 Open Access
Open Access
ARTICLE
Photodynamic therapy with TBZPy regulates the PI3K/AKT and endoplasmic reticulum stress-related PERK/eIF2α pathways in HeLa cells
1 The Second Clinical Medical College of Lanzhou University, Lanzhou University Second Hospital, Lanzhou, 730000, China
2 Department of Dermatology, Lanzhou University Second Hospital, Lanzhou, 730000, China
3 Department of Dermatology and Venereology, Guangdong Women’s and Children’s Hospital, Southern Medical University, Guangzhou, 510000, China
* Corresponding Authors: ZHIWEN ZHANG. Email: ; MUZHOU TENG. Email:
BIOCELL 2023, 47(8), 1783-1791. https://doi.org/10.32604/biocell.2023.028056
Received 28 November 2022; Accepted 06 May 2023; Issue published 28 August 2023
Abstract
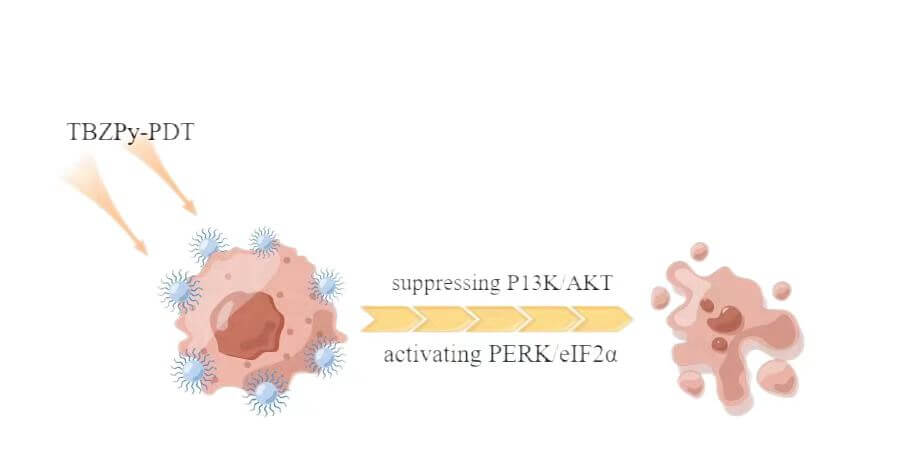
Background: ((1-triphenylaminebenzo[c][1,2,5] thiadiazole-4-yl) styryl)-1-methylpyridin methylpyridin-1-ium iodide salt (TBZPy) is a novel photosensitizer that displays excellent photodynamic properties. However, There are few reports on the mechanism of action of the TBZPy photodynamic. Previous studies revealed that photodynamic therapy (PDT) could induce endoplasmic reticulum stress by acting on the endoplasmic reticulum. Therefore, in this study, we investigated the effects of endoplasmic reticulum stress induced by TBZPy-PDT in treating High-risk human papillomavirus (HR-HPV) infection and their underlying mechanisms. Methods: The human cervical cancer cell line HeLa (containing whole genome of HR-HPV18) was treated with TBZPy-PDT. Cell migration, invasion, and colony-forming ability were evaluated using wound-healing, Transwell invasion, and colony-forming assays, respectively. Through western blot analysis, we determined the level of expression of the PI3K/AKT and PERK/eIF2α pathway proteins and the proteins associated with calcium trafficking and apoptosis. The calcium levels in the cytoplasm were detected via flow cytometry. Results: The result shows that TBZPy-PDT could inhibite the migration, invasion, and colony forming ability of infected HeLa cells by downregulating the PI3K/AKT pathway in vitro. And we found that TBZPy-PDT induced endoplasmic reticulum stress-specific apoptosis via the PERK/eIF2α pathway. Moreover, TBZPy-PDT increased the levels of calcium and calmodulin, while decreasing the levels of endoplasmic reticulum calcium-binding proteins. Conclusions: TBZPy-PDT is effective on treating human papillomavirus-infected cells. Targeting the PI3K/AKT and PERK/eIF2α pathways and the endoplasmic reticulum stress process may help improve the effects of TBZPy-PDT for treating high-risk human papillomavirus infection.Graphic Abstract
Keywords
Cervical cancer is one of the most common cancer types among women. Persistent infection with a high-risk HPV type is a significant risk factor for cervical cancer. HPV-infected individuals are also at risk of developing cervical intraepithelial neoplasia (CIN), which may develop into cervical cancer if left untreated. CIN is typically treated by invasive methods, including large-loop excision of the transformation zone (LEEP/LLETZ), cold knife conization(CKC), laser evaporation, and cryotherapy. However, these treatments carry serious adverse effects such as bleeding, endometriosis, cervical stenosis, and an increased risk of miscarriage. Photodynamic therapy (PDT) has emerged as a highly-selective and non-invasive alternative for CIN treatment; with a low risk of severe systemic complications after treatment, and preservation of reproductive function (Istomin et al., 2010; Unanyan et al., 2021). The efficiency of PDT is directly linked to the efficacy of photosensitizers used in the treatment (Li et al., 2021b). Photosensitizers can selectively accumulate in target lesions, and when excited by laser irradiation, produce reactive oxygen species that cause cytotoxic effects on target cancer cells (Casas, 2020).
Traditional photosensitizers, such as cyanine compounds and porphyrins, were shown to be effective in treatment (Gunaydin et al., 2021; Malacarne et al., 2021). However, some traditional photosensitizers undergo aggregation-induced quenching at high concentrations or are present in an aggregated state in aqueous media, which sharply decreases the photochemical efficiency and significantly weakens the single oxygen generation ability (Hu et al., 2018). In contrast, the aggregation-induced luminescence effect is a phenomenon in which the fluorescence is significantly increased in an aggregated or solid state (Chen et al., 2016). A photosensitizer with aggregation-induced luminescence can produce stronger fluorescence emission (Wan et al., 2020). TBZPy has been recently was developed as a novel photosensitizer with aggregation-induced emission ability. When applied to cancer or CIN models in vitro and in vivo, TBZPy showed good fluorescence emission, produced high levels of reactive oxygen species, and exerted strong cytotoxicity (Li et al., 2022). We previously reported that the use of TBZPy-PDT leads to a reduction in mitochondrial membrane potential, cell viability, and the induction of apoptosis in HeLa cells (Li et al., 2021a). However, the specific mechanisms underlying the effects of TBZPy are still not fully understood.
Endoplasmic reticulum Stress (ERS) has been regarded as a critical mechanism in PDT-mediated cell death (Chen et al., 2020; He et al., 2020), and is required to induce immunogenic cell death (Gomes-da-Silva et al., 2018). The endoplasmic reticulum plays vital roles in protein folding, lipogenesis, and the maintenance of calcium homeostasis. When the endoplasmic reticulum is exposed to stimuli such as hypoxia, oxidative stress, and variations in calcium concentrations, adaptive responses called ERS are initiated (Cao et al., 2021a; Das et al., 2021). Accumulation of unfolded or misfolded proteins in the lumen of the reticulum can induce an unfolded protein stress response. ERS regulates cell proliferation and apoptosis via several pathways, including the protein kinase-like endoplasmic reticulum kinase (PERK) pathway (Cao et al., 2021b).
The endoplasmic reticulum is also a major store of calcium ions (calcium) in cells. Stressors such as reactive oxygen species or ultraviolet radiation may cause calcium release calcium from the reticulum, which subsequently affects cell proliferation and induces apoptosis. Calcium release inhibits the function of calcium-dependent chaperones and thus leads to abnormal accumulation of misfolded or unfolded proteins in the lumen, and further increases the stress on the organelle (Nyberg and Espinosa, 2016; Zhang et al., 2019). Thus, the endoplasmic reticulum mediates inter- and intra-cellular signal transduction by modifying the cytoplasmic calcium concentration and an increase in calcium concentration leads to the activation of downstream signaling pathways (Yong et al., 2021).
The phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway is an essential cell signaling pathway involved in various physiological conditions in mammals. Activation of the PI3K/AKT pathway was previously shown to inhibit ERS and cell apoptosis. In contrast, PERK phosphorylation triggered by PI3K/AKT pathway activation was shown to inhibit the phosphorylation of eIF2α. The phosphorylation of PERK-eIF2α, in turn, inhibits Akt activity.
Here, we investigated the mechanisms involved in the effect of TBZPy-PDT on HeLa cells. Specifically, we aimed to clarify the ERS mechanism upon application of TBZPy-PDT and to assess its potential for clinical use in treating CIN and cervical cancer.
HeLa cells, kindly provided by Dr. Chun Cai Gu (Nan Fang Hospital, Southern Medical University, Guangzhou, China). HeLa cells were incubated in RPMI 1640 medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA). HeLa cells, and cell culture reagents were prepared following protocols described previously. Fluo-4 AM and Apoptosis Analysis Kit were provided by Beyotime (Shanghai, China); BD Matrigel™ Basement Membrane Matrix (No. 356234) was purchased from BD Biosciences (New Jersey, USA). Anti-BIP, (1:3000), anti-caspase-12 (1:2000), and anti-caspase-3 (1:1000) were purchased from Proteintech (Wuhan, China). Anti-CHOP, (1:2000) and anti-β-actin (1:1000) were obtained from Cell Signaling Technology (Danvers, MA, USA). TBZPy was synthesized using a previously published method.
HeLa cells were seeded in 6-well plates. Groups of cells were as follows: control (no treatment), light (400–600 nm, light power 30 mW, 5 min), TBZPy, and TBZPy-PDT (TBZPy+Light). TBZPy was first applied to cells in the TBZPy and TBZPy-PDT groups at a final concentration of 10 µM for 24 h. Next, TBZPy-PDT and laser groups were irradiated with a LED light source. After discarding the PBS, HeLa cells were cultured for 24 h in the dark.
The cells were placed in control or TBZPy-PDT groups. After the corresponding treatments, HeLa cells were suspended and collected. The cell count was determined, 2 × 103/mL HeLa cells and 200 µL of cell suspension were added to each well in a 96-well plate (400 cells/well), with 300 µL of culture medium. The plates were cultured at 37°C in a humidified incubator containing 5% CO2. After colonies were formed from a single HeLa cell after 10 days, the culture was rinsed with Phosphate Buffer Saline (Beyotime, Shanghai, China), and 200 µL crystal violet dye solution (Beyotime, Shanghai, China) was added to each well. After 20 min, the 96-well plates were washed with slow-flowing tap water and dried. Images of clones formed were taken using an enzyme-linked immunospot instrument (Autoimmun Diagnostika, Strassberg, Germany).
First, serum-free IMDM (HyClone, Logan, UT, USA) was diluted with Matrigel at a volume ratio of 1:3. Then, 40 μl of diluted Matrigel was added to the Transwell chamber(Corning, NY, USA). HeLa cells (density of approximately 1 × 106 mL) were digested and placed into a single-cell suspension. Following the determination of the cell density, 1 × 105 cells were extracted. The cells were suspended in 100 μL serum-free IMDM medium, and added to the middle upper chamber, while 600 μL IMDM complete medium was added into the lower chamber. HeLa cells were removed from the upper chamber using cotton swabs 12 h after treatment. The cells were fixed with 4% paraformaldehyde for 15 min and washed once with PBS. The chamber was stained with 0.1% crystal violet for 20 min and cleaned with PBS. Images of cell invasion were taken under a light microscope. Five fields were randomly selected for cell count analysis and comparison.
Wound healing assay and assessment of cell migration rate
Four groups of cells were established: control, Light, TBZPy, and TBZPy-PDT. HeLa cells were seeded into 6-well plates. After scratching the cell layer with the head of the aseptic gun, each group was treated as described in the section “PhotoDynamic Therapy”. After 24 h, wound healing was monitored using an inverted fluorescence microscope. The scratch width of eight sites in each group was measured using Image Pro-Plus software (Image-Pro Plus, v 6.0).
First, HeLa cells were incubated with the photosensitizer, (TBZPy) for 24 h to determine the intracellular localization of TBZPy. Subsequently, ER-TrackerTM green (Invitrogen, Paisley, UK) at a concentration of 500 nmol/L was used to label the intracellular localization of the endoplasmic reticulum. Finally, DAPI (Beyotime, Shanghai, China) was used to stain the nuclei. Images of stained cells were taken using laser confocal microscopy (DMI6000B; Leica, Wetzlar, Germany).
Cells in control, Light-, TBZPy-, and TBZPy-PDT groups were seeded in 6-well plates at 3 × 105 cells/well concentration, and cultured overnight. After washing with PBS, the cells were collected, stained with an Annexin V-FITC/PI apoptosis assay kit (Becton Dickinson, New Jersey, USA), and analyzed by flow cytometry (BD Biosciences, New Jersey, USA).
This assay was performed according to a previously published protocol. Four groups of cells were established: control, Light, TBZPy, and TBZPy-PDT. Collected cells were placed in a radioimmunoprecipitation assay buffer (Beyotime, Shanghai, China). First, proteins measured using a bicinchoninic acid assay kit (BOSTER, Wuhan, China) were isolated by electrophoresis on a 10% sodium dodecyl sulfate-polyacrylamide gel (Thermofisher, MA, USA). The proteins were then transferred onto polyvinylidene difluoride membranes (Beyotime, Shanghai, China). Next, the membranes were blocked with 5% defatted milk, and then incubated with primary antibodies and gently shaken overnight at 4°C. Then, membranes were washed with PBS three times and further incubated with the 1:5000 diluted HRP-coupled secondary antibody for 1 h. Finally, the protein bands were visualized and analyzed using ImageJ software (version 2.0.0).
Fluo-4 AM calcium (Thermofisher, MA, USA) indicator was diluted with PBS to a concentration of 2 µM. HeLa cells were collected after each treatment and washed three times with PBS. Fluo-4 AM (200 µL) was added to the working solution, and HeLa cells were incubated at 37°C for 30 min. Each group was washed three times with PBS. Fluorescence (488 nm) was detected using flow cytometry (BD Biosciences, New Jersey, USA).
Results were expressed as average ± SD. All experiments were repeated three times. SPSS (version 20.0) was used for statistical analysis. Student’s t-test was used to compare the means of two groups of experimental data. p < 0.05 was considered statistically significant.
TBZPy-PDT inhibited migration, invasion, and colony formation activities of HeLa cells
We first examined the effects of TBZPy-PDT on the migration, invasion, and colony formation activities of HeLa cells, which are infected with HR-HPV18. HeLa cells were incubated with TBZPy (5 µM) for 24 h and then irradiated. The migration abilities of HeLa cells were evaluated using a wound healing assay. As shown in Figs. 1A and 1B, TBZPy-PDT significantly reduced the wound closure rate compared to the control (NC) group. The invasion abilities of cells were assessed using the Transwell® invasion assay. The number of HeLa cells that penetrated the Matrigel was significantly lower in the TBZPy-PDT group than that in the NC group (Fig. 1C), indicating that TBZPy-PDT attenuated the invasion ability of HeLa cells. The colony-forming abilities of cells were measured using a colony-forming assay. TBZPy-PDT also markedly inhibited the colony formation of HeLa cells (Figs. 1D and 1E). These results suggest that TBZPy-PDT suppressed the migration, invasion, and colony formation activities of HeLa cells.
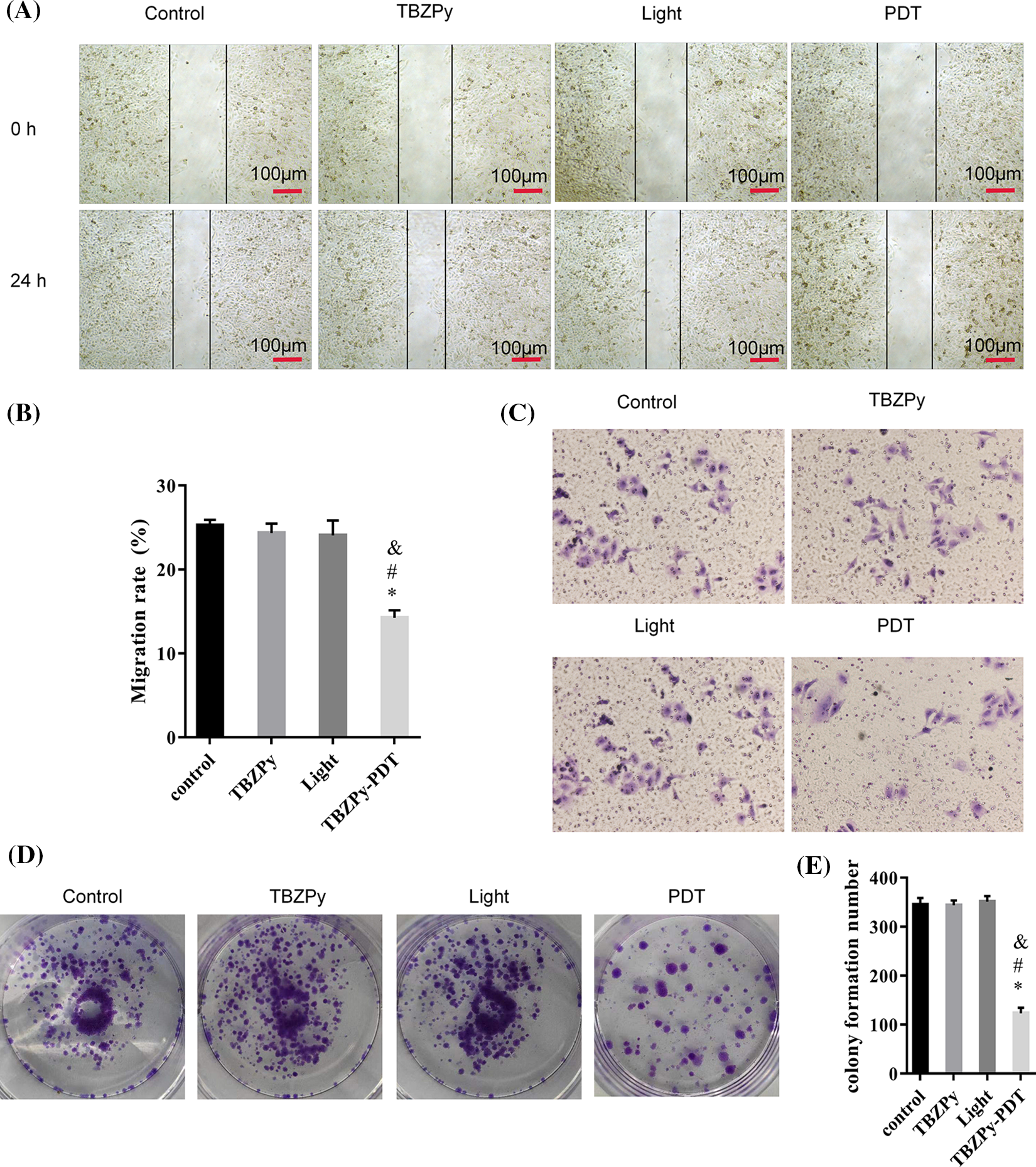
Figure 1: TBZPy-PDT inhibited the migration, invasion, and colony-forming activities of HeLa cells. (A) Representative images of wound-healing in HeLa cells at 0 and 24 h. HeLa cells were divided into four groups: control, TBZPy, Light, and TBZPy-PDT. (B) Quantification of cell migration rates in the four groups at 24 h (mean ± SD, n = 3). Migration rate (%) = (A0–An)/A0 × 100, where A0 is the initial wound width and An is the width at the metering point. (C) Representative images of HeLa cell invasion in the four groups at 24 h. (D) Images showing colony formation. (E) Quantification of colony formation in the above groups (mean ± SD, n = 3). *p < 0.05 compared with the control group; #p < 0.05 compared with the TBZPy group; &p < 0.05 compared with the Light group.
TBZPy-PDT suppressed the PI3K/AKT pathway in HeLa cells
We next investigated the effects of TBZPy-PDT on the PI3K/AKT pathway, which plays a critical role in the proliferation, invasion, and migration of cells (Jiang et al., 2020; Liu et al., 2021; Xie et al., 2019). Moreover, the PI3K/AKT pathway acts as an upstream mechanism mediating the ER stress and PERK/eIF2α signaling pathways, which are involved in TBZPy-PDT-induced apoptosis (Mounir et al., 2011). Therefore, we explored the effects of TBZPy-PDT on key downstream signaling proteins, including p-PI3K, PI3K, p-AKT, and AKT in HeLa cells by western blot analysis. As shown in Figs. 2A and 2B, TBZPy-PDT significantly decreased the expression levels of p-PI3K and p-AKT compared to the NC group. In addition, knockdown of PI3K significantly increased the apoptotic rates in HeLa cells (Fig. 2C). These results indicate that TBZPy-PDT attenuated the PI3K/AKT pathway in HeLa cells, which led to promotion cell apoptosis and inhibition of ER stress and PERK/eIF2α signaling pathways.
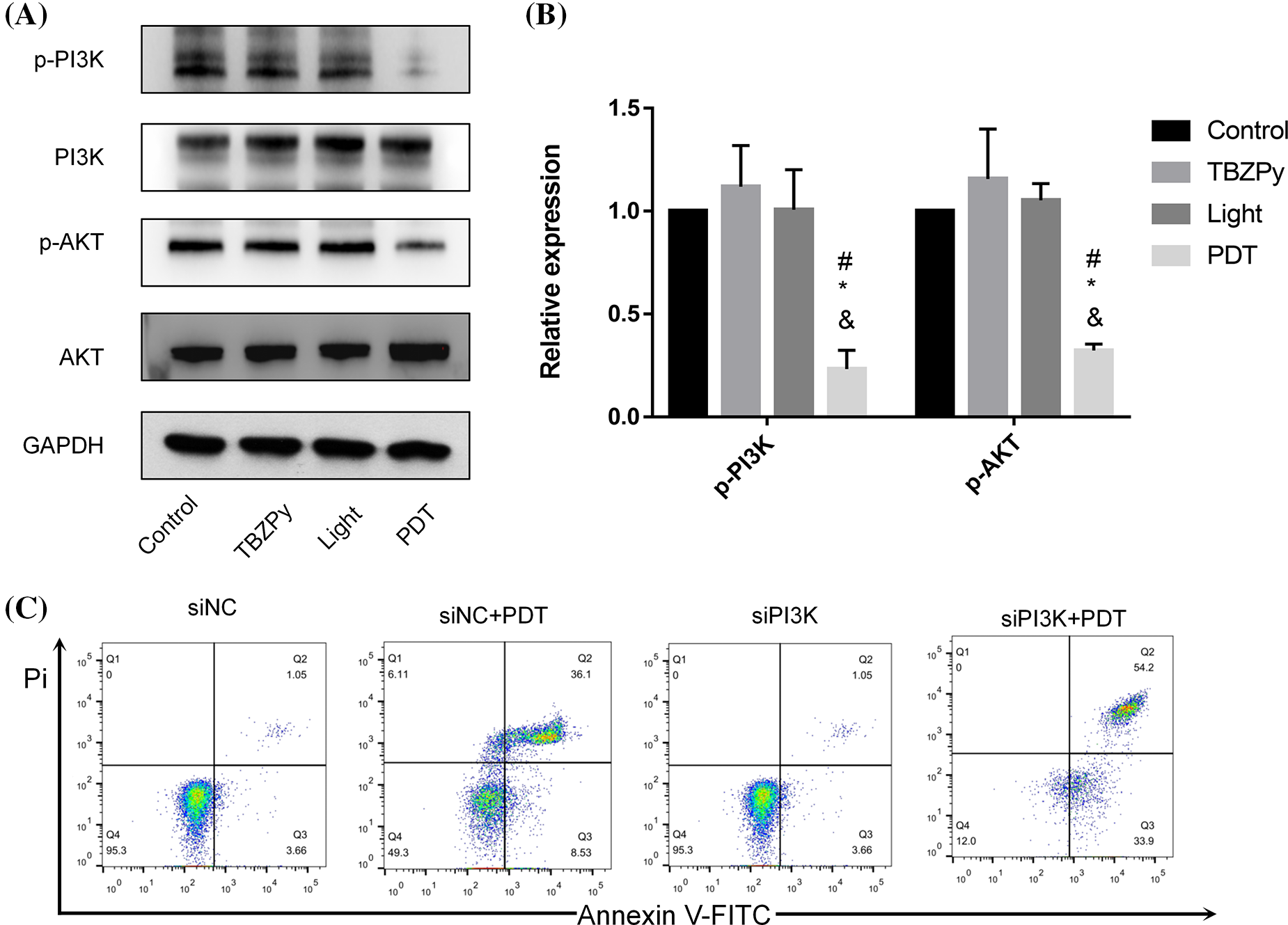
Figure 2: (TBZPy-PDT) suppressed (PI3K/AKT) pathways in HeLa cells. (A) Expression levels of the PI3K, p-PI3K, AKT, and p-AKT in the control, TBZPy, Light, and TBZPy-PDT groups. (B) Quantification of expression levels of the p-PI3K, p-AKT, proteins in the four groups. *p < 0.05 compared with the control group; #p < 0.05 compared with the TBZPy group; &p < 0.05 compared with the Light group. (C) Flow cytometry profile of apoptotic rates in HeLa cells with or without PI3K knockdown.
TBZPy-PDT triggered ERS via the PERK/eIF2α signaling pathway
We then examined the effects of TBZPy-PDT on ER stress, which is a cellular response to various stimuli that disrupt ER homeostasis and cause accumulation of unfolded or misfolded proteins in the ER lumen (Grebenová et al., 2003). ER stress activates three major signaling pathways: PERK/eIF2α, IRE1/XBP1, and ATF6 (Coker-Gurkan et al., 2021; Walter and Ron, 2011). Among them, PERK/eIF2α is considered to be the main pathway involved in PDT-induced apoptosis (Chen et al., 2019). Therefore, we investigated the effects of TBZPy-PDT on this pathway and its downstream targets. First, we observed the co-localization of TBZPy and ER in HeLa cells by fluorescence microscopy. As shown in Fig. 3A, the red fluorescent signal of TBZPy overlapped with the green fluorescent signal of the ER, indicating that TBZPy-PDT acted on the ER. Next, we measured the expression levels of ER stress markers, including BIP and CHOP, by western blot analysis. As shown in Figs. 3B and 3C, TBZPy-PDT significantly increased the expression levels of BIP and CHOP compared to the NC group. We also measured the expression levels of PERK and eIF2α phosphorylation by western blot analysis. As shown in Figs. 3D and 3E, TBZPy-PDT significantly increased the expression levels of p-PERK and p-eIF2α compared to the NC group. These results indicate that TBZPy-PDT triggered ER stress via the PERK/eIF2α signaling pathway. Moreover, we measured the expression levels of cleaved caspase-3 and -12 by western blot analysis. As shown in Figs. 3F and 3G, TBZPy-PDT significantly increased the expression levels of cleaved caspase-3 and -12 compared to the NC group. These results suggest that TBZPy-PDT induced ER stress-specific apoptosis via the PERK/eIF2α pathway. Furthermore, we used the PERK inhibitor GSK2606414 to block the PERK/eIF2α/CHOP pathway and evaluated its effects on TBZPy-PDT-treated HeLa cells. As shown in Fig. 3H, GSK2606414 significantly attenuated the inhibitory effect of TBZPy-PDT on HeLa cells and reduced the apoptosis rate. These results confirm that PERK/eIF2α/CHOP pathway mediated TBZPy-PDT-induced apoptosis.
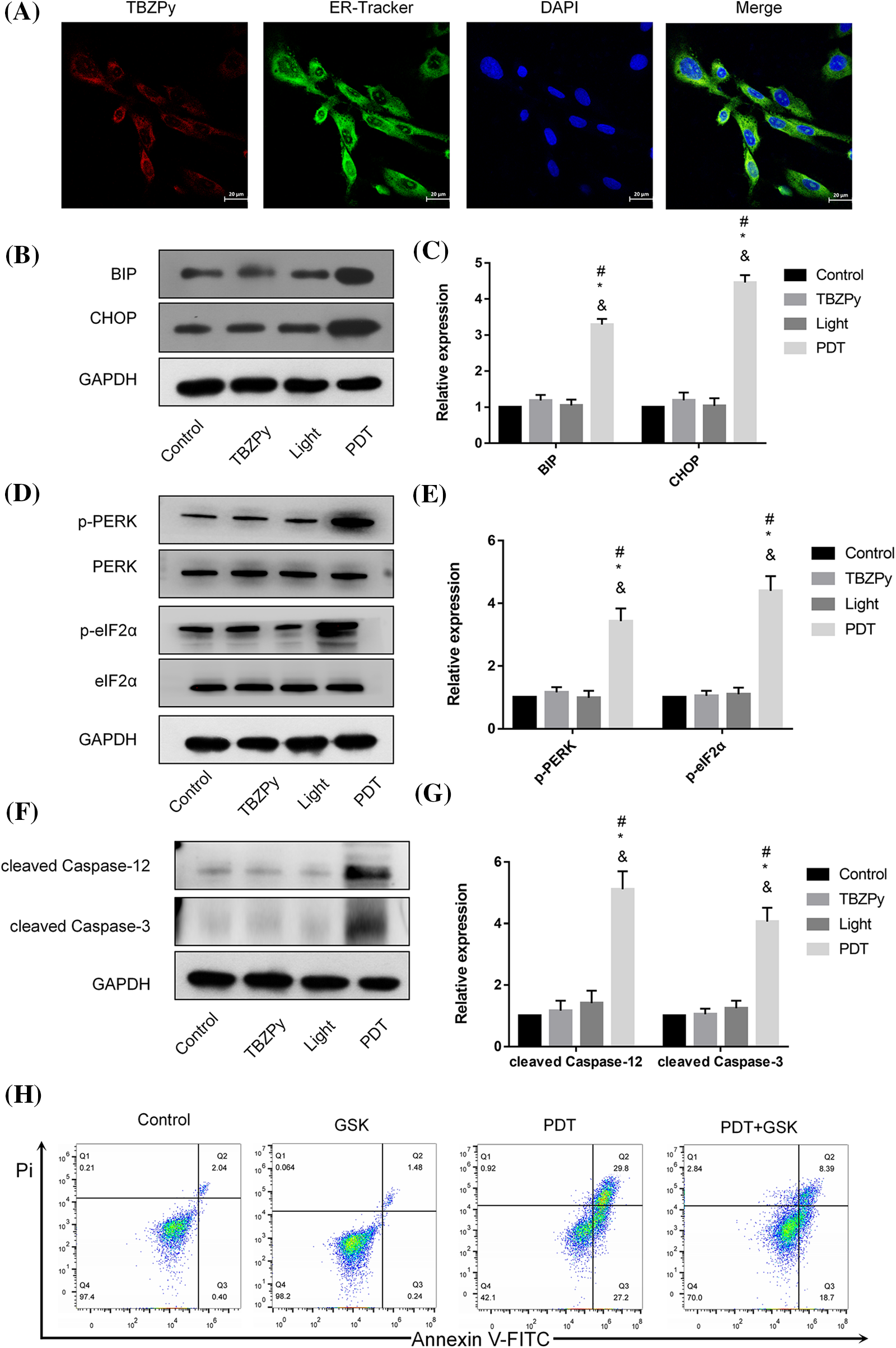
Figure 3: TBZPy-PDT triggered endoplasmic reticulum stress (ERS) via PERK/eIF2α/BIP/CHOP signaling pathway, and ERS-specific apoptosis via the activation of caspase-3 and -12. (A) The intracellular localization of TBZPy was observed by ER-Tracker™ green staining (×400). (B, C) Expression levels of BIP and CHOP in control, TBZPy, Light, and TBZPy-PDT groups. (D, E) Expression levels of PERK, p-PERK, eIF2α, p-eIF2α proteins. (F, G) Expression levels of the cleaved caspase-3 and cleaved caspase-12 and cell apoptosis. (H) Pre-treatment with 2 μM GSK2606414 alleviated cell apoptosis in HeLa cells that was increased after treatment with TBZPy-PDT. *p < 0.05 compared with the control group; #p < 0.05 compared with the TBZPy group; &p < 0.05 compared with the Light group.
TBZPy-PDT increased the levels of calmodulin and calcium in the cytoplasm while decreasing levels of calcium-binding proteins in the endoplasmic reticulum
We then examined the effects of TBZPy-PDT on calcium homeostasis, which is an important aspect of ER stress. Calcium is an essential second messenger that regulates various cellular processes such as proliferation, differentiation, migration, and apoptosis (Berridge et al., 2000). The ER is a major intracellular calcium store that maintains calcium balance and modulates calcium signaling. ER stress can cause calcium efflux from the ER to the cytoplasm, which can activate caspase-12 and other apoptotic factors (Nakagawa et al., 2000). Moreover, ER stress can affect the expression levels of calcium-binding chaperones in the ER, such as ERp57 and ERp72, which are critical for protein folding and quality control (Ni and Lee, 2007). Calmodulin is a calcium sensor that detects fluctuations in calcium levels and modulates downstream pathways (Mukherjee et al., 2019). Therefore, we investigated the effects of TBZPy-PDT on cytosolic Ca2+ concentration, endoplasmic calcium-binding chaperones, and calmodulin by western blot analysis and flow cytometry. As shown in Figs. 4A and 4B, TBZPy-PDT significantly increased the expression level of calmodulin and the cytosolic Ca2+ concentration compared to the NC group. In contrast, TBZPy-PDT significantly decreased the expression levels of ERp57 and ERp72 compared to the NC group (Figs. 4C and 4D). These results indicate that TBZPy-PDT modulated the levels of calmodulin and calcium in the cytoplasm while decreasing levels of calcium-binding proteins in the ER.
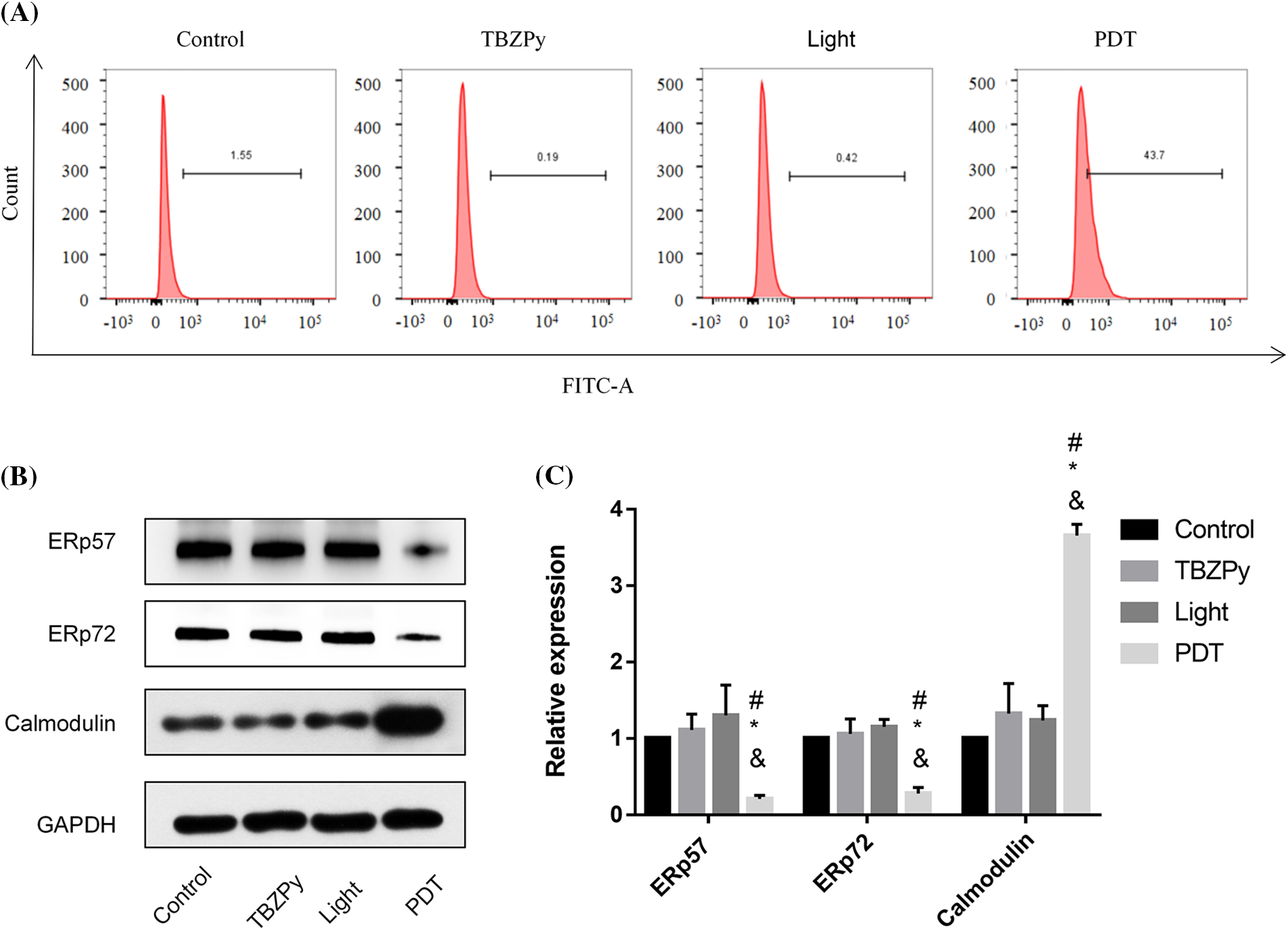
Figure 4: TBZPy-PDT led to increases in levels of Ca2+ and calmodulin, and a decrease in levels of ERp57 and ERp72 in HeLa cells. (A) Effects of TBZPy-PDT on calcium concentration in HeLa Cells. Groups were set as control, TBZPy, Light, and TBZPy-PDTgroups. (B) and (C) show western blotting and quantification analysis of ERp57, ERp72, and calmodulin protein expressions after HeLa cells were treatedwith TBZPy-PDT, and then cultured for 24 h. *p < 0.05 compared with the control group; #p < 0.05 compared with the TBZPy group; & p < 0.05 compared with the Light group.
TBZPy is a novel photosensitizer that exhibits good fluorescence properties and high levels of singlet oxygen production in anoxic environments. In this study, we investigated the effects and mechanisms of TBZPy-PDT on HR-HPV infection using HeLa cells as a model system. We found that TBZPy-PDT inhibited the migration, invasion, and colony formation activities of HeLa cells by suppressing the PI3K/AKT pathway. We also found that TBZPy-PDT induced ER stress-specific apoptosis via the PERK/eIF2α pathway. Moreover, TBZPy-PDT modulated the levels of calmodulin and calcium in the cytoplasm while decreasing levels of calcium-binding proteins in the ER.
Our findings are consistent with previous studies that showed that PDT could modulate calcium homeostasis and affect calcium-related proteins (Grebenová et al., 2003; Li et al., 2021b; Wang et al., 2021). Calcium is an essential second messenger that regulates various cellular processes such as proliferation, differentiation, migration, and apoptosis (Berridge et al., 2000). The ER is a major intracellular calcium store that maintains calcium balance and modulates calcium signaling. ER stress can cause calcium efflux from the ER to the cytoplasm, which can activate caspase-12 and other apoptotic factors (Nakagawa et al., 2000). Moreover, ER stress can affect the expression levels of calcium-binding chaperones in the ER, such as ERp57 and ERp72, which are critical for protein folding and quality control (Ni and Lee, 2007). Calmodulin is a calcium sensor that detects fluctuations in calcium levels and modulates downstream pathways (Mukherjee et al., 2019). In our study, we demonstrated that TBZPy-PDT increased the expression level of calmodulin and the cytosolic Ca2+ concentration while decreasing the expression levels of ERp57 and ERp72, indicating that TBZPy-PDT modulated the levels of calmodulin and calcium in the cytoplasm while decreasing levels of calcium-binding proteins in the ER. These results suggest that TBZPy-PDT might affect calcium trafficking and apoptosis through modulating calcium-related proteins.
We investigated the mechanisms associated with the effect of TBZPy PDT on ERS in HeLa cells. Our study demonstrates that TBZPy may be used to treat CIN and cervical cancer. Targeting the PI3K/AKT and PERK/eIF2α pathways, and the endoplasmic reticulum stress mechanisms may help improve the effects of TBZPy-PDT for treating CIN and cervical cancer. TBZPy-PDT offers a cervical cancer treatment alternative for patients with fertility issues and specific lesions.
Funding Statement: This work was supported by the Natural Science Foundation of Gansu Province (22JR5RA496, 22JR5RA955), Talent Innovation and Entrepreneurship Project of Lanzhou City (2022-RC-49), Talent Innovation and Entrepreneurship Project of Chengguan District (2022-rc-7), Foundation of Lanzhou University Second Hospital (CYXZ2022-22), Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2021-QN-A02), Fundamental Research Funds for the Central Universities (lzujbky-2022-50), Project of Gansu Province Health Commission (GSWSKY2022-02) Innovation Fund for Colleges and Universities (2021B-046).
Author Contributions: YFL, JZ, YTF and HDX designed thestudy and analyzed the data. KXK, YLZ, ZWZ, and YML conducted experiments and collected data. MZT was responsible for manuscript drafting. All authors read and approved the final draft of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Berridge MJ, Lipp P, Bootman MD (2000). The versatility and universality of calcium signalling. Nature Reviews Molecular Cell Biology 1: 11–21. [Google Scholar] [PubMed]
Cao S, Tang J, Huang Y, Li G, Li Z, Cai W, Yuan Y, Liu J, Huang X, Zhang H (2021a). The road of solid tumor survival: From drug-induced endoplasmic reticulum stress to drug resistance. Frontiers in Molecular Biosciences 8: 620514. https://doi.org/10.3389/fmolb.2021.620514 [Google Scholar] [PubMed] [CrossRef]
Cao L, Zhang J, Du Y, Sun M, Xiang Y, Sheng Y, Ren X, Shao J (2021b). Selenite induced breast cancer MCF7 cells apoptosis through endoplasmic reticulum stress and oxidative stress pathway. Chemico-Biological Interactions 349: 109651. https://doi.org/10.1016/j.cbi.2021.109651 [Google Scholar] [PubMed] [CrossRef]
Casas A (2020). Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: A review. Cancer Letters 490: 165–173. https://doi.org/10.1016/j.canlet.2020.06.008 [Google Scholar] [PubMed] [CrossRef]
Chen MT, Huang RL, Ou LJ, Chen YN, Men L, Chang X, Wang L, Yang YZ, Zhang Z (2019). Pollen typhae total flavone inhibits endoplasmic reticulum stress-induced apoptosis in human aortic-vascular smooth muscle cells through down-regulating PERK-eIF2α-ATF4-CHOP pathway. Chinese Journal of Integrative Medicine 25: 604–612. [Google Scholar] [PubMed]
Chen Y, Yin H, Tao Y, Zhong S, Yu H, Li J, Bai Z, Ou Y (2020). Antitumor effects and mechanisms of pyropheophorbide‐α methyl ester‐mediated photodynamic therapy on the human osteosarcoma cell line MG‐63. International Journal of Molecular Medicine 45: 971–982. https://doi.org/10.3892/ijmm.2020.4494 [Google Scholar] [PubMed] [CrossRef]
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J, Wen X (2016). The PI3K/AKT pathway in the pathogenesis of prostate cancer. Frontiers in Bioscience 21: 1084–1091. https://doi.org/10.2741/4443 [Google Scholar] [PubMed] [CrossRef]
Coker-Gurkan A, Can E, Sahin S, Obakan-Yerlikaya P, Arisan ED (2021). Atiprimod triggered apoptotic cell death via acting on PERK/eIF2α/ATF4/CHOP and STAT3/NF-κB axis in MDA-MB-231 and MDA-MB-468 breast cancer cells. Molecular Biology Reports 47: 5233–5247. https://doi.org/10.1007/s11033-021-06528-1 [Google Scholar] [PubMed] [CrossRef]
Das S, Mondal A, Samanta J, Chakraborty S, Sengupta A (2021). Unfolded protein response during cardiovascular disorders: A tilt towards pro-survival and cellular homeostasis. Molecular and Cellular Biochemistry 476: 4061–4080. https://doi.org/10.1007/s11010-021-04223-0 [Google Scholar] [PubMed] [CrossRef]
Gomes-da-Silva LC, Zhao L, Bezu L, Zhou H, Sauvat A et al. (2018). Photodynamic therapy with redaporfin targets the endoplasmic reticulum and Golgi apparatus. The EMBO Journal 37: 2033. https://doi.org/10.15252/embj.201798354 [Google Scholar] [PubMed] [CrossRef]
Grebenová D, Kuzelová K, Smetana K, Pluskalová M, Cajthamlová H, Marinov I, Fuchs O, Soucek J, Jarolím P, Hrkal Z (2003). Mitochondrial and endoplasmic reticulum stress-induced apoptotic pathways are activated by 5-aminolevulinic acid-based photodynamic therapy in HL60 leukemia cells. Journal of Photochemistry and Photobiology B-Bioloqy 69: 71–85. https://doi.org/10.1016/S1011-1344(02)00410-4 [Google Scholar] [PubMed] [CrossRef]
Gunaydin G, Gedik ME, Ayan S (2021). Photodynamic therapy-current limitations and novel approaches. Frontiers in Chemistry 9: 691697. https://doi.org/10.3389/fchem.2021.691697 [Google Scholar] [PubMed] [CrossRef]
He C, Xia J, Gao Y, Chen Z, Wan X (2020). Chlorin A-mediated photodynamic therapy induced apoptosis in human cholangiocarcinoma cells via impaired autophagy flux. American Journal of Translational Research 12: 5080–5094. [Google Scholar] [PubMed]
Hu F, Xu S, Liu B (2018). Photosensitizers with aggregation-induced emission: Materials and biomedical applications. Advanced Materials 30: e1801350. https://doi.org/10.1002/adma.201801350 [Google Scholar] [PubMed] [CrossRef]
Istomin YP, Lapzevich TP, Chalau VN, Shliakhtsin SV, Trukhachova TV (2010). Photodynamic therapy of cervical intraepithelial neoplasia grades II and III with photolon. Photodiagnosis and Photodynamic Therapy 7: 144–151. https://doi.org/10.1016/j.pdpdt.2010.06.005 [Google Scholar] [PubMed] [CrossRef]
Jiang N, Dai Q, Su X, Fu J, Feng X, Peng J (2020). Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Molecular Biology Reports 47: 4587–4629. https://doi.org/10.1007/s11033-020-05435-1 [Google Scholar] [PubMed] [CrossRef]
Li Z, Teng M, Wang Y, Feng Y, Xiao Z et al. (2021a). Dihydroartemisinin administration improves the effectiveness of 5-aminolevulinic acid-mediated photodynamic therapy for the treatment of high-risk human papillomavirus infection. Photodiagnosis and Photodynamic Therapy 33: 102078. https://doi.org/10.1016/j.pdpdt.2020.102078 [Google Scholar] [PubMed] [CrossRef]
Li Z, Teng M, Wang Y, Wang Q, Feng Y, Xiao Z, Li C, Zeng K (2021b). The mechanism of 5-aminolevulinic acid photodynamic therapy in promoting endoplasmic reticulum stress in the treatment of HR-HPV-infected HeLa cells. Photodermatology Photoimmunology & Photomedicine 37: 348–359. https://doi.org/10.1111/phpp.12663 [Google Scholar] [PubMed] [CrossRef]
Li Z, Xiao Z, Feng Y, Wang Q, Teng M (2022). Mechanism of a new photosensitizer (TBZPy) in the treatment of high-risk human papillomavirus-related diseases. Photodiagnosis and Photodynamic Therapy 37: 102591. https://doi.org/10.1016/j.pdpdt.2021.102591 [Google Scholar] [PubMed] [CrossRef]
Liu L, Xu Z, Yu B, Tao L, Cao Y (2021). Berbamine Inhibits cell proliferation and migration and induces cell death of lung cancer cells via regulating c-Maf, PI3K/Akt, and MDM2-P53 pathways. Evidence-Based Complementary and Alternative Medicine 2021: 5517143. https://doi.org/10.1155/2021/5517143 [Google Scholar] [PubMed] [CrossRef]
Malacarne MC, Banfi S, Rugiero M, Caruso E (2021). Drug delivery systems for the photodynamic application of two photosensitizers belonging to the porphyrin family. Photochemical and Photobiological Sciences 20: 1011–1025. https://doi.org/10.1007/s43630-021-00076-0 [Google Scholar] [PubMed] [CrossRef]
Mounir Z, Krishnamoorthy JL, Wang S, Papadopoulou B, Campbell S, Muller WJ, Hatzoglou M, Koromilas AE (2011). Akt determines cell fate through inhibition of the PERK-eIF2α phosphorylation pathway. Science Signaling 4: ra62. [Google Scholar]
Mukherjee R, Bhattacharya A, Sau A, Basu S, Chakrabarti S, Chakrabarti O (2019). Calmodulin regulates MGRN1-GP78 interaction mediated ubiquitin proteasomal degradation system. The FASEB Journal 33: 1927–1945. https://doi.org/10.1096/fj.201701413RRR [Google Scholar] [PubMed] [CrossRef]
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000). Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403: 98–103. https://doi.org/10.1038/47513 [Google Scholar] [PubMed] [CrossRef]
Ni M, Lee AS (2007). ER chaperones in mammalian development and human diseases. FEBS Letters 581: 3641–3651. https://doi.org/10.1016/j.febslet.2007.04.045 [Google Scholar] [PubMed] [CrossRef]
Nyberg WA, Espinosa A (2016). Imiquimod induces ER stress and Ca2+ influx independently of TLR7 and TLR8. Biochemical and Biophysical Research Communications 473: 789–794. https://doi.org/10.1016/j.bbrc.2016.03.080 [Google Scholar] [PubMed] [CrossRef]
Unanyan A, Pivazyan L, Davydova J, Murvatova K, Khrapkova A, Movsisyan R, Ishchenko A, Ishchenko A (2021). Efficacy of photodynamic therapy in women with HSIL, LSIL and early stage squamous cervical cancer: A systematic review and meta-analysis. Photodiagnosis and Photodynamic Therapy 36: 102530. https://doi.org/10.1016/j.pdpdt.2021.102530 [Google Scholar] [PubMed] [CrossRef]
Walter P, Ron D (2011). The unfolded protein response: From stress pathway to homeostatic regulation. Science 334: 1081–1086. [Google Scholar] [PubMed]
Wan Q, Zhang RY, Zhuang ZY, Li YX, Huang YH, Wang ZM, Zhang WJ, Hou JQ, Tang BZ (2020). Molecular engineering to boost AIE-active free radical photogenerators and enable high-performance photodynamic therapy under hypoxia. Advanced Functional Materials 30: 2002057. https://doi.org/10.1002/adfm.202002057 [Google Scholar] [CrossRef]
Wang S, Li C, Sun P, Shi J, Wu X et al. (2021). PCV2 triggers PK-15 cell apoptosis through the PLC-IP3R-Ca2+ signaling pathway. Frontiers in Microbiology 12: 674907. https://doi.org/10.3389/fmicb.2021.674907 [Google Scholar] [PubMed] [CrossRef]
Xie J, Wang S, Li Z, Ao C, Wang J, Wang L, Peng X, Zeng K (2019). 5-aminolevulinic acid photodynamic therapy reduces HPV viral load via autophagy and apoptosis by modulating Ras/Raf/MEK/ERK and PI3K/AKT pathways in HeLa cells. Journal of Photochemistry and Photobiology B-Bioloqy 194: 46–55. https://doi.org/10.1016/j.jphotobiol.2019.03.012 [Google Scholar] [PubMed] [CrossRef]
Yong J, Johnson JD, Arvan P, Han J, Kaufman RJ (2021). Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus. Nature Reviews Endocrinology 17: 455–467. https://doi.org/10.1038/s41574-021-00510-4 [Google Scholar] [PubMed] [CrossRef]
Zhang Z, Zhang L, Zhou L, Lei Y, Zhang Y, Huang C (2019). Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biology 25: 101047. https://doi.org/10.1016/j.redox.2018.11.005 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools