 Open Access
Open Access
REVIEW
Ketone bodies and inflammation modulation: A mini-review on ketogenic diet’s potential mechanisms in mood disorders
1 College of Physical Education, Jilin University, Changchun, 130012, China
2 China Japan Union Hospital, Jilin University, Changchun, 130012, China
3 Health Nutrition, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1130032, Japan
4 Faculty of Sport Sciences, Waseda University, Tokorozawa, 3591192, Japan
* Corresponding Authors: SIHUI MA. Email: ; HUIJUAN JIA. Email:
(This article belongs to the Special Issue: Neuroimmune Interactions at the Crossroads of Health and Disease)
BIOCELL 2023, 47(8), 1897-1906. https://doi.org/10.32604/biocell.2023.027632
Received 07 November 2022; Accepted 15 March 2023; Issue published 28 August 2023
Abstract
Mental disorders such as depression and anxiety inflict significant burdens on individuals and society. Commonly prescribed treatments often involve cognitive therapy and medications. However, for patients resistant to these conventional methods, alternative therapies like the Ketogenic Diet (KD) offer a promising avenue. KD and its key metabolite, β-hydroxybutyrate (BHB), have been hypothesized to alleviate mental disorders through anti-inflammatory actions, a crucial pathway in the pathophysiology of depression. This mini-review examines 15 clinical trials exploring the influence of KD and BHB on inflammation and their potential roles in managing mental disorders. Both human and animal studies were scrutinized to elucidate possible cellular and molecular mechanisms. Out of the 15 trials, 10 reported reduced levels of at least one inflammatory mediator or mRNA post KD or BHB treatment, while two observed an elevation in anti-inflammatory agents. These findings suggest that KD and BHB could modulate cellular inflammatory pathways, highlighting their potential for therapeutic application in mental disorders.Keywords
Supplementary Material
Supplementary Material FileList of Abbreviations
| 5-HT | Serotonin |
| ALOX | Arachidonate 5-Lipoxygenase |
| BHB | β-hydroxybutyrate |
| bw | Body weight |
| CMD | Common mental disorders |
| COX | Cyclooxygenase |
| CRP | C-reactive protein |
| ELISA | Enzyme-linked immunosorbent assay |
| FoxO1 | Forkhead O1 |
| GAD | Generalized anxiety disorder |
| HDAC | Histone deacetylase |
| IDO | Indoleamine 2,3 dioxygenase |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| KD | Ketogenic diet |
| LPS | Lipopolysaccharide |
| MAOA | Type A monoamine oxidase |
| MCP | Monocyte chemotactic protein |
| MnSOD | Manganese Superoxide Dismutase |
| NF-κB | Nuclear factor-kappa B |
| NLRP | NLR family pyrin domain containing |
| PI3K | Phosphoinositide 3-kinases |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| SCOT | Succinyl-CoA:3-ketoacid-CoA transferase |
| TNF | Tumor necrosis factor |
| TRP | Tryptophan |
Common mental disorders (CMD) refer to two main diagnostic categories: depressive and anxiety disorders. According to the World Health Organization, in 2015, the proportion of the global population with depression was estimated to be 4.4%. The prevalence was more common among females (5.1%) than males (3.6%) (World Health Organization, 2017). The proportion of anxiety disorders was estimated to be 3.6%, which also appears to be more common among females (4.6%) than males (2.6%) (World Health Organization, 2017). Both these mental disorders are a leading cause of disability, imposing a substantial burden on individuals and society (Friedrich, 2017; Stein and Craske, 2017).
While cognitive therapy and medications are the first-line treatment for CMD, 10%–30% of the patients do not respond to antidepressant medicines. Additionally, they exhibit symptoms such as declined physical health and social/occupational function, or even suicidal intentions (Pilkington, 2018). Therefore, alternative and complementary therapies for the prevention and treatment of CMD are urgently needed.
Though the pathology of CMD is not fully understood, the mutual and functional role of the immune system in the development of CMD symptomology has been highlighted by recent findings. Inflammation, mainly mediated by cytokines and chemokines that are secreted by immune cells, is generally considered a defense mechanism upon infection. However, chronic and excessive inflammation may interfere with synaptic remodeling, transcription, and epigenetics. It may also harm the integrity of neuronal function, influence neurocircuitry and/or neurotransmitter systems, and produce behavioral alternations (van Velzen et al., 2017). Mounting evidence indicates that inflammatory cytokines are responsible for the development of CMD. For instance, epidemiological studies using data collected from 147,478 individuals from the UK Biobank and 2,905 from the Netherlands Study of Depression and Anxiety indicate that the inflammation level was associated with core depressive and anxiety symptoms of low mood, anhedonia, and other symptoms (van Eeden, 2022; Milaneschi et al., 2021). In animal model studies, evidence shows that activated immune responses are observed in many CMD animal models, and treatment with several kinds of cytokines could produce depressive and anxiety-like behaviors (Camara et al., 2013; Murray et al., 2013). Furthermore, CMD occurs more frequently in those who already have medical disorders associated with immune dysfunction (Gałecki and Talarowska, 2018). Therefore, anti-inflammatory agents and treatments are considered effective ways to fight CMD.
The ketogenic diet (KD) is a dietary regimen that contains high fat and low carbohydrate content. It is an established treatment for refractory epilepsy, including some inflammation-induced epileptic encephalopathies (Ma and Suzuki, 2019).
Nowadays, ketone supplementations are available for use, and people can use the supplementations to achieve nutritional ketosis easily (Kovács et al., 2019). There are two primary forms of ketone supplements, ketone salts, and ketone esters. Both will be metabolized to β-hydroxybutyrate (BHB) after absorption (O’Malley et al., 2017; Hashim and VanItallie, 2014). The anti-inflammatory properties of BHB are attracting increasing attention. However, the efficacy and underlying mechanisms of KD and BHB are not fully understood. Therefore, this review aims to discuss the therapeutic utility of KD and ketone supplementations, as substitutions for KD, in CMD treatment, focusing on their anti-inflammatory properties.
Abstracts of publications identified in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Ovid (including Medline, PsychINFO, NURSING, and EMBASE), Scopus, and EBSCO (including CINAHL) were searched and reviewed for relevant papers. The search took place on 22 August 2022 and was not restricted by publication date. Two searches included (1) evaluations on the status of inflammation in mood disorders using animal models, and (2) the anti-inflammation properties of KD or BHB in patients. The exact search terms were depression (Title/Abstract) OR depressive symptom (Title/Abstract) OR anxiety (Title/Abstract) OR anxiety symptom (Title/Abstract) AND (ketogenic diet (Title/Abstract) OR ketone body (Title/Abstract) OR ketone ester (Title/Abstract) OR ketone diester (Title/Abstract) OR ketone salt (Title/Abstract) OR ketone supplements (Title/Abstract) OR ketosis (Title/Abstract) for (1), and, inflammation (Title/Abstract) OR inflammatory (Title/Abstract) OR cytokine (Title/Abstract) OR anti-inflammation (Title/Abstract)) AND (ketogenic diet (Title/Abstract) OR ketone body (Title/Abstract) OR ketone ester (Title/Abstract) OR ketone diester (Title/Abstract) OR ketone salt (Title/Abstract) OR ketone supplements (Title/Abstract) OR ketosis (Title/Abstract)) for (2) and each part was carried out in a single search. Due to limited evidence, the first search was concluded using narrative descriptions. The second search was carried out based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) search strategy (Page et al., 2021), and subsequent reference narrowing is described in Fig. 1. The Prism checklist can be found as Suppl. Table S1 in the supplementary file. Two authors carried out the search and screen separately using the Covidence system (https://www.covidence.org/). The flow chart of the selection of studies is shown in Fig. 1.
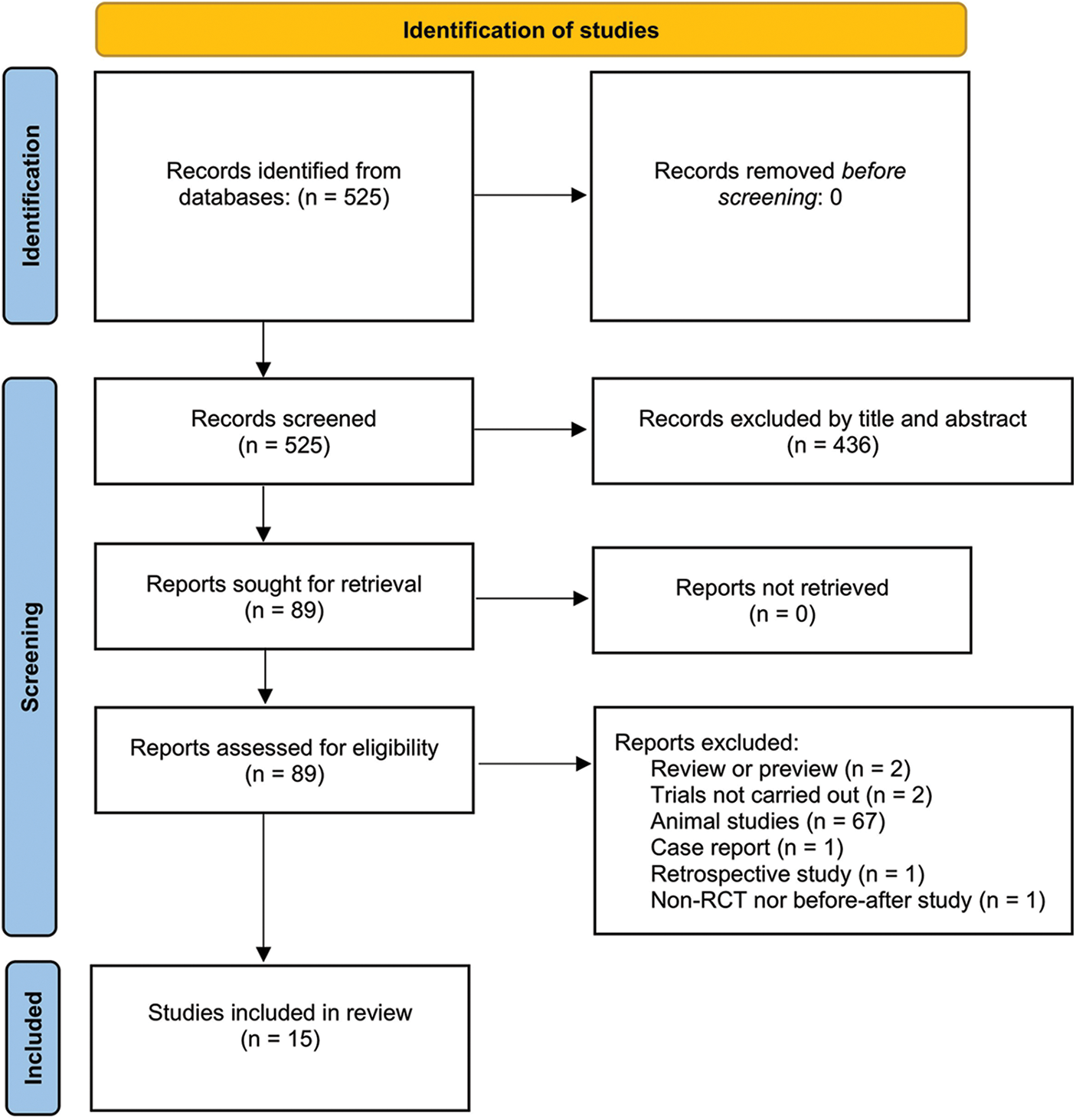
Figure 1: PRISMA flow chart of the study.
Inclusion and exclusion criteria
For search (2), randomized crossover or parallel controlled trials assessing the impact of KD or BHB on human inflammation status measures were included in the current analysis. There were no exclusion criteria for study duration, diet, ketone supplement type or dose, or participants’ sex and age, sample size, or health status. Studies assessing the impact in animal models were separated from human trials and analyzed separately. Studies of case reports were excluded.
Data were extracted from 15 studies that were selected to meet the inclusion and exclusion criteria. Intervention type and duration, sample characterization, subject status, study design, assay, and inflammatory mediators were extracted from each study to provide descriptive characteristics of participants. For animal studies, the findings have been discussed in a non-systematic way as an elaboration on the proposed mechanisms.
For the results of search (2), based on the PRISMA principles shown in Fig. 1, we retrieved 15 articles that have been summarized in Table 1.
Among the studies, 13 studies included KD as an intervention (Bock et al., 2018; Bosco et al., 2018; Castaldo et al., 2021; Rhyu and Cho, 2014; Cipryan et al., 2020a, 2020b; Paoli et al., 2021; Bertoli et al., 2015; Kong et al., 2020; Khodabakhshi et al., 2021; Rosenbaum et al., 2019; Monda et al., 2020; Shaw et al., 2020), while only 2 studies employed ketone body administration as an intervention (Shaw et al., 2020; Martin-Arrowsmith et al., 2020). Alteration of multiple cytokines or other inflammatory mediators such as adiponectin, resistin, etc., were reported.
Among the studies, 10 of 15 (66.7%), reported at least one inflammatory mediator (C-reactive protein (CRP), leptin, interleukin (IL)-1β, IL-2, IL-6, IL-12p40, interferon (IFN)-γ and/or tumor necrosis factor (TNF)-α) (Bosco et al., 2018; Castaldo et al., 2021; Cipryan et al., 2020b, 2020a; Kong et al., 2020; Shaw et al., 2020; Khodabakhshi et al., 2021, Monda et al., 2020) or the mRNA of the indicated inflammatory mediators (IFN-γ mRNA, Shaw et al., 2020) was decreased by KD or BHB intervention. However, 3 studies reported that no changes were observed (Bock et al., 2018; Rhyu and Cho, 2014;). Further, 2 reported that an anti-inflammation mediator (adiponectin and/or IL-10) was increased (Rosenbaum et al., 2019; Monda et al., 2020; Bertoli et al., 2015). The selected studies conducted on humans showed that KD has great potential in treating inflammatory diseases, showing the potential to be utilized as a treatment for CMD based on the inflammation hypothesis of the pathology of CMD. However, the evidence of BHB is far from convincing, and further studies are warranted. The details of the above studies are listed as Table 1.
Inflammation is our body’s defense system protecting us against infection, cellular damage, and other harmful agents, both exogenously and endogenously (Suzuki, 2018a; Suzuki et al., 2020). During inflammation, cytokines play a significant role in mediating cellular signaling and immunological responses. Cytokines are a category of small proteins (5~20 kDa) secreted by a broad range of cells, including immune cells such as macrophages, lymphocytes, mast cells, endothelial cells, fibroblasts, myocytes, adipocytes, and other kinds of cells (Ma et al., 2020; Suzuki, 2018b). The subcategories of cytokines include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factors, all involved in fighting off infections and other immune responses (Aw et al., 2018). However, inflammation that acts against wrong stimuli may induce unwanted cytokine production, thus bringing undesired consequences. A large body of studies has reported that pro-inflammatory or inflammatory cytokines increase in CMD patients (Hou et al., 2017; Gelman, 2019; Zou et al., 2018; Martinez et al., 2018; Jia et al., 2019; Petralia et al., 2019). The evidence on the cytokine secretion profile collected from papers published in the recent five years is depicted in Fig. 2.
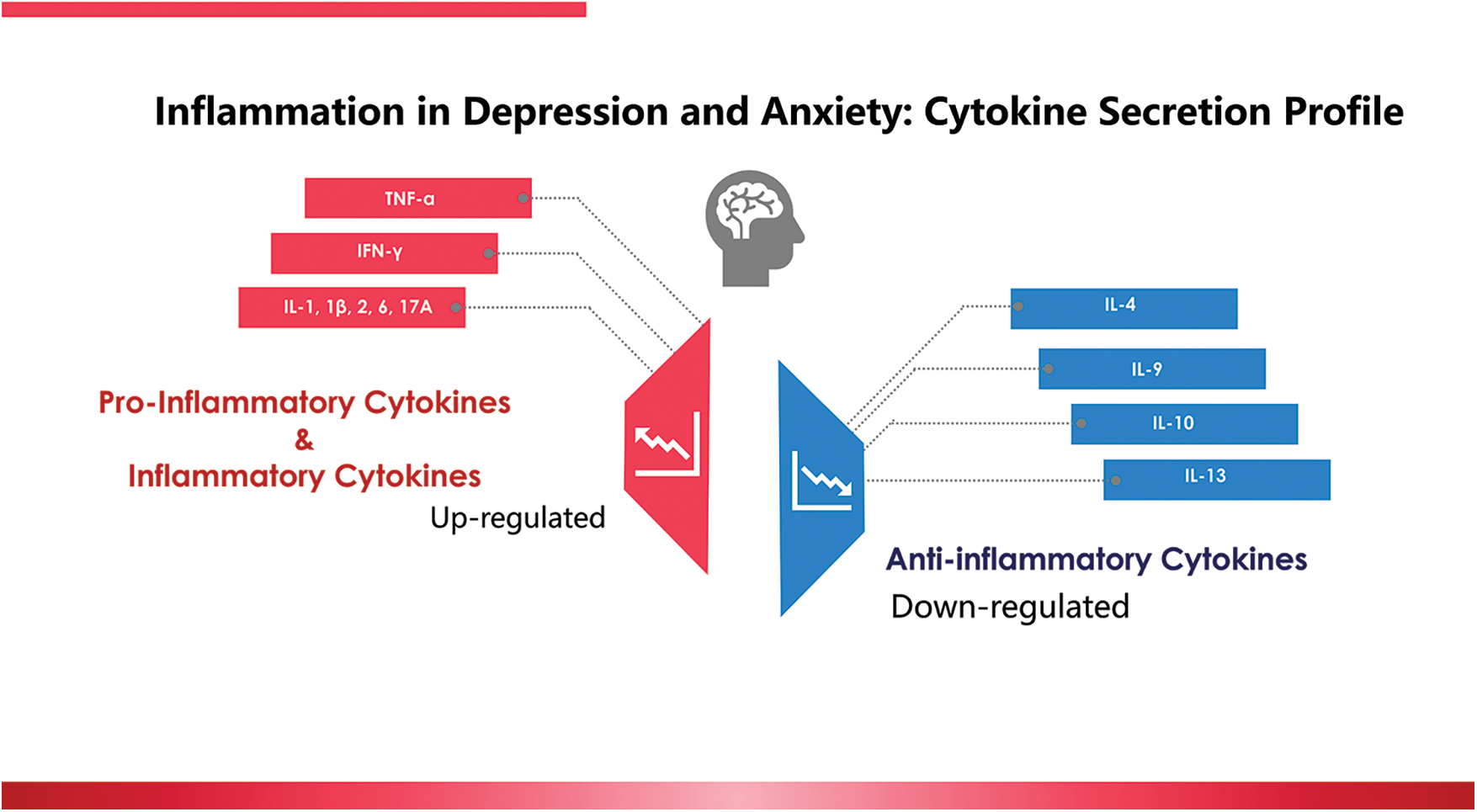
Figure 2: Inflammation in depression and anxiety: the cytokine secretion profile reported in the recent five years.
According to a case-controlled study conducted in generalized anxiety disorder (GAD) patients, significantly higher ratios of TNF-α/IL-10, TNF-α/IL-4, IFN-γ/IL-10, and IFN-γ/IL-4 were found in the GAD group compared to the control group (patient group: n = 54; control group: n = 64) (Hou et al., 2017). In pregnant women with severe anxiety and depression, the levels of Th1-(IL-6, TNF-α, IL-2, IFN-γ), Th17-(IL-17A), and Th2-(IL-9, IL-10, and IL-13) were higher than those in the control group (n = 139, patient group; n = 40, control group) (Gelman, 2019). In one report, patients with major depressive disorders had significantly higher levels of IL-1β, IL-10, and TNF-α but significantly lower levels of IL-8 (n = 117, patient group; n = 102, control group) (Zou et al., 2018). In another report, IL-1, IL-6, TNF-α, IFN-γ, and IL-10 were found to be associated with depression scores in people with alcohol and drug use disorder (n = 80, patient) (Martinez et al., 2018). Further, a significantly positive correlation between serum cortisol levels and Hamilton Depression Rating Scale scores was observed in 89 male depression patients (Jia et al., 2019). In women with postpartum depression, significant fluctuation of TNF-α and IL-18 were found (Petralia et al., 2019). Therefore, targeting pro-inflammatory and inflammatory cytokines and their signaling pathways might be novel strategies to treat CMD.
Although the mechanism of inflammation in CMD has not been fully explained, the loss of regulation of inflammatory agents and neurotransmitters seems to be the key. The imbalance of neurotransmitters, including serotonin (5-HT), norepinephrine, and dopamine has been reported and treated to be the reason for CMD (Yan, 2018; Naoi et al., 2018). Meanwhile, inflammatory cytokines trigger neuroinflammation, microglial activation, and disturbance of neurotransmitters, resulting in CMD pathophysiological process (Morris et al., 2016). Let us consider the loss of the regulation process of 5-HT as an example. According to the cytokine theory, the initiation of stress increases the production of cytokines, including TNFs, and ILs. IFNs may contribute to the pathophysiological process of CMD. Increased levels of the abovementioned inflammatory cytokines may activate the production of the indoleamine 2,3 dioxygenase (IDO), with subsequent production of tryptophan (TRP) catabolites along the IDO pathway, decreasing the availability of TRP and serotonin and contributing to the progress of CMD (Morris et al., 2016). Conversely, phytochemical supplementation that suppresses pro-inflammatory cytokines, thus inhibiting type A monoamine oxidase (MAOA), has been reported to be efficient in relieving depression (Yan, 2018). Therefore, the use of anti-inflammatory compounds and diets that exhibit anti-inflammatory properties may be a strategy for CMD treatment.
Introduced as a treatment for refractory epilepsy in the first place, the KD has been reported for its anti-inflammatory properties in recent years. This provides a potential contributing role in treating CMDs, based on the inflammation hypothesis of the pathology of CMD. For example, in a lipopolysaccharide (LPS)-induced inflammation model in rats, a 2-week administration of KD attenuated LPS-induced fever, and reduced blood and hippocampal IL-1β concentration to alleviate inflammation (Barua et al., 2018). In another rodent inflammation model built by inducing a hind paw inflammation, a KD significantly reduced decreased paw swelling, plasma extravasation, and the peripheral inflammatory response (Ruskin et al., 2021). In a paper published in 2016, after a one-month feeding period of KD mice then received LPS by intraperitoneal injection it was reported that KD mice had significantly low expression of nuclear factor-kappa B (NF-κB), IL-6, and TNF-α (Nandivada et al., 2016). These studies showed that a KD has excellent potential in serving as an anti-inflammation therapy for inflammation. In the neuroscience field, KD also attracts great attention. In a memory impairment and central nervous system-inflammation murine model, pre-feeding of a one-week KD (12.2 g polyunsaturated fatty acids in 100 g per KD) decreased circulating inflammatory cytokines including IL-1β, IL-6, TNF-α, IL-12, IL-17A, IFN-γ, MCP-1, MIP-1α and MIP-1β (Kim et al., 2012). In a spinal cord injury rat model, KD suppressed the NF-κB pathway and the expression of TNF-α, IL-1β, and IFN-γ (Lu et al., 2018). In another mouse model, motor dysfunction was induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment, and pre-treatment of KD decreased IL-1β, IL-6, TNF-α (Yang and Cheng, 2010).
The anti-inflammatory properties of a KD may be attributed to the ability to activate peroxisome proliferator-activated receptor-gamma (Jeong et al., 2011; Zhang et al., 2018), subsequently decreasing systematic inflammation (Zhang et al., 2018). Furthermore, increasing evidence shows that the enhanced levels of BHB, the metabolite of fatty acids during a KD, may contribute to playing an anti-inflammatory role (Prattichizzo et al., 2018; Zitvogel et al., 2017).
Although clinical trials on patients with common mental disorders aiming to elicit the anti-inflammatory properties of BHB have not yet been carried out, animal studies have provided some clues. For example, exogenous ketone body administration has been shown to decrease anxiety-related behaviors evaluated by an open-arm system in rodents (83-days sub-chronic beta-hydroxybutyrate-mineral salt supplementation) (Kovács et al., 2018), possibly by interacting with the Adenosine A1 receptor (Kovács et al., 2018). In another study, BHB administration has also been shown to reduce depressive-like behaviors in rodents as evaluated by chronic unpredictable stress and sucrose preference experimental methods (Kovács et al., 2018). In situ BHB administration has been reported to suppress macrophage/microglia activation (Huang et al., 2018). Although the underlying mechanisms have not been elucidated yet, one of the possible mechanisms to ameliorate depressive and anxiety symptoms is by suppressing inflammation (Kong et al., 2017).
The anti-inflammatory properties of BHB have been reported extensively. A study conducted in a rodent model revealed the protective effect of BHB on neuroinflammation. In this study, repeated BHB administration reduced depressive and anxiety behaviors in animals undergoing chronic unpredictable stress (Kong et al., 2017). Additionally, pre-treatment of BHB reduced hippocampal IL-1β and TNF-α (Yamanashi et al., 2017). BHB could alleviate inflammation by attenuating NLR family pyrin domain containing (NLRP)-3 inflammasome formation in IL-1β/IL-18 over-expression mice (Yamanashi et al., 2017). Suppression of the activation of the NLRP3 inflammasome by preventing K+ efflux and reducing apoptosis-associated speck-like protein with a caspase-recruitment domain (ASC) oligomerization and speck formation in human monocytes has also been documented (Kajitani et al., 2020). In BV2 cells, BHB supplementation inhibited LPS-induced inflammatory responses and NLRP3 inflammasome protein level, shifting the activation of macrophages/microglia from the proinflammatory M1 to the anti-inflammatory M2a type (Deng et al., 2021). In both mice and human neutrophils separated from the blood, BHB suppressed IL-1β production by inhibiting both priming and assembling procedures in the activation of the NLRP3 inflammasome (Youm et al., 2015). On the other hand, when the ketolytic, rate-limiting enzyme SCOT (succinyl-CoA:3-ketoacid-CoA transferase 1; encoded by Oxct1) was deleted, markers of sterile inflammation and macrophage infiltration were shown to be attenuated in mice that underwent transverse aortic constriction surgery. Further, the NLRP3 expression was also reduced, indicating that elevated circulating BHB might be linked to reduced inflammation through an NLRP3-mediating manner (Goldberg et al., 2017).
Calorie restriction is well known for its anti-inflammation activities (Ottaviano and Zaman, 2023). Other studies also reported that BHB might exert its anti-inflammatory effects as a calorie restriction mimic (Kim et al., 2019). In one report, BHB administration upregulated Forkhead Box1 (FoxO1) and its target genes catalase/manganese superoxide dismutase (MnSOD). Both play critical roles in quenching inflammation-induced reactive oxygen species, thus ameliorating renal inflammation in aging rats (Kim et al., 2019) by down-regulating TNFSF6, TNF-α, PI3K, NF-κB and toll-like receptor 1 on LPS-stimulated macrophages (Qiao et al., 2020). Another role of BHB during anti-inflammation might be as the inhibitor of histone deacetylase (HDAC), as HDAC inhibition is well-known to have anti-inflammatory effects. For example, in HEK293 cells, HDAC activity decreased with the elevation of BHB concentration, indicating that BHB function as an HDAC inhibitor is dose-dependent (Shimazu et al., 2013). In the same study, it was also shown that dietary BHB exhibited protective effects on cells against oxidative stress via up-regulation of FoxO3a, which shares a similar function as FoxO1, and its target gene, catalase and mitochondrial MnSOD2 (Shimazu et al., 2013). To summarize, numerous studies also show the potential role of BHB in the treatment of CMD. However, since most of the studies are conducted in animals, and the mechanisms are speculative, further studies are urgently encouraged to validate the safety, effectiveness, and mechanisms of BHB application for human CMD treatment.
The anti-inflammatory properties of KD or BHB, the biomarker metabolite during KD administration, are attracting the attention of researchers. In this review, we summarized 15 clinical trials that employed KD or BHB to study their effects on the prevention and treatment of inflammation. Most of them documented favorable results. A KD or BHB-based therapy may thus contribute to relieving neuroinflammation and depressive and anxiety behaviors and have the potential to become a part of the CMD-treatment strategy.
Funding Statement: Department of Science and Technology of Jilin Province, No. 20210402019GH, Fundamental Research Funds of Jilin University, Seed Fund, No. 2021ZZ021. Jilin Province Education Science Planning Project, No. GH21006 and the Fundamental Research Funds for the Central Universities, No. 2022CXTD03.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Yan Zheng, Sihui Ma; data collection: Yan Zheng, Sihui Ma; analysis and interpretation of results: Yan Zheng, Sihui Ma; draft manuscript preparation: Yan Zheng, Sihui Ma, Katsuhiko Suzuki, Hisanori Kato, and Huijuan Jia. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at DOI: 10.32604/biocell.2023.027632.
References
Aw NH, Canetti E, Suzuki K, Goh J (2018). Monocyte subsets in atherosclerosis and modification with exercise in humans. Antioxidants 7: 196. https://doi.org/10.3390/antiox7120196 [Google Scholar] [PubMed] [CrossRef]
Barua CC, Haloi P, Saikia B, Sulakhiya K, Pathak DC, Tamuli S, Rizavi H, Ren X (2018). Zanthoxylum alatum abrogates lipopolysaccharide-induced depression-like behaviours in mice by modulating neuroinflammation and monoamine neurotransmitters in the hippocampus. Pharmaceutical Biology 56: 245–252. https://doi.org/10.1080/13880209.2017.1391298 [Google Scholar] [PubMed] [CrossRef]
Bertoli S, Neri IG, Trentani C, Ferraris C, de Amicis R, Battezzati A, Veggiotti P, de Giorgis V, Tagliabue A (2015). Short-term effects of ketogenic diet on anthropometric parameters, body fat distribution, and inflammatory cytokine production in GLUT1 deficiency syndrome. Nutrition 31: 981–987. https://doi.org/10.1016/j.nut.2015.02.017 [Google Scholar] [PubMed] [CrossRef]
Bock M, Karber M, Kuhn H (2018). Ketogenic diets attenuate cyclooxygenase and lipoxygenase gene expression in multiple sclerosis. eBioMedicine 36: 293–303. https://doi.org/10.1016/j.ebiom.2018.08.057 [Google Scholar] [PubMed] [CrossRef]
Bosco G, Rizzato A, Quartesan S, Camporesi E, Mangar D et al. (2018). Effects of the ketogenic diet in overweight divers breathing enriched air nitrox. Scientific Reports 8: 2655. https://doi.org/10.1038/s41598-018-20933-w [Google Scholar] [PubMed] [CrossRef]
Camara ML, Corrigan F, Jaehne EJ, Jawahar MC, Anscomb H, Koerner H, Baune B (2013). TNF-α and its receptors modulate complex behaviours and neurotrophins in transgenic mice. Psychoneuroendocrinology 38: 3102–3114. https://doi.org/10.1016/j.psyneuen.2013.09.010 [Google Scholar] [PubMed] [CrossRef]
Castaldo G, Pagano I, Grimaldi M, Marino C, Molettieri P et al. (2021). Effect of very-low-calorie ketogenic diet on psoriasis patients: A nuclear magnetic resonance-based metabolomic study. Journal of Proteome Research 20: 1509–1521. https://doi.org/10.1021/acs.jproteome.0c00646 [Google Scholar] [PubMed] [CrossRef]
Cipryan L, Dostal T, Plews DJ, Hofmann P, Laursen PB (2020a). Adiponectin/leptin ratio increases after a 12-week very low-carbohydrate, high-fat diet, and exercise training in healthy individuals: A non-randomized, parallel design study. Nutrition Research 87: 22–30. https://doi.org/10.1016/j.nutres.2020.12.012 [Google Scholar] [PubMed] [CrossRef]
Cipryan L, Maffetone PB, Plews DJ, Laursen PB (2020b). Effects of a four-week very low-carbohydrate high-fat diet on biomarkers of inflammation: Non-randomised parallel-group study. Nutrition and Health 26: 35–42. https://doi.org/10.1177/0260106020903206 [Google Scholar] [PubMed] [CrossRef]
Deng Y, Xie M, Li Q, Xu X, Ou W et al. (2021). Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circulation Research 128: 232–245. https://doi.org/10.1161/CIRCRESAHA.120.317933 [Google Scholar] [PubMed] [CrossRef]
Friedrich MJ (2017). Depression is the leading cause of disability around the world. JAMA 317: 1517. https://doi.org/10.1001/jama.2017.3826 [Google Scholar] [PubMed] [CrossRef]
Gałecki P, Talarowska M (2018). Inflammatory theory of depression. Psychiatria Polska 52: 437–447. https://doi.org/10.12740/PP/76863 [Google Scholar] [PubMed] [CrossRef]
Gelman PL (2019). The cytokine profile of women with severe anxiety and depression during pregnancy. BMC Psychiatry 19: 104. https://doi.org/10.1186/s12888-019-2087-6 [Google Scholar] [PubMed] [CrossRef]
Goldberg EL, Asher JL, Molony RD, Shaw AC, Zeiss CJ, Wang C, Morozova-Roche LA, Herzog RI, Iwasaki A, Dixit VD (2017). β-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Reports 18: 2077–2087. https://doi.org/10.1016/j.celrep.2017.02.004 [Google Scholar] [PubMed] [CrossRef]
Hashim SA, VanItallie TB (2014). Ketone body therapy: From the ketogenic diet to the oral administration of ketone ester. Journal of Lipid Research 55: 1818–1826. https://doi.org/10.1194/jlr.R046599 [Google Scholar] [PubMed] [CrossRef]
Hou R, Garner M, Holmes C, Osmond C, Teeling J, Lau L, Baldwin DS (2017). Peripheral inflammatory cytokines and immune balance in generalised anxiety disorder: Case-controlled study. Brain, Behavior, and Immunity 62: 212–218. https://doi.org/10.1016/j.bbi.2017.01.021 [Google Scholar] [PubMed] [CrossRef]
Huang C, Wang P, Xu X, Zhang Y, Gong Y et al. (2018). The ketone body metabolite β-hydroxybutyrate induces an antidepression-associated ramification of microglia via HDACs inhibition-triggered Akt-small RhoGTPase activation. Glia 66: 256–278. https://doi.org/10.1002/glia.23241 [Google Scholar] [PubMed] [CrossRef]
Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS (2011). Ketogenic diet-induced peroxisome proliferator-activated receptor-γ activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Experimental Neurology 232: 195–202. https://doi.org/10.1016/j.expneurol.2011.09.001 [Google Scholar] [PubMed] [CrossRef]
Jia Y, Liu L, Sheng C, Cheng Z, Cui L et al. (2019). Increased serum levels of cortisol and inflammatory cytokines in people with depression. The Journal of Nervous and Mental Diseases 207: 271–276. https://doi.org/10.1097/NMD.0000000000000957 [Google Scholar] [PubMed] [CrossRef]
Kajitani N, Iwata M, Miura A, Tsunetomi K, Yamanashi T et al. (2020). Prefrontal cortex infusion of beta-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, produces antidepressant-like effects in a rodent model of depression. Neuropsychopharmacolpgy Reports 40: 157–165. https://doi.org/10.1002/npr2.12099 [Google Scholar] [PubMed] [CrossRef]
Khodabakhshi A, Akbari ME, Mirzaei HR, Seyfried TN, Kalamian M, Davoodi SH (2021). Effects of Ketogenic metabolic therapy on patients with breast cancer: A randomized controlled clinical trial. Clinical Nutrition 40: 751–758. https://doi.org/10.1016/j.clnu.2020.06.028 [Google Scholar] [PubMed] [CrossRef]
Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM (2012). Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One 7: e35476. https://doi.org/10.1371/journal.pone.0035476 [Google Scholar] [PubMed] [CrossRef]
Kim DH, Park MH, Ha S, Bang EJ, Lee Y, Lee AK, Lee J, Yu BP, Chung HY (2019). Anti-inflammatory action of β-hydroxybutyrate via modulation of PGC-1α and FoxO1, mimicking calorie restriction. Aging 11: 1283–1304. https://doi.org/10.18632/aging.101838 [Google Scholar] [PubMed] [CrossRef]
Kong G, Huang Z, Ji W, Wang X, Liu J, Wu X, Huang Z, Li R, Zhu Q (2017). The ketone metabolite β-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class 1 histone deacetylases. Journal of Neurotrauma 34: 2645–2655. https://doi.org/10.1089/neu.2017.5192 [Google Scholar] [PubMed] [CrossRef]
Kong Z, Sun S, Shi Q, Zhang H, Tong TK, Nie J (2020). Short-term ketogenic diet improves abdominal obesity in overweight/obese Chinese young females. Frontiers of Physiology 11: 856. https://doi.org/10.3389/fphys.2020.00856 [Google Scholar] [PubMed] [CrossRef]
Kovács Z, D’Agostino DP, Ari C (2018). Anxiolytic effect of exogenous ketone supplementation is abolished by adenosine A1 receptor inhibition in Wistar Albino Glaxo/Rijswijk rats. Frontiers in Behavioral Neuroscience 12: 29. https://doi.org/10.3389/fnbeh.2018.00029 [Google Scholar] [PubMed] [CrossRef]
Kovács Z, D’Agostino DP, Diamond D, Kindy MS, Rogers C, Ari C (2019). Therapeutic potential of exogenous ketone supplement induced ketosis in the treatment of psychiatric disorders: Review of current literature. Frontiers in Psychiatry 10: 363. https://doi.org/10.3389/fpsyt.2019.00363 [Google Scholar] [PubMed] [CrossRef]
Lu Y, Yang YY, Zhou MW, Liu N, Xing HY, Liu XX, Li F (2018). Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neuroscience Letters 683: 13–18. https://doi.org/10.1016/j.neulet.2018.06.016 [Google Scholar] [PubMed] [CrossRef]
Ma S, Suzuki K (2019). Keto-adaptation and endurance exercise capacity, fatigue recovery, and exercise-induced muscle and organ damage prevention: A narrative review. Sports 7: 40. https://doi.org/10.3390/sports7020040 [Google Scholar] [PubMed] [CrossRef]
Ma S, Tominaga T, Kanda K, Sugama K, Omae C, Hashimoto S, Aoyama K, Yoshikai Y, Suzuki K (2020). Effects of an 8-week protein supplementation regimen with hyperimmunized cow milk on exercise-induced organ damage and inflammation in male runners: A randomized, placebo controlled, cross-over study. Biomedicines 8: 51. https://doi.org/10.3390/biomedicines8030051 [Google Scholar] [PubMed] [CrossRef]
Martin-Arrowsmith PW, Lov J, Dai J, Morais JA, Churchward-Venne TA (2020). Ketone monoester supplementation does not expedite the recovery of indices of muscle damage after eccentric exercise. Frontiers in Nutrition 7: 607299. https://doi.org/10.3389/fnut.2020.607299 [Google Scholar] [PubMed] [CrossRef]
Martinez P, Lien L, Zemore S, Bramness JG, Neupane SP (2018). Circulating cytokine levels are associated with symptoms of depression and anxiety among people with alcohol and drug use disorders. Journal of Neuroimmunology 318: 80–86. https://doi.org/10.1016/j.jneuroim.2018.02.011 [Google Scholar] [PubMed] [CrossRef]
Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, Burgess S, Penninx BWJH, Khandaker GM (2021). Association of inflammation with depression and anxiety: Evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Molecular Psychiatry 26: 7393–7402. https://doi.org/10.1038/s41380-021-01188-w [Google Scholar] [PubMed] [CrossRef]
Monda V, Polito R, Lovino A, Finaldi A, Valenzano A et al. (2020). Short-term physiological effects of a very low-calorie ketogenic diet: Efects on adiponectin levels and inflammatory states. International Journal of Molecular Sciences 21: 3228. https://doi.org/10.3390/ijms21093228 [Google Scholar] [PubMed] [CrossRef]
Morris GF, Carvalho A, Anderson G, Galecki P, Maes M (2016). The many neuroprogressive actions of tryptophan catabolites (TRYCATs) that may be associated with the pathophysiology of neuro-immune disorders. Current Pharmaceutical Design 22: 963–977. https://doi.org/10.2174/1381612822666151215102420 [Google Scholar] [PubMed] [CrossRef]
Murray CL, Obiang P, Bannerman D, Cunningham C (2013). Endogenous IL-1 in cognitive function and anxiety: A study in IL-1RI−/− mice. PLoS One 10: e78385. https://doi.org/10.1371/journal.pone.0078385 [Google Scholar] [PubMed] [CrossRef]
Nandivada P, Fell GL, Pan AH, Nose V, Ling PR, Bistrian BR, Puder M (2016). Eucaloric ketogenic diet reduces hypoglycemia and inflammation in mice with endotoxemia. Lipids 51: 703–714. https://doi.org/10.1007/s11745-016-4156-7 [Google Scholar] [PubMed] [CrossRef]
Naoi M, Maruyama W, Shamoto-Nagai M (2018). Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: From neurotransmitter imbalance to impaired neurogenesis. Journal of Neural Transmission 125: 53–66. https://doi.org/10.1007/s00702-017-1709-8 [Google Scholar] [PubMed] [CrossRef]
Ottaviano K, Zaman JA (2023). Diet and Inflammation in Obesity: Prevention and Management in Inflammation and Obesity, pp. 213–231. Cambridge: Academic Press. [Google Scholar]
O’Malley T, Myette-Cote E, Durrer C, Little JP (2017). Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Applied Physiology, Nutrition, and Metabolism 42: 1031–1035. https://doi.org/10.1139/apnm-2016-0641 [Google Scholar] [PubMed] [CrossRef]
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC et al. (2021). The PRISMA, 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: n71. https://doi.org/10.1136/bmj.n71 [Google Scholar] [PubMed] [CrossRef]
Paoli A, Cenci L, Pompei P, Sahin N, Bianco A, Neri M, Caprio M, Moro T (2021). Effects of two months of very low carbohydrate ketogenic diet on body composition, muscle strength, muscle area, and blood parameters in competitive natural body builders. Nutrients 13: 374. https://doi.org/10.3390/nu13020374 [Google Scholar] [PubMed] [CrossRef]
Petralia MC, Mazzon E, Fagone P, Falzone L, Bramanti P, Nicoletti F, Basile MS (2019). Retrospective follow-up analysis of the transcriptomic patterns of cytokines, cytokine receptors and chemokines at preconception and during pregnancy, in women with post-partum depression. Experimental and Therapeutic Medicine 18: 2055–2062. https://doi.org/10.3892/etm.2019.7774 [Google Scholar] [PubMed] [CrossRef]
Pilkington K (2018). Current research on complementary and alternative medicine (CAM) in the treatment of depression: Evidence-based review. In: Understanding Depression, vol. 2, pp. 317–328. Singapore: Springer. [Google Scholar]
Prattichizzo F, Nigris VD, Micheloni S, Sala LL, Ceriello A (2018). Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: Is low-grade inflammation the neglected component? Diabetes. Obesity and Metabolism 20: 2515–2522. https://doi.org/10.1111/dom.13488 [Google Scholar] [PubMed] [CrossRef]
Qiao G, Lv T, Zhang M, Chen P, Sun Q, Zhang J, Li Q (2020). β-Hydroxybutyrate (β-HB) exerts anti-Inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated macrophages in Liza haematocheila. Fish and Shellfish Immunology 107: 444–451. https://doi.org/10.1016/j.fsi.2020.11.005 [Google Scholar] [PubMed] [CrossRef]
Rhyu H, Cho SY (2014). The effect of weight loss by ketogenic diet on the body composition, performance-related physical fitness factors and cytokines of Taekwondo athletes. Journal of Exercise Rehabilitation 10: 326–331. https://doi.org/10.12965/jer.140160 [Google Scholar] [PubMed] [CrossRef]
Rosenbaum M, Hall KD, Guo J, Ravussin E, Mayer LS, Reitman ML, Smith SR, Walsh BT, Leibel RL (2019). Glucose and lipid homeostasis and inflammation in humans following an isocaloric ketogenic diet. Obesity 27: 971–981. https://doi.org/10.1002/oby.22468 [Google Scholar] [PubMed] [CrossRef]
Ruskin DN, Sturdevant IC, Wyss LS, Masino SA (2021). Ketogenic diet effects on inflammatory allodynia and ongoing pain in rodents. Scientific Reports 11: 725. https://doi.org/10.1038/s41598-020-80727-x [Google Scholar] [PubMed] [CrossRef]
Shaw DM, Merien F, Braakhuis A, Keaney L, Dulson DK (2020). Acute hyperketonaemia alters T-cell-related cytokine gene expression within stimulated peripheral blood mononuclear cells following prolonged exercise. European Journal of Applied Physiology 120: 191–202. https://doi.org/10.1007/s00421-019-04263-x [Google Scholar] [PubMed] [CrossRef]
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K et al. (2013). Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339: 211–214. https://doi.org/10.1126/science.1227166 [Google Scholar] [PubMed] [CrossRef]
Stein MB, Craske MG (2017). Treating anxiety in 2017: Optimizing care to improve outcomes. JAMA 318: 235–236. https://doi.org/10.1001/jama.2017.6996 [Google Scholar] [PubMed] [CrossRef]
Suzuki K (2018a). Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 9: 223. https://doi.org/10.3390/biom9060223 [Google Scholar] [PubMed] [CrossRef]
Suzuki K (2018b). Cytokine response to exercise and its modulation. Antioxidants 7: 17. https://doi.org/10.3390/antiox7010017 [Google Scholar] [CrossRef]
Suzuki K, Tominaga T, Ruhee RT, Ma S (2020). Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants 9: 401. https://doi.org/10.3390/antiox9050401 [Google Scholar] [PubMed] [CrossRef]
van Eeden W (2022). Basal and LPS-stimulated inflammatory markers and the course of individual symptoms of depression and anxiety. European Psychiatry 65: S259. https://doi.org/10.1192/j.eurpsy.2022.666 [Google Scholar] [CrossRef]
van Velzen LS, Wijdeveld M, Black CN, van Tol MJ, van der Wee NJA, Veltman DJ, Penninx BWJH, Schmaal L (2017). Oxidative stress and brain morphology in individuals with depression, anxiety and healthy controls. Progress in Neuro-Psychopharmacology and Biological Psychiatry 76: 140–144. https://doi.org/10.1016/j.pnpbp.2017.02.017 [Google Scholar] [PubMed] [CrossRef]
World Health Organization (2017). Depression and other common mental disorders: Global health estimates. Geneva: World Health Organization, No. WHO/MSD/MER/2017.2. [Google Scholar]
Yamanashi T, Iwata M, Kamiya N, Tsunetomi K, Kajitani N et al. (2017). Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Scientific Reports 7: 7677. https://doi.org/10.1038/s41598-017-08055-1 [Google Scholar] [PubMed] [CrossRef]
Yan Q (2018). Neuroimmune imbalances and yin-yang dynamics in stress, anxiety, and depression. Psychoneuroimmunology: Methods and Protocols 1781: 77–85. https://doi.org/10.1007/978-1-4939-7828-1_5 [Google Scholar] [PubMed] [CrossRef]
Yang X, Cheng B (2010). Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. Journal of Molecular Neuroscience 42: 145–153. https://doi.org/10.1007/s12031-010-9336-y [Google Scholar] [PubMed] [CrossRef]
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M et al. (2015). The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nature Medicine 21: 263–269. https://doi.org/10.1038/nm.3804 [Google Scholar] [PubMed] [CrossRef]
Zhang Q, Xu L, Xia J, Wang D, Qian M, Ding S (2018). treatment of diabetic mice with a combination of ketogenic diet and aerobic exercise via modulations of PPARs gene programs. PPAR Research 2018: 4827643. https://doi.org/10.1155/2018/4827643 [Google Scholar] [PubMed] [CrossRef]
Zitvogel L, Pietrocola F, Kroemer G (2017). Nutrition, inflammation and cancer. Nature Immunology 18: 843–850. https://doi.org/10.1038/ni.3754 [Google Scholar] [PubMed] [CrossRef]
Zou W, Feng R, Yang Y (2018). Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS One 13: e0197267. https://doi.org/10.1371/journal.pone.0197267 [Google Scholar] [PubMed] [CrossRef]
Supplementary Materials
TABLE S1. Prisma checklist
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF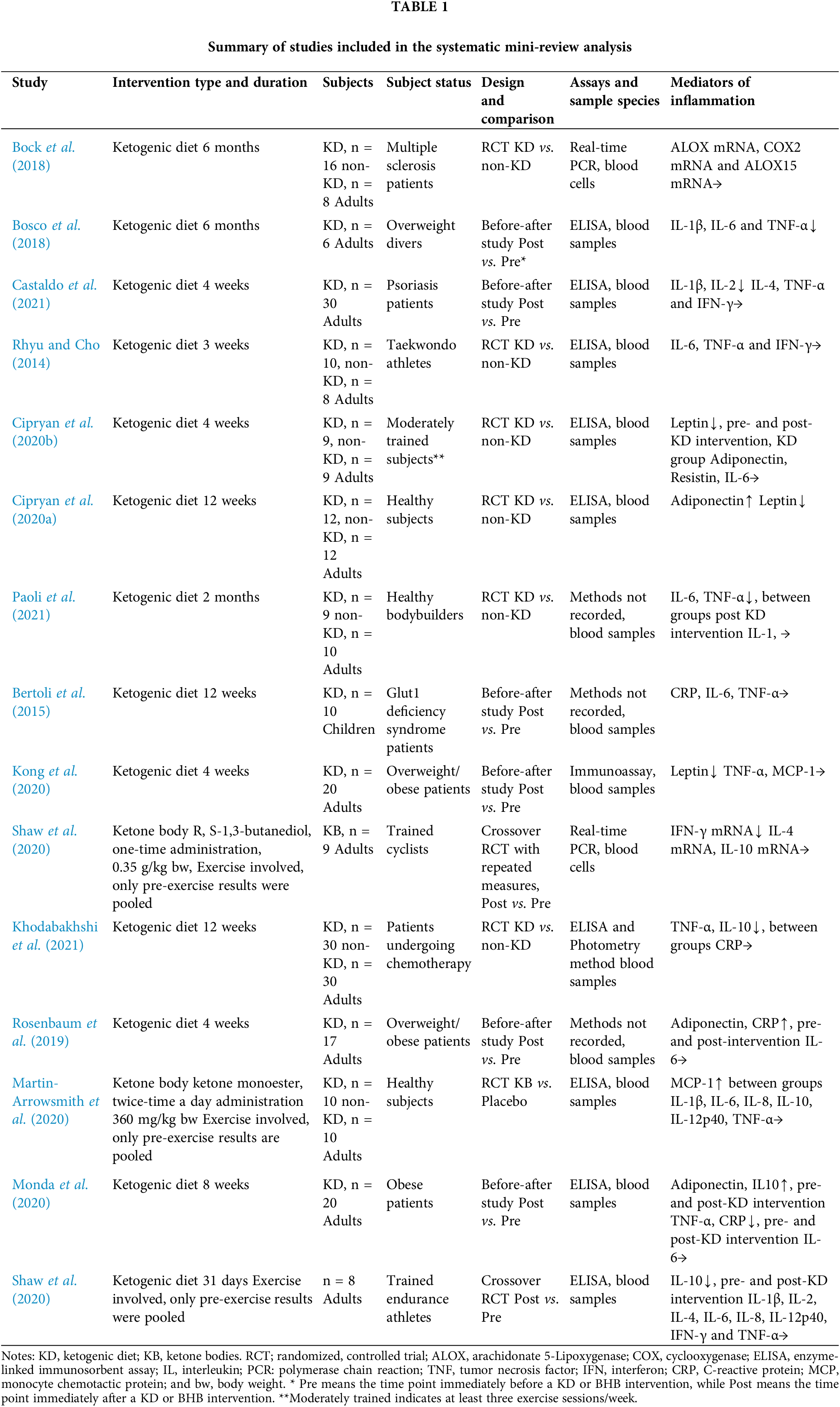
 Downloads
Downloads
 Citation Tools
Citation Tools