 Open Access
Open Access
REVIEW
The progress of combination therapy with immune checkpoint inhibitors in breast cancer
Department of Radiology, Zhejiang University Hospital, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
* Corresponding Author: JUNWEI WENG. Email:
BIOCELL 2023, 47(6), 1199-1211. https://doi.org/10.32604/biocell.2023.028516
Received 22 December 2022; Accepted 02 February 2023; Issue published 19 May 2023
Abstract
Immunotherapy targets the dysfunctional immune system to induce cancer cell killing by CD8-positive T cells. Immune checkpoint inhibitors (ICIs), specifically anti-PD-1 antibodies, anti-PD-L1 antibodies, and anti-CTLA4 antibodies, have revolutionized the management of many malignancies due to their significant role in generating a durable clinical response. However, clinical data suggest that response rates to ICI monotherapy are low due to the immunologically silent characteristics of breast cancer (BC). Chemotherapy, surgery, radiotherapy, and targeted therapy were recently reported to alter the tumor microenvironment and enhance the ICI response. Some clinical studies supported that ICIs, in combination with other treatment strategies, show superior efficacy in BC control, especially triple-negative breast cancer. Therefore, seeking a reasonable combination therapy is a promising way to improve ICI response. The present review highlights the clinical efficacy of ICIs treatment options in combination with standard-of-care therapies, such as chemotherapy and targeted therapy.Keywords
Breast cancer (BC) is the most commonly diagnosed cancer (30% of new cancer cases in women each year) and was the second leading cause of death due to cancer (15% of female cancer-related deaths annually) in women worldwide in 2020 (Siegel et al., 2022; Wilkinson and Gathani, 2022). Most patients with early-stage BC are effectively treated with surgical resection, and the average five-year overall survival rate for women with non-metastatic invasive BC is 90% (Kashyap et al., 2022). However, there are only a few treatment strategies for patients with unresectable BC or who develop distant metastatic disease (Kashyap et al., 2022; Loibl et al., 2021). Chemotherapy and/or radiotherapy provide only modest clinical benefits for these patients, and their five-year overall survival rate is less than 30% (Kashyap et al., 2022; Riggio et al., 2021). Most patients with BC die due to BC progression (Emens, 2018). Increasing evidence shows that the immune system plays a crucial role in the progression of various cancers by facilitating immune escape and decreasing the responses to standard BC treatment (Savas et al., 2016). Altering the immune system is effective for tumor control (Farkona et al., 2016).
Immune checkpoints are essential for maintaining self-tolerance, which is necessary to prevent damage to normal tissue in the immune response to infection (Morad et al., 2021). Tumors use a variety of mechanisms to evade immune detection and eradication, including activation of inhibitory pathways controlled by immune checkpoints (Bagchi et al., 2021). Therefore, targeting these dysfunctional immune checkpoints is a promising therapeutic strategy for tumor control. The most successful immune checkpoints are cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed cell death-1 (PD-1), and its ligand (PD-L1), which could improve the cytotoxicity and proliferative capacity of tumor-infiltrating lymphocytes (TILs). Inhibitors developed to target the above immune checkpoints have been reported to induce durable objective responses in multiple cancers by disrupting the interaction between immune checkpoints and their receptors, including BC (Doroshow et al., 2021). However, clinical data show that response rates to immune checkpoint inhibitor (ICI) monotherapy are low due to the immunologically silent characteristics of BC (Thomas et al., 2021). Increasing data show that systemic therapy, such as chemotherapy and radiotherapy, helps reshape the tumor microenvironment into an immune-favorable phenotype by altering the infiltration of TILs and Treg cells. More clinical studies support that combining ICIs and chemotherapy or radiotherapy is effective for BC control (Schmid et al., 2018). Therefore, seeking a rational combination of ICIs with other treatments is a promising direction for BC control in the future (Ulas et al., 2021). The present review highlights the clinical efficacy of ICIs in combination with standard therapies, such as chemotherapy and targeted therapy (Fig. 1).
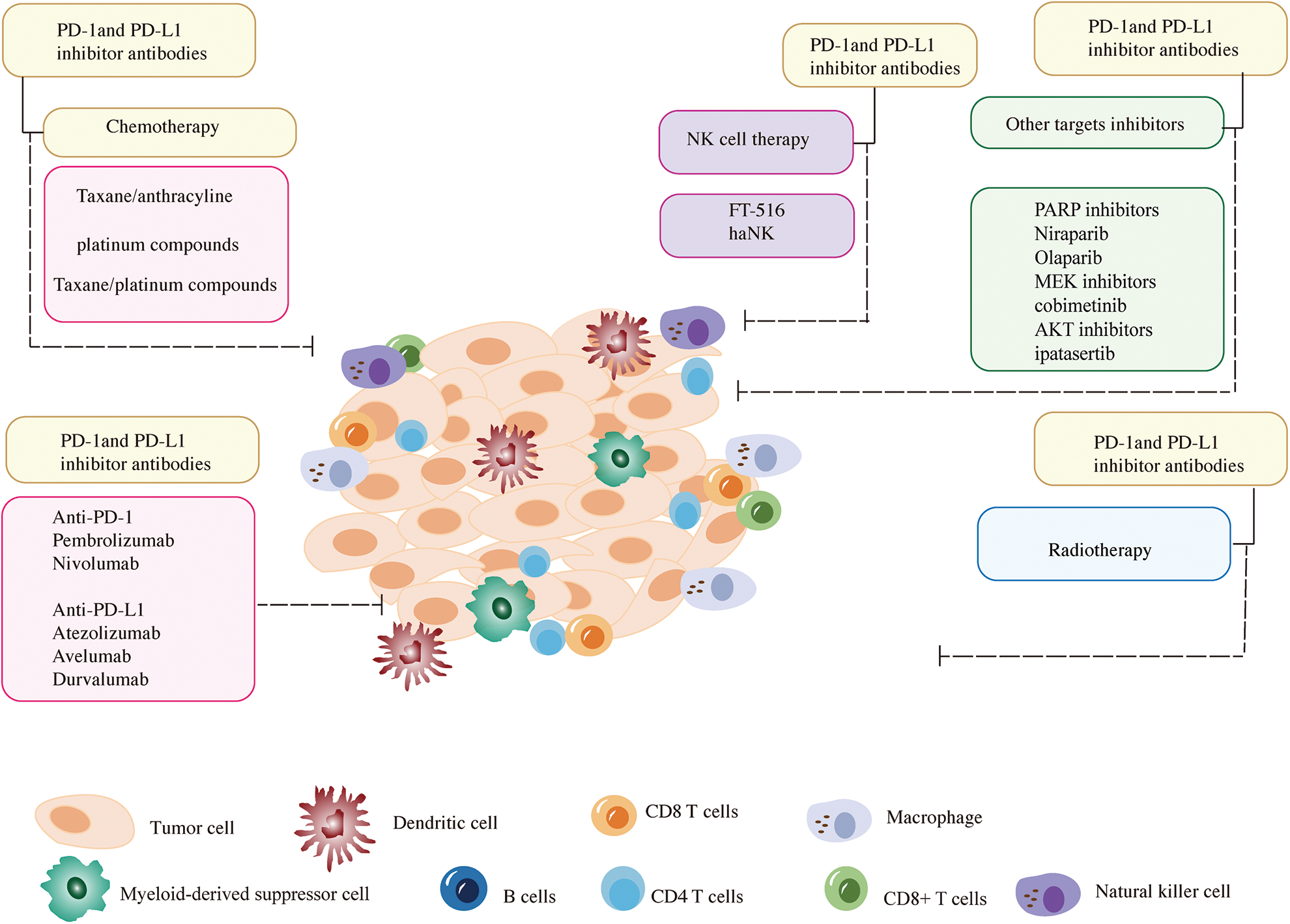
Figure 1: A summary of the combinations of immune checkpoint inhibitors and other breast cancer treatment strategies discussed in this review. The represented inhibitors of anti-PD-1 and anti-PD-L1 are listed at the bottom of the left corner. The current common combination methods of anti-PD-1 and anti-PD-L1 with chemotherapy are shown in the upper left corner. The combination methods of anti-PD-1 and anti-PD-L1 inhibitors with NK cells or other targeted inhibitors are listed in the upper right corner. In addition, the combination of anti-PD-1 and anti-PD-L1 inhibitors with radiotherapy is exhibited at the bottom of the right corner.
Immune Checkpoint Inhibitor-Chemotherapy Combination Treatment
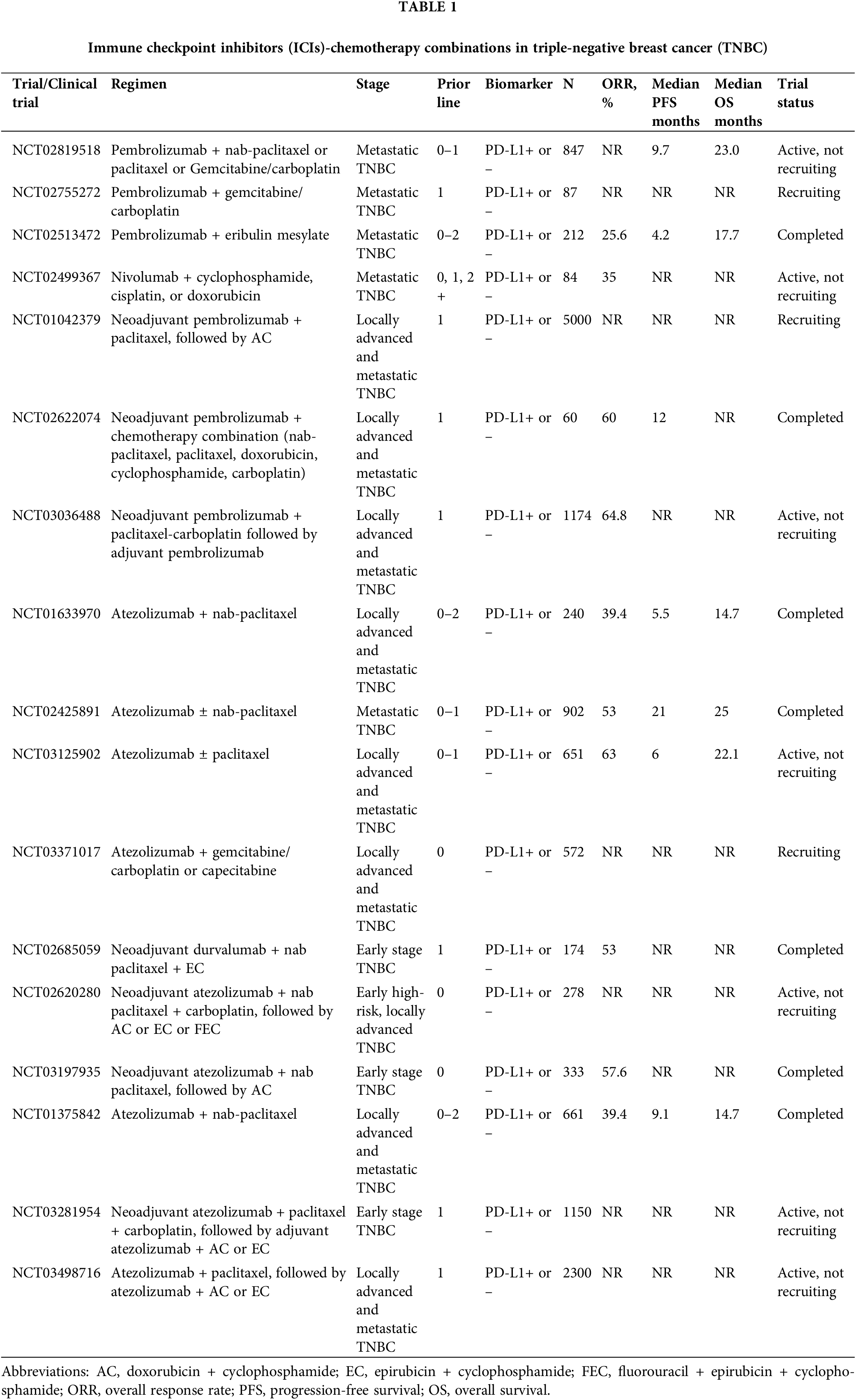
Chemotherapeutic agents, such as oxaliplatin and 5-fluorouracil, induce macrophages to release inflammatory cytokines, increase the expression of MHC class I molecules, enhance tumor-associated antigen expression, and promote dendritic cell activation, potentially augment the immune response following or during ICI treatment (Kgd et al., 2022; Najmeh et al., 2018). Therefore, the combination of ICIs and chemotherapy is a more promising treatment method for metastatic triple-negative breast cancer (mTNBC) control than ICI monotherapy (Table 1).
Programmed cell death-1 antibody-chemotherapy combination treatment
Taxanes and anthracyclines are the most common chemotherapy agents in the treatment of early and metastatic BC (Ghersi et al., 2005). Anthracycline-based regimens decrease BC mortality by 20%–30% (Hatzis et al., 2011; Jasra and Anampa, 2018). The I-SPY 2 (NCT01042379) study evaluated pathologic complete response (pCR) rates in erb-B2 receptor tyrosine kinase (ERBB2)-negative BC or TNBC patients who received neoadjuvant chemotherapy with pembrolizumab and taxanes or anthracyclines at an early stage. This combination treatment strategy increased the pCR rate from 17% to 44% (Schmid et al., 2020b). The KEYNOTE-522 trial (NCT03036488) reported that the addition of pembrolizumab to paclitaxel plus carboplatin and an anthracycline plus cyclophosphamide as neoadjuvant therapy followed by surgery plus an additional nine cycles of adjuvant pembrolizumab improved pCR rates from 51.2% to 64.8% and 18-month event-free survival (EFS) rates from 85.3% to 91.3%. Patients in this trial with node-negative TNBC had a smaller benefit, which suggests that the risk of this five-drug regimen is greater than the benefit of early node-negative TNBC patients and TNBC patients with high TIL levels, who have a good prognosis under the current standard regimen. The pCR rate of early BC patients who received combination therapy before surgery was significantly higher than patients who received chemotherapy alone, regardless of PD-L1 expression (Schmid et al., 2020a). Several ongoing trials will further elucidate the role of chemotherapy in combination with ICIs in early BC, and detailed information is provided in Table 1.
KEYNOTE-173 (NCT02622074) trial was an international phase Ib, open-label, multicohort study which evaluated six chemotherapy (nab-paclitaxel or paclitaxel, anthracycline, cyclophosphamide, carboplatin, and doxorubicin) plus pembrolizumab regimens as neoadjuvant treatment for patients with high-risk, early-stage TNBC. This study confirmed that the toxicity profile of this combination treatment strategy was similar to that of pembrolizumab and chemotherapy individually, including neutropenia, nausea, anemia, and febrile neutropenia, with a pCR rate of 60% across all treatment cohorts. This study also revealed that patients with higher pre-treatment PD-L1 expression were associated with better outcomes (Schmid et al., 2020b). The KEYNOTE-355 (NCT02819518) was a randomized, placebo-controlled, double-blind, phase 3 clinical trial that recently reported that first-line chemotherapy (paclitaxel, gemcitabine + carboplatin, or nab-paclitaxel) with pembrolizumab significantly improved progression-free survival (PFS) compared to chemotherapy alone in patients with mTNBC expressing PD-L1 with a combined positive score ≥10, which was defined as the ratio of all PD-L1-expressing cells (including tumor cells, lymphocytes, and macrophages) to the number of all tumor cells (Cortes et al., 2020). Eribulin is a halicassicin class of antineoplastic non-taxane inhibitors of microtubule dynamics that inhibit transforming growth factor β, and it exerts a powerful function in BC control. The clinical activity of the combination of eribulin and pembrolizumab was first evaluated in the KEYNOTE-150 (NCT02513472) study, and this study revealed a significant improvement in ORR (26.4%) and median PFS (4.1 months) in these patients (Tolaney et al., 2021). The subsequent randomized IMpassion130 phase III trial (NCT00388726) also revealed that overall survival (OS) was significantly improved in patients who underwent eribulin treatment (Cortes et al., 2011). BR-076 (NCT02755272) is a phase 2 clinical trial on pembrolizumab in combination with gemcitabine/carboplatin in mTNBC that is ongoing.
Nivolumab is a human IgG4 monoclonal antibody that blocks PD-1 to inhibit signals that prevent the activation of T cells from attacking the cancer (Fong and Cunningham, 2021). Nivolumab has been used to treat various solid tumors, such as melanoma, lung cancer, renal cell carcinoma, and colon cancer (Carlino et al., 2021). Some clinical trials evaluated the safety and clinical efficiency of nivolumab monotherapy in BC treatment (Brahmer et al., 2012). The most common drug-related adverse events were fatigue, infusion reactions, diarrhea, arthralgia, rash, nausea, pruritus, and headache, but these side effects were endurable (Brahmer et al., 2012). A single-center phase 2 clinical trial TONIC (NCT02499367) evaluated the efficacy of nivolumab in pretreated mTNBC (cyclophosphamide, cisplatin, and doxorubicin). Notably, the objective response rate (ORR) of nivolumab plus doxorubicin was 35%, compared to 23% for cisplatin and 17% for patients who did not receive chemotherapy. This trial also detected an upregulation of immune-related genes involved in PD-1/PD-L1 (programmed death ligand 1) and T-cell cytotoxicity pathways after doxorubicin and cisplatin induction. The results demonstrated that JAK-STAT and TNF-α signaling were enriched. Taken together, the clinical and translational data of this study indicate that short-term doxorubicin and cisplatin treatment may induce a more favorable tumor microenvironment and increase the likelihood of response to PD-1 blockade in TNBC, which also suggests that chemotherapy preconditioning induces inflammatory tumor microenvironments (Voorwerk et al., 2019). Several ongoing trials will elucidate the efficacy of the combination of ICIs and nivolumab for BC, but the results have not been determined.
Programmed cell death-ligand 1 antibody-chemotherapy combination treatment
Atezolizumab, avelumab, and durvalumab selectively target PD-L1 to prevent the interaction between PD-1 (CD279) and B7-1, which reverses T-cell suppression (Schmid et al., 2018). The safety and clinical efficacy of these three anti-PD-L1 antibodies have been reported in BC (Emens et al., 2021). NCT01375842 is a multicohort phase I study involving 116 patients with mTNBC that evaluated the efficacy of atezolizumab monotherapy in BC. Atezolizumab treatment prolonged the ORR (5 of 21 [24%]), with a median OS of 17.6 months. This clinical trial also demonstrated that patients with PD-L1 overexpression and more tumor-infiltrating immune cells had higher ORRs and longer OS (12% [11 of 91]; 10.1 [95% CI, 7.0–13.8] months, respectively) than patients with fewer immune cells (0 of 21; 6.0 [95% CI, 2.6–12.6] months, respectively) (Emens et al., 2019). GP28328 (NCT01633970) was a multicenter and multicohort phase 1b study of atezolizumab plus chemotherapy in the treatment of advanced solid tumors, which showed that the atezolizumab plus nanoparticle albumin-bound (nab) paclitaxel group had a better ORR (53.8% vs. 30.0%) and PFS (8.6 vs. 5.1 months). OS also increased significantly in the atezolizumab plus nab-paclitaxel group compared to nab-paclitaxel monotherapy (24.2 vs. 12.4 months) (Adams et al., 2019). The IMpassion130 (NCT02425891) trial showed that the combination of atezolizumab with first-line nab-paclitaxel for metastatic TNBC significantly improved PFS and showed a clinically meaningful effect on OS in patients with PD-L1-positive tumors. The intention-to-treat analysis showed that the median PFS was 7.2 months with atezolizumab plus nab-paclitaxel compared to 5.5 months with placebo plus nab-paclitaxel. For patients with PD-L1–positive tumors, the median PFS was 7.5 and 5.0 months, respectively. The intention-to-treat analysis revealed that the median OS increased to 21.3 months, and the median OS was 25.0 and 15.5 months, respectively, in patients with PD-L1–positive tumors (Emens et al., 2021). Based on these results, the Food and Drug Administration (FDA) granted accelerated approval to atezolizumab on March 8, 2019 (TECENTRIQ, Genentech Inc., South San Francisco, CA, USA) plus nab-paclitaxel adult patients with unresectable locally advanced or mTNBC whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥1% of the tumor area), as determined by an FDA-approved test. The subsequent IMpassion131 study (NCT03125902) evaluated the safety and efficacy of atezolizumab plus paclitaxel as a first-line therapy in patients with locally advanced or mTNBC. However, this trial showed that this combination treatment strategy did not improve PFS or OS vs. paclitaxel alone (Miles et al., 2021). IMpassion132 (NCT03371017) is a multinational, double-blind placebo-controlled two-arm randomized phase III trial comparing atezolizumab plus chemotherapy (capecitabine [mandatory in platinum-pretreated patients] or gemcitabine/carboplatin) vs. placebo plus chemotherapy in early relapsing TNBC (Cortés et al., 2019). The primary endpoint of IMpassion132 is OS in the intent-to-treat population. Median OS will be estimated in the atezolizumab and placebo groups using Kaplan–Meier methodology, but the result is pending. Compared to atezolizumab, there is limited information on the effect of durvalumab combined with chemotherapy in early TNBC treatment. A randomized phase II study, the GeparNuevo trial (NCT02685059), investigated the safety and efficacy of the combination of durvalumab and anthracycline taxane-based neoadjuvant therapy in early TNBC. The results indicated that durvalumab plus anthracycline taxane-based neoadjuvant therapy provided significantly improved survival (95.2% vs. 83.5%) despite a modest pCR (53.4% vs. 44.2%) (Loibl et al., 2022b). The NeoTRIPaPDL1 (NCT02620280) clinical trial is a phase III study that also revealed that neoadjuvant atezolizumab plus carboplatin and nab-paclitaxel followed by adjuvant chemotherapy in early-stage high-risk or locally advanced TNBC did not significantly increase the rate of pCR in women with TNBC (48.6% vs. 44.4%; p = 0.48) (Gianni et al., 2022). Additional studies are needed to clarify the optimal duration and sequence of ICIs in early TNBC treatment.
The placebo-controlled NSABP B-59 trial (NCT03281954) and the IMpassion030 (NCT03498716) trial evaluated the efficacy, safety, and pharmacokinetics of adjuvant atezolizumab in combination with chemotherapy, such as paclitaxel, doxorubicin, epirubicin, and cyclophosphamide, in patients with stage II–III TNBC (Pérez-García et al., 2020; Adams et al., 2019). Another small trial is examining whether the combination of atezolizumab and neoadjuvant chemotherapy provides clinical benefits for advanced TNBC patients. The IMpassion031 trial (NCT03197935) evaluated whether the addition of atezolizumab to the novel adjuvant nab-paclitaxel, doxorubicin, and cyclophosphamide resulted in a higher pCR rate compared to placebo. Interim analysis showed that atezolizumab combined with the sequence nab-paclitaxel and anthracycline chemotherapy prolonged OS and PFS in patients with early TNBC. Patients receiving atezolizumab in combination with chemotherapy had a pCR rate of 57.6% compared to 41.1% in patients who did not receive atezolizumab. Among PD-L1-positive patients, the pCR rate reached 69% in patients who received atezolizumab plus chemotherapy and 49% in patients receiving placebo plus chemotherapy (Mittendorf et al., 2020). Two other locally advanced TNBC studies are ongoing to evaluate the effect of PD-L1 blocking chemotherapy in adjuvant therapy. The phase III randomized trial A-Brave (NCT02926196) is examining whether 1 year of adjuvant therapy improves disease-free survival (DFS) compared with high-risk primary TNBC patients who have completed treatment, including surgery following neoadjuvant chemotherapy. The study focused on avelumab in patients with high-risk or residual disease, but the results are pending (Table 1).
Immune Checkpoint Inhibitor-Surgery Combination Treatment
Surgery has been the primary modality for the treatment of BC for centuries since it was first performed by Halsted in 1882 (Matsen and Neumayer, 2013). Surgery was recently reported to alter immune function by switching to a Th2 immune response, activating Treg cells, recruiting MDSCs, and inhibiting natural killer cells (NK) and T-cell function (Loibl et al., 2021; Miles et al., 2021; Popa and Georgescu, 2017). Therefore, the perioperative phase is considered the best time to improve immunity and reduce tumor recurrence and metastasis (Forde et al., 2018). However, no trial has directly evaluated the safety and clinical efficacy of ICIs in combination with surgery. We conclude from the above five clinical trials, I-SPY 2, GeparNuevo, KEYNOTE-173, KEYNOTE-522, and NeoTRIPaPDL1, that surgery after the combination of ICIs and chemotherapy prolonged the pCR and OS regardless of PD-L1 expression. However, the combination of the treatment plan, operation time, and postoperative treatment must be further discussed (Loibl et al., 2022a, 2022b). Four ongoing clinical studies are examining the efficacy of the combination of ICIs and surgery for patients with early BC (Table 2).
Immune Checkpoint Inhibitor-Radiotherapy Combination Treatment
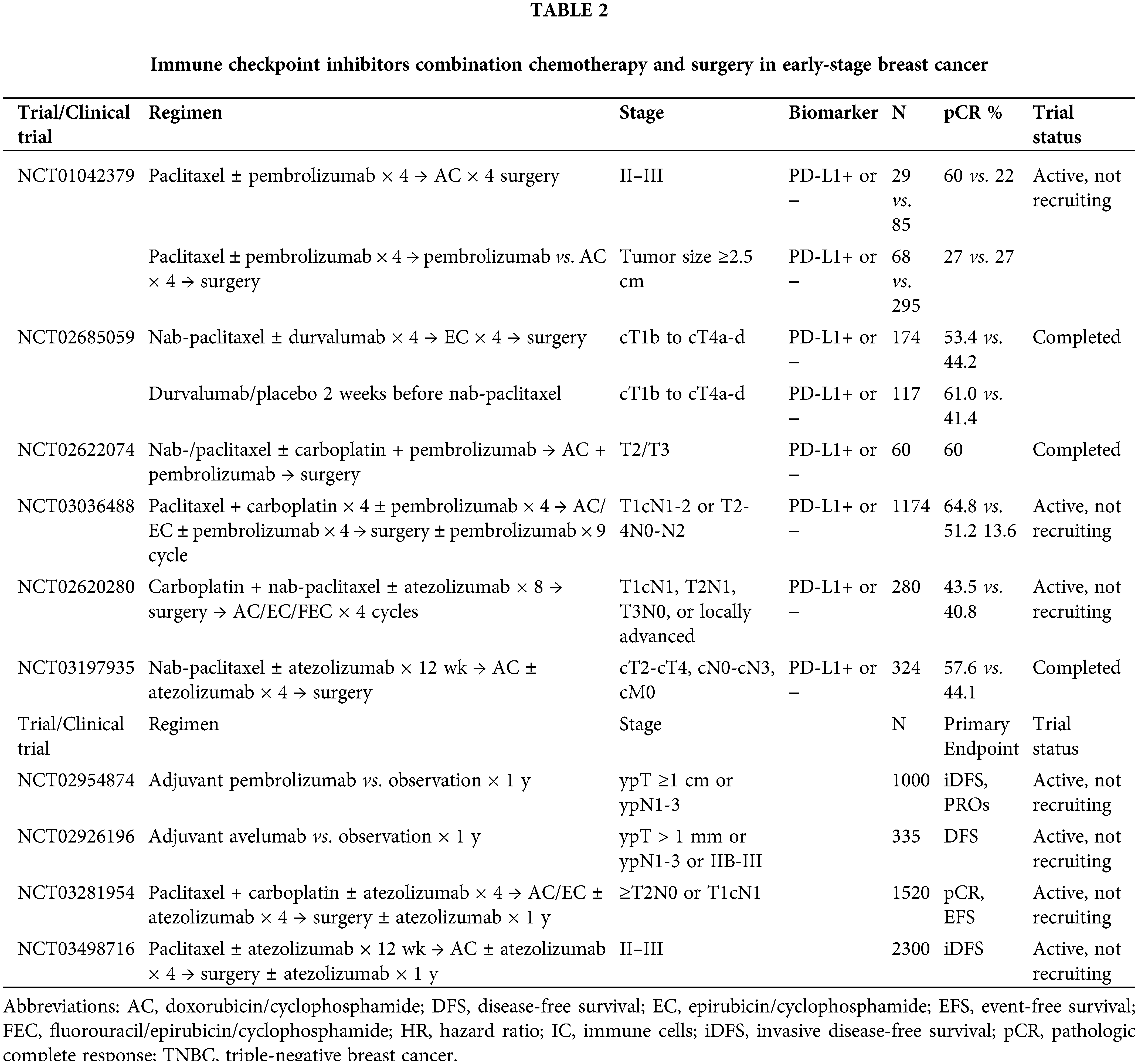
Radiotherapy is the standard treatment for BC, and it reduces local recurrence and improves OS in early to locally advanced BC (Dandawate et al., 2016). The role of radiotherapy in BC management continues to evolve. For patients with early-stage breast cancer, low-grade whole breast irradiation after breast-conserving surgery is the standard of care (Zi et al., 2017). Radiotherapy is associated with tumor DNA damage, which leads to the release of antigens and danger signals that promote antigen presentation and tumor-specific T-cell activation, and RT enhances the anti-tumor activity of ICIs (Bradley and Mendenhall, 2018; Lhuillier et al., 2021; Zi et al., 2017).
Rudqvist et al. (2018) demonstrated that the combination of anti-CTLA-4 antibodies and RT (12 Gy of irradiation) significantly inhibited BC growth. They also found that CTLA-4 blockade with one fraction of 12 Gy increased mouse survival time but did not lead to statistically significant tumor control compared with radiotherapy alone (Rudqvist et al., 2018). Tremelimumab is a fully human monoclonal antibody against CTLA-4 (Santa-Maria et al., 2017) that has been used for the control of various solid tumors, including melanoma, hepatocellular carcinoma, and non-small cell lung cancer (Ulas et al., 2021). However, the role of tremelimumab in BC control has been poorly studied. The KCSG BR17-04 trial is a phase II trial that enrolled 119 patients with hormone receptor (HR)-positive metastatic BC to examine the efficacy and safety of combined durvalumab and tremelimumab in BC control. This combination showed modest activity and good tolerability in these patients (Moon et al., 2022). The combination of radiotherapy and tremelimumab was first administered to six patients with advanced BC. The results of this trial showed that the best curative response was a stable disease, with one case of stable disease lasting more than six months. One patient lived for more than eight years, and the median OS increased to 50.8 months. Following 1 week of treatment, the proliferation of peripheral blood mononuclear cells increased in five patients, and the Treg number increased simultaneously (Jiang et al., 2019). This study confirmed that patients with advanced BC benefited from the combination of tremelimumab and RT. A subsequent single-institution study (NCT02563925) confirmed the safety and efficacy of RT and concurrent tremelimumab ± HER2-directed therapy with trastuzumab for patients with BC brain metastases (Page et al., 2022). A phase II clinical trial enrolled 17 patients with advanced TNBC with distant metastases to assess the clinical efficacy of pembrolizumab plus RT. The ORR reached 17.6% in this study, in which three patients achieved complete response (CR), and one patient achieved stable disease (SD) (Ho et al., 2020). These clinical studies suggested that the combination of RT and ICIs provided benefits for patients with BC. However, the radiation doses, fractionation, and delivery schedules have not been validated. The Memorial Sloan Kettering Cancer Center and Cedars-Sinai Medical Center performed a trial to examine the efficacy of the combination of pembrolizumab and five fractions of 6 Gy in metastatic TNBC patients. A similar study is ongoing in different solid tumors, including metastatic melanoma, BC, and pancreatic cancer, comparing two irradiation schedules of three fractions of 8 Gy of irradiation or one fraction of 17 Gy in combination with tremelimumab and durvalumab (Craig et al., 2021). NCT02303366 is a phase 1 study that examined the safety and biological effects of pembrolizumab and 1 fraction of 20 Gy in BC (Zi et al., 2017). The Netherlands Cancer Institute is using 20 Gy one-part induction therapy in TNBC patients, low-dose doxorubicin, cyclophosphamide or cisplatin, or no nivolumab induction therapy (Table 3).
Immune Checkpoint Inhibitor-Targeted Therapy Combination Treatment
Immune checkpoint inhibitor-poly (ADP-ribose) polymerase inhibitor combination treatment
Poly (ADP-Ribose) polymerase inhibitors (PARPis) induce cell death by targeting the homologous recombination repair pathway in cells with BRCA1/2 mutation, and PARPis are approved for TNBC patients with germline mutations in BRCA1/2 (Shen et al., 2019). Increasing evidence supports the potential for the combination of PARPis and ICIs to induce a stronger anti-tumor immune response in these TNBC patient subpopulations due to the activation of infiltrating T cells following PARPi induction of cell death and the release of tumor antigens (Li et al., 2020). PARPis contributed to PD-L1 overexpression in cell lines and animal models, which supports the anti-tumor activity of the combination of PD1/PD-L1 inhibitors and PARPis in the treatment of BC (Jiao et al., 2017). PARPi monotherapy prolonged the PFS and OS of TNBC patients with BRCA1/2 mutations (Tung et al., 2020). However, whether PARPis combined with ICIs are safe and improve clinical efficacy remains to be discussed.
An open-label, multicenter, phase 1/2, basket study, the MEDIOLA trial (NCT02734004) was the first study to assess the safety and clinical activity of olaparib in combination with durvalumab in patients with germline BRCA1-mutated or BRCA2-mutated mBC (Domchek et al., 2020). The results of this clinical trial showed that 11 (32%) patients experienced grade 3 or worse adverse events, of which the most common were anemia (four [12%]), neutropenia (three [9%]), and pancreatitis (two [6%]). Three (9%) patients were discontinued due to adverse events, and four (12%) patients experienced a total of six serious adverse events. There were no treatment-related deaths. Twenty-four of the 30 patients who were eligible for activity analysis had disease control at 12 weeks (Domchek et al., 2020). The phase II TOPACIO trial (NCT02657889) revealed that the combination of niraparib plus pembrolizumab achieved an ORR of 21% and a disease control rate (DCR) of 49% in patients with advanced TNBC (Vinayak et al., 2019). Within the limits of cross-trial comparisons, this ORR was slightly lower than the ORRs of 55% and 62% associated with single-agent PARP inhibitor therapy in patients with TNBC and germline BRCA mutations in the OlympiAD (NCT02000622) and EMBRACA (NCT01945775) trials, respectively. The MEDIOLA trial (NCT02734004) of the doublet or triplet combination of durvalumab, olaparib, and VEGFR inhibitors in mTNBC with germline BRCA mutations demonstrated an ORR of 58.8%, which was more similar to single-agent PARP therapy, with a median PFS of 4.9 months (Domchek et al., 2020). Several clinical trials have evaluated the clinical efficacy of the combination of PD-L1 inhibition and PARPis in mTNBC. For example, ongoing larger trials of olaparib with atezolizumab (NCT02849496) or durvalumab (NCT03167619) are in progress. Cyclin-dependent kinase (CDK) inhibitors boosted effector T-cell activity and inhibited Treg proliferation, which led to fibroblast-derived proinflammatory cytokine secretion and enhanced cell surface antigen presentation (Roskoski, 2019). CDK inhibitors sensitize BC cells to PARPis, which may further augment the treatment response to ICIs (Johnson et al., 2011). It would be interesting to examine the clinical benefits of combining PARPis, ICIs, and CDK inhibitors.
Immune checkpoint-inhibitor-anti-angiogenic therapy combination treatment
Low-dose anti-angiogenic therapy normalizes blood vessels, encourages CD8+ T-cell and B-cell infiltration, increases PD-1 expression in tumor cells, and enhances anti-PD-1 therapeutic effectiveness. Previous trials showed that anti-angiogenesis or anti-PD-1/PD-L1 monotherapy only showed modest effects on TNBC. Preclinical studies demonstrated that anti-angiogenic therapy sensitized BC to PD-1/PD-L1 blockade by reshaping the tumor microenvironment (Wang et al., 2020). Therefore, patients with TNBC may benefit from the combination of ICIs and anti-angiogenesis therapy. Camrelizumab, in combination with apatinib, has shown promising efficacy and a manageable safety profile in patients with advanced TNBC (Liu et al., 2020). NCT04303741 also revealed the clinical efficacy of camrelizumab, famitinib, and eribulin in heavily pretreated patients with advanced TNBC. The ORR was 37.0% (17/46, 95% CI 23.2–52.5), the DCR was 87.0% (40/46, 95% CI 73.7–95.1), and the PFS was 8.1 (95% CI 4.6–10.3) months (Li et al., 2022; Liu et al., 2022; Wu et al., 2022).
Immune checkpoint inhibitors-other target combination treatment
The COLET (NCT02322814) study assessed the combination of the MEK1/2 inhibitor cobimetinib, atezolizumab, and paclitaxel/nab-paclitaxel as first-line therapy for locally advanced TNB or mTNBC. Interim analysis revealed an ORR of 34% in combination with paclitaxel and 29% with nab-paclitaxel (Brufsky et al., 2019). Two phase-II trials are underway to evaluate the safety and efficacy of pembrolizumab/avelumab in combination with binimetinib (NCT03106415 and NCT03971409) in patients with locally advanced TNBC or mTNBC (Chumsri et al., 2020). Enobosarm (GTx-024) was used with pembrolizumab to treat luminal androgen receptor-positive TNBC. The adverse effects of combining enobosarm with pembrolizumab are well tolerated, with a 25% moderate response at 16 weeks regardless of PD-L1 expression (Yuan et al., 2021). However, the trial ended early when the supply of the GTx-024 drug was interrupted. Clinical trials of AR+ TNBC combined with ICIs and AR-targeted therapy will be investigated in the future (Lehmann et al., 2020; Yuan et al., 2021). A nonrandomized, open-label, multicohort phase 1b study (NCT02779751) investigated the safety and efficacy of abemaciclib plus pembrolizumab with/without anastrozole in patients with hormone receptor-positive (HR+) human epidermal growth factor receptor 2-negative (HER2-) BC. The ORR and DCR were 23.1/28.6% and 84.6/82.1%, respectively, and the median PFS and OS were 8.9 and 26.3 months, respectively (Rugo et al., 2022).
AKT inhibitors, in combination with ICIs, have also attracted considerable interest and are considered another important class of targeted therapies for BC control in combination with ICIs (Hua et al., 2021). PTEN is a well-known tumor suppressor and a negative regulator of AKT (Álvarez-Garcia et al., 2019). AKT inhibitors lead to immunotherapy resistance and promote tumor-specific lymphocyte amplification (de Bono et al., 2019). This evidence supports the potential promising efficacy of the combination of ICIs and AKT inhibitors in BC treatment. A phase Ib trial (NCT03800836) evaluated the combination of ipatasertib (IPAT), atezolizumab, and taxane as first-line therapy for locally advanced TNBC or mTNBC. The results demonstrated an impressive ORR of 73%, with similar responses regardless of PIK3CA/AKT1/PTEN alteration status and PD-L1 expression (Heeke and Tan, 2021). These data also led to the addition of a paclitaxel, ipatasertib, and atezolizumab arm in the larger phase III IPATunity130 trial (NCT03337724), and the AKT inhibitor capivasertib in combination with paclitaxel and durvalumab is being investigated in the BEGONIA trial (NCT03742102) (Turner et al., 2022).
Immune Checkpoint Inhibitor-Natural Killer Cell Combination Treatment
More than 40 years ago, it was discovered that NK are lymphocytes with the ability to dissolve tumor cells. Preclinical studies showed that NK cells modulated the immune response by secreting chemokines and cytokines and releasing cytotoxic particles containing granulocytes and perforins, which cause target cell death (Terrén et al., 2019). The release of the stress-inducing ligands MHC class I polypeptide-related sequence A (MICA) and MICB by tumor cells leads to the downregulation of NKG2D receptors and decreased sensitivity of NK cells, which leads to immune escape (Liu et al., 2021; Shimasaki et al., 2020; Xie et al., 2020). These results also provide a theoretical basis for the combined application of NK cells and ICIs. The ongoing QUILT-3.067 (NCT03387085) trial is evaluating the safety and efficacy of the combination of NK cells and ICIs in patients with refractory, metastatic, or unresectable TNBC tumors. The study is unique in design because it combines avelumab with high-affinity NK (haNK) cell therapy, IL-15 cytokine administration, cancer vaccines, and metronomic chemoradiation to stimulate the innate and adaptive immune systems. Interim results of nine patients demonstrated an overall response rate of 67%, with a disease control response rate of 78% and a CR rate of 22%. Notably, the duration of the treatment responses with a median PFS of 13.7 months is very promising compared to the historical PFS of 3 months (Nangia et al., 2019). NCT04551885 is examining the efficacy of the combination of avelumab and FT-516 in patients with advanced TNBC, but the results of the data are not clear.
Accumulating evidence corroborates that ICI monotherapy, PD-1/PD-L1 antibodies, and CTLA-4 antibodies produce only modest or low response in BC control due to its immunological silencing characteristics. Finding appropriate ways to alter the BC immune microenvironment is critical to improving the efficacy of ICIs. Increasing evidence shows that chemotherapy, surgery, radiation, targeted therapy, and cellular immunotherapy reshape the tumor microenvironment. The safety and efficacy of the combination of ICIs and these treatment strategies are summarized in this review. These clinical trials show that many unanswered questions remain about the combination of ICIs and other treatment strategies. However, the identity of reliable biomarkers for predicting response, the most effective combination of ICIs and chemotherapy drugs, the combination of ICIs and other strategies that may be used as first-line therapy, and the dose of radiotherapy that will have the most benefit for patients are not known. Many ongoing clinical trials are being performed to help select the best treatment for BC patients.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm the following contributions to the paper. Manuscript drafting: Kaimin Fan; manuscript revision and editing: Kaimin Fan and Junwei Weng; preparation of figure and tables: Kaimin Fan and Junwei Weng. Both authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Adams S, Diamond JR, Hamilton E, Pohlmann PR, Tolaney SM et al. (2019). Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: A phase 1b clinical trial. JAMA Oncology 5: 334–342. https://doi.org/10.1001/jamaoncol.2018.5152 [Google Scholar] [PubMed] [CrossRef]
Álvarez-Garcia V, Tawil Y, Wise HM, Leslie NR (2019). Mechanisms of PTEN loss in cancer: It’s all about diversity. Seminars in Cancer Biology 59: 66–79. https://doi.org/10.1016/j.semcancer.2019.02.001 [Google Scholar] [PubMed] [CrossRef]
Bagchi S, Yuan R, Engleman EG (2021). Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annual Review of Pathology: Mechanisms of Disease 16: 223–249. https://doi.org/10.1146/annurev-pathol-042020-042741 [Google Scholar] [PubMed] [CrossRef]
Bradley JA, Mendenhall NP (2018). Novel radiotherapy techniques for breast cancer. Annual Review of Medicine 69: 277–288. https://doi.org/10.1146/annurev-med-042716-103422 [Google Scholar] [PubMed] [CrossRef]
Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL et al. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England Journal of Medicine 366: 2455–2465. https://doi.org/10.1056/NEJMoa1200694 [Google Scholar] [PubMed] [CrossRef]
Brufsky A, Kim SB, Zvirbule Z, Dirix LY, Eniu AE et al. (2019). Phase II COLET study: Atezolizumab (A) + cobimetinib (C) + paclitaxel (P)/nab-paclitaxel (nP) as first-line (1L) treatment (tx) for patients (pts) with locally advanced or metastatic triple-negative breast cancer (mTNBC). Journal of Clinical Oncology 37: 1013. https://doi.org/10.1200/JCO.2019.37.15_suppl.1013 [Google Scholar] [CrossRef]
Carlino MS, Larkin J, Long GV (2021). Immune checkpoint inhibitors in melanoma. The Lancet 398: 1002–1014. https://doi.org/10.1016/S0140-6736(21)01206-X [Google Scholar] [PubMed] [CrossRef]
Chumsri S, Polley MY, Mathur P, Reis A, Tenner KS, Weidner M, Advani P (2020). Phase I results of the phase I/II study of pembrolizumab in combination with binimetinib in patients with unresectable locally advanced or metastatic triple-negative breast cancer. Journal of Clinical Oncology 38: 78–83. https://doi.org/10.1200/JCO.2020.38.5_suppl.78 [Google Scholar] [CrossRef]
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA et al. (2020). Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396: 1817–1828. https://doi.org/10.1016/S0140-6736(20)32531-9 [Google Scholar] [PubMed] [CrossRef]
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT et al. (2011). Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACEA phase 3 open-label randomised study. Lancet 377: 914–923. https://doi.org/10.1016/S0140-6736(11)60070-6 [Google Scholar] [PubMed] [CrossRef]
Cortés J, André F, Gonçalves A, Kümmel S, Martín M, Schmid P, Schuetz F (2019). IMpassion132 phase III trial: Atezolizumab and chemotherapy in early relapsing metastatic triple-negative breast cancer. Future Oncology 15: 1951–1961. https://doi.org/10.2217/fon-2019-0059 [Google Scholar] [PubMed] [CrossRef]
Craig DJ, Nanavaty NS, Devanaboyina M, Stanbery L, Hamouda D, Edelman G, Dworkin L, Nemunaitis JJ (2021). The abscopal effect of radiation therapy. Future Oncology 17: 1683–1694. https://doi.org/10.2217/fon-2020-0994 [Google Scholar] [PubMed] [CrossRef]
Dandawate PR, Subramaniam D, Jensen RA, Anant S (2016). Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Seminars in Cancer Biology 40–41: 192–208. https://doi.org/10.1016/j.semcancer.2016.09.001 [Google Scholar] [PubMed] [CrossRef]
de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S et al. (2019). Randomized phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clinical Cancer Research 25: 928–936. https://doi.org/10.1158/1078-0432.CCR-18-0981 [Google Scholar] [PubMed] [CrossRef]
Domchek SM, Postel VS, Im SA, Park YH, Delord JP et al. (2020). Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLAAn open-label, multicentre, phase 1/2, basket study. The Lancet Oncology 21: 1155–1164. https://doi.org/10.1016/S1470-2045(20)30324-7 [Google Scholar] [PubMed] [CrossRef]
Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM et al. (2021). PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nature Reviews Clinical Oncology 18: 345–362. https://doi.org/10.1038/s41571-021-00473-5 [Google Scholar] [PubMed] [CrossRef]
Emens LA (2018). Breast cancer immunotherapy: Facts and hopes. Clinical Cancer Research 24: 511–520. https://doi.org/10.1158/1078-0432.CCR-16-3001 [Google Scholar] [PubMed] [CrossRef]
Emens LA, Adams S, Barrios CH, Diéras V, Iwata H et al. (2021). First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Annals of Oncology 32: 983–993. https://doi.org/10.1016/j.annonc.2021.05.355 [Google Scholar] [PubMed] [CrossRef]
Emens LA, Cruz C, Eder JP, Braiteh F, Chung C et al. (2019). Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncology 5: 74–82. https://doi.org/10.1001/jamaoncol.2018.4224 [Google Scholar] [PubMed] [CrossRef]
Farkona S, Diamandis EP, Blasutig IM (2016). Cancer immunotherapy: The beginning of the end of cancer? BMC Medicine 14: 73. https://doi.org/10.1186/s12916-016-0623-5 [Google Scholar] [PubMed] [CrossRef]
Fong C, Cunningham D (2021). Chemotherapy with nivolumab in advanced gastro-oesophageal adenocarcinoma. The Lancet 398: 2–3. https://doi.org/10.1016/S0140-6736(21)00988-0 [Google Scholar] [PubMed] [CrossRef]
Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR et al. (2018). Neoadjuvant PD-1 blockade in resectable lung cancer. The New England Journal of Medicine 378: 1976–1986. https://doi.org/10.1056/NEJMoa1716078 [Google Scholar] [PubMed] [CrossRef]
Ghersi D, Wilcken N, Simes J, Donoghue E (2005). Taxane containing regimens for metastatic breast cancer. The Cochrane Database of Systematic Reviews CD003366. https://doi.org/10.1002/14651858.CD003366.pub2 [Google Scholar] [PubMed] [CrossRef]
Gianni L, Huang CS, Egle D, Bermejo B, Zamagni C et al. (2022). Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Annals of Oncology 33: 534–543. https://doi.org/10.1016/j.annonc.2022.02.004 [Google Scholar] [PubMed] [CrossRef]
Hatzis C, Pusztai L, Valero V, Booser DJ, Esserman L et al. (2011). A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA 305: 1873–1881. https://doi.org/10.1001/jama.2011.593 [Google Scholar] [PubMed] [CrossRef]
Heeke AL, Tan AR (2021). Checkpoint inhibitor therapy for metastatic triple-negative breast cancer. Cancer Metastasis Reviews 40: 537–547. https://doi.org/10.1007/s10555-021-09972-4 [Google Scholar] [PubMed] [CrossRef]
Ho AY, Barker CA, Arnold BB, Powell SN, Hu ZI et al. (2020). A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 126: 850–860. https://doi.org/10.1002/cncr.32599 [Google Scholar] [PubMed] [CrossRef]
Hua H, Zhang H, Chen J, Wang J, Liu J, Jiang Y (2021). Targeting Akt in cancer for precision therapy. Journal of Hematology and Oncology 14: 128. https://doi.org/10.1186/s13045-021-01137-8 [Google Scholar] [PubMed] [CrossRef]
Jasra S, Anampa J (2018). Anthracycline use for early stage breast cancer in the modern Era: A review. Current Treatment Options in Oncology 19: 30. https://doi.org/10.1007/s11864-018-0547-8 [Google Scholar] [PubMed] [CrossRef]
Jiang DM, Fyles A, Nguyen LT, Neel BG, Sacher A, Rottapel R, Wang BX, Ohashi PS, Sridhar SS (2019). Phase I study of local radiation and tremelimumab in patients with inoperable locally recurrent or metastatic breast cancer. Oncotarget 10: 2947–2958. https://doi.org/10.18632/oncotarget.26893 [Google Scholar] [PubMed] [CrossRef]
Jiao SP, Xia WY, Yamaguchi HH, Wei YK, Chen MK et al. (2017). PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clinical Cancer Research 23: 3711–3720. https://doi.org/10.1158/1078-0432.CCR-16-3215 [Google Scholar] [PubMed] [CrossRef]
Johnson N, Li YC, Walton ZE, Cheng KA, Li D et al. (2011). Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nature Medicine 17: 875–882. https://doi.org/10.1038/nm.2377 [Google Scholar] [PubMed] [CrossRef]
Kashyap D, Pal D, Sharma R, Garg VK, Goel N et al. (2022). Global increase in breast cancer incidence: Risk factors and preventive measures. BioMed Research International 2022: 9605439. https://doi.org/10.1155/2022/9605439 [Google Scholar] [PubMed] [CrossRef]
Kgd F, Ea E, Ksds F, Jpm L, Fdcl P, Odl P, Fq C, Jam N, Wilke DV (2022). Chromomycin A5 induces bona fide immunogenic cell death in melanoma. Frontiers in Immunology 13: 941757. https://doi.org/10.3389/fimmu.2022.941757 [Google Scholar] [PubMed] [CrossRef]
Lehmann BD, Abramson VG, Sanders ME, Mayer EL, Haddad TC et al. (2020). TBCRC 032 IB/II multicenter study: Molecular insights to AR antagonist and PI3K inhibitor efficacy in patients with AR+ metastatic triple-negative breast cancer. Clinical Cancer Research 26: 2111–2123. https://doi.org/10.1158/1078-0432.CCR-19-2170 [Google Scholar] [PubMed] [CrossRef]
Lhuillier C, Rudqvist NP, Yamazaki T, Zhang T, Charpentier M et al. (2021). Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. The Journal of Clinical Investigation 131: 138740. https://doi.org/10.1172/JCI138740 [Google Scholar] [PubMed] [CrossRef]
Li C, Jiang YZ, Wu SY, Wu J, Di GH et al. (2022). Famitinib with camrelizumab and nab-paclitaxel for advanced immunomodulatory triple-negative breast cancer (FUTURE-C-PlusAn open-label, single-arm, phase II trial. Clinical Cancer Research 28: 2807–2817. https://doi.org/10.1158/1078-0432.CCR-21-4313 [Google Scholar] [PubMed] [CrossRef]
Li H, Liu ZY, Wu N, Chen YC, Cheng Q, Wang J (2020). PARP inhibitor resistance: The underlying mechanisms and clinical implications. Molecular Cancer 19: 107. https://doi.org/10.1186/s12943-020-01227-0 [Google Scholar] [PubMed] [CrossRef]
Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J (2021). NK cell-based cancer immunotherapy: From basic biology to clinical development. Journal of Hematology and Oncology 14: 7. https://doi.org/10.1186/s13045-020-01014-w [Google Scholar] [PubMed] [CrossRef]
Liu J, Liu Q, Li Y, Li Q, Su F et al. (2020). Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: An open-label phase II trial. Journal for Immunotherapy of Cancer 8: e000696. https://doi.org/10.1136/jitc-2020-000696 [Google Scholar] [PubMed] [CrossRef]
Liu J, Wang Y, Tian Z, Lin Y, Li H et al. (2022). Multicenter phase II trial of Camrelizumab combined with Apatinib and Eribulin in heavily pretreated patients with advanced triple-negative breast cancer. Nature Communications 13: 3011. https://doi.org/10.1038/s41467-022-30569-0 [Google Scholar] [PubMed] [CrossRef]
Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G (2021). Breast cancer. Lancet 397: 1750–1769. https://doi.org/10.1016/S0140-6736(20)32381-3 [Google Scholar] [PubMed] [CrossRef]
Loibl S, Schneeweiss A, Huober J, Braun M, Rey J et al. (2022a). Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Annals of Oncology 33: 1149–1158. https://doi.org/10.1016/j.annonc.2022.07.1940 [Google Scholar] [PubMed] [CrossRef]
Loibl S, Untch M, Burchardi N, Huober J, Sinn BV et al. (2022b). A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Annals of Oncology 30: 1279–1288. https://doi.org/10.1093/annonc/mdz158 [Google Scholar] [PubMed] [CrossRef]
Matsen CB, Neumayer LA (2013). Breast cancer: A review for the general surgeon. JAMA Surgery 148: 971–980. https://doi.org/10.1001/jamasurg.2013.3393 [Google Scholar] [PubMed] [CrossRef]
Miles D, Gligorov J, André F, Cameron D, Schneeweiss A et al. (2021). Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Annals of Oncology 32: 994–1004. https://doi.org/10.1016/j.annonc.2021.05.801 [Google Scholar] [PubMed] [CrossRef]
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH et al. (2020). Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031A randomised, double-blind, phase 3 trial. Lancet 396: 1090–1100. https://doi.org/10.1016/S0140-6736(20)31953-X [Google Scholar] [PubMed] [CrossRef]
Moon YW, Kim E, Kim MH, Kim GM, Kim SG, Chae Y, Lee J (2022). Abstract P1-19-03: Phase II trial of durvalumab and tremelimumab in the hormone receptor-positive metastatic breast cancer with high tumor mutational burden selected by whole exome sequencing: Korean cancer study group trial (KCSG BR17-04). Cancer Research 82: P1–19–03. https://doi.org/10.1158/1538-7445.SABCS21-P1-19-03 [Google Scholar] [CrossRef]
Morad G, Helmink BA, Sharma P, Wargo JA (2021). Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184: 5309–5337. https://doi.org/10.1016/j.cell.2021.09.020 [Google Scholar] [PubMed] [CrossRef]
Najmeh K, Jamshid H, Afshin N, Roobina B, Morteza H, Mahboubeh A, Nasim K, Mahsa B, Reza M, Seyed Alireza R (2018). Myeloid-derived suppressor cells elimination by 5-fluorouracil increased dendritic cell-based vaccine function and improved immunity in tumor mice. Iranian Journal of Allergy, Asthma, and Immunology 17: 47–55. https://pubmed.ncbi.nlm.nih.gov/29512369 [Google Scholar]
Nangia CS, Kistler M, Sender LS, Lee JH, Jones FR, Jafari O, Soon-Shiong P (2019). Innate and adaptive immunotherapy: An orchestration of immunogenic cell death by overcoming immune suppression and activating NK and T-cell therapy in patients with third line or greater TNBC. Journal of Clinical Oncology 37: 12566–12569. https://doi.org/10.1200/JCO.2019.37.15_suppl.e12566 [Google Scholar] [CrossRef]
Page DB, Beal K, Linch SN, Spinelli KJ, Rodine M et al. (2022). Brain radiotherapy, tremelimumab-mediated CTLA-4-directed blockade +/− trastuzumab in patients with breast cancer brain metastases. NPJ Breast Cancer 8: 50–57. https://doi.org/10.1038/s41523-022-00404-2 [Google Scholar] [PubMed] [CrossRef]
Popa F, Georgescu AV (2017). Abdominal wall reconstruction after flap surgery and the effect on the immune system. BioMed Research International 2017: 2421585. https://doi.org/10.1155/2017/2421585 [Google Scholar] [PubMed] [CrossRef]
Pérez-García J, Soberino J, Racca F, Gion M, Stradella A, Cortés J (2020). Atezolizumab in the treatment of metastatic triple-negative breast cancer. Expert Opinion on Biological Therapy 20: 981–989. https://doi.org/10.1080/14712598.2020.1769063 [Google Scholar] [PubMed] [CrossRef]
Riggio AI, Varley KE, Welm AL (2021). The lingering mysteries of metastatic recurrence in breast cancer. British Journal of Cancer 124: 13–26. https://doi.org/10.1038/s41416-020-01161-4 [Google Scholar] [PubMed] [CrossRef]
Roskoski R (2019). Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacological Research 139: 471–488. https://doi.org/10.1016/j.phrs.2018.11.035 [Google Scholar] [PubMed] [CrossRef]
Rudqvist NP, Pilones KA, Lhuillier C, Wennerberg E, Sidhom JW et al. (2018). Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T cells. Cancer Immunology Research 6: 139–150. https://doi.org/10.1158/2326-6066.CIR-17-0134 [Google Scholar] [PubMed] [CrossRef]
Rugo HS, Kabos P, Beck JT, Jerusalem G, Wildiers H et al. (2022). Abemaciclib in combination with pembrolizumab for HR+, HER2− metastatic breast cancer: Phase 1b study. NPJ Breast Cancer 8: 118. https://doi.org/10.1038/s41523-022-00482-2 [Google Scholar] [PubMed] [CrossRef]
Santa-Maria CA, Kato T, Park JH, Flaum LE, Jain S, Tellez C, Stein RM (2017). Durvalumab and tremelimumab in metastatic breast cancer (MBCImmunotherapy and immunopharmacogenomic dynamics. Journal of Clinical Oncology 35: 3052. https://doi.org/10.1200/JCO.2017.35.15_suppl.3052 [Google Scholar] [CrossRef]
Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, Loi S (2016). Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nature Reviews Clinical Oncology 13: 228–241. https://doi.org/10.1038/nrclinonc.2015.215 [Google Scholar] [PubMed] [CrossRef]
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH et al. (2018). Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. The New England Journal of Medicine 379: 2108–2121. https://doi.org/10.1056/NEJMoa1809615 [Google Scholar] [PubMed] [CrossRef]
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S et al. (2020a). Pembrolizumab for early triple-negative breast cancer. The New England Journal of Medicine 382: 810–821. https://doi.org/10.1056/NEJMoa1910549 [Google Scholar] [PubMed] [CrossRef]
Schmid P, Salgado R, Park YH, Muñoz-Couselo E, Kim SB et al. (2020b). Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Annals of Oncology 31: 569–581. https://doi.org/10.1016/j.annonc.2020.01.072 [Google Scholar] [PubMed] [CrossRef]
Shen JF, Zhao W, Ju ZL, Wang LL, Peng Y et al. (2019). PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Research 79: 311–319. https://doi.org/10.1158/0008-5472.CAN-18-1003 [Google Scholar] [PubMed] [CrossRef]
Shimasaki N, Jain A, Campana D (2020). NK cells for cancer immunotherapy. Nature Reviews Drug Discovery 19: 200–218. https://doi.org/10.1038/s41573-019-0052-1 [Google Scholar] [PubMed] [CrossRef]
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022). Cancer statistics, 2022. CA: A Cancer Journal for Clinicians 72: 7–33. https://doi.org/10.3322/caac.21708 [Google Scholar] [PubMed] [CrossRef]
Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F (2019). NK cell metabolism and tumor microenvironment. Frontiers in Immunology 10: 2278. https://doi.org/10.3389/fimmu.2019.02278 [Google Scholar] [PubMed] [CrossRef]
Thomas R, Al-Khadairi G, Decock J (2021). Immune checkpoint inhibitors in triple negative breast cancer treatment: promising future prospects. Frontiers in Oncology 10: 600573. https://doi.org/10.3389/fonc.2020.600573 [Google Scholar] [PubMed] [CrossRef]
Tolaney SM, Kalinsky K, Kaklamani VG, D’Adamo DR, Aktan G et al. (2021). Eribulin Plus pembrolizumab in patients with metastatic triple-negative breast cancer (ENHANCE 1A phase Ib/II study. Clinical Cancer Research 27: 3061–3068. https://doi.org/10.1158/1078-0432.CCR-20-4726 [Google Scholar] [PubMed] [CrossRef]
Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R et al. (2020). TBCRC 048: Phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. Journal of Clinical Oncology 38: 4274–4282. https://doi.org/10.1200/JCO.20.02151 [Google Scholar] [PubMed] [CrossRef]
Turner N, Dent RA, O’Shaughnessy J, Kim SB, Isakoff SJ et al. (2022). Ipatasertib plus paclitaxel for PIK3CA/AKT1/PTEN-altered hormone receptor-positive HER2-negative advanced breast cancer: Primary results from cohort B of the IPATunity130 randomized phase 3 trial. Breast Cancer Research and Treatment 191: 565–576. https://doi.org/10.1007/s10549-021-06450-x [Google Scholar] [PubMed] [CrossRef]
Ulas EB, Dickhoff C, Schneiders FL, Senan S, Bahce I (2021). Neoadjuvant immune checkpoint inhibitors in resectable non-small-cell lung cancer: A systematic review. ESMO Open 6: 100244. https://doi.org/10.1016/j.esmoop.2021.100244 [Google Scholar] [PubMed] [CrossRef]
Vinayak S, Tolaney SM, Schwartzberg L, Mita M, McCann G et al. (2019). Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncology 5: 1132–1140. https://doi.org/10.1001/jamaoncol.2019.1029 [Google Scholar] [PubMed] [CrossRef]
Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK et al. (2019). Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nature Medicine 25: 920–928. https://doi.org/10.1038/s41591-019-0432-4 [Google Scholar] [PubMed] [CrossRef]
Wang Q, Gao J, Di W, Wu X (2020). Anti-angiogenesis therapy overcomes the innate resistance to PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models. Cancer Immunology, Immunotherapy CII 69: 1781–1799. https://doi.org/10.1007/s00262-020-02576-x [Google Scholar] [PubMed] [CrossRef]
Wilkinson L, Gathani T (2022). Understanding breast cancer as a global health concern. The British Journal of Radiology 95: 20211033. https://doi.org/10.1259/bjr.20211033 [Google Scholar] [PubMed] [CrossRef]
Wu SY, Xu Y, Chen L, Fan L, Ma XY et al. (2022). Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: Concept exploration and biomarker analysis in the FUTURE-C-Plus trial. Molecular Cancer 21: 84–89. https://doi.org/10.1186/s12943-022-01536-6 [Google Scholar] [PubMed] [CrossRef]
Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J (2020). CAR-NK cells: A promising cellular immunotherapy for cancer. eBioMedicine 59: 102975. https://doi.org/10.1016/j.ebiom.2020.102975 [Google Scholar] [PubMed] [CrossRef]
Yuan Y, Lee JS, Yost SE, Frankel PH, Ruel C et al. (2021). A phase II clinical trial of pembrolizumab and enobosarm in patients with androgen receptor-positive metastatic triple-negative breast cancer. The Oncologist 26: 99–e217. https://doi.org/10.1002/onco.13583 [Google Scholar] [PubMed] [CrossRef]
Zi H, Ay H, Hl M (2017). Combined radiation therapy and immune checkpoint blockade therapy for breast cancer. International Journal of Radiation Oncology, Biology, Physics 99: 153–164. https://doi.org/10.1016/j.ijrobp.2017.05.029 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools