 Open Access
Open Access
REVIEW
Molecular mechanisms and cellular process in signal transduction pathway related to air pollutants in obstructive lung diseases: A mini-review
Division of Allergy and Respiratory Medicine, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, 170 Jomaru-ro, Bucheon-si, Gyeonggi-do, 14584, Korea
* Corresponding Author: AN-SOO JANG. Email:
BIOCELL 2023, 47(8), 1703-1708. https://doi.org/10.32604/biocell.2023.028975
Received 20 January 2023; Accepted 06 May 2023; Issue published 28 August 2023
Abstract
Exposure to air pollutants such as PM10, PM2.5, PM0.1, O3, CO, NO2, and SO2, and biological pollutants are important factors causing the evolution and furtherance of obstructive lung diseases (OLD), including asthma and chronic obstructive pulmonary disease (COPD). Asthma is the most frequent chronic inflammatory airway disease, characterized by breathlessness, wheezing, chest tightness, and cough, together with the presence of exaggerated expiratory airflow fluctuation that varies over time. COPD is a heterogeneous lung condition characterized by chronic respiratory symptoms such as dyspnea, cough, expectoration, and/or exacerbations due to abnormalities of the airways and/or alveoli that cause persistent, often progressive, airflow obstruction. Understanding the molecular mechanisms and cellular processes based on the development of OLD on exposure to air pollutants will provide insights into the solution of pathogenesis, prevention, and treatment of these conditions. The molecular mechanisms and cellular process involved in signal transduction pathway plays a role in the binding of extracellular signaling molecules and ligands to receptors placed on the cell surface or on the inner side cell that trigger inflammation that occurs, especially when something important enters the cell to bring into a cascade response. This binding then alters the cell metabolism, shape, and gene expression in the airway. This review aimed to reveal the effect of air pollutants on the molecular mechanisms and cellular processes involved in the signal transduction pathways in OLD.Keywords
Air pollutants like particulate matter (PM), ozone, and biological pollutants penetrate deep into the airways and reach the small air-containing compartment of the lungs where the terminal bronchioles and from which airway gases are exchanged with the capillaries entering the bloodstream to trigger airway inflammation (Lee et al., 2021). Air pollutants compose a mixture of PMs and gases.
Ambient air pollution, including ozone, nitric dioxide (NO2), sulfur dioxide (SO2), and PM is a serious problem to community health (Xie et al., 2016; Yin et al., 2017; McCormack et al., 2008).
Asthma is the most common chronic inflammatory airway disease, characterized by cardinal symptoms of breathlessness, wheezing, chest tightness, and cough, along with exaggerated expiratory airflow fluctuation that varies over time (Reddel et al., 2022). Chronic obstructive pulmonary disease (COPD) is a heterogeneous lung condition that presents chronic respiratory symptoms (dyspnea, cough, expectoration and/or exacerbations) caused by abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) leading to persistent, often progressive, airflow obstruction (Agustí et al., 2023).
Public health research has revealed that long-term exposure to high levels of air pollutants increases the development and progression of obstructive lung diseases (OLD), including asthma (Li et al., 2003) and COPD (Sint et al., 2008). Inhalation of PM2.5, ozone, NO2, and SO2 can traverse the nose, and enter the respiratory terminal unit after arriving at the lower respiratory tract (Cassee et al., 2002; Albert et al., 2002).
Accumulation of PM2.5 in lung alveoli leads to lung inflammation by promoting reactive oxygen species (ROS) production and cytokine secretion, such as interleukin (IL)-1 and IL-6 (Wang et al., 2018). Animal experiments have demonstrated that exposure to air pollutants results in a greater generation of pulmonary ROS and inflammation according to pollutants concentration (Jiang et al., 2018; Wang et al., 2019b). The increase in the quantity or variety of pollutants in the lungs gradually incites the symptoms of respiratory diseases like asthma and COPD.
Signal transduction is the process by which a substance obtained through an enzymatic process or substantial signal is carried through a cell via a series of molecular events, most generally protein phosphorylation catalyzed by protein kinases, leading to a cell-mediated response (Bradshaw and Dennis, 2010). The response can then alter the cell's shape, metabolism, and gene expression. The ligand binding in a receptor initiates a biochemical cascade known as a signaling pathway. The majority of signal transduction pathways involve the binding of signaling molecules to receptors that generate significant events inside the cell. The interaction of a signaling molecule with a receptor causes a modification in the conformation of the receptor. Most ligands like growth factors, cytokines, and neurotransmitters are soluble molecules in the extracellular medium that bind to cell surface receptors. Ligands binding to their receptors in important signaling pathways can cause a modification of second messengers and finally lead to altered cellular responses (Bradshaw and Dennis, 2010). Mitogen-activated protein kinase (MAPK) is one of several proteins in the cell that sends a signal from a receptor on the cell surface to the DNA in the cell nucleus (Orton et al., 2005).
Proteins and non-protein molecules like ions and phospholipids play important roles in a signaling pathway. In this review, we present the effect of air pollutants on signal transduction pathways in OLD.
Long-term diesel exhaust particles (DEPs) as PM 2.5 exposure may increase AHR, inflammation, lung fibrosis, and goblet cell hyperplasia in a mouse model (Kim et al., 2016). Moreover, co-exposure to ozone and DEP has an additive effect on airway hyperresponsiveness by modulation of IL-4 and interferon-gamma in a mouse model of asthma (Jang et al., 2005).
PM2.5 induce the production of ROS through the NOD-like receptor protein 3 (NLRP3) inflammasome, which triggers the activation of caspase-1 and the subsequent activation of IL-1β, which can cause airway inflammation (Borthwick, 2016; Xu et al., 2019; Jia et al., 2021; Liu et al., 2022a, 2022b). Exposure to PM2.5 decreases miR-331 expression through the ROS/phosphoinositide 3-kinases/Protein kinase B (ROS/PI3K/Akt) pathway, leading to the activation and increased expression of IκB kinase (IKK-β) and nuclear factor kappa B (NFκB) in human airway epithelial cells (Song et al., 2017). PM2.5 induces the transformation of macrophages into foam cells in the RAW264.7 cell line by upregulating the expression of the toll-like receptor 4/myeloid differentiation primary response 88/NFκB (TLR4/MyD88/NFκB) pathway; these activated macrophages cause airway inflammation (Geng et al., 2019; Guan et al., 2022).
Mucin 5AC (Muc5ac) is related to the pathophysiology, therapy, and prognosis of bronchial asthma. PM2.5 entering the airway can irritate and weaken or gradually destroy the bronchial wall, leading to Muc5ac gene activation and secretion. The Notch signaling pathway affects the Muc5ac secretion animal model of asthma, whose respiratory airways are exposed to PM2.5. Jagged1, Jagged2, Notch3, and Notch4 altered the expression of Hes1, which affects TNF-α cytokine when PM2.5 trigger airway secretion via Muc5ac (Liu et al., 2022c).
The lungs exposed to smoke and air pollutants produced ROS. Low levels of intracellular ROS regulate cell metabolism, and the NADPH oxidases act as signal transduction mediators by causing oxidative modifications of histones, enzymes, and transcription factors. Redox signaling is also controlled by ROS in mitochondria, the endoplasmic reticulum, and the inner side of the nucleus. Low levels of intracellular ROS are maintained through the enzymatic and non-enzymatic antioxidants (Michaeloudes et al., 2021). Wood smoke particle mediates airway epithelial responses through NFκB signaling, which has a direct influence over proinflammatory gene expression (Gupta et al., 2021).
PM2.5 increases the expression and activity of sirtuin 2 (SIRT2) in lung tissues. Subsequently, SIRT2 causes the phosphorylation and acetylation of p65, activation of the NF-κB signaling pathway, and leads to an increase in airway inflammation, mucus secretion by goblet cells, and moving faster tracheal anatomical change (Liu et al., 2021).
Benzo(a)pyrene (BaP) is an omnipresent air pollutant that can aggravate lung diseases. The over-secretion of airway mucus and Muc5ac as a result of air pollution is associated with aryl hydrocarbon receptor/mitochondrial ROS/extracellular signal-regulated kinase (ERK) pathway activation (Sun et al., 2021).
Treg cell-specific Notch1 receptor code affects different Treg cell-responses in allergic and autoimmune diseases. In individuals with asthma, Notch4, Wnt, and Hippo were upregulated in circulating Treg cells as a function of disease severity and were associated with reduced Treg cell-mediated suppression (Harb et al., 2020).
PM2.5 caused changes in the viable activity of human airway smooth muscle cells with altered secretion of kallikrein 14, bradykinin 2 receptor, bradykinin, and cytosol calcium, indicating that kallikrein plays an important role in PM2.5-induced airway hyperreactivity and inflammation (Cao et al., 2020). PM can affect an acute aggravation of airway diseases such as asthma and COPD, increasing the severity of symptoms like cough, shortness of breath, and mortality. PM can also heighten airway inflammation through the TLR2/NF-κB/NLRP3 signaling pathway (Dai et al., 2020; Morales-Rubio et al., 2022).
PM2.5 increase secretion of collagen 1, connective tissue growth factor, IL-6, and heme oxygenase-1 through the transforming growth factor-β1/hers against decapentaplegic homolog 3 (TGF-β1/Smad3) pathway, causing the progression of airway fibrosis in an animal model of asthma after exposure of PM2.5 and water-soluble components (Wu et al., 2021).
SO2 produces ROS and activates the TLR4/NF-κB pathway and can further augment IL-4 and IL-5 cytokine secretion and eosinophilic airway inflammation (Zhang et al., 2021a, 2021b). Nano-SiO2 particles in the animal model of asthma might synergistically activate IgE-sensitization of mast cells through the MAPK signaling pathway and ERK1/2 phosphorylation, and nano-SiO2 particles could incite airway inflammation (Yang et al., 2022).
Chronic Obstructive Pulmonary Disease (COPD)
COPD is a major global public health problem, and smoking is a major cause. Therefore cigarette smoke (CS)-associated with COPD needs to be studied for the mechanism and potential therapeutic targets (Wiegman et al., 2022). CS-induced oxidative stress plays a crucial role in the pathophysiology of COPD, characterized by bronchial obstruction, chronic airway inflammation, and alveolar destruction. Ozone is a gaseous air pollutant produced from the photochemical interaction of NO and organic compounds. Acute ozone exposure brings about airway responsiveness and neutrophilic inflammation. Chronic ozone exposure in mice activates oxidative pathways causing chronic lung inflammation and structural change of alveoli similar to those observed in CS-induced COPD (Wiegman et al., 2022).
CS activates cyclooxygenase-2 (COX-2), an inducible enzyme that synthesizes prostaglandin E2 (PGE2) and contributes to airway inflammation. Overexpression of the COX-2 gene is related to airway inflammation, invasion, metastasis, and epithelial-mesenchymal transition (EMT). CS extracts promoted EMT in airway epithelial cells through the COX-2/MMP/β-catenin pathway (Agraval et al., 2022; Vogelstein and Kinzler, 2004).
Exposure to PM2.5 contributes to airway remodeling as a key feature of COPD. The Wnt/β-catenin pathway activation can lead to airway remodeling. Exposure to PM2.5 induces the proliferation of human bronchial smooth muscle cells, contributing to airway remodeling through the Wnt5a/β-catenin signaling in vivo and in vitro, suggesting that the regulation of Wnt5a/β-catenin signaling might be a target for the treatment of COPD (Zou et al., 2021).
PM2.5 alters the normal structure and function of the airway epithelium, causing epithelial barrier dysfunction. Src homology domain 2-containing protein tyrosine phosphatase 2 (Shp2) has been implicated in respiratory diseases through the ERK1/2 MAPK signaling pathway (Zhang et al., 2021b).
PM2.5, with a large specific surface area, includes various organic matter, bacteria, heavy metals, and minerals and is an important factor in the development and progression of respiratory diseases such as asthma and COPD. The miR-140-5p/TLR4 signaling pathway, after exposure to PM2.5, mediated the inflammatory change in 16HBE cells. The differential expression and activation of various miRNA and TLR4/NF-κB signaling induced by PM2.5 exposure implicates PM2.5 in the pathogenesis of asthma and COPD (Chen et al., 2021).
Smoking is a major cause of COPD through various mechanisms, such as affecting the respiratory mucus-ciliary transport system, impairing the sensitivity of cough reflex, and inducing lung inflammation. The ROS produced by smoke can affect the membrane integrity and organelles in the airways and trigger a stress response with concomitant release of mitochondrial ROS, DNA, and proteases, leading to fibrosis (Wiegman et al., 2022).
ROS and DNA in mitochondria activate the NLRP3 inflammasome and can accelerate cell death pathways such as those involving caspases, resulting in airway inflammation, alveolar septa destruction, and lung fibrosis (Wiegman et al., 2022).
Hydrogen sulfide (H2S) affects the development of a variety of lung diseases. H2S treatment alleviated CS-induced COPD through inhibition of the TGF-β1/Smad pathway, improved lung function, and reduced histopathological changes and airway remodeling in a COPD model as a result of CS exposure (Wang et al., 2020). Airway remodeling, one of the key features of COPD, is associated with EMT in the small airways of smokers and patients with COPD. Sirtuin 1 (SIRT1) can reduce oxidative stress and modulate EMT. In one study, H2S prevented CS-induced airway remodeling in mice by changing oxidative stress and EMT, which was partially mitigated by SIRT1 activation (Guan et al., 2019).
PM2.5 and smoking are common contributors to COPD. The exposure of combined PM2.5 and CS/CSE induced pulmonary inflammation and Wnt5a expression in an experimental model using in vitro and in vivo. PM2.5 make worse CS/CSE-induced lung inflammation through the Wnt5a-ERK pathway in COPD (Wang et al., 2019b).
The enormous increase in diesel vehicles can cause serious health problems due to the production of DEPs. DEPs absorbed in the respiratory tract can indirectly lead to an increase in the expression of MUC5AC and MUC5B via TLR4, ERK1/2, p38 MAPK, and NF-κB signaling pathways in human airway epithelial cells (Na et al., 2019).
Biomass fuel smoke activates mucous cell metaplasia and mucus secretion, contributing to COPD. PM2.5 induce the expression of Muc5ac via epithelial growth factor receptor (EGFR)-ERK signaling, in an EGFR ligand-dependent mechanism (Huang et al., 2017).
Pyroptosis, a type of programmed cell death mediated by caspases-1 or -11, may play a crucial role in epithelial injury and airway remodeling, thereby aggravating or inducing asthma and COPD by TLR4/NF-κB pathway (Wang et al., 2022b). FGF10 inhibits oxidative stress-mediated pyroptosis via the PI3K/Akt/Nrf2 pathway (Liu et al., 2022b; Wang et al., 2022).
Air pollutants such as PM, ozone, NO2, SO2, and biological contaminants cause airway inflammation, remodeling, and fibrosis in the respiratory tract via various signal pathways (Fig. 1). These findings suggest that blocking certain signaling pathways can be a potential therapeutic target for airway diseases caused by pollutants, such as asthma and COPD.
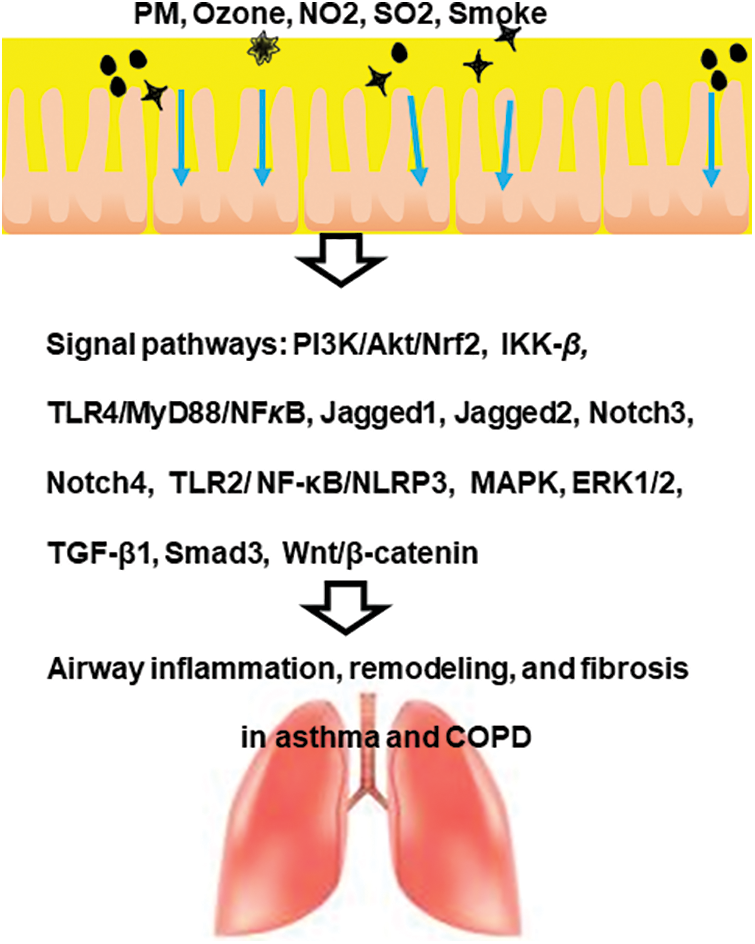
Figure 1: A scheme of the impact of air pollutants on signaling pathways in obstructive lung diseases such as asthma and chronic obstructive pulmonary disease (COPD).
Acknowledgement: I thank the constant support provided by SoonChunHyang University (Korea) for this study.
Funding Statement: I acknowledge the funding provided by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2020R1A2C1006506).
Author Contributions: The author confirms sole responsibility for the following: article conception and manuscript preparation.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The author declares that they have no conflicts of interest to report regarding the present study.
References
Agraval H, Sharma JR, Prakash N, Yadav UCS (2022). Fisetin suppresses cigarette smoke extract-induced epithelial to mesenchymal transition of airway epithelial cells through regulating COX-2/MMPs/β-catenin pathway. Chemico-Biological Interactions 351: 109771. https://doi.org/10.1016/j.cbi.2021.109771 [Google Scholar] [PubMed] [CrossRef]
Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A et al. (2023). Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. The European Respiratory Journal 61: 2300239. https://doi.org/10.1183/13993003.00239-2023 [Google Scholar] [PubMed] [CrossRef]
Alberts B, Lewis J, Raff M, Roberts K, Walter P (2002). Molecular biology of the cell (4th ed.). New York: Garland Science. [Google Scholar]
Borthwick LA (2016). The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Seminars in Immunopathology 38: 517–534. https://doi.org/10.1007/s00281-016-0559-z [Google Scholar] [PubMed] [CrossRef]
Bradshaw RA, Dennis EA (2010). Handbook of Cell Signaling (2nd ed.). Amsterdam, Netherlands: Academic Press. [Google Scholar]
Cao X, Wang M, Li J, Luo Y, Li R, Yan X, Zhang H (2020). Fine particulate atter increases airway hyperresponsiveness through kallikrein-bradykinin pathway. Ecotoxicology and Environmental Safety 195: 110491. https://doi.org/10.1016/j.ecoenv.2020.110491 [Google Scholar] [PubMed] [CrossRef]
Cassee FR, Muijser H, Duistermaat E, Freijer JJ, Geerse KB, Marijnissen JC, Arts JH (2002). Particle size-dependent total mass deposition in lungs determines inhalation toxicity of cadmium chloride aerosols in rats. Application of a multiple path dosimetry model. Archives of Toxicology 76: 277–286. https://doi.org/10.1007/s00204-002-0344-8 [Google Scholar] [PubMed] [CrossRef]
Chen X, Deng T, Huo T, Dong F, Deng J (2021). MiR-140-5p/TLR4/NF-κB signaling pathway: Crucial role in inflammatory response in 16HBE cells induced by dust fall PM2.5. Ecotoxicology and Environmental Safety 208: 111414. https://doi.org/10.1016/j.ecoenv.2020.111414 [Google Scholar] [PubMed] [CrossRef]
Dai MY, Chen FF, Wang Y, Wang MZ, Lv YX, Liu RY (2020). Particulate matters induce acute exacerbation of allergic airway inflammation via the TLR2/NF-κB/NLRP3 signaling pathway. Toxicology Letters 321: 146–154. https://doi.org/10.1016/j.toxlet.2019.12.013 [Google Scholar] [PubMed] [CrossRef]
Geng J, Liu H, Ge P, Hu T, Zhang Y, Zhang X, Xu B, Wang B, Xie J (2019). PM2.5 promotes plaque vulnerability at different stages of atherosclerosis and the formation of foam cells via TLR4/MyD88/NFκB pathway. Ecotoxicology and Environmental Safety 176: 76–84. https://doi.org/10.1016/j.ecoenv.2019.03.068 [Google Scholar] [PubMed] [CrossRef]
Guan Y, Li L, Kan L, Xie Q (2022). Inhalation of salvianolic acid B prevents fine particulate matter-induced acute airway inflammation and oxidative stress by downregulating the LTR4/MyD88/NLRP3 pathway. Oxidative Medicine and Cellular Longevity 2022: 5044356. https://doi.org/10.1155/2022/5044356 [Google Scholar] [PubMed] [CrossRef]
Guan R, Wang J, Cai Z, Li Z, Wang L et al. (2019). Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biology 28: 101356. https://doi.org/10.1016/j.redox.2019.101356 [Google Scholar] [PubMed] [CrossRef]
Gupta A, Sasse SK, Gruca MA, Sanford L, Dowell RD, Gerber AN (2021). Deconvolution of multiplexed transcriptional responses to wood smoke particles defines rapid aryl hydrocarbon receptor signaling dynamics. Journal of Biological Chemistry 297: 101147. https://doi.org/10.1016/j.jbc.2021.101147 [Google Scholar] [PubMed] [CrossRef]
Harb H, Stephen-Victor E, Crestani E, Benamar M, Massoud A et al. (2020). A regulatory T cell Notch4-GDF15 axis licenses tissue inflammation in asthma. Nature Immunology 21: 1359–1370. https://doi.org/10.1038/s41590-020-0777-3 [Google Scholar] [PubMed] [CrossRef]
Huang L, Pu J, He F, Liao B, Hao B et al. (2017). Positive feedback of the amphiregulin-EGFR-ERK pathway mediates PM2.5 from wood smoke-induced MUC5AC expression in epithelial cells. Scientific Reports 7: 11084. https://doi.org/10.1038/s41598-017-11541-1 [Google Scholar] [PubMed] [CrossRef]
Jang AS, Choi IS, Takizawa H, Rhim T, Lee JH, Park SW, Park CS (2005). Additive effect of diesel exhaust particulates and ozone on airway hyperresponsiveness and inflammation in a mouse model of asthma. Journal of Korean Medical Science 20: 759–763. https://doi.org/10.3346/jkms.2005.20.5.759 [Google Scholar] [PubMed] [CrossRef]
Jia H, Liu Y, Guo D, He W, Zhao L, Xia S (2021). PM2.5-induced pulmonary inflammation via activating of the NLRP3/caspase-1 signaling pathway. Environmental Toxicology 36: 298–307. https://doi.org/10.1002/tox.23035 [Google Scholar] [PubMed] [CrossRef]
Jiang S, Zhou J, Zhang J, Du X, Zeng X et al. (2018). The severity of lung injury and metabolic disorders induced by ambient PM2.5 exposure is associated with cumulative dose. Inhalation Toxicology 30: 239–246. https://doi.org/10.1080/08958378.2018.1508258 [Google Scholar] [PubMed] [CrossRef]
Kim BG, Lee PH, Lee SH, Kim YE, Shin MY et al. (2016). Long-term effects of diesel exhaust particles on airway inflammation and remodeling in a mouse model. Allergy Asthma and Immunological Research 8: 246–256. https://doi.org/10.4168/aair.2016.8.3.246 [Google Scholar] [PubMed] [CrossRef]
Lee PH, Park S, Lee YG, Choi SM, An MH, Jang AS (2021). The impact of environmental pollutants on barrier dysfunction in respiratory disease. Allergy Asthma and Immunological Research 13: 850–862. https://doi.org/10.4168/aair.2021.13.6.850 [Google Scholar] [PubMed] [CrossRef]
Li N, Hao M, Phalen RF, Hinds WC, Nel AE (2003). Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clinical Immunology 109: 250–265. https://doi.org/10.1016/j.clim.2003.08.006 [Google Scholar] [PubMed] [CrossRef]
Liu K, Hua S, Song L (2022a). PM2.5 exposure and asthma development: The key role of oxidative stress. Oxidative Medicine and Cellular Longevity 2022: 1–12. https://doi.org/10.1155/2022/3618806 [Google Scholar] [PubMed] [CrossRef]
Liu L, Shi Q, Wang K, Qian Y, Zhou L, Bellusci S, Chen C, Dong N (2022b). Fibroblast growth factor 10 protects against particulate matter-induced lung injury by inhibiting oxidative stress-mediated pyroptosis via the PI3K/Akt/Nrf2 signaling pathway. International Immunopharmacology 113: 109398. https://doi.org/10.1016/j.intimp.2022.109398 [Google Scholar] [PubMed] [CrossRef]
Liu M, Shi Z, Yin Y, Wang Y, Mu N, Li C, Ma H, Wang Q (2021). Particulate matter 2.5 triggers airway inflammation and bronchial hyperresponsiveness in mice by activating the SIRT2-p65 pathway. Frontiers in Medicine 15: 750–766. https://doi.org/10.1007/s11684-021-0839-4 [Google Scholar] [PubMed] [CrossRef]
Liu Y, Zhou L, Wu H, Wang Y, Danzengluobu, Zhang B (2022c). Role of notch signaling pathway in Muc5ac secretion induced by atmospheric PM2.5 in rats. Ecotoxicology and Environment Safety 229: 113052. https://doi.org/10.1016/j.ecoenv.2021.113052 [Google Scholar] [PubMed] [CrossRef]
McCormack MC, Breysse PN, Hansel NN, Matsui EC, Tonorezos ES, Curtin-Brosnan J, Williams DL, Buckley TJ, Eggleston PA, Diette GB (2008). Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environmental Research 106: 148–155. https://doi.org/10.1016/j.envres.2007.08.012 [Google Scholar] [PubMed] [CrossRef]
Michaeloudes C, Abubakar-Waziri H, Lakhdar R, Raby K, Dixey P, Adcock IM, Mumby S, Bhavsar PK, Chung KF (2021). Molecular mechanisms of oxidative stress in asthma. Molecular Aspects of Medicine 85: 101026. https://doi.org/10.1016/j.mam.2021.101026 [Google Scholar] [PubMed] [CrossRef]
Morales-Rubio R, Amador-Muñoz O, Rosas-Pérez I, Sánchez-Pérez Y, García-Cuéllar C, Segura-Medina P, Osornio-Vargas Á., de Vizcaya-Ruiz A (2022). PM2.5 induces airway hyperresponsiveness and inflammation via the AhR pathway in a sensitized Guinea pig asthma-like model. Toxicology 465: 153026. https://doi.org/10.1016/j.tox.2021.153026 [Google Scholar] [PubMed] [CrossRef]
Na HG, Kim YD, Choi YS, Bae CH, Song SY (2019). Diesel exhaust particles elevate MUC5AC and MUC5B expression via the TLR4-mediated activation of ERK1/2, p38 MAPK, and NF-κB signaling pathways in human airway epithelial cells. Biochemical and Biophysical Research Communications 512: 53–59. https://doi.org/10.1016/j.bbrc.2019.02.146 [Google Scholar] [PubMed] [CrossRef]
Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W (2005). Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochemical Journal 392: 249–261. https://doi.org/10.1042/BJ20050908 [Google Scholar] [PubMed] [CrossRef]
Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG et al. (2022). Global initiative for asthma strategy 2021: Executive summary and rationale for key changes. American Journal of Respiratory and Critical Care Medicine 205:17–35. https://doi.org/10.1164/rccm.202109-2205PP [Google Scholar] [PubMed] [CrossRef]
Sint T, Donohue JF, Ghio AJ (2008). Ambient air pollution particles and the acute exacerbation of chronic obstructive pulmonary disease. Inhalation Toxicology 20: 25–29. https://doi.org/10.1080/08958370701758759 [Google Scholar] [PubMed] [CrossRef]
Song L, Li D, Li X, Ma L, Bai X, Wen Z, Zhang X, Chen D, Peng L (2017). Exposure to PM2.5 induces aberrant activation of NF-κB in human airway epithelial cells by downregulating miR-331 expression. Environmental Toxicology and Pharmacology 50: 192–199. https://doi.org/10.1016/j.etap.2017.02.011 [Google Scholar] [PubMed] [CrossRef]
Sun Y, Shi Z, Lin Y, Zhang M, Liu J et al. (2021). Benzo(a)pyrene induces MUC5AC expression through the AhR/mitochondrial ROS/ERK pathway in airway epithelial cells. Ecotoxicology and Environment Safety 210: 111857. https://doi.org/10.1016/j.ecoenv.2020.111857 [Google Scholar] [PubMed] [CrossRef]
Vogelstein B, Kinzler KW (2004). Cancer genes and the pathways they control. Nature Medicine 10: 789–799. https://doi.org/10.1038/nm1087 [Google Scholar] [PubMed] [CrossRef]
Wang L, Meng J, Wang C, Wang Y, Yang C, Li Y (2022). Hydrogen sulfide attenuates cigarette smoke-induced pyroptosis through the TLR4/NF-κB signaling pathway. International Journal of Molecular Medicine 49: 56. https://doi.org/10.3892/ijmm.2022.5112 [Google Scholar] [PubMed] [CrossRef]
Wang L, Meng J, Wang C, Yang C, Wang Y et al. (2020). Hydrogen sulfide alleviates cigarette smoke-induced COPD through inhibition of the TGF-β1/smad pathway. Experimental Biology and Medicine 245: 190–200. https://doi.org/10.1177/1535370220904342 [Google Scholar] [PubMed] [CrossRef]
Wang H, Shen X, Liu J, Wu C, Gao J, Zhang Z, Zhang F, Ding W, Lu Z (2019a). The effect of exposure time and concentration of airborne PM2.5 on lung injury in mice: A transcriptome analysis. Redox Biology 26: 101264. https://doi.org/10.1016/j.redox.2019.101264 [Google Scholar] [PubMed] [CrossRef]
Wang H, Shen X, Tian G, Shi X, Huang W et al. (2018). AMPKα2 deficiency exacerbates long-term PM2.5 exposure-induced lung injury and cardiac dysfunction. Free Radical Biology and Medicine 121: 202–214. https://doi.org/10.1016/j.freeradbiomed.2018.05.008 [Google Scholar] [PubMed] [CrossRef]
Wang Z, Zhao J, Wang T, Du X, Xie J (2019b). Fine-particulate matter aggravates cigarette smoke extract-induced airway inflammation via Wnt5a-ERK pathway in COPD. International Journal of Chronic Obstructive Pulmononary Disease 14: 979–994. https://doi.org/10.2147/COPD.S195794 [Google Scholar] [PubMed] [CrossRef]
Wiegman CH, Li F, Ryffel B, Togbe D, Chung KF (2022). Oxidative stress in ozone-induced chronic lung inflammation and emphysema: A facet of chronic obstructive pulmonary disease. Frontiers in Immunology 11: 1957. https://doi.org/10.3389/fimmu.2020.01957 [Google Scholar] [PubMed] [CrossRef]
Wu H, Wang D, Shi H, Liu N, Wang C, Tian J, Wang X, Zhang Z (2021). PM2.5 and water-soluble components induce airway fibrosis through TGF-β1/Smad3 signaling pathway in asthmatic rats. Molecular Immunology 137: 1–10. https://doi.org/10.1016/j.molimm.2021.06.005 [Google Scholar] [PubMed] [CrossRef]
Xie Y, Dai H, Dong H, Hanaoka T, Masui T (2016). Economic impacts from PM2.5 pollution-related health effects in China: A provincial-level analysis. Environmental Science and Technology 50: 4836–4843. https://doi.org/10.1021/acs.est.5b05576 [Google Scholar] [PubMed] [CrossRef]
Xu Z, Ding W, Deng X (2019). PM2.5, fine particulate matter: A novel player in the epithelial-mesenchymal transition? Frontiers in Physiology 10: 1404. https://doi.org/10.3389/fphys.2019.01404 [Google Scholar] [PubMed] [CrossRef]
Yang YS, Cao MD, Wang A, Liu QM, Zhu DX et al. (2022). Nano-silica particles synergistically IgE-mediated mast cell activation exacerbating allergic inflammation in mice. Frontiers in Immunology 13: 911300. https://doi.org/10.3389/fimmu.2022.911300 [Google Scholar] [PubMed] [CrossRef]
Yin H, Pizzol M, Xu L (2017). External costs of PM2.5 pollution in Beijing, China: Uncertainty analysis of multiple health impacts and costs. Environmental Pollution 226: 356–369. https://doi.org/10.1016/j.envpol.2017.02.029 [Google Scholar] [PubMed] [CrossRef]
Zhang L, Yi H, Sang N (2021a). Sulfur dioxide-induced exacerbation of airway inflammation via reactive oxygen species production and the toll-like receptor 4/nuclear factor-κB pathway in asthmatic mice. Toxicology and Industrial Health 37: 564–572. https://doi.org/10.1177/07482337211033136 [Google Scholar] [PubMed] [CrossRef]
Zhang Y, Zhang L, Chen W, Zhang Y, Wang X, Dong Y, Zhang W, Lin X (2021b). Shp2 regulates PM2.5-induced airway epithelial barrier dysfunction by modulating ERK1/2 signaling pathway. Toxicological Letters 350: 62–70. https://doi.org/10.1016/j.toxlet.2021.07.002 [Google Scholar] [PubMed] [CrossRef]
Zou W, Wang X, Sun R, Hu J, Ye D, Bai G, Liu S, Hong W, Guo M, Ran P (2021). PM2.5 induces airway remodeling in chronic obstructive pulmonary diseases via the Wnt5a/β-catenin pathway. International Journal of Chronic Obstructive Pulmononary Disease 16: 3285–3295. https://doi.org/10.2147/COPD.S334439 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools