 Open Access
Open Access
ARTICLE
Scutellarin alleviates complete freund’s adjuvant-induced rheumatoid arthritis in mice by regulating the Keap1/Nrf2/HO-1 pathway
1 Department of Pharmacy, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, 311201, China
2 Department of Nursing, Community Healthcare Center of Shushan Street, Hangzhou, 311201, China
* Corresponding Author: XIAOYING ZHANG. Email:
(This article belongs to the Special Issue: Natural Products for Chronic Inflammatory Diseases: Pharmacology and Toxicology)
BIOCELL 2023, 47(6), 1307-1316. https://doi.org/10.32604/biocell.2023.028714
Received 04 January 2023; Accepted 06 February 2023; Issue published 19 May 2023
Abstract
Scutellarin (SCU) is a herbal flavonoid glucuronide with multiple pharmacological activities, including anti-oxidant, anti-inflammation, vascular relaxation, anti-platelet, and myocardial protection. However, the effect of SCU on complete Freund’s adjuvant (CFA)-induced rheumatoid arthritis (RA) had not been studied. In this study, we investigated the beneficial effects of SCU in the CFA-induced RA mice model and the anti-arthritic activity was evaluated by paw edema. Enzyme-linked immunosorbent assay (ELISA) was carried out to evaluate the plasma levels of immunoglobulin (Ig)G, IgE, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, receptor activator of nuclear factor-κB ligand (RANKL), and osteoprotegerin (OPG). Histological slides were prepared from the harvested paws of mice to determine the pathological changes in the joints. The proportions of T helper type 1 (Th1) and T helper type 2 (Th2) cells of CD4+ T lymphocyte subsets were analyzed by flow cytometry. The expression of Kelch-like ECH-associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) was analyzed using real-time quantitative PCR (RT-qPCR) and western blotting assays. The present study demonstrated that SCU prevented CFA-induced RA, and inhibited the expression of inflammation factors, IgG, IgE, TNF-α, IL-1β, and IL-6. While SCU also reduced the RANKL level, it increased OPG expression in RA mice. The Th1/Th2 ratio was significantly lower in mice treated with SCU. Additionally, HO-1 expression was reduced while the expression of Keap1 and Nrf2 was elevated following SCU treatment. Results provide preliminary evidence to employ SCU in arthritis treatment which might be related to the regulation of Th1/Th2 balance and the Keap1/Nrf2/HO-1 pathway.Keywords
Rheumatoid arthritis (RA) is a chronic inflammatory disease of joints, which causes joint deformation, cartilage erosion, and functional limitation (Firestein, 2003; Jiang et al., 2021). RA is a globally distributed disease with a prevalence of approximately 0.5%–2% (Wu et al., 2022). As a disabling disease, RA patients require chronic, if not lifetime treatments to alleviate the symptoms. Disease-modifying antirheumatic drugs (DMARDs) are conventional drugs used to treat RA. RA can be treated with a single drug or a combination of more than one drug (Zhao et al., 2022). A lot of studies contribute to exploring the pathogenesis of RA and finding effective drugs to cure RA patients. Although non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to prevent RA progression and maintain joint function, prolonged use of NSAIDs may lead to upper gastrointestinal bleeding, nephrotoxicity, and other adverse effects (Crofford, 2013; Sostres et al., 2010; Wynne and Long, 1996). Thus, more and more researchers have focused on developing novel therapeutic agents. Currently, some biological DMARDs are also being recommended. For example, natural bioactive ingredients are expected to work as reliable complementary approaches for RA treatment due to their good safety profile and medicinal synergy effects. In recent decades, several bioactive molecules have been discovered for RA control (Zhang et al., 2020; Funk et al., 2009).
Scutellarin (SCU) is a flavonoid extracted from Erigeron breviscapus, which has anti-oxidative and anti-inflammatory properties and can protect the cardiovascular system (Wang and Ma, 2018). Previous studies reported that SCU showed a protective role in cartilage destruction. For example, Luo et al. (2020) demonstrated that SCU could prevent osteoarthritis (OA) progression by inhibiting inflammation. In another study, SCU was found to ameliorate OA by inhibiting the Wnt/β-catenin and mitogen-activated protein kinase (MAPK) signaling pathways (Liu et al., 2020). The nuclear factor (NF)-κB and phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathways were also reported to be involved in the protective effects of SCU in OA (Wang et al., 2019). In addition, SCU could inhibit collagen-induced arthritis (CIA) by lowering toll-like receptor 4 (TLR4)/NF-κB mediated inflammation (Zhang et al., 2017). Recent evidence demonstrated that SCU could activate the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) signaling pathway and increase the expression of the antioxidant protein heme oxygenase-1 (HO-1) (Liu et al., 2019). In hepatocytes, SCU could inhibit kelch ECK associating protein 1 (Keap1), an endogenous inhibitor of Nrf2 (Mukerjee et al., 2022), and upregulate the expression of Nrf2 and HO-1 (Wu and Jia, 2019). However, to our knowledge, few studies have reported the relationship between SCU and CFA-induced RA.
In the present study, we examined the potential effects of SCU on CFA-induced RA by evaluating inflammation and osteoclast differentiation. Moreover, the underlying mechanism of SCU against CFA-induced RA involving the Keap1/Nrf2/HO-1 pathway was also probed. This can provide a direction for the action mechanism and drug research of the anti-RA effect of SCU. Therefore, this study may provide a new treatment strategy for RA and a reference for clinical application and development of research on SCU.
Rheumatoid arthritis model construction and treatment
The experimental protocol and animal ethics procedures were approved by the Institutional Animal Care and Use Committee, Ningbo No.6 Hospital. The animal use license number is SYXK (Jiangsu) 2018-0027. A total of 24 male C57BL/6 mice weighing 25–30 g were purchased from the Academy of Zhejiang Province Medical Sciences and kept on a 12 h light/dark cycle at a constant temperature (25°C ± 2°C) and humidity (60%–70%). Mice were provided a standard diet and water ad libitum. Mice were arbitrarily divided into four groups (n = 6 each). Group I mice served as the control. The model was constructed as previously described (Wilhelm et al., 2021). The mice were injected with 0.1 mL of complete Freund’s adjuvant (CFA) by the intraplantar route in the right hind paws to induce RA. After seven days of injection, mice in group II (Model) were administered with 0.9% sterile saline by gavage for 21 days. Further, mice in group III (CFA + SCU) were treated with 20 mg/kg SCU and group IV (CFA + leflunomide) were treated with 4 mg/kg leflunomide (positive control). At the end of the experimental period (28 days), all mice were anesthetized and sacrificed by chloroform anesthesia. The serum, spleen, and synovium samples were obtained for further biochemical and histopathological experiments.
Bodyweight and footpad volume measurements
The body weight of mice was measured with a precision balance every 3 days and the alterations were calculated by comparing them to the initial body weight. The hind footpad volumes were measured using the footpad volume measuring instrument (Muromachi Kikai, Japan) every 3 days. The swelling rate was calculated by the following formula: swelling rate (%) = (footpad volume after treatment – footpad volume before treatment)/footpad volume before treatment × 100%.
After the mice were euthanized, the spleens were expunged immediately and processed in phosphate buffer saline (PBS). The spleens were weighed and the indexes were calculated using the expression: organ weight/body weight × 10. After weighing, the spleens were digested into single cells for subsequent experiments.
Inflammatory marker assessment
The collected blood samples were centrifuged at 6,000 rpm for 15 min to obtain sera. The levels of immunoglobulin (Ig) IgG (mlbio, ml037601-J), IgE (mlbio, ml037602), tumor necrosis factor (TNF)-α (mlbio, ml002095), interleukin (IL)-1β (mlbio, ml063132), IL-6 (mlbio, ml063159), receptor activator of NF-κB ligand (RANKL; mlbio, ml037743), and osteoprotegerin (OPG; Abcam, ab203365) were measured with corresponding enzyme-linked immune sorbent assay (ELISA) kits according to the instructions. The synovium tissues were homogenized for measuring the levels of RANKL and OPG.
The joints of the hind paw tissues were processed with 4% paraformaldehyde for 24 h and decalcified with 10% formic acid solution. After being embedded in paraffin and sliced into the 5 μm thick sections, the samples were stained with hematoxylin for 5 min and eosin for 2 min. After being dehydrated with gradient alcohol, the samples were permeated and sealed with xylene and neutral gum, respectively. The whole field views of hematoxylin and eosin (H&E)-stained slices were captured using a light microscope (Olympus, Japan).
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was used to study apoptosis in synovium tissue. After routine dewaxing and rehydration, the sections were treated with proteinase K for 15 min at 37°C and then incubated with a TUNEL mixture (Beyotime, C1086) for 60 min in the dark according to the manufacturer’s protocol. 4′,6-diamidino-2-phenylindole (DAPI) staining (Beyotime, 1 µg/mL) was applied for nuclear staining. The TUNEL-positive cells were measured under the microscope (Nikon C1 System, Nikon Corporation).
The percentages of IFN-γ+ T cells and IL-4+ T cells and the Th1/Th2 ratio were determined by flow cytometry with a FACS Canto II (BD Biosciences). The following antibodies were used for flow cytometry: Fluorescein isothiocyanate (FITC)-anti-CD4, PerCP-Cy5.5-IFNγ, and IL-4-APC. Isolated spleen cells were treated with ionomycin (750 ng/ml, Sigma), phorbol 12-myristate 13-acetate (200 ng/ml, Sigma), and BD GolgiPlug (BD Biosciences) for 5 h in a cell incubator (37°C, 5% CO2). Then, the cells were incubated with the aforementioned antibodies in the dark for 30 min. The data were analyzed by the Flowjo software (Tree Star, Korea).
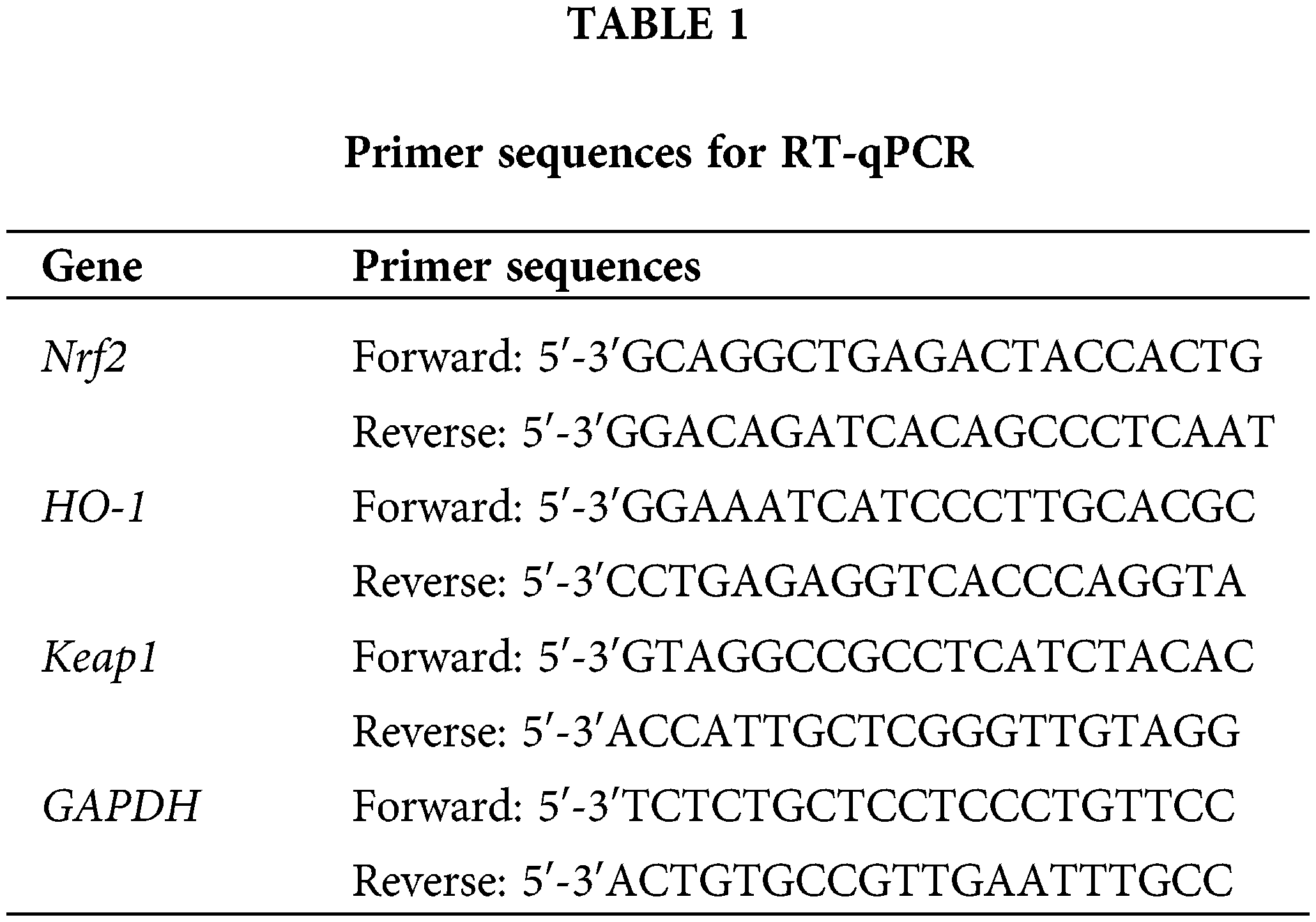
Reverse transcription-quantitative polymerase chain reaction
Real-Time Quantitative Reverse Transcription PCR (RT-qPCR) analysis was performed as previously reported (Xie et al., 2020). Total RNA was extracted from mice synovial tissue using Trizol reagent (Invitrogen, CA, USA). The RNA was reverse transcribed into cDNA using FastKing First-strand cDNA Synthesis Mix (Tiangen, Beijing, China). RT-qPCR was performed using SYBR Green PCR Master Mix (Lifeint, Xiamen, China) on the MX3000P Real-Time system (Stratagene, Carlsbad, CA, USA). The composition of the PCR reaction mixture is presented in Table 1. The qPCR reaction conditions applied were: 95°C for 3 min, followed by 40 cycles of 95°C for 12 s and 62°C for 40 s. The primer sequences for RT-qPCR are shown in Table 1. GAPDH was used as an internal reference gene, and relative mRNA expression was calculated by the 2−∆∆Ct method.
Western blot analysis was carried out according to a previously published method (Shen et al., 2021). Total protein was extracted from tissues using radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China). Protein concentration was detected using a BCA protein assay kit (Beyotime). Then, 25 µg of protein was separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE: 10% separating gel and 5% concentrating gel). The separated protein was transferred to a polyvinylidene difluoride (PVDF) membrane and blocked in 5% skim milk for 1 h at room temperature. The PVDF membrane was incubated with the corresponding primary antibodies overnight at 4°C. The primary antibodies used in this study are listed below: Nrf2 (1:1,000; cat. no. ab137550; Abcam), HO-1 (1:1,000; cat. no. ab52947; Abcam), and Keap1 (1:2,000; cat. no. ab227828; Abcam). Following that, the PVDF membrane was incubated with goat anti-rabbit IgG H&L secondary antibodies (1:10,000; cat. no. ab6721; Abcam) for 60 min at room temperature. Protein bands were visualized using ECL (Thermo, MA, USA) and subsequently analyzed with ImageJ software v 1.8.0 (National Institutes of Health, MD, USA).
All of the statistical examinations were analyzed using GraphPad Prism 8.0 software (GraphPad Software, CA, USA). Data were illustrated as mean ± standard deviation (SD). A one-way ANOVA was used to statistically compare differences among more than two groups, followed by Tukey’s test. Data were regarded as statistically significant differences if p < 0.05.
SCU improved clinical responses of RA mice
To explore the effect of SCU on regulating the occurrence and development of RA in vivo, the RA mouse model was successfully constructed by CFA induction and administrated with SCU or leflunomide. Firstly, we measured the volume of the paw and the body weight of the mice and evaluated the arthritis index according to the degree of joint redness and swelling. As shown in Figs. 1A–1C, the body weights of the RA model mice were significantly lower than that of control mice (p < 0.001), and the paw volume and arthritis index were significantly increased (p < 0.001). Leflunomide, a Food and Drug Administration (FDA)-approved medication indicated for the treatment of RA, was employed as a positive control. Compared with the model mice, RA mice treated with SCU or leflunomide showed higher body weight, and significantly decreased paw swelling volume and arthritis index (p < 0.05; Figs. 1A–1C).
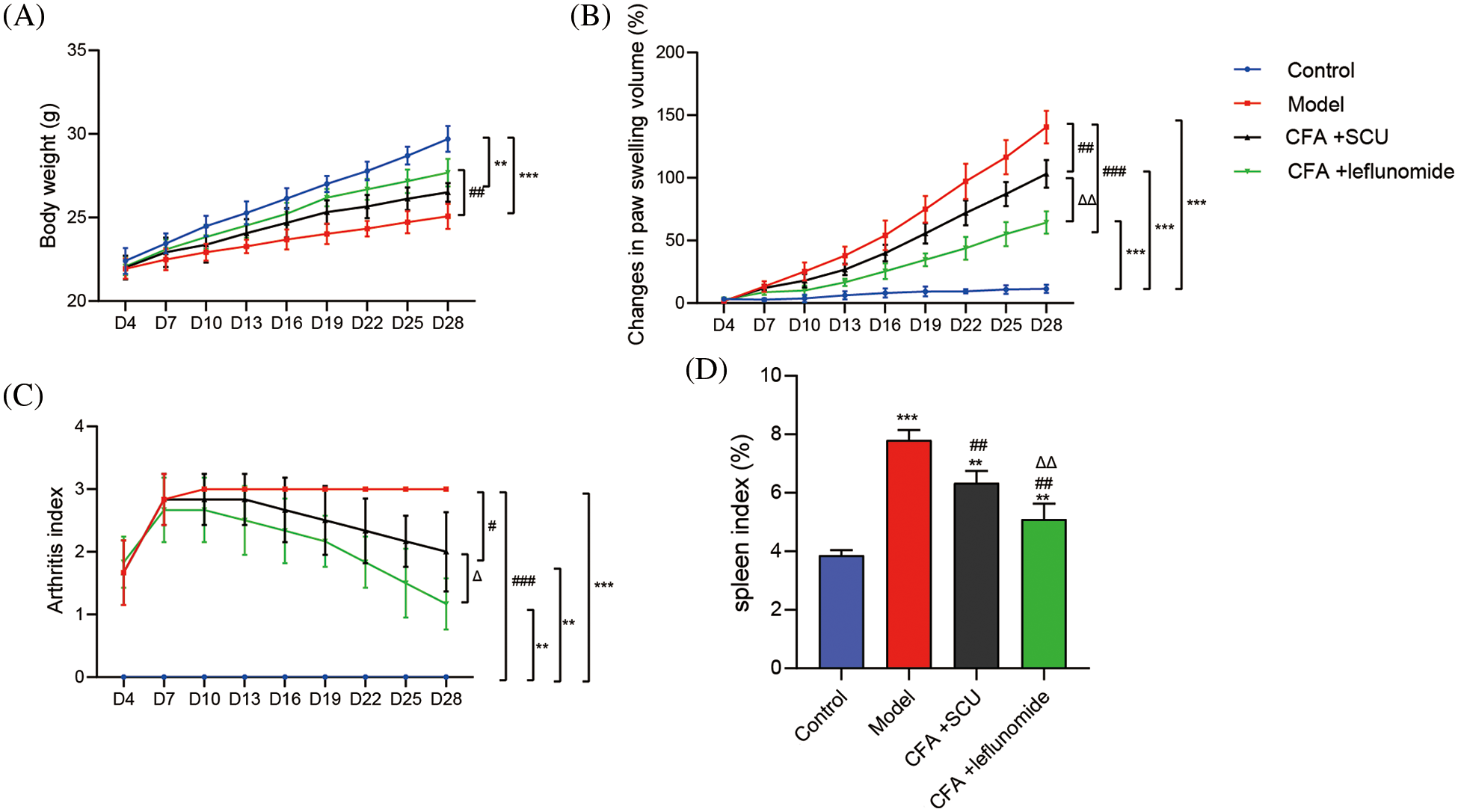
Figure 1: Scutellarin (SCU) improved clinical responses in rheumatoid arthritis (RA) mice. (A) Effects of SCU on the body weight. (B) Effects of SCU on the paw swelling volume. (C) Effects of SCU on the arthritis index. (D) Effects of SCU on the spleen index. The average content in each group was expressed as the mean ± square deviation (SD) (n = 6). **p < 0.01 and ***p < 0.001 vs. the control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. the model group; ∆p < 0.05 and ∆∆p < 0.01 vs. the complete Freund’s adjuvant (CFA) + SCU group.
The spleen is an important immune organ, which can systematically reflect the immune functional status. As shown in Fig. 1D, a significantly higher spleen index was found in the RA model mice compared to the control mice (p < 0.001). The treatment of SCU or leflunomide significantly reduced the spleen index of RA mice, indicating SCU could exert a certain degree of immunosuppression (p < 0.01; Fig. 1D).
SCU relieved the histological damage and chondrocyte apoptosis in RA mice
The morphology of joint tissues harvested from mice was detected by HE staining. In the control group, the articular surface of the mice was smooth and intact. Obvious histopathological changes were found in the RA model group. Rough and degenerated cartilage surfaces and abnormal hyperplastic synovium were observed accompanied by abundant inflammatory cell infiltration in the joint cavity in RA mice. Treatment with SCU or leflunomide reduced the degree of cartilage destruction, inflammatory cell infiltration, and synovial epithelium hyperplasia of RA mice (Fig. 2A). In addition, chondrocyte apoptosis was evaluated by TUNEL staining after the mice were sacrificed. As shown in Fig. 2B, only a few apoptotic cells were observed in the joint tissue of control mice while the RA model mice showed a substantial number of apoptotic cells. Upon treatment using SCU or leflunomide, chondrocyte apoptosis was significantly suppressed in the RA mice.
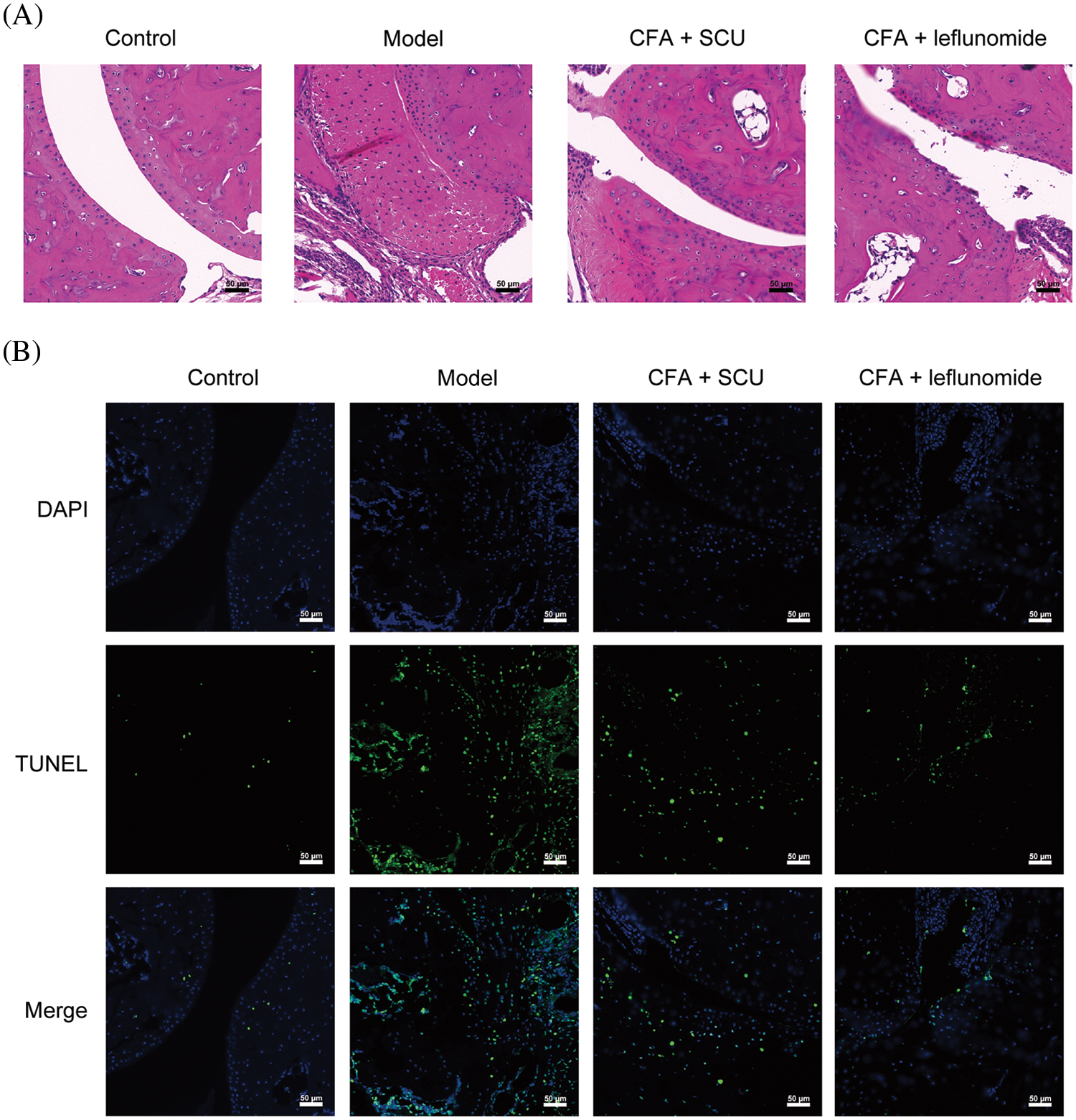
Figure 2: Scutellarin (SCU) relieved the histological damage and chondrocytes apoptosis in rheumatoid arthritis (RA) mice. (A) Effects of SCU on histological damage. (B) Chondrocyte apoptosis in RA mice was detected by the TUNEL assay (scale bar = 50 μm); CFA: complete Freund’s adjuvant.
SCU inhibited serum immunoglobulins and cytokines in RA mice
Then the serum inflammatory factors of RA mice were detected by ELISA as discussed. As shown in Figs. 3A–3E, the concentrations of immunoglobulins (IgG and IgE) and inflammatory cytokines (TNF-α, IL-1β, and IL-6) in the serum samples of RA mice were significantly higher than that of control mice (p < 0.001). However, these immunoglobulins and inflammatory cytokines levels in the serum samples of RA mice remarkably dropped after SCU or leflunomide treatment (p < 0.01; Figs. 3A–3E). It was noticed that SCU showed a similar inhibitory effect on immunoglobulin levels like with leflunomide. Results revealed that SCU could inhibit the inflammatory responses in RA mice.
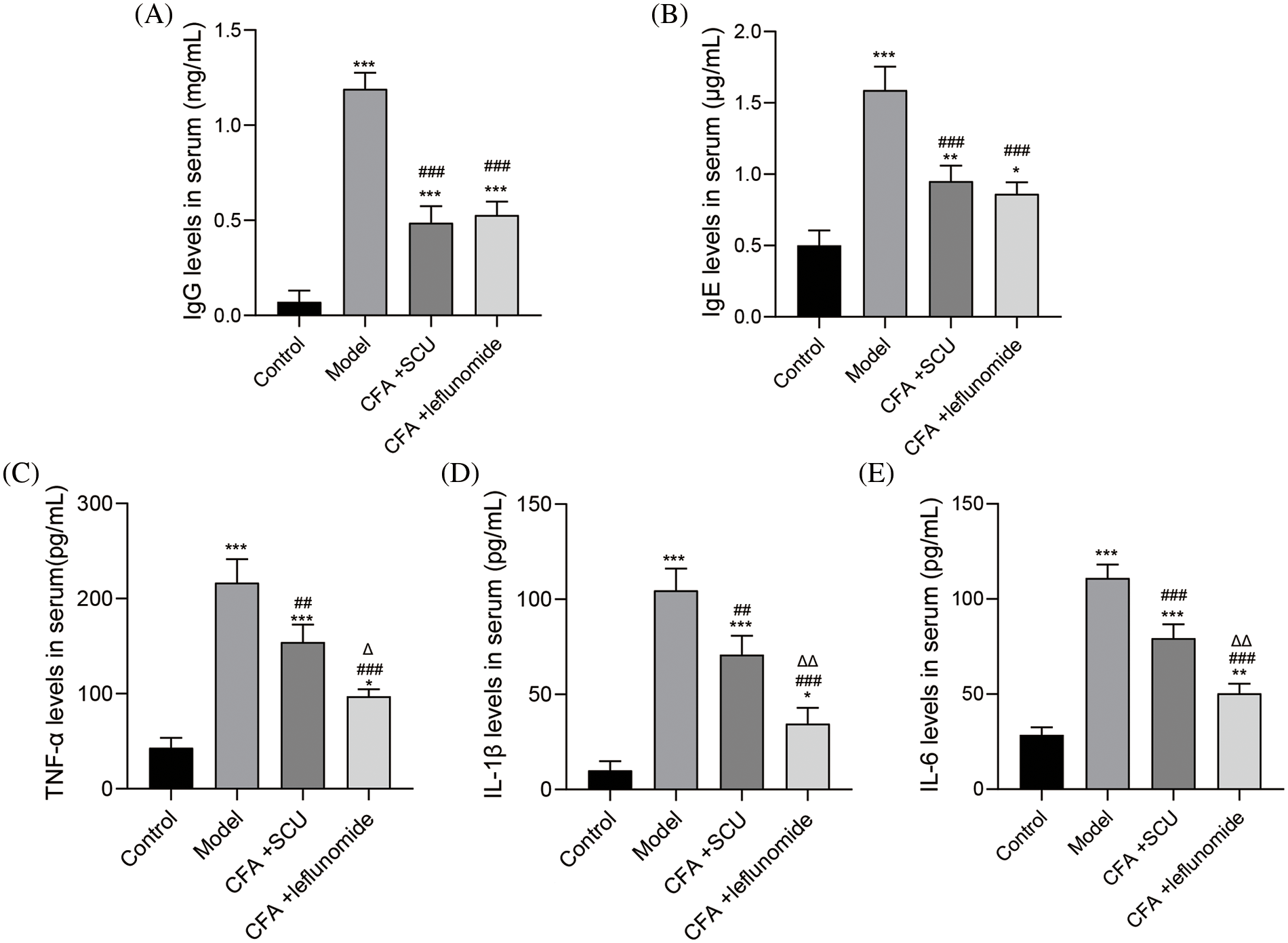
Figure 3: Scutellarin (SCU) inhibited serum immunoglobulins and cytokines in rheumatoid arthritis (RA) mice. (A–E) Immunoglobulin (Ig)G, IgE, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 levels in each group. The average level in each group was expressed as the mean ± SD (n = 6). *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group; ##p < 0.01 and ###p < 0.001 vs. the model group; ∆p < 0.05 and ∆∆p < 0.01 vs. the complete Freund’s adjuvant (CFA) + SCU group.
SCU improved the balance of Th1 and Th2 cells in spleens of RA mice
Th1 and Th2 cells play important roles in the pathogenesis of RA (Piazza et al., 2022). We hence analyzed the Th1 and Th2 cell profiles in mouse spleens by flow cytometry. Results showed that the percentages of IFN-γ+ T cells and IL-4+ T cells were higher in the RA model group than those in the control group. This was also observed for the Th1/Th2 ratio (p < 0.001; Figs. 4A–4D). Compared with the RA model mice, the frequency of IFN-γ+ T cells and IL-4+ T cells were significantly decreased in RA mice treated with SCU or leflunomide (p < 0.01) with SCU showing better efficiency in inhibiting IFN-γ+ T cells (p < 0.01). In addition, the Th1/Th2 ratio in the CFA + leflunomide group was significantly higher than the CFA + SCU group (p < 0.01), which was similar to the RA model group. However, SCU treatment significantly reduced the Th1/Th2 ratio (Fig. 4D).
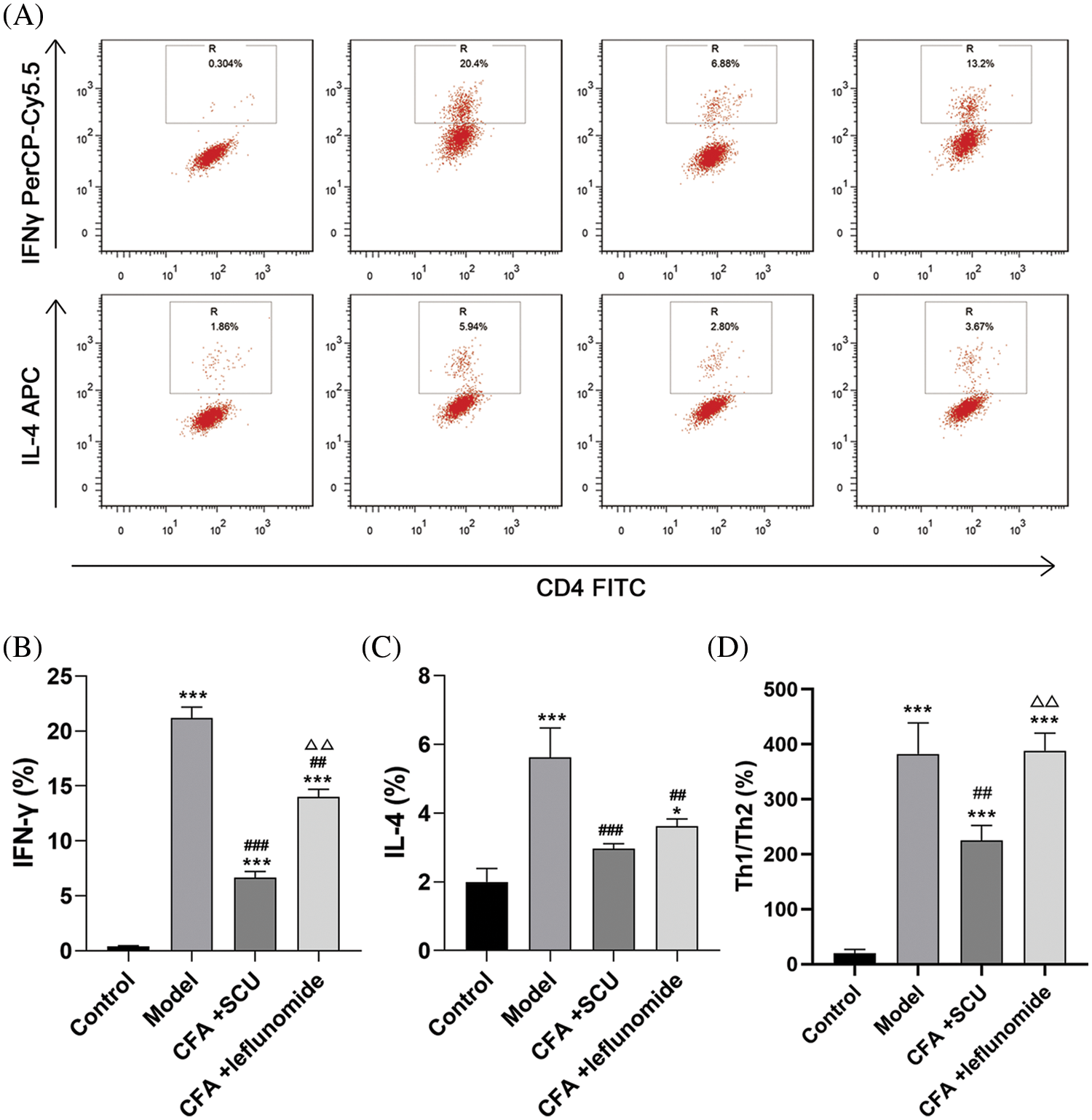
Figure 4: The effects of scutellarin (SCU) on the percentage of T helper type 1 (Th1) and T helper type 2 (Th2) cells in mouse spleens. (A) CD4 antibody was used to isolate T-helper (Th) cells. (B–D) Expression of interferon-gamma (IFN-γ) and interleukin-4 (IL-4) and the Th1/Th2 ratio were analyzed. The average content in each group was expressed as the mean ± SD (n = 6). *p < 0.05 and ***p < 0.001 vs. the control group; ##p < 0.01 and ###p < 0.001 vs. the model group; ∆∆p < 0.01 vs. the complete Freund’s adjuvant (CFA) + SCU group.
SCU inhibited RANKL and OPG levels in RA mice
The vital involvement of cytokines RANKL and OPG in osteoclast differentiation and activation is known (Lacey et al., 1998). To further investigate the effect of SCU on RA, the serum and tissue levels of RANKL and OPG were examined by ELISA. As shown in Figs. 5A–5D, the serum and tissues of RA mice documented increased RANKL and decreased OPG levels (p < 0.001). SCU or leflunomide treatment significantly downregulated RANKL levels in serum and tissues of RA mice while it significantly up-regulated OPG (p < 0.01). Further, there was no statistically significant difference in the effects of SCU and leflunomide on levels of RANKL and OPG.
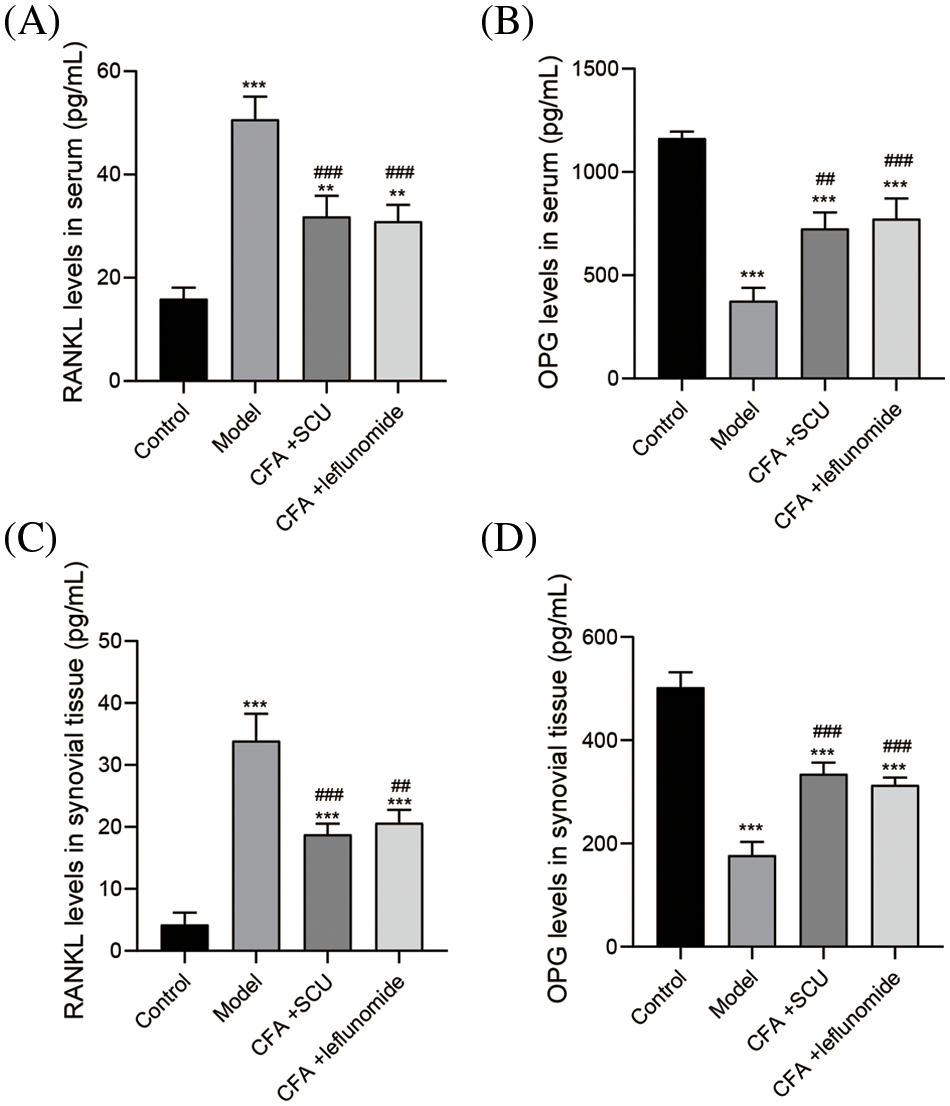
Figure 5: Scutellarin (SCU) inhibited receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) levels in RA mice. (A and B) Effects of SCU on the serum levels of RANKL and OPG. (C and D) Effects of SCU on the tissue levels of RANKL and OPG. The average content in each group was expressed as the mean ± SD (n = 6). **p < 0.01 and ***p < 0.001 vs. the control group; ##p < 0.01 and ###p < 0.001 vs. the model group.
SCU induced the Keap1/Nrf2/HO-1 signaling pathway in RA mice
Maintaining high levels of HO-1 is a promising strategy to protect against inflammation and arthritis (Poulet and Beier, 2016). Hence, we explored whether SCU regulates the Keap1/Nrf2/HO-1 signaling pathways to exert an anti-oxidation function in RA mice. As shown in Figs. 6A and 6B, significantly upregulated Keap1 level and downregulated Nrf2 and HO-1 levels were observed in the model group (p < 0.001). Further, SCU or leflunomide treatment significantly inhibited the mRNA and protein expression of Keap1 in synovium tissues of RA mice (p < 0.01). Compared with the leflunomide treatment, SCU exhibited more significant inhibition on the expression of Keap1 (p < 0.05). As Keap1 is an inhibitor of Nrf2, our observations showed that SCU or leflunomide treatment induced the expression of Nrf2 and HO-1 compared to the RA model group (p < 0.05; Figs. 6A and 6B). Further, SCU exerted a better efficiency than leflunomide in increasing the HO-1 expression (p < 0.05).
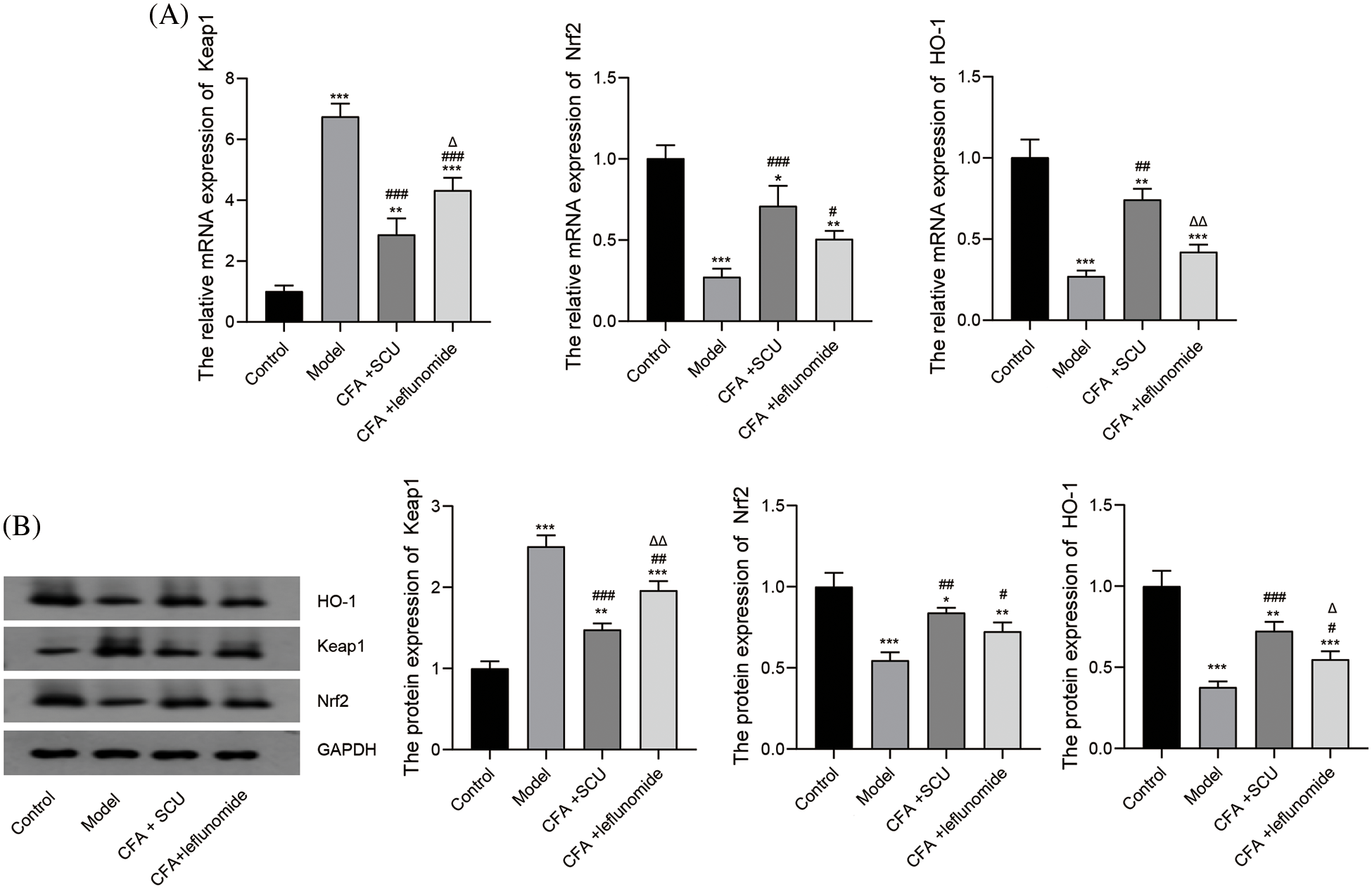
Figure 6: Analyzing the mechanism of the anti-arthritis effects of scutellarin (SCU). (A) Relative mRNA expressions of Kelch-like ECH-associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) were detected by real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR). (B) Protein expression levels of Keap1, Nrf2, and HO-1 were detected by western blotting. The average content in each group was expressed as the mean ± SD (n = 6). *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. the model group; ∆p < 0.05 and ∆∆p < 0.01 vs. the complete Freund’s adjuvant (CFA) + SCU group.
SCU is a major effective constituent of the Chinese medicine Erigeron breviscapus and has been verified to inhibit collagen-induced arthritis (Zhang et al., 2017). In the present study, we evaluated the therapeutic potential of SCU in a CFA-induced RA mouse model and also explored the underlying mechanism of SCU on RA. We found that SCU could alleviate the arthritis score and inflammatory response in CFA-induced RA mice. Furthermore, this protective effect was related to the regulation of the Th1/Th2 ratio and the Keap1/Nrf2/HO-1 pathway.
CFA is used in most procedures for the experimental induction of RA as it induces changes similar to RA in humans (Youssef et al., 2022). The induction of arthritis causes some pathological alterations, such as inflammatory synovitis with enhanced infiltration of inflammatory cells, followed by cartilage degradation and joint deformation (Robinson et al., 2016). In our study, there were obvious histopathological changes in the RA model mice inclusive of the degenerated cartilage surface and abnormal hyperplastic synovium, accompanied by abundant inflammatory cell infiltration in the joint cavity. Further, increased apoptosis of chondrocytes and destroyed dynamic balance between cell proliferation and death rates were reported in RA patients (Faramarzi et al., 2022). The chondrocytes are decreased substantially, accompanied by gradual degradation of extracellular matrix (ECM), eventually leading to degradation of articular cartilage. In the present study, we found abundant apoptotic chondrocytes in the synovium of RA mice. These results indicate the successful construction of the RA mouse model. Several studies have reported the protective effect of SCU on arthritis (Yang et al., 2022; Zhang et al., 2017). Our results revealed that SCU treatment alleviated inflammatory cell infiltration and synovium hyperplasia and reduced chondrocyte apoptosis in RA mice, which indicates that SCU has a therapeutic effect on RA.
Inflammatory responses play a major role in the physiopathology of RA, and high levels of immunoglobulins (IgA, IgE, IgD, and IgG) and pro-inflammatory cytokines (such as TNF-α, IL-1β, IL-17, IL-22, and IL-6) are observed in the synovial fluid and the serum (Achudhan et al., 2021; Shu et al., 2022; Gioud-Paquet et al., 1987). This increase in immunoglobulins and cytokines is partly correlated with the severity of cartilage destruction. CFA treatment can also increase pro-inflammatory cytokine levels in the serum and synovial fluid. Zhang et al. (2017) demonstrated that SCU could inhibit inflammatory cytokines levels in collagen-induced arthritis mice. Similarly, our findings also revealed that SCU treatment significantly reduced CFA-induced IgG, IgE, TNF-α, IL-1β, and IL-6 levels in RA mice, indicating that administration of SCU could inhibit the inflammatory response in RA mice.
Studies have shown that Th1 cells and their secreted cytokines are involved in joint inflammation, whereas Th2 cells and their secreted cytokine IL-4 help alleviate inflammatory responses (Cutolo et al., 2022; Xie et al., 2022). The Th1/Th2 cell ratio is implicated to be important in the pathogenesis of RA (Bao et al., 2022). Further, an imbalance of Th1 and Th2 cells is commonly observed in RA patients (Li et al., 2021). IFN-γ is an important pro-inflammatory cytokine secreted by Th1 cells in RA, which promotes the inflammatory response in RA by activating macrophages. Hence, for evaluating the anti-inflammatory effect of SCU, we analyzed percentages of Th1 and Th2 cells and the Th1/Th2 ratio in the mice spleens by flow cytometry. The treatment with SCU significantly decreased the percentage of IFN-γ+ Th1 cells and the Th1/Th2 ratio. These results suggest that SCU regulated the balance of Th1/Th2 cells in RA mice. The above data reveals that SCU ameliorates RA by inhibiting the inflammatory response in the mice models.
A precise balance between bone formation and resorption is required for joint functioning. The protein RANKL can induce the differentiation of progenitor cells into mature osteoclasts while OPG can inhibit RANKL function conversely (Tan et al., 2017; Wang et al., 2017). Our data showed that the administration of SCU increased RANKL expression and decreased OPG expression, which revealed the regulating effect of SCU on bone metabolism.
HO-1 is a member of the heat shock protein family and is an important anti-inflammatory, antioxidative, and cytoprotective enzyme regulated by the activation of Nrf2 (Zeng et al., 2022). A previous study demonstrated that the HO-1/Nrf-2 signaling pathway was involved in the inflammatory response of RA (Zeng et al., 2022). An important finding of the present study relates to the SCU-mediated upregulation of HO-1 expression that was associated with a reduced in vivo inflammatory response. The results were consistent with a previous report that HO-1 is upregulated by the transcriptional factor Nrf2 (Na and Surh, 2014). Puppala et al. (2022) revealed that perillyl alcohol alleviated RA by regulating the Keap1/Nrf2 signaling pathway. Our data showed that SCU significantly promoted the degradation of Keap1, an endogenous inhibitor of Nrf2. Considering that HO-1 is a regulator of the inflammatory response, it is conceivable that the upregulation of HO-1 by SCU was involved in regulating the inflammatory response in RA through the activation of the Keap1/Nrf2/HO-1 pathway.
Our results indicated the therapeutic effects of SCU on RA evidenced by the improvement of clinical responses and histological damage and reduced chondrocyte apoptosis in RA model mice treated with SCU. SCU also ameliorated the inflammatory response and decreased the Th1/Th2 ratio in RA mice. Additionally, this study demonstrated that the mechanism of SCU inhibiting the CFA-induced RA may involve the regulation of the Keap1/Nrf2/HO-1 pathway. Nevertheless, more experiments are needed to validate the relationship between SCU and the Keap1/Nrf2/HO-1 pathway in RA. In addition, the regulating effects of SCU on the balance between osteoblast and osteoclast need further investigation. Our results revealed that SCU treatment may help in the clinical treatment of RA, while more clinical studies need to be conducted.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper as follows: Study conception and design: JL, QW, and XZ; data collection: JL and QW; analysis and interpretation of results: JL, QW, and XZ; draft manuscript preparation: JL. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval: All animal protocols were approved by the Animal Experimental Ethics Committee of The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University. The animal use license number is SYXK (Jiangsu) 2018-0027.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Achudhan D, Liu S, Lin Y, Huang C, Tsai C, Ko C, Chiang I, Kuo Y, Tang C (2021). Antcin K inhibits TNF-α, IL-1β and IL-8 expression in synovial fibroblasts and ameliorates cartilage degradation: Implications for the treatment of rheumatoid arthritis. Frontiers in Immunology 12: 790925. https://doi.org/10.3389/fimmu.2021.790925 [Google Scholar] [PubMed] [CrossRef]
Bao Y, Peng J, Yang K, Wang C, Guo Y, Guo Z, Du S (2022). Therapeutic effects of Chinese medicine Di-Long (Pheretima vulgaris) on rheumatoid arthritis through inhibiting NF-κB activation and regulating Th1/Th2 balance. Biomedicine & Pharmacotherapy 147: 112643. https://doi.org/10.1016/j.biopha.2022.112643 [Google Scholar] [PubMed] [CrossRef]
Crofford LJ (2013). Use of NSAIDs in treating patients with arthritis. Arthritis Research & Therapy 15: 1–10. https://doi.org/10.1186/ar4174 [Google Scholar] [PubMed] [CrossRef]
Cutolo M, Campitiello R, Gotelli E, Soldano S (2022). The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Frontiers in Immunology 13: 867260. https://doi.org/10.3389/fimmu.2022.867260 [Google Scholar] [PubMed] [CrossRef]
Faramarzi F, Zafari P, Alimohammadi M, Golpour M, Ghaffari S, Rafiei A (2022). Inhibitory effects of cold atmospheric plasma on inflammation and tumor-like feature of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Inflammation 45: 2433–2448. https://doi.org/10.1007/s10753-022-01703-3 [Google Scholar] [PubMed] [CrossRef]
Firestein GS (2003). Evolving concepts of rheumatoid arthritis. Nature 423: 356–361. https://doi.org/10.1038/nature01661 [Google Scholar] [PubMed] [CrossRef]
Funk JL, Frye JB, Oyarzo JN, Timmermann BN (2009). Comparative effects of two gingerol-containing Zingiber officinale extracts on experimental rheumatoid arthritis. Journal of Natural Products 72: 403–407. https://doi.org/10.1021/np8006183 [Google Scholar] [PubMed] [CrossRef]
Gioud-Paquet M, Auvinet M, Raffin T, Girard P, Bouvier M, Lejeune E, Monier JC (1987). IgM rheumatoid factor (RFIgA RF, IgE RF, and IgG RF detected by ELISA in rheumatoid arthritis. Annals of the Rheumatic Diseases 46: 65–71. https://doi.org/10.1136/ard.46.1.65 [Google Scholar] [PubMed] [CrossRef]
Jiang Q, Wang X, Huang E, Wang Q, Wen C, Yang G, Lu L, Cui D (2021). Inflammasome and its therapeutic targeting in rheumatoid arthritis. Frontiers in Immunology 12: 816839. https://doi.org/10.3389/fimmu.2021.816839 [Google Scholar] [PubMed] [CrossRef]
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR et al. (1998). Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176. https://doi.org/10.1016/S0092-8674(00)81569-X [Google Scholar] [PubMed] [CrossRef]
Li Y, Jie Y, Wang X, Lu J (2021). Serum IL-35 is decreased in overweight patients with rheumatoid arthritis: Its correlation with Th1/Th2/Th17-related cytokines. BMC Immunology 22: 42. https://doi.org/10.1186/s12865-021-00431-x [Google Scholar] [PubMed] [CrossRef]
Liu F, Li L, Lu W, Ding Z, Huang W et al. (2020). Scutellarin ameliorates cartilage degeneration in osteoarthritis by inhibiting the Wnt/β-catenin and MAPK signaling pathways. International Immunopharmacology 78: 105954. https://doi.org/10.1016/j.intimp.2019.105954 [Google Scholar] [PubMed] [CrossRef]
Liu Y, Wang J, Zhang X, Wang L, Hao T, Cheng Y, Wang D (2019). Scutellarin exerts hypoglycemic and renal protective effects in db/db mice via the Nrf2/HO-1 signaling pathway. Oxidative Medicine and Cellular Longevity 2019: 1354345. https://doi.org/10.1155/2019/1354345 [Google Scholar] [PubMed] [CrossRef]
Luo Z, Hu Z, Bian Y, Su W, Li X, Li S, Wu J, Shi L, et al.(2020). Scutellarin attenuates the IL-1β-induced inflammation in mouse chondrocytes and prevents osteoarthritic progression. Frontiers in Pharmacology 11: 107. [Google Scholar] [PubMed]
Mukerjee N, Al-Khafaji K, Maitra S, Suhail Wadi J, Sachdeva P et al. (2022). Recognizing novel drugs against Keap1 in Alzheimer’s disease using machine learning grounded computational studies. Frontiers in Molecular Neuroscience 15: 1036552. https://doi.org/10.3389/fnmol.2022.1036552 [Google Scholar] [PubMed] [CrossRef]
Na HK, Surh YJ (2014). Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radical Biology and Medicine 67: 353–365. https://doi.org/10.1016/j.freeradbiomed.2013.10.819 [Google Scholar] [PubMed] [CrossRef]
Piazza S, Fumagalli M, Martinelli G, Pozzoli C, Maranta N, Angarano M, Sangiovanni E, Dell’Agli MJM (2022). Hydrolyzable tannins in the management of Th1, Th2 and Th17 inflammatory-related diseases. Molecules 27: 7593. https://doi.org/10.3390/molecules27217593 [Google Scholar] [PubMed] [CrossRef]
Poulet B, Beier F (2016). Targeting oxidative stress to reduce osteoarthritis. Arthritis Research & Therapy 18: 32. https://doi.org/10.1186/s13075-015-0908-7 [Google Scholar] [PubMed] [CrossRef]
Puppala E, Jain S, Saha P, Rachamalla M, Np S et al. (2022). Perillyl alcohol attenuates rheumatoid arthritis via regulating TLR4/NF-κB and Keap1/Nrf2 signaling pathways: A comprehensive study onin-vitro and in-vivo experimental models. Phytomedicine 97: 153926. https://doi.org/10.1016/j.phymed.2022.153926 [Google Scholar] [PubMed] [CrossRef]
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J (2016). Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nature Reviews Rheumatology 12: 580–592. https://doi.org/10.1038/nrrheum.2016.136 [Google Scholar] [PubMed] [CrossRef]
Shen P, Lin W, Ba X, Huang Y, Chen Z, Han L, Qin K, Huang Y, Tu S (2021). Quercetin-mediated SIRT1 activation attenuates collagen-induced mice arthritis. Journal of Ethnopharmacology 279: 114213. https://doi.org/10.1016/j.jep.2021.114213 [Google Scholar] [PubMed] [CrossRef]
Shu C, Chen J, Lv M, Xi Y, Zheng J, Xu X (2022). Plumbagin relieves rheumatoid arthritis through nuclear factor kappa-B (NF-κB) pathway. Bioengineered 13: 13632–13642. https://doi.org/10.1080/21655979.2022.2081756 [Google Scholar] [PubMed] [CrossRef]
Sostres C, Gargallo CJ, Arroyo MT, Lanas A (2010). Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Practice & Research Clinical Gastroenterology 24: 121–132. https://doi.org/10.1016/j.bpg.2009.11.005 [Google Scholar] [PubMed] [CrossRef]
Tan EM, Li L, Indran IR, Chew N, Yong EL (2017). TRAF6 mediates suppression of osteoclastogenesis and prevention of ovariectomy-induced bone loss by a novel prenylflavonoid. Journal of Bone and Mineral Research 32: 846–860. https://doi.org/10.1002/jbmr.3031 [Google Scholar] [PubMed] [CrossRef]
Wang W, Li J, Li F, Peng J, Xu M et al. (2019). Scutellarin suppresses cartilage destruction in osteoarthritis mouse model by inhibiting the NF-κB and PI3K/AKT signaling pathways. International Immunopharmacology 77: 105928. https://doi.org/10.1016/j.intimp.2019.105928 [Google Scholar] [PubMed] [CrossRef]
Wang X, Liu S, Cao L, Zhang T, Yue D et al. (2017). miR-29a-3p suppresses cell proliferation and migration by downregulating IGF1R in hepatocellular carcinoma. Oncotarget 8: 86592–86603. https://doi.org/10.18632/oncotarget.21246 [Google Scholar] [PubMed] [CrossRef]
Wang L, Ma Q (2018). Clinical benefits and pharmacology of scutellarin: A comprehensive review. Pharmacology & Therapeutics 190: 105–127. https://doi.org/10.1016/j.pharmthera.2018.05.006 [Google Scholar] [PubMed] [CrossRef]
Wilhelm E, Soares P, Reis A, Motta K, Lemos B et al. (2021). Se-[(2,2-Dimethyl-1,3-dioxolan-4-yl)methyl] 4chlorobenzoselenolate attenuates inflammatory response, nociception, and affective disorders related to rheumatoid arthritis in mice. ACS Chemical Neuroscience 12: 3760–3771. https://doi.org/10.1021/acschemneuro.1c00512 [Google Scholar] [PubMed] [CrossRef]
Wu H, Jia L (2019). Scutellarin attenuates hypoxia/reoxygenation injury in hepatocytes by inhibiting apoptosis and oxidative stress through regulating Keap1/Nrf2/ARE signaling. Bioscience Reports 39: BSR20192501. https://doi.org/10.1042/BSR20192501 [Google Scholar] [PubMed] [CrossRef]
Wu D, Luo Y, Li T, Zhao X, Lv T et al. (2022). Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Frontiers in Immunology 13: 1051082. https://doi.org/10.3389/fimmu.2022.1051082 [Google Scholar] [PubMed] [CrossRef]
Wynne H, Long A (1996). Patient awareness of the adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs). British Journal of Clinical Pharmacology 42: 253–256. https://doi.org/10.1046/j.1365-2125.1996.41420.x [Google Scholar] [PubMed] [CrossRef]
Xie C, Jiang J, Liu J, Yuan G, Zhao Z (2020). Ginkgolide B attenuates collagen-induced rheumatoid arthritis and regulates fibroblast-like synoviocytes-mediated apoptosis and inflammation. Annals of Translational Medicine 8: 1497. https://doi.org/10.21037/atm-20-6420 [Google Scholar] [PubMed] [CrossRef]
Xie J, Wang J, Bai H, He J, Jia R et al. (2022). A decreased absolute number of T cells in patients with active rheumatoid arthritis is associated with elevated serum osteopontin levels with disease progression. Advances in Therapy 39: 3280–3291. https://doi.org/10.1007/s12325-022-02171-9 [Google Scholar] [PubMed] [CrossRef]
Yang H, Wang Z, Wang L, Li Y, Guo J et al. (2022). Scutellarin ameliorates osteoarthritis by protecting chondrocytes and subchondral bone microstructure by inactivating NF-κB/MAPK signal transduction. Biomedicine & Pharmacotherapy 155: 113781. https://doi.org/10.1016/j.biopha.2022.113781 [Google Scholar] [PubMed] [CrossRef]
Youssef M, Abdel-Reheim M, Morsy M, El-Daly M, Atwa G, Yahya G, Cavalu S, Saber S, Ahmed Gaafar AG (2022). Ameliorative effect of dabigatran on CFA-induced rheumatoid arthritis via modulating kallikrein-kinin system in rats. International Journal of Molecular Sciences 23: 10297. https://doi.org/10.3390/ijms231810297 [Google Scholar] [PubMed] [CrossRef]
Zeng Z, Yan K, Liu W (2022). Specneuzhenide ameliorate complete freund adjuvant induced arthritis in rats: Involvement of NF-κB and HO-1/Nrf-2 pathway. Journal of Oleo Science 71: 551–561. https://doi.org/10.5650/jos.ess21413 [Google Scholar] [PubMed] [CrossRef]
Zhang L, Sun S, Li W, Zhang W, Wang X, Yang SY (2017). Effect of Scutellarin inhibits collagen‐induced arthritis through TLR4/NF‐κB‐mediated inflammation. Molecular Medicine Reports 16: 5555–5560. https://doi.org/10.3892/mmr.2017.7292 [Google Scholar] [PubMed] [CrossRef]
Zhang Y, Wang S, Song S, Yang X, Jin G (2020). Ginsenoside Rg3 alleviates complete freund’s adjuvant-induced rheumatoid arthritis in mice by regulating CD4+CD25+Foxp3+ Treg cells. Journal of Agricultural and Food Chemistry 68: 4893–4902. https://doi.org/10.1021/acs.jafc.0c01473 [Google Scholar] [PubMed] [CrossRef]
Zhao T, Yang Q, Xi Y, Xie Z, Shen J, Li Z, Li Z, Qin D (2022). Ferroptosis in rheumatoid arthritis: A potential therapeutic strategy. Frontiers in Immunology 13: 779585. https://doi.org/10.3389/fimmu.2022.779585 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools