 Open Access
Open Access
ARTICLE
MiR-520f-3p inhibits epithelial-mesenchymal transition of colorectal cancer cells by targeting Yes-associated protein 1
1 Department of Gastroenterology, The First Clinical Medical College of Shanxi Medical University, Taiyuan, 030001, China
2 The First Clinical Medical School, Shanxi Medical University, Taiyuan, 030001, China
* Corresponding Author: Jinchun Liu,
(This article belongs to the Special Issue: Non-Coding RNAs in the Regulation of Human Cancers)
BIOCELL 2023, 47(8), 1803-1810. https://doi.org/10.32604/biocell.2023.029516
Received 23 February 2023; Accepted 02 April 2023; Issue published 28 August 2023
Abstract
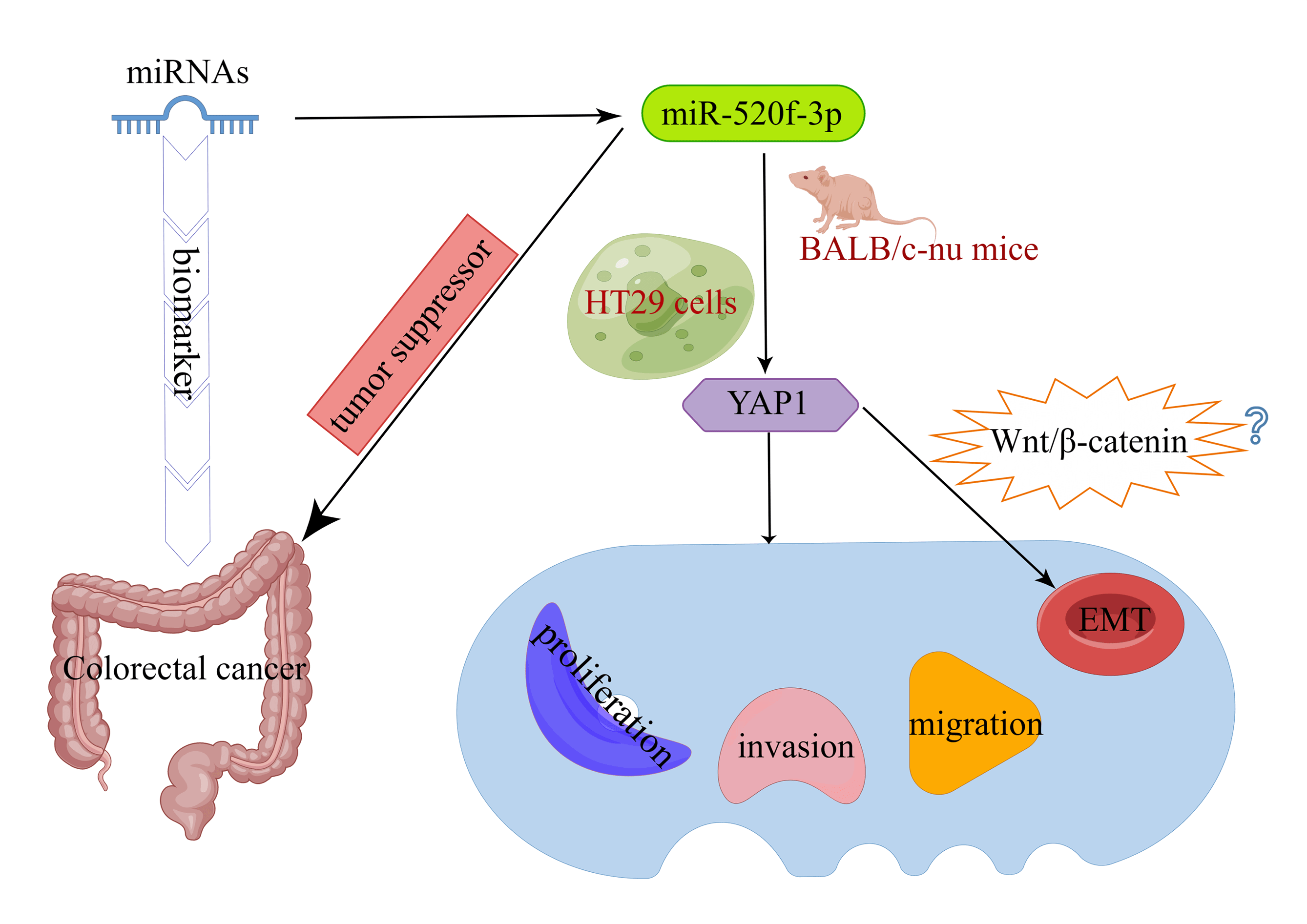
Background: Colorectal cancer (CRC) is one of the most common malignancies. Early diagnosis is the key to effective treatment of CRC. Since microRNAs (miRNAs) can be used as biomarkers of CRC, the objective of this work was to examine the effect of miR-520f-3p, which targets YAP1 (Yes-associated protein 1), on the ability of CRC cells to proliferate, invade, migrate, and undergo epithelial-mesenchymal transition (EMT). Methods: A miR-520f-3p mimic was used to overexpress miR-520f-3p in HT29 cells. To establish the tumor-bearing mouse model, transfected HT29 cells were subcutaneously implanted into BALB/c-nu nude mice, and YAP1 and miR-520f-3p levels were determined using qRT‒PCR. The viability, invasion ability, and migration ability of cells were evaluated by CCK-8, Transwell, and wound healing assays. Apoptosis was detected by flow cytometry and TUNEL assays. The regulatory link between miR-520f-3p and the YAP1 gene was examined by dual-luciferase reporter assay. Tumor tissues with positive Ki-67 expression were identified by immunohistochemistry. Vimentin, E-cadherin, and YAP1 expression were evaluated by western blotting. Results: MiR-520f-3p overexpression could inhibit proliferation, invasion, migration, and EMT and induce apoptosis in HT29 cells. YAP1 was found as a target of miR-520f-3p. The inhibitory effects of miR-520f-3p on proliferation, invasion, migration, and EMT may be reversed by overexpressing YAP1. In tumor-bearing mice, miR- 520f-3p overexpression reduced the Ki-67 level, increased apoptosis, and prevented tumor development and spread. Conclusion: By targeting YAP1, miR-520f-3p may be capable of suppressing CRC cell proliferation, invasion, migration, and EMT, providing a novel therapeutic target for the disease.Graphic Abstract
Keywords
Among gastrointestinal malignancies, colorectal cancer (CRC) has the second-highest morbidity and mortality rates, second only to gastric and esophageal cancers (Goncalves-Ribeiro et al., 2016). Despite significant advancements in surgical procedures, the 5-year survival rate and surgical cure rate for CRC have consistently been relatively low, approximately 50%, in recent years (Lievre et al., 2006). Patients with CRC still have a significant risk of local recurrence after surgery. Therefore, for the current therapeutic management of CRC, it has become vital to enhance the understanding of the pathological process of CRC and conduct research on specific innovative biomarkers for early clinical diagnosis and prognostic evaluation of CRC (Aan et al., 2016).
MicroRNAs (miRNAs), molecules like siRNAs, play a number of roles in the regulation of cell growth and developmental processes (Li et al., 2016). MiRNAs are thought to function as oncogenes and are closely linked to tumor formation (Gregory et al., 2008). They are crucial in the development of CRC and in colorectal carcinogenesis. Following the clinical discovery of abnormal levels of miR-92 in the plasma of CRC patients, it has been considered a biomarker for CRC screening (Ng et al., 2009). Additionally, the expression of miR-21, miR-31, miR-143, and miR-145 is strongly correlated with clinicopathological features of CRC (Slaby et al., 2007). Among these miRNAs, miR-143 functions in the inhibition of CRC proliferation and promotes apoptosis (Borralho et al., 2009). MiR-520f-3p, which exerts suppressive effects in a number of malignancies, is another important miRNA. MiR-520f-3p targets sex-determining region Y box protein 9 to inhibit gastric cancer proliferation (Chen et al., 2020) and also downregulates leucine zipper 1, another mechanism by which it promotes the malignancy of gliomas (Xiao et al., 2019). However, the function of miR-520f-3p in CRC is uncertain. In this article, we focus on the role and mechanism of action of miR-520f-3p in colon cancer.
Several characteristics of cancer malignancy, such as tumor invasion, metastasis development, and therapy resistance, are correlated with epithelial-mesenchymal transition (EMT) (Li et al., 2015). YAP1 (Yes Associated Protein 1) has been extensively documented to be involved in the EMT process (Wierzbicki and Rybarczyk, 2015). YAP1 affects the Wnt/β-catenin signaling pathway to control the growth of glioma cells (Reis et al., 2012). However, it is unknown whether miR-520f-3p can influence CRC cell motility, invasion, and EMT by targeting YAP1. In this study, we examined EMT in CRC cells by transfecting miR-520f-3p mimic in HT29 cells. We discovered that miR-520f-3p acted as a tumor suppressor by targeting YAP1, thus preventing the growth of tumors and inhibiting EMT in CRC cells.
The HT29 cell line was obtained from the American Type Culture Collection (ATCC, Manassas, USA). Cells were cultured at 37°C in DMEM containing 10% fetal bovine serum (FBS) and 5% CO2. Cell fusion was considered complete when the confluence was at least 80%. Cells in the logarithmic growth phase were selected for subsequent experimental studies. The miR-520f-3p mimic and its nonspecific negative control (NC) oligos, the miR-520f-3p agomir and its NC, and pcDNA 3.0-YAP1 and pcDNA-NC were provided by GenePharma (Shanghai Scigrace Biotech Co., Ltd., Shanghai, China).
HT29 cells were transfected with the NC and the miR-520f-3p mimic. The study had three groups: the control group, the NC group, and the miR-520f-3p mimic group.
Three groups were used in the experiment: the miR-520f-3p mimic group, the miR-520f-3p mimic + pcDNA-NC group, and the miR-520f-3p mimic + pcDNA-YAP1 group.
Cell counting kit-8 (CCK-8) assay
The 96-well plates used for the growth of the transfected cells were seeded at a density of 2 × 104 cells in 1 mL per well. A volume of 100 μL of cell culture medium was added to each well. After incubating the cells at room temperature for 12, 24, and 48 h, the cell viability in the plate was evaluated. Following a 2-h treatment at 37°C with 10 μL of CCK8 reagent (GlpBio, Montclair, USA) in each well, the optical density (OD) at 450 nm was measured using a SpectraMax Mini-microplate reader (Thermo Fisher Scientific™, Waltham, USA).
Six-well plates with 2 × 105 cells in each well were used for cell culture. The cells were incubated in a serum-free medium for 24 h. The Transwell device (Corning, New York, USA) was placed in a CO2 incubator for 24 h at 37°C after 300 μL of cell suspension was added to the upper chamber of the device, which contained a membrane coated with matrix gel, and 500 μL of RPMI-1640 medium (Sigma‒Aldrich, Saint Louis, USA) containing 10% FBS was added to the lower chamber. Cells on the bottom surface of the chamber membrane were fixed with methanol for 20 min, followed by 20 min of staining with 0.1% crystal violet and 20 min of rinsing with phosphate-buffered saline (PBS). Five randomly selected fields were examined under an inverted microscope (Leica, Heidelberg, Germany) to count the invaded cells.
Cells (2 × 105) were seeded into 6-well plates for culture, and the cells were incubated in a serum-free medium for 24 h. Then, the cells were observed under a microscope, and the initial scratch width was recorded. After 24 h of incubation, the scratch width was observed and recorded again.
The Annexin-V-APC Detection Kit (Procell Life Science & Technology Co., Ltd., Wuhan, China) instructions were followed to identify apoptosis. After 48 h of transfection, HT29 cells from each group were collected, and 1 × 106 cells were resuspended in 100 μL of dye solution, shaken, and mixed before being added to 5 μL of Annexin-V-APC solution. Following a 15 min incubation at room temperature, 5 μL of propidium iodide was added, and the solution was carefully mixed and incubated for 5 min at 4°C in the dark. The cells were analyzed by an Attune flow cytometer (Thermo Fisher Scientific™, Waltham, USA) with a 488 nm excitation laser within 1 h.
Dual-luciferase reporter assay
The target sequences of YAP1 and miR-520f-3p were predicted using TargetScan. Both the wild-type (wt-YAP1) and mutant (mut-YAP1) sequences were inserted into the pSI-Check2 expression vector and co-transfected with the NC mimic or miR-520f-3p mimic. Finally, a Renilla-Firefly Luciferase Dual Assay Kit (MedChemExpress, New Jersey, USA) was used to measure the luciferase activity of the cells.
Quantitative reverse transcription–polymerase chain reaction (qRT‒PCR)
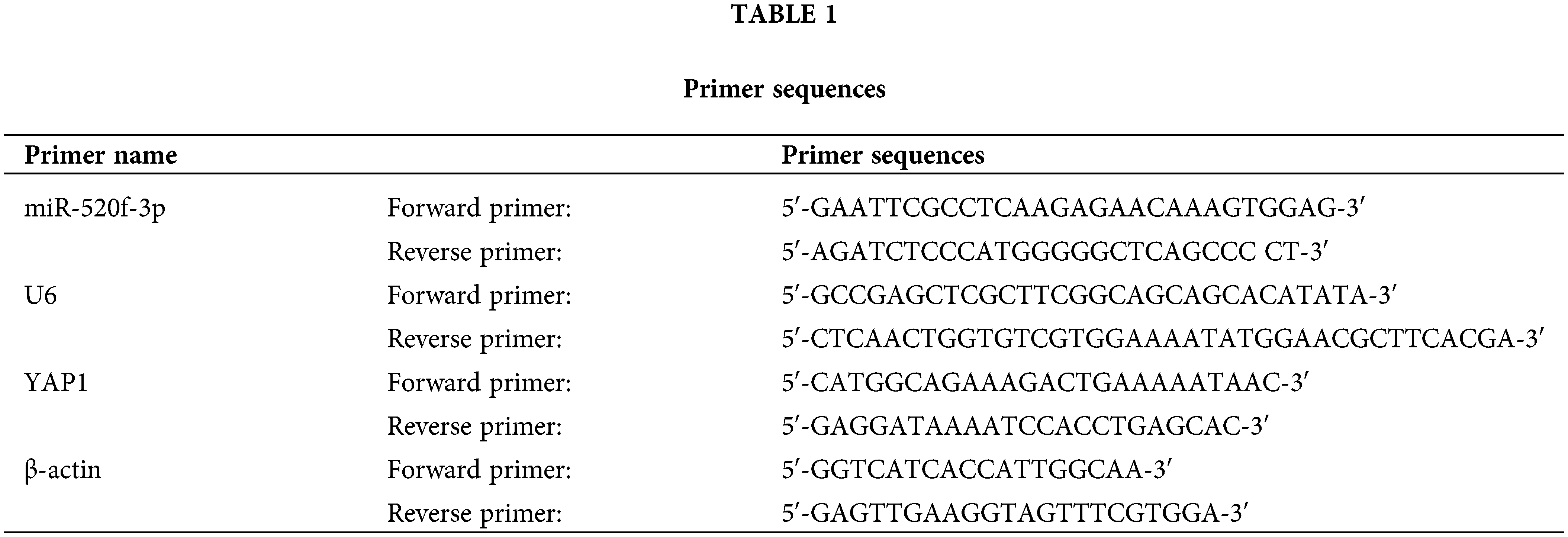
Total RNA was extracted from tumor tissues and CRC cells using TRIzol reagent (Thermo Fisher Scientific™, Waltham, USA) and then reverse-transcribed to cDNA. QPCR was performed using cDNA as the template. The reaction was carried out as follows: denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 15 s, annealing at 60°C for 30 s, extension at 72°C for 40 s, 55°C for 15 s, and 72°C for 40 s. Table 1 lists each primer sequence (MBL Beijing Biotech Co., Ltd., Beijing, China) in full. At the end of the experiment, the Ct values were estimated, and the 2−ΔΔCt method was used to determine the levels of miR-520f-3p and YAP1.
Total protein was extracted from HT29 cells and tumor tissues using RIPA buffer (Solarbio, Beijing, China). The protein concentration in each group was detected using the BCA protein quantification kit (Solarbio, Beijing, China). The proteins were then separated by SDS‒PAGE and transferred to PVDF membranes. The membrane was incubated with primary antibodies specific for E-cadherin (Abcam, Cambridge, UK), vimentin (Abcam, Cambridge, UK), and YAP1 (Abcam, Cambridge, UK) at 4°C overnight after blocking with 5% skim milk for 2 h. After that, the membrane was incubated for 1 h at room temperature with the appropriate secondary antibody. Protein bands were visualized using an enhanced chemiluminescence kit (Thermo Fisher Scientific™, Waltham, USA). The grayscale values of the target protein bands were analyzed using ImageJ software. For internal reference, β-actin was used.
Female BALB/c-nu mice (4–5 weeks old and weight 18–22 g) were provided from Shanxi University of Chinese Medicine (Charles River, Beijing, China). The mice were housed in an SPF-rated animal facility with a 12 h light-dark cycle, a temperature range of 22°C–24°C, and humidity in the range of 40%–60%. In the animal facility, the mice were provided clean drinking water and food. In this study, all the animal experiments were carried out in conformity with ethical guidelines for the use of laboratory animals (Medical Ethics Committee of Shanxi Medical University, ID: 2021-220). HT29 cells were transfected with the NC agomir or miR-520f-3p agomir. Then, the transfected HT29 cells (1 × 107 cells/mL) were injected subcutaneously to establish the tumorigenic nude mice model. The tumor volume was measured every seven days for a total of three times. These nude mice were euthanized after 21 days, and the tumor tissue was used for later tests. The mice were categorized into two groups: the NC agomir and miR-520f-3p agomir groups. Twelve mice were included in each group.
As previously mentioned, tumor tissues were embedded in paraffin, sliced into sections, and successively dehydrated with varying percentages of alcohol (75%, 85%, 95%, and 100%, v/v). After that, the sections underwent three PBS washes and were soaked in deionized water containing 3% H2O2 for 20 min. Following another PBS wash, the sections were blocked with goat serum for 15 min. Next, the sections were incubated overnight with a primary antibody against Ki-67 (Abcam, Cambridge, UK) at 4°C. After being incubated with the secondary antibody at 37°C for 2 h, the sections were stained with DAB (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) for 5–7 min. The slices were first rinsed in PBS for 5 min, re-stained with hematoxylin (Beyotime Biotechnology, Shanghai, China) for 2 min, treated with fractionated alcohol in hydrochloric acid for 1 min, and then rinsed in running water for 10 min. The slices were dried naturally and sealed, and finally, were photographed for documentation after positive protein expression had been observed under an optical microscope (Leica, Heidelberg, Germany).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
The standard procedure involved dewaxing, hydrating, and incubating the sections for 20 min at room temperature with 20 μg/mL protease K solution before three washes with PBS. Following a 1-h incubation in TUNEL reaction solution at 37°C, the sections were thoroughly washed three times with PBS. Then, 50 μL of HRP-streptavidin was added, and the sections were incubated at room temperature for 30 min. In addition, DAPI (Thermo Fisher Scientific™, Waltham, USA) and SYTO™ 9 Green Fluorescent Nucleic Acid Stain (Thermo Fisher Scientific™, Waltham, USA) were used to stain the sections after washing with PBS. A fluorescence microscope (Leica, Heidelberg, Germany) was used to visualize apoptotic cells.
All data are expressed as the means ± standard deviations (SD). SPSS 20.0 software was used for statistical evaluation. One-way ANOVA (including the least significant difference test or Dunnett’s test) was used to compare differences among various groups. Statistical significance is indicated as follows: *p < 0.05, **p < 0.01.
Inhibition of the growth and epithelial-mesenchymal transition of colorectal cancer cells due to miR-520f-3p overexpression
When HT29 cells were transfected with the miR-520f-3p mimic, the expression of miR-520f-3p was noticeably increased (Fig. 1A). The CCK-8 assay results miR-520f-3p overexpression inhibited the proliferation of HT29 cells (Fig. 1B). The flow cytometry results (Fig. 1C) suggested that miR-520f-3p overexpression dramatically induced apoptosis in HT29 cells. According to the Transwell and wound healing assay results (Figs. 1D and 1E), overexpression of miR-520f-3p markedly inhibited the invasion and migration of HT29 cells. Furthermore, western blotting (Fig. 1F) showed that overexpression of miR-520f-3p downregulated vimentin and upregulated E-cadherin. In summary, overexpression of miR-520f-3p could inhibit the proliferation, invasion, migration, and EMT of HT29 cells and promote their apoptosis.
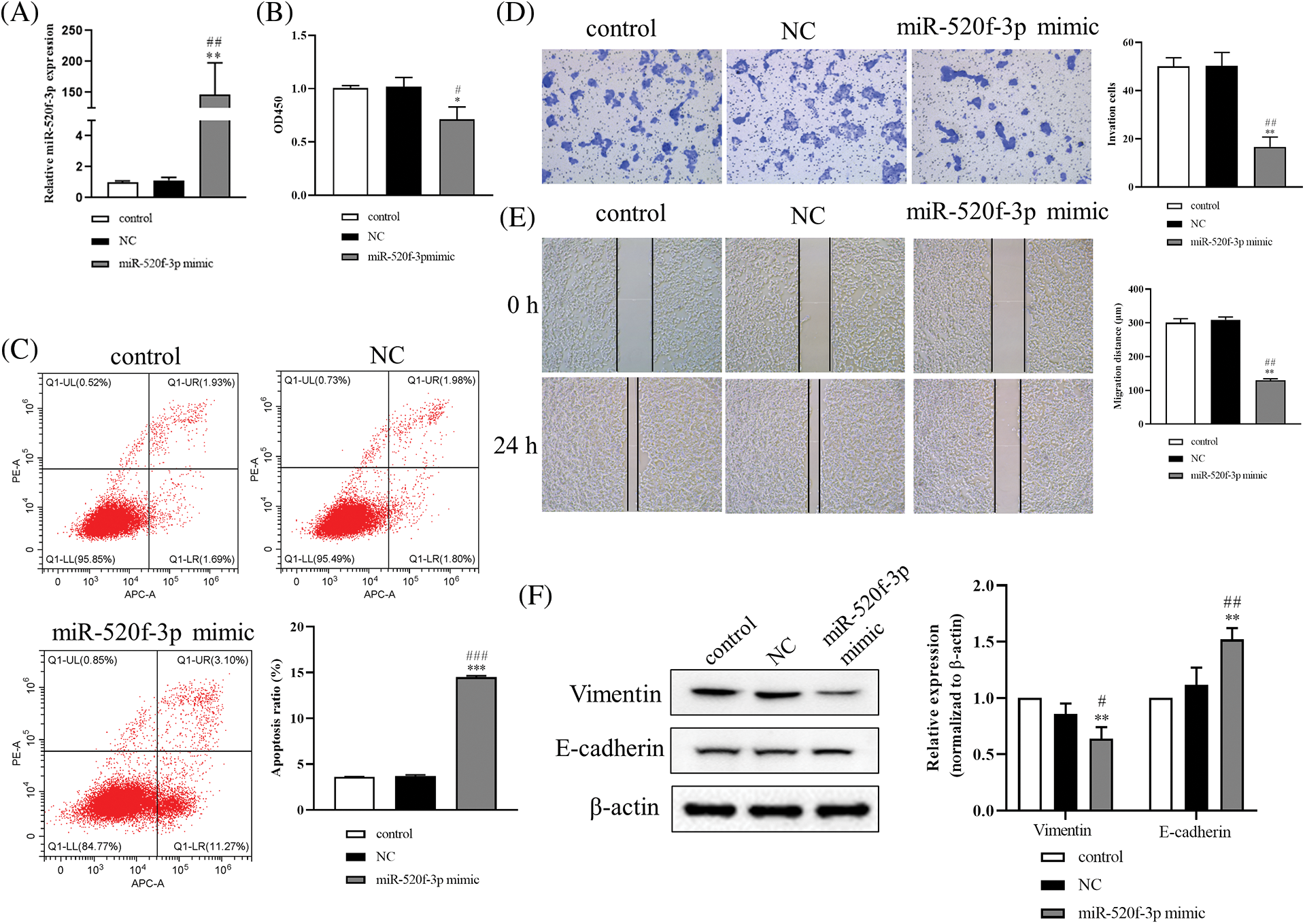
Figure 1: Overexpression of miR-520f-3p inhibited the growth and epithelial-mesenchymal transition of colorectal cancer cells. (A) Quantitative reverse transcription-polymerase chain reaction to determine the level of miR-520f-3p in HT29 cells. (B) A Cell Counting Kit-8 assay was used to determine the proliferation of HT29 cells. The OD450 value was determined. (C) Flow cytometry to determine the apoptosis rate. (D) Transwell invasion assay in HT29 cells. Scale bar, 50 μm. (E) Wound healing migration assay in HT29 cells. Scale bar, 50 μm. (F) Western blotting to detect vimentin and E-cadherin. The values are expressed as mean ± SD, n = 3 per group. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. the NC group.
Yes-associated protein 1 as the target gene of miR-520f-3p
To further understand the underlying molecular mechanism of miR-520f-3p, we examined any potential targeted regulatory link between miR-520f-3p and YAP1. The 3′UTR of YAP1 contained a nucleotide sequence complementary to miR-520f-3p (Fig. 2A). The dual-luciferase reporter assay indicated a significant decrease in luciferase activity after co-transfection of wt-YAP1-3′UTR with the miR-520f-3p mimic, whereas co-transfection of mut-YAP1-3′UTR with the miR-520f-3p mimic did not alter the luciferase activity (Fig. 2B). Then, qRT-PCR (Fig. 2C) and western blotting, respectively, (Fig. 2D) revealed that YAP1 protein and mRNA expression were considerably downregulated by the miR-520f-3p mimic, according to the findings. In summary, miR-520f-3p had a negative regulatory interaction with YAP1 in HT29 cells.
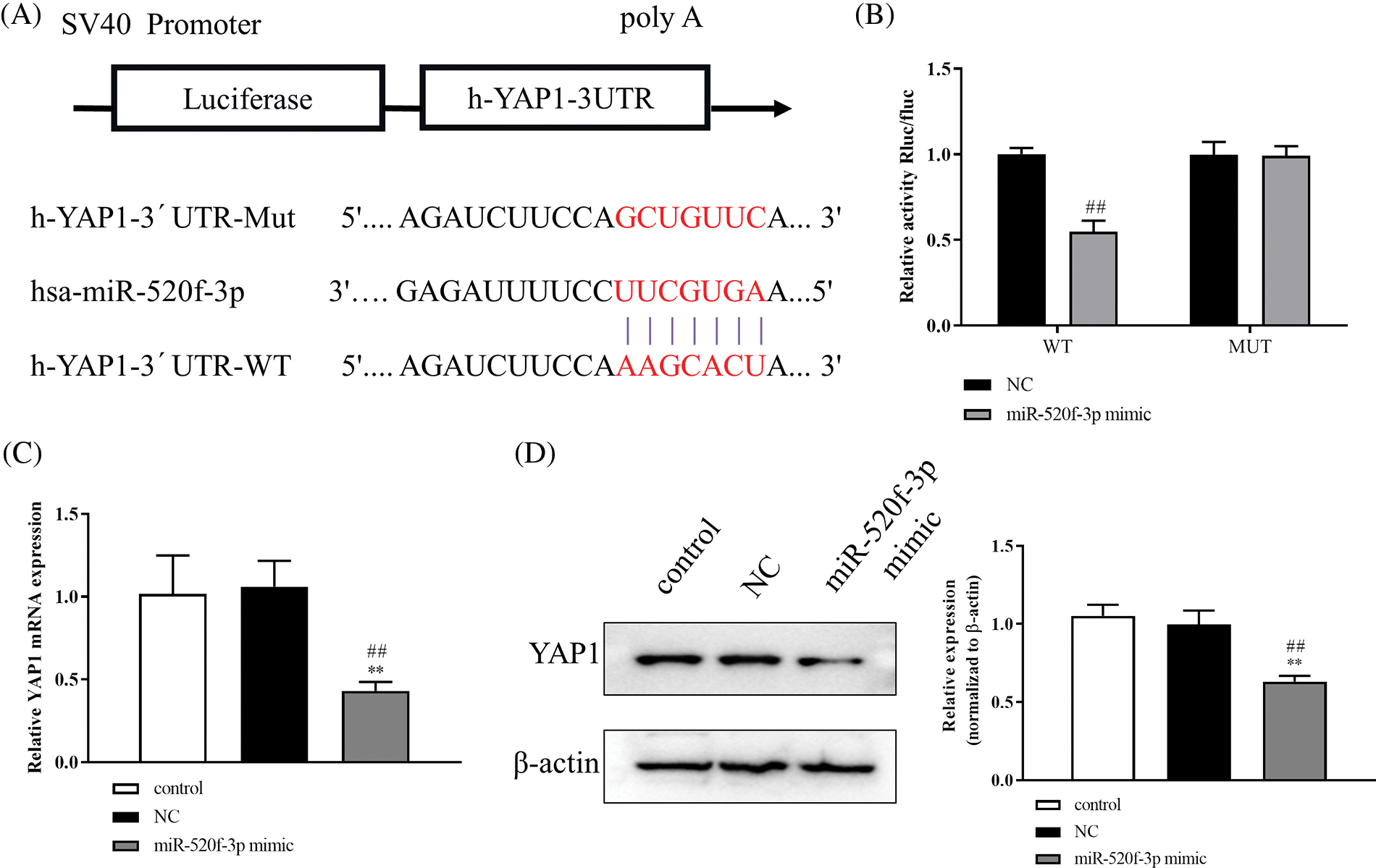
Figure 2: YAP1 is the target gene for miR-520f-3p. (A) The 3′UTR of YAP1 contains a nucleotide sequence complementary to miR-520f-3p. The red bases indicate the binding sites. (B) Dual-luciferase reporter assay. (C) Quantitative reverse transcription-polymerase chain reaction to measure YAP1 miRNA level in HT29 cells. (D) Western blotting to measure the level of Yes-associated protein 1 (YAP1). The values are expressed as mean ± SD, n = 3 per group. **p < 0.01 vs. the control group; ##p < 0.01 vs. the NC group.
The proliferation, apoptosis, invasion, and epithelial-mesenchymal transition of colorectal cancer cells are controlled by the miR-520f-3p target YAP1
The results from the CCK-8 assay showed significantly higher HT29 cell proliferation in the miR-520f-3p mimic+pcDNA-YAP1 group than in the miR-520f-3p mimic+pcDNA-NC group (Fig. 3A). Further, flow cytometry (Fig. 3B) showed reduced apoptosis rate of HT29 cells in the miR-520f-3p mimic+pcDNA-YAP1 group compared to the miR-520f-3p mimic+pcDNA-NC group. The Transwell assay results (Fig. 3C) suggested that the invasion ability of HT29 cells in the miR-520f-3p mimic+pcDNA-YAP1 group was significantly increased. The levels of vimentin protein increased and that of E-cadherin decreased in the miR-520f-3p mimic+pcDNA-YAP1 group compared to the miR-520f-3p mimic+pcDNA-NC group, according to western blot analysis (Fig. 3D). These findings demonstrated that miR-520f-3p could target YAP1 to regulate the proliferation, apoptosis, invasion, and EMT of HT29 cells.
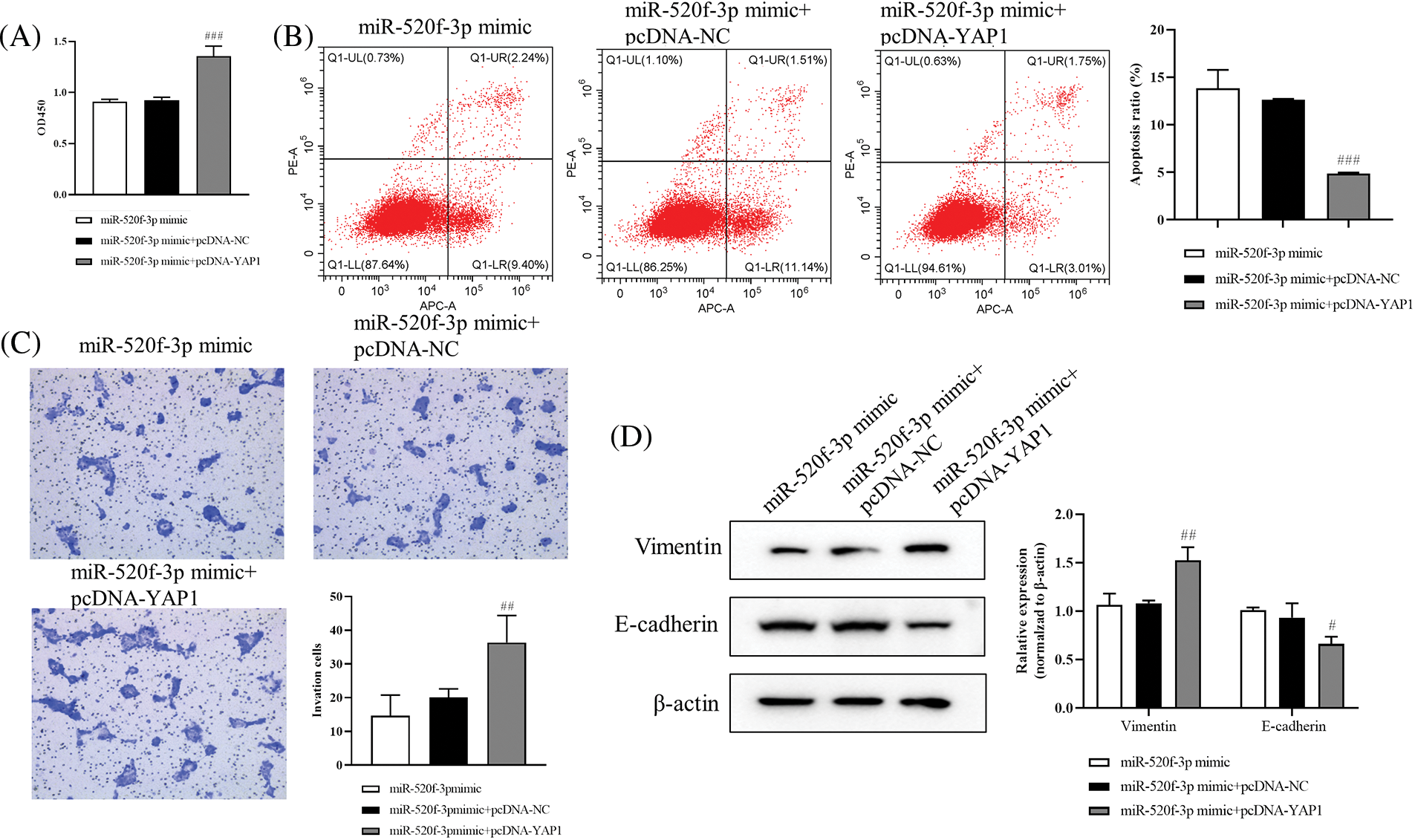
Figure 3: The proliferation, apoptosis, invasion, and epithelial-mesenchymal transition of colorectal cancer cells are controlled by the miR-520f-3p target YAP1. (A) The CCK-8 assay was used to evaluate the proliferation of HT29 cells. The OD450 value was determined. (B) Flow cytometry to determine the apoptosis rate. (C) Transwell invasion assay for HT29 cells. Scale bar, 50 μm. (D) Western blotting for vimentin and E-cadherin expression in HT29 cells. The values are expressed as the means ± SD, n = 3 per group. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. the miR-520f-3p mimic+pcDNA-NC group.
Effect of miR-520f-3p overexpression on tumor development and metastasis in colorectal cancer tumor-bearing mice
To investigate the effects of miR-520f-3p overexpression on tumor development and metastasis in mice with CRC, HT29 cells transfected with the NC agomir or miR-520f-3p agomir and were subcutaneously administered into mice to establish the tumor-bearing mouse model. Compared to the miR-520f-3p agomir group, the tumor volume in the NC agomir group increased steadily after 21 days (Fig. 4A); however, the body weights of the mice did not change significantly. The apoptosis rate in the miR-520f-3p agomir group increased significantly, as shown by the TUNEL assay (Fig. 4B). The qRT‒PCR results showed that compared to the NC agomir group, miR-520f-3p expression in the miR-520f-3p agomir group increased significantly (Fig. 4C). Malignant tumor development and growth are facilitated by the nuclear protein Ki-67. Immunohistochemical analysis revealed that the tumor tissue in the miR-520f-3p agomir group was considerably less positively stained, indicating Ki-67expression (Fig. 4D). Thus, miR-520f-3p overexpression prevented tumor development and metastasis in CRC tumor-bearing mice.
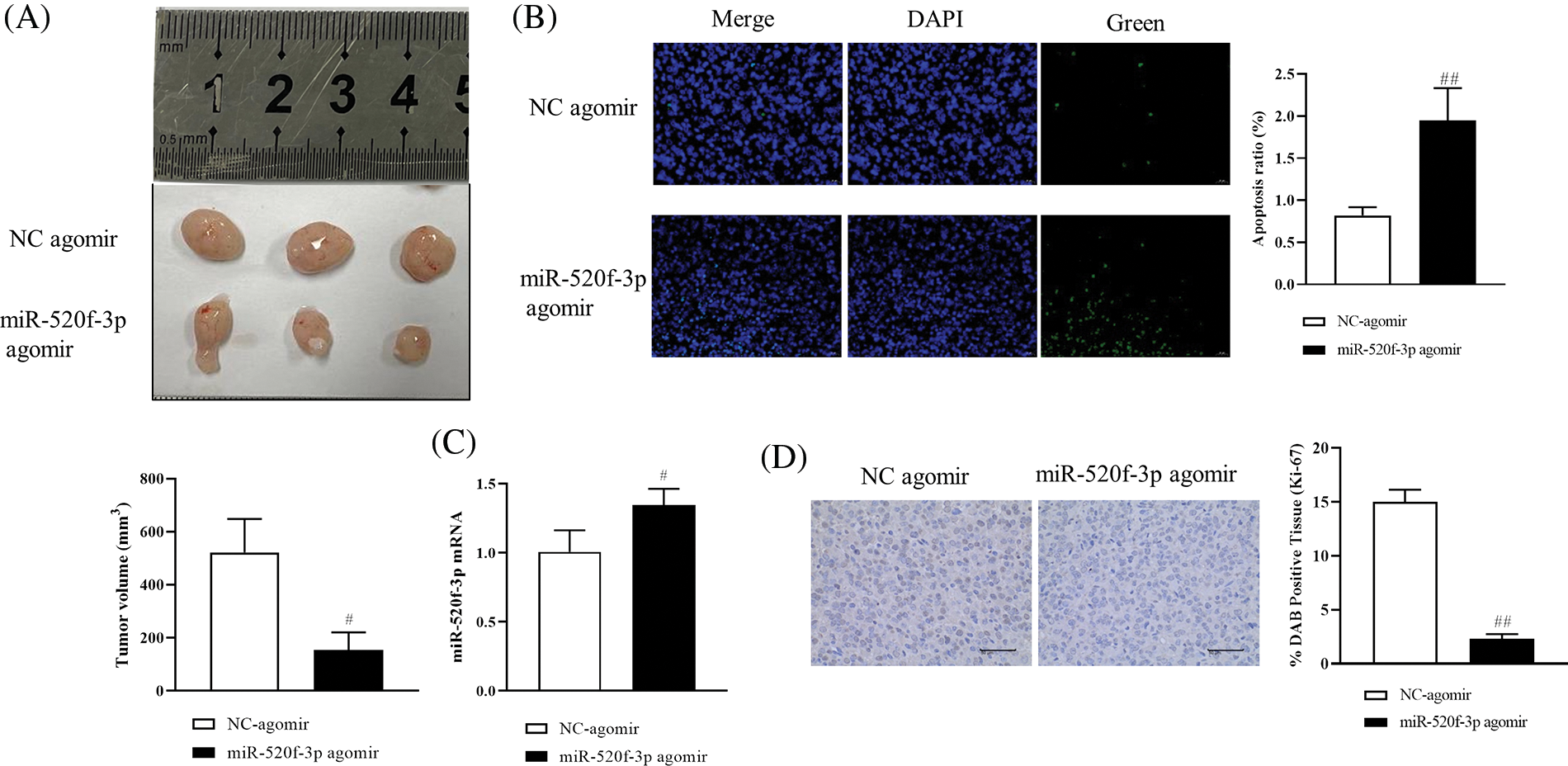
Figure 4: Effect of miR-520f-3p overexpression on tumor development and metastasis in colorectal cancer tumor-bearing mice. (A) The tumor volume was calculated as follows: (long diameter × short diameter × short diameter × 1/2). (B) Apoptotic cells (%) were detected by a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay. Scale bar, 20 μm. (C) The mRNA level of miR-520f-3p, as determined by quantitative reverse transcription-polymerase chain reaction. (D) Immunohistochemical analysis to detect the expression of Ki-67 in tumor tissues. Scale bar, 50 μm. The values are expressed as the means ± SD, n = 12 per group. #p < 0.05, ##p < 0.01 vs. the NC agomir group.
CRC is one of the more prevalent gastrointestinal malignancies (Li et al., 2021). In recent years, with changes in people’s lifestyles and dietary habits, its incidence has been increasing significantly (Bray et al., 2018; Baidoun et al., 2021). CRCs develop and spread through a multistep, multi-mechanistic process. Recent research has demonstrated a substantial correlation between miR-520f-3p expression and the development of CRC. The current work showed that overexpression of miR-520f-3p in CRC cells could substantially inhibit their ability to proliferate, migrate, invade, and undergo EMT and could even lead to their apoptosis. In addition, overexpression of YAP1, a target gene of miR-520f-3p, abolished the suppressive effects of the miR-520f-3p mimic on HT29 cell proliferation, migration, invasion, and EMT. Thus, miR-155-5p could exert tumor-suppressive effects by targeting YAP1. MiR-520f-3p can participate in biological processes through specific targets or signaling pathways (Drusco et al., 2018; Lu et al., 2021). In cholangiocarcinoma (CCA), suppression of SNHG20 expression was shown to control miR-520f-3p-mediated proliferation (Guan et al., 2020). Additionally, the lncRNA WEE2-AS1 contributes to glioblastoma development by acting as a molecular sponge for miR-520f-3p (Lin et al., 2021). In this study, we discovered that overexpression of miR-520f-3p inhibited HT29 cell proliferation, migration, and invasion, suggesting that miR-520f-3p expression inhibits the development of malignant behaviors in CRC cells and is inversely correlated with tumorigenic activities. EMT is a critical process for cancer cell metastasis (Lin et al., 2019). Certain involved proteins or miRNAs can regulate the EMT process, causing ovarian cancer to invade and spread (Braga et al., 2020; Yue et al., 2021; Liu et al., 2019). The findings of this study suggest that miR-520f-3p overexpression controlled the expression of EMT biomarkers, upregulating E-cadherin and downregulating vimentin expression. YAP1 has emerged as a promising candidate for cancer therapy because it plays an important role in modulating a number of oncogenic processes and inhibiting their activity (Wang et al., 2017). YAP1 overexpression also promotes EMT in breast cancer (Zhang et al., 2016), pancreatic cancer (Ling et al., 2017), CRC (Yuan et al., 2016), and hepatocellular carcinoma (Wang et al., 2016). YAP1, a target of miR-195, is downregulated by miR-195, which slows the development of CRC (Sun et al., 2017). The current study findings were in line with those of earlier investigations, which demonstrated that miR-520f-3p negatively targets to control YAP1. According to the study, modifying YAP1 expression could counteract the effect of miR-520f-3p on CRC cell migration, invasion, apoptosis, and EMT. These results showed that YAP1 overexpression increased the level of vimentin protein while decreasing the level of E-cadherin protein in the miR-520f-3p mimic-treated HT29 cells.
CRC cell proliferation and apoptosis were, respectively, suppressed and enhanced as a result of overexpression of miR-520f-3pin in vivo. In the tumor-bearing mouse model, the growth of tumors in mice in the miR-520f-3p agomir group was slower than that in the NC agomir group mice. The miR-520f-3p agomir promoted apoptosis in tumor cells. In colon cancer, Ki67 is the best molecular indicator for evaluating the proliferative activity of cancer cells. The rate of positive Ki-67 expression in the miR-520f-3p agomir group was noticeably lower than that in the NC agomir group.
The limitation of this study is that the mechanism by which miR-520f-3p inhibits the proliferation, invasion, migration, and EMT of CRC cells is not discussed in depth. Abnormal Wnt/β-catenin activation can directly drive the progression and metastasis of CRC, but whether miR-520f-3p can be exploited to treat CRC through this signaling pathway remains unclear. YAP1 is widely reported to be involved in the process of EMT. The Wnt pathway is also a critical pathway regulating EMT. β-Catenin is an important biomarker for detecting Wnt activation. The Hippo/YAP pathway interacts with the Wnt/β-catenin pathway in multiple ways, synergistically regulating tumorigenesis. The Hippo pathway affects the expression of Wnt target genes by altering the transcriptional activity of β-catenin. YAP1 is an important molecule involved in the transcription of components of the Hippo pathway. In future research, we will explore whether miR-520f-3p inhibits tumor growth and EMT in CRC cells by targeting YAP1 to inhibit the Wnt/β-catenin signaling pathway.
In summary, the current research showed that miR-520f-3p suppresses the growth and EMT of CRC cells by targeting YAP1. The study might identify miR-520f-3p and YAP1 as novel therapeutic targets for CRC.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Lijun Jiang: the conception and design of the manuscript; Jinchun Liu: administrative support; Wenmin Ji: provided the study materials; Yajie Gong: data collection and assembly; Jiajun Li: data analysis and interpretation. All authors participated in writing the manuscript and have read and approved the submitted manuscript.
Availability of Data and Materials: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Animal Medical Ethics Committee of Shanxi Medical University (ID: 2021-220).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Aan DSW, van Leeuwen BL, Elferink MA, de Bock GH (2016). The evaluation of more lymph nodes in colon cancer is associated with improved survival in patients of all ages. PLoS One 11: e155608. https://doi.org/10.1371/journal.pone.0155608 [Google Scholar] [PubMed] [CrossRef]
Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G et al. (2021). Colorectal cancer epidemiology: Recent trends and impact on outcomes. Current Drug Targets 22: 998–1009. https://doi.org/10.2174/18735592MTEx9NTk2y [Google Scholar] [CrossRef]
Borralho PM, Kren BT, Castro RE, Moreira da Silva IB, Steer CJ et al. (2009). MicroRNA-143 reduces viability and increases sensitivity to 5-fluorouracil in HCT116 human colorectal cancer cells. FEBS Journal 276: 6689–6700. https://doi.org/10.1111/j.1742-4658.2009.07383.x [Google Scholar] [PubMed] [CrossRef]
Braga EA, Fridman MV, Moscovtsev AA, Filippova EA, Dmitriev AA et al. (2020). LncRNAs in ovarian cancer progression, metastasis, and main pathways: CeRNA and alternative mechanisms. International Journal of Molecular Sciences 21: 8855. https://doi.org/10.3390/ijms21228855 [Google Scholar] [PubMed] [CrossRef]
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68: 394–424. https://doi.org/10.3322/caac.21492 [Google Scholar] [PubMed] [CrossRef]
Chen JQ, Huang ZP, Li HF, Ou YL, Huo F et al. (2020). MicroRNA-520f-3p inhibits proliferation of gastric cancer cells via targeting SOX9 and thereby inactivating Wnt signaling. Scientific Reports 10: 6197. https://doi.org/10.1038/s41598-020-63279-y [Google Scholar] [PubMed] [CrossRef]
Drusco A, Fadda P, Nigita G, Fassan M, Bottoni A et al. (2018). Circulating micrornas predict survival of patients with tumors of glial origin. eBioMedicine 30: 105–112. https://doi.org/10.1016/j.ebiom.2018.03.022 [Google Scholar] [PubMed] [CrossRef]
Goncalves-Ribeiro S, Diaz-Maroto NG, Berdiel-Acer M, Soriano A, Guardiola J et al. (2016). Carcinoma-associated fibroblasts affect sensitivity to oxaliplatin and 5FU in colorectal cancer cells. Oncotarget 7: 59766–59780. https://doi.org/10.18632/oncotarget.11121 [Google Scholar] [PubMed] [CrossRef]
Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A et al. (2008). The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature Cell Biology 10: 593–601. https://doi.org/10.1038/ncb1722 [Google Scholar] [PubMed] [CrossRef]
Guan C, Zhao Y, Wang W, Hu Z, Liu L, et al. (2020). Knockdown of lncRNA SNHG20 suppressed the proliferation of cholangiocarcinoma by sponging miR-520f-3p. Cancer Biotherapy and Radiopharmaceuticals. https://doi.org/10.1089/cbr.2020.4042 [Google Scholar] [PubMed] [CrossRef]
Li W, Chen Y, Zhang J, Hong L, Yuan N et al. (2015). IKBKE upregulation is positively associated with squamous cell carcinoma of the lung in vivo and malignant transformation of human bronchial epithelial cells in vitro. Medical Science Monitor 21: 1577–1586. https://doi.org/10.12659/MSM.893815 [Google Scholar] [PubMed] [CrossRef]
Li J, Ma X, Chakravarti D, Shalapour S, DePinho RA (2021). Genetic and biological hallmarks of colorectal cancer. Genes & Development 35: 787–820. https://doi.org/10.1101/gad.348226.120 [Google Scholar] [PubMed] [CrossRef]
Li C, Xu N, Li YQ, Wang Y, Zhu ZT (2016). Inhibition of SW620 human colon cancer cells by upregulating miRNA-145. World Journal of Gastroenterology 22: 2771–2778. https://doi.org/10.3748/wjg.v22.i9.2771 [Google Scholar] [PubMed] [CrossRef]
Lievre A, Bachet JB, Le Corre D, Boige V, Landi B et al. (2006). KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Research 66: 3992–3995. https://doi.org/10.1158/0008-5472.CAN-06-0191 [Google Scholar] [PubMed] [CrossRef]
Lin X, Wang S, Sun M, Zhang C, Wei C et al. (2019). Correction to: MiR-195-5p/NOTCH2-mediated EMT modulates IL-4 secretion in colorectal cancer to affect M2-like TAM polarization. Journal of Hematology & Oncology 12: 122. https://doi.org/10.1186/s13045-019-0810-x [Google Scholar] [PubMed] [CrossRef]
Lin H, Zuo D, He J, Ji T, Wang J et al. (2021). Long noncoding RNA WEE2-AS1 plays an oncogenic role in glioblastoma by functioning as a molecular sponge for microRNA-520f-3p. Oncology Research 28: 591–603. https://doi.org/10.3727/096504020X15982623243955 [Google Scholar] [PubMed] [CrossRef]
Ling HH, Kuo CC, Lin BX, Huang YH, Lin CW (2017). Elevation of YAP promotes the epithelial-mesenchymal transition and tumor aggressiveness in colorectal cancer. Experimental Cell Research 350: 218–225. https://doi.org/10.1016/j.yexcr.2016.11.024 [Google Scholar] [PubMed] [CrossRef]
Liu L, Wu N, Wang Y, Zhang X, Xia B et al. (2019). TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K/AKT oncogenic signaling. Journal of Experimental & Clinical Cancer Research 38: 106. https://doi.org/10.1186/s13046-019-1061-y [Google Scholar] [PubMed] [CrossRef]
Lu G, Tian S, Sun Y, Dong J, Wang N et al. (2021). NEK9, a novel effector of IL-6/STAT3, regulates metastasis of gastric cancer by targeting ARHGEF2 phosphorylation. Theranostics 11: 2460–2474. https://doi.org/10.7150/thno.53169 [Google Scholar] [PubMed] [CrossRef]
Ng EK, Chong WW, Jin H, Lam EK, Shin VY et al. (2009). Differential expression of microRNAs in plasma of patients with colorectal cancer: A potential marker for colorectal cancer screening. Gut 58: 1375–1381. https://doi.org/10.1136/gut.2008.167817 [Google Scholar] [PubMed] [CrossRef]
Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J et al. (2012). Endothelial Wnt/beta-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. Journal of Experimental Medicine 209: 1611–1627. https://doi.org/10.1084/jem.20111580 [Google Scholar] [PubMed] [CrossRef]
Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D et al. (2007). Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology 72: 397–402. https://doi.org/10.1159/000113489 [Google Scholar] [PubMed] [CrossRef]
Sun M, Song H, Wang S, Zhang C, Zheng L et al. (2017). Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer. Journal of Hematology & Oncology 10: 79. https://doi.org/10.1186/s13045-017-0445-8 [Google Scholar] [PubMed] [CrossRef]
Wang S, Li H, Wang G, Zhang T, Fu B et al. (2016). Yes-associated protein (YAP) expression is involved in epithelial-mesenchymal transition in hepatocellular carcinoma. Clinical & Translational Oncology 18: 172–177. https://doi.org/10.1007/s12094-015-1353-4 [Google Scholar] [PubMed] [CrossRef]
Wang Z, Liu P, Zhou X, Wang T, Feng X et al. (2017). Endothelin promotes colorectal tumorigenesis by activating YAP/TAZ. Cancer Research 77: 2413–2423. https://doi.org/10.1158/0008-5472.CAN-16-3229 [Google Scholar] [PubMed] [CrossRef]
Wierzbicki PM, Rybarczyk A (2015). The Hippo pathway in colorectal cancer. Folia Histochemica et Cytobiologica 53: 105–119. https://doi.org/10.5603/FHC.a2015.0015 [Google Scholar] [PubMed] [CrossRef]
Xiao Y, Sun Y, Liu G, Zhao J, Gao Y et al. (2019). Androgen receptor (AR)/miR-520f-3p/SOX9 signaling is involved in altering hepatocellular carcinoma (HCC) cell sensitivity to the Sorafenib therapy under hypoxia via increasing cancer stem cells phenotype. Cancer Letters 444: 175–187. https://doi.org/10.1016/j.canlet.2018.11.004 [Google Scholar] [PubMed] [CrossRef]
Yuan Y, Li D, Li H, Wang L, Tian G et al. (2016). YAP overexpression promotes the epithelial-mesenchymal transition and chemoresistance in pancreatic cancer cells. Molecular Medicine Reports 13: 237–242. https://doi.org/10.3892/mmr.2015.4550 [Google Scholar] [PubMed] [CrossRef]
Yue H, Li W, Chen R, Wang J, Lu X et al. (2021). Stromal POSTN induced by TGF-β1 facilitates the migration and invasion of ovarian cancer. Gynecologic Oncology 160: 530–538. https://doi.org/10.1016/j.ygyno.2020.11.026 [Google Scholar] [PubMed] [CrossRef]
Zhang X, Liu X, Luo J, Xiao W, Ye X et al. (2016). Notch3 inhibits epithelial-mesenchymal transition by activating Kibra-mediated Hippo/YAP signaling in breast cancer epithelial cells. Oncogenesis 5: e269. https://doi.org/10.1038/oncsis.2016.67 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools