 Open Access
Open Access
ARTICLE
SR-BI expression regulates the gastric cancer tumor immune microenvironment and is associated with poor prognosis
1 The First Clinical Medical College of Zhejiang Chinese Medical University, Hangzhou, 310006, China
2 Department of General Surgery, HwaMei Hospital, University of Chinese Academy of Sciences, Ningbo, 315010, China
3 The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institutes of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, 310022, China
4 Zhejiang Key Lab of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer, Zhejiang Cancer Hospital, Hangzhou, 310022, China
5 Department of Chinese Surgery, Linping District Hospital of Traditional Chinese Medicine, Hangzhou, 311100, China
6 Department of Gastrointestinal Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325099, China
* Corresponding Authors: HUA RUAN. Email: ; JIANCHENG SUN. Email:
# These authors contributed equally to this work
BIOCELL 2023, 47(5), 991-1002. https://doi.org/10.32604/biocell.2023.028587
Received 27 December 2022; Accepted 13 January 2023; Issue published 10 April 2023
Abstract
Aim: Scavenger receptor class B, type I (SR-BI) is an integral plasma membrane protein that has been reported to be overexpressed in various malignancies, such as renal cancer, breast cancer, and prostate cancer, and is an independent prognostic factor. However, the clinical value and expression of SR-BI in GC are unknown. Our research aimed to explore the role of SR-BI in combination with immune markers as a diagnostic and prognostic marker for gastric cancer (GC). Methods: GC tissues, paracancerous tissues, and clinicopathological data of 149 patients were collected. The expression level of SR-BI, Tumor-infiltrating lymphocytes (TILs), and PD-L1 were evaluated by immunohistochemistry (IHC). The associations of the SR-BI staining intensity with clinicopathological features and immune markers were determined by the chi-square test. Univariate and multivariate COX regression analyses were used to evaluate independent prognostic factors. Kaplan–Meier analyses were performed to plot the survival curve. Results: Our results indicated that SR-BI was expressed at higher levels in tumor tissues than in adjacent paracancerous tissues (p < 0.001), and patients with high levels of SR-BI expression had a worse prognosis. Univariate and multivariate analyses revealed that high SR-BI expression was an independent factor for poor prognosis. The chi-square test determined that the expression of SR-BI was negatively correlated with CD4+ T cells and CD8+ T cells (CD4+ T cells, p = 0.013; CD8+ T cells, p = 0.021), and positively correlated with PD-L1 (p = 0.022). Finally, survival analysis revealed that CD4+ T cells were associated with the prognosis of GC patients (p = 0.019), and the combined survival analysis of SR-BI and CD4+ T cells was also statistically significant (p = 0.030). Conclusion: SR-BI is highly expressed in GC tissue and associated with poor prognosis. Moreover, SR-BI can also regulate the GC tumor immune microenvironment.Keywords
Gastric cancer (GC) is one of the most common malignancies worldwide. According to 2020 data, the incidence and mortality rates of GC rank 5th and 4th, respectively, among all cancers. More than 1,000,000 new GC cases and approximately 769,000 GC deaths were estimated to occur globally in 2020 (Sung et al., 2021). Early diagnosis of GC relies primarily on imaging, serum tumor marker profiling, endoscopy, and tissue biopsy. Current methods for the early diagnosis of GC are limited, and most GC patients are thus in advanced stages at the time of diagnosis, resulting in a poor prognosis (Smyth et al., 2020). At present, surgery remains the main method of GC treatment. However, due to the limited means of early screening and diagnosis of GC, approximately 1/3rd of patients miss the opportunity for surgery at the first visit. In addition to surgical treatment, other current commonly used treatment methods, such as radiotherapy, chemotherapy, targeted therapy, and immunotherapy, offer certain benefits for the prognosis of GC patients. However, the toxic side effects and resistance of drugs limit their prolonged use. Therefore, improvements in the early diagnosis of GC and the identification of new therapeutic targets are urgently needed.
Scavenger receptor class B type I (SR-BI) is an integral plasma membrane protein (Ryu et al., 2020) that was first isolated and identified by Calvo and Vega (1993). It mediates the selective uptake and reverse transport of cholesterol and plays an essential function in lipoprotein metabolism (Nakagawa-Toyama et al., 2005; Yang et al., 2016). Numerous previous studies demonstrated that SR-BI is expressed at significantly higher levels in tumor tissues than in paracancerous tissues in solid tumors such as breast cancer, renal cell carcinoma, prostate cancer, and liver cancer (Zhu et al., 2021; Traughber et al., 2020; Yuan et al., 2016; Xu et al., 2017). For instance, a study reported that low expression of SR-BI inhibited the proliferation and invasion of clear cell renal cell carcinoma cells and decreased the expression of Akt pathway components (Xu et al., 2018). Another study confirmed that knockdown of SR-BI expression in the MDAMB-468 and MCF-7 breast cancer cell lines could inhibit tumor cell proliferation and promote tumor cell apoptosis (Karimi and Karami Tehrani, 2021). Furthermore, the anti-inflammatory activity of SR-BI could promote tumor growth while the proinflammatory activity of SR-B1 could enhance antitumor immune responses (Zhu et al., 2009). Thus, SR-BI is identified as pro-oncogenic, highly expressed in a variety of tumors, and associated with the tumor immune response. However, its expression and clinical significance in GC are still unclear and deserve further investigation.
Tumor-infiltrating lymphocytes (TILs), including CD3+ T cells, CD4+ T cells, and CD8+ T cells, represent the host’s immunological response to cancer cells. Several studies have reported that they may play differential roles in different types of tumors (Liu et al., 2022; Uppal et al., 2020). Programmed Cell Death Ligand 1 (PD-L1) is a cancer cell surface marker that, when overexpressed, avoids T-cell-related targeting and leads to the evasion of GC by the immune system surveillance. According to a recent analysis, approximately 40% of patients with gastric tumors that express PD-L1 have a lower rate of metastasis but worse overall survival (OS) outcomes, and immunotherapy may be a better treatment option for this population (Sexton et al., 2020). Clinical trials have used multiple PD-L1 immunohistochemical assays to assess the use of PD-L1 expression on tumor cells, immune cells, or both as a biomarker for predicting immunotherapeutic responses (Paver et al., 2021). In a study on whether TILs can predict clinical prognosis in GC, CD3+ T cells and CD8+ T cells remained independent prognostic factors in multivariate survival analysis (Lee et al., 2008). Therefore, in this study, immunohistochemical staining of CD3+ T cells, CD4+ T cells, CD8+ T cells, and PD-L1 in tissue specimens from 149 patients with GC who underwent radical surgical resection was performed to quantify the numbers of infiltrating cells in the tumors and to explore the role of SR-BI in the GC tumor immune microenvironment.
To elaborate, this study aimed to explore the differential expression of SR-BI through tissue microarray analyses of 149 GC patients and to analyze the correlations of both TILs (CD3+ T cells, CD4+ T cells, and CD8+ T cells) and PD-L1 to further explore the diagnostic and prognostic value of SR-BI in GC and its role in the GC immune microenvironment. This study found that SR-BI is an oncogenic driver that can predict patient survival time in our dataset and regulate the tumor immune microenvironment in GC. SR-BI may be important for guiding immunotherapy in GC patients.
One hundred forty-nine patients with GC who underwent surgical resection between January 2013 and December 2017 in the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) were recruited for this study. The patient inclusion criteria were as follows: (I) pathological diagnosis of GC; (II) complete clinical data; (III) did not receive anticancer therapy (radiotherapy, chemotherapy, or biotherapy) before surgery; and (IV) complete survival follow-up data. The patient exclusion criteria were as follows: (I) occurrence of other types of malignant tumors within 5 years and (II) metastases from other tumor types. Survival information was obtained by telephone follow-up and medical records. OS was defined as the duration from surgery to death or last follow-up.
The tumor tissues and paracancerous tissues were surgically resected, fixed in formalin, and paraffin-embedded. Two pathologists independently screened and selected representative tumor tissues and adjacent paracancerous tissues to construct tissue chips. The slices were dewaxed, rinsed three times with PBS (biosharp, Guangzhou, China)for 5 min each, rinsed twice with distilled water, and then subjected to antigen retrieval. Then, the slices were incubated overnight with the primary antibodies (SR-BI (21277-1-AP, Proteintech, Chicago, IL, USA); CD3 (ab16669, Abcam, Cambridge, MA, USA); CD4 (ab133616, Abcam, Cambridge, MA, USA); CD8 (ab17147, Abcam, Cambridge, MA, USA); PD-L1 (SK006, DAKO, Glostrup, Denmark)) at 4°C and washed with PBS 3 times for 5 min each. Then, goat anti-rabbit IgG H&L (Biotin) (1:1000, Abcam, Cambridge, MA, USA)/goat anti-mouse IgG H&L (Biotin) (1:500, Abcam, Cambridge, MA, USA) was added to the tissue chip for 30 min, and the chip was then washed with PBS thrice for 5 min for each wash. Then, nuclei were counterstained with a DAB chromogenic and hematoxylin kit (ZSGB-BIO Corp., Shanghai, China), after which the tissue chip was dehydrated and sealed with neutral gel.
Interpretation of staining results
The immunohistochemical staining results were interpreted by two experienced physicians from the pathology department. The formula for the H-score was as follows: H-score = ∑ (IS × AP), where IS represents the staining intensity and AP represents the percentage of positively stained tumor cells, producing a membrane score ranging between 0 and 12. The intensity of tumor cell staining was determined based on an IS value between 0 and 3 (0, no staining; 1, weak staining; 2, intermediate staining; 3, strong staining). The AP value depended on the percentage of positively stained cells as follows: 0 (0%), 1 (1%–25%), 2 (26%–50%), 3 (51%–75%), and 4 (76%–100%). The median H-score (H-score = 6) was set as the cutoff value, and SR-BI expression was divided into high and low groups. The expression of PD-L1 was expressed by the combined positive score (CPS) as follows: CPS = [PD-L1-positive cells ((tumor cells, lymphocytes, macrophages)/total tumor cells)] * 100. Among them, CPS ≥ 10 was regarded as positive (Schoemig-Markiefka et al., 2021). Pathologists assessed TILs by observing and recording the number of all corresponding lymphocytes in the entire magnification field of view. The median was used as the cutoff value to stratify patients into high and low-expression groups.
Statistical Analysis SPSS 25.0 software was used to perform statistical analyses of the collected results. Continuous variables were summarized as medians and quartiles, while categorical variables were presented as counts and percentages. Differences in SR-BI expression and clinicopathological features, TILs, and PD-L1 expression were analyzed using the chi-square test or Fisher’s exact test. Survival curves were drawn by the Kaplan–Meier method, and effects were determined by univariate and multivariate COX regression analyses. Prognostic factors in GC patients as well as hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were calculated simultaneously. A p-value < 0.05 indicated a statistically significant difference.
General clinicopathological data
The median age of the study population was 62 years; 106 (71.1%) males and 43 (28.9%) females were included. The distribution of tumor sites was analyzed, revealing 93 cases (62.4%) of distal GC, 46 cases (30.9%) of proximal GC, and only 10 cases (6.7%) of total GC. Among the 149 patients, 85.9% were classified as TNM stage III-IV, and 21 cases (14.1%) were classified as stage I-II. All GC patients were followed up until August 2021, during which 81 patients died. More general clinicopathological data are shown in Table 1.
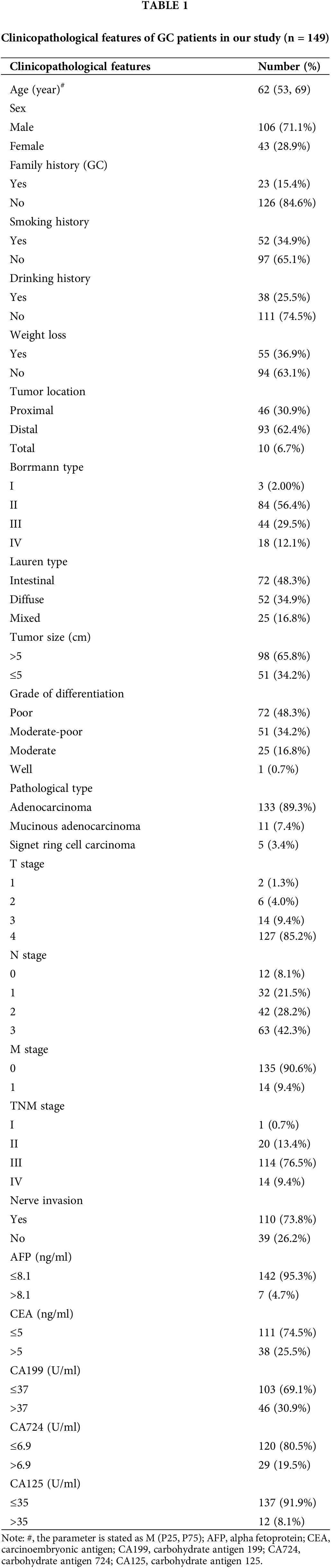
High expression of SR-BI in GC tissues
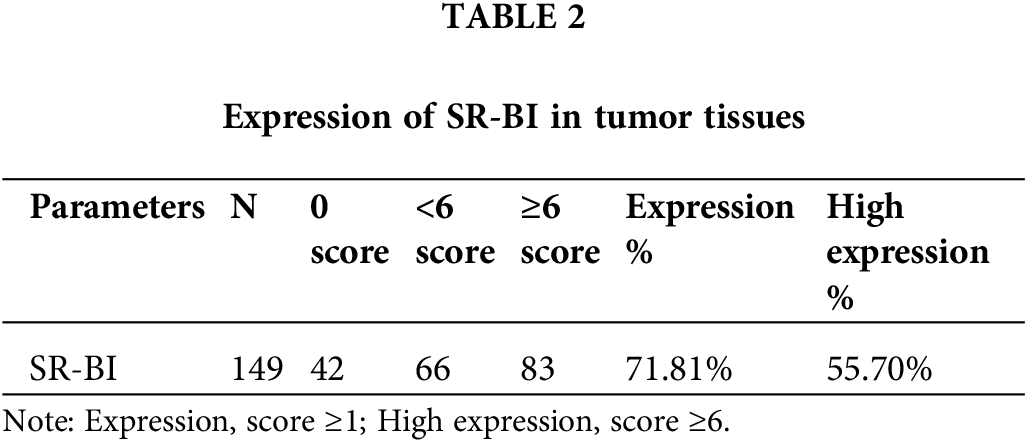
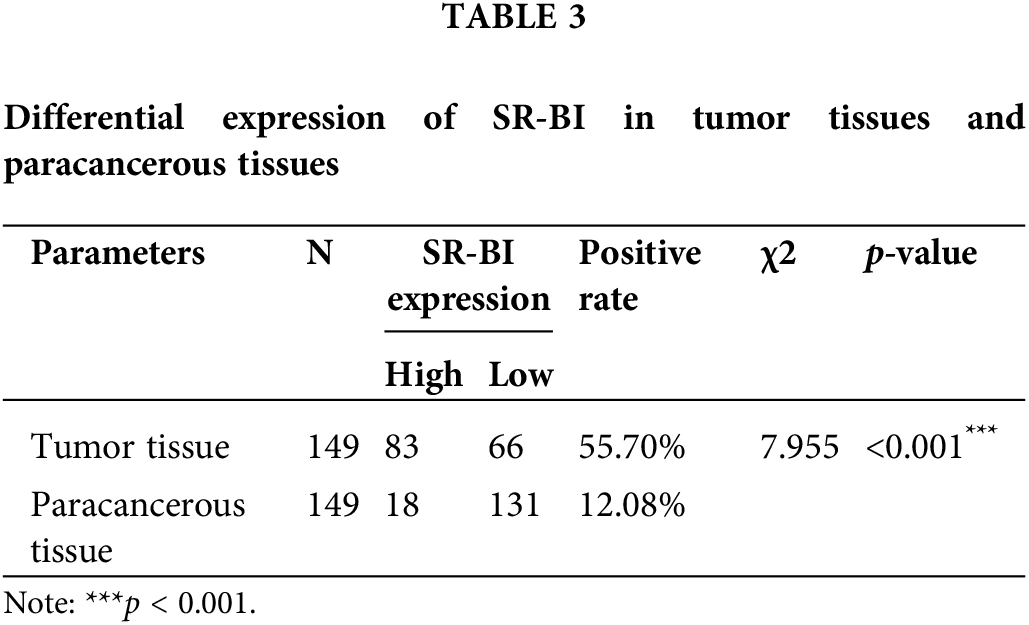
As shown in Fig. 1A, SR-BI was mainly expressed on the cell membrane. In tumor tissues, 107 of the 149 samples (71.81%) exhibited SR-BI expression. According to the H-score formula, 3 cases had 2 points, 7 cases obtained 3 points, 14 cases got 4 points, 19 cases had 6 points, 62 cases obtained 8 points, and 2 had 12 points. According to the median SR-BI expression score of 6 points, an H-score = 6 points was set as the cutoff value. A score <6 points was used to define the low SR-BI expression group and a score ≥6 points was used to define the high-expression group. The specific expression levels of SR-BI in tumor tissues are shown in Table 2. Moreover, we found that SR-BI had a certain expression pattern in paracancerous tissues, among which 131 samples had a low expression, and only 18 samples had a high expression. SR-BI expression was significantly different in tumor tissues (55.7% vs. 12.08% p < 0.001). The specific distribution is shown in Table 3 and Fig. 1B.
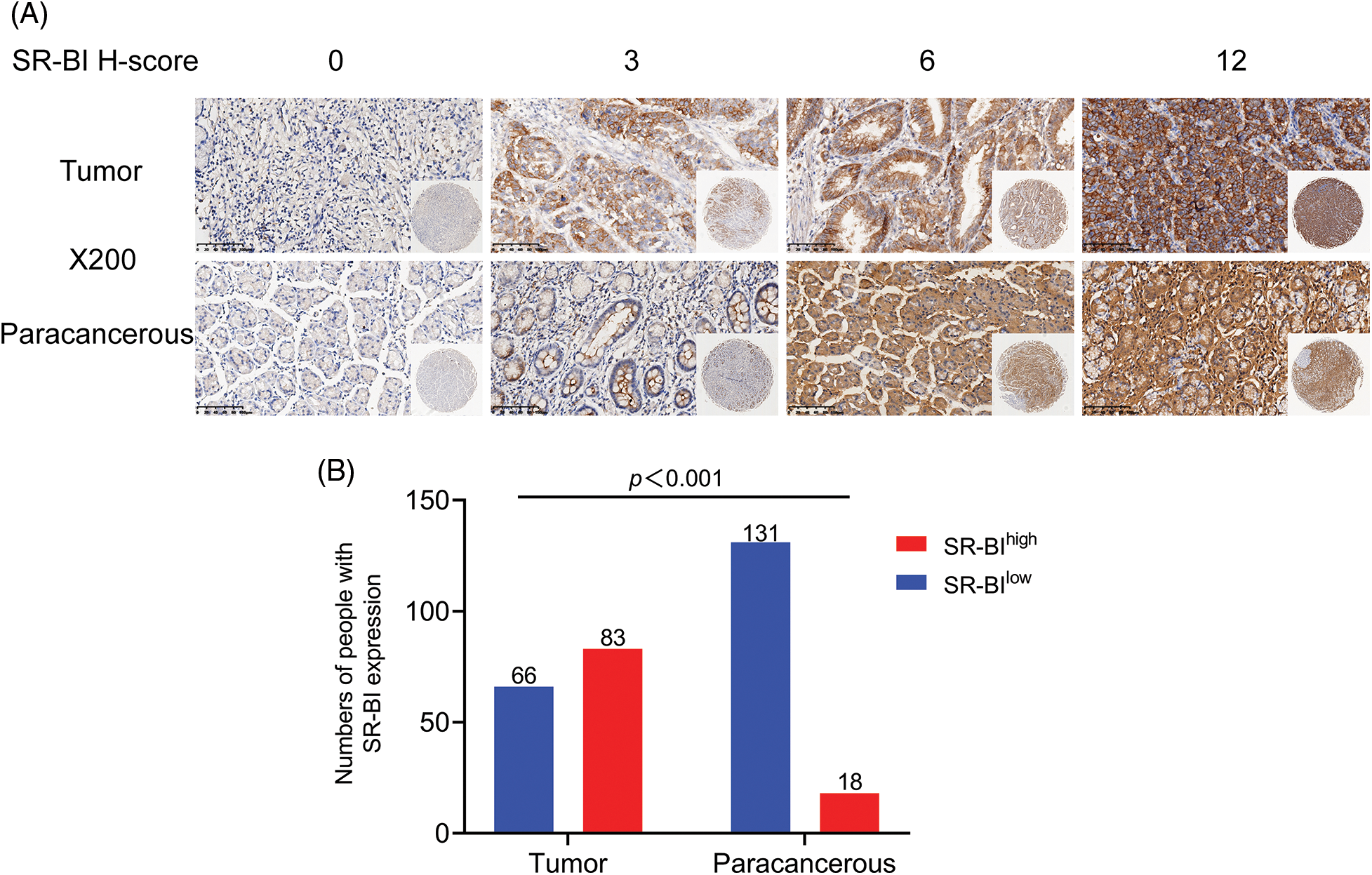
Figure 1: SR-BI is highly expressed in GC tissues. Immunohistochemical staining of SR-BI in tumor tissues and paracancerous tissues (scale bars: ×200 and 1:20 μm) (A). Differential expression of SR-BI in tumor tissues and paracancerous tissues (B).
Relationships between SR-BI expression and clinicopathological features
The correlations between SR-BI expression and clinicopathological characteristics in GC were statistically analyzed using the chi-square test or Fisher’s exact test, as shown in Table 4. Only the carcinoembryonic antigen (CEA) (p = 0.027) levels were found to differ between the two groups, while the other parameters (such as age, sex, smoking history, drinking history, family history, TNM stage, Lauren type, Borrmann type, tumor size, and tumor location) did not vary between the groups. These results indicated that the expression of SR-BI was associated with the CEA level but not with the patient’s age, sex, smoking history, drinking history, family history, TNM stage, Lauren type, Borrmann type, tumor size, or tumor location, among other parameters.
SR-BI expression is negatively correlated with CD4+ T cells and CD8+ T cells and positively correlated with PD-L1
The numbers of TILs (CD3+ T cells, CD4+ T cells, CD8+ T cells) and PD-L1 expression in tumor tissues were determined by IHC staining (Fig. 2). The median number of TILs was set as the cutoff value for division into high and low groups. The chi-square test was used to compare the correlations between the above indicators and the expression of SR-BI. As shown in Fig. 3 and Table 5, the expression of SR-BI was negatively correlated with the CD4+ and CD8+ T cell numbers (p = 0.013, p = 0.021) but not with the number of CD3+ T cells (p = 0.052). The expression of SR-BI was positively correlated with PD-L1, and the PD-L1 positivity rate was higher when SR-BI was highly expressed; the difference was statistically significant (p = 0.022).
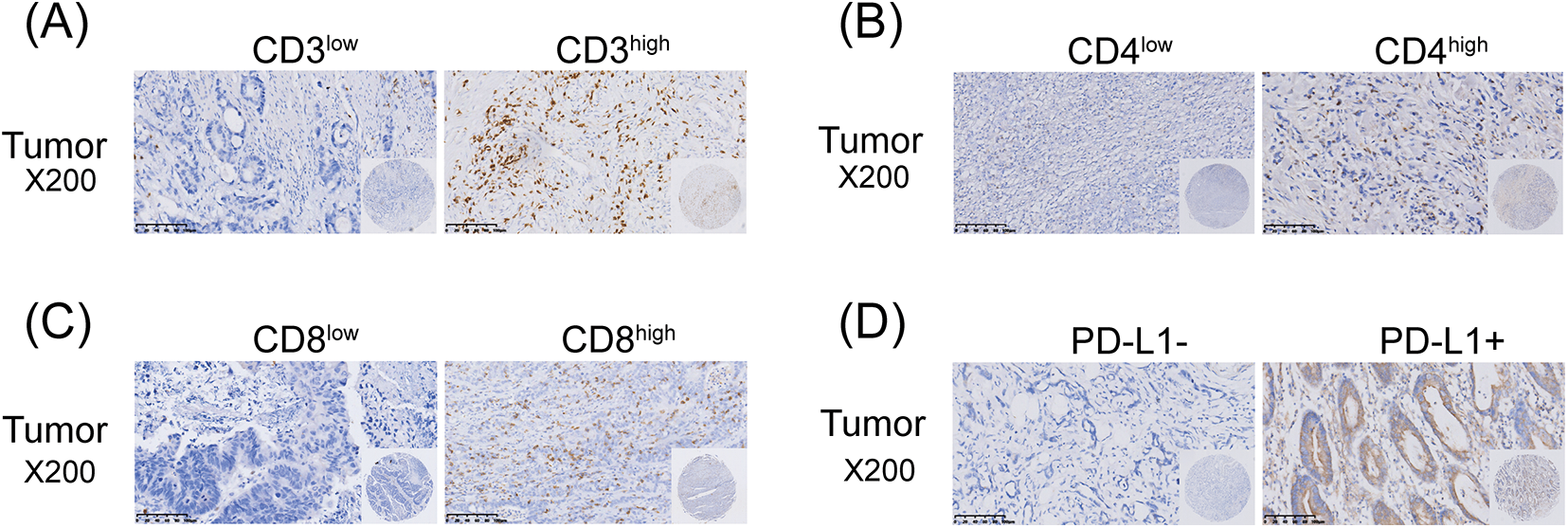
Figure 2: Immunohistochemical staining of TILs and PD-L1 in GC tissues (scale bars: ×200 and 1:20 μm). The Immunohistochemical staining of CD3+ T cells (A); The Immunohistochemical staining of CD4+ T cells (B); The Immunohistochemical staining of CD8+ T cells (C); The Immunohistochemical staining of PD-L1 (D).
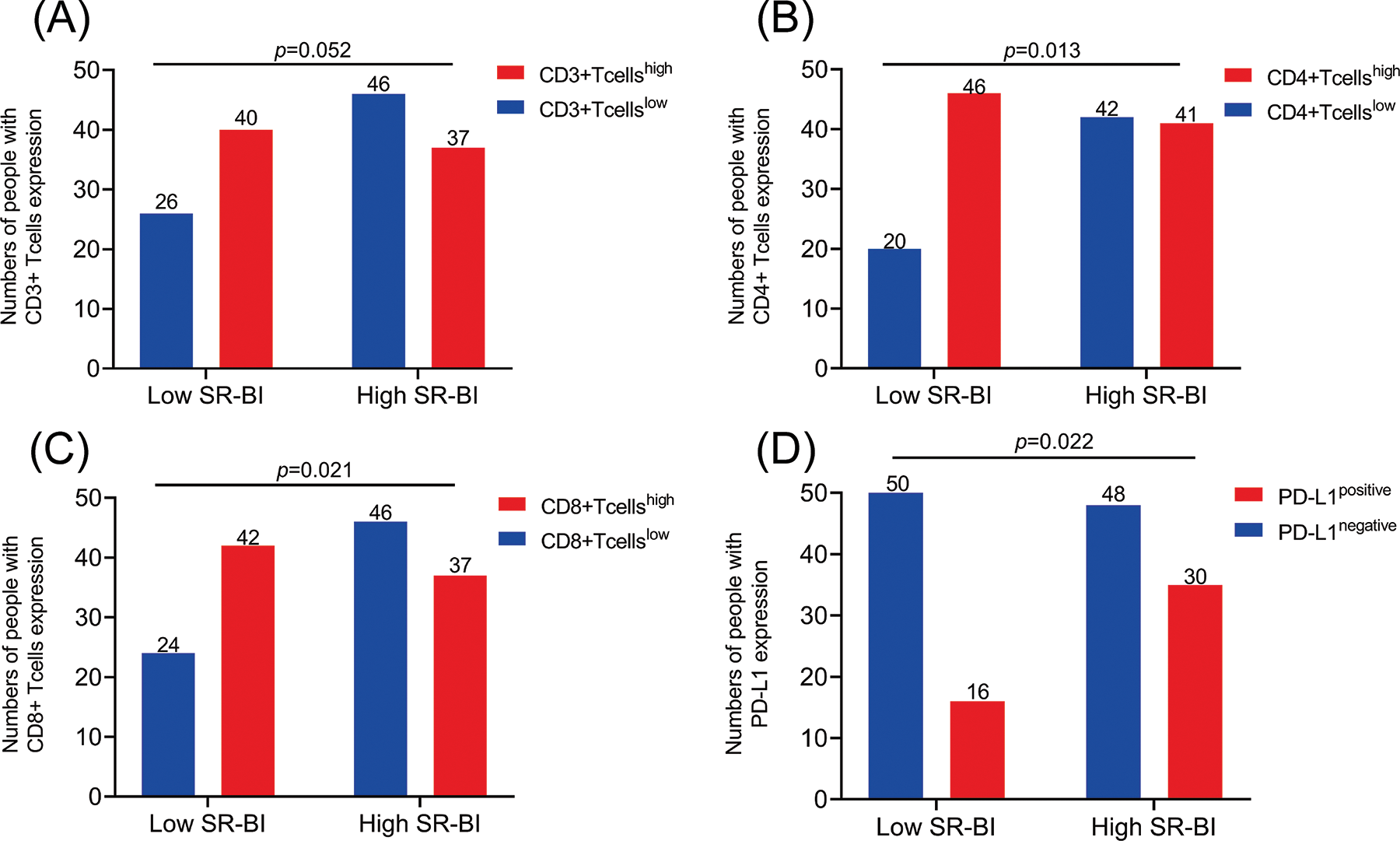
Figure 3: Correlations between SR-BI Expression and the levels of TILs and PD-L1 in GC. The expression of SR-BI was not correlated with the numbers of CD3 T+ cells (A); The expression of SR-BI was negatively correlated with CD4 T+ cells (B); The expression of SR-BI was negatively correlated with CD8 T+ cells (C); The expression of SR-BI was positively correlated with PD-L1 (D).
SR-BI and CD4+ T cells are key prognostic factors in GC
As shown in Fig. 4A, Kaplan–Meier survival curves indicated that compared with that of patients in the low expression group, the prognosis of patients with high expression of SR-BI was worse (p = 0.021). The 3-year OS of patients in the low SR-BI expression group was 68.1%, while that of patients in the high SR-BI expression group was only 49.2%. Similarly, Kaplan–Meier survival analysis was performed to compare the OS rates according to the levels of CD3+ T cells, CD4+ T cells, CD8+ T cells, and PD-L1 (Figs. 4B–4E). The results suggested that only the number of CD4+ T cells affected the prognosis of patients (p = 0.019). The 3-year OS of the group with high CD4+ T cell numbers was 66.6%, while it was only 48.3% in the group with low CD4+ T cell numbers. Finally, SR-BI and CD4+ T cells were combined to generate a survival curve and all patients (n = 149) were divided into four groups according to their expression. As shown in Fig. 4F, the group with the high expression of SR-BI combined with low CD4+ T cell numbers had the worst prognosis while the group with low expression of SR-BI combined with high CD4+ T cell numbers had the best prognosis. The differences in OS among the four groups were statistically significant (p = 0.030).
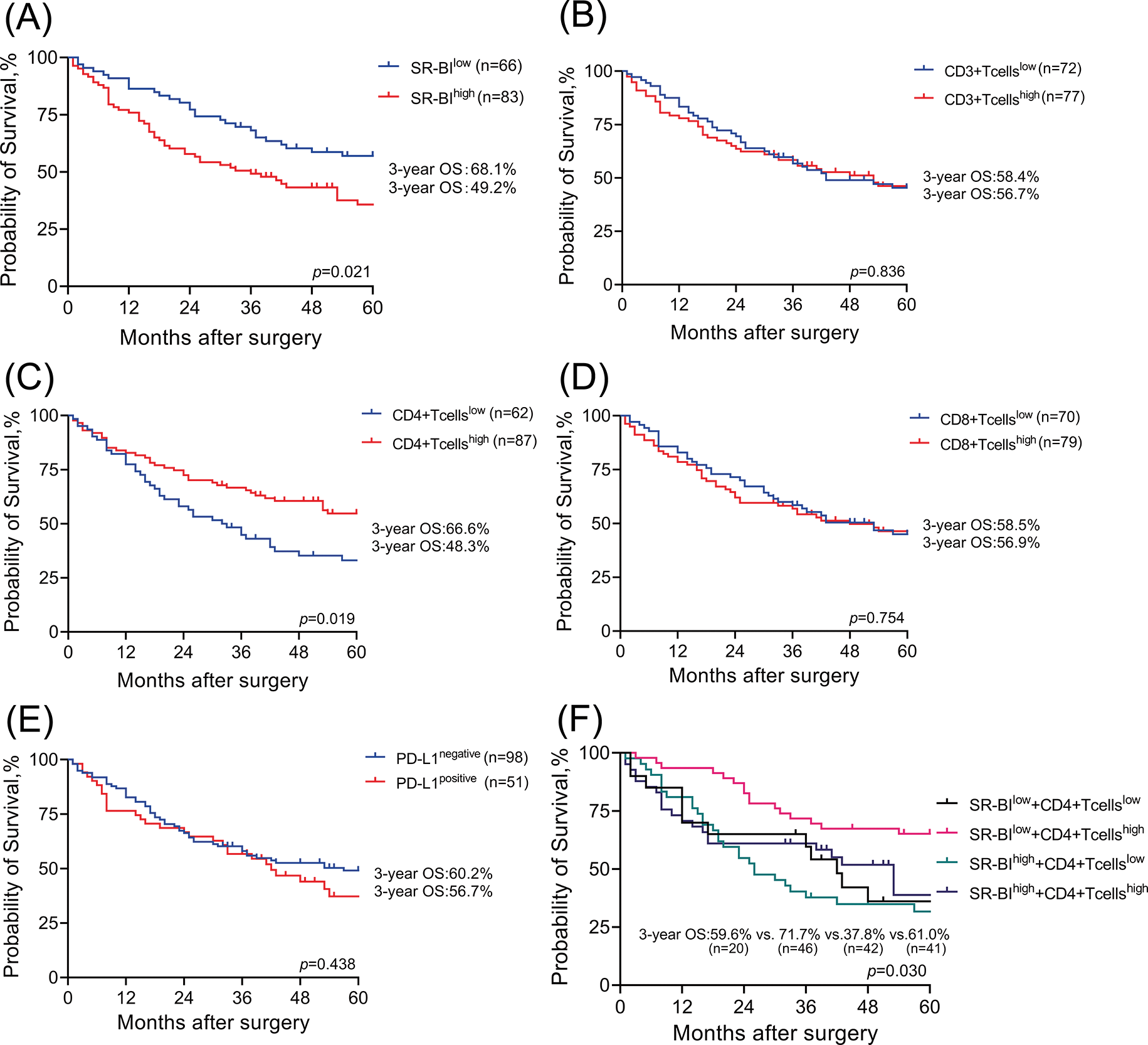
Figure 4: Survival analysis of SR-BI and TILs. Kaplan–Meier OS analyses of SR-BI in tumor tissues (A); The 3-year OS is 68.1% and 49.2%. Kaplan–Meier OS analyses of CD3 T+ cells in tumor tissues (B); The 3-year OS is 58.4% and 56.7%. Kaplan–Meier OS analyses of CD4 T+ cells in tumor tissues (C); The 3-year OS is 66.6% and 48.3%. Kaplan–Meier OS analyses of CD8 T+ cells in tumor tissues (D); The 3-year OS is 58.5% and 56.9%. Kaplan–Meier OS analyses of PD-L1 in tumor tissues (E); The 3-year OS is 60.2% and 56.7%. Kaplan–Meier OS analyses of SR-BI combined with CD4+ T cells in tumor tissue (F); The 3-year OS is 59.6%, 71.7%, 37.8% and 61.0%.
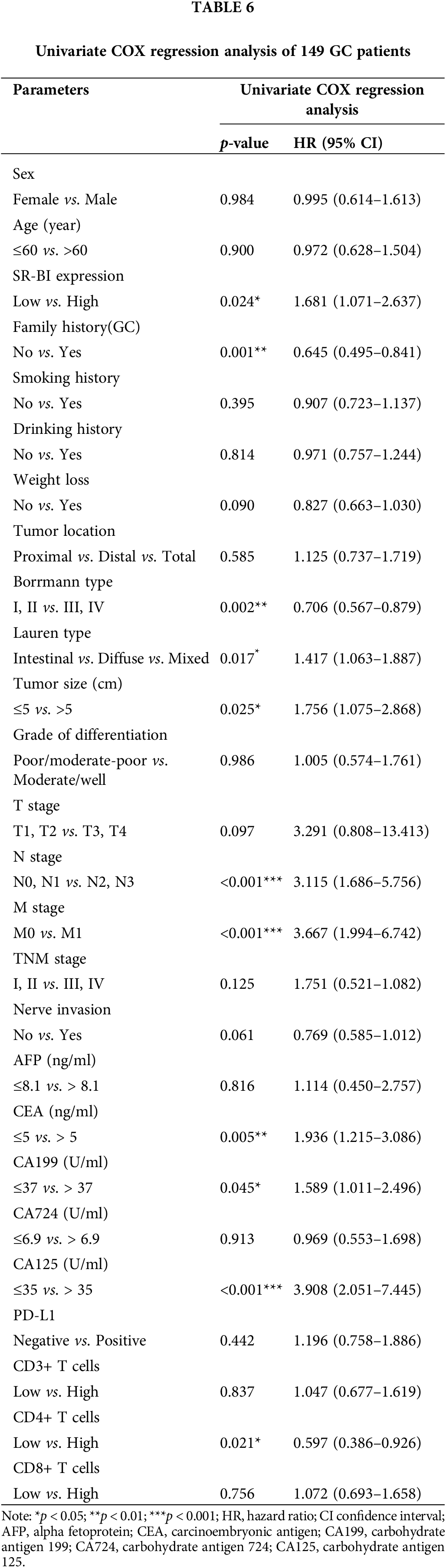
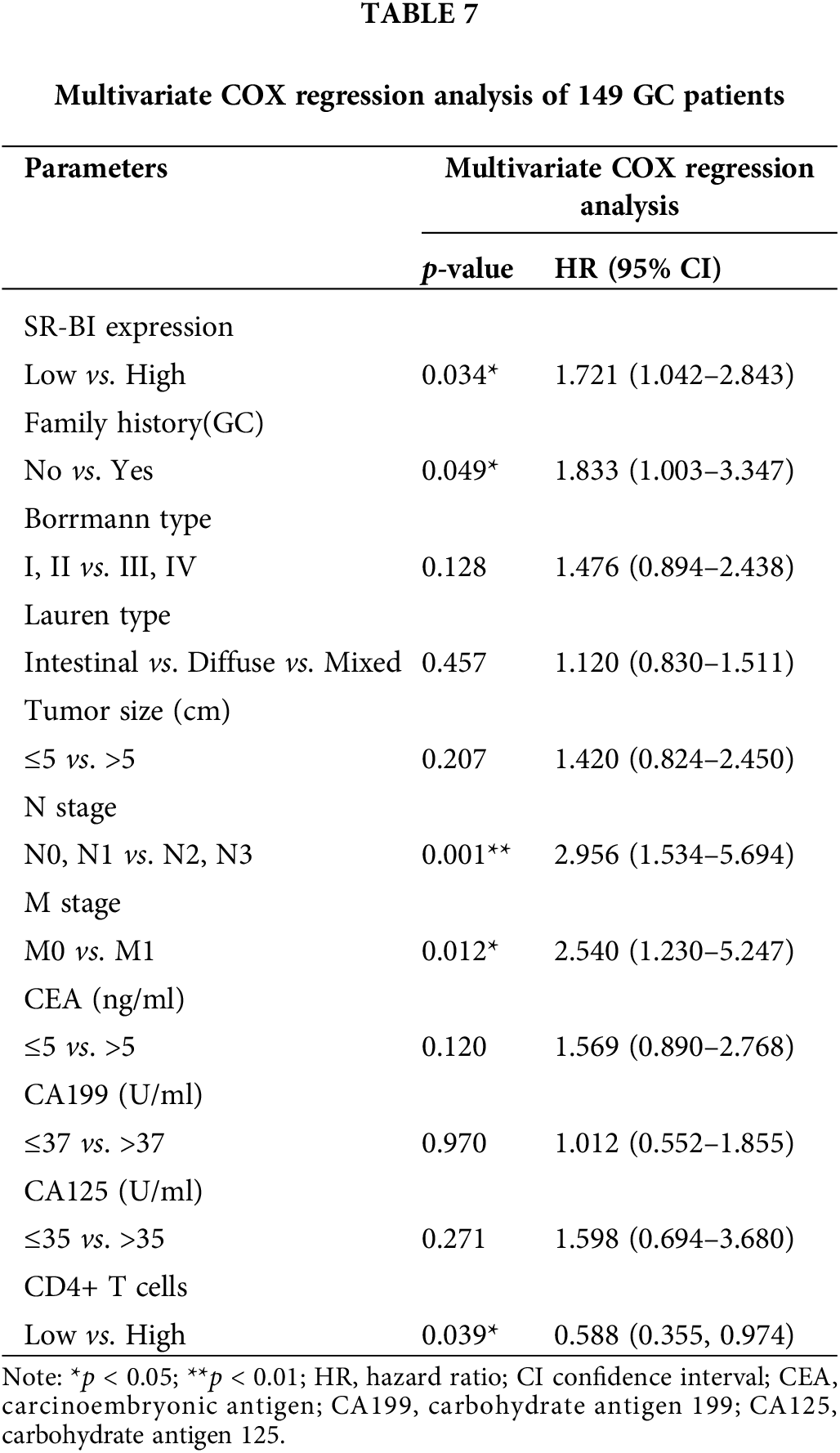
Furthermore, the general data, pathological data, and immune-related indicators were included in the univariate COX regression analysis (Table 6). The results suggested that the SR-BI expression level (p = 0.024), number of CD4+ T cells (p = 0.021), GC family history (p = 0.001), Borrmann type (p = 0.002), tumor size (p = 0.025), N stage (p < 0.001), M stage (p < 0.001), CEA level (p = 0.005), CA199 level (p = 0.045), CA125 level (p < 0.001) and Lauren type (p = 0.017) are prognostic factors for OS. Subsequently, according to the results of univariate analysis, we selected the indicators with p < 0.05 and included them in the multivariate COX regression analysis (summarized in Table 7). A high expression of SR-BI (p = 0.034), a family history of GC (p = 0.049), a high N stage (p = 0.001), the M1 stage (p = 0.012), and low CD4+ T cell numbers (p = 0.039) were independent factors for poor GC prognosis. Thus, both the univariate and multivariate analyses indicated that SR-BI expression and CD4+ T cell numbers were key prognostic factors for GC patients.
The development of molecular biology and genomic techniques has gradually provided new opportunities to elucidate the mechanisms underlying tumorigenesis and development. It is now possible to prevent, diagnose and treat malignancies through the discovery and intervention of new molecular targets. GC, especially advanced GC, has limited treatment strategies and a dismal prognosis. Since early gastroscopic screening in China is not as popular as in Japan, South Korea, or Western countries, most patients are already in advanced stages when they consult a doctor and have thus missed the best treatment opportunity. The current conventional treatment strategy for GC is surgery and perioperative chemotherapy (Smyth et al., 2020; Thrift and El-Serag, 2020). In recent years, immunotherapy has also shown good therapeutic value in GC in various studies (Xie et al., 2021).
SR-BI is a multiligand membrane receptor, and an increasing number of studies have confirmed that it is highly expressed in solid tumors, such as breast cancer (Li et al., 2016) and lung cancer (Feng et al., 2018), and is associated with poor prognosis. In addition, SR-BI is involved in tumor proliferation, invasion, angiogenesis, and signal regulation. Danilo et al. (2013) found that high-density lipoprotein (HDL) and SR-BI in combination could activate the PI3K/Akt and MAPK signaling pathways. This PI3K/Akt activation is an important signaling mechanism for tumor angiogenesis (Xue et al., 2021). Furthermore, another study confirmed that SR-BI was upregulated in hepatocellular carcinoma that enhanced the proliferation and invasion of hepatocellular carcinoma cells through miR-497 (Zhu et al., 2021). In this context, we designed this study to investigate the differential expression of SR-BI in GC and its correlation with the tumor microenvironment.
In this study, the expression of SR-BI in GC and paracancerous tissues was assessed by IHC, revealing a higher SR-BI expression in GC tissues compared with paracancerous tissues. Among the 149 cases of GC, 83 had high SR-BI expression (55.7%) as opposed to only 18 (12.08%) that had high SR-BI expression in the corresponding adjacent tissue. The differential expression of SR-BI in GC was consistent with breast cancer and prostate cancer (Li et al., 2016; Schorghofer et al., 2015), which both showed high expression in tumor tissues, suggesting that SR-BI may be a cancer driver gene. Further, survival analysis showed that the 3-year OS of patients with high SR-BI expression was significantly lower than that of patients with low SR-BI expression. Both univariate and multivariate analyses indicated that high expression of SR-BI was an independent factor for poor prognosis in GC patients. The resulting association of SR-BI expression with poor prognosis is even more consistent with that in other cancers and is even more indicative of the possible involvement of SR-BI in the development of cancer, leading to a poor prognosis.
Previous studies have shown that members of the scavenger receptor (SR) family can control the activation of homeostatic immune responses and function as coreceptors in initiating effector immune responses when macromolecules are exposed to threats that may disrupt host homeostasis (Yu et al., 2015). Shetty et al. (2011) showed that Stablin-1 (a member of the SR class H family) in hepatic sinusoidal endothelial cells could preferentially promote CD4+FoxP3+ regulatory T cells (Tregs) in the liver tissue of patients with hepatocellular carcinoma to recruit immune effectors, and thereby inactivate immune effector functions and promote tumor escape. Another study found that the lentiviral vector-mediated siRNA-mediated knockdown of SRA/CD204 enhanced the ability of wild-type dendritic cells (DCs) to stimulate the expansion and activation of ideal or established melanoma antigen-specific CD8+ T cells. SRA/CD204-silenced DCs were used to produce antigen-targeted vaccines, and the antitumor immune activity against tumor formation and metastasis was significantly improved (Yi et al., 2011). In addition, SR-BI-mediated signaling could induce the release of potent proinflammatory cytokines and chemokines, such as TNF-α, IL-1β, and IL-8, which activate monocytes, neutrophils, and T cells, thereby contributing to fine-tuning of the immune response (Vasquez et al., 2017; Zheng et al., 2014).
Despite the close correlation between the SR family and the immune microenvironment, SR-BI has received little attention in this context. Thus, this study further explored the correlation between SR-BI and the immune microenvironment. Current immunotherapeutic approaches are aimed at stimulating or inhibiting lymphocyte activity based on observed lymphocyte–tumor interactions. This confirms that TILs and the expression of PD-L1 and receptors such as HER2, ER, and PR affect the responses of patients to immunotherapy (Nelson et al., 2021). In this study, we evaluated the levels of CD8+ T cells, CD3+ T cells, CD4+ T cells, and PD-L1 in GC tumor tissue by immunohistochemistry and found that the PD-L1 positivity rate was higher when SR-BI was highly expressed. At the same time, the numbers of CD4+ T cells and CD8+ T cells decreased significantly when SR-BI was highly expressed. Based on this, we speculate that the expression of SR-BI impacts the GC immune microenvironment. The increase in the expression of SR-BI may inhibit the numbers of CD8+ T cells and CD4+ T cells and increase the expression of PD-L1, which would ultimately affect a patient’s response to immunotherapy.
Wang et al. (2018) evaluated the levels of PD-L1 and T-cell infiltration in advanced GC and found that PD-L1 expression and increased CD8+ T-cell density were associated with improved prognosis. Further, multivariate COX regression analysis showed that CD8+ T cells were independent predictors of OS. In addition, a meta-analysis found that high levels of CD8+ T, CD3+ T, and CD4+ T-cell infiltration in tumor tissue were correlated with a better OS for GC patients (Zhang et al., 2020). However, the prognostic value of PD-L1 and GC is still controversial, with some studies documenting that PD-L1 expression is associated with good prognosis while others report that PD-L1 expression is associated with poor prognosis or has no correlation at all with prognosis (Qiu and Du, 2021; Pereira et al., 2021; Fang et al., 2017; Gu et al., 2017). Therefore, this study investigated the prognostic significance of PD-L1 and TILs. Kaplan–Meier analysis was used to plot the survival curves of CD3+ T cells, CD4+ T cells, CD8+ T cells, and PD-L1 expression. Patients with high CD4+ T cell numbers had a better prognosis, while the numbers of CD3+ T cells and CD8+ T cells and the level of PD-L1 had no effect on prognosis. Finally, we analyzed the survival curve of patients generated by SR-BI and CD4+ T cell numbers in combination and found that patients with low SR-BI expression combined with high CD4+ T cell numbers had the best prognosis, while those with high SR-BI expression combined with low CD4+ T cell numbers had the worst prognosis. This result indicated that the co-interaction of SR-BI and CD4+ T cells may have some influence on the prognosis of GC. While this further confirms that SR-BI may be involved in the regulation of GC tumor microenvironment, the specific mechanism still needs further study.
Nevertheless, this study has some limitations. Firstly, the sample size was not sufficiently large, and all GC patients were from one medical center. The sample size can be further expanded in the future. Secondly, this study analyzed only the expression differences in SR-BI in tumor tissues and the correlations with prognosis at a superficial level and did not involve in-depth mechanistic research. Subsequently, cell line and animal experiments must be designed to explore the mechanism underlying the impact of SR-BI on tumorigenesis and the specific role of SR-BI in the immune microenvironment.
SR-BI is highly expressed in GC tissue and is associated with poor prognosis. Moreover, SR-BI can regulate the GC tumor immune microenvironment and has the potential as a diagnostic, immunotherapeutic, and prognostic marker in GC.
Funding Statement: This study was supported by the Natural Science Foundation of Zhejiang Province (HDMY22H160008), the Medical Science and Technology Project of Zhejiang Province (2022KY114), and the National Natural Science Foundation of China (82204828).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: LY, HR and JCS; data collection: PCY, ZHB, YHX, CH, and RLZ; analysis and interpretation of results: YW and SQC; draft manuscript preparation: YW and SQC All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Ethics Approval: The study was approved by the research ethics committee of the Cancer Hospital of Zhejiang Cancer Hospital (IRB-2021-431). Informed consent was obtained from the subjects for the tissue samples and clinical information involved in the study.
Conflicts of Interest:: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Calvo D, Vega MA (1993). Identification, primary structure, and distribution of CLA-1, a novel member of the CD36/LIMPII gene family. The Journal of Biological Chemistry 268: 18929–18935. https://doi.org/10.1016/S0021-9258(17)46716-0 [Google Scholar] [CrossRef]
Danilo C, Gutierrez-Pajares JL, Mainieri MA, Mercier I, Lisanti MP, Frank PG (2013). Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Research 15: R87. https://doi.org/10.1186/bcr3483 [Google Scholar] [PubMed] [CrossRef]
Fang W, Chen Y, Sheng J, Zhou T, Zhang Y, Zhan J, Liu L, Huang J, Peng P, Zhang L (2017). Association between PD-L1 expression on tumour-infiltrating lymphocytes and overall survival in patients with gastric cancer. Journal of Cancer 8: 1579–1585. https://doi.org/10.7150/jca.18729 [Google Scholar] [PubMed] [CrossRef]
Feng H, Wang M, Wu C, Yu J, Wang D, Ma J, Han J (2018). High scavenger receptor class B type I expression is related to tumor aggressiveness and poor prognosis in lung adenocarcinoma: A STROBE compliant article. Medicine (Baltimore) 97: e0203. https://doi.org/10.1097/MD.0000000000010203 [Google Scholar] [PubMed] [CrossRef]
Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X (2017). PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One 12: e0182692. https://doi.org/10.1371/journal.pone.0182692 [Google Scholar] [PubMed] [CrossRef]
Karimi N, Karami Tehrani FS (2021). Expression of S.R.-B1 receptor in breast cancer cell lines, MDAMB-468 and MCF-7: Effect on cell proliferation and apoptosis. Iranian Journal of Basic Medical Sciences 24: 1069–1077. https://doi.org/10.22038/ijbms.2021.56752.12674 [Google Scholar] [PubMed] [CrossRef]
Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH (2008). Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. British Journal of Cancer 99: 1704–1711. https://doi.org/10.1038/sj.bjc.6604738 [Google Scholar] [PubMed] [CrossRef]
Li J, Wang J, Li M, Yin L, Li XA, Zhang TG (2016). Up-regulated expression of scavenger receptor class B type 1 (SR-B1) is associated with malignant behaviors and poor prognosis of breast cancer. Pathology Research and Practice 212: 555–559. https://doi.org/10.1016/j.prp.2016.03.011 [Google Scholar] [PubMed] [CrossRef]
Liu W, Chen G, Zhang C, Liao X, Xie J, Liang T, Liao W, Song L, Zhang X (2022). Prognostic significance of tumor-infiltrating lymphocytes and macrophages in nasopharyngeal carcinoma: A systematic review and meta-analysis. European Archives of Oto-Rhino-Laryngology 279: 25–35. https://doi.org/10.1007/s00405-021-06879-2 [Google Scholar] [PubMed] [CrossRef]
Nakagawa-Toyama Y, Hirano K, Tsujii K, Nishida M, Miyagawa J, Sakai N, Yamashita S (2005). Human scavenger receptor class B type I is expressed with cell-specific fashion in both initial and terminal site of reverse cholesterol transport. Atherosclerosis 183: 75–83. https://doi.org/10.1016/j.atherosclerosis.2005.02.035 [Google Scholar] [PubMed] [CrossRef]
Nelson MA, Ngamcherdtrakul W, Luoh SW, Yantasee W (2021). Prognostic and therapeutic role of tumor-infiltrating lymphocyte subtypes in breast cancer. Cancer and Metastasis Reviews 40: 519–536. https://doi.org/10.1007/s10555-021-09968-0 [Google Scholar] [PubMed] [CrossRef]
Paver EC, Cooper WA, Colebatch AJ, Ferguson PM, Hill SK et al. (2021). Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 53: 141–156. https://doi.org/10.1016/j.pathol.2020.10.007 [Google Scholar] [PubMed] [CrossRef]
Pereira MA, de Castria TB, Ramos M, Dias AR, Cardili L, de Moraes RDR, Zilberstein B, Nahas SC, Ribeiro Jr U, de Mello ES (2021). Cytotoxic T-lymphocyte-associated protein 4 in gastric cancer: Prognosis and association with PD-L1 expression. Journal of Surgical Oncology 124: 1040–1050. https://doi.org/10.1002/jso.26604 [Google Scholar] [PubMed] [CrossRef]
Qiu Z, Du Y (2021). Clinicopathological and prognostic significance of programmed death ligant-1 expression in gastric cancer: A meta-analysis. Journal of Gastrointestinal Oncology 12: 112–120. https://doi.org/10.21037/jgo-20-568 [Google Scholar] [PubMed] [CrossRef]
Ryu S, Howland A, Song B, Youn C, Song PI (2020). Scavenger receptor class A to E involved in various cancers. Chonnam Medical Journal 56: 1–5. https://doi.org/10.4068/cmj.2020.56.1.1 [Google Scholar] [PubMed] [CrossRef]
Schoemig-Markiefka B, Eschbach J, Scheel AH, Pamuk A, Rueschoff J et al. (2021). Optimized PD-L1 scoring of gastric cancer. Gastric Cancer 24: 1115–1122. https://doi.org/10.1007/s10120-021-01195-4 [Google Scholar] [PubMed] [CrossRef]
Schorghofer D, Kinslechner K, Preitschopf A, Schutz B, Rohrl C, Hengstschlager M, Stangl H, Mikula M (2015). The HDL receptor S.R.-BI is associated with human prostate cancer progression and plays a possible role in establishing androgen independence. Reproductive Biology and Endocrinology 13: 88. https://doi.org/10.1186/s12958-015-0087-z [Google Scholar] [PubMed] [CrossRef]
Sexton RE, Al Hallak MN, Diab M, Azmi AS (2020). Gastric cancer: A comprehensive review of current and future treatment strategies. Cancer and Metastasis Reviews 39: 1179–1203. https://doi.org/10.1007/s10555-020-09925-3 [Google Scholar] [PubMed] [CrossRef]
Shetty S, Weston CJ, Oo YH, Westerlund N, Stamataki Z et al. (2011). Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. Journal of Immunology 186: 4147–4155. https://doi.org/10.4049/jimmunol.1002961 [Google Scholar] [PubMed] [CrossRef]
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F (2020). Gastric cancer. Lancet 396: 635–648. https://doi.org/10.1016/S0140-6736(20)31288-5 [Google Scholar] [PubMed] [CrossRef]
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca: A Cancer Journal for Clinicians 71: 209–249. https://doi.org/10.3322/caac.21660 [Google Scholar] [PubMed] [CrossRef]
Thrift AP, El-Serag HB (2020). Burden of gastric cancer. Clinical Gastroenterology and Hepatology 18: 534–542. https://doi.org/10.1016/j.cgh.2019.07.045 [Google Scholar] [PubMed] [CrossRef]
Traughber CA, Opoku E, Brubaker G, Major J, Lu H et al. (2020). Uptake of high-density lipoprotein by scavenger receptor class B type 1 is associated with prostate cancer proliferation and tumor progression in mice. The Journal of Biological Chemistry 295: 8252–8261. https://doi.org/10.1074/jbc.RA120.013694 [Google Scholar] [PubMed] [CrossRef]
Uppal A, Dehal A, Chang SC, Barrak D, Naeini Y, Jalas JR, Bilchik AJ (2020). The immune microenvironment impacts survival in western patients with gastric adenocarcinoma. Journal of Gastrointestinal Surgery 24: 28–38. https://doi.org/10.1007/s11605-019-04403-w [Google Scholar] [PubMed] [CrossRef]
Vasquez M, Simoes I, Consuegra-Fernandez M, Aranda F, Lozano F, Berraondo P (2017). Exploiting scavenger receptors in cancer immunotherapy: Lessons from CD5 and SR-B1. European Journal of Immunology 47: 1108–1118. https://doi.org/10.1002/eji.201646903 [Google Scholar] [PubMed] [CrossRef]
Wang Y, Zhu C, Song W, Li J, Zhao G, Cao H (2018). PD-L1 expression and CD8+ T cell infiltration predict a favorable prognosis in advanced gastric cancer. Journal of Immunology Research, 2018: 4180517. https://doi.org/10.1155/2018/4180517 [Google Scholar] [PubMed] [CrossRef]
Xie J, Fu L, Jin L (2021). Immunotherapy of gastric cancer: Past, future perspective and challenges. Pathology Research and Practice 218: 153322. https://doi.org/10.1016/j.prp.2020.153322 [Google Scholar] [PubMed] [CrossRef]
Xu G, Lou N, Xu Y, Shi H, Ruan H et al. (2017). Diagnostic and prognostic value of scavenger receptor class B type 1 in clear cell renal cell carcinoma. Tumour Biology 39: 1010428317699110. https://doi.org/10.1177/1010428317699110 [Google Scholar] [PubMed] [CrossRef]
Xu GH, Lou N, Shi HC, Xu YC, Ruan HL et al. (2018). Up-regulation of SR-B1 promotes progression and serves as a prognostic biomarker in clear cell renal cell carcinoma. BMC Cancer 18: 88. https://doi.org/10.1186/s12885-017-3761-z [Google Scholar] [PubMed] [CrossRef]
Xue C, Li G, Lu J, Li L (2021). Crosstalk between circRNAs and the PI3K/AKT signaling pathway in cancer progression. Signal Transduction and Targeted Therapy 6: 400. https://doi.org/10.1038/s41392-021-00788-w [Google Scholar] [PubMed] [CrossRef]
Yang X, Sethi A, Yanek LR, Knapper C, Nordestgaard BG, Tybjaerg-Hansen A, Becker DM, Mathias RA, Remaley AT, Becker LC (2016). SCARB1 gene variants are associated with the phenotype of combined high high-density lipoprotein cholesterol and high lipoprotein (a). Circulation. Cardiovascular Genetics 9: 408–418. https://doi.org/10.1161/CIRCGENETICS.116.001402 [Google Scholar] [PubMed] [CrossRef]
Yi H, Guo C, Yu X, Gao P, Qian J, Zuo D, Manjili MH, Fisher PB, Subjeck JR, Wang XY (2011). Targeting the immunoregulator SRA/CD204 potentiates specific dendritic cell vaccine-induced T-cell response and antitumor immunity. Cancer Research 71: 6611–6620. https://doi.org/10.1158/0008-5472.CAN-11-1801 [Google Scholar] [PubMed] [CrossRef]
Yu X, Guo C, Fisher PB, Subjeck JR, Wang XY (2015). Scavenger receptors: Emerging roles in cancer biology and immunology. Advances in Cancer Research 128: 309–364. https://doi.org/10.1016/bs.acr.2015.04.004 [Google Scholar] [PubMed] [CrossRef]
Yuan B, Wu C, Wang X, Wang D, Liu H, Guo L, Li XA, Han J, Feng H (2016). High scavenger receptor class B type I expression is related to tumor aggressiveness and poor prognosis in breast cancer. Tumour Biology 37: 3581–3588. https://doi.org/10.1007/s13277-015-4141-4 [Google Scholar] [PubMed] [CrossRef]
Zhang N, Cao M, Duan Y, Bai H, Li X, Wang Y (2020). Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: A meta-analysis and experimental validation. Archives of Medical Science 16: 1092–1103. https://doi.org/10.5114/aoms.2019.86101 [Google Scholar] [PubMed] [CrossRef]
Zheng Z, Ai J, Li XA (2014). Scavenger receptor class B type I and immune dysfunctions. Current Opinion in Endocrinology Diabetes and Obesity 21: 121–128. https://doi.org/10.1097/MED.0000000000000046 [Google Scholar] [PubMed] [CrossRef]
Zhu P, Liu X, Treml LS, Cancro MP, Freedman BD (2009). Mechanism and regulatory function of CpG signaling via scavenger receptor B1 in primary B cells. The Journal of Biological Chemistry 284: 22878–22887. https://doi.org/10.1074/jbc.M109.018580 [Google Scholar] [PubMed] [CrossRef]
Zhu S, Cao S, Yang W, Che J, Li D, Pei R, Ding Y (2021). The maturation of tumor suppressor miR-497 in hepatocellular carcinoma is inhibited by oncogenic circRNA SCARB1. Cancer Management and Research 13: 5751–5759. https://doi.org/10.2147/CMAR.S304125 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools