 Open Access
Open Access
REVIEW
Control of tendon cell fate in the embryonic limb: A molecular perspective
Departamento de Medicina Genómica y Toxicología Ambiental, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México Ciudad Universitaria, Apartado Postal 70228, México, DF 04510, México
* Corresponding Authors: JESSICA CRISTINA MARÍN-LLERA. Email: ; JESÚS CHIMAL-MONROY. Email:
(This article belongs to the Special Issue: Cellular and Molecular Toxicology in Reproductive and Developmental Biology)
BIOCELL 2023, 47(3), 465-471. https://doi.org/10.32604/biocell.2023.024625
Received 02 June 2022; Accepted 05 September 2022; Issue published 03 January 2023
Abstract
The molecular cascade underlying tendon formation starts when progenitor cells begin to express the Scleraxis (Scx) gene. Scx knockout mice develop some but not all tendons, suggesting that additional factors are necessary for tendon commitment, maintenance, and differentiation. Other transcription factors, such as Mohawk (Mkx) or early growth response (Egr), maintain Scx expression and extracellular matrix formation during fibrillogenesis. The inhibition of wingless and int-related protein signaling is necessary and sufficient to induce the expression of Scx. Once the commitment of tenogenic lineage occurs, transforming growth factor-beta (TGFβ) induces the Scx gene expression, becoming involved in the maintenance of tendon cell fate. From this point of view, we discussed two phases of the tenogenic process during limb development: dependent and independent of mechanical forces. Finally, we highlight the importance of understanding embryonic tendon development to improve therapeutic strategies in regenerative medicines for tendons.Keywords
The formation of the musculoskeletal system during limb development is a paradigmatic model for studying cell differentiation, morphogenesis, and patterning. At the onset of limb formation, cartilage and tendon progenitor cells arise from the lateral plate mesoderm while the limb bud forms. Concomitant with the establishment of the limb primordium, the commitment of mesodermal cells is controlled by three signaling centers that coordinate the spatial distribution and patterning of differentiating tissue. The apical ectodermal ridge (AER) regulates the proximo-distal axis and limb outgrowth, maintaining the cells underneath the AER in a multipotent, proliferative state; this region is referred to as the undifferentiated zone. The dorsal and ventral ectoderm coordinate to establish the limb’s dorsal and ventral polarity. Finally, the zone of polarizing activity provides the pattern formed according to the anterior and posterior polarity of the limb (McQueen and Towers, 2020; Marin-Llera et al., 2019).
The fine-tuned control of proliferation and differentiation influenced by signals from the ectoderm forms the distinct anatomical regions of the limb and its tissue components (Fig. 1A). In each anatomically-distinct area, the differentiation of mesodermal cells initiates once signals from the ectoderm cease (McQueen and Towers, 2020; Cooper et al., 2011; Dudley et al., 2002). Besides, mesodermal cells are kept under a proliferative, undifferentiated state by the action of fibroblast growth factor (FGF) and wingless and int-related protein (WNT) signaling (ten Berge et al., 2008). Cartilage commitment occurs at the core of the limb and gives rise to skeletal elements. In contrast, tendon differentiation occurs between these skeletal elements and the ectodermal surface of the limb (Fig. 1B) (Hurle et al., 1990).
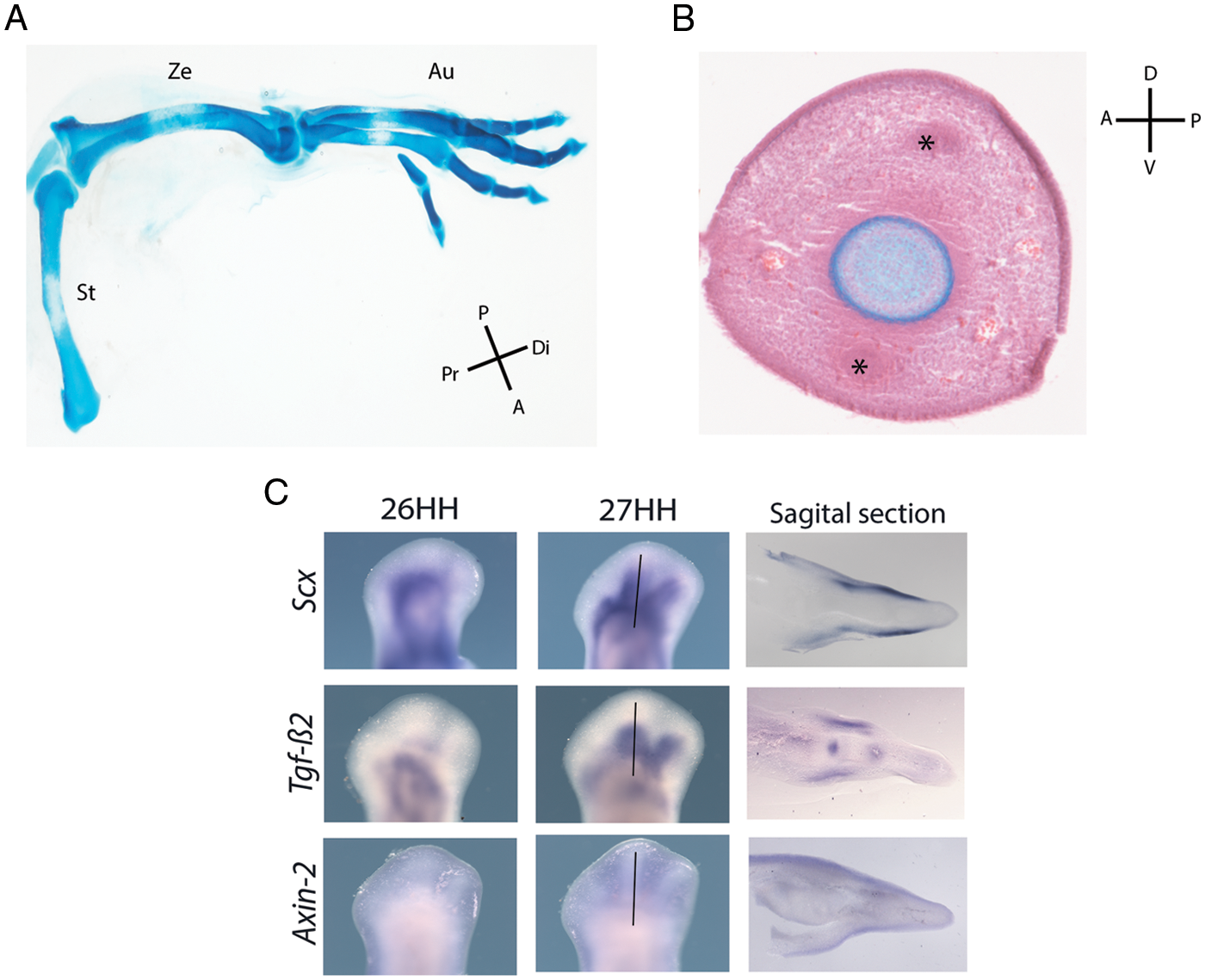
Figure 1: Limb anatomy and tendon cell fate. (A) Anatomical regions of a chicken hindlimb stained with Alcian blue. The most proximal element, the stylopod (St), is identified as a single skeletal element; two central skeletal elements in the zeugopod (Ze), and the autopod (Au), are characterized by the most distal and highly segmented skeletal elements. Proximal (Pr), distal (Di), posterior (P), and anterior (A). (B) Transversal section of a chicken digit stained with Alcian blue and hematoxylin and eosin. Undifferentiated mesodermal cells, blood vessels, and ectoderm surround the skeletal element. The asterisks denote the ventral and dorsal tendon blastema, positioned between the ectoderm and the skeletal element. Dorsal (D); ventral (V); anterior (A), and posterior (P). (C) In situ hybridization showing the expression pattern of Scx, Tgfb2, and Axin2 in whole-mount chicken hindlimbs at 26 and 27 HH stages and sagittal section at stage 27 HH (see the black line as a reference) (Scx: Scleraxis; Tgfb2: transforming growth factor beta 2; Axin2: a protein involved in the negative control of WNT β-catenin signaling).
Tendons are difficult to heal due to their relatively acellular and avascular nature. After an injury, tendons form scar tissue and ectopic bone without regenerating the original tendon structure with low mechanical properties. Numerous efforts to promote tendon healing techniques such as PRP (platelet-rich plasma), stem cells, scaffolds, gene therapy, gel and cell sheets, and scaffolds have been well documented (Lakhani et al., 2021; He et al., 2022). However, in-depth knowledge of cellular and molecular processes during tendon development is essential to improve therapeutic strategies.
Since the early cellular processes underlying tendon formation in the limb have been characterized (Hurle et al., 1990), an early molecular marker characteristic of this developmental progression has been identified (Schweitzer et al., 2001). Therefore, the mechanisms that dictate the specification of mesodermal cells in tendon progenitor cells warrant further investigation.
From histological to molecular events during tendon formation
Tendons connect skeletal muscles to bones and transmit the mechanical force of muscular contraction to produce movement, whereas the ligaments align bones within joints to maintain their stability (Murchison et al., 2007). Tendons are connective tissues rich in extracellular matrix (ECM) components, mainly collagen types I and III, tenascin, and fibronectin (Hurle et al., 1990).
The first histological event observed during tendon differentiation is the formation of an ECM scaffold, particularly in the long autopodial tendons that correspond to extensor (dorsal) and flexor (ventral) tendons in developing autopod chick embryos (Hurle et al., 1990). Together with the establishment of digital rays during tendon development, the formation of a thick ectodermal-mesodermal lamina rich in tenascin occurs. Finally, mesodermal cells condensate to originate the tendon blastema around this lamina (Hurle et al., 1990).
The earliest molecular steps of tenogenesis involve the recruitment of progenitor cells and are muscle-independent. In contrast, once the tendon tissue is established, the subsequent tendon development depends on the presence of muscle; in its absence, tendon development is arrested. Thus, the first stage of tendon differentiation does not need the mechanical load; the second stage does (reviewed by Felsenthal and Zelzer, 2017).
Sox9 (SRY-Box transcription factor 9) and Scleraxis (Scx; bHLH transcription factor) are the master genes that regulate chondrogenesis (Akiyama et al., 2002; Akiyama et al., 2005) and tenogenesis, respectively (Schweitzer et al., 2001; Liu et al., 2021). Chondrocytes and tenocytes differentiate from a common precursor expressing both master genes (Sox9+/Scx+) (Sugimoto et al., 2013). When Sox9+/Scx+ cells enter the tendon differentiation program, Sox9 ceases to be expressed while Scx expression is maintained (Blitz et al., 2013). In contrast, precursor cells near the skeletal elements become Sox9+, differentiating down the cartilage lineage; the expression of Sox9 is initially maintained while Scx ceases to be expressed (Takimoto et al., 2012; Blitz et al., 2013; Sugimoto et al., 2013). Mesodermal cells commit to the tendon fate after WNT signals emanating from the dorsal and ventral ectoderm stop receiving (ten Berge et al., 2008). In this sense, the molecular processes that trigger tenogenic differentiation start with Scx expression, as observed in all tendon precursors of the limb (Schweitzer et al., 2001).
Transforming growth factor-beta (TGFβ) family members are expressed in the tendon blastema (Merino et al., 1998; Pryce et al., 2009). Although the onset of the tendon differentiation program occurs in conditional deletion of TgfbR2 in cells expressing paired-related homeobox 1 (PRRX1) and double mutants for Tgfb2−/−/Tgfb3−/− (Pryce et al., 2009), most tendons and ligaments are lost after, suggesting a role for TGFβ in maintaining Scx expression. Thus, its role appears as a permissive factor that induces Scx gene expression in committed cells to the tenogenic lineage or maintains the tendon cell fate (Garcia-Lee et al., 2021; Pryce et al., 2009). However, its participation in the recruitment of new progenitors cannot be ruled out because of the robust role of TGFβ in inducing Scx gene expression in a short time (Pryce et al., 2009). Interestingly, Scx is needed to form long tendons and those responsible for transmitting musculoskeletal force in the limbs, trunk, and tail, but not the tendons anchoring muscle (Murchison et al., 2007). Thus, other transcription factors may be required to induce tendon differentiation independently of Scx gene expression. Transcriptomic analysis of developing mouse limb tendon cells and gain and loss of function experiments suggest that TGFβ via the suppressor of mothers against decapentaplegic (SMAD 2/3) signaling is sufficient to induce Scx expression during tendon development in mouse limb explants and C3H10T1/2 cells (Havis et al., 2014).
Interestingly, the overexpression of Sox9 in tenocytes promotes its conversion to chondrocytes (Soeda et al., 2010; Takimoto et al., 2012). Thus, the ability of precursor cells to start the chondrogenesis or tenogenesis program depends on the inducer. However, TGFβ induces the ectopic expression of Sox9 and Scx gene expression when implanted in the third interdigit in chick embryos or micromass cultures, suggesting that cell fate between chondrogenesis or tenogenesis is finely regulated via two SMAD-interacting proteins, transforming growth-interacting factor (TGIF) and ski novel gene (SnoN), that negatively regulate the TGFβ signaling pathway. TGIF directs precursor cells to enter the tendon differentiation program instead of chondrogenesis (Lorda-Diez et al., 2009). Thus, cells may commit to following either the tenogenic or chondrogenic differentiation program in response to the TGFβ signaling threshold.
Role of early growth response (EGR) and Mohawk (MKX) in the tendon differentiation program
The homeodomain protein MKX and zinc-finger protein EGR are involved in tendon development (Ito et al., 2010; Liu et al., 2010; Lejard et al., 2011; Guerquin et al., 2013). Mkx gene is expressed after the expression of Scx occurs. Knocking out Mkx does not affect the formation of tendons but causes defects in type I collagen fibrils and other ECM components such as lumican, decorin, and fibromodulin, which affects the growth and mass of tendons (Ito et al., 2010). Therefore, while SCX is necessary for the onset of tenogenesis in some tendons, MKX is not required to ensure its differentiation. Given that MKX promotes the expression of Scx by binding to the Tgfb2 promoter, Mkx gene expression is required during tendon development (Liu et al., 2015). Also, MKX regulates Sox9 by repressing its expression (Suzuki et al., 2016). Consequently, chondrocyte differentiation is inhibited, as demonstrated in Mkx−/− rats undergoing cell transdifferentiation from tenocytes to chondrocytes, which leads to early tendon ossification (Suzuki et al., 2016).
EGR1 and EGR2 are two DNA-binding proteins involved in embryonic tendon formation. Their genes share sequence homology with the Stripe gene expressed in the tendons of Drosophila (Lejard et al., 2011). Egr1 and Egr2 null and double Egr1/2 mutant mice demonstrate that both control tendon type I collagen transcription and fibrillogenesis. Furthermore, both Egr1 and Egr2 are sufficient to induce Scx gene expression. However, double Egr1/2 mutant mice do not exhibit a tendon phenotype; Scx gene expression is reduced but not inhibited, suggesting that Egr1/2 is not involved in the onset of tendon differentiation (Lejard et al., 2011). Remarkably, the TGFβ signaling pathway is activated after the overexpression of Egr1 in cell culture, like MKX, since Egr1 is enriched at the Tgfb2 promoter (Guerquin et al., 2013). Given that TGFβ also induces Egr gene expression in chicken limbs in vivo (Lejard et al., 2011), these data support that TGFβ can induce tenogenesis. This signaling pathway seems sufficient but not necessary for initiating tendon differentiation (Garcia-Lee et al., 2021).
Role of the ectoderm and wingless and int-related protein signaling in the onset of the tendon differentiation program
As mentioned above, tendons are positioned between the ectoderm and skeletal elements during limb development, and the first histological evidence of tendon formation is observed between both tissues (Hurle et al., 1990). Thus, signals proceeding from the dorsal and ventral ectoderm of the embryonic limb and skeletal elements may be required to control cell differentiation and its proper location in the limb. Tendon tissue formation is disrupted after removing the ectoderm due to the reduced area of Scx gene expression (Schweitzer et al., 2001). Besides, removing the ectoderm extends the formation of cartilage and connective tissue but not muscle (Geetha-Loganathan et al., 2010). Interestingly, the ectoderm’s molecular signals that inhibit Scx expression belong to the bone morphogenetic protein family (BMP). The inhibition of BMP signaling after applying Noggin, an antagonist of BMP, extends the expression area of the Scx gene and inhibits the molecular markers of muscle (Schweitzer et al., 2001). Another possibility that Scx gene expression is lost is because cell death occurs after ectoderm removal (Fernandez-Teran et al., 2013); progenitor cells are depleted. However, all this data reflects that the ectoderm regulates both the position of tendons and muscles.
Although BMP signaling plays an essential role in controlling cell differentiation of muscle, tendon, and cartilage (reviewed in Wang et al., 2014), other studies indicate that WNT signaling from the ectoderm regulates the onset of tendon differentiation (ten Berge et al., 2008). The WNT signaling pathway is among the signals expressed in the ectodermal tissue. WNT β-catenin signaling regulates connective tissue formation, while the sub-ectodermal mesenchyme is maintained as a pool of progenitors. After sub-ectodermal cells are far away from the WNT-ectodermal signals, presumably Wnt6, the progenitors start the expression of Scx or Sox9, probably through WNT-mediated centripetal patterning of the limb by the surface ectoderm (Geetha-Loganathan et al., 2010). Cells differentiate into tendons or cartilage depending on their proximity to the ectoderm as the primary source of WNT signaling with influence on the mesodermal tissue (ten Berge et al., 2008). Skeletal elements are present in the most central region of the limb, and the tendon is established in the area between skeletal elements and the ectoderm (Hurle et al., 1990). In this context, undifferentiated cells commit to the tendon differentiation program after the WNT signaling inhibition or after the treatment with TGFβ (Garcia-Lee et al., 2021).
Interestingly, when WNT and TGFβ signaling pathways are simultaneously inhibited, the Scx gene expression is observed (Garcia-Lee et al., 2021). This suggests that the molecular cascade of tendon differentiation begins at a certain distance from the ectoderm when the negative influence of WNT signaling in mesodermal tissue is abolished or reduced. Furthermore, TGFβ signaling is permissive as it induces the expression of Axin2; its genic product is involved in the negative control of WNT β-catenin signaling (Garcia-Lee et al., 2021) and also involved in the maintenance of the tendon cell fate (Tan et al., 2020).
Tendon maintenance depends on the mechanical load
The second stage of tendon development is mechanical load dependence. Thus, it requires the presence of muscle and its contraction. In chick embryos, zeugopod or autopod tendon development is arrested in muscle-less limbs. In contrast, in muscle-less limbs of mouse embryos, autopod tendons are normal, and zeugopod tendons are lost (Gaut and Duprez, 2016; Felsenthal and Zelzer, 2017; Bobzin et al., 2021). Interestingly, the zeugopod tendons in mice with muscular dysgenesis do not degenerate but are smaller than normal, with lower Scx gene expression than in normal animals (Gaut and Duprez, 2016; Felsenthal and Zelzer, 2017). The mechanical stimulation in tendon stem/progenitor cells (TSPC) in culture regulates the expression of genes involved in the homeostasis of the TSPC niche, such as ECM and integrin receptors resulting in the control of the expression matrix metalloproteinases (Popov et al., 2015). TGFβ is involved in the maintenance of the tendon development program and possibly in the recruitment of tendon progenitors (Pryce et al., 2009). These authors propose that TGFβ from the muscles may be necessary to maintain tendon progenitors (Pryce et al., 2009). The presence of muscle or muscular contractions promotes the disruption of the ECM of tendons, promoting TGFβ releasing from ECM that also regulates ECM homeostasis and Scx gene expression (Felsenthal and Zelzer, 2017). Mechanical load regulates Egr1 and Mkx expression: the higher mechanical load increases their expression; in contrast, the lower mechanical load reduces it (Gaut et al., 2016; Kayama et al., 2016). Because of mechanical load, EGR1/2 and MKX maintain tendon cell fate by activating the expression of Scx and Col1a1 genes during development (Guerquin et al., 2013). Egr1 is enriched at the Tgfb2 promoter (Guerquin et al., 2013), and because mechanical load regulates EGR1, TGFβ signaling participates in the maintenance of tendon cell identity (Havis and Duprez, 2020; Tan et al., 2020). On the other hand, Mkx knockout mice cannot maintain tenogenic gene expression after mechanical stimuli presenting hindlimb tendons with heterotopic ossification (Kayama et al., 2016; Liu et al., 2019).
The expression patterns of Scx, Tgfb2, and Axin2 enable determining the induction and maintenance signals underlying tendon differentiation in a temporal-spatial manner (Fig. 1C). Tendon development is a highly orchestrated process with deep-seated compensatory mechanisms. This process initially depends on the inhibition of WNT signaling as it is sufficient and necessary for Scx gene expression (Figs. 2A and 2B). In contrast, TGFβ signaling is permissive in inducing and maintaining the tendon differentiation process by promoting Axin2 gene expression and Mkx and Erg1/2 loop regulation; also, the function of TGFβ in the maintenance depends on the mechanical load during tendon development (Figs. 2C and 2D).
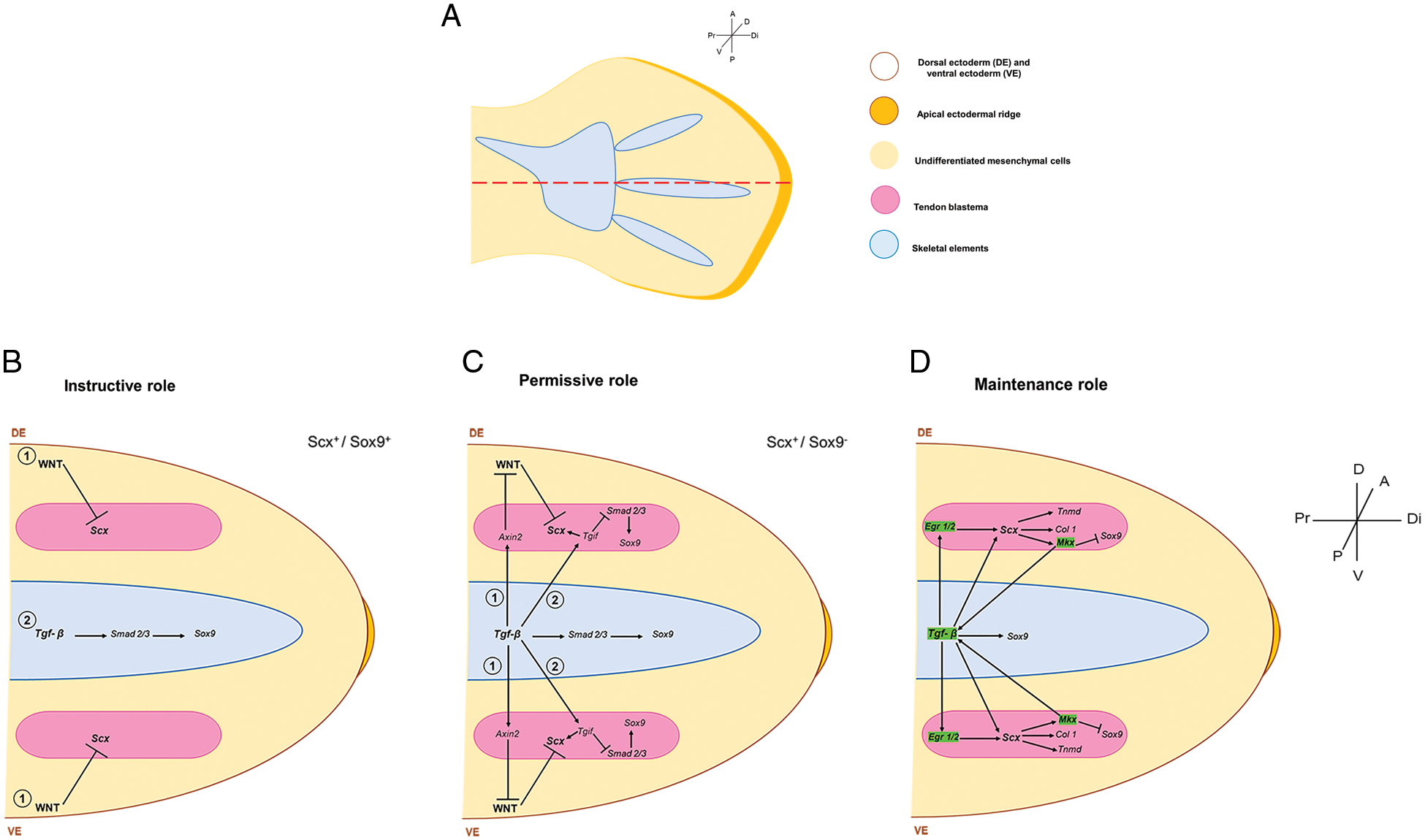
Figure 2: A model of induction and maintenance of the tendon program. (A) Schematic representation of the position of the sagittal section (dotted red line) shown in the models of B-D in a 28 HH chicken hindlimb. Models represent the induction and maintenance of dorsal and, presumably, ventral blastemas. (B) (1) Instructive induction of tendon fate initially depends on inhibiting WNT signaling as it is sufficient and necessary for Scx gene expression. (2) The TGFβ signal induces Sox9 gene expression during the formation of the skeletal elements. (C) Following Scx induction in progenitor cells, the signal emanating from the skeletal elements plays a permissive role in inducing (1) Axin and (2) Tgif to promote Scx gene expression. (D) For maintenance, TGFβ and EGR1/2 cooperate to control tendon differentiation, while Mkx promotes Scx expression by binding to the Tgfb2 promoter. The mechanical load induces green-highlighted genes during tendon development. Thus, TGFβ plays a role in maintaining the tendon differentiation program once established the tendon fate. (Scx: Scleraxis; Mkx: Mohawk transcription factor; Tgfb2: transforming growth factor beta 2; Axin2: a protein involved in the negative control of WNT β-catenin signaling).
The literature demonstrates the scientific community’s interest in a safe therapeutic approach with high tendon regenerative potential. Although many aspects of tendon development have been described, more specific elements are continuously being discovered that require constant further exploration; for example, Dact proteins are suggested as adaptor proteins that modulate WNT and TGFβ signaling during limb development (Sensiate et al., 2014). The complete signaling pathway that drives tendon formation is still unknown. Future studies in this arena should provide insight into Scx-mediated control of tenogenic differentiation, regulation of tendon migration to their insertion sites in muscles, and the spatial organization of tendon fibers. Translating regenerative tendon therapies from bench to bed requires a deeper understanding of the cellular processes during embryonic tendon development (Ideo et al., 2020). This knowledge would provide cues to promote tendon regeneration by improving therapeutic strategies following the routes of tendon developmental programs.
Author Contributions: JCM-L and JC-M wrote the manuscript, and CAJ-C and JCM-L prepared the figures. All authors approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: This work was supported by the Dirección General de Asuntos del Personal Académico (DGAPA)-Universidad Nacional Autónoma de México [Grant No. IN213314] and Consejo Nacional de Ciencia y Tecnología (CONACyT) [Grant No. 1887 CONACyT-Fronteras de la Ciencia] awarded to JC-M.
Conflicts of Interest: The authors declare that they have no conflicts of interest.
References
Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes & Development 16: 2813–2828. DOI 10.1101/gad.1017802. [Google Scholar] [CrossRef]
Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N et al. (2005). Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proceedings of the National Academy of Sciences 102: 14665–14670. DOI 10.1073/pnas.0504750102. [Google Scholar] [CrossRef]
ten Berge D, Brugmann SA, Helms JA, Nusse R (2008). Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development 135: 3247–3257. DOI 10.1242/dev.023176. [Google Scholar] [CrossRef]
Blitz E, Sharir A, Akiyama H, Zelzer E (2013). Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140: 2680–2690. DOI 10.1242/dev.093906. [Google Scholar] [CrossRef]
Bobzin L, Roberts RR, Chen HJ, Crump JG, Merrill AE (2021). Development and maintenance of tendons and ligaments. Development 148: dev186916. DOI 10.1242/dev.186916. [Google Scholar] [CrossRef]
Cooper KL, Hu JK, ten Berge D, Fernandez-Teran M, Ros MA, Tabin CJ (2011). Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science 332: 1083–1086. DOI 10.1126/science.1199499. [Google Scholar] [CrossRef]
Dudley AT, Ros MA, Tabin CJ (2002). A re-examination of proximodistal patterning during vertebrate limb development. Nature 418: 539–544. DOI 10.1038/nature00945. [Google Scholar] [CrossRef]
Felsenthal N, Zelzer E (2017). Mechanical regulation of musculoskeletal system development. Development 144: 4271–4283. DOI 10.1242/dev.151266. [Google Scholar] [CrossRef]
Fernandez-Teran M, Ros MA, Mariani FV (2013). Evidence that the limb bud ectoderm is required for survival of the underlying mesoderm. Developmental Biology 381: 341–352. DOI 10.1016/j.ydbio.2013.06.032. [Google Scholar] [CrossRef]
Garcia-Lee V, Díaz-Hernandez ME, Chimal-Monroy J (2021). Inhibition of WNT/β-catenin is necessary and sufficient to induce Scx expression in developing tendons of chicken limb. The International Journal of Developmental Biology 65: 395–401. DOI 10.1387/ijdb.200166jc. [Google Scholar] [CrossRef]
Gaut L, Duprez D (2016). Tendon development and diseases. Wiley Interdisciplinary Reviews: Developmental Biology 5: 5–23. DOI 10.1002/wdev.201. [Google Scholar] [CrossRef]
Gaut L, Robert N, Delalande A, Bonnin MA, Pichon C, Duprez D (2016). EGR1 regulates transcription downstream of mechanical signals during tendon formation and healing. PLoS One 11: e0166237. DOI 10.1371/journal.pone.0166237. [Google Scholar] [CrossRef]
Geetha-Loganathan P, Nimmagadda S, Christ B, Huang R, Scaal M (2010). Ectodermal Wnt6 is an early negative regulator of limb chondrogenesis in the chicken embryo. BMC Developmental Biology 10: 32. DOI 10.1186/1471-213X-10-32. [Google Scholar] [CrossRef]
Guerquin MJ, Charvet B, Nourissat G, Havis E, Ronsin O et al. (2013). Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. Journal of Clinical Investigation 123: 3564–3576. DOI 10.1172/JCI67521. [Google Scholar] [CrossRef]
Havis E, Bonnin MA, Olivera-Martinez I, Nazaret N, Ruggiu M et al. (2014). Transcriptomic analysis of mouse limb tendon cells during development. Development 141: 3683–3696. DOI 10.1242/dev.108654. [Google Scholar] [CrossRef]
He P, Ruan D, Huang Z, Wang C, Xu Y et al. (2022). Comparison of tendon development versus tendon healing and regeneration. Frontiers in Cell and Developmental Biology 10: 821667. DOI 10.3389/fcell.2022.821667. [Google Scholar] [CrossRef]
Havis E, Duprez D (2020). EGR1 transcription factor is a multifaceted regulator of matrix production in tendons and other connective tissues. International Journal of Molecular Sciences 21: 1664. DOI 10.3390/ijms21051664. [Google Scholar] [CrossRef]
Hurle JM, Ros MA, Gañan Y, Macias D, Critchlow M, Hinchliffe JR (1990). Experimental analysis of the role of ECM in the patterning of the distal tendons of the developing limb bud. Cell Differentiation and Development 30: 97–108. DOI 10.1016/0922-3371(90)90078-B. [Google Scholar] [CrossRef]
Ideo K, Tokunaga T, Shukunami C, Takimoto A, Yoshimoto Y et al. (2020). Role of Scx+/Sox9+ cells as potential progenitor cells for postnatal supraspinatus enthesis formation and healing after injury in mice. PLoS One 15: e0242286. DOI 10.1371/journal.pone.0242286. [Google Scholar] [CrossRef]
Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T et al. (2010). The Mohawk homeobox gene is a critical regulator of tendon differentiation. PNAS 107: 10538–10542. DOI 10.1073/pnas.1000525107. [Google Scholar] [CrossRef]
Kayama T, Mori M, Ito Y, Matsushima T, Nakamichi R, Suzuki H, Ichinose S, Saito M, Marumo K, Asahara H (2016). Gtf2ird1-dependent mohawk expression regulates mechanosensing properties of the tendon. Molecular and Cellular Biology 36: 1297–1309. DOI 10.1128/MCB.00950-15. [Google Scholar] [CrossRef]
Lakhani A, Sharma E, Kapila A, Khatri K (2021). Known data on applied regenerative medicine in tendon healing. Bioinformation 17: 514–527. DOI 10.6026/97320630017514. [Google Scholar] [CrossRef]
Lejard V, Blais F, Guerquin MJ, Bonnet A, Bonnin M-A et al. (2011). EGR1 and EGR2 involvement in vertebrate tendon differentiation. Journal of Biological Chemistry 286: 5855–5867. DOI 10.1074/jbc.M110.153106. [Google Scholar] [CrossRef]
Liu H, Zhang C, Zhu S, Lu P, Zhu T et al. (2015). Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFβ signaling pathway. Stem Cells 33: 443–455. DOI 10.1002/stem.1866. [Google Scholar] [CrossRef]
Liu H, Xu J, Jiang R (2019). Mkx-deficient mice exhibit Hedgehog signaling-dependent ectopic ossification in the Achilles tendons. Journal of Bone and Mineral Research 34: 557–569. DOI 10.1002/jbmr.3630. [Google Scholar] [CrossRef]
Liu H, Xu J, Lan Y, Lim H-W, Jiang R (2021). The scleraxis transcription factor directly regulates multiple distinct molecular and cellular processes during early tendon cell differentiation. Frontiers in Cell and Developmental Biology 9: 654397. DOI 10.3389/fcell.2021.654397. [Google Scholar] [CrossRef]
Liu W, Watson SS, Lan Y, Keene DR, Ovitt CE, Liu H, Schweitzer R, Jiang R (2010). The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Molecular and Cellular Biology 30: 4797–4807. DOI 10.1128/MCB.00207-10. [Google Scholar] [CrossRef]
Lorda-Diez CI, Montero JA, Martinez-Cue C, Garcia-Porrero JA, Hurle JM (2009). Transforming growth factors β coordinate cartilage and tendon differentiation in the developing limb mesenchyme. Journal of Biological Chemistry 284: 29988–29996. DOI 10.1074/jbc.M109.014811. [Google Scholar] [CrossRef]
Marin-Llera JC, Garciadiego-Cazares D, Chimal-Monroy J (2019). Understanding the cellular and molecular mechanisms that control early cell fate decisions during appendicular skeletogenesis. Frontiers in Genetics 10: 1–17. DOI 10.3389/fgene.2019.00977. [Google Scholar] [CrossRef]
McQueen C, Towers M (2020). Establishing the pattern of the vertebrate limb. Development 47: dev177956. DOI 10.1242/dev.177956. [Google Scholar] [CrossRef]
Merino R, Ganan Y, Macias D, Economides AN, Sampath KT, Hurle JM (1998). Morphogenesis of digits in the avian limb is controlled by FGFs, TGFbetas, and noggin through BMP signaling. Developmental Biology 200: 35–45. DOI 10.1006/dbio.1998.8946. [Google Scholar] [CrossRef]
Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R (2007). Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134: 2697–2708. DOI 10.1242/dev.001933. [Google Scholar] [CrossRef]
Popov C, Burggraf M, Kreja L, Ignatius A, Schieker M, Docheva D (2015). Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Molecular Biology 16: 6. DOI 10.1186/s12867-015-0036-6. [Google Scholar] [CrossRef]
Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R (2009). Recruitment and maintenance of tendon progenitors by TGFβ signaling are essential for tendon formation. Development 136: 1351–1361. DOI 10.1242/dev.027342. [Google Scholar] [CrossRef]
Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin C (2001). Analysis of the tendon cell fate. Development 128: 3855–3866. DOI 10.1242/dev.128.19.3855. [Google Scholar] [CrossRef]
Sensiate LA, Sobreira DR, Da Veiga FC, Peterlini DJ, Pedrosa AV et al. (2014). Dact gene expression profiles suggest a role for this gene family in integrating Wnt and TGF-β signaling pathways during chicken limb development. Developmental Dynamics 243: 428–439. DOI 10.1002/dvdy.23948. [Google Scholar] [CrossRef]
Soeda T, Deng JM, de Crombrugghe B, Behringer RR, Nakamura T, Akiyama H (2010). Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis 48: 635–644. DOI 10.1002/dvg.20667. [Google Scholar] [CrossRef]
Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, Hiraki Y, Shukunami C (2013). Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140: 2280–2288. DOI 10.1242/dev.096354. [Google Scholar] [CrossRef]
Suzuki H, Ito Y, Shinohara M, Yamashita S, Ichinose S et al. (2016). Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proceedings of the National Academy of Sciences 113: 7840–7845. DOI 10.1073/pnas.1522054113. [Google Scholar] [CrossRef]
Takimoto A, Oro M, Hiraki Y, Shukunami C (2012). Direct conversion of tenocytes into chondrocytes by Sox9. Experimental Cell Research 318: 1492–1507. DOI 10.1016/j.yexcr.2012.04.002. [Google Scholar] [CrossRef]
Tan G-K, Pryce BA, Stabio A, Brigande JV, Wang CJ, Xia Z, Tufa SF, Keene DR, Schweitzer R (2020). Tgfβ signaling is critical for maintenance of the tendon cell fate. eLlife 9: e52695. DOI 10.7554/eLife.52695. [Google Scholar] [CrossRef]
Wang RN, Green J, Wang Z, Deng Y, Qiao M et al. (2014). Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes & Diseases 1: 87–105. DOI 10.1016/j.gendis.2014.07.005. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools