 Open Access
Open Access
ARTICLE
LIM1863 is useful to explore collective cancer cell migration, and the group of heterogeneous cells undergoing collective migration behaves like a supracellular unit
1 Department of Radiotherapy, Hebei Province Hospital of Chinese Medicine, Hebei University of Chinese Medicine, Shijiazhuang, China
2 Department of General Surgery, Chongqing General Hospital, Chongqing, China
3 Key Laboratory of Integrated Chinese and Western Medicine for Gastroenterology Research (Hebei), Shijiazhuang, China
* Corresponding Authors: JIANMING HE. Email: ; XI LIANG. Email:
BIOCELL 2023, 47(12), 2671-2680. https://doi.org/10.32604/biocell.2023.043494
Received 04 July 2023; Accepted 26 September 2023; Issue published 27 December 2023
Abstract
Introduction: Collective cancer cell migration (CCCM) and epithelial-to-mesenchymal transition (EMT) play key roles in metastasis. This study reports that the colorectal carcinoma cell line LIM1863 is useful for the study of CCCM and EMT. Methods: Hematoxylin and eosin staining, scanning electron microscopy, transmission electron microscopy, and western blot analysis were performed. Results: LIM1863 automatically grew as spheroids in suspension and had important typical epithelial properties, including several layers of cells arranged around a central lumen, apical-basal polarity, and types of cell-cell junctions. Treatment with a combination of both TGF beta 1 and TNF alpha induced definite and distinct EMT, a spheroid changing phenotype to form a monolayer high-confluent patch without lumen, without polarity. Spontaneous CCCM occurred in spheroids. Flat EMT cells adhered to the base of a dish, exhibited persistent movement as a cluster of cells, and then shed, resulting in a cluster. All cells from one cluster undergoing CCCM died. Otherwise, all cells undergoing EMT disappeared and almost all cells located in the cell reservoir survived and proliferated. Conclusion: LIM1863 is an excellent cell line to study CCCM and EMT. The group of heterogeneous cells undergoing CCCM behaves like a supracellular unit.Keywords
Metastasis is responsible for the majority of cancer morbidity and mortality and is estimated to account for 90% of cancer-related deaths (Jonckheere et al., 2022). It is a complex, multistep process whereby cancer cells shed from primary tumors, then survive and proliferate at secondary sites to colonize distant metastatic lesions (VanderVorst et al., 2019). Epithelial-to-mesenchymal transition (EMT) and migration play central roles in the process of epithelial cancer metastasis. Migratory modes may be classified into two major subtypes: single cancer cell migration and collective cancer cell migration (CCCM), where multiple adherent cancer cells move as a coordinated unit in a sheet or cluster with directional multicellular movement (VanderVorst et al., 2019). In CCCM of epithelial cancer, proposed the “leader–follower” model, leader cells occupy the leading edge and are supposed to be EMT cells. Follower cells are located in the cell reservoir and their features are basically consistent with cells from primary cancer lesions (Shellard and Mayor, 2019; Qin et al., 2021).
CCCM is a highly discussed and very current topic, but it is far from clear. Monolayer cells and spheroids embedded in extracellular matrices are two commonly used in vitro models (Saenz-de-Santa-Maria et al., 2020; Meyer et al., 2021; Ogoke et al., 2021). Monolayer cells cannot well recapitulate three-dimensional architecture, for example, intercellular adhesion, of CCCM in vivo. Spheroids embedded in extracellular matrices are a complicated system, and CCCM is usually caused by external cues such as cell-extracellular matrix (ECM) interactions and soluble chemoattractants because extracellular matrices contain several types of ECM and several growth factors, such as transforming growth factor (TGF), epidermal growth factor (EGF), fibroblast growth factor (FGF) (Saenz-de-Santa-Maria et al., 2020; Ogoke et al., 2021). LIM1863, a colorectal carcinoma cell line established by Whitehead et al. (1987), is unique. It automatically grows as spheroids in suspension without any special treatment. One spheroid has at least two types of cells: morphologically mature goblet and columnar cells (Whitehead et al., 1987). Here, we reported that LIM1863 is useful to explore EMT, CCCM, particularly spontaneous CCCM, and interesting phenomena were presented.
The human colorectal cancer cell line LIM1863 was kindly shared by Dr. Robert H Whitehead (Ludwig Institute for Cancer Research, Australia) and was cultured in RPMI-1640 medium (Hyclone, Logan, USA) containing 100 mL/L fetal bovine serum (FBS) (Gibco, Grand Island, USA), 1 mg/L insulin (Sigma Aldrich, St Louis, USA), 100,000 IU/L penicillin, 100 mg/L streptomycin (Gibco) with 5% CO2 at 37°C in a normal petri dish or 6-well plate as previously described (Whitehead et al., 1987).
To observe the process of spontaneous CCCM, LIM1863 spheroids were vigorously pipetted and washed with phosphate-buffered saline (PBS) once. Spheroids were propagated in a well of a 6-well plate with 3 mL normal medium with a subcultivation ratio of 1:4. Two-thirds of suspended spheroids were discarded, and 2 mL medium was replaced on day 5. After that, 2 mL medium was replaced every other day and two-thirds of spheroids were discarded every 4 to 6 days. To observe the fate of detached clusters undergoing CCCM, LIM1863 spheroids were propagated in a well of a 96-well plate with 0.2 mL normal medium with a subcultivation ratio of 1:6. Half suspended spheroids were discarded and half medium was replaced on day 5. Two days later, all suspended spheroids in the well in which CCCM occurred was discarded and 0.1 mL normal medium was added. Half the medium was replaced every week or before detachment using a micropipette.
To explore the rate of CCCM, LIM1863 spheroids were washed with PBS twice, and the single cell suspension was prepared by incubating with 0.25% trypsin-Ethylenediaminetetraacetic acid (Gibco) at 37°C for 10 min, washing with normal medium once, PBS twice. Then, 106 LIM1863 cells were added to the wells of a 6-well plate with 3 mL normal medium. Cells assembled automatically as spheroids in suspension within 1 day and kept growing. Two mL medium was replaced on day 5 and then, 2 mL medium was replaced every other day. Spheroids undergoing collective migration were counted on day 10. Spheroids in the serum-free group were cultured with RPMI-1640 medium (Hyclone) containing 1 mg/L insulin (Sigma Aldrich), 105 IU/L penicillin, 100 mg/L streptomycin (Gibco) and spheroids in the glucose-free group were cultured with glucose-free RPMI-1640 medium (Gibco, cat.: 11879020) containing 100 mL/L FBS, 1 mg/L insulin (Sigma Aldrich), 105 IU/L penicillin, 100 mg/L streptomycin (Gibco). Treatment was the same as the control group. In the starvation group, 106 LIM1863 cells were incubated in the wells of a 6-well plate with 1.5 mL normal medium; 0.75 mL medium was replaced on day 5 and then, 0.75 mL medium was replaced every other day. Each group had six replicates.
The human colon carcinoma cell line HCT 116 and the human colon adenocarcinoma cell line HT29 were obtained from the Cell Bank, Chinese Academy of Science and were routinely grown in McCoy’s 5A (Gibco) as we previously described (Liang et al., 2015). Spheroids were prepared by using the liquid overlay technique, as we previously described (Liang et al., 2015). In brief, exponentially-growing cells were seeded into a 6-well plate that was previously coated with 2% agarose. Plates were gently horizontally swirled for 10 min every 6 h for the first 24 h on an orbital shaker. Half of the medium was refreshed every day (Liang et al., 2015). Photograph or samples were taken on day 6. HCT 116 and HT29 were authenticated by short tandem repeat profiling.
In the EMT assay, 106 LIM1863 cells were added in a well of a 6-well plate with 3 mL normal medium. Cells assembled automatically as spheroids in suspension within 1 day and kept growing. One day later, 300 μL tumor necrosis factor-alpha (TNF-α) (Sangon Biotech, cat.: C600021, Shanghai, China) at 100 ng/mL and 300 μL transforming growth factor beta 1 (TGF-β1) (Peprotech, Rocky Hill, USA) at 20 ng/mL were added (Bates and Mercurio, 2003; Vincan et al., 2007). Photographs or samples were taken on day 3.
Hematoxylin and eosin (HE) staining
One million LIM1863 cells were added in a well of a 6-well plate with 3 mL normal medium and cultured for 5 days. LIM1863 spheroids were embedded with paraffin. HCT 116 spheroids and HT29 spheroids were prepared as mentioned above and embedded with OCT. Paraffin-embedded or OCT-embedded spheroids were cut into 6 μm sections. Sections were routinely stained with HE (He et al., 2016).
Colorectal tumors were induced in C57BL/6 mice by azoxymethane (Sigma Aldrich)-dextran sodium sulfate (MP Biomedicals, molecular weight 36, −50 kDa. Irvine, USA) as we previously described (He et al., 2015; Liang et al., 2019). In brief, mice were purchased from GemPharmatech Co. Ltd. (Chengdu, China) and housed in a specific pathogen-free animal facility in plastic cages in a temperature-controlled room (22 ± 1°C) with a daylight cycle from 6 AM to 6 PM. Male mice, (6–7-week-old; 20–25 gram weight), were given a single intraperitoneal injection of 10 mg/kg body weight azoxymethane (AOM). Starting at a week after injection, animals received dextran sodium sulfate (DSS) for 7 days via free access to drinking water containing 2% DSS. Then, mice were fed with regular water. Mice were sacrificed 18 weeks after AOM treatment using an overdose of isoflurane inhalation followed by cervical dislocation. Colorectal tumors were fixed in 10% formalin/PBS, and paraffin-embedded samples were sectioned at a thickness of 6 µm. Sample slides were routinely stained with HE.
LIM1863 spheroids were fixed in 4% paraformaldehyde. Fixed samples were embedded in paraffin and sectioned at a thickness of 6 μm using a paraffin microtome (Leica RM 2135, Leica, Germany). Sample slides were routinely stained with Alcian blue (Kinoshita et al., 2019).
Slides preparation for the scanning electron microscopy
Sample slides were routinely prepared as previously described (Avenarius et al., 2018). In brief, LIM1863 spheroids were fixed in 2.5% glutaraldehyde and then in 1% osmium tetroxide. Samples were mounted on stubs using colloidal silver paste and were observed by scanning electron microscopy (AMRay 1000B, Bedford, USA).
Slides preparation for the transmission electron microscopy
Sample slides were routinely prepared as previously described (Liang et al., 2015). In brief, LIM1863 spheroids were fixed in 2.5% glutaraldehyde and then in 1% osmium tetroxide. Samples were dehydrated using graded alcohol. Ultrathin sections were stained with uranium acetate and lead citrate. Sample slides were observed using a transmission electron microscope (TECNAI10, Philip, Holland).
The animal study was approved by the Ethics Committee of Hebei Province Hospital of Chinese Medicine (HBZY2019-KY-169-01) (Liang et al., 2019).
Medical images from three patients were presented here and all patients signed informed consent for publication. This study was approved by the Ethics Committee of Hebei Province Hospital of Chinese Medicine (HBZY2022-KY-032-01) and was carried out in accordance with the associated guidelines (Mu et al., 2015; Liang et al., 2019).
Cells were lysed as we previously described (He et al., 2009). In brief, cells were washed with PBS once, 2.0 × 106 cells were lysed at 4°C in 200 μL 2 × sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) protein loading buffer (Beyotime Co., Beijing, China cat.: P0015B) with Protease Inhibitor Cocktail (Sigma Aldrich, cat.: P8340), Phosphatase Inhibitor Cocktail 2 (Sigma Aldrich, cat.: P5726), Phosphatase Inhibitor Cocktail 3 (Sigma Aldrich, cat.: P0044) for 5 min. Cell lysates were boiled at 96°C for 3 min and sonicated. Lysates were centrifuged at 1,000 g for 2 min at 4°C and the supernatant was collected as cell protein.
Cell protein (2 µL) was mixed with 10 μL 1 × SDS-PAGE protein loading buffer (Beyotime Co. cat.: P0015A) and was resolved by 10% SDS/PAGE and blotted on nitrocellulose membranes (Bio-Rad) as we previously described (Liang et al., 2015). Nitrocellulose membranes were blocked by incubation with 5% BSA/PBS/T at room temperature for 30 min. Membranes were incubated overnight with specific primary antibodies at 4°C. After incubation with secondary antibodies at 37°C for 40 min, immunoreactive proteins were visualized using the Enhanced Chemiluminescent Substrate (Thermo Scientific, Pittsburgh, USA cat.: 34094).
E-cadherin antibody was from Abcam Inc., Cambridge, USA (cat.: ab1416, 1:1,000); antibodies against β-catenin (cat.: 9582, 1:1000), β-actin (cat.: 3700, 1:1000), and horseradish peroxidase-linked secondary antibodies (cat.: 7076, 1:1000; cat.: 7074, 1:1000) were from Cell Signaling Technology, Berkeley, USA.
Numbers in the text were mean ± standard error of the mean. Statistical differences between the two groups were analyzed by one-way ANOVA using GraphPad Prism 8.0.2 (GraphPad Software, San Diego, USA). A p-value < 0.05 was considered statistically significant.
LIM1863 spheroids mimic colorectal cancer in vivo well and maintains typical epithelial properties
LIM1863 automatically grew as spheroids in suspension (Fig. 1A). HE staining showed that a spheroid consisted of layers of cells arranged around a central lumen. Nuclei were at the outer rim in the order, which indicates that LIM1863 cells were polarized (Fig. 1B). Those epithelial characteristics in LIM1863 spheroids were the same as in colorectal cancer lesions in vivo (Fig. 1C). Results of the scanning electron microscopy showed that spheroids consisted of layers of cells arranged around a central lumen (Fig. 1D). Alcian-blue staining showed that all lumens were alcian blue positive (Fig. 1E). Types of cell-cell junctions were observed by transmission electron microscopy (Fig. 1F). Those indicate that LIM1863 spheroids mimic colorectal cancer in vivo well and maintains typical epithelial properties.
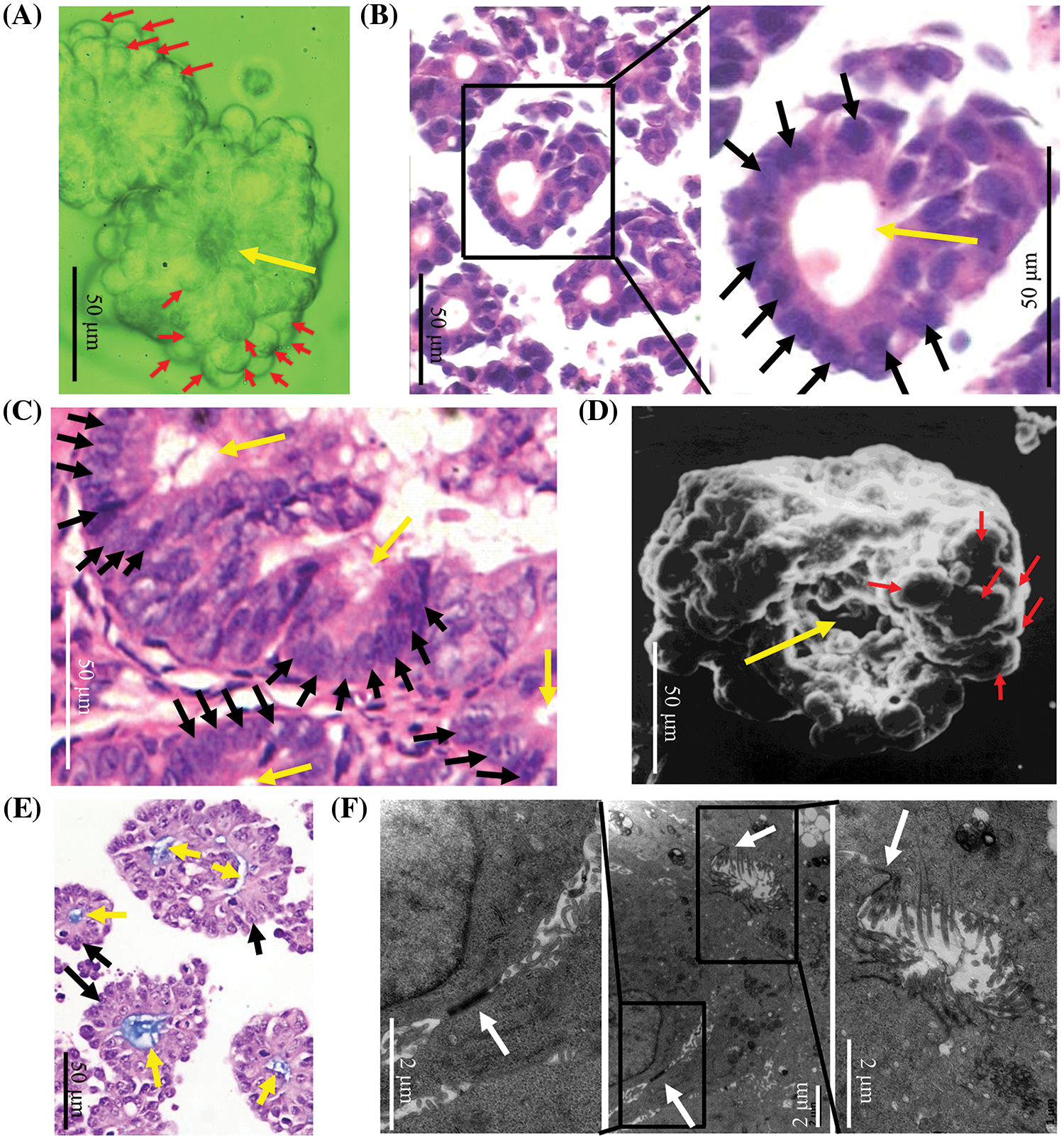
Figure 1: A LIM1863 spheroid mimicking a small colorectal cancer lesion in vivo. (A) LIM1863 in suspension. (B) Hematoxylin and eosin (HE) staining of LIM1863. (C) HE staining of colorectal cancer in mice. (D) A spheroid was observed by scanning electron microscopy. (E) Alcian-blue staining of LIM1863. (F) Types of cell-cell junctions observed under the transmission electron microscopy. Red arrows, cells; yellow arrows, lumens; black arrows, nuclei; white arrows, cell-cell junctions.
LIM1863 cells were treated with a combination of both TGF-β1 and TNF-α for 3 days. For 24 to 36 h, a LIM1863 spheroid changed phenotype to form a monolayer as a high-confluent patch without any lumen or lumen-like structure. Cells became flattened and nuclei were in a state of disorder. Those indicate that cells lose apical-basal polarity and organization (Figs. 2A and 2B). E-cadherin, an epithelial marker, was decreased in LIM1863 when grown in monolayers (Bates and Mercurio, 2003; Vincan et al., 2007). That was replicated here. Western blotting showed that the level of E-cadherin in LIM1863 spheroids was higher than in monolayers. The level of β-catenin, a mesenchymal marker, in LIM1863 spheroids, was lower than in monolayers (Fig. 2C). These indicate that a combination of both TGF-β1 and TNF-α induced LIM1863 to undergo EMT.
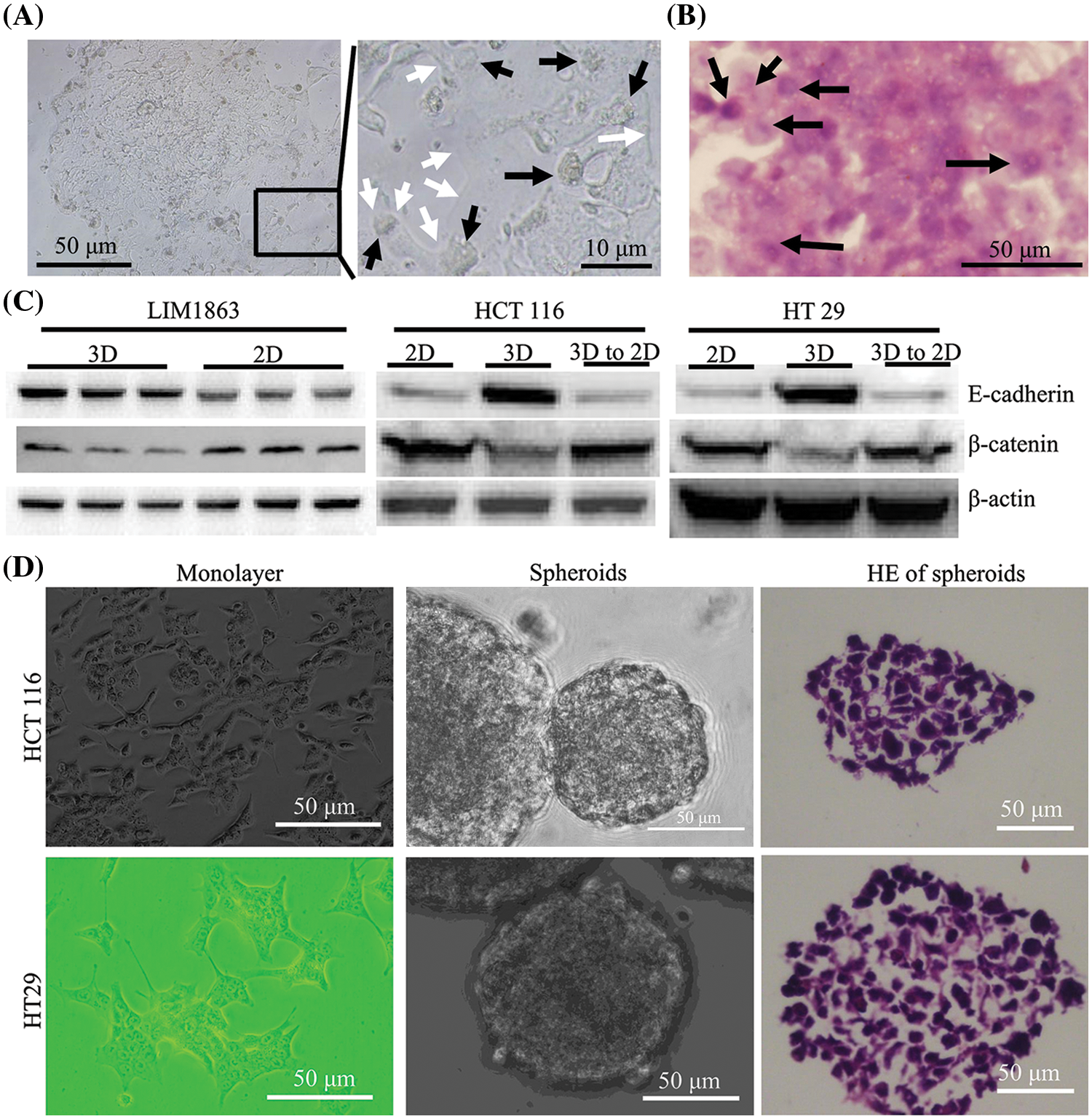
Figure 2: Epithelial Mesenchymal transition (EMT) in LIM1863 and other colorectal cancer cell lines. (A) Treatment with a combination of both TGF-β1 and TNF-α induced LIM1863 cells to form a monolayer as a high-confluent patch. (B) Hematoxylin and eosin staining LIM1863 EMT cells. (C) Western blotting. Each group had three replicates. (D) HCT 116 and HT29. Black arrows, nuclei; white arrows, cell membranes.
HCT 116 and HT29 automatically grew monolayers. When seeded under non-adhesive conditions, dispersed cells aggregated automatically and formed spheroids. Lumens were extremely rare and nuclei were in a state of disorder (Fig. 2D). We previously reported that E-cadherin was increased in spheroids and decreased when monolayers grew. The expression level of β-catenin was decreased in spheroids and increased when monolayers grew (Liang et al., 2015, 2016). These findings were replicated here (Fig. 2C), indicating that when cells grow as spheroids, they acquire some epithelial properties and when HCT 116 or HT29 switch from spheroids to monolayers, they undergo EMT.
Therefore, LIM1863 spheroids have several important epithelial properties that almost all other colorectal cancer lines do not have, including automatically growing as spheroids, a central lumen, cell organization, apical-basal polarity, etc. Together with definite and distinct changes in growth pattern, morphology, function, and molecular markers, LIM1863 maintains important typical epithelial properties as colorectal cancer cells in vivo and is ideal for studying EMT.
LIM1863 is useful in the study of collective cancer cell migration
CCCM spontaneously occurred in LIM1863 spheroids (Fig. 3). To observe the process of spontaneous CCCM, LIM1863 cells were treated as Fig. 3A. A few cells in the spheroid adhered the base of a dish to grow as a monolayer, became flattened, moved out and formed leading and protruding edges (Figs. 3B and 4). The nuclei of those flat cells were not at the outer rim in order anymore (Fig. 4). Those flat cells were motile and persistently kept moving forward in different directions, followed by a cluster of cells. Those suggest that leader cells should undergo EMT. Leader cells, together with follower cells, formed a “finger-like” structure. No obvious morphological difference between follower cells and cells in original spheroids was observed (Figs. 3B and 4). The morphological difference between leaders and followers was definite and distinct. One spheroid had several or more “finger-like” structures caused by CCCM. Two to 20 days later, LIM1863 leader cells and their followers shed, resulting in a cluster (Fig. 3B). Those appeared similar to cancer lesions in vivo (Figs. 5A–5D). In vivo, one cancer lesion had several or more “finger-like” protrusions (Figs. 5A–5D). Discrete cancer cell clusters were observed around a colorectal cancer lesion (Fig. 5A). Hence, LIM1863 is an excellent cell line to study CCCM, particularly spontaneous CCCM.
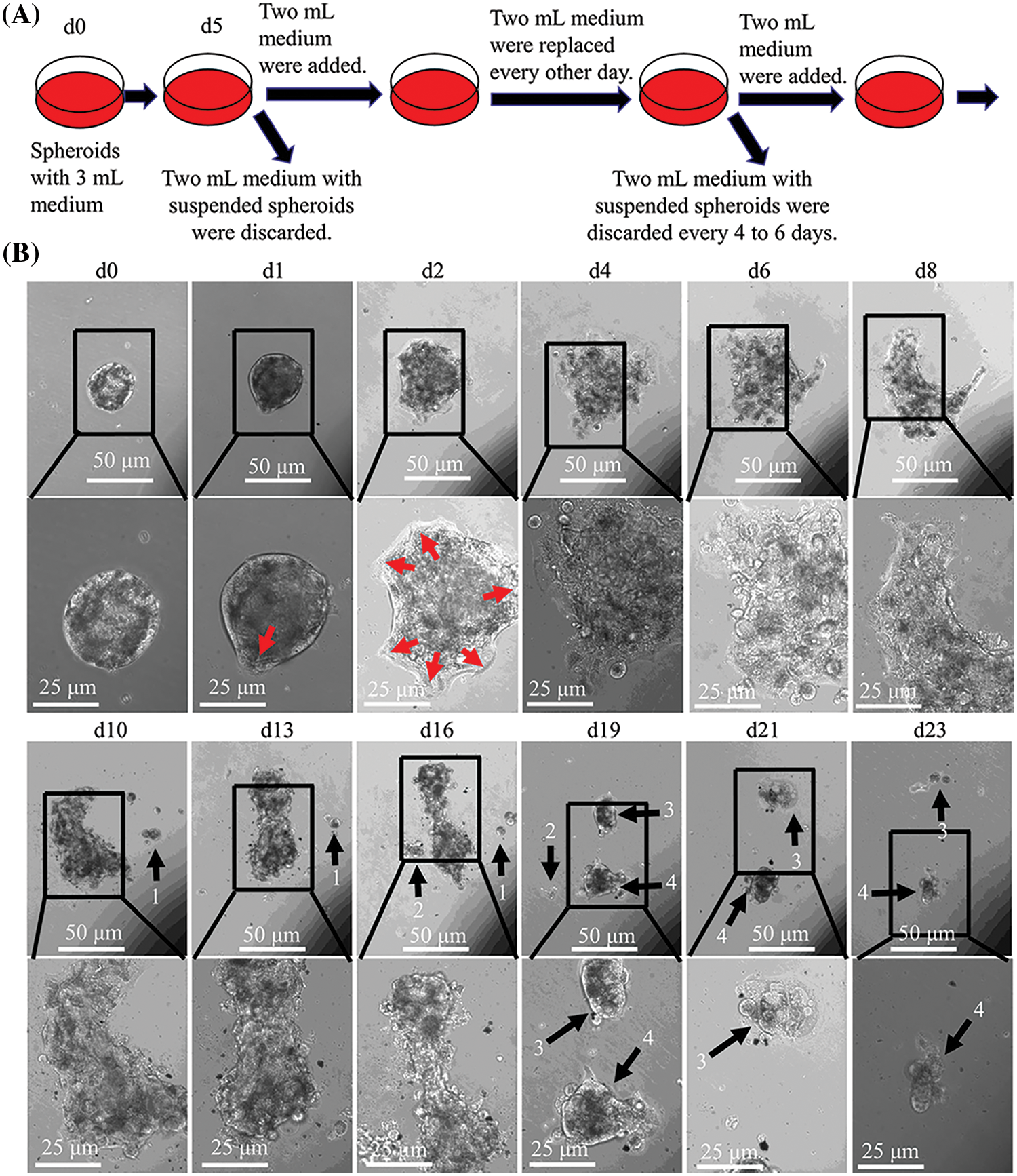
Figure 3: The process of spontaneous collective cancer cell migration (CCCM) in LIM1863 spheroids. (A) The diagram illustrates the procedure for observation of the process of spontaneous CCCM. (B) The process of spontaneous CCCM. One spheroid had several “finger-like” structures (red arrows). Cell clusters (black arrows) shedding from the spheroid persistently kept moving and died out.
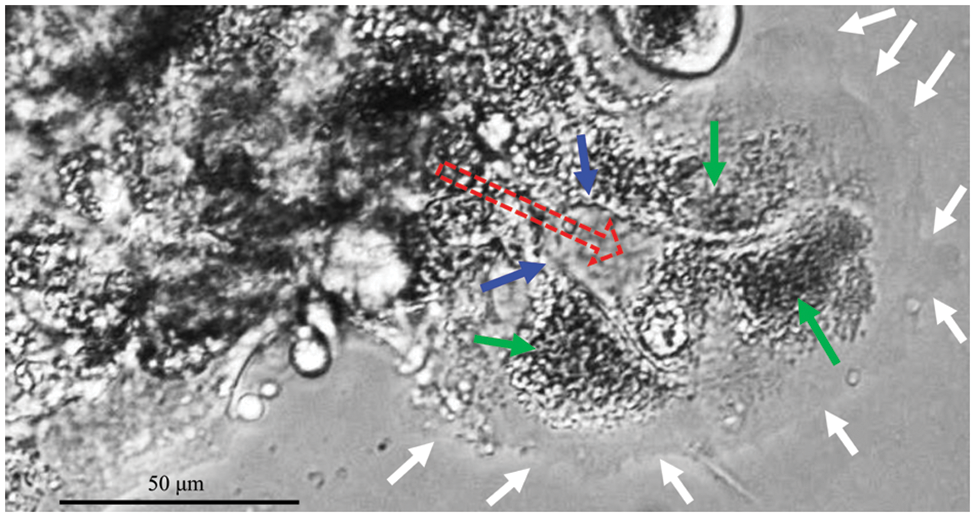
Figure 4: “Finger-like” structures. Red arrows, moving direction; white arrows, cell membranes (also the front border of the “Finger-like” structure); green arrows, nuclei; blue arrows, borders among cells (also cell membranes).
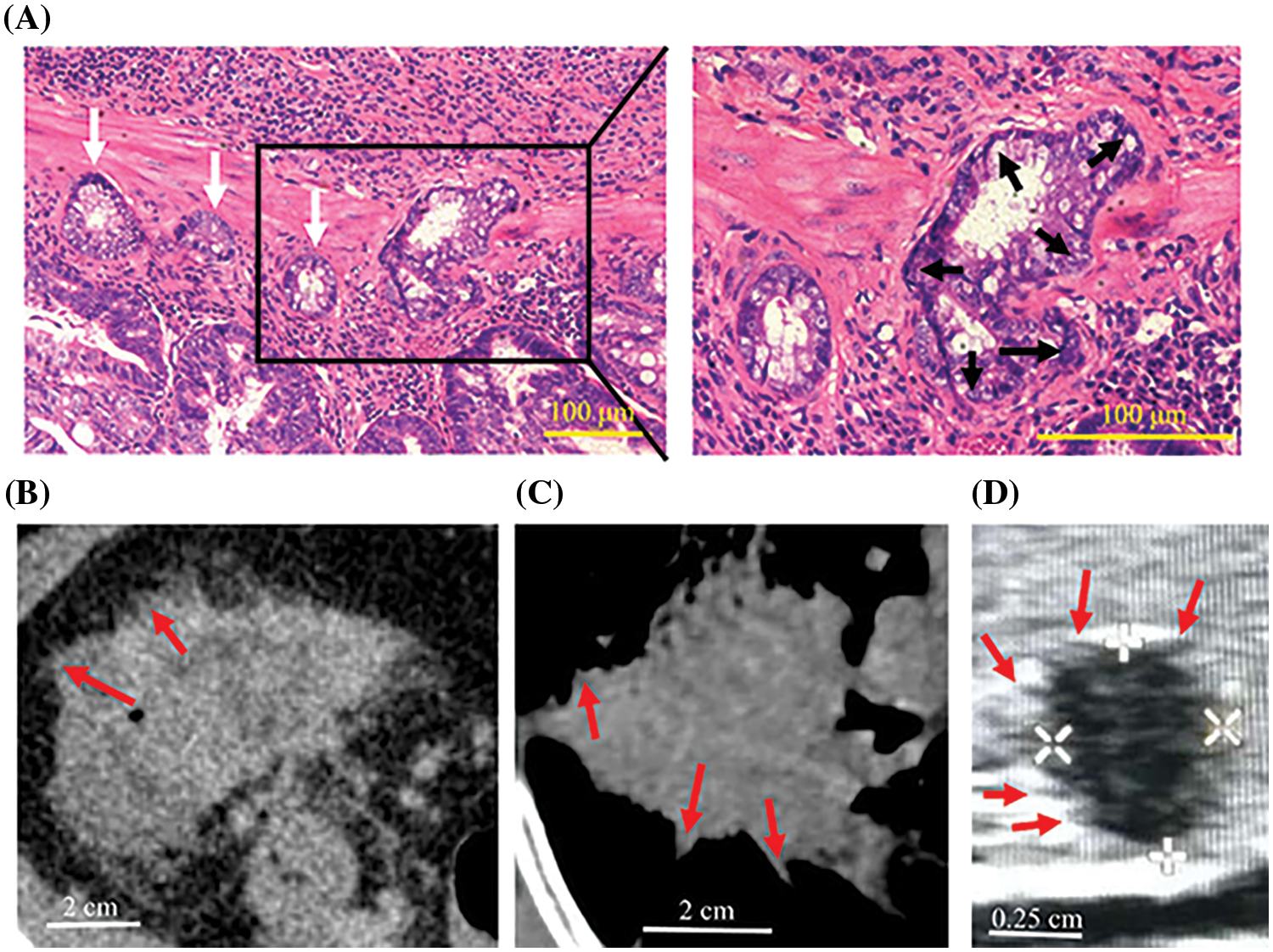
Figure 5: Peritumoral deposits and spiculations in vivo. (A) Colorectal cancer lesions in mice had several “finger-like” structures (black arrows) and cancer cell clusters (white arrows) were scattered around the primary tumor. (B–D) CT of colorectal cancer (B), CT of lung cancer (C) and ultrasonography of thyroid cancer (D) in humans showing spiculations (red arrows).
All cells from one cluster undergoing collective cancer cell migration die; otherwise, almost all follower cells, survive
The ratio of spheroids undergoing spontaneous CCCM was very low. To explore the rate of CCCM, LIM1863 cells were treated as Fig. 6A. In a well of a 6-well plate, 106 LIM1863 cells (about 10,000 spheroids) were cultured. The number of spheroids undergoing spontaneous CCCM in one well was 5.3 ± 1.2. Starvation induced CCCM. The number of spheroids undergoing CCCM in the starvation group was significantly higher than in the control group. However, deprivation of serum or glucose did not significantly change the ratio (Fig. 6B).
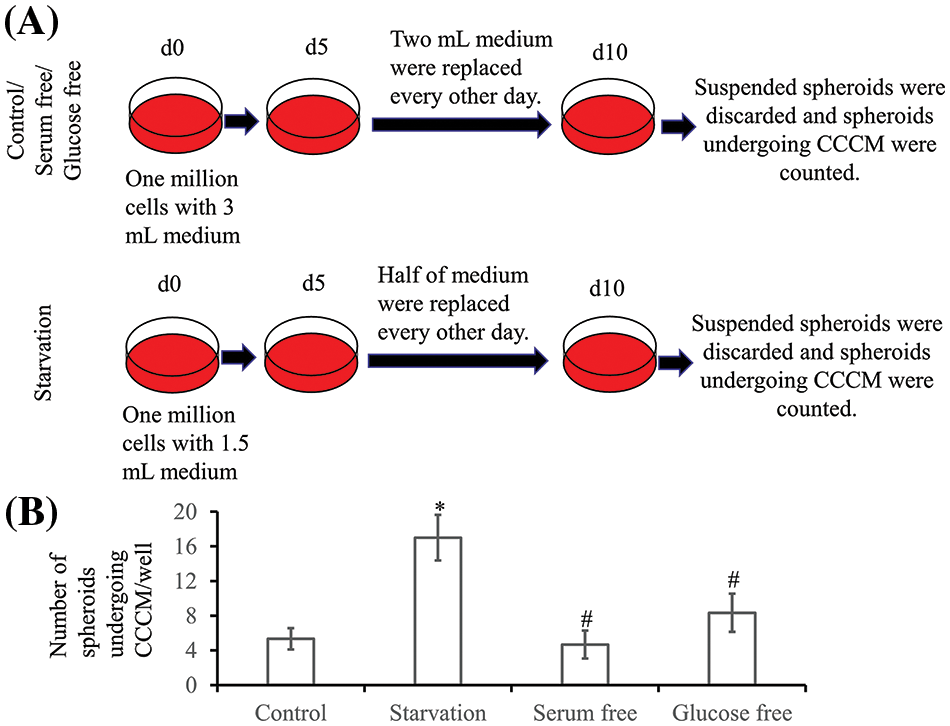
Figure 6: Collective cancer cell migration (CCCM) in LIM1863. (A) The diagram illustrates the procedure for exploration of the rate of CCCM. (B) Numbers of spheroids undergoing CCCM in one well (n = 6; *p < 0.05 vs. Control; #p > 0.05 vs. Control).
The spheroids undergoing CCCM were separated into several clusters at last. Each cluster was formed by leader cells and their followers. Leader cells adhered to the dish base and kept moving. Beyond our expectation, the number of cells in one cluster was continuously decreasing while the cluster was collectively migrating, and finally, all cells died (Fig. 3B). Time durations for their death varied enormously, but usually was several days. All cells in number 1 cluster to number 4 died within 10 days after shedding or separation (Fig. 3B).
A few spheroids/clusters in which spontaneous CCCM occurred survived. Under this condition, EMT cells disappeared in those spheroids within 10 days. Then, cells in the reservoir survived and proliferated, and the spheroid grew in suspension again. Usually, that happened within 2 weeks after EMT occurred (Fig. 7A). Among 12 spheroids undergoing CCCM, 3 spheroids survived and proliferated. EMT cells in those 3 spheroids disappeared within 10 days.
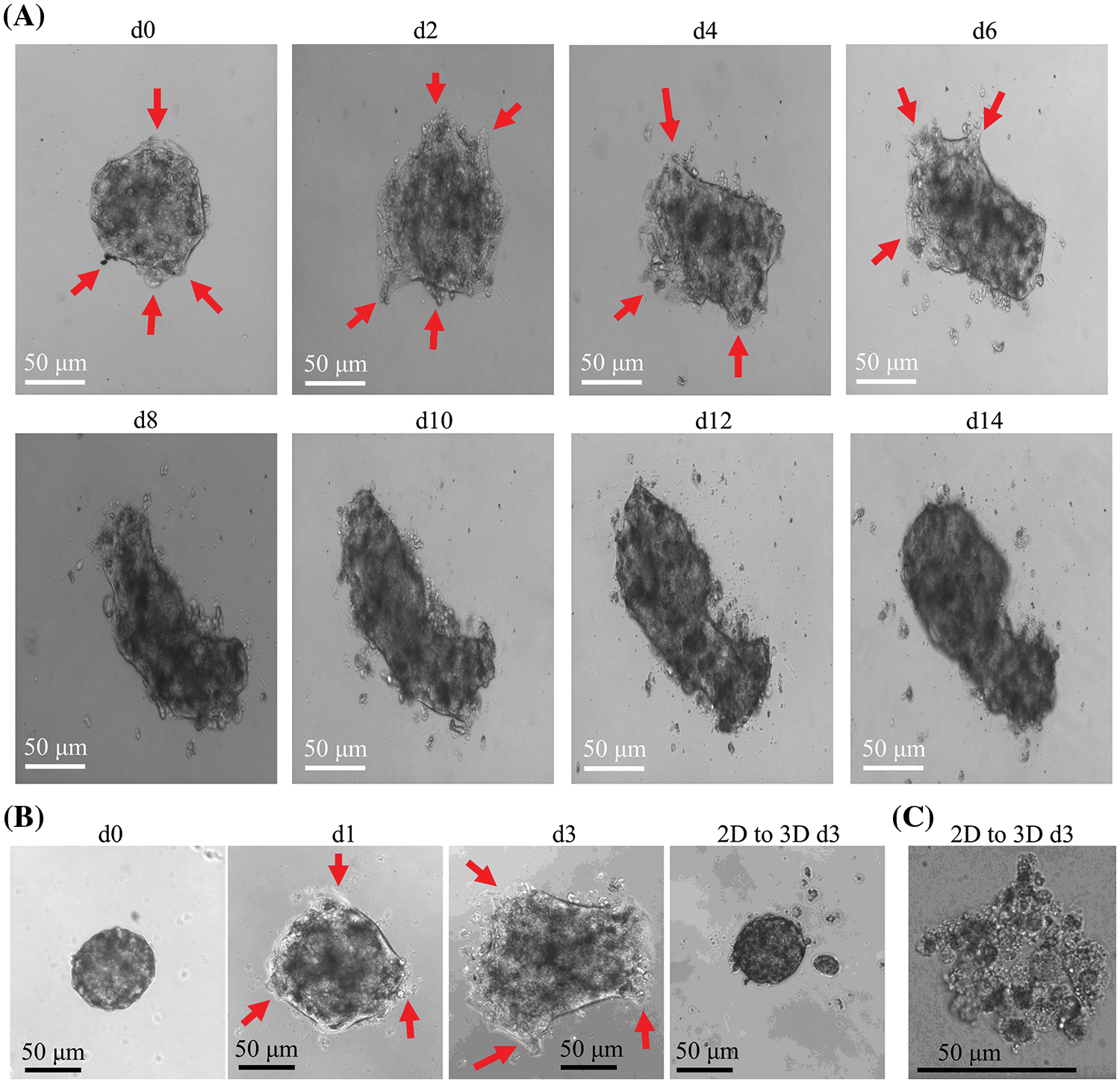
Figure 7: Collective cancer cell migration (CCCM) in LIM1863. (A) Spheroids in which CCCM occurred survived. (B and C) Detachment of spheroids at an early stage saved the spheroid (B), but at a very late stage, it failed (C). *p < 0.05 vs. control; #p > 0.05 vs. control. Red arrows: “finger-like” structures.
If the spheroid undergoing CCCM was detached from the dish base by gently pipetting with micropipette at an early stage, within 10 days after EMT initiation and more than 80% cells were alive, the spheroid would grow in suspension as normal and almost all cells survived (Fig. 7B). If the spheroid or cluster was detached at a very late stage (the cluster shed or separated for more than 5 days and the alive cells decreased to 30%.), the spheroid or cluster died out gradually (Fig. 7C). Those led to the hypothesis that the group of heterogeneous colorectal cancer cells in one cluster undergoing CCCM might face the same fate, either all survival or all death. That means all cells in one CCCM cluster behave like a supracellular unit.
EMT has been proposed as the critical mechanism for the acquisition of the metastatic phenotype by epithelial cancer cells both in single-cell migration and in collective cell migration (Saenz-de-Santa-Maria et al., 2020). It is a complex biological process that allows epithelial cancer cells to undergo morphological transformation into a mesenchymal phenotype to acquire invasive potential, including loss of apical-basal polarity and junctional architecture, and acquire mesenchymal characteristics, such as increased motility (Antony et al., 2019). Apical-basal polarity is one of the most important characteristics of epithelial cells. Loss of apical-basal polarity is a milestone for EMT but that could be simulated extremely rarely in models in vitro (Antony et al., 2019). Compared to colorectal epithelial cells in vivo, most of colorectal cancer cell lines, for example, HCT 116 and HT29, lose apical-basal polarity, organization, cell-cell junctions and change molecular expression levels when automatically growing as a monolayer in vitro (Liang et al., 2016; Antony et al., 2019; Jiang et al., 2021; Jonckheere et al., 2022; Shao et al., 2022). Unlike most other colorectal cancer cell lines, LIM1863 maintains important typical epithelial properties, including layers of cells arranged around a central lumen, apical-basal polarity, having types of cell-cell junctions, and so on. These observations suggest that LIM1863 is an epithelial cancer cell line, while most other colorectal cancer cell lines might be epithelial-to-mesenchymal transformed cancer cell lines rather than epithelial cancer cell lines. Furthermore, together with definite and distinct changes in growth pattern, morphology (particularly loss of apical-basal polarity), function, and molecular markers during EMT, LIM1863 is ideal for studying EMT.
EMT occurs spontaneously in a small part of LIM1863 cells and usually leads to CCCM. In this study, less than 1% of spheroids underwent CCCM, while other spheroids in the same well did not. So, EMT and CCCM should be induced by internal cues rather than external cues. According to the topology within the migrating cell ensemble, EMT cells, always occupying the leading edge, are assumed to be “leader” cells and the other distinct cell populations are assumed to be “follower” cells (Qin et al., 2021). Leaders and followers can be easily discriminated by location (Shellard and Mayor, 2019). The morphological difference between leaders and followers was definite and distinct in LIM1863, while rare models have that feature. LIM1863 spheroids undergoing CCCM shares several features in common with cancer lesions in vivo, including having several “finger-like” protrusions, discrete cancer cell clusters around a cancer lesion, which may be called peritumoral deposits, tumor deposits, satellites, in transit metastases, satellite metastases, microsatellite metastases and so on (Cohen et al., 2021; Papageorgiou et al., 2021; Yosefof et al., 2022). Hence, LIM1863 is an excellent choice of cell line to study CCCM, particularly spontaneous CCCM.
Recent studies have proved that CCCM reveals worse clinical outcomes (Aceto et al., 2014; Yang et al., 2019). CCCM causes cancer cell clusters shedding from the primary tumor. Cancer cell clusters traverse the vasculature, disseminate into the bloodstream, then turn into circulating tumor cell (CTC) clusters, also known as tumor microemboli (Aceto et al., 2014; Shellard and Mayor, 2019; Yang et al., 2019; Qin et al., 2021). Accumulating evidence support the hypothesis that most of CTCs do not survive nor proliferate at secondary sites because CTCs in one patient are much more than metastases (Aceto et al., 2014; Mu et al., 2015; Jansson et al., 2016). Maheswaran et al. believe that CTCs may be poised on the verge of apoptosis (Aceto et al., 2014). Jansson et al. (2016) found that about 90% of CTCs were apoptotic (Jansson et al., 2016). This phenomenon is rarely observed in CCCM models in vitro (Saenz-de-Santa-Maria et al., 2020; Meyer et al., 2021; Ogoke et al., 2021). However, in LIM1863, most of the cells undergoing CCCM died. We hypothesize that LIM1863 spheroids might be a more relevant model of in vivo cancer migration.
One interesting phenomenon was observed in LIM1863; the group of heterogeneous colorectal cancer cells undergoing collective migration behaves like a supracellular unit. In most of the cases, all cells in clusters died. A small part of clusters could survive, and under that condition, almost all follower cells, if not all, proliferated. Since one cluster consisted of heterogeneous cells and there were tens of cells in one cluster, it may be reasonable that a portion of the cells in a cluster would survive and another portion of the cells in the same cluster would die, although that is not the case. It seems that all cells in a cluster face the same fate, either all survive or all die. Recently, a few researchers suggested a special collective migration in development and immunity, named supracellular migration (Zegers and Friedl, 2014; Shellard and Mayor, 2019). In supracellular migration, cells are organized at the tissue level, where the influence of one cell goes beyond its borders, affecting neighboring and far-away cells. The entire group of cells behaves as a supracellular unit, transmitting forces between cells, moving in the same direction with a similar speed and highly organizing cytoskeleton within supracellular entities (Friedl and Mayor, 2017; van Helvert et al., 2018; Shellard and Mayor, 2019; Bernadskaya et al., 2021; Popkova et al., 2021). Few papers have reported cancer supracellular migration. To our knowledge, no study has reported the interesting phenomenon in which a group of heterogeneous cancer cells from one cluster undergoing CCCM either all survive or all die; not much is known about the mechanism. In this study, the number of cells in one cluster decreased continuously, and when the cluster was collectively migrating, all cells died eventually (Fig. 3B). If EMT cells in the cluster disappeared, cells in the reservoir survived and proliferated (Figs. 7A and 7B). These findings suggest that cells undergoing EMT might affect all other cells in the cluster. Consequently, the migratory subtype in LIM863 may be supracellular migration rather than collective cell migration (Shellard and Mayor, 2019). The hypothesis largely remains to be elucidated and requires more evidence.
LIM1863 mimic colorectal cancer in vivo well and maintains typical epithelial properties. LIM1863 is useful in the study of CCCM and EMT. All cells from one cluster undergoing CCCM die; otherwise, almost all cells in the reservoir survive. Consequently, the migratory subtype in LIM863 may be supracellular migration rather than collective cell migration (Fig. 8).
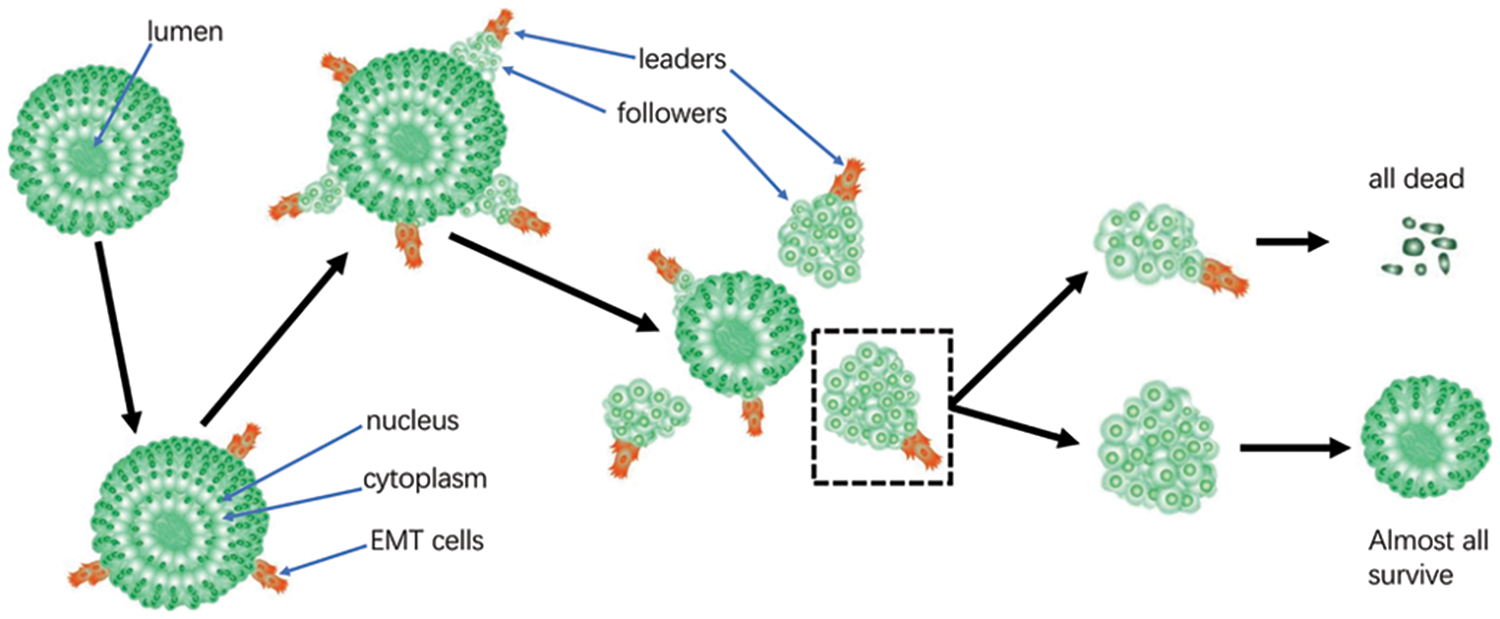
Figure 8: Schematic diagram illustrating collective cancer cell migration (CCCM) in LIM1863. Either all cells in the group of heterogeneous cells from one cluster undergoing CCCM die or all cells in the reservoir survive.
Acknowledgement: None.
Funding Statement: This work was supported by Hebei Province Key Research and Development Program (19277770D), Natural Science Foundation of Hebei Province (H2018423026), the Foundation of Health and Family Planning Commission of Hebei (20180686; 20180688), Fund of Hebei Administration of Traditional Chinese Medicine (2023020).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: X. Liang and J. He; data collection: J. Wu, X. Liang and J. He; analysis and interpretation of results: all authors; draft manuscript preparation: X. Liang and J. He. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics Approval: The animal study was approved by the Ethics Committee of Hebei Province Hospital of Chinese Medicine (HBZY2019-KY-169-01). Medical images from three patients are presented here and all patients signed informed consent for publication. This study was approved by the Ethics Committee of Hebei Province Hospital of Chinese Medicine (HBZY2022-KY-032-01) and was carried out in accordance with the Declaration of Helsinki Principles.
Conflicts of Interest: The authors declare that they have no conflicts of interest regarding the present study.
References
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS et al. (2014). Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158: 1110–1122. https://doi.org/10.1016/j.cell.2014.07.013 [Google Scholar] [PubMed] [CrossRef]
Antony J, Thiery JP, Huang RY (2019). Epithelial-to-mesenchymal transition: Lessons from development, insights into cancer and the potential of EMT-subtype based therapeutic intervention. Physical Biology 16: 041004. https://doi.org/10.1088/1478-3975/ab157a [Google Scholar] [PubMed] [CrossRef]
Avenarius MR, Jung JY, Askew C, Jones SM, Hunker KL et al. (2018). Grxcr2 is required for stereocilia morphogenesis in the cochlea. PLoS One 13: e0201713. https://doi.org/10.1371/journal.pone.0201713 [Google Scholar] [PubMed] [CrossRef]
Bates RC, Mercurio AM (2003). Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Molecular Biology of the Cell 14: 1790–1800. https://doi.org/10.1091/mbc.e02-09-0583 [Google Scholar] [PubMed] [CrossRef]
Bernadskaya YY, Yue H, Copos C, Christiaen L, Mogilner A (2021). Supracellular organization confers directionality and mechanical potency to migrating pairs of cardiopharyngeal progenitor cells. eLife 10: e70977. https://doi.org/10.7554/eLife.70977 [Google Scholar] [PubMed] [CrossRef]
Cohen R, Shi Q, Meyers J, Jin Z, Svrcek M et al. (2021). Combining tumor deposits with the number of lymph node metastases to improve the prognostic accuracy in stage III colon cancer: A post hoc analysis of the CALGB/SWOG 80702 phase III study (Alliance). Annals of Oncology 32: 1267–1275. https://doi.org/10.1016/j.annonc.2021.07.009 [Google Scholar] [PubMed] [CrossRef]
Friedl P, Mayor R (2017). Tuning collective cell migration by cell-cell junction regulation. Cold Spring Harbor Perspectives in Biology 9: a029199. https://doi.org/10.1101/cshperspect.a029199 [Google Scholar] [PubMed] [CrossRef]
He JM, Liang X, Luo F, Chen X, Xu X, Wang F, Zhang Z (2016). P53 is involved in a three-dimensional architecture-mediated decrease in chemosensitivity in colon cancer. Journal of Cancer 7: 900–909. https://doi.org/10.7150/jca.14506 [Google Scholar] [PubMed] [CrossRef]
He JM, Shin H, Wei X, Kadegowda AK, Chen R, Xie SK (2015). NPC1L1 knockout protects against colitis-asso, ciated tumorigenesis in mice. BMC Cancer 15: 189. https://doi.org/10.1186/s12885-015-1230-0 [Google Scholar] [PubMed] [CrossRef]
He JM, Wang FC, Qi HB, Li Y, Liang HJ (2009). Down-regulation of alphav integrin by retroviral delivery of small interfering RNA reduces multicellular resistance of HT29. Cancer Letters 284: 182–188. https://doi.org/10.1016/j.canlet.2009.04.023 [Google Scholar] [PubMed] [CrossRef]
Jansson S, Bendahl PO, Larsson AM, Aaltonen KE, Ryden L (2016). Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 16: 433. https://doi.org/10.1186/s12885-016-2406-y [Google Scholar] [PubMed] [CrossRef]
Jiang S, Li Q, Liu Y, Zhang H, Wang Q et al. (2021). Activation of WNT7b autocrine eases metastasis of colorectal cancer via epithelial to mesenchymal transition and predicts poor prognosis. BMC Cancer 21: 180. https://doi.org/10.1186/s12885-021-07898-2 [Google Scholar] [PubMed] [CrossRef]
Jonckheere S, Adams J, de Groote D, Campbell K, Berx G, Goossens S (2022). Epithelial-mesenchymal transition (EMT) as a therapeutic target. Cells Tissues Organs 211: 157–182. https://doi.org/10.1159/000512218 [Google Scholar] [PubMed] [CrossRef]
Kinoshita H, Hayakawa Y, Konishi M, Hata M, Tsuboi M et al. (2019). Three types of metaplasia model through Kras activation, Pten deletion, or Cdh1 deletion in the gastric epithelium. The Journal of Pathology 247: 35–47. https://doi.org/10.1002/path.5163 [Google Scholar] [PubMed] [CrossRef]
Liang X, Hu JN, He JM (2019). An optimized protocol of azoxymethane-dextran sodium sulfate induced colorectal tumor model in mice. Chinese Medical Sciences Journal 34: 270–277. https://doi.org/10.24920/003495 [Google Scholar] [PubMed] [CrossRef]
Liang X, Xu XQ, Wang FC, Chen XD, Li N, Wang CC, He JM (2015). E-cadherin knockdown increases beta-catenin reducing colorectal cancer chemosensitivity only in three-dimensional cultures. International Journal of Oncology 47: 1517–1527. https://doi.org/10.3892/ijo.2015.3137 [Google Scholar] [PubMed] [CrossRef]
Liang X, Xu XQ, Wang FC, Li N, He JM (2016). E-cadherin increasing multidrug resistance protein 1 via hypoxia-inducible factor-1α contributes to multicellular resistance in colorectal cancer. Tumour Biology 37: 425–435. https://doi.org/10.1007/s13277-015-3811-6 [Google Scholar] [PubMed] [CrossRef]
Meyer FAH, Kraus D, Glassmann A, Veit N, Winter J, Probstmeier R (2021). The Presence of Yin-Yang effects in the migration pattern of staurosporine-treated single versus collective breast carcinoma cells. International Journal of Molecular Sciences 22: 11961. https://doi.org/10.3390/ijms222111961 [Google Scholar] [PubMed] [CrossRef]
Mu Z, Wang C, Ye Z, Austin L, Civan J et al. (2015). Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Research and Treatment 154: 563–571. https://doi.org/10.1007/s10549-015-3636-4 [Google Scholar] [PubMed] [CrossRef]
Ogoke O, Yousef O, Ott C, Kalinousky A, Lin W, Shamul C, Ross S, Parashurama N (2021). Modeling liver organogenesis by recreating three-dimensional collective cell migration: A role for TGFbeta pathway. Frontiers in Bioengineering and Biotechnology 9: 621286. https://doi.org/10.3389/fbioe.2021.621286 [Google Scholar] [PubMed] [CrossRef]
Papageorgiou C, Apalla Z, Manoli SM, Lallas K, Vakirlis E, Lallas A (2021). Melanoma: Staging and follow-up. Dermatology Practical & Conceptual 11: e2021162S. https://doi.org/10.5826/dpc.11S1a162S [Google Scholar] [PubMed] [CrossRef]
Popkova A, Rauzi M, Wang X (2021). Cellular and supracellular planar polarity: A multiscale cue to elongate the drosophila egg chamber. Frontiers in Cell and Developmental Biology 9: 645235. https://doi.org/10.3389/fcell.2021.645235 [Google Scholar] [PubMed] [CrossRef]
Qin L, Yang D, Yi W, Cao H, Xiao G (2021). Roles of leader and follower cells in collective cell migration. Molecular Biology of the Cell 32: 1267–1272. https://doi.org/10.1091/mbc.E20-10-0681 [Google Scholar] [PubMed] [CrossRef]
Saenz-de-Santa-Maria I, Celada L, Chiara MD (2020). The leader position of mesenchymal cells expressing N-Cadherin in the collective migration of epithelial cancer. Cells 9: 731. https://doi.org/10.3390/cells9030731 [Google Scholar] [PubMed] [CrossRef]
Shao M, Jiang C, Yu C, Jia H, Wang Y, Mao X (2022). Capecitabine inhibits epithelial-to-mesenchymal transition and proliferation of colorectal cancer cells by mediating the RANK/RANKL pathway. Oncology Letters 23: 96. https://doi.org/10.3892/ol.2022.13216 [Google Scholar] [PubMed] [CrossRef]
Shellard A, Mayor R (2019). Supracellular migration—beyond collective cell migration. Journal of Cell Science 132: jcs226142. https://doi.org/10.1242/jcs.226142 [Google Scholar] [PubMed] [CrossRef]
van Helvert S, Storm C, Friedl P (2018). Mechanoreciprocity in cell migration. Nature Cell Biology 20: 8–20. https://doi.org/10.1038/s41556-017-0012-0 [Google Scholar] [PubMed] [CrossRef]
VanderVorst K, Dreyer CA, Konopelski SE, Lee H, Ho HH, Carraway KLIII (2019). Wnt/PCP signaling contribution to carcinoma collective cell migration and metastasis. Cancer Research 79: 1719–1729. https://doi.org/10.1158/0008-5472.CAN-18-2757 [Google Scholar] [PubMed] [CrossRef]
Vincan E, Brabletz T, Faux MC, Ramsay RG (2007). A human three-dimensional cell line model allows the study of dynamic and reversible epithelial-mesenchymal and mesenchymal-epithelial transition that underpins colorectal carcinogenesis. Cells Tissues Organs 185: 20–28. https://doi.org/10.1159/000101299 [Google Scholar] [PubMed] [CrossRef]
Whitehead RH, Jones JK, Gabriel A, Lukies RE (1987). A new colon carcinoma cell line (LIM1863) that grows as organoids with spontaneous differentiation into crypt-like structures in vitro. Cancer Research 47: 2683–2689. [Google Scholar] [PubMed]
Yang Y, Zheng H, Zhan Y, Fan S (2019). An emerging tumor invasion mechanism about the collective cell migration. American Journal of Translational Research 11: 5301–5312. [Google Scholar] [PubMed]
Yosefof E, Tzelnick S, Wallach L, Miller Y, Strenov Y, Bachar G, Shpitzer T, Mizrachi A (2022). Tumor satellites are associated with poor outcome in patients with oral cancer. The Laryngoscope 133: 336–343. https://doi.org/10.1002/lary.30156 [Google Scholar] [PubMed] [CrossRef]
Zegers MM, Friedl P (2014). Rho GTPases in collective cell migration. Small GTPases 5: e28997. https://doi.org/10.4161/sgtp.28997 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools