 Open Access
Open Access
ARTICLE
Heterogeneity beyond tumor heterogeneity—SULF2 involvement in Wnt/β-catenin signaling activation in a heterogeneous side population of liver cancer cells
1 Division of Gastroenterology & Hepatology, The University of Hong Kong-Shenzhen Hospital, Shenzhen, 518053, China
2 Shenzhen Key Laboratory of Gene and Antibody Therapy, Center for Biotechnology and Biomedicine, State Key Laboratory of Health Sciences and Technology, State Key Laboratory of Chemical Oncogenomics, Precision Medicine and Healthcare Research Center, Tsinghua-Berkeley Shenzhen Institute (TBSI), Shenzhen International Graduate School, Tsinghua University, Shenzhen, 518055, China
3 Department of Chemistry, Tsinghua University, Beijing, 100084, China
* Corresponding Authors: DONGYE YANG. Email: ; LAIQING HUANG. Email:
# These authors contributed equally to this work
(This article belongs to the Special Issue: Application of Deep Learning in Cancer)
BIOCELL 2023, 47(9), 2037-2049. https://doi.org/10.32604/biocell.2023.028863
Received 16 January 2023; Accepted 28 June 2023; Issue published 28 September 2023
Abstract
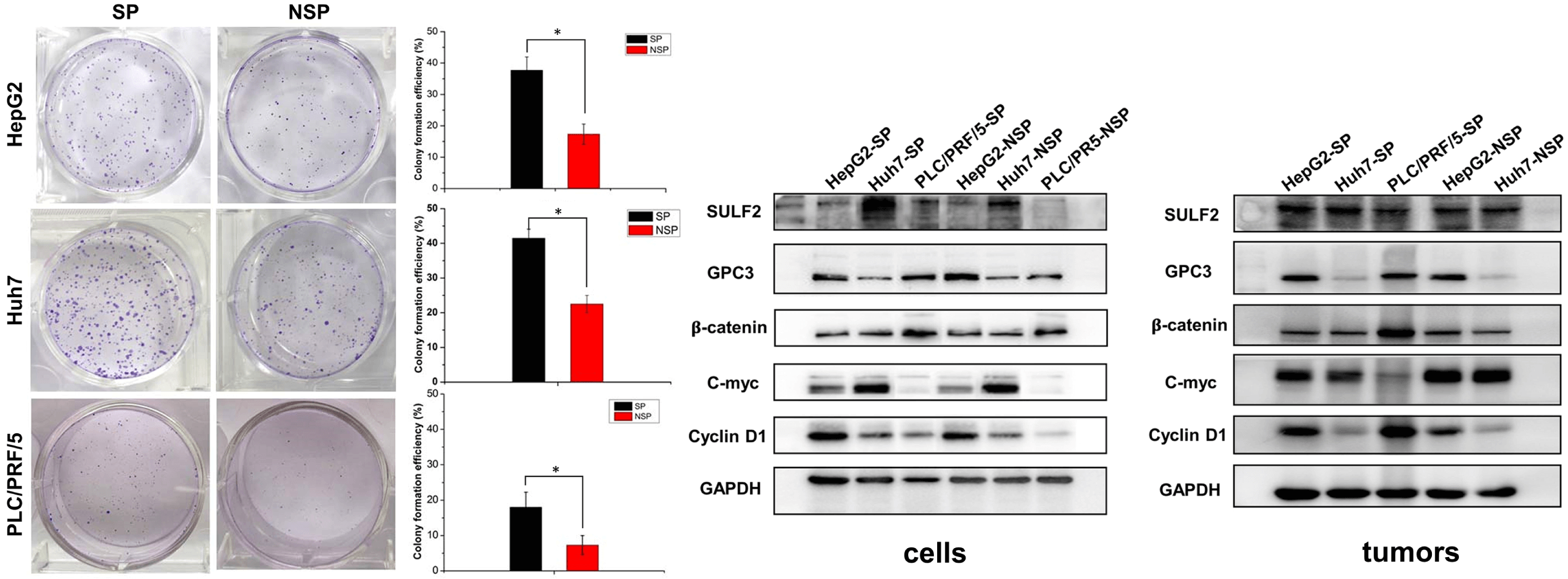
Introduction: Sulfatase 2 (SULF2), an endogenous extracellular sulfatase, can remove 6-O-sulfate groups of glucosamine residues from heparan sulfate (HS) chains to modulate the Wnt/β-catenin signaling pathway, which plays an important role in both liver carcinogenesis and embryogenesis. Side population (SP) cells are widely identified as stem-like cancer cells and are closely related to carcinoma metastasis, recurrence, and poor patient prognosis. However, the roles of SULF2 in SP cells of hepatomas are unclear, and the underlying mechanism is undefined. Objectives: This study aimed to compare the heterogeneity between SP cells and non-side population (NSP) cells derived from three different liver cancer cell lines and to elucidate the involvement of the SULF2-Wnt/β-catenin axis in liver cancer stem cells (CSCs) and its impact on the processes of carcinogenesis and invasiveness. Methods: In this work, three different liver cancer SP cells (HepG2, Huh7, and PRC/PRL/5) were sorted by flow cytometry. We also examined the migration and invasion behaviors of SP and NSP cells. To determine if this high tumorigenic potential of SP cells is correlated to SULF2, qPCR, western blotting, and immunofluorescence analysis were conducted. We also performed nude mouse xenograft experiments for in vivo analysis. Results: The results from the in vitro colony formation assay showed that SP cells exhibited a 2-fold higher colony formation efficiency compared to their NSP counterparts. The SP cells exhibited significantly higher potentials in terms of their migratory capacity and invasive ability compared to NSP cells. We found that higher expression of SULF2 in SP cells was associated with greater capabilities for clonogenicity, migration, and invasion. It was also linked to higher activation of the Wnt/β-catenin signaling pathway via stimulation of key downstream factors, particularly β-catenin, c-Myc, and cyclin D1. Further, a positive correlation between the upregulated SULF2 expression and tumorigenesis in the in vivo nude mouse xenograft models was demonstrated, highlighting that the potential underlying mechanism was Wnt/β-catenin signaling pathway activation. Conclusion: Our findings show that variable SULF2 expression was associated with differential activation of the Wnt/β-catenin signaling pathway, which could lead to behavioral differences between SP and NSP cells and also among the SP cells of the three liver cancer cell lines assessed. It was reasonably concluded that the SULF2-Wnt/β-catenin axis could play an important role in the tumorigenicity of liver cancer stem cells.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools