 Open Access
Open Access
REVIEW
Roles of miR-214 in bone physiology and disease
Department of Biotechnology, School of Bioengineering, College of Engineering and Technology, SRM Institute of Science and Technology, Kattankulathur, 603103, India
* Corresponding Author: N. Selvamurugan,
# These authors contributed equally
(This article belongs to the Special Issue: Non-Coding RNAs in the Regulation of Human Cancers)
BIOCELL 2023, 47(4), 751-760. https://doi.org/10.32604/biocell.2023.026911
Received 02 October 2022; Accepted 02 December 2022; Issue published 08 March 2023
Abstract
MicroRNAs (miRNAs) are small non-coding RNAs (ncRNAs) that regulate the expression of their target mRNAs post-transcriptionally. Since their discovery, thousands of highly conserved miRNAs have been identified and investigated for their role in human health and diseases. MiR-214 has been increasingly reported to have an association with the regulation of bone metabolism. Reports suggested that miR-214 controls the critical aspects of osteoblasts (bone-forming cells), including their differentiation, proliferation, viability, and migration. Studies have also reported the functional significance of miR-214 in bone diseases and suggested its candidature as a diagnostic and therapeutic target. Further, targeting miR-214 by other ncRNAs, such as linear ncRNAs and circular RNAs, has provided novel insights into treating bone diseases. This review briefly discusses the contemporary findings of the physiological and pathological roles of miR-214 in bone turnover. In addition, we highlight the important ncRNA/mRNA/miR-214 axes influencing osteoblast differentiation that are of therapeutic importance for the treatment of bone-related diseases.Keywords
Abbreviations
| microRNAs | (miRNAs) |
| NcRNA | Non-coding RNAs |
| MSCs | Mesenchymal stem cells |
| PTH | Parathyroid hormone |
| BMP2 | Bone morphogenetic protein2 |
| TGF-β | Transforming growth factor-beta |
| COL | Collagen |
| OC | Osteocalcin |
| OPN | Osteopontin |
| AGO | Argonaute |
| miRISC | miRNA-induced silencing complex |
| MREs | miRNA response elements |
| Dnm3os | Dynamin-3 opposite strand |
| Mgp | Matrix gla protein |
| Sox9 | SRY-box transcription factor 9 |
| TXNIP | Thioredoxin-interacting protein |
Bone is a highly dynamic organ that remodels throughout life to preserve its strength and mineral equilibrium. It mainly comprises bone-forming osteoblasts and bone-resorbing osteoclasts. The balance between bone formation and resorption remains crucial in maintaining bone remodelling and homeostasis (Katsimbri, 2017). Osteoblasts are differentiated from mesenchymal stem cells (MSCs) in the bone microenvironment via the action of various signalling molecules such as parathyroid hormone (PTH), bone morphogenetic protein2 (BMP2), and transforming growth factor-beta (TGF-β) (Krishnan et al., 2022). They secrete collagen (COL), osteocalcin (OC), osteopontin (OPN), alkaline phosphatase (ALP), and other molecules that are responsible for bone matrix mineralization (Blair et al., 2017). Osteoblast differentiation is a multistep process governed by an integrated gene expression cascade that promotes proliferation and differentiation in sequential order (Ponzetti and Rucci, 2021). Osteoblast differentiation is a very important process in bone remodelling, and any defect in this process can cause improper bone formation leading to diseases like osteoporosis.
MicroRNAs (miRNAs) are endogenous non-coding RNAs (ncRNAs, 22–25 nucleotides) that regulate the target mRNA expression post-transcriptionally. They are implicated in various physiological processes including cell growth, development, apoptosis, hormone signalling, differentiation, metabolism, and pathologies like cancer and cardiovascular diseases (Hanna et al., 2019). Several studies reported across the literature have described the process of miRNA biogenesis (Narayanan et al., 2019). miRNA biogenesis begins with synthesizing of the long primary mRNA transcript (pri-miRNA) in the nucleus catalysed by RNA polymerase II (Finnegan and Pasquinelli, 2013). The nuclear microprocessor enzyme, Drosha and Pasha (DGCR8), its cofactor, cleaves the primary transcript into a precursor hairpin loop structure (Ha and Kim, 2014). The Drosha-processed hairpin loop structure with free 5′ and 3′ ends forms the precursor miRNA duplex (pre-miRNA) that is then transported to the cytoplasm via the Exportin protein (Exp), where the cytoplasmic RNase III enzyme Dicer cleaves the hairpin loop. The 5′ guide strand is then preferably selected and loaded onto the Argonaute (AGO) complex forming the miRNA-induced silencing complex (miRISC) (O’Brien et al., 2018). The miRNA-RISC complex specifically targets certain mRNAs via binding to their miRNA response elements (MREs) that are complementary to the seed region on the miRNA. These miRNA-MRE interactions could result in complete degradation of the mRNA or inhibition of their translation, depending on the degree of complementarity between their hybridization (Finnegan and Pasquinelli, 2013; Kim et al., 2017; Pasquinelli, 2012).
Reports suggested that miRNAs could positively or negatively regulate osteoblast differentiation. For instance, Ma et al. (2019) demonstrated that miR-96 promotes osteoblast differentiation in mice by activating the Wnt signalling pathway. They identified that miR-96 targets sclerostin (SOST), a Wnt inhibitor, thus enhancing the expression of Wnt markers like Wnt1 and β-catenin. Whereas, Zhang et al. (2018) demonstrated that miR-223 inhibits osteoblast differentiation by downregulating the expression of Dehydrogenase/Reductase 3 (DHRS3), a gene involved in retinol metabolism activated during osteoblast differentiation.
Among the multiple miRNAs reported, miR-214 is well studied for its role in bone health and diseases. It has a multitude of gene targets like BMP2, activating transcription factor 4 (ATF4), and SRY-box transcription factor 4 (SOX4), which are responsible for the regulation of osteoblast differentiation. Further, miR-214 has also been implicated in bone diseases like osteoporosis and osteosarcoma (Sun et al., 2018). By targeting key molecules such as Osterix (Osx), ATF4, phosphatase and tensin homolog (Pten), β-catenin, and fibroblast growth factor receptor 1 (FGFR1), miR-214 plays an essential role in senile osteoporosis, as well as in the bone formation and resorption (Yuan et al., 2019).
Extracellular vesicles (EVs) are lipid vesicles that range from 50 nanometers to several thousand nanometers in size. Studies have identified the role of EVs in mediating cell-cell communication by transporting molecules like mRNAs, miRNAs, proteins, and lipids (Munir et al., 2020). EVs can be engineered to carry exogenous miRNAs like miR-214, thus providing a therapeutic strategy against bone diseases (Li et al., 2016). Fig. 1 illustrates the important role of miR-214 in the regulation of several molecules that are critically involved in bone remodelling. This review aims to elaborate the recent advancements in our understanding of miR-214 in the regulation of osteoblast differentiation and how it influences bone health. Further, we discuss the role of lncRNA-miR-214/circ-RNA-mRNA axes reported till date and highlight the candidature of miR-214 to serve as a therapeutic target in treating bone diseases.
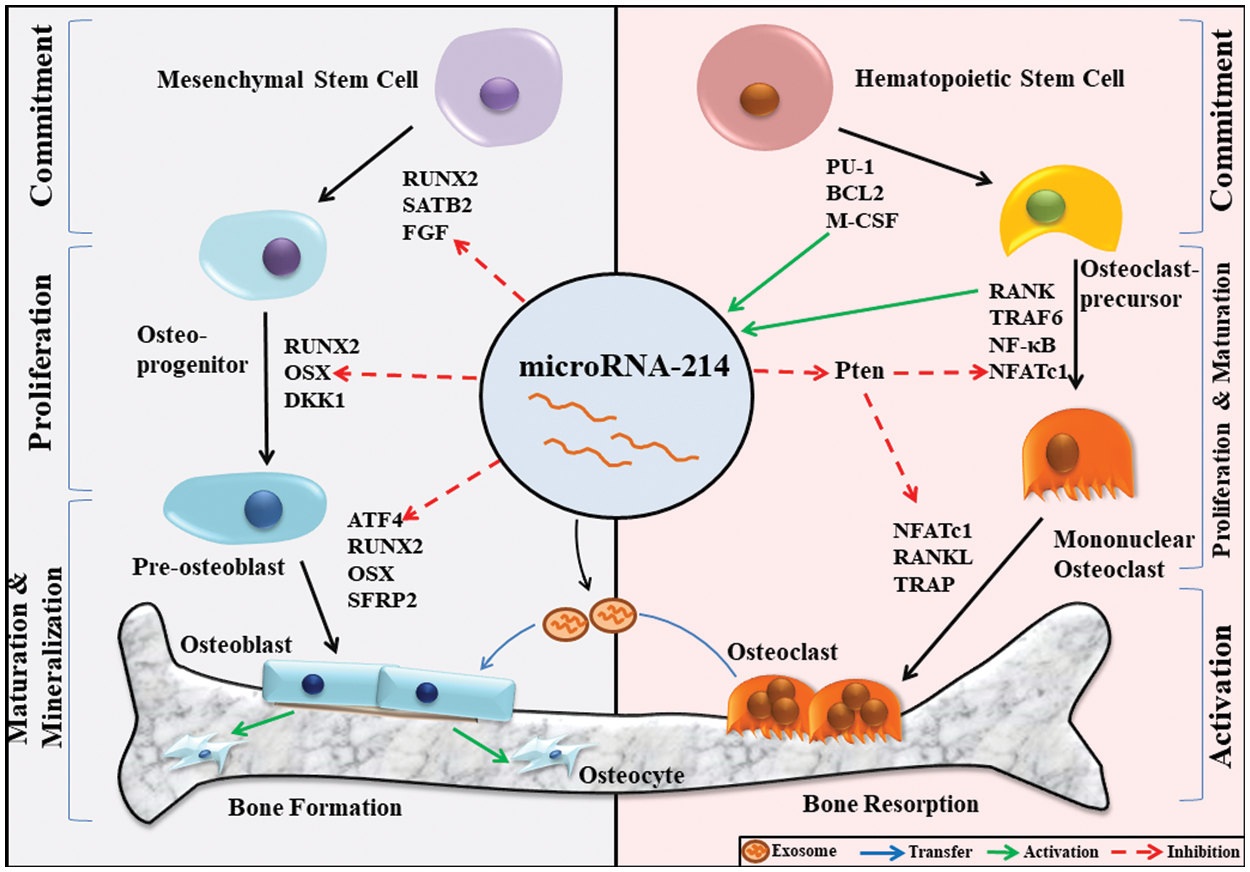
Figure 1: MiR-214-mediated regulation of bone homeostasis.
Bone homeostasis is maintained by the coupled action of both osteoblasts and osteoclasts. During osteoblast differentiation, mesenchymal stem cells differentiate to form mature osteoblasts. This process is governed by crucial transcription factors like RUNX2 (Runt-related transcription factor 2) and OSX. Because miR-214 negatively regulates FGF, OSX, and ATF4, it has the potential to regulate osteoblast differentiation at different stages of maturation. Osteoclasts are formed from the differentiation of hematopoietic stem cells. The osteoclasts markers M-CSF and RANK stimulate the expression of miR-214 in the early stages of osteoclast differentiation, which inhibits PTEN and enhances NFATc1 expression, thereby stimulating osteoclast differentiation. Further, osteoclast-derived exosomal miR-214 inhibits the differentiation of osteoblasts. Thus, miR-214 acts as a crucial regulator of bone homeostasis by acting on osteoblasts and osteoclasts.
Multiple studies have reported earlier on the critical role of miR-214 in modulating various biological processes, including tissue growth and metabolism, musculoskeletal development etc., and in certain pathological conditions, for instance, osteoporosis, cancer, and others (Amin et al., 2021). MiR-214 is coded by Dnm3os (Dynamin-3 opposite strand), an antisense ncRNA, which was previously found to cause cellular apoptosis in cervical cancer cells (Peng et al., 2017; He et al., 2020). In this section, we have emphasized the involvement of miR-214 in distinct physiological and pathological conditions.
Role of miR-214 in physiological conditions
MiR-214 is essential in stimulating and maintaining various physiological processes, specifically cellular proliferation, followed by tissue formation, which supports growth and development. Numerous studies have revealed the significance of miR-214 in regulating its downstream targets, subsequently triggering bone development (Fig. 2). It plays an imperative role in bone remodelling and chondrocyte differentiation (Iaquinta et al., 2021).
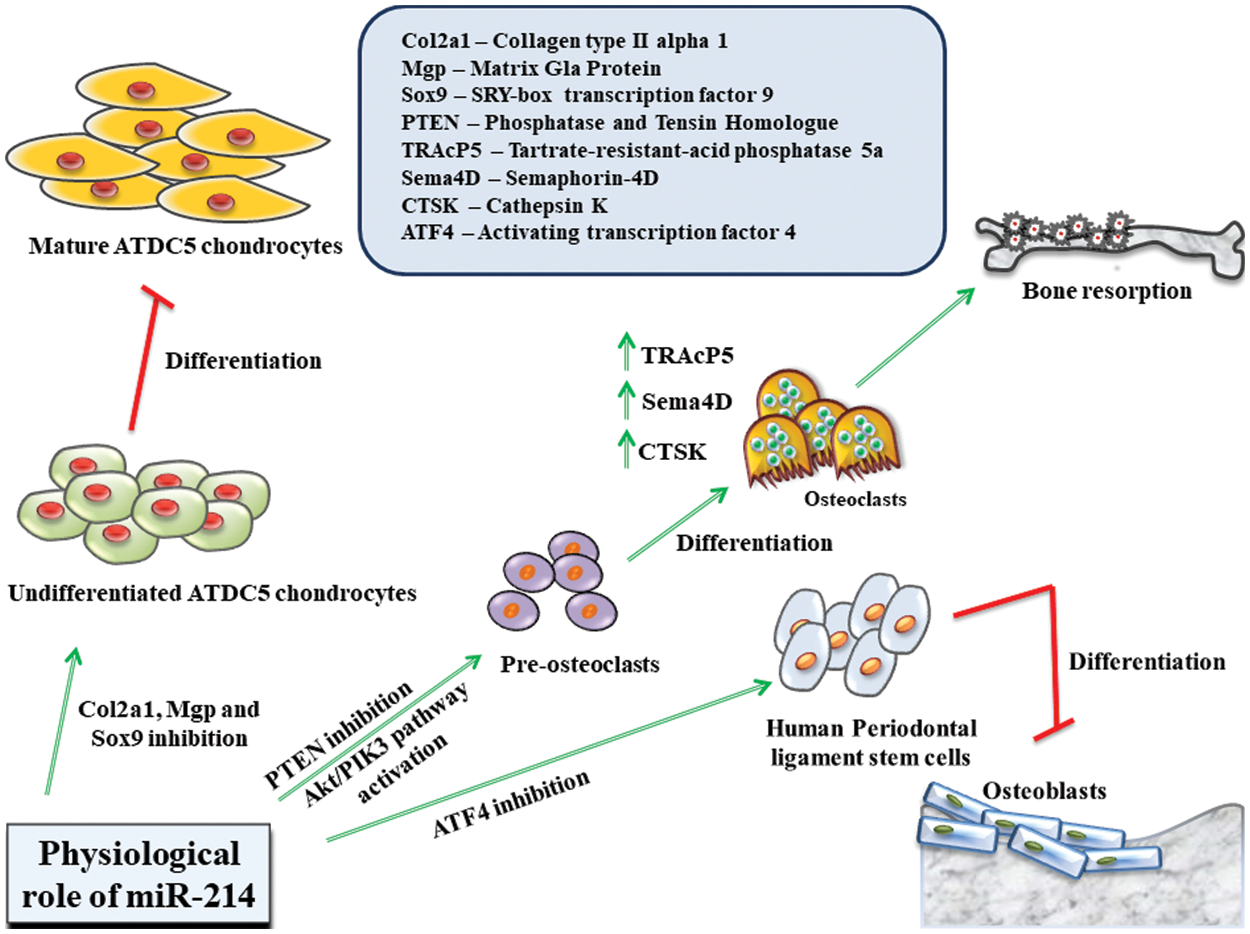
Figure 2: Role of miR-214 in regulating the physiological states of the body.
MiR-214 interacts with various intracellular downstream targets, including Col2a1, Mgp and Sox9 in ATDC5 chondrogenic cell lines, PTEN in pre-osteoclasts, ATF4 in human periodontal ligament stem cells, and aids in the development of tissues. It can either positively (indicated in green) or negatively (indicated in red) regulate cellular differentiation.
In recent years, miR-214 has been found to be involved in regulating intricate bone formation and resorption mechanisms. Yao et al. (2017) identified that miR-214 targets ATF4 to suppress the differentiation of human periodontal ligament stem cells into osteoblastic cells, thereby inhibiting bone formation via the miR-214/ATF4 axis. Likewise, in human MSCs (hMSCs), miR-214 overexpression inhibited osteogenesis by targeting β-catenin (Li et al., 2017). A study demonstrated that the downregulation of miR-214 triggered by physical exercise generated-strain on osteoblasts could stimulate osteogenesis in vivo and in vitro, followed by an upregulation of bone markers including ALP, ATF4 and Osx. However, miR-214 overexpression inhibited the expression of ALP, ATF4, and β-catenin, thus reducing osteogenic differentiation (Yuan et al., 2019). Another study by Li et al. (2016) identified that exosomal miR-214 derived from osteoclast induces osteoclast differentiation and suppresses osteoblast differentiation via targeting osteoblast-specific markers including ALP, COL, OPN, OC, and bone sialoprotein (BSP) in the osteoblastic cells, thereby reducing bone formation via increasing resorption. Their results suggested that miR-214 interacts with PTEN and triggers osteoclastogenesis via the Akt/PI3K signalling. Further, osteoclast-specific markers such as tartrate-resistant-acid phosphatase 5a (TRAcP5), semaphorin-4D (Sema4D), and cathepsin K (CTSK) were detected in serum EVs produced by osteoclasts, which could be transmitted to osteoblastic cells to suppress osteogenesis (Li et al., 2016).
Reports have also suggested the participation of miR-214 in the regulation of cartilage formation. Roberto et al. (2018) demonstrated an elevated expression of miR-214 in undifferentiated ATDC5 cells (chondrogenic cell line). Increased miR-214 was observed to target Col2a1 (collagen type II alpha 1), Mgp (Matrix gla protein), and Sox9 (SRY-box transcription factor 9). These genes regulated the differentiation of chondrogenic cells into mature chondrocytes, which was, however, restricted by the inhibition of their activities in the presence of miR-214 (Roberto et al., 2018).
Role of miR-214 in pathological conditions
The role of miR-214 in bone physiology is well recognized, as discussed above, but recent studies have shown its participation in regulating different pathological states such as osteoporosis, osteoarthritis, and cancers, including osteosarcoma and Ewing’s sarcoma (Fig. 3).
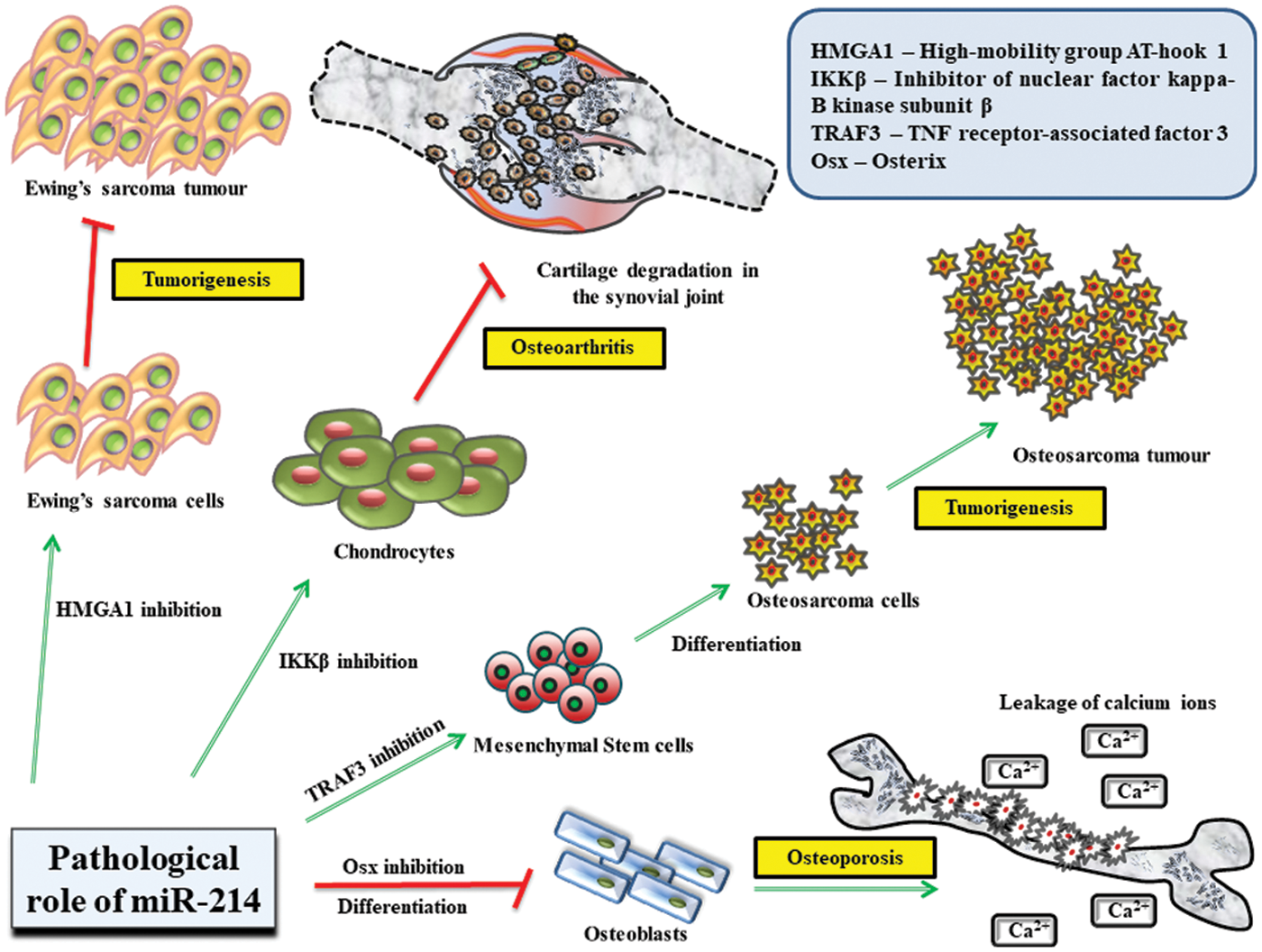
Figure 3: Role of miR-214 in regulating the pathological states of the body.
MiR-214 controls several life-threatening diseases that mostly affect bone tissue homeostasis and prominent molecular mechanisms involved in bone growth and development. It significantly inhibits HMGA1 activity in Ewing’s sarcoma cells to prevent tumor growth and TRAF3 in mesenchymal stem cells from inducing their differentiation into osteosarcoma cells. Additionally, miR-214 obstructs the functions of IKKβ in chondrocytes to impede cartilage degradation. miR-214 also restricts the expression of Osterix in osteoblasts to suppress their differentiation, which culminates in osteoporosis, followed by leakage of Ca2+ ions.
Osteoporosis is the most prominent and well-known skeletal anomaly that causes fragility in bones leading to enhanced chances of fracture occurrence (Ensrud and Crandall, 2017). miR-214 has been linked to the reduced differentiation of MSCs into osteoblasts and a rapid decline in the mineralization of the bone matrix, thereby triggering osteoporosis (Ge et al., 2017). Mohamad et al. (2019) showed the regulatory association between miR-214 and Osx in promoting primary osteoporosis, characterized by reduced bone mass and which mostly affected the trabecular and cortical bones. They identified that the expression profiles of miR-214 and Osx are inversely correlated. Further, they reported a reduction in serum calcium levels and an increase in ALP levels in osteoporotic patients (Sözen et al., 2017; Mohamad et al., 2019). Apart from that, a study found YAP1 (Yes-associated protein 1), a Hippo pathway component, to be a potential target of miR-214 in osteoporotic patients. In particular, YAP1 favours the differentiation of hMSCs into osteocytes. However, miR-214 binds to YAP1 and downregulates its expression, which hinders the differentiation of hMSCs, confirming that miR-214 inhibits YAP1, thereby inducing osteoporosis (Zhong et al., 2021).
Osteoarthritis is the most typical form of bone disability that affects and structurally modifies the whole joint. The structural modification comprises the formation of osteophytes, inflammation in the synovial portions, and degradation of cartilaginous tissues, thereby severely affecting the quality of life (Charlier et al., 2019). A study highlighted the intricate function of miR-214-3p in osteoarthritis. In chondrocytes, the Inhibitor of nuclear factor kappa-B kinase subunit β (IKKβ), an important member of the nuclear factor kappa-B (NF-kB) family, serves as an initiator of the NF-kB pathway. Activation of the NF-kB signalling pathway disrupts processes such as extracellular matrix (ECM) metabolism, followed by the degradation of chondrocytes, thus leading to osteoarthritis. However, miR-214-3p directly targeted and inhibited the activity of IKKβ to exert a chondroprotective effect that aids in preventing the disruption of ECM and chondrocyte apoptosis (Choi et al., 2019; Cao et al., 2021).
Osteosarcoma refers to bone cancer that mostly occurs in children, including adolescents, and primarily originates from a single cell in the marrow of long bones, giving rise to a large heterogeneous tumor mass (Brown et al., 2017). Recent research has identified the participation of miR-214 in regulating osteosarcoma (Rehei et al., 2018). miR-214 was found to act as an oncogene and directly regulated the expression of TNF receptor-associated factor 3 (TRAF3) in osteosarcoma cell lines and patient tissue samples. TRAF3, a member of the TRAF family of adapter proteins, is found in almost all bone cells and tissues. When miR-214 was expressed, it reduced the expression levels of TRAF3, thereby leading to osteosarcoma (Rehei et al., 2018). Similarly, Cheng et al. (2020) reported that miR-214-3p directly inhibits Fibronectin type III domain-containing protein 5 (FNDC5)/irisin in osteosarcoma cells to promote their migration and invasion. This study indicated that FNDC5 could act as a prospective inhibitor to obstruct the viability and procession of the osteosarcoma cells that are reversed by miR-214-3p (Cheng et al., 2020).
Ewing's sarcoma (EWS) refers to a malignant bone tumor, commonly occurring in the ribs, tibia, pelvis, and femur regions and majorly affects children and young adults. Histologically, EWS belongs to a diverse group of tiny round cell sarcomas, and each cell is morphologically similar to the other (Grünewald et al., 2018). A recent study suggested the regulatory role of miR-214-3p in EWS (de Feo et al., 2022). EWS has two prominent hallmarks, Cluster of differentiation 99 (CD99) and Ewing’s sarcoma-friend leukaemia integration 1 (EWS-FLI1). While EWS-FLI1 is an anomalous transcription factor, CD99 is a cell-surface protein molecule (Sen et al., 2018; Manara et al., 2016). It was determined that higher expression levels of CD99 and EWS-FLI1 are exclusively responsible for causing EWS malignancy. Typically, CD99 interacts and binds to EWS-FLI1 to promote malignancy during EWS. In addition, these hallmarks have been described to repress the activity of miR-214-3p, a tumor suppressor in EWS. miR-214-3p has been shown to target HMGA1 (High-mobility group AT-hook 1), which is a small histone protein molecule that engages in tumor growth and metastasis. miR-214-3p overexpression suppressed HMGA1 expression to restrain EWS, but this was opposed by inhibition of miR-214-3p activity in the presence of EWS-FLI1 and CD99, which led to severe EWS malignancy (de Feo et al., 2022).
Together, the findings discussed above illustrate the diverse functions of miR-214 in regulating a wide range of physiological processes in the skeletal system of our body and development at a molecular level.
MiR-214-Mediated Regulation of Osteoblast Differentiation
A repertoire of bone transcription factors, such as Runx2, Osx, ATF4, etc., were reported to regulate bone formation at the transcriptional level (Baek and Kim, 2011). Clinical evidence suggested that miR-214 could regulate bone formation under both physiological and pathological contexts via targeting the key osteogenic factors such as ATF4 (Roberto et al., 2018), Osx (Shi et al., 2013), β-catenin (Cao et al., 2017; Li et al., 2017; Zhu et al., 2017) and so on. This section outlines the molecular processes by which miR-214 influences osteoblasts and the bone microenvironment.
The Wnt/β-catenin pathway governs various crucial processes such as cell fate determination (Boland et al., 2004), embryonic development (Sidrat et al., 2021), immune responses and so on. Any dysregulation in the Wnt/β-catenin pathway has been implicated in bone-related diseases such as osteoporosis (Canalis, 2013). Enhanced activation of this pathway is correlated with increased osteoblastic differentiation of MSCs and decreased miR-214 expression. An increased level of miR-214 was observed to target β-catenin and attenuate the osteoblastic differentiation of MSCs via subduing the Wnt/β-catenin pathway (Li et al., 2017). Furthermore, Cao et al. (2017) demonstrated that miR-214 suppresses Wnt/β-catenin signalling by directly targeting β-catenin and inhibits the differentiation of periodontal ligament stem cells into osteoblasts. Likewise, miR-214-3p targeting of β-catenin inhibited the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) (Wang et al., 2019b) and delayed fracture healing (Teng et al., 2018). Additionally, high glucose-induced expression of miR-214-3p was identified to instigate bone loss in Type I diabetic mice via reducing the protein levels of β-catenin (Wang et al., 2019b).
In another study, Wang et al. (2013) observed that miR-214 overexpression negatively correlated with the expression of OCN and ALP in osteoblasts. In addition to promoting osteoclastogenesis, the osteoclast-derived exosomal miR-214-3p transferred to osteoblasts has been observed to negatively regulate osteogenesis in vivo via targeting the 3′UTR of ATF4 mRNA (Wang et al., 2013; Li et al., 2016). At the same time, Lu et al. (2017) observed that miR-214 shields MC3T3-E1 osteoblasts against H2O2–induced apoptosis by decreasing reactive oxygen species (ROS) levels via targeting ATF4. In addition, miR-214 expression was observed to be positively correlated with age, suggesting its role as a contributing factor in the prognosis of primary osteoporosis (Mohamad et al., 2019). In another study, Shi et al. (2013) concluded that overexpression of miR-214 in C2C12 myoblast cells suppresses osteogenic differentiation by inhibiting endogenous Osx protein synthesis. miR-214 was identified to bind at two sites within the Osx 3′UTR, conducive to downregulating the expression of osteoblast differentiation markers such as ALP, OC and Col1a1 (Shi et al., 2013).
A study by Yang et al. (2016) identified that miR-214 directly targets FGFR1 and attenuates the FGFR1/FGF pathway to suppress osteogenesis in vitro, while the inhibition of miR-214 using a miR-214 sponge promoted osteoblast differentiation. TNF Receptor Associated Factor 3 (TRAF3) belongs to the TNF Receptor (TNFR) family of proteins crucial for signal transduction and the activation of immune responses. Mir-29b-3p-mediated TRAF3 downregulation has previously been reported to stimulate the progression of triple-negative breast cancer cells. miR-214 targeted TRAF3 to enhance osteoclastogenesis and osteoclastic bone resorption and reduced osteoblast activity in osteolytic breast cancer bone metastasis. Additionally, the transfer of osteoclastic miR-214 to osteoblasts exerted a catabolic effect on bone formation processes (Liu et al., 2017a; Zhang et al., 2019). Guo et al. (2017) reported a negative regulation of osteogenic differentiation of BMSCs by miR-214 via direct targeting of JNK and p38 MAPK pathways. Another study by Liu et al. (2017b) identified that miR-214 targets Baculoviral IAP repeat containing 7 (BIRC7), pivotal in anti-apoptosis and maintenance of osteoblast activation, and inhibits the differentiation of human osteoblasts via promoting STAT1 expression. Additionally, miR-214 inhibited SOX4 expression in osteoblasts, thereby inducing fragility fracture and regulating fracture healing (Xin et al., 2020).
JNK, FGF, p38 MAPK, and Wnt/β-catenin pathways are some key signaling pathways that influence the proliferation and differentiation of osteoblasts. Although predominantly secreted by osteoclastic EVs, several studies have reported the functional importance of miR-214 in regulating these pathways in osteoblasts and osteoclasts, suggesting that miR-214-mediated crosstalk between osteoblasts and osteoclasts could be a crucial factor in the regulation of bone microenvironment. The mechanisms behind the crosstalk between these two functionally contrasting cell groups need to be elucidated for a better understanding of the therapeutic potential of miR-214 as a biomarker in diagnosing and treating bone degenerative diseases.
Other Non-Coding RNAs Targeting miR-214 in Osteoblasts
As discussed in the previous sections, miR-214 plays an important role in health and disease, thus making it a suitable biomarker and therapeutic target for bone-related diseases. Since RNA-based therapeutics are gaining interest, ncRNAs are currently employed as therapeutics for various diseases like cancer, cardiovascular diseases, and many more (Wang et al., 2019c; Huang et al., 2020a). ncRNAs are well-studied for their regulatory role in gene expression. Among these ncRNAs, lncRNAs and circRNAs are increasingly reported to regulate gene expression by acting as miRNA sponges (Wang et al., 2019a; Zhu et al., 2020). In this section, we discuss those ncRNAs that target miR-214 and regulate osteoblast differentiation.
Long non-coding RNAs targeting miR-214
LncRNAs are a class of ncRNAs that are greater than 200 nucleotides in length and perform several functions, including chromatin remodelling, epigenetic modifications on DNA, serving as protein scaffolds, and acting as miRNA sponges (Paraskevopoulou and Hatzigeorgiou, 2016). Over the past years, several lncRNAs have been identified to promote or suppress osteoblast differentiation. While the lncRNAs MALAT1 (metastasis-associated lung adenocarcinoma transcript 1) and H19 (imprinted maternally expressed transcript) promoted osteoblast differentiation (Xiao et al., 2017; Liang et al., 2016), lncRNAs HOTAIR (HOX transcript antisense RNA) and DANCR (differentiation antagonizing non-protein-coding RNA) inhibited osteoblast differentiation (Wei et al., 2017; Zhu and Xu, 2013). Several lncRNAs that target miR-214 have been reported to date. For instance, lncRNA KCNQ1OT1 sponged miR-214 and upregulated BMP2 expression, thereby stimulating the osteogenic differentiation of BMSCs (Wang et al., 2019a). Similarly, lncRNA XIST (X-inactive specific transcript) stimulated the osteogenic differentiation of periodontal ligament stem cells (PDLSCs) by sponging miR-214-3p (Feng et al., 2020). Stromal cell-derived factor-1 (SDF1) regulated osteoblast differentiation by stimulating BMP2 expression (Hosogane et al., 2010). A recent study demonstrated that lncRNA H19 targets miR-214-5p upon SDF1 exposure and increases BMP2 expression, hence promoting the osteoblast differentiation of BMSCs (He et al., 2021).
Li et al. (2020) demonstrated that lncRNA LOC100506178 sponged miR-214-5p and promoted BMP2 expression, subsequently favoring the differentiation of BMSCs towards osteogenic lineage. Furthermore, they identified that lncRNA LOC100506178 overexpression in BMSCs and transplantation of the same in nude mice reverses the inhibitory effect of miR-214-5p and promoted ectopic bone formation in vivo (Li et al., 2020). While miR-214 was often reported to negatively regulate osteoblast differentiation, Yang et al. (2021) identified that miR-214 could promote osteoblast differentiation by targeting thioredoxin-interacting protein (TXNIP). TXNIP is responsible for bone loss during osteoporosis, hence using a rat osteoporosis model, they found that lncRNA MEG3, which sponges miR-214, was upregulated in the osteoporosis condition. Further, siRNA-mediated silencing of lncRNA MEG3 or miR-214 overexpression led to increased expression of OPG, thereby enhancing osteoblast differentiation. Thus, lncRNA MEG3/miR-214/TXNIP is important in understanding and treating osteoporosis (Yang et al., 2021).
Long-intervening ncRNAs (lincRNAs) are a unique group of lncRNAs without protein-coding ability. LincRNAs have been recently identified and are known to play a role in several diseases, including osteosarcoma (Deniz and Erman, 2017). A recent study revealed that LINC00657 induced osteogenic differentiation of BMSCs via targeting miR-214-3p, and upregulating BMP2 expression (Li et al., 2022). Since lincRNAs are only gaining interest recently, we speculate the involvement of many novel lincRNAs that would be important regulators of osteoblast differentiation.
Circular RNAs targeting miR-214
CircRNAs are single-stranded, ncRNA molecules with persistent loop configuration and are known to regulate gene expression. They are highly stable molecules with high tissue specificity, making them ideal for therapeutics. Although circRNAs have been discovered for a long time, their role in human physiology and disease has only recently been investigated (Verduci et al., 2021). CircRNAs can sponge miRNAs and regulate osteoblast differentiation. For instance, circ_0006873, circ_FAT1, and circ_0066523 promoted osteoblast differentiation by sponging their respective target miRNAs (Ye et al., 2021; Xin et al., 2021; Lv et al., 2022).
Another study in a mice osteoporosis model demonstrated that circ_ITCH1 sponges miR-214 and upregulates the expression of YAP1. Higher expression of YAP1 corresponds to enhanced osteoblast differentiation, making circ_ITCH1 an important biomarker and a therapeutic target for the treatment of osteoporosis (Zhong et al., 2021). Runx3 is a positive mediator of osteoblast differentiation and a direct target of miR-214 (Wang et al., 2017). Circ_33287 was observed to sponge miR-214-3p and protected Runx3 from miR-214-3p mediated inhibition, thereby promoting osteogenic differentiation in maxillary sinus membrane stem cells. Further, overexpression of circ_33287 and miR-214-3p inhibition was identified to stimulate ectopic bone formation in vivo. Hence, circ_33287/miR-214-3p/Runx3 axis presents a novel molecular approach for bone regeneration in the posterior maxilla (Peng et al., 2019). Studies have demonstrated that circRNAs could regulate the proliferative and invasive capacity of osteosarcoma cells (Ji et al., 2020). Mao et al. (2021) identified the competing endogenous RNA (ceRNA) role of circ_XPR1 in osteosarcoma cells. They reported that circ_XPR1 sponges miR-214-5p and promotes the proliferative capacity of osteosarcoma cells via regulating the expression of DEAD-Box Helicase 5 (DDX5), a target of miR-214-5p. Their findings suggested that circ_XPR1/miR-214-5p/DDX5 axis may serve as an important therapeutic target for osteosarcoma (Mao et al., 2021). Table 1 summarizes the involvement of ncRNA/mir-214/mRNA axes in bone-related diseases.
The above-discussed reports highlighted the emerging role of ncRNAs targeting miR-214 in treating various bone-related disorders. However, many of these ncRNAs are still in the preclinical stage due to the challenges in developing RNA-based therapeutics. Challenges such as degradation by RNases and the inability to cross cell membranes are a few concerns that have to be addressed for the efficient development of ncRNA-based therapeutics.
MiRNAs have emerged to be pivotal in the post-transcriptional regulation of gene expression. In particular, miR-214 is extensively reported for its role in human health and diseases, especially in the musculoskeletal system. In the current review, we explained the function of miR-214 in bone biology and its effect on osteoblast differentiation. Studies reported that miR-214 regulates several aspects of osteoblasts, including their differentiation, proliferation, viability, and migration. Further, miR-214 has a key role in bone diseases like osteoporosis and osteosarcoma. The ability of miR-214 to regulate different cellular processes is due to its multitude of gene targets, thus making miR-214 a potential biomarker and therapeutic target. NcRNA-based therapeutics specifically aimed at miR-214 may tend to cure or alleviate the effects of diseases like osteoporosis. However, challenges associated with RNA-based therapeutics have to be addressed. Nevertheless, extensive studies on miR-214 might help us devise better therapeutic strategies to combat bone-related diseases.
Funding Statement: This work was supported by the Indian Council of Medical Research, India [File No. 2020-0282/SCR/ADHOC-BMS to N. S.], Department of Science & Technology [DST/INSPIRE Fellowship/2019/IF190170 to R.L. A. and DST/INSPIRE Fellowship/2021/IF210073 to I. S.].
Author Contributions: S. L., R. H. K., R. L. A., U. R. D., and S. S. wrote the manuscript. I. S. proofread and revised the content. N. S. designed and reviewed the content and secured the fund for the research.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Amin MMJ, Trevelyan CJ, Turner NA (2021). MicroRNA-214 in health and disease. Cells 10: 3274. https://doi.org/10.3390/cells10123274 [Google Scholar] [PubMed] [CrossRef]
Baek WY, Kim JE (2011). Transcriptional regulation of bone formation. Frontiers in Bioscience-Scholar 3: 126–135. https://doi.org/10.2741/s138 [Google Scholar] [PubMed] [CrossRef]
Blair HC, Larrouture QC, Li Y, Lin H, Beer-Stoltz D, Liu L, Tuan RS, Robinson LJ, Schlesinger PH, Nelson DJ (2017). Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Engineering Part B: Reviews 23: 268–280. https://doi.org/10.1089/ten.teb.2016.0454 [Google Scholar] [PubMed] [CrossRef]
Boland GM, PerkinsG Hall, TuanRS DJ (2004). Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. Journal of Cellular Biochemistry 93: 1210–1230. https://doi.org/10.1002/(ISSN)1097-4644 [Google Scholar] [CrossRef]
Brown HK, Tellez-Gabriel M, Heymann D (2017). Cancer stem cells in osteosarcoma. Cancer Letters 386: 189–195. https://doi.org/10.1016/j.canlet.2016.11.019 [Google Scholar] [PubMed] [CrossRef]
Canalis E (2013). Wnt signalling in osteoporosis: Mechanisms and novel therapeutic approaches. Nature Reviews Endocrinology 9: 575–583. https://doi.org/10.1038/nrendo.2013.154 [Google Scholar] [PubMed] [CrossRef]
Cao Y, Tang S, Nie X, Zhou Z, Ruan G, Han W, Zhu Z, Ding C (2021). Decreased miR-214-3p activates NF-κB pathway and aggravates osteoarthritis progression. eBioMedicine 65: 103283. https://doi.org/10.1016/j.ebiom.2021.103283 [Google Scholar] [PubMed] [CrossRef]
Cao F, Zhan J, Chen X, Zhang K, Lai R, Feng Z (2017). miR-214 promotes periodontal ligament stem cell osteoblastic differentiation by modulating Wnt/β-catenin signaling. Molecular Medicine Reports 16: 9301–9308. https://doi.org/10.3892/mmr.2017.7821 [Google Scholar] [PubMed] [CrossRef]
Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, Malaise M, de Seny D (2019). Chondrocyte dedifferentiation and osteoarthritis (OA). Biochemical Pharmacology 165: 49–65. https://doi.org/10.1016/j.bcp.2019.02.036 [Google Scholar] [PubMed] [CrossRef]
Chen J, Yang Y (2021). LncRNA HAGLR absorbing miR-214-3p promotes BMP2 expression and improves tibial fractures. American Journal of Translational Research 13: 11065–11080. [Google Scholar] [PubMed]
Cheng G, Xu D, Chu K, Cao Z, Sun X, Yang Y (2020). The effects of MiR-214-3p and Irisin/FNDC5 on the biological behavior of osteosarcoma cells. Cancer Biotherapy & Radiopharmaceuticals 35: 92–100. https://doi.org/10.1089/cbr.2019.2933 [Google Scholar] [PubMed] [CrossRef]
Choi MC, Jo J, Park J, Kang HK, Park Y (2019). NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells 8: 734. https://doi.org/10.3390/cells8070734 [Google Scholar] [PubMed] [CrossRef]
de Feo A, Pazzaglia L, Ciuffarin L, Mangiagli F, Pasello M et al. (2022). miR-214-3p is commonly downregulated by EWS-FLI1 and by CD99 and its restoration limits ewing sarcoma aggressiveness. Cancers 14: 1762. https://doi.org/10.3390/cancers14071762 [Google Scholar] [PubMed] [CrossRef]
Deniz E, Erman B (2017). Long non-coding RNA (lincRNAa new paradigm in gene expression control. Functional & Integrative Genomics 17: 135–143. https://doi.org/10.1007/s10142-016-0524-x [Google Scholar] [PubMed] [CrossRef]
Ensrud KE, Crandall CJ (2017). Osteoporosis. Annals of Internal Medicine 167: ITC17–ITC32. https://doi.org/10.7326/AITC201708010 [Google Scholar] [PubMed] [CrossRef]
Feng Y, Wan P, Yin L (2020). Long non-coding RNA X-inactive specific transcript (XIST) promotes osteogenic differentiation of periodontal ligament stem cells by sponging microRNA-214-3p. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 26: e918932. https://doi.org/10.12659/MSM.918932 [Google Scholar] [PubMed] [CrossRef]
Finnegan EF, Pasquinelli AE (2013). MicroRNA biogenesis: Regulating the regulators. Critical Reviews in Biochemistry and Molecular Biology 48: 51–68. https://doi.org/10.3109/10409238.2012.738643 [Google Scholar] [PubMed] [CrossRef]
Ge DW, Wang WW, Chen HT, Yang L, Cao XJ (2017). Functions of microRNAs in osteoporosis. European Review for Medical and Pharmacological Sciences 21: 4784–4789. [Google Scholar] [PubMed]
Grünewald T, Cidre-Aranaz F, Surdez D, Tomazou EM, de Álava E, Kovar H, Sorensen PH, Delattre O, Dirksen U (2018). Ewing sarcoma. Nature Reviews Disease Primers 4: 3036. https://doi.org/10.1038/s41572-018-0003-x [Google Scholar] [PubMed] [CrossRef]
Guo Y, Li L, Gao J, Chen X, Sang Q (2017). miR-214 suppresses the osteogenic differentiation of bone marrow-derived mesenchymal stem cells and these effects are mediated through the inhibition of the JNK and p38 pathways. International Journal of Molecular Medicine 39: 71–80. https://doi.org/10.3892/ijmm.2016.2826 [Google Scholar] [PubMed] [CrossRef]
Ha M, Kim VN (2014). Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology 15: 509–524. https://doi.org/10.1038/nrm3838 [Google Scholar] [PubMed] [CrossRef]
Hanna J, Hossain GS, Kocerha J (2019). The potential for microRNA therapeutics and clinical research. Frontiers in Genetics 10: 478. https://doi.org/10.3389/fgene.2019.00478 [Google Scholar] [PubMed] [CrossRef]
He Q, Li R, Hu B, Li X, Wu Y, Sun P, Jia Y, Guo Y (2021). Stromal cell-derived factor-1 promotes osteoblastic differentiation of human bone marrow mesenchymal stem cells via the lncRNA-H19/miR-214-5p/BMP2 axis. The Journal of Gene Medicine 23: e3366. https://doi.org/10.1002/jgm.3366 [Google Scholar] [PubMed] [CrossRef]
He S, Yang F, Yang M, An W, Maguire EM et al. (2020). miR-214-3p-Sufu-GLI1 is a novel regulatory axis controlling inflammatory smooth muscle cell differentiation from stem cells and neointimal hyperplasia. Stem Cell Research & Therapy 11: 465. https://doi.org/10.1186/s13287-020-01989-w [Google Scholar] [PubMed] [CrossRef]
Hosogane N, Huang Z, Rawlins BA, Liu X, Boachie-Adjei O, Boskey AL, Zhu W (2010). Stromal derived factor-1 regulates bone morphogenetic protein 2-induced osteogenic differentiation of primary mesenchymal stem cells. The International Journal of Biochemistry & Cell Biology 42: 1132–1141. https://doi.org/10.1016/j.biocel.2010.03.020 [Google Scholar] [PubMed] [CrossRef]
Huang XZ, Huang J, Li WZ, Wang JJ, Song DY, Ni JD (2020a). LncRNA-MALAT1 promotes osteogenic differentiation through regulating ATF4 by sponging miR-214: Implication of steroid-induced avascular necrosis of the femoral head. Steroids 154: 108533. https://doi.org/10.1016/j.steroids.2019.108533 [Google Scholar] [PubMed] [CrossRef]
Huang CK, Kafert-Kasting S, Thum T (2020b). Preclinical and clinical development of non-coding RNA therapeutics for cardiovascular disease. Circulation Research 126: 663–678. https://doi.org/10.1161/CIRCRESAHA.119.315856 [Google Scholar] [PubMed] [CrossRef]
Iaquinta MR, Lanzillotti C, Mazziotta C, Bononi I, Frontini F et al. (2021). The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 11: 6573–6591. https://doi.org/10.7150/thno.55664 [Google Scholar] [PubMed] [CrossRef]
Ji X, Shan L, Shen P, He M (2020). Circular RNA circ_001621 promotes osteosarcoma cells proliferation and migration by sponging miR-578 and regulating VEGF expression. Cell Death & Disease 11: 18. https://doi.org/10.1038/s41419-019-2204-y [Google Scholar] [PubMed] [CrossRef]
Jin H, Jin X, Zhang H, Wang W (2017). Circular RNA hsa-circ-0016347 promotes proliferation, invasion and metastasis of osteosarcoma cells. Oncotarget 8: 25571–25581. https://doi.org/10.18632/oncotarget.16104 [Google Scholar] [PubMed] [CrossRef]
Katsimbri P (2017). The biology of normal bone remodelling. European Journal of Cancer Care 26: e12740. https://doi.org/10.1111/ecc.12740 [Google Scholar] [PubMed] [CrossRef]
Kim B, Jeong K, Kim VN (2017). Genome-wide mapping of DROSHA cleavage sites on primary microRNAs and noncanonical substrates. Molecular Cell 66: 258–269. https://doi.org/10.1016/j.molcel.2017.03.013 [Google Scholar] [PubMed] [CrossRef]
Krishnan RH, Sadu L, Das UR, Satishkumar S, Pranav Adithya S, Saranya I, Akshaya RL, Selvamurugan N (2022). Role of p300, a histone acetyltransferase enzyme, in osteoblast differentiation. Differentiation; 124: 43–51. https://doi.org/10.1016/j.diff.2022.02.002 [Google Scholar] [PubMed] [CrossRef]
Li L, Fang J, Liu Y, Xiao L (2020). LncRNA LOC100506178 promotes osteogenic differentiation via regulating miR-214-5p-BMP2 axis in human bone marrow mesenchymal stem cells. PeerJ 8: e8909. https://doi.org/10.7717/peerj.8909 [Google Scholar] [PubMed] [CrossRef]
Li D, Liu J, Guo B, Liang C, Dang L et al. (2016). Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nature Communications 7: 10872. https://doi.org/10.1038/ncomms10872 [Google Scholar] [PubMed] [CrossRef]
Li J, Zhuang H, Wang Z, Cai J, Ma X, Chen W, Jiang X, Zhao D, Hou W, Tao Y (2022). lncRNAs MALAT1 and LINC00657 upstream to miR-214-3p/BMP2 regulate osteogenic differentiation of human mesenchymal stem cells. Molecular Biology Reports 49: 6847–6857. https://doi.org/10.1007/s11033-022-07136-3 [Google Scholar] [PubMed] [CrossRef]
Li JP, Zhuang HT, Xin MY, Zhou YL (2017). MiR-214 inhibits human mesenchymal stem cells differentiating into osteoblasts through targeting β-catenin. European Review for Medical and Pharmacological Sciences 21: 4777–4783. [Google Scholar] [PubMed]
Liang WC, Fu WM, Wang YB, Sun YX, Xu LL, Wong CW, Chan KM, Li G, Waye MM, Zhang JF (2016). H19 activates Wntsignaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Scientific Reports 6: 20121. https://doi.org/10.1038/srep20121 [Google Scholar] [PubMed] [CrossRef]
Liu J, Li D, Dang L, Liang C, Guo B et al. (2017a). Osteoclastic miR-214 targets TRAF3 to contribute to osteolytic bone metastasis of breast cancer. Scientific Reports 7: 1–13. https://doi.org/10.1038/srep40487 [Google Scholar] [PubMed] [CrossRef]
Liu J, Li Y, Luo M, Yuan Z, Liu J (2017b). MicroRNA-214 inhibits the osteogenic differentiation of human osteoblasts through the direct regulation of baculoviral IAP repeat-containing 7. Experimental Cell Research 351: 157–162. https://doi.org/10.1016/j.yexcr.2017.01.006 [Google Scholar] [PubMed] [CrossRef]
Lu XZ, Yang ZH, Zhang HJ, Zhu LL, Mao XL, Yuan Y (2017). MiR-214 protects MC3T3-E1 osteoblasts against H. European Review for Medical and Pharmacological Sciences 21: 4762–4770. [Google Scholar] [PubMed]
Lv G, Chen Y, Cheng Z, Lin L, Shen H (2022). Circ_0006873 sponges miR-142-5p to inhibit osteoblastic differentiation of hBMSCs via regulating PTEN/Akt signaling pathway. Annals of Clinical and Laboratory Science 52: 48–59. https://doi.org/10.3389/fendo.2022.945310 [Google Scholar] [CrossRef]
Ma S, Wang DD, Ma CY, Zhang YD (2019). microRNA-96 promotes osteoblast differentiation and bone formation in ankylosing spondylitis mice through activating the Wnt signaling pathway by binding to SOST. Journal of Cellular Biochemistry 120: 15429–15442. https://doi.org/10.1002/jcb.28810 [Google Scholar] [PubMed] [CrossRef]
Manara MC, Terracciano M, Mancarella C, Sciandra M, Guerzoni C et al. (2016). CD99 triggering induces methuosis of Ewing sarcoma cells through IGF-1R/RAS/Rac1 signaling. Oncotarget 7: 79925–79942. https://doi.org/10.18632/oncotarget.13160 [Google Scholar] [PubMed] [CrossRef]
Mao X, Guo S, Gao L, Li G (2021). Circ-XPR1 promotes osteosarcoma proliferation through regulating the miR-214-5p/DDX5 axis. Human Cell 34: 122–131. https://doi.org/10.1007/s13577-020-00412-z [Google Scholar] [PubMed] [CrossRef]
Mohamad N, Nabih ES, Zakaria ZM, Nagaty MM, Metwaly RG (2019). Insight into the possible role of miR-214 in primary osteoporosis via osterix. Journal of Cellular Biochemistry 120: 15518–15526. https://doi.org/10.1002/jcb.28818 [Google Scholar] [PubMed] [CrossRef]
Munir J, Yoon JK, Ryu S (2020). Therapeutic miRNA-enriched EVs: Current approaches and future prospects. Cells 9: 2271. https://doi.org/10.3390/cells9102271 [Google Scholar] [PubMed] [CrossRef]
Narayanan A, Srinaath N, Rohini M, Selvamurugan N (2019). Regulation of Runx2 by microRNAs in osteoblast differentiation. Life Sciences 232: 116676. https://doi.org/10.1016/j.lfs.2019.116676 [Google Scholar] [PubMed] [CrossRef]
O’Brien J, Hayder H, Zayed Y, Peng C (2018). Overview of microRNA biogenesis, mechanisms of actions, and circulation. Frontiers in Endocrinology 9: 402. https://doi.org/10.3389/fendo.2018.00402 [Google Scholar] [PubMed] [CrossRef]
Paraskevopoulou MD, Hatzigeorgiou AG (2016). Analyzing miRNA-LncRNA interactions. Methods in Molecular Biology 1402: 271–286. https://doi.org/10.1007/978-1-4939-3378-5 [Google Scholar] [CrossRef]
Pasquinelli AE (2012). MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nature Reviews Genetics 13: 271–282. https://doi.org/10.1038/nrg3162 [Google Scholar] [PubMed] [CrossRef]
Peng R, Men J, Ma R, Wang Q, Wang Y, Sun Y, Ren J (2017). miR-214 down-regulates ARL2 and suppresses growth and invasion of cervical cancer cells. Biochemical and Biophysical Research Communications 484: 623–630. https://doi.org/10.1016/j.bbrc.2017.01.152 [Google Scholar] [PubMed] [CrossRef]
Peng W, Zhu S, Chen J, Wang J, Rong Q, Chen S (2019). Hsa_circRNA_33287 promotes the osteogenic differentiation of maxillary sinus membrane stem cells via miR-214-3p/Runx3. Biomedicine & Pharmacotherapy 109: 1709–1717. https://doi.org/10.1016/j.biopha.2018.10.159 [Google Scholar] [PubMed] [CrossRef]
Ponzetti M, Rucci N (2021). Osteoblast differentiation and signaling: Established concepts and emerging topics. International Journal of Molecular Sciences 22: 6651. https://doi.org/10.3390/ijms22136651 [Google Scholar] [PubMed] [CrossRef]
Rehei AL, Zhang L, Fu YX, Mu WB, Yang DS, Liu Y, Zhou SJ, Younusi A (2018). MicroRNA-214 functions as an oncogene in human osteosarcoma by targeting TRAF3. European Review for Medical and Pharmacological Sciences 22: 5156–5164. https://doi.org/10.26355/eurrev_201808_15711 [Google Scholar] [PubMed] [CrossRef]
Roberto VP, Gavaia P, Nunes MJ, Rodrigues E, Cancela ML, Tiago DM (2018). Evidences for a new role of miR-214 in chondrogenesis. Scientific Reports 8: 3704. https://doi.org/10.1038/s41598-018-21735-w [Google Scholar] [PubMed] [CrossRef]
Sen N, Cross AM, Lorenzi PL, Khan J, Gryder BE, Kim S, Caplen NJ (2018). EWS-FLI1 reprograms the metabolism of Ewing sarcoma cells via positive regulation of glutamine import and serine-glycine biosynthesis. Molecular Carcinogenesis 57: 1342–1357. https://doi.org/10.1002/mc.22849 [Google Scholar] [PubMed] [CrossRef]
Shi K, Lu J, Zhao Y, Wang L, Li J, Qi B, Li H, Ma C (2013). MicroRNA-214 suppresses osteogenic differentiation of C2C12 myoblast cells by targeting Osterix. Bone 55: 487–494. https://doi.org/10.1016/j.bone.2013.04.002 [Google Scholar] [PubMed] [CrossRef]
Sidrat T, Rehman ZU, Joo MD, Lee KL, Kong IK (2021). Wnt/β-catenin pathway-mediated PPARδ expression during embryonic development differentiation and disease. International Journal of Molecular Sciences 22: 1854. https://doi.org/10.3390/ijms22041854 [Google Scholar] [PubMed] [CrossRef]
Sun Y, Kuek V, Liu Y, Tickner J, Yuan Y, Chen L, Zeng Z, Shao M, He W, Xu J (2018). miR-214 is an important regulator of the musculoskeletal metabolism and disease. Journal of Cellular Physiology 234: 231–245. https://doi.org/10.1002/jcp.26856 [Google Scholar] [PubMed] [CrossRef]
Sözen T, Özışık L, Başaran NÇ (2017). An overview and management of osteoporosis. European Journal of Rheumatology 4: 46–56. https://doi.org/10.5152/eurjrheum.2016.048 [Google Scholar] [PubMed] [CrossRef]
Teng JW, Ji PF, Zhao ZG (2018). MiR-214-3p inhibits β-catenin signaling pathway leading to delayed fracture healing. European Review for Medical and Pharmacological Sciences 22: 17–24. https://doi.org/10.26355/eurrev_201801_14095 [Google Scholar] [PubMed] [CrossRef]
Verduci L, Tarcitano E, Strano S, Yarden Y, Blandino G (2021). CircRNAs: Role in human diseases and potential use as biomarkers. Cell Death & Disease 12: 468. https://doi.org/10.1038/s41419-021-03743-3 [Google Scholar] [PubMed] [CrossRef]
Wang Y, Feng Q, Ji C, Liu X, Li L, Luo J (2017). RUNX3 plays an important role in mediating the BMP9-induced osteogenic differentiation of mesenchymal stem cells. International Journal of Molecular Medicine 40: 1991–1999. https://doi.org/10.3892/ijmm.2017.3155 [Google Scholar] [PubMed] [CrossRef]
Wang X, Guo B, Li Q, Peng J, Yang Z et al. (2013). miR-214 targets ATF4 to inhibit bone formation. Nature Medicine 19: 93–100. https://doi.org/10.1038/nm.3026 [Google Scholar] [PubMed] [CrossRef]
Wang WT, Han C, Sun YM, Chen TQ, Chen YQ (2019a). Non-coding RNAs in cancer therapy resistance and targeted drug development. Journal of Hematology& Oncology 12: 55. https://doi.org/10.1186/s13045-019-0748-z [Google Scholar] [PubMed] [CrossRef]
Wang CG, Liao Z, Xiao H, Liu H, Hu YH, Liao QD, Zhong D (2019b). LncRNA KCNQ1OT1 promoted BMP2 expression to regulate osteogenic differentiation by sponging miRNA-214. Experimental and Molecular Pathology 107: 77–84. https://doi.org/10.1016/j.yexmp.2019.01.012 [Google Scholar] [PubMed] [CrossRef]
Wang R, Zhang Y, Jin F, Li G, Sun Y, Wang X (2019c). High-glucose-induced miR-214-3p inhibits BMSCs osteogenic differentiation in type 1 diabetes mellitus. Cell Death Discovery 5: 1–11. https://doi.org/10.1038/s41420-019-0223-1 [Google Scholar] [PubMed] [CrossRef]
Wei B, Wei W, Zhao B, Guo X, Liu S (2017). Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS One 12: e0169097. https://doi.org/10.1371/journal.pone.0169097 [Google Scholar] [PubMed] [CrossRef]
Xiao X, Zhou T, Guo S, Guo C, Zhang Q, Dong N, Wang Y (2017). LncRNA MALAT1 sponges miR-204 to promote osteoblast differentiation of human aortic valve interstitial cells through upregulating Smad4. International Journal of Cardiology 243: 404–412. https://doi.org/10.1016/j.ijcard.2017.05.037 [Google Scholar] [PubMed] [CrossRef]
Xin Z, Cai D, Wang J, Ma L, Shen F, Tang C, Hu L, Sun W (2020). MiR-214 regulates fracture healing through inhibiting Sox4 and its mechanism. Journal of Musculoskeletal & Neuronal Interactions 20: 429–436. [Google Scholar]
Xin W, Yuan S, Wang B, Qian Q, Chen Y (2021). Hsa_circ_0066523 promotes the proliferation and osteogenic differentiation of bone mesenchymal stem cells by repressing PTEN. Bone & Joint Research 10: 526–535. https://doi.org/10.1302/2046-3758.108.BJR-2020-0127.R2 [Google Scholar] [PubMed] [CrossRef]
Yang L, Ge D, Cao X, Ge Y, Chen H, Wang W, Zhang H (2016). MiR-214 attenuates osteogenic differentiation of mesenchymal stem cells via targeting FGFR1. Cellular Physiology and Biochemistry 38: 809–820. https://doi.org/10.1159/000443036 [Google Scholar] [PubMed] [CrossRef]
Yang C, Gu Z, Ding R, Huang C, Li Q, Xie D, Zhang R, Qiu Y (2021). Long non-coding RNA MEG3 silencing and microRNA-214 restoration elevate osteoprotegerin expression to ameliorate osteoporosis by limiting TXNIP. Journal of Cellular and Molecular Medicine 25: 2025–2039. https://doi.org/10.1111/jcmm.16096 [Google Scholar] [PubMed] [CrossRef]
Yao Z, An W, Moming A, Tuerdi M (2022). Long non-coding RNA TUG1 knockdown repressed the viability, migration and differentiation of osteoblasts by sponging miR-214. Experimental and Therapeutic Medicine 23: 203. https://doi.org/10.3892/etm.2022.11126 [Google Scholar] [PubMed] [CrossRef]
Yao X, Wu L, Gu Z, Li J (2020). LINC01535 promotes the development of osteosarcoma through modulating miR-214-3p/KCNC4 Axis. Cancer Management and Research 12: 5575–5585. https://doi.org/10.2147/CMAR.S232757 [Google Scholar] [PubMed] [CrossRef]
Yao S, Zhao W, Ou Q, Liang L, Lin X, Wang Y (2017). MicroRNA-214 suppresses osteogenic differentiation of human periodontal ligament stem cells by targeting ATF4. Stem Cells International 2017: 3028647. https://doi.org/10.1155/2017/3028647 [Google Scholar] [PubMed] [CrossRef]
Ye Y, Ke Y, Liu L, Xiao T, Yu J (2021). CircRNA FAT1 regulates osteoblastic differentiation of periodontal ligament stem cells via miR-4781-3p/SMAD5 pathway. Stem Cells International 2021: 5177488. https://doi.org/10.1155/2021/5177488 [Google Scholar] [PubMed] [CrossRef]
Yuan Y, Guo J, Zhang L, Tong X, Zhang S et al. (2019). MiR-214 attenuates the osteogenic effects of mechanical loading on osteoblasts. International Journal of Sports Medicine 40: 931–940. https://doi.org/10.1055/a-1015-0285 [Google Scholar] [PubMed] [CrossRef]
Zhang S, Liu Y, Zheng Z, Zeng X, Liu D, Wang C, Ting K (2018). MicroRNA-223 suppresses osteoblast differentiation by inhibiting DHRS3. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 47: 667–679. https://doi.org/10.1159/000490021 [Google Scholar] [PubMed] [CrossRef]
Zhang B, Shetti D, Fan C, Wei K (2019). miR-29b-3p promotes progression of MDA-MB-231 triple-negative breast cancer cells through downregulating TRAF3. Biological Research 52: 1–12. https://doi.org/10.1186/s40659-019-0245-4 [Google Scholar] [PubMed] [CrossRef]
Zhong D, Xu GZ, Wu JZ, Liu H, Tang JY, Wang CG (2021). Circ-ITCH sponges miR-214 to promote the osteogenic differentiation in osteoporosis via upregulating YAP1. Cell Death & Disease 12: 340. https://doi.org/10.1038/s41419-021-03586-y [Google Scholar] [PubMed] [CrossRef]
Zhou Y, Li X, Yang H (2020). LINC00612 functions as a ceRNA for miR-214-5p to promote the proliferation and invasion of osteosarcoma in vitro and in vivo. Experimental Cell Research 392: 112012. https://doi.org/10.1016/j.yexcr.2020.112012 [Google Scholar] [PubMed] [CrossRef]
Zhu L, Xu PC (2013). DownregulatedLncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochemical and Biophysical Research Communications 432: 612–617. https://doi.org/10.1016/j.bbrc.2013.02.036 [Google Scholar] [PubMed] [CrossRef]
Zhu XB, Zhang ZC, Han GS, Han JZ, Qiu DP (2017). Overexpression of miR‐214 promotes the progression of human osteosarcoma by regulating the Wnt/β‐catenin signaling pathway. Molecular Medicine Reports 15: 1884–1892. https://doi.org/10.3892/mmr.2017.6203 [Google Scholar] [PubMed] [CrossRef]
Zhu X, Zhao Z, Zeng C, Chen B, Huang H et al. (2020). HNGF6A inhibits oxidative stress-induced MC3T3-E1 cell apoptosis and osteoblast phenotype inhibition by targeting Circ_0001843/miR-214 pathway. Calcified Tissue International 106: 518–532. https://doi.org/10.1007/s00223-020-00660-z [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools