] [PubMed] [CrossRef]
93. Zhang P, Shao XY, Qi GJ, Chen Q, Bu LL, Chen LJ, et al. Cdk5-dependent activation of neuronal inflammasomes in Parkinson’s disease. Mov Disord. 2016;31(3):366–76. doi:10.1002/mds.26488. [Google Scholar] [PubMed] [CrossRef]
94. Cheng X, Xu S, Zhang C, Qin K, Yan J, Shao X. The BRCC3 regulated by Cdk5 promotes the activation of neuronal NLRP3 inflammasome in Parkinson’s disease models. Biochem Biophys Res Commun. 2019;522(3):647–54. [Google Scholar] [PubMed]
95. Li Q, Wang Z, Xing H, Wang Y, Guo Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol Ther Nucleic Acids. 2021;23:1334–44. doi:10.1016/j.omtn.2021.01.022. [Google Scholar] [PubMed] [CrossRef]
96. Chierchia A, Chirico N, Boeri L, Raimondi I, Riva GA, Raimondi MT, et al. Secretome released from hydrogel-embedded adipose mesenchymal stem cells protects against the Parkinson’s disease related toxin 6-hydroxydopamine. Eur J Pharm Biopharm. 2017;121:113–20. doi:10.1016/j.ejpb.2017.09.014. [Google Scholar] [PubMed] [CrossRef]
97. Schwerk A, Altschüler J, Roch M, Gossen M, Winter C, Berg J, et al. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy. 2015;17(2):199–214. doi:10.1016/j.jcyt.2014.09.005. [Google Scholar] [PubMed] [CrossRef]
98. Lotfi MS, Kalalinia F. Flavonoids in combination with stem cells for the treatment of neurological disorders. Neurochem Res. 2023;48(11):3270–82. doi:10.1007/s11064-023-03986-w. [Google Scholar] [PubMed] [CrossRef]
99. An J, Chen B, Tian D, Guo Y, Yan Y, Yang H. Regulation of neurogenesis and neuronal differentiation by natural compounds. Curr Stem Cell Res Ther. 2022;17(8):756–71. doi:10.2174/1574888X16666210907141447. [Google Scholar] [PubMed] [CrossRef]
100. Ferdousi F, Sasaki K, Xu D, Zheng YW, Szele FG, Isoda H. Editorial: directing stem cell fate using plant extracts and their bioactive compounds. Front Cell Dev Biol. 2022;10:957601. doi:10.3389/fcell.2022.957601. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools