 Open Access
Open Access
ARTICLE
A novel mutation in ROR2 led to the loss of function of ROR2 and inhibited the osteogenic differentiation capability of bone marrow mesenchymal stem cells (BMSCs)
1 Prenatal Diagnosis Center, Shijiazhuang Obstetrics and Gynecology Hospital, Key Laboratory of Maternal and Fetal Medicine of Hebei Province, Shijiazhuang, 050011, China
2 Department of Stomatology, Fifth Medical Center of Chinese PLA General Hospital, Beijing, 100039, China
3 Institute of Hematology, Fifth Medical Center of Chinese PLA General Hospital, Beijing, 100039, China
4 Prenatal Diagnosis Center, Beijing Obstetrics and Gynecology Hospital, Beijing Maternal and Child Health Care Hospital, Capital Medical University, Beijing, 100026, China
5 Department of Orthodontics, Beijing Stomatological Hospital, Capital Medical University School of Stomatology, Capital Medical University, Beijing, 100050, China
* Corresponding Authors: JING ZHANG. Email: ; KAI YANG. Email:
# These authors contributed equally to this work
BIOCELL 2023, 47(7), 1561-1569. https://doi.org/10.32604/biocell.2023.028851
Received 11 January 2023; Accepted 20 March 2023; Issue published 21 June 2023
Abstract
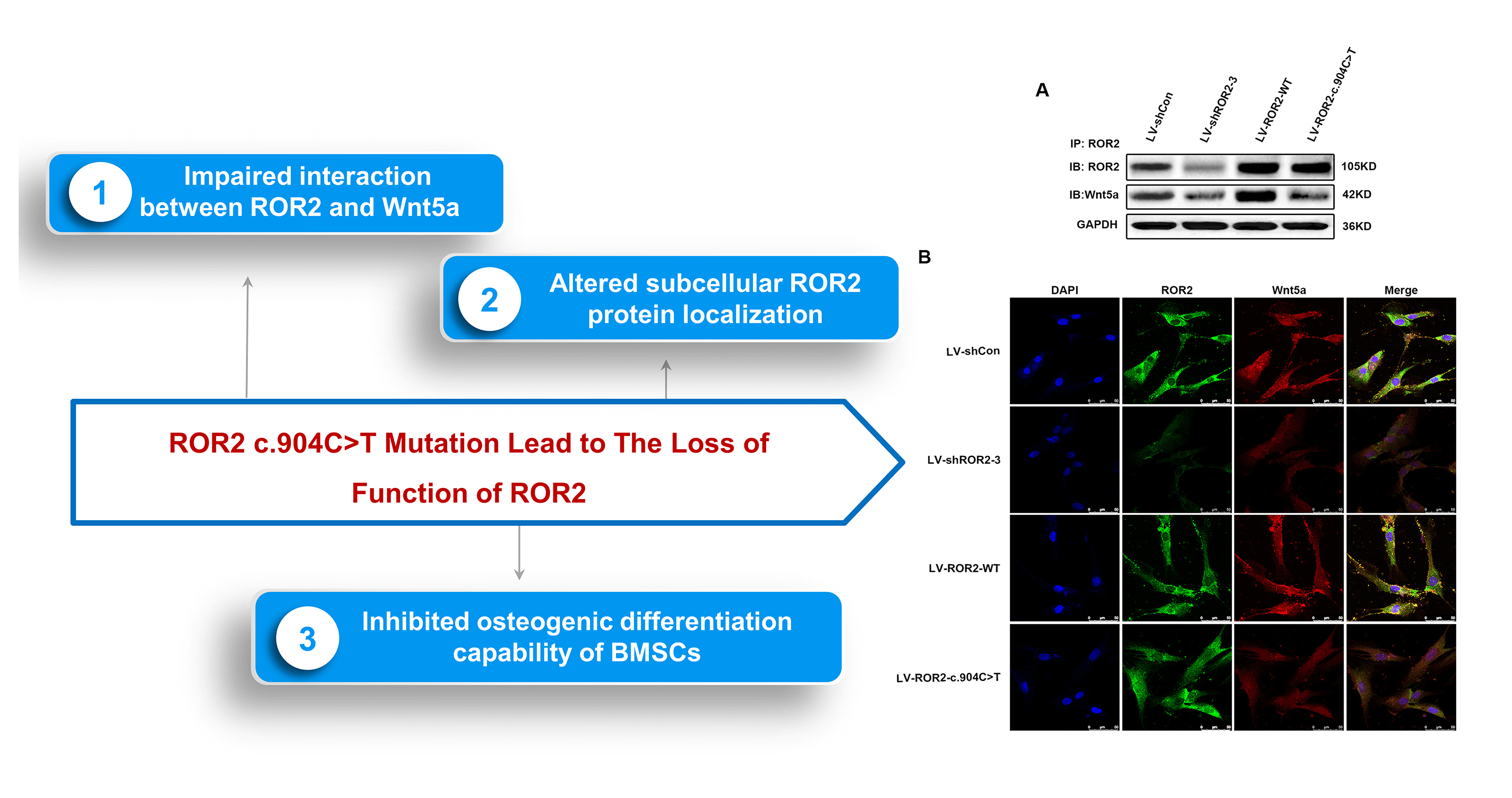
Background: Receptor tyrosine kinase-like orphan receptor 2 (ROR2) has a vital role in osteogenesis. However, the mechanism underlying the regulation of ROR2 in osteogenic differentiation is still poorly comprehended. A previous study by our research group showed that a novel compound heterozygous ROR2 variation accounted for the autosomal recessive Robinow syndrome (ARRS). This study attempted to explore the impact of the ROR2: c.904C>T variant specifically on the osteogenic differentiation of BMSCs. Methods: Coimmunoprecipitation (CoIP)-western blotting was carried out to identify the interaction between ROR2 and Wnt5a. Double-immunofluorescence staining was used for determining the expressions and co-localization of ROR2 and Wnt5a in bone marrow mesenchymal stem cells (BMSCs). Western blot (WB) analysis and quantitative reverse transcription polymerase chain reaction (RT-qPCR) were conducted to identify the expression levels of ROR2 in the BMSCs transfected with LV-shROR2 or LV-ROR2-c.904C>T. The alkaline phosphatase (ALP) activity was detected, and Alizarin Red S staining was done for evaluating the osteogenic differentiation of BMSCs. RT-qPCR was employed to identify the expression of the sphingomyelin synthase 1 (SMS1) mRNA in the BMSCs transfected with LV-shROR2 or LV-ROR2-c.904C>T and the mRNA expression levels of Runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and osteopontin (OPN). WB was performed to confirm the protein expressions of extracellular regulated protein kinases1 (ERK), P-ERK, Smad family member1/5/8 (Smad1/5/8), P-Smad1/5/8, P-P38, P38, RUNX2, OCN, and OPN in the BMSCs transfected with LV-shROR2/LV-ROR2-c.904C>T and sphingomyelin (SM). Results: The ROR2: c.904C>T mutant altered the subcellular localization of the ROR2 protein, which caused an impaired interaction between ROR2 and Wnt5a. The depletion of ROR2 restricted the osteogenic differentiation capability of BMSCs and downregulated the expression of SMS1. SM treatment could reverse the inhibition of osteoblastic differentiation in ROR2-depleted BMSCs. Conclusion: The findings of this work revealed that the ROR2: c.904C>T variant led to the loss of function of ROR2, which impaired the interaction between ROR2 and Wnt5a and also controlled the osteogenic differentiation capability of BMSCs. Furthermore, SM was revealed to be engaged in the osteoblastic differentiation of BMSCs regulated by ROR2, which renders SM a potential target in the therapy for ARRS.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools