 Open Access
Open Access
ARTICLE
Study of molecular mechanisms underlying the medicinal plant Tripterygium wilfordii-derived compound celastrol in treating diabetic nephropathy based on network pharmacology and molecular docking
1 The Second School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, 310003, China
2 Urology & Nephrology Center, Department of Nephrology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, 310014, China
3 Department of Nephrology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, 310000, China
* Corresponding Author: JUAN JIN. Email:
(This article belongs to the Special Issue: Natural Products for Chronic Inflammatory Diseases: Pharmacology and Toxicology)
BIOCELL 2023, 47(8), 1853-1867. https://doi.org/10.32604/biocell.2023.029353
Received 14 February 2023; Accepted 19 April 2023; Issue published 28 August 2023
Abstract
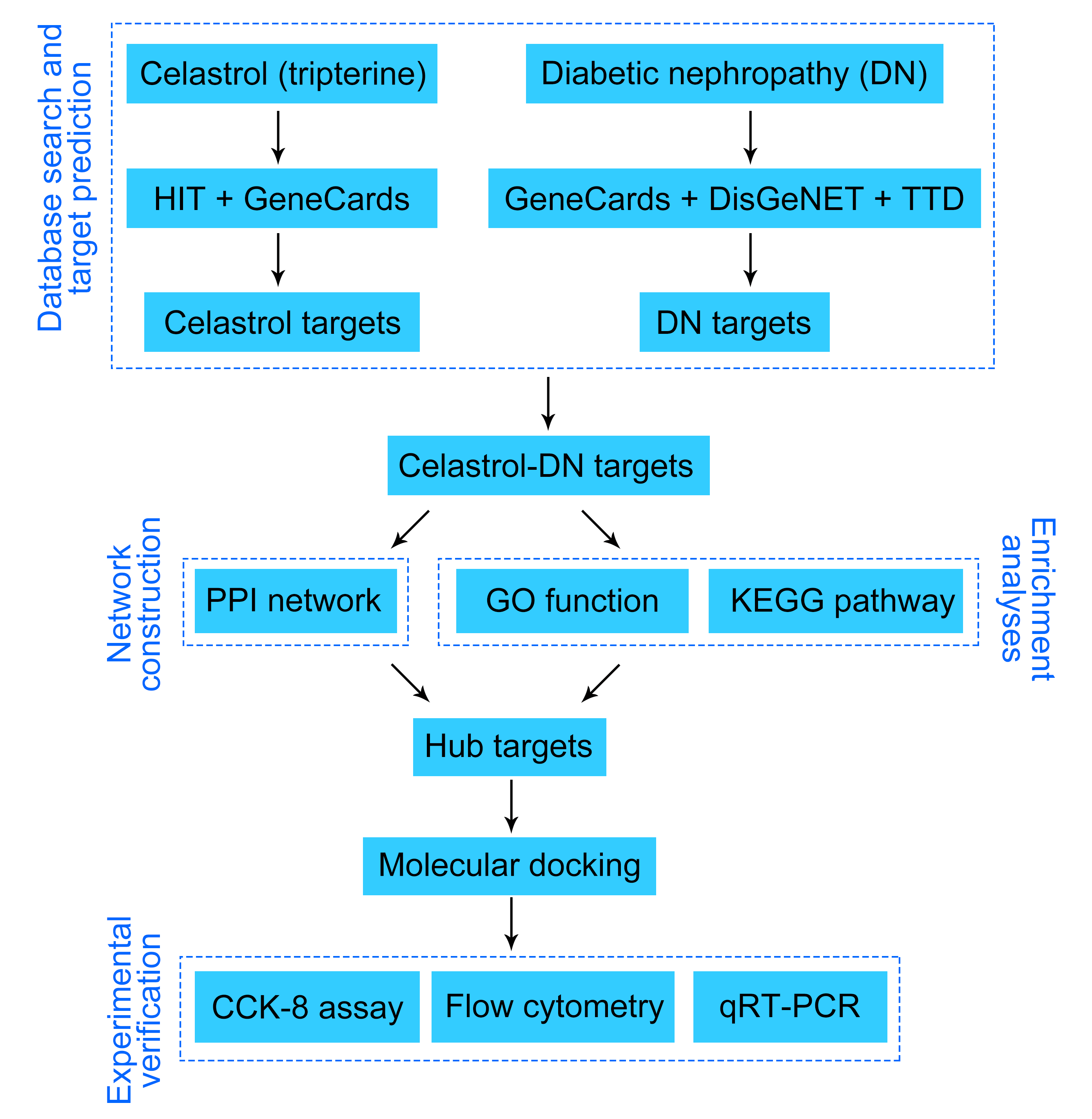
Background: Diabetic nephropathy (DN) is a serious complication of diabetes with rising prevalence worldwide. We aimed to explore the anti-DN mechanisms of the compound celastrol derived from the medicinal plant Tripterygium wilfordii. Methods: Celastrol-related targets were obtained from Herbal Ingredients’ Targets (HIT) and GeneCards databases. DN-related targets were retrieved from GeneCards, DisGeNET, and Therapeutic Targets Database (TTD). A Protein-protein interaction (PPI) network was established using the Search Tool for the Retrieval of Interacting Genes (STRING) database. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed using ClusterProfiler. The cytoHubba plugin was used to select the top 10 hub targets. Molecular docking was performed employing PyMOL and AutoDock software. Cell counting kit-8 (CCK-8) and flow cytometry assays were used to detect the viability and apoptosis of NRK-52E cells, respectively. The mRNA expression levels of mitogen-activated protein kinase 3 (MAPK3), tumor necrosis factor (TNF), and AKT serine/threonine kinase 1 (AKT1) in NRK-52E cells were assessed using quantitative real-time polymerase chain reaction (qRT-PCR). Results: We obtained sixty-six overlapping targets of celastrol and DN. GO and KEGG analyses demonstrated that the core targets of celastrol and DN were mainly involved in the inflammatory and immune response, oxidative stress, advanced glycation end products (AGEs) and their receptors (RAGEs) (AGE-RAGE), TNF, interleukin 17 (IL-17), and MAPK signaling pathways. Finally, based on the good binding activity with celastrol, MAPK3, TNF, and AKT1 were identified as the foremost targets of celastrol. We observed that celastrol enhanced the viability of high glucose (HG)-treated NRK-52E cells and inhibited apoptosis in the in vitro assays. Moreover, celastrol decreased the mRNA expression levels of MAPK3, TNF, and AKT1 in high glucose (HG)-treated NRK-52E cells. Conclusion: Celastrol may treat DN by targeting APK3, TNF, and AKT1 and regulating inflammatory responses and oxidative stress through the AGE-RAGE, TNF, IL-17, and MAPK signaling pathways.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools