 Open Access
Open Access
REVIEW
The role of periodontal disease in atherosclerotic cardiovascular disease
1 Beijing Laboratory of Oral Health, School of Basic Medicine, School of Stomatology, Capital Medical University, Beijing, 100069, China
2 Department of Biochemistry and Molecular Biology, School of Basic Medicine, Capital Medical University, Beijing, 100069, China
3 Immunology Research Center for Oral and Systemic Health, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, China
4 Research Unit of Tooth Development and Regeneration, Chinese Academy of Medical Sciences, Beijing, 100700, China
5 Department of VIP Dental Service, School of Stomatology, Capital Medical University, Beijing, 100050, China
* Corresponding Authors: JIAN ZHOU. Email: ; LEI HU. Email:
# These authors contributed equally to this article
BIOCELL 2023, 47(7), 1431-1438. https://doi.org/10.32604/biocell.2023.028217
Received 02 December 2022; Accepted 20 March 2023; Issue published 21 June 2023
Abstract
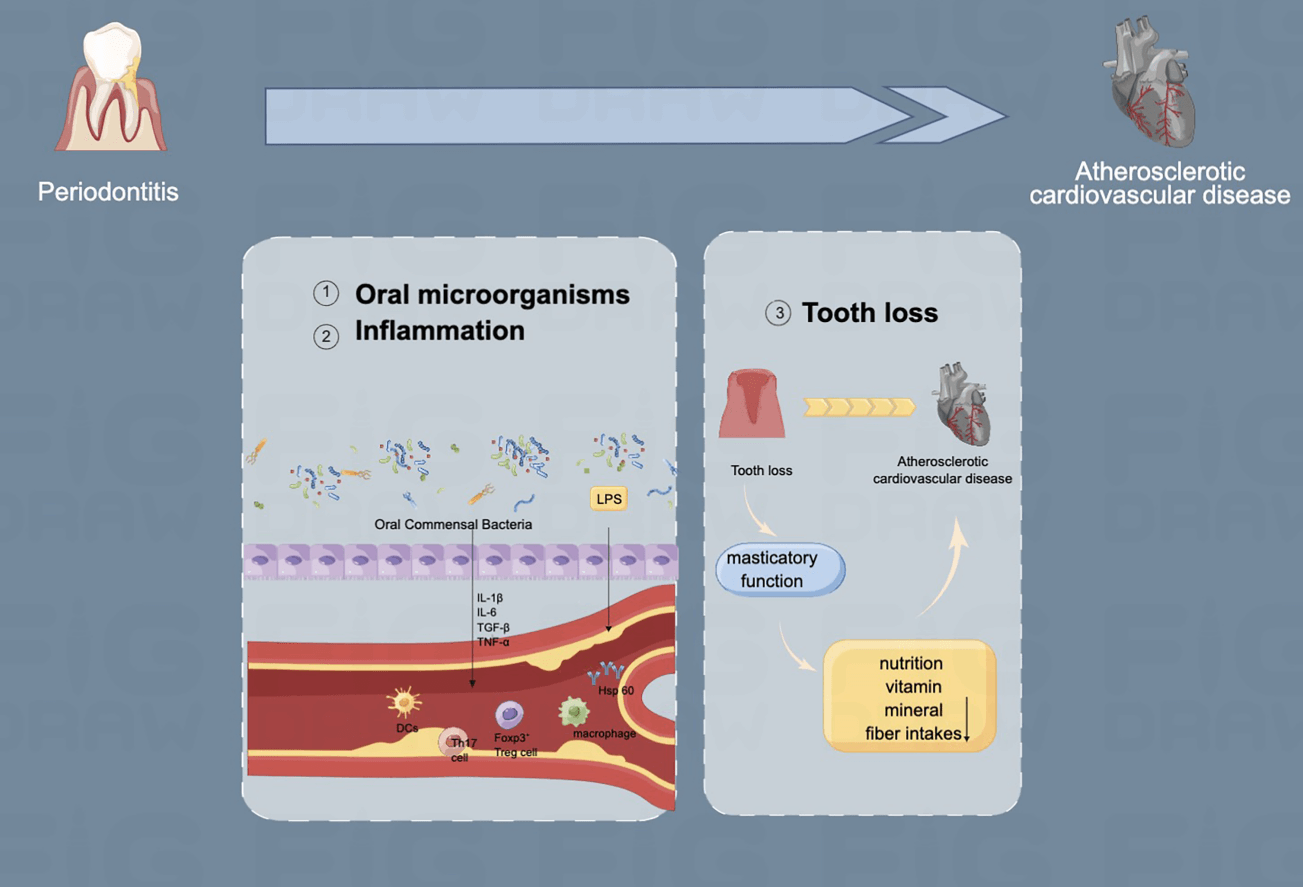
Atherosclerotic cardiovascular disease (ASCVD) includes a group of disorders of the heart and blood vessels and accounts for major morbidity and premature death worldwide. Periodontitis is a chronic inflammatory disease with the gradual destruction of supporting tissues around the teeth, including gingiva, periodontal ligament, alveolar bone, and cementum. Periodontitis has been found to potentially increase the risk of ASCVD. Generally, oral microorganisms and inflammation are the major factors for periodontitis to the incidence of ASCVD. Recently, evidence has shown that the loss of masticatory function is another important factor of periodontitis to the incidence of ASCVD. In this review, we illustrate the recent finding of the relationship between periodontitis and ASCVD, from a microscale perspective-oral microorganisms, inflammation, and tooth loss. With the high prevalence of periodontitis, it is important to add oral therapy as a regular ASCVD prevention strategy. Regular dental visits could be a helpful strategy for ASCVD patients or general medical practitioners.Graphic Abstract
Keywords
Atherosclerotic cardiovascular disease (ASCVD) contains atherosclerotic of the heart and blood vessels and is the number one cause of morbidity and mortality worldwide as well as the first for non-communicable diseases (Mendis et al., 2015; Sathiyakumar et al., 2018; Roth et al., 2020; Surma and Banach, 2021), and the mortality rate is predicted to rise to approximately 23.6 million by 2030 (Benjamin et al., 2018). Periodontitis is a chronic inflammatory illness with a high prevalence rate of 20%–50% overall (Ioannidou, 2017; Czerniuk et al., 2022). Nearly 11.2% of the world’s population is affected by the most severe form of the disease, making it the sixth most frequent human disease overall (Kassebaum et al., 2014). Periodontitis could increase the risk of ASCVD. In this review, we present the recent findings on the relationship between periodontitis and ASCVD, from a microscale perspective-oral microorganisms, inflammation, and tooth loss.
The Pathology of Periodontitis
Periodontitis is induced by oral microorganisms, mainly Gram-negative bacteria, and spirochetes. During this process, the supporting tissues around the teeth, including gingiva, periodontal ligament, alveolar bone, and cementum, deteriorate slowly. One of the typical characteristics of periodontitis is an increase in Gram-negative bacteria that induce a strong immune response depending on their pathogenic mechanisms, such as lipopolysaccharide (LPS) (Cekici et al., 2014). Additionally, some of these bacteria are capable of invading deeper tissues and inducing systemic immunity (Velsko et al., 2014). The epithelial cells act as an innate and acquired barrier against pathogens, which may be weakened by periodontal bacteria associated with chronic inflammation through epithelial-mesenchymal transition (Lee et al., 2017; Abdulkareem et al., 2018; Yamada et al., 2018). In epithelial-mesenchymal transitions, polarity, and adhesion proteins were lost, followed by the loss of epithelium-phenotype and mesenchymal-like characteristics (Kalluri and Weinberg, 2009). In turn, microulceration results in the loss of epithelial sheet coherence, allowing pathogens to penetrate the connective tissue and exposed blood vessels. Additionally, periodontal bacteria may infect host cells as a defensive strategy to evade the immune responses of the host (Deniset and Pierce, 2010). Another important characteristic of periodontitis is the infiltration of chronic inflammation. The majority of infiltrated cells include lymphocytes, neutrophils, plasmatic cells, and macrophages (Kinane et al., 2017). Periodontal ligaments are destroyed by chronic inflammation, causing the resorption of alveolar bone, which ultimately results in tooth loss.
Periodontitis Increases the Risk of Atherosclerotic Cardiovascular Disease and the Involved Mechanisms
Numerous clinical and experimental results have shown a clear link between ASCVD and periodontitis. A number of traditional risk factors are common to both periodontitis and ASCVD, such as age, smoking, diabetes mellitus, etc. Further studies show that periodontitis may be a significant risk factor for the development of atherosclerosis (Chistiakov et al., 2016), particularly the dissemination in the bloodstream of periododontopathogenic bacteria that have already been identified in atherosclerotic plaques (Ohki et al., 2012; Sasaki et al., 2021). The relationship between the two comorbidities may be explained by the following factors (Muñoz-Torres et al., 2017), including bacteremia, inflammation, and teeth loss.
Dental plaque, a complex biofilm that forms in the mouth cavity as a result of the prolonged presence of these microorganisms, causes periodontitis (Caton et al., 2018). Several microorganisms participate in the process of infection, including Porphyromonas gingivalis, Tannerella forsythia, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, and Fusobacterium nucleatum. The pathogenic bacteria that originate from inflamed periodontium can enter the body through the vascular system, spreading everywhere including distant organs and tissues, which triggers bacteremia (Herrera et al., 2020). The severity of gingival disease and the degree of transient bacteremia are correlated (Balejo et al., 2017). Bacterial infection in the blood is always a deviation from the norm, as blood is normally sterile, and it is usually transient due to the strong and powerful antimicrobial defenses of the host (Reyes et al., 2013). In patients who suffer from periodontitis, oral bacteria are the most common cause of transient bacteremia. Proteobacteria are consistently identified as being the most common microorganism in previously reported blood microbiomes profile (Païssé et al., 2016; Olde Loohuis et al., 2018). According to a study that used both molecular and traditional microbiological methods, the blood bacterial communities most closely assembled those of the skin and oral cavity; this finding revealed that species from the oral cavity comprise the majority of the viable bacterial populations in the blood (Whittle et al., 2018), and oral bacteria account for 88% of this population (Emery et al., 2020). Systemic dissemination of periodontal bacteria could potentially occur through multiple mechanisms. The bacteria in the gingival pocket are separated from the deep tissue by the gingival epithelium, known as an innate host defense system to prevent invasion. The oral cavity is characterized by high vascularity and relatively thin and friable epithelium (Leishman et al., 2010). Periodontal bacteriophages may invade oral cells through a transcellular mechanism, and reach the microcapillaries, possibly contributing to systemic bacteremia (Takeuchi et al., 2011). In addition, a dilated periodontal system can facilitate bacteremia due to inflammatory conditions in periodontitis (Priyamvara et al., 2020). Dental plaque bacteria, many of which are Gram-negative and anaerobic, seem to be easily attracted to the inflamed and ulcerated subgingival pocket epithelium (Moutsopoulos and Madianos, 2006). The formation of anaerobic bacteria biofilm leads to inflammatory processes that spread deeper into the tissue (Sufaru et al., 2022). Due to the enhanced immune cell response, an increased number of polymorphonuclear leucocytes, macrophages, and lymphocytes infiltrate the connective tissue adjacent to the periodontal pocket. The inflamed periodontium with infection is thus identified as a reservoir for gram-negative bacteria and their byproducts, including lipopolysaccharide and pro-inflammatory cytokines (Schenkein and Loos, 2013; Wang et al., 2021). Most frequently, however, physical changes in gingival tissue facilitate bacterial translocation. Any dental treatment procedure, such as scaling, root planning, and tooth extractions is established to disturb this epithelium and possibly contribute to low-level bacteremia (Bahrani-Mougeot et al., 2008; Olsen, 2008; Fan et al., 2023). Even routine oral hygiene procedures or functions (chewing, toothbrushing, and flossing) might cause gingival epithelium disruption (Lockhart et al., 2008; Tomás et al., 2012; Hajishengallis, 2015). In addition, it has been proposed, but not proven, that pathogenic bacteria can enter the bloodstream and disseminate to distant sites via phagocytic cells (the Trojan horse approach) (Carrion et al., 2012). As a consequence of this situation, the bacterial pathogen enters the leucocytes and evades microbial killing, escaping from the phagocyte after traveling to another part of the body, which is beneficial for the pathogen but causes harm to the host (Zeituni et al., 2009). Using blood samples obtained before, during, or after periodontal procedures in periodontitis patients, a systematic review based on observational studies found the most commonly detected bacteria were Viridans Streptococci, A. actinomycetemcomitans, P. gingivalis, Micromonas micros, and Actinomycete species (Horliana et al., 2014). Live bacteria in atherosclerotic plaque and atheromatous tissue samples could be detected by culturing viable P. gingivalis (Xie et al. 2020a; Hajishengallis and Chavakis, 2021). The periodontal microbe P. gingivalis is one of the most extensively studied pathogens in this regard. Intravenous injections of P. gingivalis can accelerate atherosclerosis in murine models (Xuan et al., 2017). In addition, Xie demonstrated that P. gingivalis is capable of compromising the structural integrity and inhibiting the self-repair ability of the endothelium. This is a factor thought to be of primary significance in the etiology of vascular disease (Xie et al. 2020b). Thus, evidence for biological effects comes from experimental animal studies. These studies suggest the potential mechanisms of atherogenesis, but cannot definitively prove that periodontal bacteria cause atherogenesis in humans.
Inflammatory mediators are released as a result of bacterial stimulation, in periodontal tissues that cause local pathology, such as collagen destruction and bone resorption. When oral bacteria are sown in atheroma or interact with cells in other organs, the bacteremia process causes the activation of inflammatory cells, endothelial cells, and other kinds of cells, and the creation of inflammatory mediators at distant regions. Severe periodontitis patients have higher C-reactive protein (CRP), interleukin (IL)-1, and IL-6 levels and blood neutrophil counts than healthy controls (Bokhari et al., 2012, 2014). In a prospective trial of 11,869 people, poor dental hygiene was linked to low-grade systemic inflammation and increased CVD risk (de Oliveira et al., 2010). In contrast, successful local periodontal treatment reduces inflammatory indicators systemically (Türer et al., 2017; Bajaj et al., 2018; D’Aiuto et al., 2018). LPS is a component of the membrane wall of Gram-negative bacteria, and also a kind of endotoxin produced by Gram-negative bacteria, which can induce inflammation (Rossol et al., 2011). When gram-negative bacteria are lysed in the bloodstream, LPS enters systemic circulation. Then it is recognized by the LPS binding protein (LBP) of to the host immune system. Upon binding to LPS, LBP recognizes the antigen using the CD14 co-receptor on macrophages, neutrophils, and endothelial cells. Inflammatory pathway nuclear factor-kappa beta (NF-κB) is then activated through the interaction of CD14 with toll-like receptor 4 (TLR4) and MD2 complexes (Choi et al., 2021). ASCVD events have been associated with the inflammatory pathways associated with CD14 and TLR4/NF-κB. A higher level of LPS circulating in the body is linked with elevated C-reactive protein and an increased risk of ASCVD, after adjusting for traditional risk factors (Elisa Kallio et al., 2014).
As a result of this process, there is a significant increase in endothelial cell adhesion molecules, and tumor necrosis factor-alpha (TNF-α) levels (Mann, 2011). Monocytes are captured by cellular adhesion molecules, resulting in increased endothelial permeability (Galkina and Klaus, 2007). The inflammatory mediators produced by chronic periodontitis, such as IL-1, TNF-α, CRP, prostaglandins, interleukins, and proteolyenostic enzymes like matrix metalloproteinases (MMPs), are released into the systemic circulation and can induce or exacerbate endothelial dysfunction by stimulating endothelial cells to produce other inflammatory markers (Gurav, 2014). The synthesis and bioavailability of nitric oxide (NO) can decreased in periodontitis, and have a deleterious effect on the function of the vascular endothelium and endothelium-dependent vasodilation (Moura et al., 2017). This is one of the pathways linking periodontitis to endothelial dysfunction. TNF-α is known for decreasing the ability of endothelial cells to synthesize NO and reduce the half-life of endothelial nitric oxide synthase mRNA (Horio et al., 2014). Recent research suggests that MMPs may play a key role in the instability and rupture of atherosclerotic plaques by degrading the collagen of the extracellular matrix of the fibrous layer of atheroma plaques (Brown et al., 2017). Soft connective tissues with a high rate of degraduation of extracellular matrix are prone to penetration and reshaping, and MMPs play a critical part in this process (Olejarz et al., 2020).
Atheroma formation requires both monocytes and lipoproteins to penetrate the endothelial cells to be able to initiate the process. Atheromatous lesions are created by the accumulation of lipoproteins in the intima. The permeability of dysfunctional endothelium increases and is hypothesized to strongly influence the pathophysiology of ASCVD (Zardawi et al., 2020). Endothelial dysfunction is thought to be the primary biological disorder causing ASCVD (Vanhoutte, 2009). Periodontitis can cause endothelial dysfunction in several ways. The innate immune system is triggered by the LPSs produced by the high-risk gram-negative bacteria. TLRs, which are widely distributed and found in endothelial cells, are stimulated by LPSs. The gene for the transcription factor NF-κB is activated by TLR signaling. This is followed by increased levels of TNF-α and endothelial cellular adhesion molecules (Mann, 2011).
Cellular adhesion molecules capture monocytes and as a result, increase the endothelial permeability (Galkina and Klaus, 2007). TNF-α induces increased endothelial permeability by binding with tight junction proteins (McKenzie and Ridley, 2007). By stimulating the innate immune system, chronic periodontal infections with high-risk pathogens can increase endothelium permeability.
The occurrence of accelerated arterial tissue calcification and cardiovascular disease is associated with autoimmune processes (Hansson and Hermansson, 2011; Libby, 2012; Wolf and Ley, 2019). The self-antigens heat-shock proteins (HSPs) are of special interest because they are also found in the periodontium of patients with periodontal disease, which raises the possibility that they might be the targets of the self-directed immune response in atherosclerosis (Koutouzis et al., 2009). Human HSPs are homologous to those contained in P. gingivalis and many other oral infectious bacteria (Siqueira and Rôças, 2007). Immune responses to bacterial HSP are thought to cause endothelial damage. There is also evidence that cross-reactive autoantibodies against bacterial antigens, specifically those against HSP60, promote atherosclerosis (Choi et al., 2011; Garrido-Urbani et al., 2014). In one study, cross-reactivity between P. gingivalis and human HSP60 resulted in an immediate autoimmune reaction in the vascular endothelium, which greatly accelerated atherosclerosis (Wick, 2016). As evidenced by the finding of P. gingivalis HSP-specific T-cells in laboratory samples of atherosclerosis plaque, T-cell immune responses to P. gingivalis HSP60 may contribute to atherosclerosis.
Furthermore, the peripheral blood of patients with periodontal disease was found to contain T cells that recognize HSP60 (Li et al., 2022). Recently, a study proposed that HSP60-based therapeutic strategies or vaccines may be a new immunologic approach for the prevention and treatment of atherosclerosis (Hu et al., 2018).
Untreated periodontitis may lead to the loss of teeth through the destruction of the supportive tissues of involved teeth. Even though several studies have shown evidence of an association between tooth loss and ASCVD, there are only a few related studies (Beukers et al., 2021). According to a retrospective cross-sectional study by Donders et al. (2020), the Coronary Artery Calcium (CAC) score and the number of missing teeth are statistically significantly correlated. Çetin et al. (2020) concluded that periodontitis and edentulism can be regarded as independent risk factors for ASCVD. However, when age, sex, and other well-known risk factors for ASCVD were modeled, the significant correlation was no longer present. These studies provide suggestive evidence that the relationship between tooth loss and atherosclerosis depends on their shared risk factors. Age was an essential covariate for the association between tooth loss and atherosclerosis. Elderly people are highly likely to be affected by periodontitis and ensuing tooth loss, meanwhile, the potential for atherosclerosis increases in them. The association also differed according to sex, which means that the contributions of oral inflammatory disease towards atherosclerosis and the link between tooth loss and atherosclerosis depend on gender (Asai et al., 2015). Compared with the lower values among female edentulous, the male edentulous show high atherosclerosis values (Meisel et al., 2014). The reason may be as follows: with a lower number of teeth comes a gradual decline in masticatory function, which is related to higher risks for ASCVD mortality. Tooth loss has been indicated to affect nutritional intake, poor masticatory ability obstructs nutrition and also lower vitamin, mineral, and fiber intakes (Gondivkar et al., 2019; Soliman, 2019; Tanaka et al., 2021; Riccardi et al., 2022). The studies indicate that changes in diet and nutritional intake could contribute to atherosclerosis by reducing masticatory ability due to tooth loss and reduced occlusal support caused by periodontal disease. The choice of foods that patients can ingest becomes restricted when masticatory ability comes to a decline. Nutrition plays a major role as a link between atherosclerosis and oral health. In addition, edentulous patients have been shown to eat soft food and avoid hard foods that are difficult to chew, which consequently causes less intake of vegetables and vitamins, leading to a reduced intake of antioxidant vitamins and dietary fiber (Wakai et al., 2010; Inomata et al., 2014). An increasing number of studies have detected that ingesting antioxidant vitamins and dietary fiber by eating vegetables decreases the incidence of ASCVD (Steffen et al., 2003; Okuda et al., 2015). Nutrition can be observed as a mediator in the relationship between oral and atherosclerosis.
Periodontal health could increase the risk for ASCVD through oral microorganisms, inflammation, or tooth loss, and it should be added to the existing cardiovascular risk profiles as an additional risk factor. The prevention or therapy of periodontitis can reduce serum inflammatory mediators, improve the lipid profile, and induce positive changes in other CVD surrogate measures (Herrera et al., 2020). Future studies are required to assess whether periodontal treatment is associated with a reduced presence of atherosclerosis or a reduced prevalence of atherosclerosis insociety.
Acknowledgement: This work was supported by the National Natural Science Foundation of China (82001067), the Innovation Research Team Project of Beijing Stomatological Hospital, Capital Medical University (CXTD202201), Beijing Municipal Administration of Hospitals’ Youth Program (QML20191504), Scientific Research Common Program of Beijing Municipal Commission of Education (KM202110025009) and Beijing Talents Fund (2018000021469G285).
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Lei Hu, Jian Zhou; data collection: Yifan Xu; draft manuscript preparation: Xiwei Zhao. Jinsong Wang. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Abdulkareem AA, Shelton RM, Landini G, Cooper PR, Milward MR (2018). Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial-mesenchymal transition. Journal of Periodontal Research 53: 565–574. https://doi.org/10.1111/jre.12546 [Google Scholar] [PubMed] [CrossRef]
Asai K, Yamori M, Yamazaki T, Yamaguchi A, Takahashi K, Sekine A, Kosugi S, Matsuda F, Nakayama T, Bessho K (2015). Tooth loss and atherosclerosis: The Nagahama study. Journal of Dental Research 94: 52S–58S. https://doi.org/10.1177/0022034514559127 [Google Scholar] [PubMed] [CrossRef]
Bahrani-Mougeot FK, Thornhill M, Sasser H, Marriott I, Brennan MT, Papagerakis S, Coleman S, Fox PC, Lockhart PB (2008). Systemic host immuno-inflammatory response to dental extractions and periodontitis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 106: 534–541. https://doi.org/10.1016/j.tripleo.2008.02.011 [Google Scholar] [PubMed] [CrossRef]
Bajaj JS, Matin P, White MB, Fagan A, Deeb JG, Acharya C, Dalmet SS, Sikaroodi M, Gillevet PM, Sahingur SE (2018). Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. American Journal of Physiology-Gastrointestinal and Liver Physiology 315: G824–G837. https://doi.org/10.1152/ajpgi.00230.2018 [Google Scholar] [PubMed] [CrossRef]
Balejo RDP, Cortelli JR, Costa FO, Cyrino RM, Aquino DR, Cogo-Müller K, Miranda TB, Moura SP, Cortelli SC (2017). Effects of chlorhexidine preprocedural rinse on bacteremia in periodontal patients: A randomized clinical trial. Journal of Applied Oral Science 25: 586–595. https://doi.org/10.1590/1678-7757-2017-0112 [Google Scholar] [PubMed] [CrossRef]
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR et al. (2018). Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 137: e467–e492. https://doi.org/10.1161/CIR.0000000000000558 [Google Scholar] [PubMed] [CrossRef]
Beukers NGFM, Su N, Loos BG, van der Heijden GJMG (2021). Lower number of teeth is related to higher risks for ACVD and death-systematic review and meta-analyses of survival data. Frontiers in Cardiovascular Medicine 8: 621–626. https://doi.org/10.3389/fcvm.2021.621626 [Google Scholar] [PubMed] [CrossRef]
Bokhari SAH, Khan AA, Butt AK, Azhar M, Hanif M, Izhar M, Tatakis DN (2012). Non-surgical periodontal therapy reduces coronary heart disease risk markers: A randomized controlled trial. Journal of Clinical Periodontology 39: 1065–1074. https://doi.org/10.1111/j.1600-051X.2012.01942.x [Google Scholar] [PubMed] [CrossRef]
Bokhari SAH, Khan AA, Butt AK, Hanif M, Izhar M, Tatakis DN, Ashfaq M (2014). Periodontitis in coronary heart disease patients: Strong association between bleeding on probing and systemic biomarkers. Journal of Clinical Periodontology 41: 1048–1054. https://doi.org/10.1111/jcpe.12284 [Google Scholar] [PubMed] [CrossRef]
Brown BA, Williams H, George SJ (2017). Evidence for the involvement of matrix-degrading metalloproteinases (MMPs) in atherosclerosis. Progress in Molecular Biology and Translational Science 147: 197–237. https://doi.org/10.1016/bs.pmbts.2017.01.004 [Google Scholar] [PubMed] [CrossRef]
Carrion J, Scisci E, Miles B, Sabino GJ, Zeituni AE, Gu Y, Bear A, Genco CA, Brown DL, Cutler CW (2012). Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. The Journal of Immunology 189: 3178–3187. https://doi.org/10.4049/jimmunol.1201053 [Google Scholar] [PubMed] [CrossRef]
Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS (2018). A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. Journal of Clinical Periodontology 45: S1–S8. https://doi.org/10.1111/jcpe.12935 [Google Scholar] [PubMed] [CrossRef]
Cekici A, Kantarci A, Hasturk H, van Dyke TE (2014). Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 64: 57–80. https://doi.org/10.1111/prd.12002 [Google Scholar] [PubMed] [CrossRef]
Çetin MB, Önder C, Orhan K, Kumbasar D, Serda MA, Ünsal E (2020). Relationship of periodontitis and edentulism to angiographically diagnosed coronary artery disease: A cross-sectional study. Journal of Periodontal Research 55: 895–904. https://doi.org/10.1111/jre.12782 [Google Scholar] [PubMed] [CrossRef]
Chistiakov DA, Orekhov AN, Bobryshev YV (2016). Links between atherosclerotic and periodontal disease. Experimental and Molecular Pathology 100: 220–235. https://doi.org/10.1016/j.yexmp.2016.01.006 [Google Scholar] [PubMed] [CrossRef]
Choi H, Dey AK, Priyamvara A, Aksentijevich M, Bandyopadhyay D et al. (2021). Role of periodontal infection, inflammation and immunity in atherosclerosis. Current Problems in Cardiology 46: 100638. https://doi.org/10.1016/j.cpcardiol.2020.100638 [Google Scholar] [PubMed] [CrossRef]
Choi J, Lee SY, Kim K, Choi BK, Kim MJ (2011). Identification of mono- or poly-specific monoclonal antibody to porphyromonas gingivalis heat-shock protein 60. Journal of Periodontal & Implant Science 41: 54–59. https://doi.org/10.5051/jpis.2011.41.2.54 [Google Scholar] [PubMed] [CrossRef]
Czerniuk MR, Surma S, Romańczyk M, Nowak JM, Wojtowicz A, Filipiak KJ (2022). Unexpected relationships: periodontal diseases: Atherosclerosis-plaque destabilization? From the teeth to a coronary event. Biology 11: 272. https://doi.org/10.3390/biology11020272 [Google Scholar] [PubMed] [CrossRef]
de Oliveira C, Watt R, Hamer M (2010). Toothbrushing, inflammation, and risk of cardiovascular disease: Results from Scottish Health Survey. BMJ 340: c2451. https://doi.org/10.1136/bmj.c2451 [Google Scholar] [PubMed] [CrossRef]
Deniset JF, Pierce GN (2010). Possibilities for therapeutic interventions in disrupting Chlamydophila pneumoniae involvement in atherosclerosis. Fundamental & Clinical Pharmacology 24: 607–617. https://doi.org/10.1111/j.1472-8206.2010.00863.x [Google Scholar] [PubMed] [CrossRef]
Donders HCM, IJzerman LM, Soffner M, van’t Hof AWJ, Loos BG, de Lange J (2020). Elevated coronary artery calcium scores are associated with tooth loss. PLoS One 15: e0243232. https://doi.org/10.1371/journal.pone.0243232 [Google Scholar] [PubMed] [CrossRef]
D’Aiuto F, Gkranias N, Bhowruth D, Khan T, Orlandi M et al. (2018). Systemic effects of periodontitis treatment in patients with type 2 diabetes: A 12 month, single-centre, investigator-masked, randomised trial. The Lancet Diabetes & Endocrinology 6: 954–965. https://doi.org/10.1016/S2213-8587(18)30038-X [Google Scholar] [PubMed] [CrossRef]
Elisa Kallio KA, Hätönen KA, Markku L, Veikko S, Männistö S, Pussinen PJ (2014). Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetologica 52: 395–404. https://doi.org/10.1007/s00592-014-0662-3 [Google Scholar] [PubMed] [CrossRef]
Emery DC, Cerajewska TL, Seong J, Davies M, Paterson A, Allen-Birt SJ, West NX (2020). Comparison of blood bacterial communities in periodontal health and periodontal disease. Frontiers in Cellular and Infection Microbiology 10: 577485. https://doi.org/10.3389/fcimb.2020.577485 [Google Scholar] [PubMed] [CrossRef]
Fan ZX, Tang PZ, Li C, Yang Q, Xu Y, Su C, Li L (2023). Fusobacterium nucleatum and its associated systemic diseases: Epidemiologic studies and possible mechanisms. Journal of Oral Microbiology 15: 2145729. https://doi.org/10.1080/20002297.2022.2145729 [Google Scholar] [PubMed] [CrossRef]
Galkina E, Klaus L (2007). Vascular adhesion molecules in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 27: 2292–2301. https://doi.org/10.1161/ATVBAHA.107.149179 [Google Scholar] [PubMed] [CrossRef]
Garrido-Urbani S, Meguenani M, Montecucco F, Imhof BA (2014). Immunological aspects of atherosclerosis. Seminars in Immunopathology 36: 73–91. https://doi.org/10.1007/s00281-013-0402-8 [Google Scholar] [PubMed] [CrossRef]
Gondivkar SM, Gadbail AR, Gondivkar RS, Sarode SC, Sarode GS, Patil S, Awan KH (2019). Nutrition and oral health. Disease-a-Month 65: 147–154. https://doi.org/10.1016/j.disamonth.2018.09.009 [Google Scholar] [PubMed] [CrossRef]
Gurav AN (2014). The implication of periodontitis in vascular endothelial dysfunction. European Journal of Clinical Investigation 44: 1000–1009. https://doi.org/10.1111/eci.12322 [Google Scholar] [PubMed] [CrossRef]
Hajishengallis G (2015). Periodontitis: From microbial immune subversion to systemic inflammation. Nature Reviews Immunology 15: 30–44. https://doi.org/10.1038/nri3785 [Google Scholar] [PubMed] [CrossRef]
Hajishengallis G, Chavakis T (2021). Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nature Reviews Immunology 21: 426–440. https://doi.org/10.1038/s41577-020-00488-6 [Google Scholar] [PubMed] [CrossRef]
Hansson GK, Hermansson A (2011). The immune system in atherosclerosis. Nature Immunology 12: 204–212. https://doi.org/10.1038/ni.2001 [Google Scholar] [PubMed] [CrossRef]
Herrera D, Molina A, Buhlin K, Bjorn K (2020). Periodontal diseases and association with atherosclerotic disease. Periodontology 83: 66–89. https://doi.org/10.1111/prd.12302 [Google Scholar] [PubMed] [CrossRef]
Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K et al. (2014). Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arteriosclerosis, Thrombosis, and Vascular Biology 34: 790–800. https://doi.org/10.1161/ATVBAHA.113.303116 [Google Scholar] [PubMed] [CrossRef]
Horliana ACRT, Chambrone L, Foz AM, Artese HP, Rabelo Mde s, Pannuti CM, Romito GA (2014). Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: A systematic review. PLoS One 9: e98271. https://doi.org/10.1371/journal.pone.0098271 [Google Scholar] [PubMed] [CrossRef]
Hu YY, Chen ZY, Jiang LL, Chen F, Jin RM, Cheng L (2018). Effects of oral and subcutaneous administration of HSP60 on myeloid-derived suppressor cells and atherosclerosis in ApoE−/− mice. Biochemical and Biophysical Research Communications 498: 701–706. https://doi.org/10.1016/j.bbrc.2017.10.150 [Google Scholar] [PubMed] [CrossRef]
Inomata C, Ikebe K, Kagawa R, Okubo H, Sasaki S et al. (2014). Significance of occlusal force for dietary fibre and vitamin intakes in independently living 70-year-old Japanese: From SONIC study. Journal of Dentistry 42: 556–564. https://doi.org/10.1016/j.jdent.2014.02.015 [Google Scholar] [PubMed] [CrossRef]
Ioannidou E (2017). The sex and gender intersection in chronic periodontitis. Frontiers in Public Health 5: 189. https://doi.org/10.3389/fpubh.2017.00189 [Google Scholar] [PubMed] [CrossRef]
Kalluri R, Weinberg RA (2009). The basics of epithelial-mesenchymal transition. The Journal of Clinical Investigation 119: 1420–1428. https://doi.org/10.1172/JCI39104 [Google Scholar] [PubMed] [CrossRef]
Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Murray CJL, Marcenes W (2014). Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. Journal of Dental Research 93: 1045–1053. https://doi.org/10.1177/0022034514552491 [Google Scholar] [PubMed] [CrossRef]
Kinane DF, Stathopoulou PG, Papapanou PN (2017). Periodontal diseases. Nature Reviews Disease Primers 3: 17038. https://doi.org/10.1038/nrdp.2017.38 [Google Scholar] [PubMed] [CrossRef]
Koutouzis T, Haber D, Shaddox L, Aukhil I, Wallet SM (2009). Autoreactivity of serum immunoglobulin to periodontal tissue components: A pilot study. Journal of Periodontology 80: 625–633. https://doi.org/10.1902/jop.2009.080422 [Google Scholar] [PubMed] [CrossRef]
Lee J, Roberts JS, Atanasova KR, Chowdhury N, Han K, Yilmaz Ö (2017). Human primary epithelial cells acquire an epithelial-mesenchymal-transition phenotype during long-term infection by the oral opportunistic pathogen, porphyromonas gingivalis. Frontiers in Cellular and Infection Microbiology 7: 493. https://doi.org/10.3389/fcimb.2017.00493 [Google Scholar] [PubMed] [CrossRef]
Leishman SJ, Do HL, Ford PJ (2010). Cardiovascular disease and the role of oral bacteria. Journal of Oral Microbiology 2: 5781. https://doi.org/10.3402/jom.v2i0.5781 [Google Scholar] [PubMed] [CrossRef]
Li C, Yu R, Ding YM (2022). Association between Porphyromonas Gingivalis and systemic diseases: Focus on T cells-mediated adaptive immunity. Frontiers in Cellular and Infection Microbiology 12: 1026457. https://doi.org/10.3389/fcimb.2022.1026457 [Google Scholar] [PubMed] [CrossRef]
Libby P (2012). Inflammation in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 32: 2045–2051. https://doi.org/10.1161/ATVBAHA.108.179705 [Google Scholar] [PubMed] [CrossRef]
Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK (2008). Bacteremia associated with toothbrushing and dental extraction. Circulation 117: 3118–3125. https://doi.org/10.1161/CIRCULATIONAHA.107.758524 [Google Scholar] [PubMed] [CrossRef]
Mann DL (2011). The emerging role of innate immunity in the heart and vascular system: For whom the cell tolls. Circulation Research 108: 1133–1145. https://doi.org/10.1161/CIRCRESAHA.110.226936 [Google Scholar] [PubMed] [CrossRef]
McKenzie JAG, Ridley AJ (2007). Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. Journal of Cellular Physiology 213: 221–228. https://doi.org/10.1002/(ISSN)1097-4652 [Google Scholar] [CrossRef]
Meisel P, Holtfreter B, Völzke H, Kocher T (2014). Sex differences of tooth loss and obesity on systemic markers of inflammation. Journal of Dental Research 93: 774–779. https://doi.org/10.1177/0022034514535604 [Google Scholar] [PubMed] [CrossRef]
Mendis S, Davis S, Norrving B (2015). Organizational update: The world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 46: e121–e122. https://doi.org/10.1161/STROKEAHA.115.008097 [Google Scholar] [PubMed] [CrossRef]
Moura MF, Navarro TP, Silva TA, Cota LOM, Oliveira AMSD, Costa FO (2017). Periodontitis and endothelial dysfunction: periodontal clinical parameters and levels of salivary markers interleukin-1β, tumor necrosis factor-α, matrix metalloproteinase-2, tissue inhibitor of metalloproteinases-2 complex, and nitric oxide. Journal of Periodontology 88: 778–787. https://doi.org/10.1902/jop.2017.170023 [Google Scholar] [PubMed] [CrossRef]
Moutsopoulos NM, Madianos PN (2006). Low-grade inflammation in chronic infectious diseases: Paradigm of periodontal infections. Annals of the New York Academy of Sciences 1088: 251–264. https://doi.org/10.1196/annals.1366.032 [Google Scholar] [PubMed] [CrossRef]
Muñoz-Torres FJ, Mukamal KJ, Pai JK, Willett W, Joshipura KJ (2017). Relationship between tooth loss and peripheral arterial disease among women. Journal of Clinical Periodontology 44: 989–995. https://doi.org/10.1111/jcpe.12787 [Google Scholar] [PubMed] [CrossRef]
Ohki T, Itabashi Y, Kohno T, Yoshizawa A, Nishikubo S, Watanabe S, Yamane G, Ishihara K (2012). Detection of periodontal bacteria in thrombi of patients with acute myocardial infarction by polymerase chain reaction. American Heart Journal 163: 164–167. https://doi.org/10.1016/j.ahj.2011.10.012 [Google Scholar] [PubMed] [CrossRef]
Okuda N, Miura K, Okayama A, Okamura T, Abbott RD et al. (2015). Fruit and vegetable intake and mortality from cardiovascular disease in Japan: A 24-year follow-up of the NIPPON DATA80 study. European Journal of Clinical Nutrition 9: 482–488. https://doi.org/10.1038/ejcn.2014.276 [Google Scholar] [PubMed] [CrossRef]
Olde Loohuis LM, Mangul S, Ori APS, Jospin G, Koslicki D et al. (2018). Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Translational Psychiatry 8: 96–105. https://doi.org/10.1038/s41398-018-0107-9 [Google Scholar] [PubMed] [CrossRef]
Olejarz W, Łacheta D, Kubiak-Tomaszewska G (2020). Matrix metalloproteinases as biomarkers of atherosclerotic plaque instability. International Journal of Molecular Sciences 21: 3946. https://doi.org/10.3390/ijms21113946 [Google Scholar] [PubMed] [CrossRef]
Olsen I (2008). Update on bacteraemia related to dental procedures. Transfusion and Apheresis Science 39: 173–178. https://doi.org/10.1016/j.transci.2008.06.008 [Google Scholar] [PubMed] [CrossRef]
Païssé S, Valle C, Servant F, Courtney M, Burcelin R, Amar J, Lelouvier B (2016). Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 56: 1138–1147. https://doi.org/10.1111/trf.13477 [Google Scholar] [PubMed] [CrossRef]
Priyamvara A, Dey AK, Bandyopadhyay D, Katikineni V, Zaghlol R, Basyal B, Barssoum K, Amarin R, Bhatt DL, Lavie CJ (2020). Periodontal inflammation and the risk of cardiovascular disease. Current Atherosclerosis Reports 22: 28. https://doi.org/10.1007/s11883-020-00848-6 [Google Scholar] [PubMed] [CrossRef]
Reyes L, Herrera D, Kozarov E, Roldán S, Progulske-Fox A (2013). Periodontal bacterial invasion and infection: Contribution to atherosclerotic pathology. Journal of Clinical Periodontology 40: S30–S50. https://doi.org/10.1111/jcpe.12079 [Google Scholar] [PubMed] [CrossRef]
Riccardi G, Giosuè A, Calabrese I, Vaccaro O (2022). Dietary recommendations for prevention of atherosclerosis. Cardiovascular Research 118: 1188–1204. https://doi.org/10.1093/cvr/cvab173 [Google Scholar] [PubMed] [CrossRef]
Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S (2011). LPS-induced cytokine production in human monocytes and macrophages. Critical Reviews in Immunology 31: 379–446. https://doi.org/10.1615/CritRevImmunol.v31.i5.20 [Google Scholar] [PubMed] [CrossRef]
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E et al. (2020). Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. Journal of the American College of Cardiology 76: 2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010 [Google Scholar] [PubMed] [CrossRef]
Sasaki M, Shimoyama Y, Kodama Y, Ishikawa T (2021). Tryptophanyl tRNA synthetase from human macrophages infected by porphyromonas gingivalis induces a proinflammatory response associated with atherosclerosis. Pathogens 10: 648. https://doi.org/10.3390/pathogens10121648 [Google Scholar] [PubMed] [CrossRef]
Sathiyakumar V, Kapoor K, Jones SR, Banach M, Martin SS, Toth PP (2018). Novel therapeutic targets for managing dyslipidemia. Trends in Pharmacological Sciences 39: 733–747. https://doi.org/10.1016/j.tips.2018.06.001 [Google Scholar] [PubMed] [CrossRef]
Schenkein HA, Loos BG (2013). Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. Journal of Clinical Periodontology 40: S51–S69. https://doi.org/10.1111/jcpe.12060 [Google Scholar] [PubMed] [CrossRef]
SiqueiraJr JF, Rôças IN (2007). Bacterial pathogenesis and mediators in apical periodontitis. Brazilian Dental Journal 18: 267–280. https://doi.org/10.1590/S0103-64402007000400001 [Google Scholar] [PubMed] [CrossRef]
Soliman GA (2019). Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 11: 1155. https://doi.org/10.3390/nu11051155 [Google Scholar] [PubMed] [CrossRef]
Steffen LM, JacobsJr DR, Stevens J, Shahar E, Carithers T, Folsom AR (2003). Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: The atherosclerosis risk in communities (ARIC) study. The American Journal of Clinical Nutrition 78: 383–390. https://doi.org/10.1093/ajcn/78.3.383 [Google Scholar] [PubMed] [CrossRef]
Sufaru IG, Martu MA, Solomon SM (2022). Advances in periodontal pathogens. Microorganisms 10: 1439. https://doi.org/10.3390/microorganisms10071439 [Google Scholar] [PubMed] [CrossRef]
Surma S, Banach M (2021). Fibrinogen and atherosclerotic cardiovascular diseases-review of the literature and clinical studies. International Journal of Molecular Sciences 23: 193. https://doi.org/10.3390/ijms23010193 [Google Scholar] [PubMed] [CrossRef]
Takeuchi H, Furuta N, Morisaki I, Amano A (2011). Exit of intracellular Porphyromonas gingivalis from gingival epithelial cells is mediated by endocytic recycling pathway. Cellular Microbiology 13: 677–691. https://doi.org/10.1111/j.1462-5822.2010.01564.x [Google Scholar] [PubMed] [CrossRef]
Tanaka S, Yoneoka D, Ishizuka A, Ueda P, Nakamura K, Uneyama H, Hayashi N, Shibuya K, Nomura S (2021). Projections of disability-adjusted life years for major diseases due to a change in vegetable intake in 2017–2040 in Japan. BMC Public Health 21: 770. https://doi.org/10.1186/s12889-021-10772-2 [Google Scholar] [PubMed] [CrossRef]
Tomás I, Diz P, Tobías A, Scully C, Donos N (2012). Periodontal health status and bacteraemia from daily oral activities: systematic review/meta-analysis. Journal of Clinical Periodontology 39: 213–228. https://doi.org/10.1111/j.1600-051X.2011.01784.x [Google Scholar] [PubMed] [CrossRef]
Türer ÇC, Durmuş D, Balli U, Güven B (2017). Effect of non-surgical periodontal treatment on gingival crevicular fluid and serum endocan, vascular endothelial growth factor-A, and tumor necrosis factor-alpha levels. Journal of Periodontology 88: 493–501. https://doi.org/10.1902/jop.2016.160279 [Google Scholar] [PubMed] [CrossRef]
Vanhoutte PM (2009). Endothelial dysfunction: The first step toward coronary arteriosclerosis. Circulation Journal 73: 595–601. https://doi.org/10.1253/circj.CJ-08-1169 [Google Scholar] [PubMed] [CrossRef]
Velsko IM, Chukkapalli SS, Rivera MF, Lee JY, Chen H, Zheng D, Bhattacharyya I, Gangula PR, Lucas AR, Kesavalu L (2014). Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One 9: e97811. https://doi.org/10.1371/journal.pone.0097811 [Google Scholar] [PubMed] [CrossRef]
Wakai K, Naito M, Naito T, Kojima M, Nakagaki H, Umemura O, Yokota M, Hanada N, Kawamura T (2010). Tooth loss and intakes of nutrients and foods: A nationwide survey of Japanese dentists. Community Dentistry and Oral Epidemiology 38: 43–49. https://doi.org/10.1111/j.1600-0528.2009.00512.x [Google Scholar] [PubMed] [CrossRef]
Wang W, Zheng CX, Yang JH, Li B (2021). Intersection between macrophages and periodontal pathogens in periodontitis. Journal of Leukocyte Biology 110: 577–583. https://doi.org/10.1002/JLB.4MR0421-756R [Google Scholar] [PubMed] [CrossRef]
Whittle E, Leonard MO, Harrison R, Gant TW, Tonge DP (2018). Multi-method characterization of the human circulating microbiome. Frontiers in Microbiology 9: 03266. https://doi.org/10.3389/fmicb.2018.03266 [Google Scholar] [PubMed] [CrossRef]
Wick C (2016). Tolerization against atherosclerosis using heat shock protein 60. Cell Stress & Chaperones 21: 201–211. https://doi.org/10.1007/s12192-015-0659-z [Google Scholar] [PubMed] [CrossRef]
Wolf D, Ley K (2019). Immunity and inflammation in atherosclerosis. Circulation Research 124: 315–327. https://doi.org/10.1161/CIRCRESAHA.118.313591 [Google Scholar] [PubMed] [CrossRef]
Xie MR, Tang QM, Nie JM, Zhang C, Zhou X et al. (2020a). BMAL1-downregulation aggravates porphyromonas gingivalis-induced atherosclerosis by encouraging oxidative stress. Circulation Research 126: e15–e29. https://doi.org/10.1161/CIRCRESAHA.119.315502 [Google Scholar] [PubMed] [CrossRef]
Xie MR, Tang QM, Yu SL, Sun JW, Mei F, Zhao J, Chen L (2020b). Porphyromonas gingivalis disrupts vascular endothelial homeostasis in a TLR-NF-κB axis dependent manner. International Journal of Oral Science 12: 28. https://doi.org/10.1038/s41368-020-00096-z [Google Scholar] [PubMed] [CrossRef]
Xuan Y, Qiao S, Liu GJ, Luan QX, Yu C (2017). Porphyromonas gingivalis infection accelerates atherosclerosis mediated by oxidative stress and inflammatory responses in ApoE−/− Mice. Clinical Laboratory 63: 1627–1637. https://doi.org/10.7754/Clin.Lab.2017.170410 [Google Scholar] [PubMed] [CrossRef]
Yamada M, Takahashi N, Matsuda Y, Sato K, Yokoji M et al. (2018). A bacterial metabolite ameliorates periodontal pathogen-induced gingival epithelial barrier disruption via GPR40 signaling. Scientific Reports 8: 9008. https://doi.org/10.1038/s41598-018-27408-y [Google Scholar] [PubMed] [CrossRef]
Zardawi F, Gul S, Abdulkareem A, Sha A, Yates J (2020). Association between periodontal disease and atherosclerotic cardiovascular diseases: Revisited. Frontiers in Cardiovascular Medicine 7: 625579. https://doi.org/10.3389/fcvm.2020.625579 [Google Scholar] [PubMed] [CrossRef]
Zeituni AE, Jotwani R, Carrion J, Cutler CW (2009). Targeting of DC-SIGN on human dendritic cells by minor fimbriated Porphyromonas gingivalis strains elicits a distinct effector T cell response. The Journal of Immunology 183: 5694–5704. https://doi.org/10.4049/jimmunol.0901030 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools