 Open Access
Open Access
ARTICLE
The peptide fraction of Bothrops jararaca snake venom induces toxicological effects on the male reproductive system after local envenomation in mice
1 Natural and Humanities Sciences Center, Experimental Morphophysiology Laboratory, Federal University of ABC (UFABC), São Bernardo do Campo, 09606-070, Brazil
2 Departamento de Biofísica, Universidade Federal de São Paulo, São Paulo, 04023-062, Brazil
* Corresponding Author: CARLOS ALBERTO-SILVA. Email:
(This article belongs to the Special Issue: Cellular and Molecular Toxicology in Reproductive and Developmental Biology)
BIOCELL 2023, 47(2), 289-295. https://doi.org/10.32604/biocell.2023.023787
Received 15 May 2022; Accepted 23 August 2022; Issue published 18 November 2022
Abstract
Bothrops envenomation is complex and provokes prominent local tissue damage and systemic disturbances, but little is known about their effects on the male reproductive system. After intratesticular injection, the bioactive peptide fraction (Bj-PF) obtained from Bothrops jararaca snake venom changes the structure of different stages of the seminiferous epithelium cycle in adult mice. For the first time, we investigated whether local envenomation of Bj-PF induces toxicological effects on the male reproductive system, particularly on the seminiferous epithelium and Sertoli cells. Male adult mice were treated with 0.24 mg.kg−1 by intramuscular (i.m.) injection for 24 h. The testes samples were collected for morphological and morphometric evaluation. The toxicological effects of Bj-PF were also analyzed on mitochondrial metabolism and nitrite (NO2) production in 15P-1 Sertoli cell culture. Bj-PF changed the structure and function of the seminiferous epithelium, particularly the disruption of the epithelium and the presence of degenerated germ cells in the adluminal compartment, but there were no alterations in the basal compartment. Bj-PF increased the thickness of the seminiferous epithelium and decreased the lumen diameter of the tubule. Semiquantitative histological assessment of the degree of tubule degeneration revealed that Bj-PF also increased the number of hypospermatogenic tubules compared to control. Bj-PF reduced NO2 levels in 15P-1 Sertoli cells without changing the mitochondrial metabolism. Overall, the fact that Bj-PF alters the structure and function of the seminiferous epithelium suggests that bioactive peptides found in B. jararaca snake venom can have toxicological effects on the reproductive systems of affected male mice, providing new insight into the biological characteristics of snake venom and therapeutic strategies for envenomation inflammation.Keywords
Envenomation by snakebite is a serious public health problem that the World Health Organization (WHO) considers a neglected condition (Loi, 2014). Bothrops envenomation causes prominent local tissue damage and systemic disturbances such as hemorrhage, coagulopathies, cardiovascular shock, and renal alterations (Clissa et al., 2001) by the direct action of venom components on the tissues and the release of various endogenous mediators (Russell et al., 1997).
Phospholipase A2, serine proteinase, metalloproteinase, cysteine-rich secretory protein, lectin-like protein, C-type lectin, L-amino acid oxidase, and disintegrins are among the proteins found in the venom of the Bothrops jararaca snake (Fox and Serrano, 2008). Besides these, a variety of pharmacologically active peptides have been identified, such as proline-rich oligopeptides, also known as bradykinin potentiating peptides (BPPs) from the bioactive peptide fraction (Bj-PF) of the venom (Hayashi and Camargo, 2005).
BPPs contain 5 to 13 amino acid residues with a pyroglutamic residue (<E) at the N-terminus and a proline residue at the C-terminus. BPPs longer than seven amino acids share similar features, including a high content of proline (P) residues and the tripeptide sequence Ile–Pro-Pro (IPP)at the C-terminus (Hayashi et al., 2003).
The effects of compounds from B. jararaca snake venom on the male reproductive system have also been studied by our group. BPP-10c (<ENWPHQIPP) showed inhibitory effects on the spermiogenesis without affecting the permeability of the blood-testis barrier (BTB) and the distribution of claudin-1, a protein found at the site of the BTB, in the male Swiss mice (Gilio et al., 2013). Furthermore, the effects of different peptides [BPP-10c (<ENWPHQIPP), BPP-11e (<EARPPHPPIPP), and BPP-AP (<EARPPHPPIPPAP)] were also evaluated after injection into the testicular parenchyma (Alberto-Silva et al., 2015). BPP-10c and BPP-AP showed an intense disruption of the epithelium and a high degree of seminiferous tubule degeneration. Curiously, no morphological or morphometric alterations were observed in animals treated with captopril or BPP-11e (Alberto-Silva et al., 2015).
The toxicological effects of Bj-PF obtained from B. jararaca snake venom, containing a range of <10 kDa compounds, were investigated on the testis of adult mice after intratesticular (i.t.) injection (Alberto-Silva et al., 2020). Bj-PF changed the structure and function of the seminiferous epithelium, indicating a possible inhibition of spermatozoa production; principally in the spermiogenesis stage, without altering claudin-1 distribution in the basal compartment (Alberto-Silva et al., 2020). The effects of local envenomation caused by a Bj-PF on pulmonary mechanics and lung inflammation in mice after intramuscular injection have also been reported in the literature (Alberto-Silva et al., 2022). Bj-PF increased leukocyte influx in the injected muscle region and bronchoalveolar lavage fluid (Alberto-Silva et al., 2022). Furthermore, Bj-PF altered mechanical properties in the lungs, specifically pressure dissipation against lung resistive components, total pressure variation, and static elastance (Alberto-Silva et al., 2022).
Despite significant research on the effects of snake venom on several biological systems, little is known about its effects on the male reproductive system, which allows researchers to analyze the behavior of the seminiferous epithelium in response to the injury caused by this venom.
All chemicals used in this study were of analytical reagent grade (purity higher than 95%) and purchased from Calbiochem-Novabiochem Corporation (USA), Gibco BRL (New York, USA), Fluka Chemical Corp. (Buchs, Switzerland) or Sigma-Aldrich Corporation (St. Louis, MO, USA).
Crude venom and peptide fraction of Bothrops jararaca venom
The crude venom (Bj-CV) was provided by the Laboratory of Herpetology from Butantan Institute (Sao Paulo, Brazil), and was kept at −20°C until use. The Bj-PF was obtained with the filtration system in the molecular cut-off membrane of 10 kDa (Amicon®, Merck-Millipore, Hessen, Germany) and characterized by SDS-PAGE in 15% polyacrylamide gel stained with silver nitrate, gelatinolytic activity, Fourier transform Infrared spectroscopy, and mass spectrometry to confirm the lack of proteolytic enzymes or other proteins (>10 kDa) in the venom (Alberto-Silva et al., 2020; Querobino et al., 2017).
Male Swiss mice aged between 7 to 8 weeks (body weight from 18 to 20 g) were obtained from the Federal University of ABC (São Bernardo do Campo, Brazil). Six animals were housed per cage in a 12-h light/dark cycle, with constant exhaust ventilation (Alesco®, Brazil) and receiving standardized mouse chow (Nuvital Nutrientes Ltda, Brazil) ad libitum. The experiments were carried out in accordance with the standards for the use of laboratory animals in biochemical research and were approved by local authorities (protocol number 09/2013).
Mouse 15P-1 Sertoli cells were purchased from American Type Culture Collection (ATCC®; CRL-2618TM). The cells were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (FBS) at 30°C in a humidified atmosphere containing 95% air and 5% CO2. The culture medium was replaced every two days.
Treatment of animals with Bj-PF
Albino male mice (Swiss) were assigned to groups (six animals per group) and treated with Bj-PF (0.24 mg.kg−1) diluted in sterile 0.5% NaCl (w.v−1). Bj-PF samples were injected into the gastrocnemius muscle (i.m.) using a 0.5-mL syringe and a 30-gauge needle (Ultra-Fine Short Needle, BD, Canada). The control group (n = 6) received only sterile 0.5% NaCl (w.v−1), under the same conditions reported earlier. The control group consisted of treatment with the vehicle only. The dose of Bj-PF used in the experiments was in agreement with Alberto-Silva and his collaborators (Alberto-Silva et al., 2022). After 24 h of treatment, mice were killed by CO2 asphyxiation, and testes were collected for morphological and morphometric analyses of the seminiferous epithelium.
Morphological analyses of seminiferous tubules
Testes were fixed in Bouin’s solution (4% formaldehyde with picric acid, v.v−1) for 8 h, dehydrated in increasing concentrations of alcohol (70% to 95%, v.v−1), and embedded in Paraplast® (Sigma Chemical Company, USA). Histological slices (4 μm in thickness) were stained with either hematoxylin and eosin or Mallory’s trichrome stain for morphological analysis of the seminiferous epithelium. The sections were examined using a photomicroscope (Axioskop 2, Germany), and the images were captured with a Pixera digital camera system (Pixera Corporation, USA) attached to the photomicroscope and a microcomputer (Intel® Pentium®) using the software Adobe Photoshop version 7.0.1 (Adobe Systems, USA).
Semiquantitative histopathological analysis of the seminiferous tubules
The seminiferous tubules were analyzed by semiquantitative histopathological analysis of the seminiferous tubules (Alberto-Silva et al., 2015). Seminiferous tubules of all treatments were classified into four categories: normal tubules (T1); hypospermatogenic tubules (T2); arrested maturation (T3); and tubules containing spermatogonia and Sertoli cells or Sertoli-cell-only tubules (T4). For this purpose, three 4-µm-thick sections of each testis were randomly selected, and 100 tubular cross-sections were examined from each section. Moreover, intertubular compartment morphology, in particular, Leydig cells, blood vessels, lymph vessels, fibroblasts, macrophages, and mast cells, were analyzed.
Morphometric analysis of seminiferous tubules
Eight circular or nearly circular seminiferous tubules were randomly selected in each studied stage per testis. The images captured were analyzed using the software ImageJ (National Institutes of Health, USA) to assess the diameter of the seminiferous tubules (mm), the thickness of the seminiferous epithelium (mm), and the diameter of the seminiferous tubule lumen (mm).
Effects of Bj-PF on mitochondrial metabolism in Sertoli cell
Sertoli cells (15P-1) were seeded onto 96 well plates (SPL life sciences Co., Korea) at 1 × 104 per well. The cells were treated with Bj-PF at concentrations of 0.1, 1, and 10 μg.mL−1 for 24 h of incubation. Cells untreated, which represent only one equal volume of the culture medium, or cells treated with dimethylsulfoxide (DMSO; Sigma-Aldrich, USA) 2.5% (v.v−1) diluted in the medium culture were also included in the experiments. After, the culture medium was removed and added to 50 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (0.5 mg.mL−1, Sigma-Aldrich, USA) per well. The plate was incubated for 2 h at 37°C. Then, the medium was removed and mixed with 150 μL of DMSO to dissolve the formazan crystals (Stockert et al., 2012). After 15 min of homogenization, the cell viability was determined by the absorbance of samples at 590 nm in a microplate reader (Spectramax®).
Effects of Bj-PF on nitrite (NO2) production in Sertoli cell
The NO2 concentration was evaluated using a modified Griess reagent (Sigma-Aldrich, St. Louis, MO) (Green et al., 1982). Cells were seeded in 96-well plates (Nest Biotechnology, Rahway, USA) at 1 × 104 per well and treated with Bj-PF at concentrations of 0.1, 1, and 10 μg.mL−1 for 24 h of incubation. Cells treated with Bj-CV (10 μg.mL−1), sodium nitroprusside (SNP; 1 μM), and untreated (control group) were also included in the experiments. After cell incubations, 60 µL of cell culture supernatant was mixed with 100 µL of modified Griess reagent (0.04 mg.mL−1) and then incubated for 15 min at room temperature. The NaNO2 standard curve was prepared in the range of 0.5 to 60 µM. The absorbance was determined with a microplate reader (Spectramax M3 multi-mode, Molecular Devices, CA, EUA) at 540 nm. Data were expressed as the mean ± SD of NO2 concentration (µM) in all experimental groups from the NaNO2 standard curve.
The software GraphPad Prism (version 4.0; GraphPad Software, Incorporation) was used for statistical analyses. The present study consisted of tests that produced qualitative and quantitative results. Quantitative results were analyzed by ANOVA, followed by Tukey post-test. Differences were considered significant when p < 0.05. All values were expressed as mean ± SD.
Effects of Bj-PF on seminiferous epithelium morphology
The testes of animals treated with NaCl 0.9% (mv−1) exhibited normal testicular tissue in terms of germ cell organization, maintenance of adluminal and basal compartments in the epithelium tubules, and integrity of intertubular compartments (Fig. 1A). In contrast, Bj-PF-treated testes revealed changes in the intertubular compartment, with a substantial increase in space between seminiferous tubules. Bj-PF caused the presence of atypical cells in the lumen, degenerated germ cells in the adluminal compartment, and, most importantly, epithelial disruption in the seminiferous epithelium (Fig. 1B). Despite this, no structural changes were found in the basal compartment.
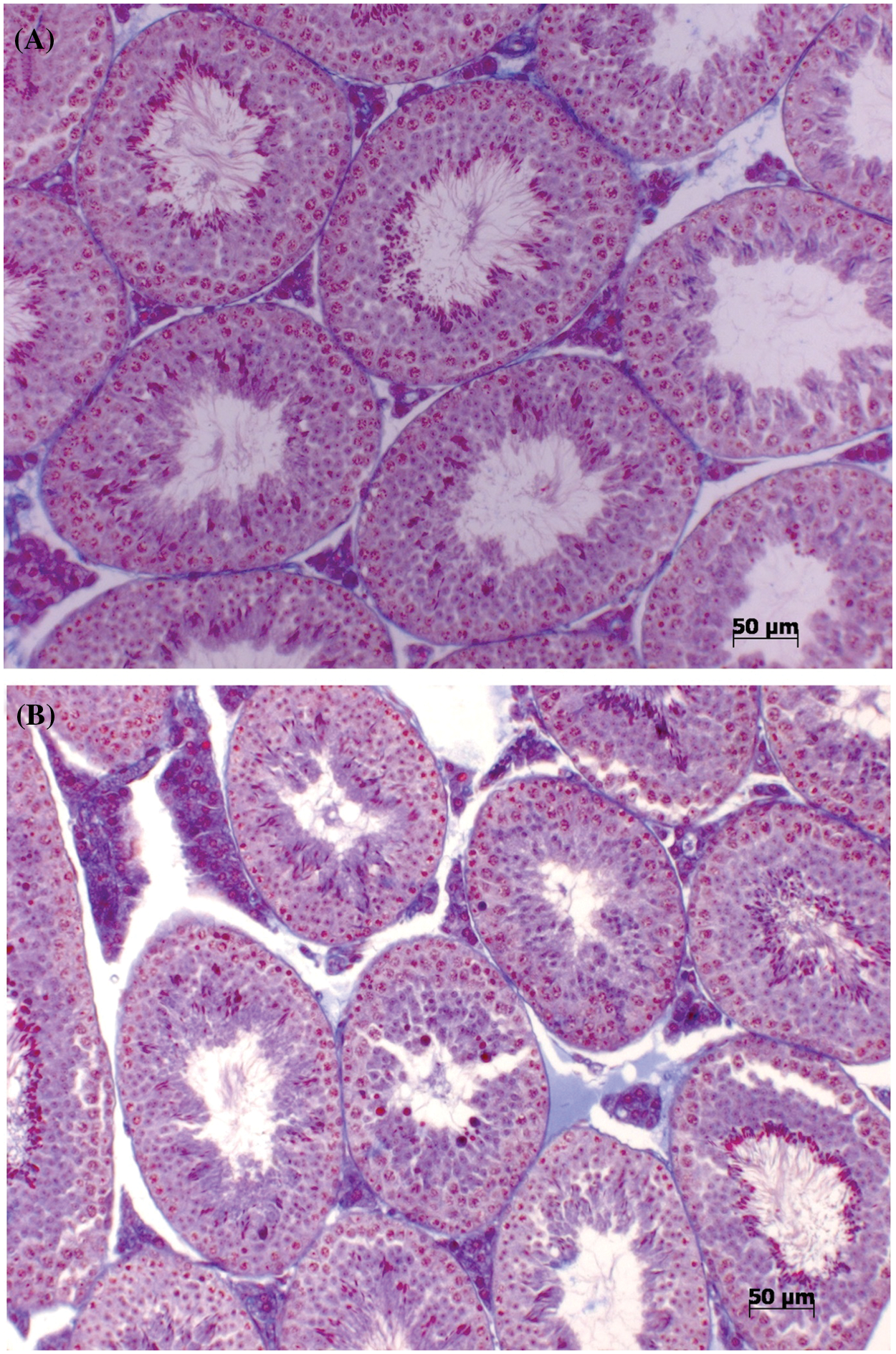
Figure 1: Effects of Bj-PF on seminiferous epithelium morphology in male adult mice after intramuscular injection. Photomicrographs of the seminiferous tubules of male adult mice treated by intramuscular injection with vehicle (A) and Bj-PF (B) stained with Mallory Tricromic. Bj-PF: peptide fraction obtained from crude venom of Bothrops jararaca snake.
Effects of Bj-PF on the degree of tubule degeneration
Bj-PF-induced tubule degeneration was identified into three categories based on the mean of tubule types present in each experimental group when compared to the control (Fig. 2). The presence of Bj-PF reduced the number of normal tubules while increasing the number of hypospermatogenic tubules. Despite this, there was no difference in the number of tubules at maturation. Also, there was no difference in the number of tubules containing spermatogonia and Sertoli cells or Sertoli-cell-only tubules in either group.
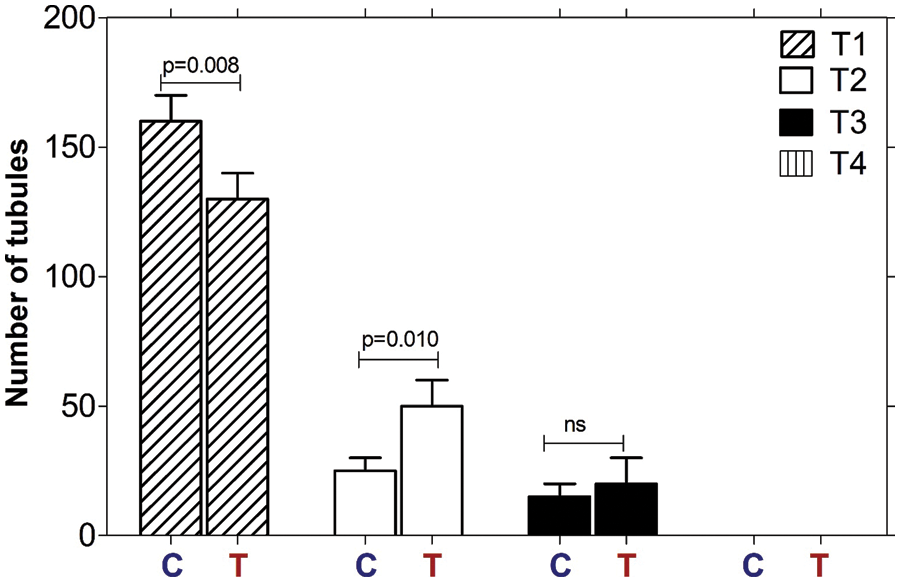
Figure 2: Semiquantitative histological assessment of the degree of tubule degeneration. Seminiferous tubules of all treatments (Vehicle and Bj-PF) were classified into four categories: normal tubules (T1); hypospermatogenic tubules (T2); arrested maturation (T3); and tubules containing spermatogonia and Sertoli cells or Sertoli-cell-only tubules (T4). Data are presented as the mean ± SD, and significant differences as assessed through one-way ANOVA followed by the Tukey post hoc test (*p < 0.05). Bj-PF: peptide fraction obtained from crude venom of Bothrops jararaca snake.
Effects of Bj-PF on morphometric parameters of seminiferous tubules
Bj-PF substantially increased the thickness of the seminiferous epithelium (p = 0.018) and decreased the diameter of the seminiferous tubule lumen (p = 0.014) when compared to the control group (Fig. 3). The diameter of tubules did not change significantly in either treatment group (p = 0.09), although there was a tendency for reduction in the Bj-PF group (Fig. 3).
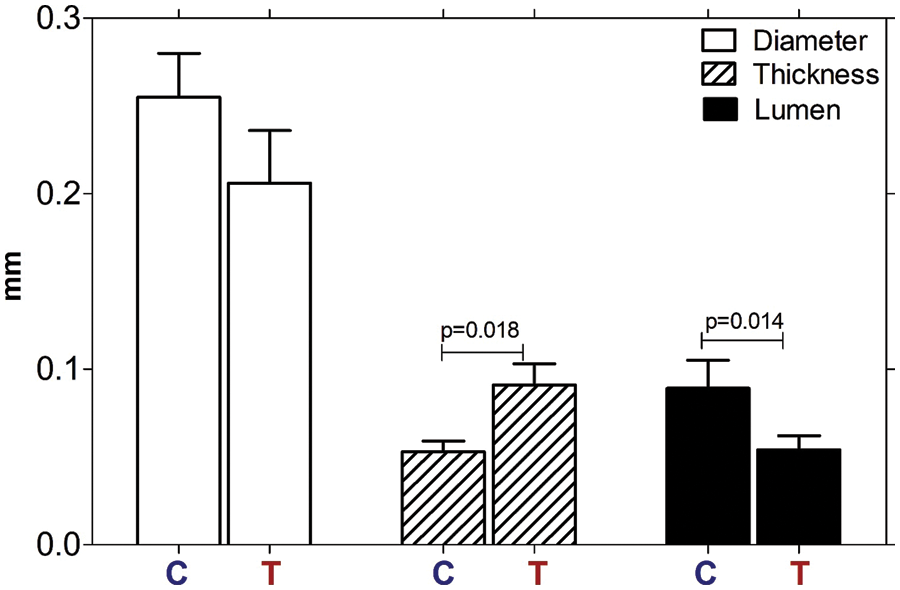
Figure 3: Morphometric analysis of seminiferous tubules of the testis of animals treated with Bj-PF. The thickness of the epithelium, diameter, and lumen of the seminiferous tubules of control and treated animals were analyzed. Values are expressed as mean ± SD and analyzed by one-way ANOVA followed by Tukey’s post-test. C: animals untreated; T: animals treated with Bj-PF. Bj-PF: peptide fraction obtained from crude venom of Bothrops jararaca snake.
Effects of Bj-PF on mitochondrial metabolism in Sertoli cell
The effects of Bj-PF on the mitochondrial metabolism of PC12 cells were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay, which has previously been published (Alberto-Silva et al., 2020; Querobino et al., 2018, 2019). Cells treated with Bj-CV only at 10 µg.mL−1 for 24 h decreased mitochondrial metabolism by 33.15 ± 12.17% when compared to the control group (Fig. 4). In contrast, Bj-PF did not change the mitochondrial metabolism of cells at all concentrations tested. DMSO, a positive control (C+), decreased the mitochondrial metabolism after 24 h compared to the control group, which is in accordance with the literature (Yi et al., 2017).
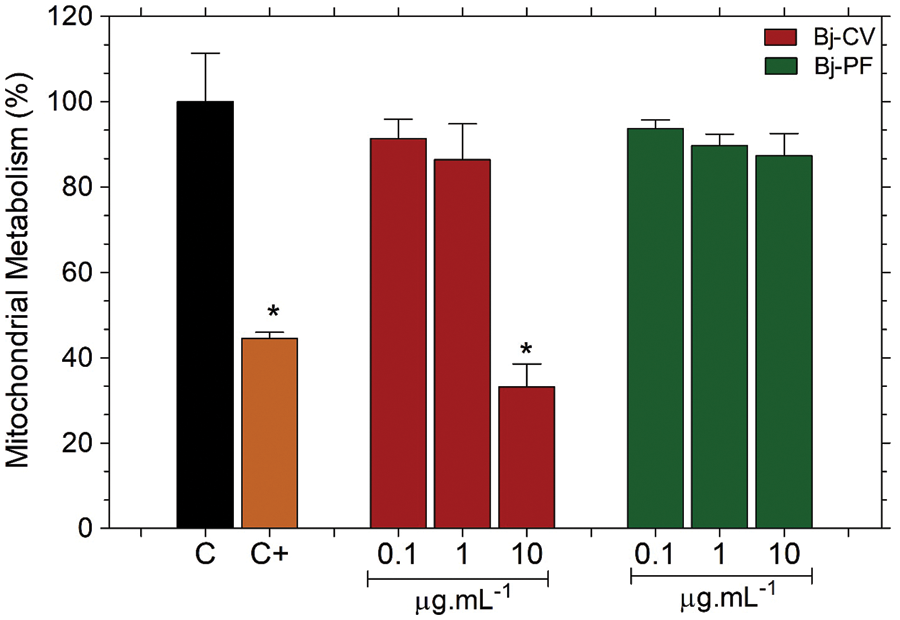
Figure 4: Effects of Bj-PF on mitochondrial metabolism of the 15P-1 Sertoli cells. Cells were seeded at 1 × 104 cells per well in a 96-well plate for 24 h and treated with Bj-PF for 24 h at 37°C. Values are expressed as mean ± SD from three independent experiments in triplicate and analyzed by one-way ANOVA followed by Tukey’s post-test. *p < 0.05 to differences among the control group. Bj-CV: crude venom of Bothrops jararaca snake; Bj-PF: peptide fraction obtained from crude venom.
Effects of Bj-PF on nitrates concentration in Sertoli cell
The nitrite levels in the culture medium of cells exposed to Bj-CV for 24 h increased (1.93 ± 0.14 μM) compared to the group control (0.93 ± 0.15 μM) (Fig. 5). In contrast, Bj-PF reduced the nitrite levels in cells treated with 1 and 10 µg.mL−1 (0.50 ± 0.03 and 0.33 ± 0.18 μM, respectively). Cells treated with the NO donor sodium nitroprusside (SNP) increased nitrite levels, according to reports in the literature (Meroni et al., 2000).
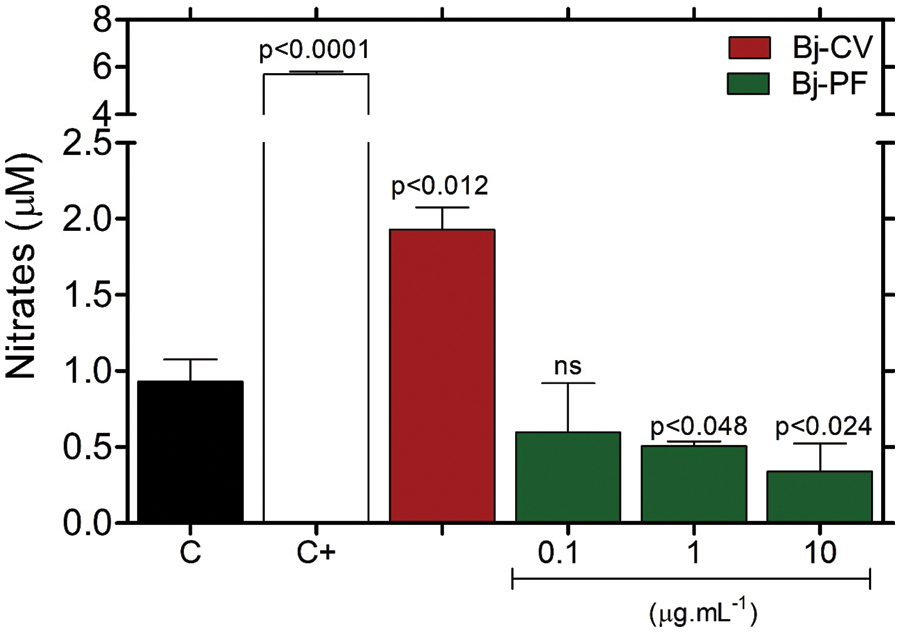
Figure 5: Effects of Bj-CV and Bj-PF on nitrite (NO2) level in the 15P-1 Sertoli cells. Cells were seeded at 1 × 104 cells per well in a 96-well plate for 24 h and treated with Bj-PF, Bj-CV, or NPS for 24 h at 37°C. Values are expressed as mean ± standard deviation from three independent experiments in triplicate and analyzed by one-way ANOVA followed by Tukey’s post-test. C: cells untreated; C+: cells treated with sodium nitroprusside; Bj-CV: crude venom of Bothrops jararaca snake; Bj-PF: peptide fraction obtained from crude venom.
Bothrops species envenomation is well understood (Alberto-Silva et al., 2022; Clissa et al., 2001; Gonçalves and Mariano, 2000; Santoro et al., 2008; Senise et al., 2015; Yamashita et al., 2014), but there has been no research on the likely impact of B. jararaca snake venom on the male reproductive system of victims. To the best of our knowledge, this is the first study to show that physiologically active peptides from B. jararaca snake venom produce testis alterations following local envenomation. In this study, we explored how a peptide fraction obtained from B. jararaca snake venom that contains a variety of smaller than 10 kDa compounds affected the male reproductive system, namely the structural and functional testis of mice following local envenomation. We show that a B. jararaca peptide fraction lacking enzymatic components can alter the structure and function of the seminiferous epithelium, specifically disrupting the epithelium and causing the presence of degenerated germ cells. This might lead to a better understanding of the clinical characteristics of envenomation as well as the discovery and application of a treatment strategy.
The cross-section of a seminiferous tubule displays a well-defined seminiferous epithelium comprised of Sertoli cells, the somatic cell type, and germ cells at various stages–where spermatogenesis occurs (Russell et al., 1993). It is described as the progression of undifferentiated spermatogonia to mature spermatozoa via three distinct stages: mitosis of spermatogonia, meiosis of spermatocytes, and spermatid maturation (spermiogenesis) (Nakata, 2019). Intratesticular injection (i.t.) has been used to describe the first action of anti-spermatogenic compounds because it optimizes the administered dosage and allows the molecule of interest to enter the testis more easily (Alberto-Silva et al., 2015; Chung et al., 2001). The effects of Bj-PF on the dynamics and structure of the seminiferous epithelium in mice after i.t. administration was reported in the literature (Alberto-Silva et al., 2020).
Histopathological analysis of the seminiferous tubules demonstrated that Bj-PF induced seminiferous tubule degeneration, decreased the number of round spermatids in stages I, V, and VII/VIII of the seminiferous epithelium, and inhibited spermatozoa production (Alberto-Silva et al., 2020). Furthermore, Alberto-Silva et al. (2020) also showed that direct administration of Bj-PF into the testis reduced the thickness of the seminiferous epithelium while increasing the diameter of the seminiferous tubule lumen. Interestingly, in contrast, we found that Bj-PF enhanced the thickness of the seminiferous epithelium while decreasing the diameter of the seminiferous tubule after local administration. Despite the presence of BTB that protects the seminiferous epithelium from invasion by molecules or cells that may disturb the process of spermatogenesis (Cheng et al., 2010), our findings suggest that local administration of Bj-PF and systemic distribution of its components might reach the intertubular compartment and cross the BTB, affecting functional characteristics of the seminiferous epithelium and explaining changes in the thickness and diameter of the seminiferous tubules, including an increase in degeneration of tubules. This concept seems to be a real possibility since BPP-10c, a peptide derived from B. jararaca snake venom and found in fraction peptide, appears to penetrate BTB, reducing spermiogenesis while not changing BTB permeability (Gilio et al., 2013).
Sertoli cells are important in initiating and controlling spermatogenesis (Gao et al., 2015) and spermiogenesis (Russell and Peterson, 1984). Failure of these cells to function leads to a loss of their capacity to maintain germ cell survival and may cause seminiferous epithelium disruption (de Kretser et al., 1998). The 15P-1 cell line was first identified as Sertoli cells based on their appearance and steroid metabolism (Rassoulzadegan et al., 1993) and were employed in multiple investigations with findings comparable to those obtained using primary Sertoli cell cultures (Alberto-Silva et al., 2020; Ghouili et al., 2018; Gu et al., 2022; Kamińska et al., 2020). For this reason, we used an in vitro culture model of 15P-1 Sertoli cells to study the effects of Bj-PF on mitochondrial metabolism and NO generation in this cell type of the seminiferous epithelium. In the present study, Bj-PF did not change the mitochondrial metabolism but reduced the NO2 levels in cells treated with 1 and 10 µg.mL−1 after 24 h of treatment. Nitric oxide (NO) regulates the cell junction dynamics in the seminiferous epithelium (Ni et al., 2019), controlling the migration of germ cells from the basal compartment to the adluminal compartment (Su et al., 2013). As a consequence, alterations in NO levels caused by Sertoli cells may explain the changes in the structure and function of seminiferous epithelium caused by Bj-PF.
In contrast to Bj-PF, Bj-CV is composed of many compounds responsible for tissue injury and hemorrhage; among them, many are cytotoxic in different cell types (de Souza et al., 2015; Querobino et al., 2018). Bj-CV (10 μg.mL−1) reduced mitochondrial metabolism of 15P-1 Sertoli cells as expected, probably by the presence of enzymatic compounds in crude venom (Fox and Serrano, 2008). Moreover, Bj-CV also increased NO2 levels in 15P-1 Sertoli cells. NO plays a relevant role in the pathophysiology of systemic alterations induced by Bj-CV, such as inflammation (Petricevich et al., 2000), and our findings suggest that crude venom may also impact the function of Sertoli cells, altering the form and function of the seminiferous epithelium.
In summary, this study has demonstrated that Bj-PF changes the structure and function of the seminiferous epithelium, suggesting that bioactive peptides found in B. jararaca snake venom could have toxicological effects on victims’ male reproductive systems, providing new insight into the biological properties of snake venom and therapeutic strategies against envenomation.
Acknowledgement: The authors would like to thank the Experimental Morphophysiology Laboratory’s technical group for their support with the histology techniques, and also the Natural and Humanities Sciences Center’s administrative-technical division for their secretarial support.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article, and original data are available from the corresponding author upon reasonable request.
Author Contribution: he authors confirm their contribution to the paper as follows: study conception and design: Carlos Alberto-Silva Author, Joyce Meire Gilio Author; data collection: Ana Carolina de Araujo Author, Rodrigo Simão Bonfim, Joyce Meire Gilio Author; analysis and interpretation of results: Carlos Alberto-Silva Author, Joyce Meire Gilio Author draft manuscript preparation: Carlos Alberto-Silva Author. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: All experimental protocols using animals were performed following the guidelines of the human use of laboratory animals of the Ethics Committee on Animal Use of Federal University of ABC (CEUA/UFABC) and were approved under protocol number 009/2013. Mouse 15P-1 Sertoli cells (CRL-2618TM, from the American Type Culture Collection-ATCC, Manassas, VA, USA) were used in mammalian cell tests. In these cases, our experiments also were developed under the application of good laboratory practice (GLP) to culture collections of cell cultures (Stevenson and Jong, 1992).
Funding Statement: This work was supported by the State of São Paulo Research Foundation (FAPESP) and the Coordination for the Improvement of Higher Education Personnel (CAPES) (Finance Code 001).
Conflicts of Interest: The authors declare that they have no conflicts of interest regarding the present study.
References
Alberto-Silva C, Franzin CS, Gilio JM, Bonfim RS, Querobino SMH (2020). Toxicological effects of bioactive peptide fractions obtained from Bothrops jararaca snake venom on the structure and function of mouse seminiferous epithelium. Journal of Venomous Animals and Toxins Including Tropical Diseases 26: 1–11. DOI 10.1590/1678-9199-jvatitd-2020-0007. [Google Scholar] [CrossRef]
Alberto-Silva C, Gilio JM, Portaro FCV, Querobino SM, Camargo ACM (2015). Angiotensin-converting enzyme inhibitors of Bothrops jararaca snake venom affect the structure of mice seminiferous epithelium. Journal of Venomous Animals and Toxins Including Tropical Diseases 21: 1425. https://www.scielo.br/j/jvatitd/a/7qDDFSk8N7SSpJbd3GHZm3v/?format=html&lang=en#. [Google Scholar]
Alberto-Silva C, Querobino SM, Melo-Silva CA, Costa MS, Franco Oliveira LV, Zamuner SR (2022). Local envenomation caused by a bioactive peptide fraction of Bothrops jararaca snake venom induces leukocyte influx in the lung and changes in pulmonary mechanics. Toxicon 207: 52–59. DOI 10.1016/j.toxicon.2022.01.001. [Google Scholar] [CrossRef]
Cheng CY, Wong EWP, Yan HHN, Mruk DD (2010). Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: New insights and advances. Molecular and Cellular Endocrinology 315: 49–56. DOI 10.1016/j.mce.2009.08.004. [Google Scholar] [CrossRef]
Chung NPY, Mruk D, Mo MY, Lee WM, Cheng CY (2001). A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biology of Reproduction 65: 1340–1351. DOI 10.1095/biolreprod65.5.1340. [Google Scholar] [CrossRef]
Clissa PB, Laing GD, Theakston RDG, Mota I, Taylor MJ, Moura-da-Silva AM (2001). The effect of jararhagin, a metalloproteinase from Bothrops jararaca venom, on pro-inflammatory cytokines released by murine peritoneal adherent cells. Toxicon 39: 1567–1573. DOI 10.1016/S0041-0101(01)00131-3. [Google Scholar] [CrossRef]
de Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N (1998). Spermatogenesis. Human Reproduction 13: 1–8. DOI 10.1093/humrep/13.suppl_1.1. [Google Scholar] [CrossRef]
de Souza LL, Stransky S, Guerra-Duarte C, Flor-Sá A, Schneider FS, Kalapothakis E, Chávez-Olórtegui C (2015). Determination of toxic activities in bothrops spp. Snake venoms using animal-free approaches: Correlation between in vitro vs. in vivo assays. Toxicological Sciences 147: 458–465. DOI 10.1093/TOXSCI/KFV140. [Google Scholar] [CrossRef]
Fox JW, Serrano SMT (2008). Exploring snake venom proteomes: Multifaceted analyses for complex toxin mixtures. Proteomics 8: 909–920. DOI 10.1002/pmic.200700777. [Google Scholar] [CrossRef]
Gao Y, Mruk DD, Cheng CY (2015). Sertoli cells are the target of environmental toxicants in the testis-A mechanistic and therapeutic insight. Expert Opinion on Therapeutic Targets 19: 1073–1090. DOI 10.1517/14728222.2015.1039513. [Google Scholar] [CrossRef]
Ghouili F, Roumaud P, Martin LJ (2018). Gja1 expression is regulated by cooperation between SOX8/SOX9 and cJUN transcription factors in TM4 and 15P-1 Sertoli cell lines. Molecular Reproduction and Development 85: 875–886. DOI 10.1002/mrd.23049. [Google Scholar] [CrossRef]
Gilio JM, Portaro FC, Borella MI, Lameu C, Camargo AC, Alberto-Silva C (2013). A bradykinin-potentiating peptide (BPP-10c) from Bothrops jararaca induces changes in seminiferous tubules. Journal of Venomous Animals and Toxins including Tropical Diseases 19: 28. DOI 10.1186/1678-9199-19-28. [Google Scholar] [CrossRef]
Gonçalves LRC, Mariano M (2000). Local haemorrhage induced by Bothrops jararaca venom: Relationship to neurogenic inflammation. Mediators of Inflammation 9: 101–107. DOI 10.1080/096293500411569. [Google Scholar] [CrossRef]
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry 126: 131–138. DOI 10.1016/0003-2697(82)90118-X. [Google Scholar] [CrossRef]
Gu JY, Fu ZB, Chen JL, Liu YJ, Cao XZ, Sun Y (2022). Endotoxin tolerance induced by Porphyromonas gingivalis lipopolysaccharide alters macrophage polarization. Microbial Pathogenesis 164: 105448. DOI 10.1016/j.micpath.2022.105448. [Google Scholar] [CrossRef]
Hayashi MAF, Camargo ACM (2005). The Bradykinin-potentiating peptides from venom gland and brain of Bothrops jararaca contain highly site specific inhibitors of the somatic angiotensin-converting enzyme. Toxicon 45: 1163–1170. DOI 10.1016/j.toxicon.2005.02.017. [Google Scholar] [CrossRef]
Hayashi MAF, Murbach AF, Ianzer D, Portaro FCV, Prezoto BC et al. (2003). The C-type natriuretic peptide precursor of snake brain contains highly specific inhibitors of the angiotensin-converting enzyme. Journal of Neurochemistry 85: 969–977. DOI 10.1046/j.1471-4159.2003.01743.x. [Google Scholar] [CrossRef]
Kamińska A, Marek S, Pardyak L, Brzoskwinia M, Bilinska B, Hejmej A (2020). Crosstalk between Androgen-ZIP9 signaling and Notch pathway in rodent Sertoli cells. International Journal of Molecular Sciences 21: 1–22. DOI 10.3390/ijms21218275. [Google Scholar] [CrossRef]
Loi M (2014). World Health Organization World Health Reports. In: Michalos AC (eds.Encyclopedia of Quality of Life and Well-Being Research. Dordrecht: Springer. DOI 10.1007/978-94-007-0753-5_3285. [Google Scholar] [CrossRef]
Meroni SB, Suburo AM, Cigorraga SB (2000). Interleukin-1beta regulates nitric oxide production and gamma-glutamyl transpeptidase activity in sertoli cells. Journal of Andrology 21: 855–861. DOI 10.1002/j.1939-4640.2000.tb03416.x. [Google Scholar] [CrossRef]
Nakata H (2019). Morphology of mouse seminiferous tubules. Anatomical Science International 94: 1–10. DOI 10.1007/s12565-018-0455-9. [Google Scholar] [CrossRef]
Ni F-D, Hao SL, Yang WX (2019). Multiple signaling pathways in Sertoli cells: Recent findings in spermatogenesis. Cell Death and Disease 10: 1–15. DOI 10.1038/s41419-019-1782-z. [Google Scholar] [CrossRef]
Petricevich VL, Teixeira CFP, Tambourgi DV, Gutiérrez JM (2000). Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon 38: 1253–1266. DOI 10.1016/S0041-0101(99)00227-5. [Google Scholar] [CrossRef]
Querobino SM, Carrettiero DC, Costa MS, Alberto-Silva C (2017). Neuroprotective property of low molecular weight fraction from B. jararaca snake venom in H2O2-induced cytotoxicity in cultured hippocampal cells. Toxicon 129: 134–143. DOI 10.1016/j.toxicon.2017.02.015. [Google Scholar] [CrossRef]
Querobino SM, Costa MS, Alberto-Silva C (2019). Protective effects of distinct proline-rich oligopeptides from B. jararaca snake venom against oxidative stress-induced neurotoxicity. Toxicon 167: 29–37. DOI 10.1016/j.toxicon.2019.06.012. [Google Scholar] [CrossRef]
Querobino SM, Ribeiro CAJ, Alberto-Silva C (2018). Bradykinin-potentiating PEPTIDE-10C, an argininosuccinate synthetase activator, protects against H2O2-induced oxidative stress in SH-SY5Y neuroblastoma cells. Peptides 103: 90–97. DOI 10.1016/j.peptides.2018.03.017. [Google Scholar] [CrossRef]
Rassoulzadegan M, Paquis-Flucklinger V, Bertino B, Sage J, Jasin M, Miyagawa K, van Heyningen V, Besmer P, Cuzin F (1993). Transmeiotic differentiation of male germ cells in culture. Cell 75: 997–1006. DOI 10.1016/0092-8674(93)90543-Y. [Google Scholar] [CrossRef]
Russell FE, Walter FG, Bey TA, Fernandez MC (1997). Snakes and snakebite in Central America. Toxicon 35: 1469–1522. DOI 10.1016/S0041-0101(96)00209-7. [Google Scholar] [CrossRef]
Russell LD, Ettlin RA, Hikim APS, Clegg ED (1993). Histological and histopathological evaluation of the testis. International Journal of Andrology 16: 83. DOI 10.1111/j.1365-2605.1993.tb01156.x. [Google Scholar] [CrossRef]
Russell LD, Peterson RN (1984). Determination of the elongate spermatid-Sertoli cell ratio in various mammals. Journal of Reproduction and Fertility 70: 635–641. DOI 10.1530/jrf.0.0700635. [Google Scholar] [CrossRef]
Santoro ML, Sano-Martins IS, Fan HW, Cardoso JLC, Theakston RDG, Warrell DA (2008). Haematological evaluation of patients bitten by the jararaca, Bothrops jararaca, in Brazil. Toxicon 51: 1440–1448. DOI 10.1016/j.toxicon.2008.03.018. [Google Scholar] [CrossRef]
Senise LV, Yamashita KM, Santoro ML (2015). Bothrops jararaca envenomation: Pathogenesis of hemostatic disturbances and intravascular hemolysis. Experimental Biology and Medicine 240: 1528–1536. DOI 10.1177/1535370215590818. [Google Scholar] [CrossRef]
Stevenson RE, Jong SC (1992). Application of good laboratory practice (GLP) to culture collections of microbial and cell cultures. World Journal of Microbiology & Biotechnology 8: 229–235. DOI 10.1007/BF01201869. [Google Scholar] [CrossRef]
Stockert JC, Blázquez-Castro A, Cañete M, Horobin RW, Villanueva Á. (2012). MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochemica 114: 785–796. DOI 10.1016/j.acthis.2012.01.006. [Google Scholar] [CrossRef]
Su W, Mruk DD, Cheng CY (2013). Regulation of actin dynamics and protein trafficking during spermatogenesis-Insights into a complex process. Critical Reviews in Biochemistry and Molecular Biology 48: 153–172. DOI 10.3109/10409238.2012.758084. [Google Scholar] [CrossRef]
Yamashita KM, Alves AF, Barbaro KC, Santoro ML (2014). Bothrops jararaca venom metalloproteinases are essential for coagulopathy and increase plasma tissue factor levels during envenomation. PLoS Neglected Tropical Diseases 8: e2814. DOI 10.1371/journal.pntd.0002814. [Google Scholar] [CrossRef]
Yi X, Liu M, Luo Q, Zhuo H, Cao H, Wang J, Han Y (2017). Toxic effects of dimethyl sulfoxide on red blood cells, platelets, and vascular endothelial cells in vitro. FEBS Open Bio 7: 485–494. DOI 10.1002/2211-5463.12193. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools