 Open Access
Open Access
ARTICLE
Clinical implication of naive and memory T cells in locally advanced cervical cancer: A proxy for tumor biology and short-term response prediction
1 Key Laboratory of Cancer Immunotherapy and Radiotherapy, Chinese Academy of Medical Sciences, Affiliated Cancer Hospital of Xinjiang Medical
University, Urumqi, China
2 Key Laboratory of Oncology of Xinjiang Uyghur Autonomous Region, Affiliated Cancer Hospital of Xinjiang Medical University, Urumqi, China
3 State Key Laboratory of Pathogenesis, Prevention, and Treatment of High Incidence Diseases in Central Asia, The Third Affiliated Hospital of Xinjiang Medical
University, Urumqi, China
4 Chinese Academy of Medical Sciences Oxford Institute (CAMS Oxford Institute), University of Oxford, Oxford, UK
* Corresponding Author: Ruozheng Wang,
BIOCELL 2023, 47(6), 1365-1375. https://doi.org/10.32604/biocell.2023.027201
Received 19 October 2022; Accepted 16 January 2023; Issue published 19 May 2023
Abstract
Background: This study was designed to investigate the feasibility of tumor-infiltrating immune cells with different phenotypic characteristics for predicting short-term clinical responses in patients with locally advanced cervical cancer (LACC). Methods: Thirty-four patients who received concurrent chemoradiotherapy and twenty-one patients who merely underwent radiotherapy were enrolled in this study. We retrospectively analyzed the T cell markers (i.e., CD3, CD4, CD8), memory markers (i.e., CD45, CCR7), and differentiation markers (i.e., CD27) in the peripheral blood and tumor tissues of patients with LACC before treatment based on flow cytometry. We also analyzed the relationship of T cell subsets between peripheral blood and tumor tissues, and their correlation with complete response or partial response. Results: The percentage of central memory CD8+ TCM (CD8+ CD45RA− CD27+ CCR7+ ) cells in LACC patients was significantly lower than that of the control group. The percentage of CD8+ TN in the peripheral blood of LACC patients was significantly higher than that of tumor tissues. CD8+ TEM in the peripheral blood was significantly lower than that of tumor tissues. The percentage of CD8+ TN and CD8+ TCM in human papillomavirus (HPV) positive samples was significantly higher than that of HPV-negative samples. Similarly, the percentage of CD8+ TCM in tumor tissues was significantly higher in cancer tissue samples with lymph nodes compared with those without. Conclusion: A higher proportion of CD4+ TCM and a lower proportion of CD8+ TN in the tumor microenvironment of LACC may contribute to the therapy response prediction.Keywords
Cervical cancer, ranked as the fourth most common cancer among the female population worldwide (Ferlay et al., 2019), is one of the major causes of cancer-related death (Bray et al., 2018). The prognosis of locally advanced cervical cancer (LACC) patients is poor with a high risk of recurrence (Chhabra, 2018) and an extremely lower 5-year survival rate due to a lack of effective treatment strategies (Chhabra, 2015).
Dysregulation of the immune system is a hallmark of cancer (Hanahan and Weinberg, 2011). Increasing evidence indicates that naive T cells and memory T cells-mediated host immune response plays a key role in the pathogenesis of cancer (Wörmann et al., 2014). Memory T cells have been confirmed to mediate the tumor microenvironment in anti-tumor immunity (Hu and Wang, 2017). Phenotypic expression of cell surface proteins (e.g., CCR7, CD45RA, and CD27) has been utilized to study the differentiation of memory T cells. Expression of the costimulatory molecules (e.g., CD27 and CD28, CD45RA or CD45RO) is associated with the function of naive, memory, and effector CD8+T cells (Hamann et al., 1997; Tomiyama et al., 2002). Additionally, flow cytometry analysisfor CD45RA and either CD27 or CCR7 contributes to the identification of TN (CD45RA+/CD27+/CCR7+), TEM (CD45RA−/CD27−/CCR7−), and TCM (CD45RA−/CD27+/CCR7+), respectively (Van Braeckel-Budimir et al., 2018; Weekes et al., 1999). Moreover, the stepwise differentiation of human memory CD4+T cells by CCR7 and CD27 indicated that these markers contributed to the identification of the distinct maturational stages of CD4 memory T cells with different functional activities (Fritsch et al., 2005). Furthermore, van Lier and colleagues defined two kinds of CD8 memory T-cell subsets based on the expression of CD45 isoforms (i.e., CD45RA and CD45RO) that lacked immediate cytolytic function, and CD45RA+CD27− effector cells with a low proliferative capacity and high perforin and cytotoxicity (Hamann et al., 1997). Such a technique is reported to provide a more precise determination of the maturational stages of T lymphocytes with different functions. In this study, three cell surface markers (i.e., CD45RA, CD27, and CCR7) were utilized to determine the stages of CD4 and CD8 memory T cells. Then, we investigated the feasibility of using tumor-infiltrating immune cells with different markers for predicting short-term clinical responses in LACC patients who underwent chemoradiotherapy and radiotherapy, respectively.
Fifty-five LACC patients (34–83 yrs, median: 52 yrs; ICD-10, code C53.900) admitted to our hospital between August 2016 and April 2019 were recruited in this study. All the patients received no treatment before admission. The inclusion criteria were as follows: (a) pathologically confirmed with cervical squamous cell carcinoma; (b) at a stage of IB2-IVA by FIGO staging system; (c) aged >18 years old with a Karnofsky Performance Scale (KPS) of ≥70 points; (d) received no human papillomavirus (HPV) vaccination before; (e) showing no contraindications to radiotherapy or chemotherapy. The following patients should be excluded: (a) complicated with other neoplastic diseases, autoimmune-related diseases, as well as acute and chronic infectious diseases; (b) those with a history of transplantation. Twenty-six healthy subjects, who underwent physical examination in the Healthcare Center of our hospital at the same time, served as normal control. The clinical staging of each patient was based on the FIGO staging system (2009 Edition) (Pecorelli, 2009). Written informed consent was obtained from each patient and health control before entering the study. This study was approved by the Institutional Ethics Committee of the Affiliated Cancer Hospital of Xinjiang Medical University (No. K-2019001).
All patients received external irradiation and intracavity brachytherapy, and the individualized treatment plan was formulated according to the 2019 National Comprehensive Cancer Network (NCCN) Cervical Cancer Guidelines (Koh et al., 2019), as well as the economic situation and personal desire of the patients. Radiotherapy mode was as follows: intensity modulated radiotherapy (in vitro irradiation) +Ir192 high dose rate intracavitary post-loading therapy (intracavitary irradiation). Synchronous chemotherapy methods were as follows: single cisplatin (30 mg/m2) via intravenous infusion, once a week, 4–6 cycles of chemotherapy; or paclitaxel (135 mg/m2, d1) combined with cisplatin (50 mg/m2, d1-3, via intravenous infusion), 3 weeks per cycle.
Peripheral blood samples (5 mL) were collected from each subject before any treatment, followed by lymphocyte assessment using a routine clinical flow cytometry assay (Lambert et al., 2020). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from fresh EDTA anticoagulant blood based on the Ficoll-Hypaque density gradient centrifugation. Cervical cancer tissue samples were obtained using the biopsy forceps, and then were cut into fragments (1–3 mm) with sterile surgical scissors and placed into a C tube (BD Biosciences) filled with RPIM 1640 (5 ml, Sigma-Aldrich) supplemented with enzymes in the tumor ionization kit (BD Biosciences). Single-cell suspension was obtained with a 70 μm cell filter, followed by rinsing twice with R10. Flow cytometry was performed to analyze the CD45RA, CD27, and CCR7 on CD4+T and CD8+T cells in the peripheral blood from LACC and healthy controls, as well as tumor biopsies from LACC patients. Flow cytometry data were analyzed using the BD FACS Diva flow cytometry analysis software.
Multi-chromatic flow cytometry staining
T cell differentiation phenotype was discriminated by the surface markers CD27, CCR7, and CD45RA. Cells derived from tumor tissues or PBMCs were stained with LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific, Waltham, MA, USA) for 20 min on ice before surface staining with conjugated antibodies in FACS washing buffer for another 20 min and fixed with 1×CellFix solution (BD Biosciences). Data were acquired using the BD LSR Fortessa™ cell analyzer (BD Biosciences). In order to ensure the quality of the FACS data, samples with viable CD3+T of less than 10,000 cells were ruled out.
Statistical analysis was conducted with the SPSS software (25.0 version). The figures were drawn using the GraphPad Prism Software (8.0 version). A Z-test was used for comparison of the measurement data. Data that were not normally distributed were compared using the Rank sum test. Paired sample t-test was performed to analyze the distribution difference of naive and memory T cells between tumor tissues and peripheral blood samples. Univariate and multivariate logistic regression analyses were used to evaluate the relationship between naive or memory T cell subsets and short-term responses. In addition, the odds ratio (OR) with 95% confidence interval (CI) was calculated. All the tests were performed at least in triplicate. p < 0.05 was considered to be statistically significant.
Clinicopathological characteristics
All the 55 cases received radiotherapy, among which 34 received concurrent chemoradiotherapy. For the patients who underwent concurrent chemoradiotherapy, most patients received cisplatin-based chemotherapy, such as cisplatin monotherapy or cisplatin combined with paclitaxel. The clinicopathological characteristics of the LACC patients and healthy control are summarized in Table 1.
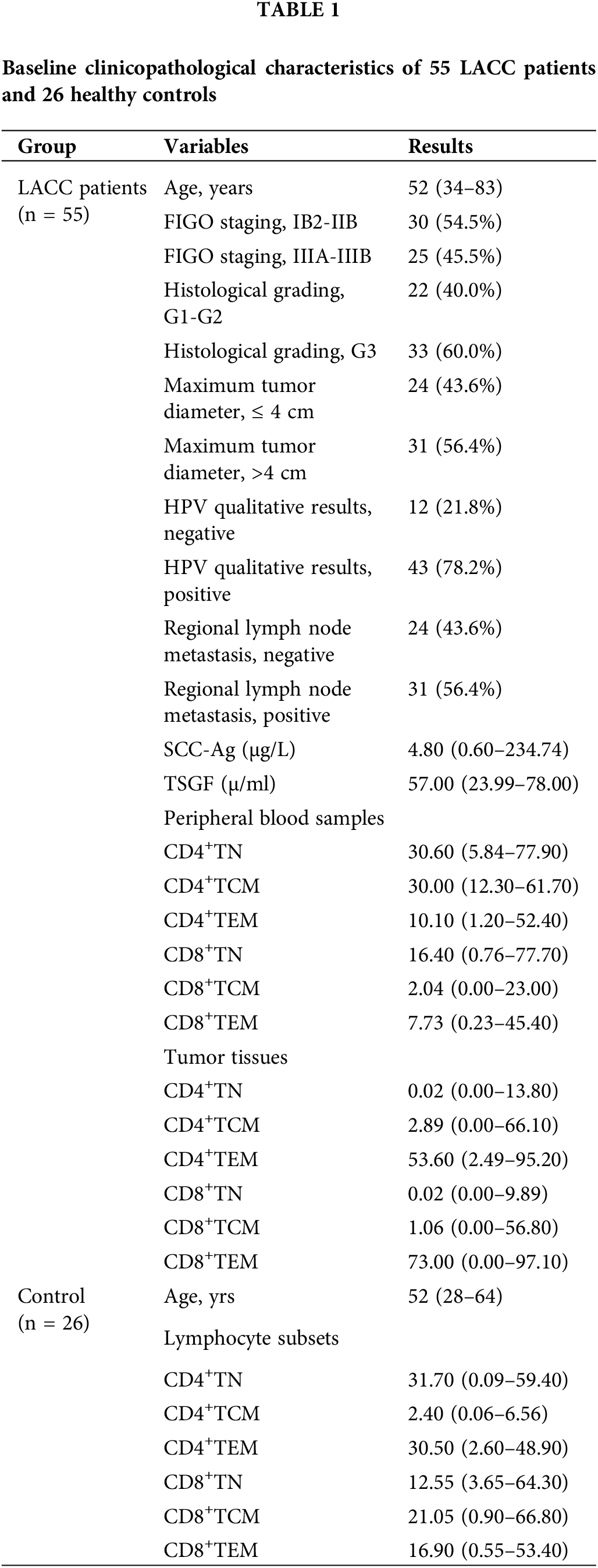
Characterization of memory CD4 and CD8 T cells
We determined the expression of CD45RA, CD27, and CCR7 on CD4+T and CD8+T cells in the tumor biopsy samples of LACC patients, as well as the peripheral blood samples of LACC and healthy controls. Fig. 1 shows the representative images for CD45RA, CD27, and CCR7 in CD4+T cells (i.e., CD4+CD45RA+T cells, CD4+CCR7+T cells, and CD4+CD27+T cells) and CD8+T cells (CD8+CD45RA+T cells, CD8+CCR7+T cells, and CD8+CD27+T cells), respectively.
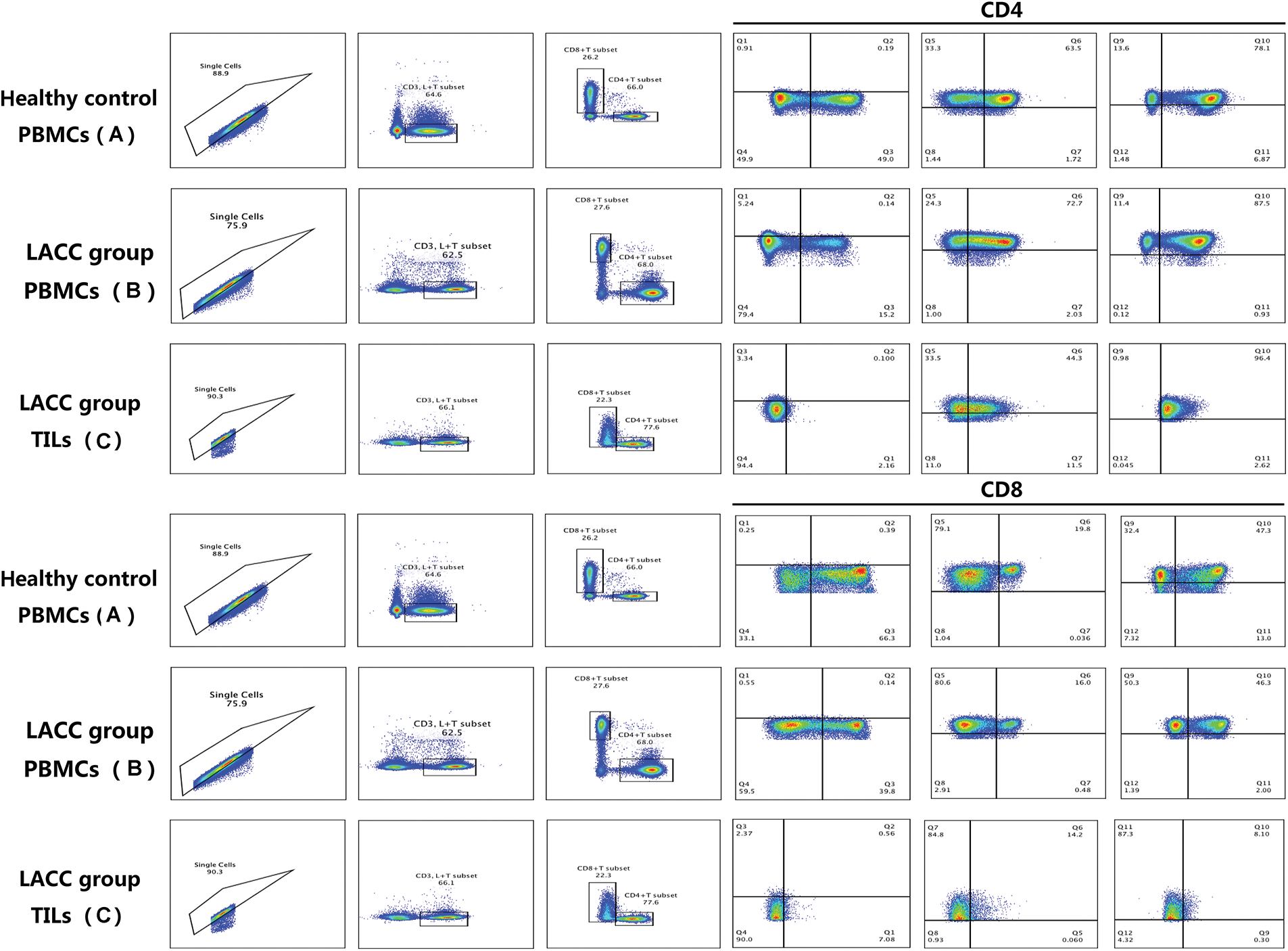
Figure 1: Flow cytometric analyses on the CD45RA, CD27, and CCR7 in CD4+T and CD8+T cells in the peripheral blood of healthy controls, as well as the blood and tumor of LACC patients. The frequency of the different T-cell subgroups were then calculated by the function of Boolean Combination Gates in FlowJo software.
Comparison of the naive and memory T cell subsets between LACC patients and healthy controls
Upon characterizing the memory CD4 and CD8 T cells in the peripheral blood, we then compared the naive and memory T cells in the blood samples of the LACC group and healthy control. The changes in CD4+TN, CD4+TCM, CD4+TEM, CD8+TN, and CD8+TEM showed no statistical differences between the LACC group and health control. The percentage of the central memory CD8+TCM (CD8+CD45RA−CD27+CCR7+) cells was significantly lower than that of the control group (3.26 ± 3.02 vs. 5.41 ± 4.80, p = 0.005, Fig. 2).
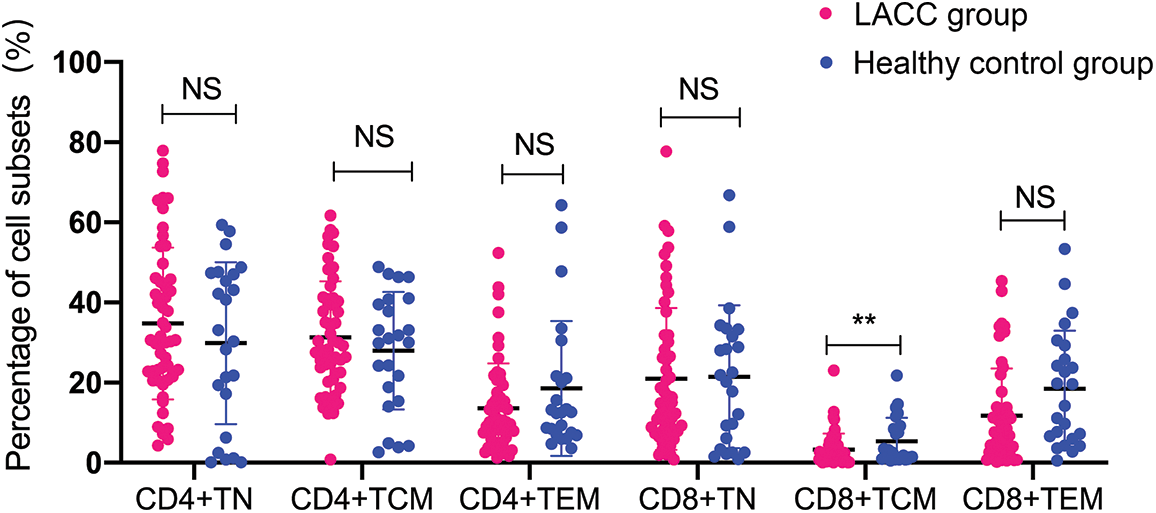
Figure 2: Frequency of naive and memory T cell in CD4+T and CD8+T cells in the peripheral blood of 55 LACC patients and 26 healthy controls. The p value was determined using the unpaired Z-test. The expression level of the naive and memory CD4+T and CD8+T cells was expressed as a percentage of the total CD4 and CD8 T cells. **p < 0.01; NS, no significance.
Comparison of the naive and memory T cell subsets between the cervical tumor tissue and peripheral blood in the LACC patients
Then, we compared the naïve and memory T cells between the tumor tissues and blood samples in the LACC patients. The percentage of CD8+TCM showed no statistical differences between the peripheral blood and tumor tissue of the LACC patients. The percentage of CD4+TN and CD4+TCM in the peripheral blood of the LACC patients was significantly higher than in the tumor tissues (CD4+TN: 34.76 ± 18.93 vs. 2.29 ± 0.78, p < 0.001; CD4+TCM: 31.26 ± 14.03 vs. 14.86 ± 8.83, p < 0.001). The percentage of CD4+TEM in the peripheral blood was significantly lower than that of the tumor tissues (13.60 ± 11.21 vs. 46.52 ± 29.46, p < 0.001). The percentage of CD8+TN in the peripheral blood was significantly higher than that in the tumor tissues (20.93 ± 17.68 vs. 1.65 ± 0.48, p < 0.001). CD8+TEM in the peripheral blood was significantly lower than that of tumor tissue (11.67 ± 8.86 vs. 61.73 ± 31.25, p < 0.001, Fig. 3).
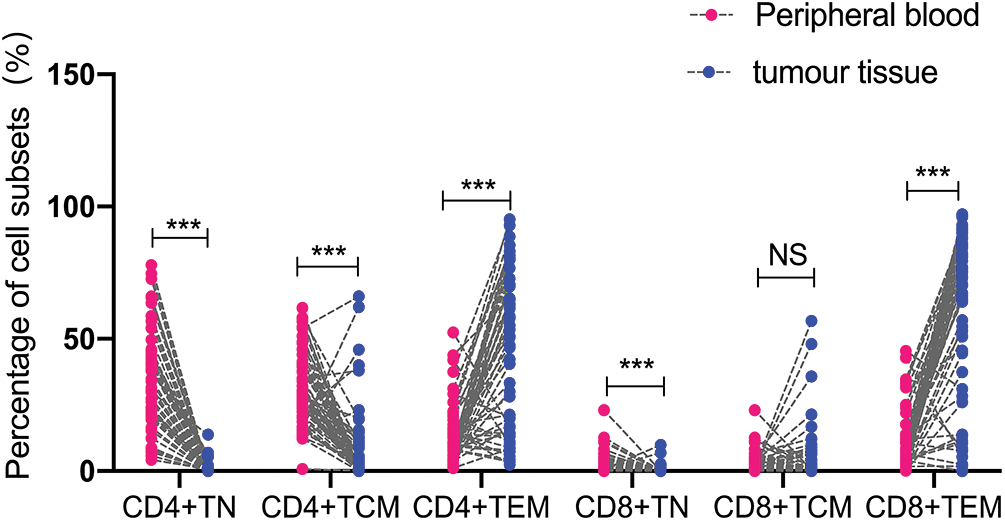
Figure 3: Comparison of naive and memory T cell levels in the peripheral blood and tumor tissue of 55 LACC patients. The level of naive or memory on CD4+T cells or CD8+T cells was expressed as a percentage of the total CD4 and CD8 T cells. The p value was determined using an unpaired Z-test. ***p < 0.001; NS, no significance.
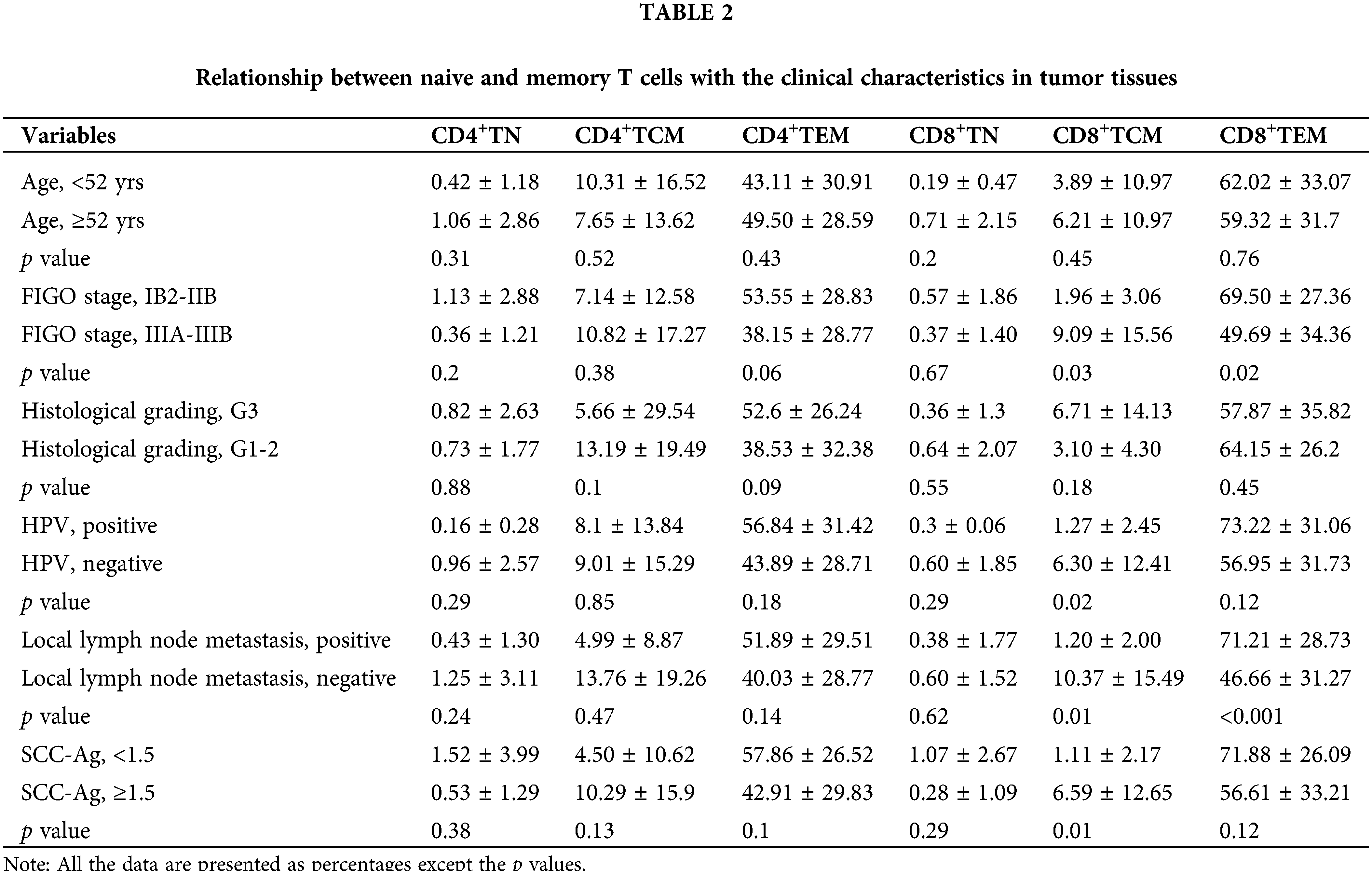
CD8+TCM was related to FIGO stage, HPV, local lymph node metastasis, and SCC-Ag in cervical cancer tissues
In this section, we determined the relationship between the proportion of naive and memory T cells with the clinical characteristics of tumor tissues, including age, FIGO stage, histological grading, HPV and lymph node metastasis, as well as SCC-Ag. Briefly, there were no statistical differences in the proportion of CD4+TN, CD4+TCM, and CD4+TEM between these with different clinical characteristics (p > 0.05, Table 2). In contrast, the percentage of CD8+TCM was significantly higher in the LACC patients at IIIA and IIIB stages than those at the IB2 and IIB stage (9.09 ± 15.56% vs. 1.96 ± 3.06%, p = 0.03). The percentage of CD8+TEM in the patients at IIIA-IIIB stage was significantly lower than those at the IB2-IIB stage (49.69 ± 34.36% vs. 69.50 ± 27.36%, p = 0.02). The HPV-negative samples showed a higher CD8+TCM level compared with that in the HPV-positive samples (6.30 ± 12.41% vs. 1.27 ± 2.45%, p = 0.02). Compared with the local LNM positive samples, the CD8+TCM level was significantly higher in the negative samples (10.37 ± 15.49% vs. 1.20 ± 2.00%, p = 0.01), while the CD8+TEM level was significantly lower (46.66 ± 31.27% vs. 71.21 ± 28.73%, p < 0.001). For the tumor samples with SCC-Ag of ≥1.5, the proportion of CD8+TCM was significantly higher compared with the samples with SCC-Ag of <1.5 (6.59 ± 12.65% vs. 1.11 ± 2.17%, p = 0.01).
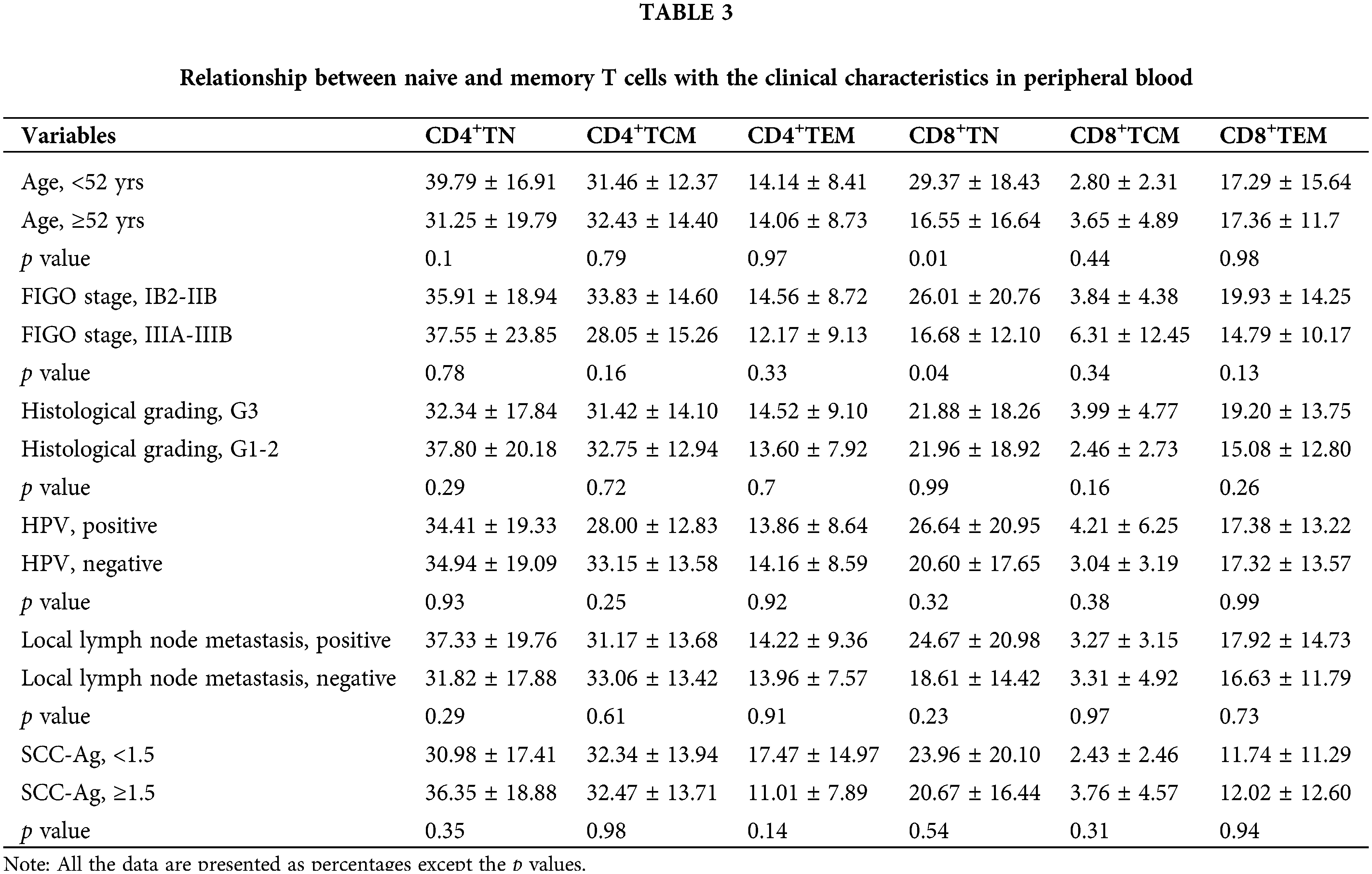
For the relationship between the proportions of naive and memory T cells with the clinical characteristics in peripheral blood samples, CD4+TN, CD4+TCM, and CD4+TEM showed no statistical differences between the samples with different characteristics (p > 0.05, Table 3). The CD8+TN proportion in the blood obtained from those aged higher than 52 yrs was significantly lower than that in those aged less than 52 yrs (16.55 ± 16.64 vs. 29.37 ± 18.43. p = 0.01). Besides, the proportion of CD8+TN in the blood obtained from those with FIGO stages of IIIA-IIIB was significantly lower than that of those with IB2-IIB (16.68 ± 12.10 vs. 26.01 ± 20.76, p = 0.04).
Factors screened for predicting short-term response
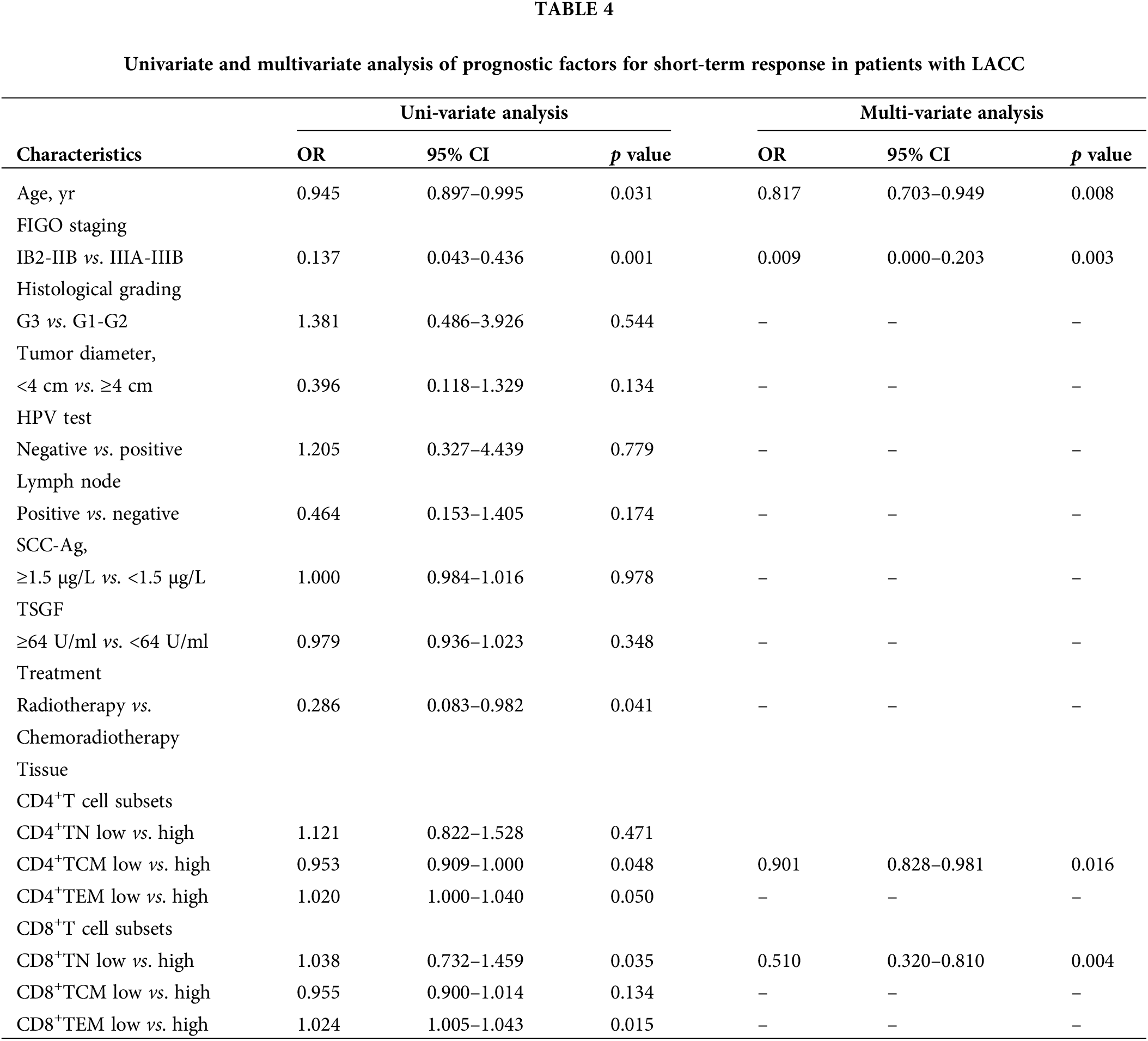
We determined the factors that functioned in predicting short-term response among the LACC patients. Logistic regression analysis revealed that patients aged <52 yrs showed a better short-term response compared with those aged ≥52 yrs (OR: 0.945, 95% CI: 0.897–0.995, p = 0.031). Patients at a FIGO staging of IIIA-IIIB had a poorer short-term response compared to those of IB2-IIB stage (OR: 0.137, 95% CI: 0.043–0.436, p = 0.001). There was significant difference in short-term response in low vs. high CD4+TCM subsets (OR: 0.953, 95% CI 0.909–1.000, p = 0.048) and there was also association between CD8+TN subsets and short-term response (OR: 1.038, 95% CI 0.732–1.459, p = 0.035, Table 4).
Efficiency of naive and memory T cells in tumor as prediction markers for the short-term response in LACC
To test the efficiency of the naïve and memory T cells as prediction markers for LACC, we evaluated the short-term response based on the clinical data and CT/MRI by referring to RECISIT (version 1.1) one month after the first-line CCRT. Thirty-four cases (61.8%) showed complete remission (CR), and 21 (38.2%) showed partial remission (PR). An ROC analysis was employed to determine the efficiency of naive/memory T cell phenotypes in distinguishing CR and PR among the patients. Naive and memory T-cell subsets in tumor tissues was effective in predicting short-term CR and PR in the LACC patients (Fig. 4). The area under the curve (AUC) of CD4+TN and CD8+TN cell subsets was 0.997 and 0.993, respectively. The sensitivity of CD4+TCM, CD4+TEM, and CD8+TEM was 61.8%, 76.5%, and 79.4%, respectively (Table 5). According to the cut-off value, the target T cell subsets in the tumor tissue were divided into the lower group and the higher group. The CD4+TN, CD4+TCM, CD4+TEM, CD4+TN, CD4+TCM, and CD4+TEM in the peripheral blood were not feasible for use in distinguishing the CR and PR for the LACC patients (Fig. 5).
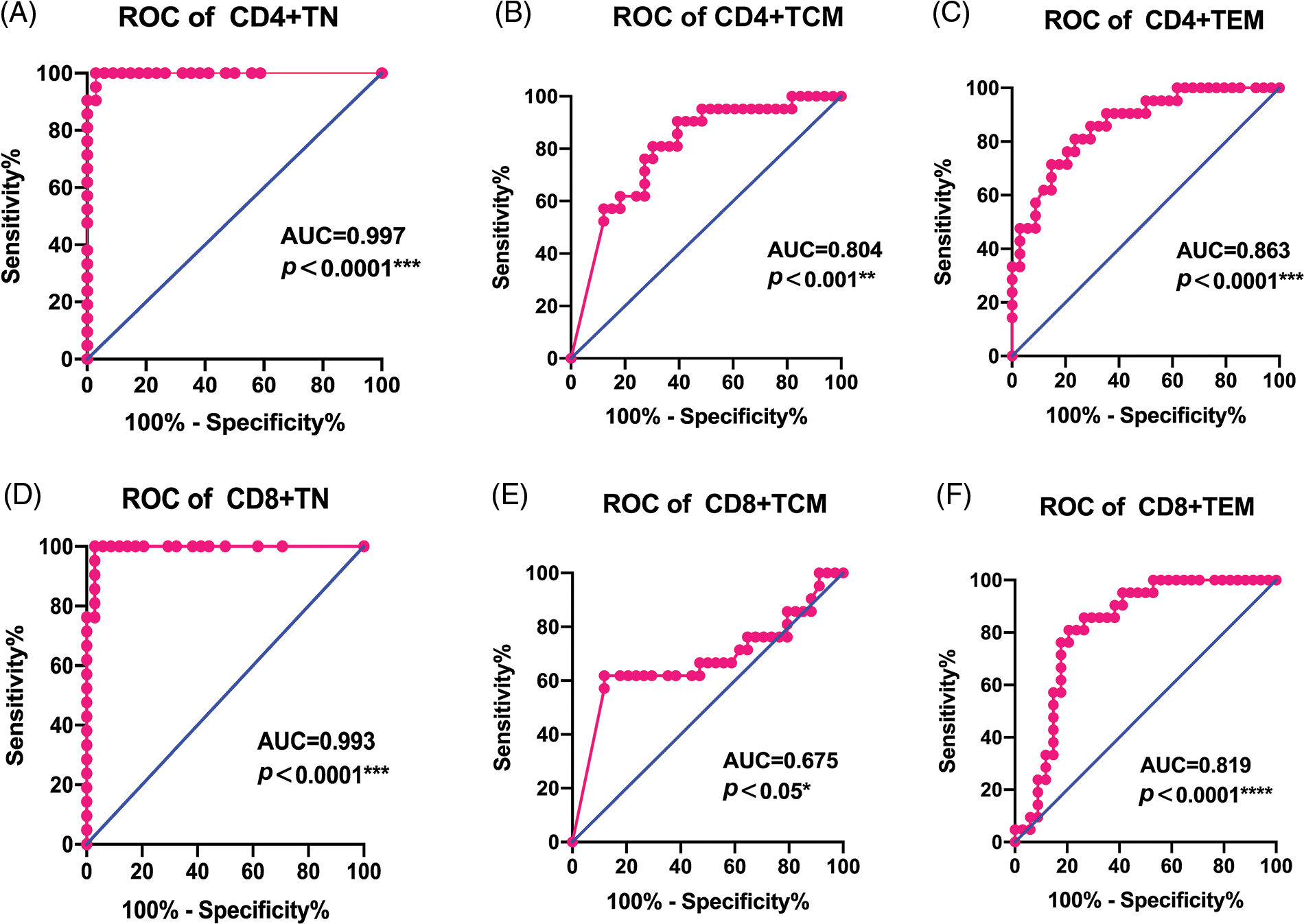
Figure 4: ROC curves of immune cell for discriminating complete response and partial response for CCRT in tumor tissues. (A) CD4+TN, (B) CD4+TCM, (C) CD4+TEM (D) CD8+TN, (E) CD8+TCM, (F) CD8+TEM.
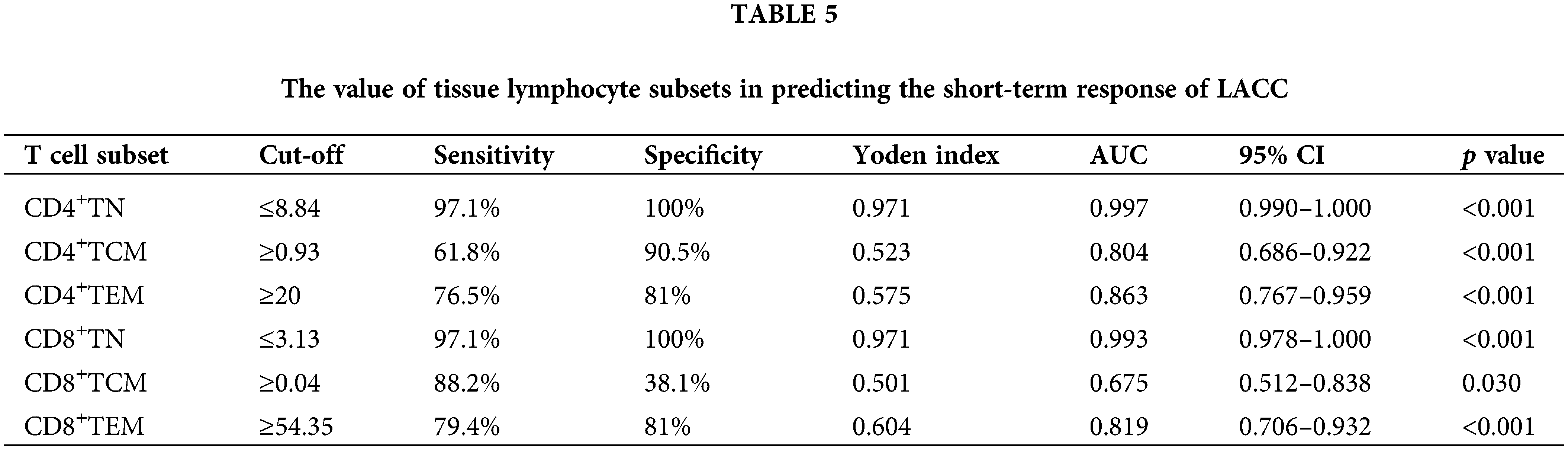
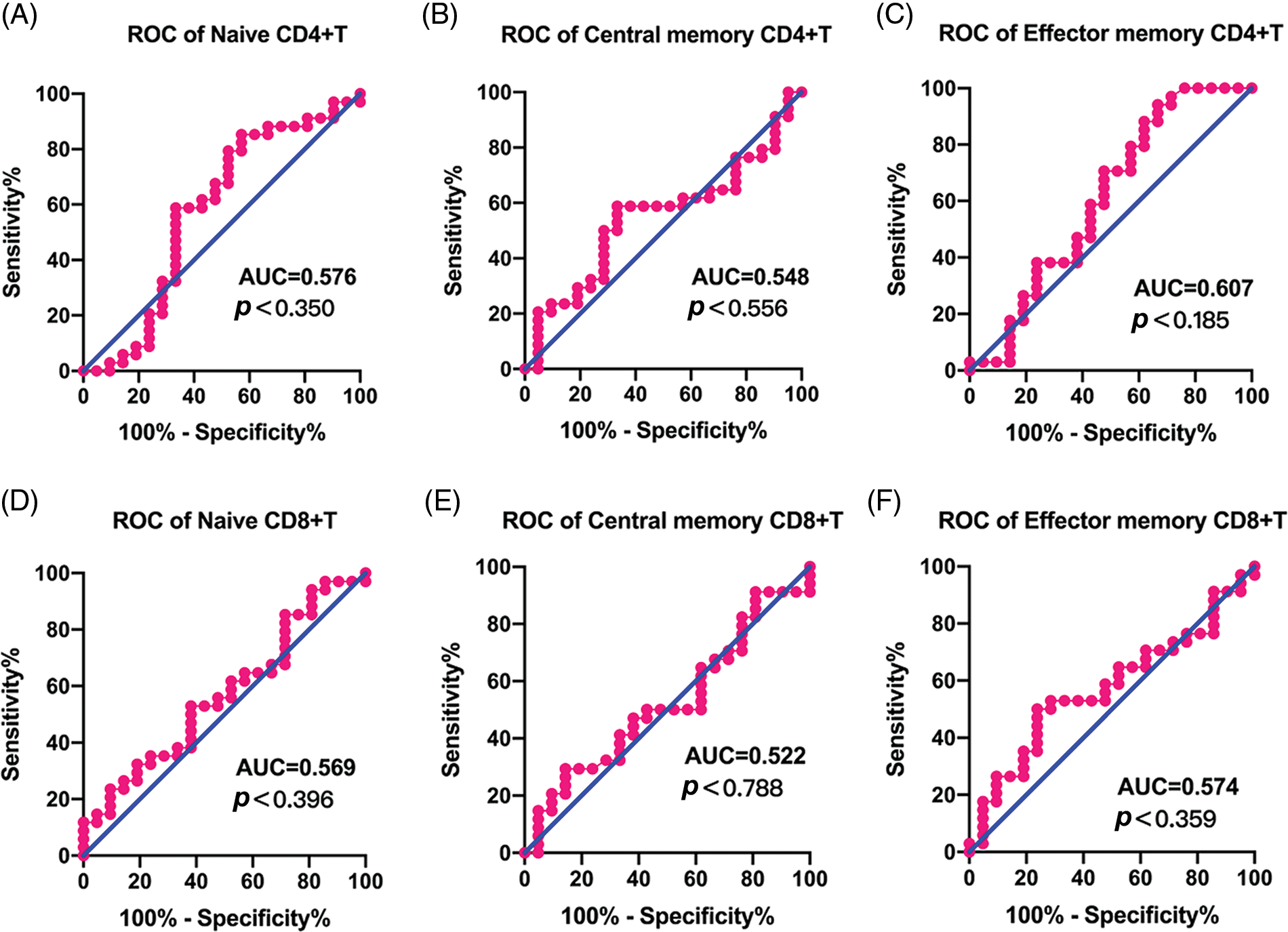
Figure 5: ROC curves of immune cell to discriminate complete response and partial response for CCRT in peripheral blood. (A) CD4+TN, (B) CD4+TCM, (C) CD4+TEM, (D) CD8+TN, (E) CD8+TCM, (F) CD8+TEM.
Cervical cancer is an immunogenic tumor correlated with the function of various immunocytes and inflammatory cells including naive T cells and memory T cells. However, their prognostic roles in the pathogenesis of LACC are still not well defined (Crespo et al., 2018). In a previous study, naive T cells expressing CD45RA that were usually functionally quiescent (Sallusto et al., 2004) would respond to stimuli, which subsequently produced a high level of chemokines (e.g., CXCL8). This mediated neutrophil migration to tumor tissues and promoted the tumor growth (Crespo et al., 2018). In this study, we focused on the expression of naive, central memory, and effector memory T cell subsets in the peripheral blood and tumor tissues of LACC patients. Additionally, we further explored its correlation with their clinical pathology. Our data showed that there were no differences in the percentages of CD4+ or CD8+T cells in the blood samples between LACC patients and healthy controls, except the CD8+TCM. In contrast, the percentages of CD4+ and CD8+ showed statistical differences between the peripheral blood and tumor tissues of LACC patients, except CD8+TCM. This phenomenon indicates a local defect in recruiting central memory CD8+T cells in the peripheral blood of LACC patients, which may be involved in the escape of tumor cells from the immune response.
The homeostasis of memory T-cell pool is under strict control, regardless of loss or over-production of memory T cells (Ucar et al., 2017). Generally, the pools will re-equilibrate to a normal number after increased input into the pool is caused by immune responses. Afterwards, it would lead to an expansion of the set threshold after T cell loss. In addition, a significant increase was seen in the proportion of memory T cells. Nevertheless, the absolute number of memory T cells showed no changes, which emphasizes that memory T cells did not ‘spill over’ into the void left in the presence of naive T cell loss (Akondy et al., 2017). The CD4+T cells functioned as central regulators for humoral and cellular immune responses. Absence of CD4+T cell subsets was associated with the compromising of CD8+T cell response and CD8+T cell memory, together with downregulating the effector responses. On this basis, it is reasonable to speculate that CD4+T cells are likely to be involved in the maintenance and control of protective immune responses. Furthermore, according to a global analysis of T-cell clones specific for Epstein-Barr virus or tetanus toxoid in human peripheral blood, the memory CD4+T-cell repertoire may be more heterogeneous than that of the memory CD8+T-cell pool. In addition, epigenetic evidence of age-associated differentiation was more pronounced for CD8+T cells than for CD4+T cells (Provinciali et al., 2009). A possible explanation was that central memory or stem-like memory CD8 T cells could revert back to a largely naive phenotype, which then contaminated the naive compartment (Sallusto et al., 1999). As CD4+T cell subsets contain more complex populations (e.g., Th1, Th2, Treg, and Th17), more studies are required to illustrate the relationship between CD4+T subsets and LACC. The remarkable diversity of CD4+T cells is linked to their challenging roles in the orchestration of immune responses. The CD4+T cells existed as multiple functionally and phenotypically distinct subsets, while the memory CD4+T cells, even within a single subset, persisted in interchangeable distinct states of differentiation. Such persistence may maximize their effectiveness upon re-encountering a pathogen. In this study, the CD4+TN and CD4+TCM in peripheral blood were significantly higher than those in tumor tissues, while the CD4+TEM in peripheral blood was significantly lower than those in tumor tissues.
No pre-existing immunity or adaptive immune response has been reported to rely on the naive lymphocytes (Almeida et al., 2001). CD8+T cells play a pivotal role in clearing intracellular pathogens and combating tumors. The hallmark of adaptive T immunity is the naive program, which actively maintains CD8+T cell quiescence until receiving appropriate activation signals. Indeed, a cardinal feature of T cell-mediated immunity is the establishment of immunological memory, where memory CD8+T cells are capable of rapidly responding to re-infection (Bourgeois et al., 2005). Reactivating CD8+T cell immunity against tumors using targeted checkpoint blockade therapy has led to the revolutionization of cancer treatment (Hu et al., 2020). In the past decades, the quiescence of immature T cells was in a “default” or “quiescent” state (Lalvani et al., 1997). However, it is now clear that maintenance of the CD8+T cell naivety is in an active process, which was speculated to evolve with the adaptive immune system. This thereby created obstacles to the default receptors and memory recognition programs for hosts under dynamic equilibrium conditions. In fact, receptor and memory CD8+T cells showed functional characteristics that were usually attributed to innate immunity. On this basis, a key difference between adaptive immunity and innate immunity was the presence of a real “infantile state”. For the quiescence in naive T cells, a threshold was required to be overcome for optimal CD8+T cell activation. Dysregulation of these restraint programs resulted in the lowering or removal of this activation threshold, which contributed to the development of autoimmunity or excessive antigen-specific responses to intracellular pathogens. Thus, in the design of novel vaccine strategies to induce CD8+T cell activation and subsequent robust responses, there should be a consideration towards targeting the negative regulators. Our data showed that the proportion of CD8+TCM and CD8+TEM cells in tumor tissues was correlated with FIGO stage, and the proportion of CD8+TEM cells was also correlated with HPV test, local lymph node metastasis, and SCC-Ag. Compared with patients at IB2-IIB, patients with IIIA-IIIB had a higher proportion of CD8+TCM cells and a lower proportion of CD8+TEM cells (p < 0.05). The proportion of CD8+TEM in tumor tissue was negatively correlated with HPV test and local lymph node metastasis, and positively correlated with SCC-Ag level. Moreover, in the patients with an HPV-positive status, higher percentages of CD8+TCM cells were observed compared with patients with an HPV-negative status. These findings could be an indication for the presence of anti-HPV immunity as has been described previously (Heusinkveld et al., 2012). HPV-specific immunity may contribute to the improved prognosis for patients with HPV-positive LACC. Notably, the CD8+TN ratio in the peripheral blood of patients with FIGO stage IIIA-IIIB was higher than that of patients with stage IB2-IIB. Compared with patients aged ≥52 years old, patients aged <52 years old had a higher proportion of CD8+TN cells in their peripheral blood. All these contributed to the development of regimens based on the immune response of the T cells to the malignancies.
It was noted that the percentage of effector memory T cells increased from stages IB2 and IIB tumor patients and subsequently declined again in stages IIIA and IIIB. However, naive T cells in the peripheral blood of LACC patients showed an opposite trend. It can be envisioned that a small tumor load could be expected to show limited effects on the immune system in LACC patients, while with the increase of tumor load in locally advanced or even advanced stages, tumors may exert immune suppressive effects, leading to a decline in effector T cells. In addition, we also found a decrease in the proportion of CD8+TEM in the LACC patients with regional lymph node metastasis, as regional lymph node metastasis is also associated with a higher tumor burden. Therefore, we concluded that a higher tumor load before initial treatment may indicate a deficiency in the autoimmune function. As we described before, both naive and memory T cells showed their prognostic roles in various tumors, but few studies have been performed in cervical cancer. Our data showed that there was no correlation between CD4+TN, CD4+TCM, CD4+TEM, CD8+TN, CD8+TCM, and CD8+TEM in peripheral blood and CD4+TN, CD4+TEM, CD8+TCM, and CD8+TEM in tumor tissues with the short-term response of LACC patients. However, a higher CD4+TCM baseline and a lower CD8+TN were positively correlated with a better short-term response. In addition, among these six T cell subsets, only CD4+TCM (OR: 0.953, 95% CI 0.909–1.000, p = 0.048) and CD8+TN showed independent prognostic impacts in tumor tissues according to the multivariate analysis (OR: 1.038, 95% CI 0.732–1.459, p = 0.035). Intriguingly, most of the data reported in the previous literature were derived from peripheral blood, which involved the ratio of immature to memory T cells (Liu et al., 2019; Yang et al., 2017). In this study, the elevated CD4+TCM and CD8+TN in tumor tissues was correlated with a better short-term response. These results suggest that naive and memory T cells were more suitable to reveal the systemic inflammatory response to LACC. In line with previous studies (Provinciali et al., 2009; Saule et al., 2006), our data demonstrated that there was an increase in the number of circulating memory T cells, especially CD4+ memory T cells. Besides, the naive T cells were negatively correlated with the age. These facilitated an understanding of the characterization of the CD4+ and CD8+ cells and their roles in predicting the prognosis of LACC.
There are some limitations in this study. First, the sample size of the patients involved in determining the change of naïve and memory T cells during the first-line treatment was not large. Second, it was not clarified whether there was an inherent relationship between the increased proportion of CD4+TCM and the decreased proportion of CD8+TN in tumor microenvironment. Third, an external validation cohort is still required in the future to make the results more convincing.
CD4+TCM and CD8+TN showed independent effects on the prognosis of LACC patients. A higher proportion of CD4+TCM and a lower proportion of CD8+TN in the tumor microenvironment of LACC may provide a simple and easily implemented tool for predicting the response after treatment. Therefore, these two cell subsets can be used as biomarkers to predict the short-term response of LACC patients.
Acknowledgement: We thank all the patients and healthy women for participating in this study and donating their blood and tissue samples.
Funding Statement: This work was supported by the Project of the Central Government Guiding Local Science and Technology under Grant Number ZYYD2022B18.
Author Contributions: The authors confirm contributions to the paper as follows: study conception and design, WRZ; data collection, WYT, FPW, and FYN; analysis and interpretation of results, YX and PYC; draft manuscript preparation, WYT. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: All patients and healthy women provided written informed consent before participating in the study. This study was approved by the Institutional Ethics Committee of Affiliated Cancer Hospital of Xinjiang Medical University (No. K-2019001).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT et al. (2017). Origin and differentiation of human memory CD8 T cells after vaccination. Nature 552: 362–367. https://doi.org/10.1038/nature24633 [Google Scholar] [PubMed] [CrossRef]
Almeida AR, Borghans JA, Freitas AA (2001). T cell homeostasis: Thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors. Journal of Experimental Medicine 194: 591–599. https://doi.org/10.1084/jem.194.5.591 [Google Scholar] [PubMed] [CrossRef]
Bourgeois C, Kassiotis G, Stockinger B (2005). A major role for memory CD4 T cells in the control of lymphopenia-induced proliferation of naive CD4 T cells. The Journal of Immunology 174: 5316–5323. https://doi.org/10.4049/jimmunol.174.9.5316 [Google Scholar] [PubMed] [CrossRef]
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68: 394–424. https://doi.org/10.3322/caac.21492 [Google Scholar] [PubMed] [CrossRef]
Chhabra R (2015). Cervical cancer stem cells: Opportunities and challenges. Journal of Cancer Research and Clinical Oncology 141: 1889–1897. https://doi.org/10.1007/s00432-014-1905-y [Google Scholar] [PubMed] [CrossRef]
Chhabra R (2018). let-7i-5p, miR-181a-2-3p and EGF/PI3K/SOX2 axis coordinate to maintain cancer stem cell population in cervical cancer. Scientific Reports 8: 7840. https://doi.org/10.1038/s41598-018-26292-w [Google Scholar] [PubMed] [CrossRef]
Crespo J, Wu K, Li W, Kryczek I, Maj T, Vatan L, Wei S, Opipari AW, Zou W (2018). Human naive T cells express functional CXCL8 and promote tumorigenesis. The Journal of Immunology 201: 814–820. https://doi.org/10.4049/jimmunol.1700755 [Google Scholar] [PubMed] [CrossRef]
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F (2019). Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer 144: 1941–1953. https://doi.org/10.1002/ijc.31937 [Google Scholar] [PubMed] [CrossRef]
Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE (2005). Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. The Journal of Immunology 175: 6489–6497. https://doi.org/10.4049/jimmunol.175.10.6489 [Google Scholar] [PubMed] [CrossRef]
Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA (1997). Phenotypic and functional separation of memory and effector human CD8+T cells. Journal of Experimental Medicine 186: 1407–1418. https://doi.org/10.1084/jem.186.9.1407 [Google Scholar] [PubMed] [CrossRef]
Hanahan D, Weinberg RA (2011). Hallmarks of cancer: The next generation. Cell 144: 646–674. https://doi.org/10.1016/j.cell.2011.02.013 [Google Scholar] [PubMed] [CrossRef]
Heusinkveld M, Goedemans R, Briet RJ, Gelderblom H, Nortier JW, Gorter A, Smit VT, Langeveld AP, Jansen JC, van der Burg SH (2012). Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. International Journal of Cancer 131: E74–E85. https://doi.org/10.1002/ijc.26497 [Google Scholar] [PubMed] [CrossRef]
Hu B, Jadhav RR, Gustafson CE, Le Saux S, Ye Z, Li X, Tian L, Weyand CM, Goronzy JJ (2020). Distinct age-related epigenetic signatures in CD4 and CD8 T cells. Frontiers in Immunology 11: 585168. https://doi.org/10.3389/fimmu.2020.585168 [Google Scholar] [PubMed] [CrossRef]
Hu G, Wang S (2017). Tumor-infiltrating CD45RO+ memory T lymphocytes predict favorable clinical outcome in solid tumors. Scientific Reports 7: 10376. https://doi.org/10.1038/s41598-017-11122-2 [Google Scholar] [PubMed] [CrossRef]
Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM et al. (2019). Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. Cervical Cancer Version 17: 64–84. https://doi.org/10.6004/jnccn.2019.0001 [Google Scholar] [PubMed] [CrossRef]
Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ (1997). Rapid effector function in CD8+ memory T cells. Journal of Experimental Medicine 186: 859–865. https://doi.org/10.1084/jem.186.6.859 [Google Scholar] [PubMed] [CrossRef]
Lambert C, Yanikkaya Demirel G, Keller T, Preijers F, Psarra K, Schiemann M, Özçürümez M, Sack U (2020). Flow cytometric analyses of lymphocyte markers in immune oncology: A comprehensive guidance for validation practice according to laws and standards. Frontiers in Immunology 11: 2169. https://doi.org/10.3389/fimmu.2020.02169 [Google Scholar] [PubMed] [CrossRef]
Liu C, Hu Q, Xu B, Hu X, Su H, Li Q, Zhang X, Yue J, Yu J (2019). Peripheral memory and naïve T cells in non-small cell lung cancer patients with lung metastases undergoing stereotactic body radiotherapy: Predictors of early tumor response. Cancer Cell International 19: 121. https://doi.org/10.1186/s12935-019-0839-5 [Google Scholar] [PubMed] [CrossRef]
Pecorelli S (2009). Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. International Journal of Obstetrics & Gynaecology 105: 103–104. https://doi.org/10.1016/j.ijgo.2009.02.012 [Google Scholar] [PubMed] [CrossRef]
Provinciali M, Moresi R, Donnini A, Lisa RM (2009). Reference values for CD4+ and CD8+ T lymphocytes with naïve or memory phenotype and their association with mortality in the elderly. Gerontologia 55: 314–321. https://doi.org/10.1159/000199451 [Google Scholar] [PubMed] [CrossRef]
Sallusto F, Geginat J, Lanzavecchia A (2004). Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annual Review of Immunology 22: 745–763. https://doi.org/10.1146/annurev.immunol.22.012703.104702 [Google Scholar] [PubMed] [CrossRef]
Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A (1999). Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. https://doi.org/10.1038/44385 [Google Scholar] [PubMed] [CrossRef]
Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M (2006). Accumulation of memory T cells from childhood to old age: Central and effector memory cells in CD4+ versus effector memory and terminally differentiated memory cells in CD8+ compartment. Mechanisms of Ageing and Development 127: 274–281. https://doi.org/10.1016/j.mad.2005.11.001 [Google Scholar] [PubMed] [CrossRef]
Tomiyama H, Matsuda T, Takiguchi M (2002). Differentiation of human CD8+ T cells from a memory to memory/effector phenotype. The Journal of Immunology 168: 5538–5550. https://doi.org/10.4049/jimmunol.168.11.5538 [Google Scholar] [PubMed] [CrossRef]
Ucar D, Márquez EJ, Chung CH, Marches R, Rossi RJ et al. (2017). The chromatin accessibility signature of human immune aging stems from CD8+ T cells. Journal of Experimental Medicine 214: 3123–3144. https://doi.org/10.1084/jem.20170416 [Google Scholar] [PubMed] [CrossRef]
van Braeckel-Budimir N, Varga SM, Badovinac VP, Harty JT (2018). Repeated antigen exposure extends the durability of influenza-specific lung-resident memory CD8+ T cells and heterosubtypic immunity. Cell Reports 24: 3374–3382.e3373. https://doi.org/10.1016/j.celrep.2018.08.073 [Google Scholar] [PubMed] [CrossRef]
Weekes MP, Wills MR, Mynard K, Hicks R, Sissons JG, Carmichael AJ (1999). Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28- CD8+ T-cell population. Immunology 98: 443–449. https://doi.org/10.1046/j.1365-2567.1999.00901.x [Google Scholar] [PubMed] [CrossRef]
Wörmann SM, Diakopoulos KN, Lesina M, Algül H (2014). The immune network in pancreatic cancer development and progression. Oncogene 33: 2956–2967. https://doi.org/10.1038/onc.2013.257 [Google Scholar] [PubMed] [CrossRef]
Yang P, Ma J, Yang X, Li W (2017). Peripheral CD4+ naïve/memory ratio is an independent predictor of survival in non-small cell lung cancer. Oncotarget 8: 83650–83659. https://doi.org/10.18632/oncotarget.19330 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools