 Open Access
Open Access
ARTICLE
UCHL5 inhibits U251 glioma cell proliferation and tumor growth via stabilizing and deubiquitinating PTEN
1 Guangdong Provincial Key Laboratory of Regional Immunity and Diseases, Department of Neurosurgery, Shenzhen Second People’s Hospital, The first Affiliated Hospital of Shenzhen University, Shenzhen University Medical School, Shenzhen University, Shenzhen, 518055, China
2 Institute of Biological Therapy, Department of Immunology, Shenzhen University, Shenzhen, 518055, China
3 Department of Neurosurgery, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310058, China
* Corresponding Author: WEILIN CHEN. Email:
# These authors contributed equally to this work
BIOCELL 2023, 47(12), 2617-2625. https://doi.org/10.32604/biocell.2023.042476
Received 31 May 2023; Accepted 23 October 2023; Issue published 27 December 2023
Abstract
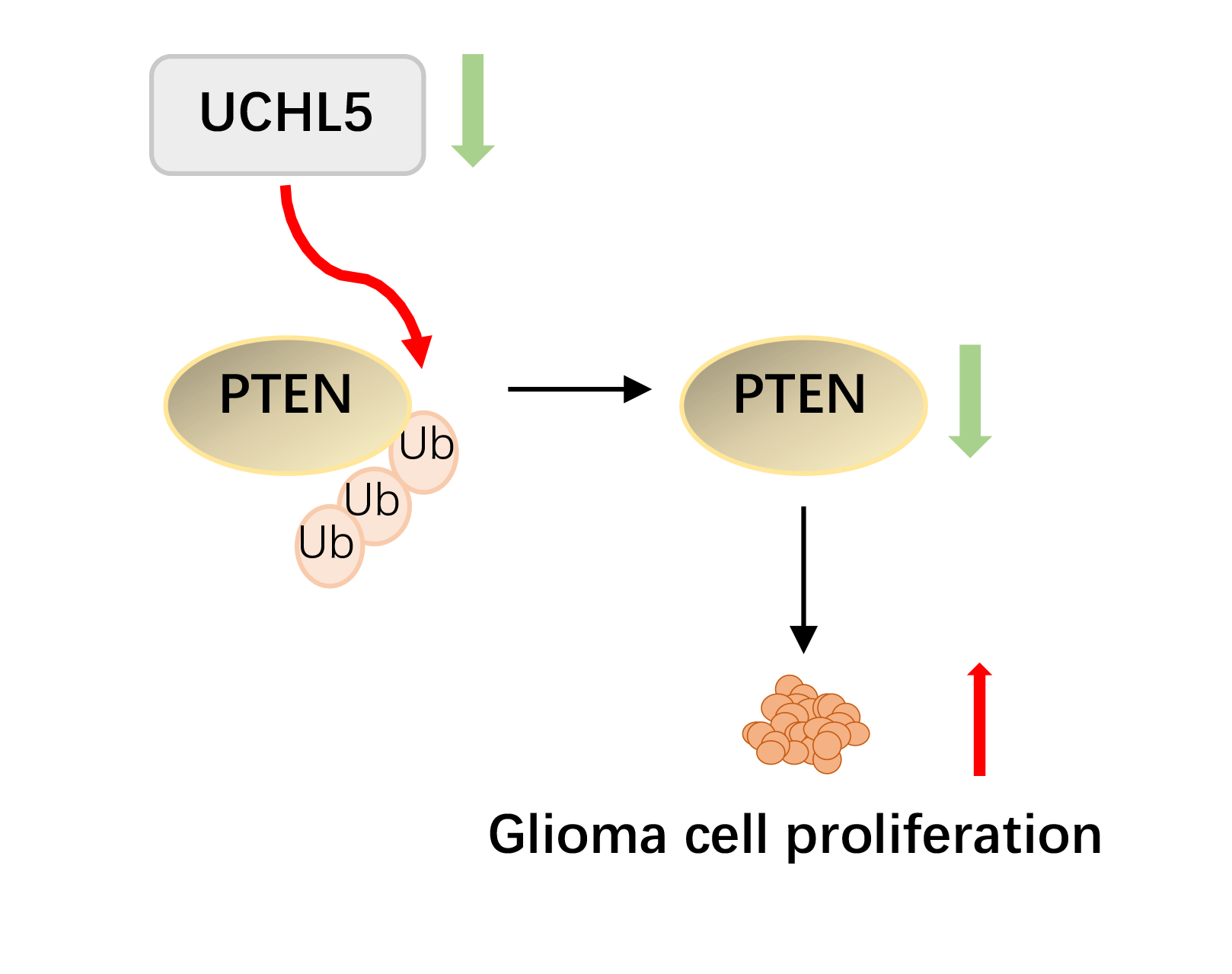
Background: Glioma is the most common primary brain tumor. Exploration of new tumorigenesis mechanism of glioma is critical to determine more effective treatment targets as well as to develop effective prognosis methods that can enhance the treatment efficacy. We previously demonstrated that the deubiquitinase biquitin carboxyl-terminal hydrolase L5 (UCHL5) was downregulated in human glioma. However, the effect and mechanism of UCHL5 on the proliferation of glioma cells remains unknown. Methods: Transfection of siRNA was used to knockdown the expression of UCHL5 in U251 cells. The 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay, Edu assay, and colony formation assay were employed to identify the effect of UCHL5 on the proliferation of U251 glioma cells. Western blotting and quantitative real-time PCR were carried out to detect the interaction of UCHL5 and PTEN. The effect of UCHL5 on the growth of glioma in vivo was evaluated in nude mice. Then Immunohistochemistry (IHC) were performed to analysis the expression of UCHL5 and PTEN in human glioma tissues. Results: Here, we have reported that silencing of UCHL5 could promote the proliferation of U251 glioma cells through MTT assay, Edu assay, and colony formation assay. Mechanically, we revealed that UCHL5 stabilizes the phosphatase and tensin homolog (PTEN) expression by deubiquitination, thereby inhibiting cell proliferation in U251 cells. Tumor xenograft experiments further demonstrated that silencing the UCHL5 expression could accelerate U251 cell growth in vivo. Finally, in human glioma tissue microarray, the positive correlation between UCHL5 and PTEN expression was confirmed through IHC assay. Conclusion: UCHL5 restrains the proliferation of U251 glioma cells by stabilizing and deubiquitinating PTEN. Our findings provide ideas for developing enhanced targeted PTEN therapy for patients with glioma.Graphic Abstract
Keywords
Glioma accounts for over 36% of all primary central nervous system tumors, making it the most common primary brain tumor (Grauwet and Chiocca, 2016). According to the histological classification of gliomas from grades I to IV by the World Health Organization (WHO) classification 2016, the most malignant glioma is glioblastoma (GBM), which belongs to grade IV (Louis et al., 2016). GBM is a malignant solid tumor that is prone to recurrence, accounting for 57% of all gliomas, with a median survival rate of only 14–16 months following the current standard-of-care treatment (Hosseinalizadeh et al., 2023), which includes surgery followed by ionizing radiation and chemotherapy with temozolomide (Nicholson and Fine, 2021). It is therefore urgent to explore more highly efficient therapies for GBM patients. Recently, with the emergence of several targeted treatment alternatives, the use of biomarkers that guide specific targeted therapies is extremely attractive (Capper et al., 2018; Kristensen et al., 2019; Yang et al., 2022a). It is important to delve deeper into the novel molecular mechanism of GBM to unveil more potent therapeutic targets and advance the development of efficacious prognostic methodologies. We believe that these endeavors will undoubtedly enhance the therapeutic outcomes for patients grappling with GBM.
Ubiquitin C-terminal hydrolase-L5 (UCHL5)/Uch37, is a member of the deubiquitinating enzymes (DUBs) family that remove the post-translational modification ubiquitin (Ub) chains from varieties of protein substrates (Fang and Shen, 2017). UCHL5 is abnormally expressed in numerous human tumors, including urothelial carcinoma (Chow et al., 2022), lung cancer (Wang et al., 2022b), and pancreatic adenocarcinoma (Yang et al., 2022b). UCHL5 plays multiple roles in different stages of the development of human cancers by rescuing the substrate proteins from proteasome-dependent degradation or regulating gene transcription (Cao et al., 2022; Yao et al., 2008). In pancreatic adenocarcinoma, elevated UCHL5 levels promote cell proliferation, colony formation, and migration through deubiquitination and stabilizing of ELK3 (ETS Transcription Factor 3) proteins (Yang et al., 2022b). UCHL5 was reported to accelerate the progression of bladder cancer cells by activating c-Myc (Cao et al., 2022). In our previous study, we demonstrated that UCHL5 inhibits glioma cell migration and invasion by limiting the SNRPF expression (Ge et al., 2017). However, it remains unclear whether the UCHL5 expression influences glioma cell proliferation.
In the present study, we demonstrated that UCHL5 inhibited the proliferation of U251 glioma cells both in vitro and in vivo. Mechanically, UCHL5 stabilized PTEN protein by deubiquitinating it. Our cumulative findings indicate that UCHL5 could inhibit the proliferation of glioma cells expressing PTEN protein, thereby providing a rationale to target PTEN in glioma therapy.
Cell culture, transfection, and infection
The cell lines U251 and HEK293T were purchased from the Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in DMEM supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) at 37°C under a 5% CO2 atmosphere. MG132 (#M8699, Sigma-Aldrich, Merck, kGaA, Darmstadt, Germany), chloroquine (#C6628, Sigma-Aldrich Merck, kGaA, Darmstadt, Germany), and b-AP15 (#S4920, Selleck, Shanghai, China) were used at the specified conditions. Plasmid DNA and siRNA were transfected using JetPrime (Polyplus Transfection, New York, NY, USA). Lentivirus packaging and cell infection experiments were conducted as described elsewhere (Ge et al., 2017).
Plasmids, ShRNA, and antibodies
Flag-tagged PTEN and Myc-tagged UCHL5 were constructed at our laboratory. HA-tagged ubiquitin, K48-ubiquitin and K63-ubiquitin were received as a kind gift from Prof. Xuetao Cao (Nankai University, Tianjin, China). shRNA3 (GGAGACTGTATCAATTAGATTTCAAGAGAATCTAATTCATACAGTCTCCTTTTTT) targeting UCHL5 (NM00119261) were inserted into lentivirus vector pLent-4 in 1 shRNA-GFP-Puro (Vigene Inc., Shangdong, China) (Ge et al., 2017). The open reading frame of UCHL5 was cloned into pLent-EF1a-FH-CMV-GFP (Vigene Inc., Shandong, China). These constructs were confirmed by DNA sequencing. The following antibodies were used in the experiment: anti-UCHL5 (Abcam, Cambridge, MA, USA), anti-PTEN (CST, Danvers, MA, USA), anti-HA (ProteinTech, Wuhan, China), anti-Flag (GenScript, Piscataway, NJ, USA), anti-MYC (GenScript, Piscataway, NJ, USA), anti-ubiquitin (CST, Danvers, MA, USA), and anti-GAPDH (ProteinTech, Wuhan, China).
U251 cells (2 × 105) were seeded into a 12-well plate overnight. Next, 20 nM of small-interfering RNAs (siRNAs) targeting UCHL5 were added to 100 μL of JetPrime buffer, followed by the addition of 3 μL transfection reagents and then vortexing and incubating for 10 min. The resultant mixture was then added to the experimental cells. After 48 or 72 h of transfection, the cells were harvested and analyzed by quantitative real-time polymerase chain reaction (PCR) or Western blotting.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from the cell lysates using TRIzol agent (Takara, Beijing, China). cDNA was produced by using a reverse transcription kit (Yeason Biotech, Shanghai, China). The mRNA expression levels of the related genes were determined by using the HifffTM qPCR SYBR Green Master Mix Kit (Yeason Biotech, Shanghai, China). Data were normalized to the GAPDH expression. UCHL5, forward primer: GAGTGGTGCCTCATGGAAAG, reverse primer: CAAGTCGGGAGTCCTGAACC; GAPDH, forward primer: GAGTCAACGGATTTGGTCGT, reverse primer: GACAAGCTTCCCGTTCTCAG; PTEN, forward primer: TGGATTCGACTTAGACTTGACCT, reverse primer: GGTGGGTTATGGTCTTCAAAAGG.
The protein were extracted from cells with cell lysis buffer supplemented with a protease inhibitor cocktail. The protein samples were separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After blocking in 5% non-fat milk or 5% bovine serum albumin (BSA) dissolved in TBST buffer for 1 h at room temperature, the membranes were incubated with first antibodies for overnight at 4°C. Then, the membranes were washed thrice to five with TBST at 10-min intervals and incubated with the specific secondary antibody for 1 h at room temperature. Enhanced chemiluminescence (ECL) chromogenic substrate (Fdbio science, Hangzhou, China) was used to expose the protein bands.
Plasmid transfected cells were harvested at 24–30 h post-transfection. SiRNA-transfected cells were harvested at 72 h post-transfection. Whole-cell extracts were lysed in an immunoprecipitation buffer. The cell lysis was centrifugalized for 10 min under 12,000 × g at 4°C. Then, the supernatants were incubated with Flag-M2 magnetic beads (Sigma-Aldrich, Merck, kGaA, Darmstadt, Germany) or protein A/G Plus-Agarose immunoprecipitation reagent (Santa Cruz Biotechnology, Dallas, Texas, USA) together with 1 μg anti-HA or PTEN antibody overnight at 4°C. The magnetic beads or agaroses were washed five times with immunoprecipitation washing buffer (composition: 0.5% Nonidet-P40, 100 mM NaCl, 1 mM EDTA, 20 mM Tris-Cl; pH 8.0). Then, the magnetic beads or agaroses were boiled for 10 min with SDS sample-loading buffer and subjected to Western blotting.
The cells expressing UCHL5 siRNA were seeded into a 96-well plate for 24, 48, 72, and 96 h. Then, 10 μL of MTT reagent was mixed into each well and allowed to react for 4 h. Then, 100 μL of dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Merck, kGaA, Darmstadt, Germany) was used to resolve the formazan to stop the reaction. The data were detected at an optical density value of 570 nm.
UCHL5 siRNA or shRNA were transfected int U251 cells. The cells containing siRNA or shRNA (1000 cells/well) were seeded into a 12-well plate. After 5–7 days, the cells were fixed with methyl alcohol for 0.5 h. Subsequently, the colonies were stained with Giemsa for 15 min, washed with sterile water, and then photographed. The obtained colony numbers were counted by Image J software (Wisconsin, USA).
The cells were exposed to 10 μM of 5-ethynyl-20-deoxyuridine (Edu) (Yeason Biotech, Shanghai, China) for 2 h at 37°C, followed by fixing with 4% paraformaldehyde for 20 min and washing with PBS containing 3% BSA. The cells were treated with 0.5% Triton-X-100 for 20 min, followed by reacting with 500 μL of 1× Apollo® reaction cocktail for 30 min. Finally, the DNA of cells were stained with 500 μL of Hoechst 33342 (Yeason Biotech, Shanghai, China).
Tissue microarrays (TMA or tissue chips) containing 67 tissue cores were obtained from the Department of Neurosurgery of the Second Affiliated Hospital, Zhejiang University, School of Medicine (Hangzhou, China). Studies involving human participants were reviewed and approved by the ethics committee of Zhejiang University (Approval number: 2020-620). The patients provided their written informed consent for their participation in the present study. The TMA was blocked with 5% BSA and incubated with primary antibody overnight at 4°C. The slides were incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibody and then treated by a 3,3′-diaminobenzidine (DAB) substrate kit (Abcam, Cambridge, MA, USA) for 3 min at room temperature, followed by counterstaining with hematoxylin and observing under a light microscope.
BALB/c nude mice (female, age: 5–6 weeks) were purchased from Guangdong Medical Laboratory Animal Center (Guangdong, China). The mice were randomly assigned to two groups and housed in SPF-breeding units. Then, 5 × 106 cells expressing shNC or shUCHL5 were subcutaneously injected into the mice (n = 3/group). The tumor volume was recorded every 2 days. The tumors were removed and measured 22 days after the mice were euthanized. The calculation formula of tumor volume is as follows: V = 1/2 ab2 mm3 (a: major axis; b: minor axis). The animal study was reviewed and approved by the ethics committee of Zhejiang University (Approval number: 2020-892).
Statistical analysis for the comparisons between groups was conducted using Student’s t-tests. A two-sided Chi-square test and Fisher’s exact test were employed to determine the association between UCHL5 and PTEN. Data analysis was conducted using GRAPHPAD PRISM 7 software (GraphPad Software, San Diego, CA, USA).
Knockdown of UCHL5 promotes U251 cell proliferation
In our previous study, the UCHL5 was demonstrated to inhibit the migration and invasion of human glioma cells (Ge et al., 2017). In order to further research the role of UCHL5 on the proliferation of human glioma cells, we interfered the UCHL5 expression in U251 cells by transfecting with UCHL5 siRNA or shRNA, followed by the EdU, MTT, and colony formation assays. Western blotting results revealed that the expression levels of UCHL5 protein were reduced in the U251 cells transfected with UCHL5 siRNA (siUCHL5) (Fig. 1A). qRT-PCR results indicated that the mRNA levels were reduced by almost 50% in the U251 cells with siUCHL5 (Fig. 1B). EdU staining exhibited that the numbers of DNA synthesis in U251 cells transfected with siUCHL5 were more than that in the control U251 cells (Fig. 1C). MTT assay elicited that the viability of U251 cells was increased by the knockdown of UCHL5 (Fig. 1D). In addition, U251 cells were infected with a lentivirus-encoding negative control or UCHL5 shRNA (shUCHL5). Western blotting assay was performed to detect the protein levels of UCHL5. The result showed that the expression levels of UCHL5 protein were downregulated by transfecting with shUCHL5 (Fig. 1E). qRT-PCR showed that UCHL5 mRNA were also knocked down by the shUCHL5 (Fig. 1F). According to the results of the EdU assay, U251 cells expressing shUCHL5 exhibited more numbers of DNA synthesis than U251 cells expressing shNC (Fig. 1G). MTT assay revealed the knockdown of UCHL5 promoted the proliferation of U251 cells (Fig. 1H). The number of colonies formed by U251 cells expressing shUCHL5 was significantly higher than the number of control cells (Fig. 1I). Together, the above data supported that the silence of UCHL5 accelerated the proliferation of U251 cells.
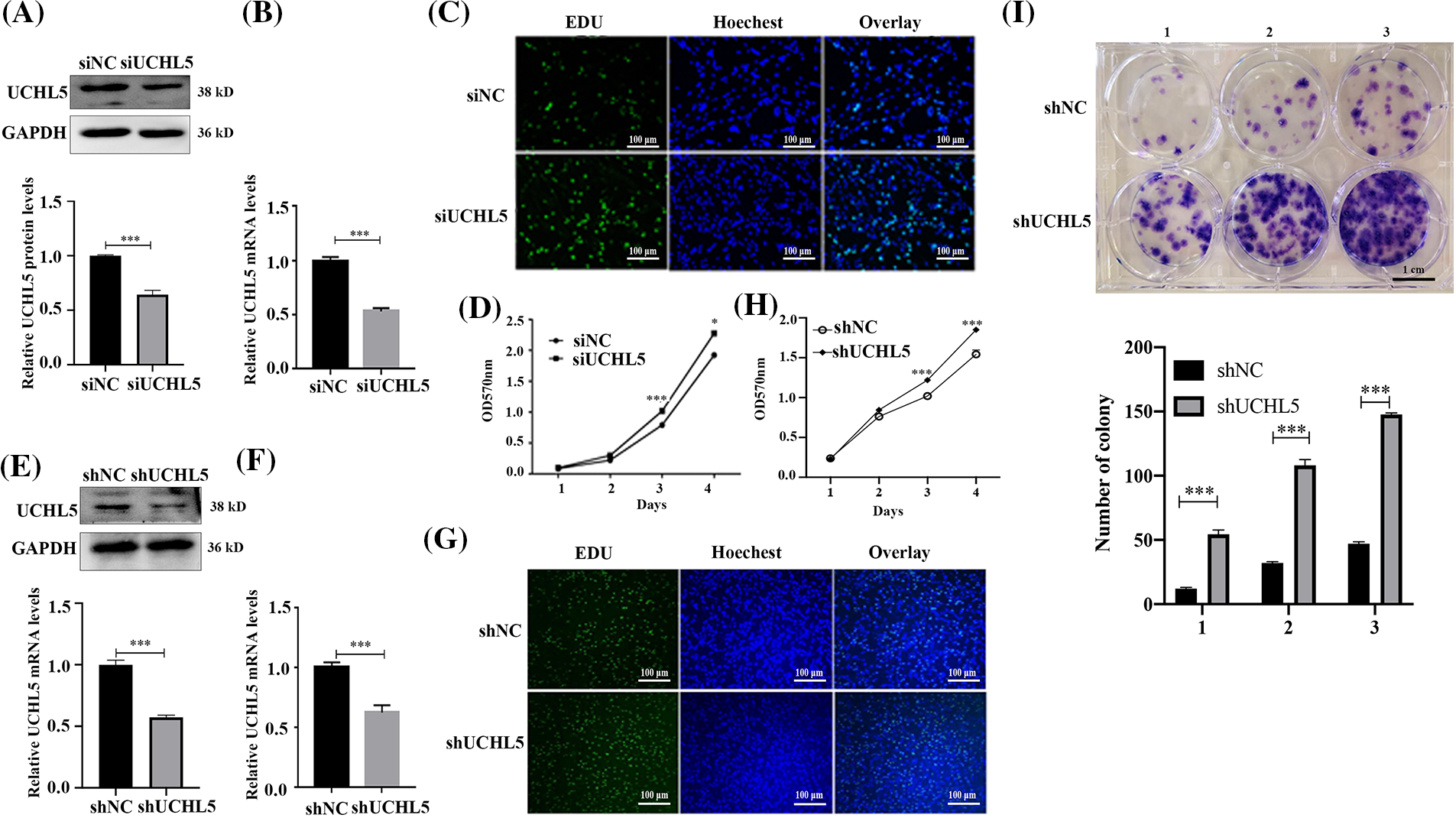
Figure 1: Silencing of UCHL5 promoted U251 cell proliferation. (A and B) The expression of UCHL5 was determined by Western blotting and qRT-PCR in U251 cells transfected with negative control siRNA (siNC) or UCHL5-siRNA (siUCHL5). (C and D) Cell proliferation detected by Edu staining and MTT assay in U251 cells transfected with siNC or siUCHL5. Scale bar = 100 μm. (E and F) The expression of UCHL5 was detected by Western blotting and qRT-PCR in U251 cells infected with lentiviruses encoding negative control or UCHL5-targeting shRNA (shNC or shUCHL5). (G) Cell proliferation was detected using Edu staining in U251 cells transfected with shNC or shUCHL5. (H) Cell proliferation was detected by MTT assay in U251 cells infected with lentiviruses encoding shNC or shUCHL5. (I) Cell proliferation was detected through colony formation assay in U251 cells transfected with shNC or shUCHL5. Scale bar = 1 cm. (A–I) Each bar represents the mean ± SD for biological triplicate experiments. *p < 0.05, ***p < 0.001, Student’s t-test.
UCHL5 stabilizes the PTEN expression
A phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which is a tumor suppressor gene, has been shown to inhibit tumor progression in gliomas (Khan et al., 2018). We detected the mRNA and protein levels of PTEN in the U251 cells transfected with siUCHL5. The results showed that the mRNA levels of PTEN were unchanged after the knockdown of UCHL5 in U251 cells (Fig. 2A). However, the protein expression of PTEN was lower in the U251 cells expressing siUCHL5 (Fig. 2B). In addition, we infected U251 cells with lentivirus expressing UCHL5 shRNA (shUCHL5) to knock down the UCHL5 expression. In the U251 cell expressing shUCHL5, the mRNA levels of PTEN showed no difference from that in U251 cells expressing negative-control shRNA (shNC) (Fig. 2C). The protein levels of PTEN were lower in the shUCHL5-U251 cells when compared with that in the control cells (Fig. 2D).
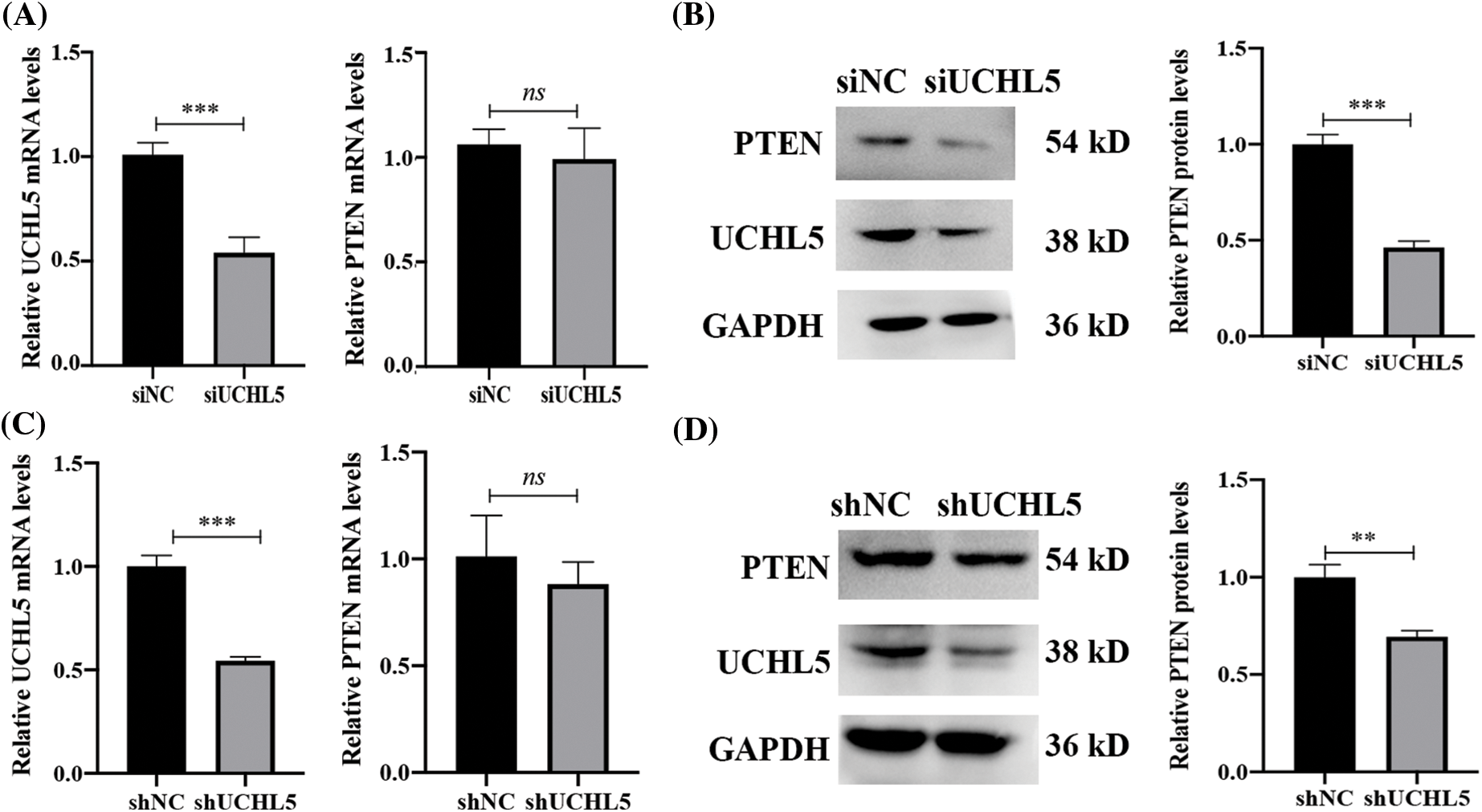
Figure 2: UCHL5 stabilizes PTEN expression. (A and B) The expression of UCHL5 and PTEN were determined by qRT-PCR and Western blotting in U251 cells transfected with negative-control siRNA (siNC) or UCHL5-siRNA (siUCHL5). (C and D) The expression levels of UCHL5 and PTEN were detected by qRT-PCR and Western blotting in U251 cells infected with lentivirus expressing shNC or shUCHL5. Each bar represents the mean ± SD for biological triplicate experiments. **p < 0.01, ***p < 0.001, ns: no significant, Student’s t-test.
In eukaryotic cells, protein degradation involves two major pathways—the ubiquitin-proteasome pathway and lysosomal proteolysis. To investigate the degradation pathway involved in the regulation of the PTEN expression by UCHL5 downregulation, we transfected siUCHL5 into U251 cells, followed by treatment with proteasome inhibitor MG132 or lysosome inhibitor chloroquine (CQ). Western blotting revealed that MG132, rather than CQ increase the PTEN protein levels upon UCHL5 expression silencing (Fig. 3A). The data indicated that UCHL5 stabilized the PTEN expression in the ubiquitin-proteasome pathway. Moreover, we treated U251 cells with b-AP15 to inhibit the deubiquitinating activity of UCHL5 and detected the expression of PTEN. The results indicated that the expression of PTEN was downregulated in U251 cells after treatment with b-AP15 (Fig. 3B). Next, Co-immunoprecipitation (Co-IP) assay was performed to determine the interaction of UCHL5 and PTEN. As shown in Fig. 3C, UCHL5 could bind with PTEN (Fig. 3C). Furthermore, we detected whether UCHL5 affects the ubiquitination levels of PTEN. As shown in Fig. 3D, silencing the UCHL5 expression in U251 cells upregulated the endogenous ubiquitination levels of PTEN. To reflect the de-ubiquitinating effect of UCHL5 on PTEN, we further detected the corresponding effect in HEK293T cells, which is conducive to transfection plasmids and widely applied in the study of the effect of ubiquitinase/deubiquitinase on substrate proteins (Li et al., 2022; Zhang et al., 2022). Myc-tagged UCHL5 (Myc-UCHL5), HA-tagged ubiquitin (HA-Ub), and Flag-tagged PTEN (Flag-PTEN) plasmids were co-transfected into HEK293T cells to detect PTEN ubiquitination. Co-IP assay exhibited that the overexpression of UCHL5 decreased PTEN ubiquitination (Fig. 3E). Moreover, both the K48-linked ployubiquitination chain and K63-linked ployubiquitination chain could be removed from the PTEN protein by UCHL5 (Fig. 3F). These results together suggest that UCHL5 stabilized the expression of PTEN via deubiquitination.
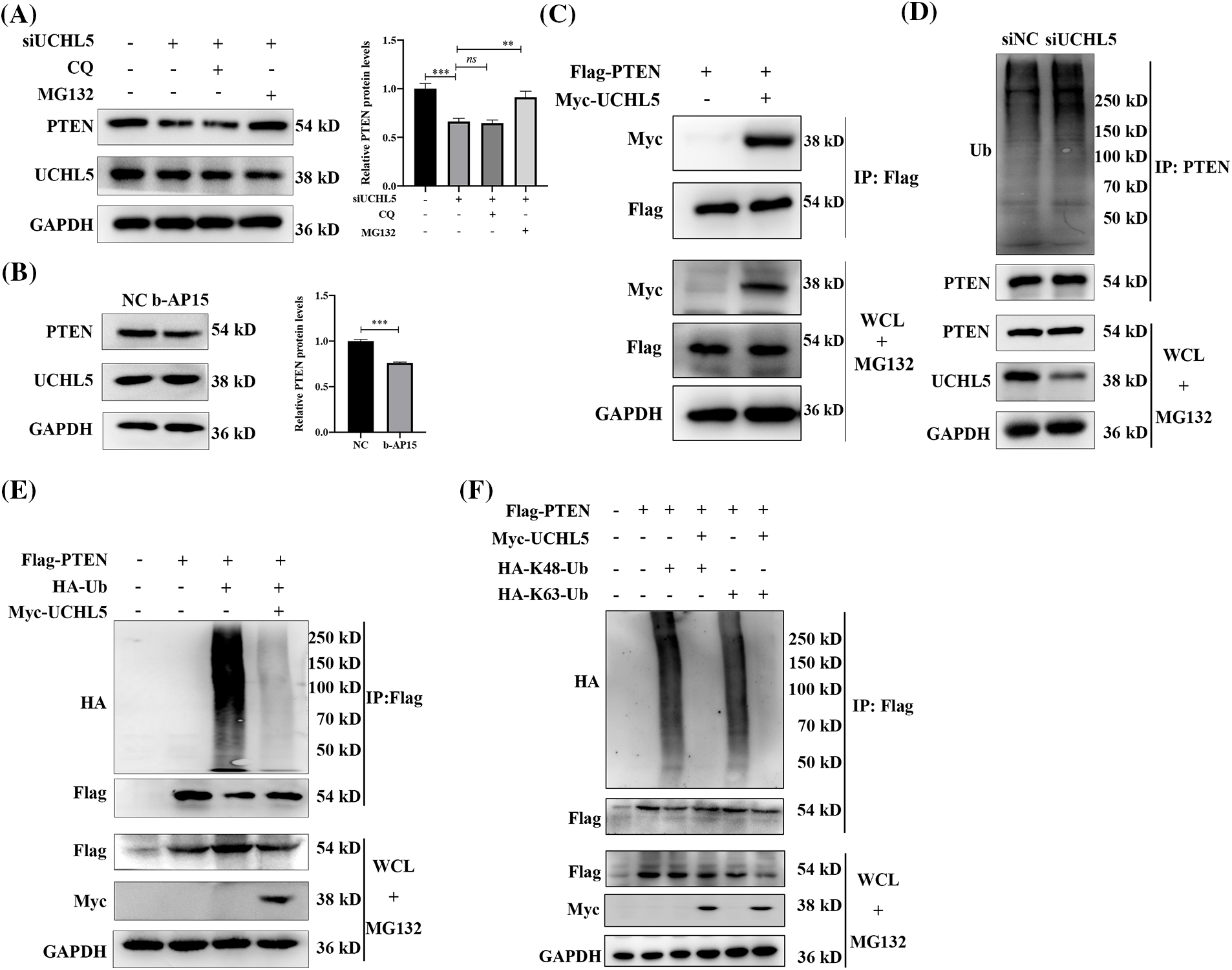
Figure 3: UCHL5 deubiquitinated PTEN. (A) U251 cells were treated with negative cotrol siRNA (siNC) or UCHL5 siRNA (siUCHL5) for 60 h. MG132 inhibitor (10 μM) or CQ inhibitor (50 μM) were added for 6 h before harvesting the cells. The expression of UCHL5 and PTEN protein was detected by Western blotting. Each bar represents the mean ± SD for biological triplicate experiments. ***p < 0.001, **p < 0.01, ns: no significant, Student’s t-test. (B) The protein levels of UCHL5 and PTEN were detected by Western blotting in U251 cells treated without or with b-AP15 for 6 h. Each bar represents the mean ± SD for biological triplicate experiments. ***p < 0.001, **p < 0.01, ns: no significant, Student’s t-test. (C) HEK293T cells were co-transfected with the Flag-tagged PTEN (Flag-PTEN) and Myc-tagged UCHL5 (Myc-UCHL5) for 30 h. Cell lysates were immunoprecipitated (IP) with Flag-M2 magnetic beads and immunoblotted with the anti-Myc and anti-Flag antibodies. (D) Cell lysates were immunoprecipitated with an anti-PTEN antibody after the U251 cells transfected with siUCHL5 for 72 h. Endogenous ubiquitination levels were detected by Western blotting. (E and F) HEK293T cells were co-transfected with the indicated plasmids for 30 h. CO-IP and Western blotting assay were performed to detect the ubiquitination levels of PTEN. WCL, whole cell lysates.
Knockdown of UCHL5 promotes the growth of glioma xenograft
To determine the role of UCHL5 in the development of human glioma in vivo, we constructed and selected U251 cells stably expressing shUCHL5 or shNC. The U251 cells expressing shUCHL5 or shNC were re-suspended in 0.1 mL PBS and implanted subcutaneously into the flanks of BALB/c nude mice by, respectively. At 22 days after the inoculation of the cells, the tumor size of shUCHL5-U251 cells was larger than that of the U251 cells containing shNC (Figs. 4A and 4B). The tumor volume was measured every 2 days after the tumor cell injection. The tumor volume was calculated as follows: V = 1/2 ab2 mm3 (a: major axis; b: minor axis). The result indicated that the volumes of xenograft derived from shUCHL5-U251 cells were larger than that obtained from the negative control U251 cells (Fig. 4C). Taken together, these findings imply that the knockdown of UCHL5 promoted the proliferation of glioma xenograft in mice.
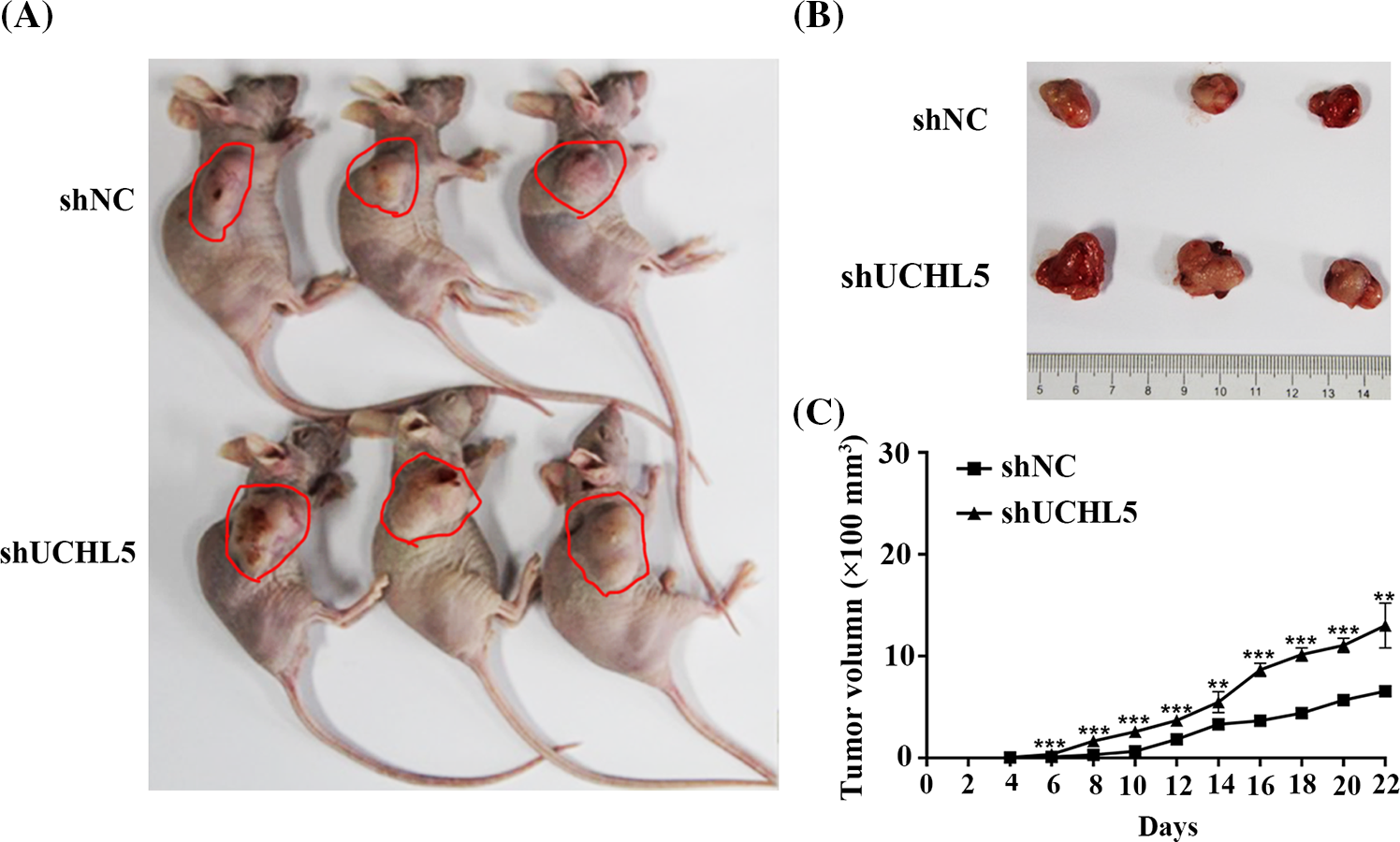
Figure 4: Knockdown of UCHL5 promoted the growth of glioma xenograft in nude mice. U251 cells stably expressing negative-control shRNA (shNC) or UCHL5 shRNA (shUCHL5) were injected into the right flanks of BALB/C nude mice. The tumor volumes were measured every 2 days. Mice photos (A), tumor images (B), and growth curves (C) were obtained on day 22 after the inoculation. n = 3 biologically independent tumor samples for each group. The data are presented as mean values ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t-test. The red circles are used to point out tumors.
UCHL5 expression positively correlated with PTEN in human glioma
To investigate the relationship between UCHL5 and PTEN expression, we analyzed the clinical samples of human glioma patients. Immunohistochemical tests of the TMA after incubation with UCHL5 and PTEN antibody revealed that the glioma sample with a high expression of UCHL5 protein also showed a high expression of PTEN (Fig. 5A). Similarly, the glioma sample with a poor expression of UCHL5 protein also showed a poor expression of PTEN (Fig. 5B). Moreover, the protein levels of PTEN were positively correlated with the UCHL5 expression (Fig. 5C).
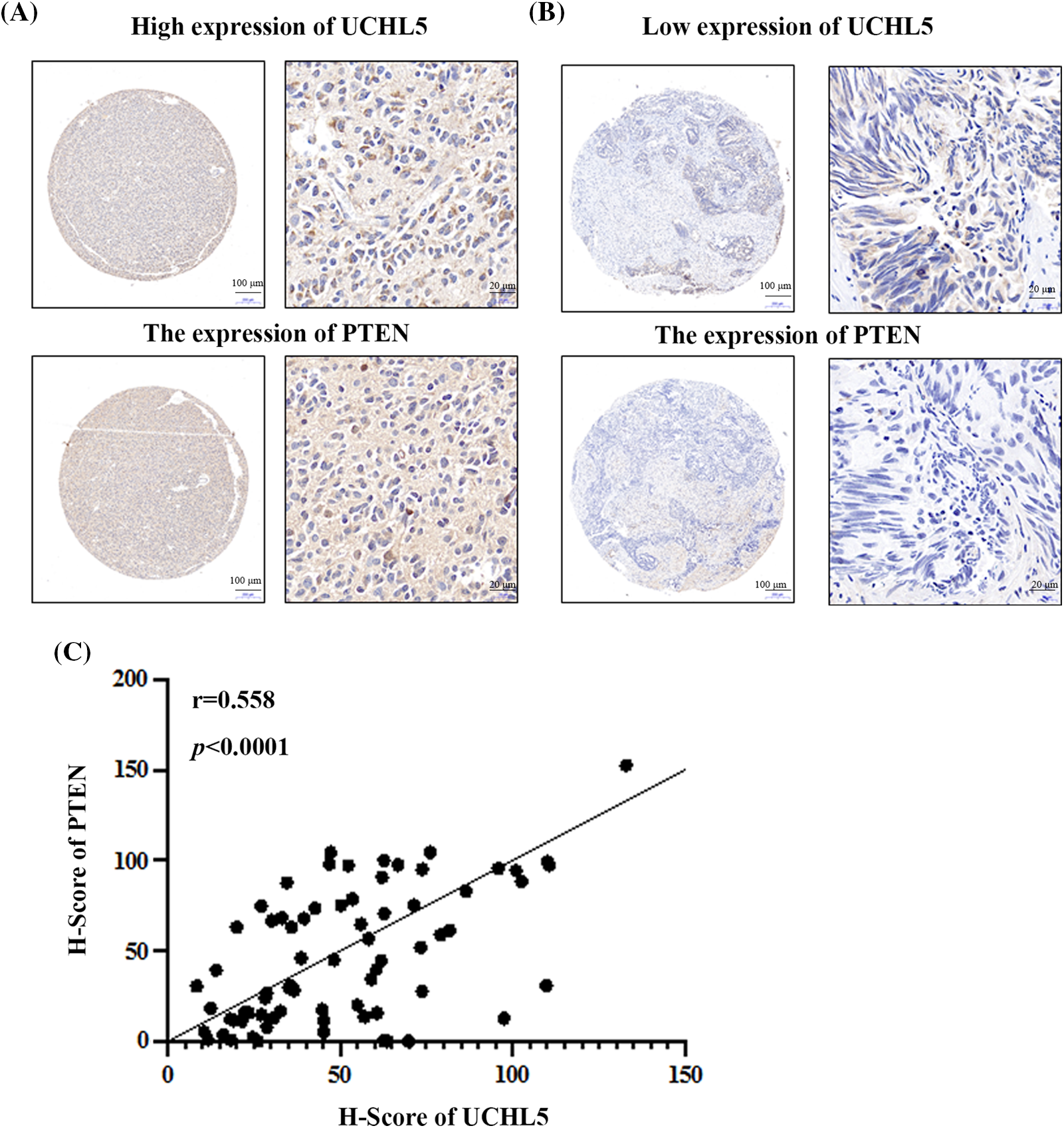
Figure 5: UCHL5 expression positively correlated with the PTEN expression in clinical glioma tissue samples obtained from patients. (A) and (B) The expression levels of UCHL5 and PTEN protein in human glioma tissue microarrays (TMA) were detected by IHC using the UCHL5 and PTEN antibodies. Scale bar, 100 or 20 μm, as indicated. (C) Statistical analysis of the correlation between UCHL5 expression and PTEN expression in glioma TMA (n = 63).
GBM accounts for almost 80% of all malignant brain tumors (Weller et al., 2015). The survival period of only 5.5% of GBM patients is beyond 5 years after the current standard-of-care treatment (Cantrell et al., 2019; Molinaro et al., 2019). The malignant proliferation contributes to this pessimistic survival data. The ubiquitin-proteasomal system is involved in multiple cellular processes, including cell proliferation (Jang, 2018). Several studies have concentrated on the modulation of protein ubiquitination and deubiquitination as a mechanism of glioma control (Hede et al., 2014; Jin et al., 2017; Maksoud, 2021). TRIM59, a ubiquitin ligase, induced ubiquitination and degradation of tumor suppressive histone variant macroH2A1, which results in the suppression of glioma growth (Sang et al., 2019). USP5, a deubiquitinating enzyme, promoted the proliferation of GBM by stabilizing Cyclin D1 (Li et al., 2021). USP39 was demonstrated that accelerate the progression of glioma by deubiquitinating and stabilizing Cyclin B1 protein (Xiao et al., 2023). Numerous deubiquitinating enzymes (DUB) genes are expressed abnormally in human glioma samples, including USP20, USP31, and USP18 (Tang et al., 2017). In the previous study, UCHL5 was poorly expressed in human glioma samples and that it inhibited the migration and invasion of glioma cells by downregulating small nuclear ribonucleoprotein polypeptide F (SNRPF) (Ge et al., 2017). However, the deubiquitylation function of UCHL5 in the development of glioma remains unclear, necessitating further study about the role of UCHL5 in human glioma. In this study, we found that the knockdown of UCHL5 could accelerate the proliferation of U251 glioma cells both in vitro and in nude mice, indicating that UCHL5 is a promising targeted protein in the exploration of new therapy for inhibiting glioma cells’ proliferation.
PTEN is a tumor suppressor gene that classically dampens the PI3K/AKT/mTOR growth-promoting signaling cascade. Abnormally low PTEN expression is considered to predict the malignant progression of gliomas, and PTEN can act as a biomarker to predict the prognosis of patients with gliomas (Khan et al., 2018). The loss of PTEN has been implicated as a cause of resistance to therapies. We therefore speculated that the effect of UCHL5 on U251 glioma cells was determined by the PTEN expression. Obviously, the PTEN expression was downregulated by the knockdown of UCHL5 in U251 glioma cells. In the clinical glioma samples obtained from patients, the expression of PTEN and UCHL5 showed a positive correlation. PTEN acts as a tumor suppressor and is frequently disrupted in several types of human cancers (Lee et al., 2018). Ubiquitination/deubiquitination is one of the major molecular mechanisms regulating protein stability, subcellular localization, and lipid phosphatase activity of PTEN (Christine et al., 2022; Wang et al., 2022a). As reported, OUT domain-containing protein 3 (OTUD3) remarkably upregulated the PTEN levels and suppressed tumorigenesis (Yuan et al., 2015). Ubiquitin-specific protease (USP) 7 reduced monoubiquitination of PTEN and interacted with it to restrict PTEN localization, thereby inducing apoptosis in cancer cells (Wu et al., 2014; Yeasmin Khusbu et al., 2018). Moreover, USP10 activated PTEN by removing the K63-linked polyubiquitnation and suppressed the AKT/mTOR signaling, thereby inhibiting non-small cell lung cancer cell proliferation (He et al., 2021). Our data indicated that the polyubiquitin chain of PTEN could be removed by UCHL5, thereby implying that UCHL5 acts as a deubiquitinase of PTEN. Ub chains have eight different types, including lysine 6 (K6), lysine 11 (K11), lysine 27 (K27), lysine 29 (K29), lysine 33 (K33), lysine 48 (K48), lysine 63 (K63) and methionine 1 (M1). Among them, the K48- and K63-linked polyUb chains were widely studied. The K48 chains mainly responsible for the degradation of substrate protein. The K63 chains were related to the activation of substrate protein (Sun and Zhang, 2022). In the present study, both the K48 and K63 linked polyubiquitin chains in PTEN could be released by UCHL5, indicating UCHL5 might regulated the stabilization and activation of PTEN. The increasing molecular regulating mechanism of PTEN undoubtedly suggests the possible design of novel cancer therapies.
Overall, this study is the first to report UCHL5 as a deubiquitinase of PTEN. Considering that UCHL5 is downregulated in glioma cells and inhibits U251 glioma cell proliferation, the restoration of UCHL5 may provide a new strategy for the treatment of glioma patients with PTEN deficiency. Future research should focus on precisely define the interaction of UCHL5 and PTEN and to reveal the PTEN downstream targets in the tumorigenesis of glioma.
In summary, UCHL5 knockdown promoted the proliferation of U251 glioma cells in vitro and the growth of U251 cells in vivo. Mechanically, UCHL5 stabilized the PTEN expression by deubiquitinating it. Moreover, the protein levels of UCHL5 and PTEN showed a positive correlation in the human glioma TMA. The present findings demonstrated that UCHL5 acts as a PTEN deubiquitinase to stabilize the PTEN expression, which induces the inhibiting effect on the cells proliferation. The cumulative data implies that the selective elevation of UCHL5 represents an extremely promising therapy for targeting PTEN in human glioma.
Acknowledgement: The authors thank the Instrument Analysis Center of Shenzhen University for providing instrument support and Jiafeng GE (Zhejiang University) for providing technology support.
Funding Statement: This work was supported by grants from the Shenzhen Science and Technology Innovation Commission Grant (Nos. JCYJ20180507182253653 and JCYJ20190808172201639), Guangdong Province Basic and Applied Basic Research Fund (No. 2022A1515111143).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Weilin CHEN, Yue XIAO; data collection: Wenjing MA, Weiwei HU, Guodong HUANG; analysis and interpretation of results: Xinyi CHEN, Qianqian DI, Xibao ZHAO; draft manuscript preparation: Weilin CHEN, Yue XIAO. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: The animal study was reviewed and approved by the Ethics Committee of Shenzhen University Medical School (Approved number: 2020-892). The studies involving human participants were reviewed and approved by the Ethics Committee of Zhejiang University (Approved number: 2020-620). The patients provided their written informed consent to participate in this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Cantrell JN, Waddle MR, Rotman M, Peterson JL, Ruiz-Garcia H, Heckman MG, Quinones-Hinojosa A, Rosenfeld SS, Brown PD, Trifiletti DM (2019). Progress toward long-term survivors of glioblastoma. Mayo Clinic Proceedings 94: 1278–1286. https://doi.org/10.1016/j.mayocp.2018.11.031 [Google Scholar] [PubMed] [CrossRef]
Cao Y, Yan X, Bai X, Tang F, Si P et al. (2022). UCHL5 promotes proliferation and migration of bladder cancer cells by activating c-Myc via AKT/mTOR signaling. Cancers 14: 5538. https://doi.org/10.3390/cancers14225538 [Google Scholar] [PubMed] [CrossRef]
Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D et al. (2018). DNA methylation-based classification of central nervous system tumours. Nature 555: 469–474. https://doi.org/10.1038/nature26000 [Google Scholar] [PubMed] [CrossRef]
Chow PM, Dong JR, Chang YW, Kuo KL, Lin WC, Liu SH, Huang KH (2022). The UCHL5 inhibitor b-AP15 overcomes cisplatin resistance via suppression of cancer stemness in urothelial carcinoma. Molecular Therapy-Oncolytics 26: 387–398. https://doi.org/10.1016/j.omto.2022.08.004 [Google Scholar] [PubMed] [CrossRef]
Christine A, Park MK, Song SJ, Song MS (2022). The equilibrium of tumor suppression: DUBs as active regulators of PTEN. Experimental & Molecular Medicine 54: 1814–1821. https://doi.org/10.1038/s12276-022-00887-w [Google Scholar] [PubMed] [CrossRef]
Fang Y, Shen X (2017). Ubiquitin carboxyl-terminal hydrolases: Involvement in cancer progression and clinical implications. Cancer and Metastasis Reviews 36: 669–682. https://doi.org/10.1007/s10555-017-9702-0 [Google Scholar] [PubMed] [CrossRef]
Ge J, Hu W, Zhou H, Yu J, Sun C, Chen W (2017). Ubiquitin carboxyl-terminal hydrolase isozyme L5 inhibits human glioma cell migration and invasion via downregulating SNRPF. Oncotarget 8: 113635–113649. https://doi.org/10.18632/oncotarget.23071 [Google Scholar] [PubMed] [CrossRef]
Grauwet K, Chiocca EA (2016). Glioma and microglia, a double entendre. Nature Immunology 17: 1240–1242. https://doi.org/10.1038/ni.3586 [Google Scholar] [PubMed] [CrossRef]
He Y, Jiang S, Mao C, Zheng H, Cao B, Zhang Z, Zhao J, Zeng Y, Mao X (2021). The deubiquitinase USP10 restores PTEN activity and inhibits non-small cell lung cancer cell proliferation. Journal of Biological Chemistry 297: 101088. https://doi.org/10.1016/j.jbc.2021.101088 [Google Scholar] [PubMed] [CrossRef]
Hede SM, Savov V, Weishaupt H, Sangfelt O, Swartling FJ (2014). Oncoprotein stabilization in brain tumors. Oncogene 33: 4709–4721. https://doi.org/10.1038/onc.2013.445 [Google Scholar] [PubMed] [CrossRef]
Hosseinalizadeh H, Ebrahimi A, Tavakoli A, Monavari SH (2023). Glioblastoma as a novel drug repositioning target: Updated state. Anti-Cancer Agents in Medicinal Chemistry 23: 1253–1264. https://doi.org/10.2174/1871520623666230202163112 [Google Scholar] [PubMed] [CrossRef]
Jang HH (2018). Regulation of protein degradation by proteasomes in cancer. Journal of Cancer Prevention 23: 153–161. https://doi.org/10.15430/JCP.2018.23.4.153 [Google Scholar] [PubMed] [CrossRef]
Jin WL, Mao XY, Qiu GZ (2017). Targeting deubiquitinating enzymes in glioblastoma multiforme: Expectations and challenges. Medicinal Research Reviews 37: 627–661. https://doi.org/10.1002/med.21421 [Google Scholar] [PubMed] [CrossRef]
Khan IN, Ullah N, Hussein D, Saini KS (2018). Current and emerging biomarkers in tumors of the central nervous system: Possible diagnostic, prognostic and therapeutic applications. Seminars in Cancer Biology 52: 85–102. https://doi.org/10.1016/j.semcancer.2017.07.004 [Google Scholar] [PubMed] [CrossRef]
Kristensen BW, Priesterbach-Ackley LP, Petersen JK, Wesseling P (2019). Molecular pathology of tumors of the central nervous system. Annals of Oncology 30: 1265–1278. https://doi.org/10.1093/annonc/mdz164 [Google Scholar] [PubMed] [CrossRef]
Lee YR, Chen M, Pandolfi PP (2018). The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nature Reviews Molecular Cell Biology 19: 547–562. https://doi.org/10.1038/s41580-018-0015-0 [Google Scholar] [PubMed] [CrossRef]
Li Y, Wang T, Wan Q, Wang Q, Chen Z et al. (2022). TRAF4 maintains deubiquitination of caveolin-1 to drive glioblastoma stemness and temozolomide resistance. Cancer Research 82: 3573–3587. https://doi.org/10.1158/0008-5472.CAN-21-3882 [Google Scholar] [PubMed] [CrossRef]
Li G, Yang T, Chen Y, Bao J, Wu D et al. (2021). USP5 sustains the proliferation of glioblastoma through stabilization of cyclinD1. Frontiers in Pharmacology 12: 720307. https://doi.org/10.3389/fphar.2021.720307 [Google Scholar] [PubMed] [CrossRef]
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016). The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathologica 131: 803–820. https://doi.org/10.1007/s00401-016-1545-1 [Google Scholar] [PubMed] [CrossRef]
Maksoud S (2021). The role of the ubiquitin proteasome system in glioma: Analysis emphasizing the main molecular players and therapeutic strategies identified in glioblastoma multiforme. Molecular Neurobiology 58: 3252–3269. https://doi.org/10.1007/s12035-021-02339-4 [Google Scholar] [PubMed] [CrossRef]
Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR (2019). Genetic and molecular epidemiology of adult diffuse glioma. Nature Reviews Neurology 15: 405–417. https://doi.org/10.1038/s41582-019-0220-2 [Google Scholar] [PubMed] [CrossRef]
Nicholson JG, Fine HA (2021). Diffuse glioma heterogeneity and its therapeutic implications. Cancer Discovery 11: 575–590. https://doi.org/10.1158/2159-8290.CD-20-1474 [Google Scholar] [PubMed] [CrossRef]
Sang Y, Li Y, Zhang Y, Alvarez AA, Yu B, Zhang W, Hu B, Cheng SY, Feng H (2019). CDK5-dependent phosphorylation and nuclear translocation of TRIM59 promotes macroH2A1 ubiquitination and tumorigenicity. Nature Communications 10: 4013. https://doi.org/10.1038/s41467-019-12001-2 [Google Scholar] [PubMed] [CrossRef]
Sun M, Zhang X (2022). Current methodologies in protein ubiquitination characterization: From ubiquitinated protein to ubiquitin chain architecture. Cell & Bioscience 12: 126. https://doi.org/10.1186/s13578-022-00870-y [Google Scholar] [PubMed] [CrossRef]
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z (2017). GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Research 45: W98–W102. https://doi.org/10.1093/nar/gkx247 [Google Scholar] [PubMed] [CrossRef]
Wang K, Liu J, Li YL, Li JP, Zhang R (2022a). Ubiquitination/de-ubiquitination: A promising therapeutic target for PTEN reactivation in cancer. Biochimica et Biophysica Acta-Reviews on Cancer 1877: 188723. https://doi.org/10.1016/j.bbcan.2022.188723 [Google Scholar] [PubMed] [CrossRef]
Wang S, Wang T, Yang Q, Cheng S, Liu F et al. (2022b). Proteasomal deubiquitylase activity enhances cell surface recycling of the epidermal growth factor receptor in non-small cell lung cancer. Cellular Oncology 45: 951–965. https://doi.org/10.1007/s13402-022-00699-0 [Google Scholar] [PubMed] [CrossRef]
Weller M, Wick W, Aldape K, Brada M, Berger M et al. (2015). Glioma. Nature Reviews Disease Primers 1: 15017. https://doi.org/10.1038/nrdp.2015.17 [Google Scholar] [PubMed] [CrossRef]
Wu Y, Zhou H, Wu K, Lee S, Li R, Liu X (2014). PTEN phosphorylation and nuclear export mediate free fatty acid-induced oxidative stress. Antioxidants & Redox Signaling 20: 1382–1395. https://doi.org/10.1089/ars.2013.5498 [Google Scholar] [PubMed] [CrossRef]
Xiao Y, Chen X, Hu W, Ma W, Di Q, Tang H, Zhao X, Huang G, Chen W (2023). USP39-mediated deubiquitination of Cyclin B1 promotes tumor cell proliferation and glioma progression. Translational Oncology 34: 101713. https://doi.org/10.1016/j.tranon.2023.101713 [Google Scholar] [PubMed] [CrossRef]
Yang Y, Cao L, Guo Z, Gu H, Zhang K, Qiu Z (2022b). Deubiquitinase UCHL5 stabilizes ELK3 to potentiate cancer stemness and tumor progression in pancreatic adenocarcinoma (PAAD). Experimental Cell Research 421: 113402. https://doi.org/10.1016/j.yexcr.2022.113402 [Google Scholar] [PubMed] [CrossRef]
Yang K, Wu Z, Zhang H, Zhang N, Wu W et al. (2022a). Glioma targeted therapy: Insight into future of molecular approaches. Molecular Cancer 21: 39. https://doi.org/10.1186/s12943-022-01513-z [Google Scholar] [PubMed] [CrossRef]
Yao T, Song L, Jin J, Cai Y, Takahashi H et al. (2008). Distinct modes of regulation of the Uch37 deubiquitinating enzyme in the proteasome and in the Ino80 chromatin-remodeling complex. Molecular Cell 31: 909–917. https://doi.org/10.1016/j.molcel.2008.08.027 [Google Scholar] [PubMed] [CrossRef]
Yeasmin Khusbu F, Chen FZ, Chen HC (2018). Targeting ubiquitin specific protease 7 in cancer: A deubiquitinase with great prospects. Cell Biochemistry & Function 36: 244–254. https://doi.org/10.1002/cbf.3336 [Google Scholar] [PubMed] [CrossRef]
Yuan L, Lv Y, Li H, Gao H, Song S et al. (2015). Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nature Cell Biology 17: 1169–1181. https://doi.org/10.1038/ncb3218 [Google Scholar] [PubMed] [CrossRef]
Zhang A, Huang Z, Tao W, Zhai K, Wu Q, Rich JN, Zhou W, Bao S (2022). USP33 deubiquitinates and stabilizes HIF-2alpha to promote hypoxia response in glioma stem cells. The EMBO Journal 41: e109187. https://doi.org/10.15252/embj.2021109187 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools