 Open Access
Open Access
ARTICLE
Evaluation of combined detection of nuclear factor erythroid 2-related factor 2 and glutathione peroxidase 4 in primary hepatic carcinoma and preliminary exploration of pathogenesis
Department of Hepatobiliary Surgery, The Second Hospital of Nanjing, Nanjing Hospital Affiliated to Nanjing University of Traditional Chinese Medicine, Nanjing, 210003, China
* Corresponding Author: AIDONG GU. Email:
BIOCELL 2023, 47(12), 2609-2615. https://doi.org/10.32604/biocell.2023.042472
Received 31 May 2023; Accepted 26 July 2023; Issue published 27 December 2023
Abstract
Objective: This study aims to analyze the clinical significance and mechanism of nuclear factor erythroid 2-related factor 2 (NRF2) and glutathione peroxidase 4 (GPX4) in primary hepatic carcinoma (PHC). Methods: The expression of NRF2 and GPX4 in peripheral blood of patients with PHC was determined to analyze the diagnostic value of the two combined for PHC. The prognostic significance of NRF2 and GPX4 was evaluated by 3-year follow-up. Human liver epithelial cells THLE-2 and human hepatocellular carcinoma cells HepG2 were purchased, and the expression of NRF2 and GPX4 in the cells was determined. NRF2 and GPX4 aberrant expression vectors were constructed and transfected into HepG2, and changes in cell proliferation and invasion capabilities were observed. Results: The expression of NRF2 and GPX4 in patients with PHC was higher than that in patients with LC or VH (p < 0.05), and the two indicators combined was excellent in diagnosing PHC. Moreover, patients with high expression of NRF2 and GPX4 had a higher risk of death (p < 0.05). In in vitro experiments, both NRF2 and GPX4 expression was elevated in HepG2 (p < 0.05). HepG2 activity was enhanced by increasing the expression of the two, vice versa (p < 0.05). Conclusion: NRF2 and GPX4 combined is excellent in diagnosing PHC, and promotes the malignant development of PHC.Graphic Abstract
Keywords
Primary hepatic carcinoma (PHC), whose incidence has been increasing worldwide in recent years, has become the fourth most common malignant tumor and the second leading cause of tumor death across the world, seriously threatening the safety of patients (Lu et al., 2022). Timely radical surgery can effectively save lives of patients with early-stage PHC (Gilles et al., 2022). However, once the disease has progressed to the middle and advanced stages or has invaded or metastasized, radical surgery is often no longer a cure, leaving high prognostic mortality of PHC (Nagaraju et al., 2022). A highly efficient diagnostic method or treatment option is urgently needed for early-stage PHC clinically to reduce its threat.
Ferroptosis is an indispensable participant in the pathogenesis of many malignant tumors, including PHC (Chen et al., 2022). Ferroptosis is an iron-dependent and non-apoptotic regulated cell death characterized by the accumulation of intracellular lipid reactive oxygen species; it is associated with multiple tumors Field's development and therapeutic response (Capelletti et al., 2020). Nuclear factor erythroid 2-related factor 2 (NRF2) and glutathione peroxidase 4 (GPX4), as extremely classical ferroptosis factors, have been proven to play an important role in malignant tumors such as gastric and lung cancers and regulate a variety of biological behaviors of tumor cells, including oxidative stress, proliferation, and differentiation (Bellezza et al., 2018; Bersuker et al., 2019). In breast cancer, NRF2 inhibits the transcription of the estrogen receptor-related receptor 1 gene by binding to its promoter sequence, thereby reducing the degradation of RhoA protein and enhancing the migration, invasion, and proliferation of breast cancer cells (Kasai et al., 2020). Besides, NRF2 also inhibits E-cadherin expression, up-regulates N-cadherin expression in tumor cells, and allows tumor cells to lose epithelial properties and transform into mesenchymal cells (Sajadimajd and Khazaei, 2018). However, the effects of NRF2 and GPX4 on PHC remain unclear. Recently, some preliminary studies suggest a potential link between NRF2, GPX4, and liver injury (Lee et al., 2020; Yang et al., 2022). Hence, the two indicators may also be significant for diagnosing and treating PHC.
To verify our hypothesis and provide a new reference basis for future diagnosis and treatment of PHC, we preliminarily analyzed the clinical significance and mechanism of NRF2 and GPX4 in PHC. The results are as follows.
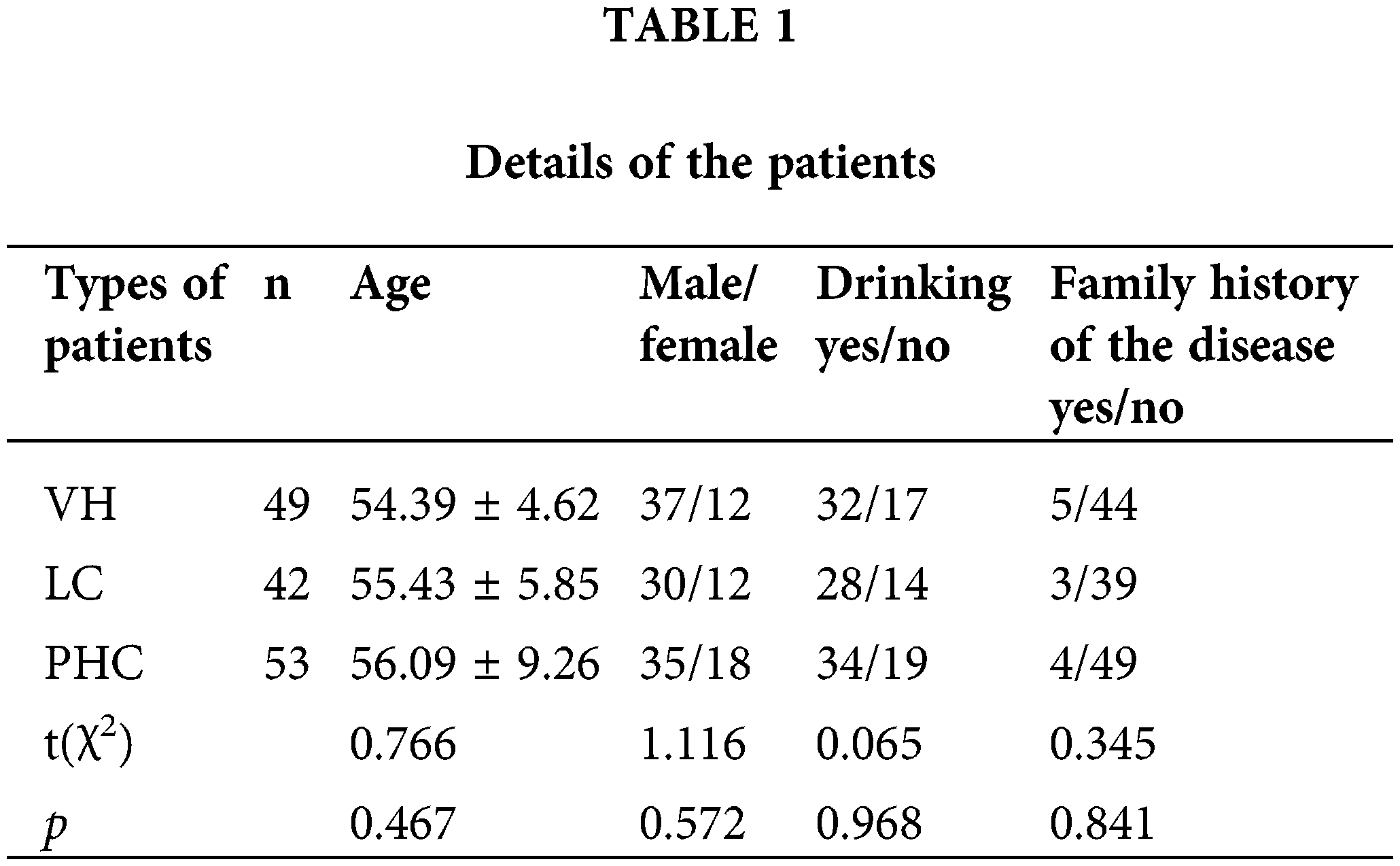
In this study, 53 patients with PHC, 42 with liver cirrhosis (LC), and 49 with viral hepatitis (VH) admitted to our hospital from April 2019 to February 2020 were enrolled. The total number of study subjects was 144. Their data are detailed in Table 1, with no significant differences in clinical data (p > 0.05). The ethics committee of our hospital has approved the study, and all subjects have signed the informed consent form.
Inclusion and exclusion criteria
Inclusion criteria: patients diagnosed with PHC, LC, or VH by pathology tests (liver function test); patients with complete medical history; patients aged >18 years; patients willing to cooperate with the study. Exclusion criteria: patients complicated with other tumors, cardiovascular and cerebrovascular diseases, or organ failure in addition to the disease investigated.
Determination of nuclear factor erythroid 2-related factor 2 and glutathione peroxidase 4 expression
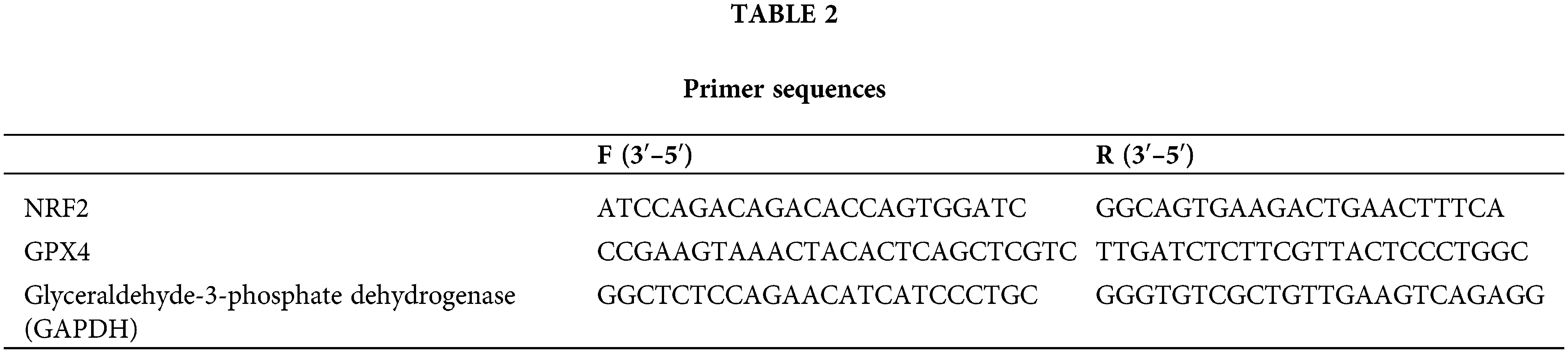
Fasting peripheral blood samples were collected from patients in the three groups at admission. Total RNA was extracted according to the instructions for using the Trizol kit (ThermoFisher Scientific, Shanghai, China) and reverse transcribed into complementary DNA cDNA at 37°C for 15 min and at 85°C for 5 s. The SYBR Green quantitative polymerase chain reaction (PCR) kit (ThermoFisher Scientific, Shanghai, China) was used to prepare 25 μL system reaction liquid for PCR reaction, with the reaction conditions of 95°C for 30 s, 95°C for 5 s, and 60°C for 30 s, 40 cycles in total. Primer sequences were obtained from Beijing Huanzhong Ruiqi Technology Co., Ltd. (Beijing, China) as described in Table 2.
Patients with PHC were followed up for 3 years. The follow-up was carried out by regular review, with an interval between two visits not more than 3 months. The 3-year survival of patients with PHC was statistically analyzed, and the survival curve was drawn.
Human liver epithelial cells THLE-2 and human hepatocellular carcinoma cells HepG2 were purchased from BeNa Culture Collection (Beijing, China) and cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum and 1% bispecific antibody in an environment containing 5% CO2 at 37°C.
Determination of nuclear factor erythroid 2-related factor 2 and glutathione peroxidase 4 expression
Proteins were extracted by cell lysis, separated by electrophoresis in 10% polyacrylamide gel (ThermoFisher Scientific, Shanghai, China), and transferred to polyvinylidene fluoride membrane (Sigma-Aldrich, Shanghai, China) for 90 min. The membrane was blocked with 5% skimmed milk powder at room temperature for 1 h, added with NRF2 (1: 1000, ab137550, Abcam), GPX4 (1: 1000, ab125066, Abcam), and GAPDH (1: 2000, ab8245, Abcam) antibodies and left overnight at 4°C, brought to room temperature the next day, washed, added with secondary antibody (1: 5000, ab205718, Abcam), incubated in a shaker at room temperature for 2 h, and washed. Then, luminous liquid (Yeasen Biotechnology (Shanghai) Co., Ltd., Shanghai, China) was added for color development and photo taking, and the protein bands were analyzed by Image J. Antibodies were purchased from abacm (Cambridge, UK).
HepG2 in the logarithmic growth phase was cultured in 10% inactivated serum DMEM (Sigma-Aldrich, Shanghai, China) for 48 h. Lentiviral vectors with silenced NRF2 and GPX4 were separately transfected into HepG2 and labeled as si-NRF2 group and si-GPX4 group, respectively, according to the instruction for using lentivirus (Commissioned by Yunzhou Bio-technology Co., Ltd., Guangzhou, China). Lentiviral vectors for increased expression of NRF2 or GPX4 were separately transfected into HepG2 and labeled as el-NRF2 group and el-GPX 4 group, respectively. In addition, HepG2 transfected with corresponding NRF2 and GPX4 empty vectors were considered as NC-NRF2 group and NC-GPX4 group, respectively. The expression of NRF2 and GPX4 genes was determined by PCR to verify the success rate of transfection, and the method was the same as above.
Cells of each group were inoculated in 6-well plates (500 cells/well), and the culture medium was changed every 2 d. The cells were fixed with 4% paraformaldehyde (Sigma-Aldrich, Shanghai, China) at 48 h of culture, stained with 1% crystal violet for 30 min, dried, and photographed. The number of colonies was counted.
Matrigel gel (Sigma-Aldrich, Shanghai, China) was mixed with DMEM at a ratio of 1: 8, and 20 μL of the mixture was added into the Transwell chamber (ThermoFisher Scientific, Shanghai, China) in a cell culture incubator overnight. Then, 500 μL of medium containing 10% FBS was added into 24-well plates, and 200 μL of cell suspension containing 1 × 105 cells per mL prepared with the serum-free medium was added in the upper chamber. After 48 h, the chamber was removed, fixed with methanol, stained with crystal violet, and observed under an inverted microscope. The number of cells was statistically analyzed.
All experiments in this study were repeated three times, and the results were averaged. SPSS 23.0 software (IBM, New York, USA) was used for statistical analysis. Enumeration data [n (%)] was compared by chi-square test, and the results of measurement (χ ± s) were compared by independent sample t-test, analysis of variance, and LSD test. Combined detection was performed by binary logistic regression analysis to obtain the joint formula, which was then analyzed by ROC curve. The survival rate was calculated by the Kaplan-Meier method and compared by the Log-rank test. A value with p < 0.05 was considered statistically significant.
Clinical significance of nuclear factor erythroid 2-related factor 2 and glutathione peroxidase 4
The expression of NRF2 and GPX4 mRNA in the peripheral blood of patients with PHC was higher than those of patients with LC or VH (p < 0.05), depicted in Figs. 1A and 1B. Subsequently, binary logistic regression analysis was performed with PHC and LC as independent variables and NRF2 and GPX4 expression levels as covariates, and the joint formula Log (p) = −10.967 + 3.548 × NRF2 + 3.199 × GPX4 was obtained. According to ROC, when the Log (p) was >0.6170, the sensitivity and specificity for predicting PHC in patients with LC were respectively 75.47% and 90.48% (p < 0.05). Using PHC and VH as independent variables, the joint formula was Log (p) = −8.439 + 2.379 × NRF2 + 2.760 × GPX4. When the Log (p) was >0.4246, the sensitivity and specificity for predicting PHC in patients with VH were respectively 83.02% and 71.43% (p < 0.05), depicted in Figs. 1C and 1D.
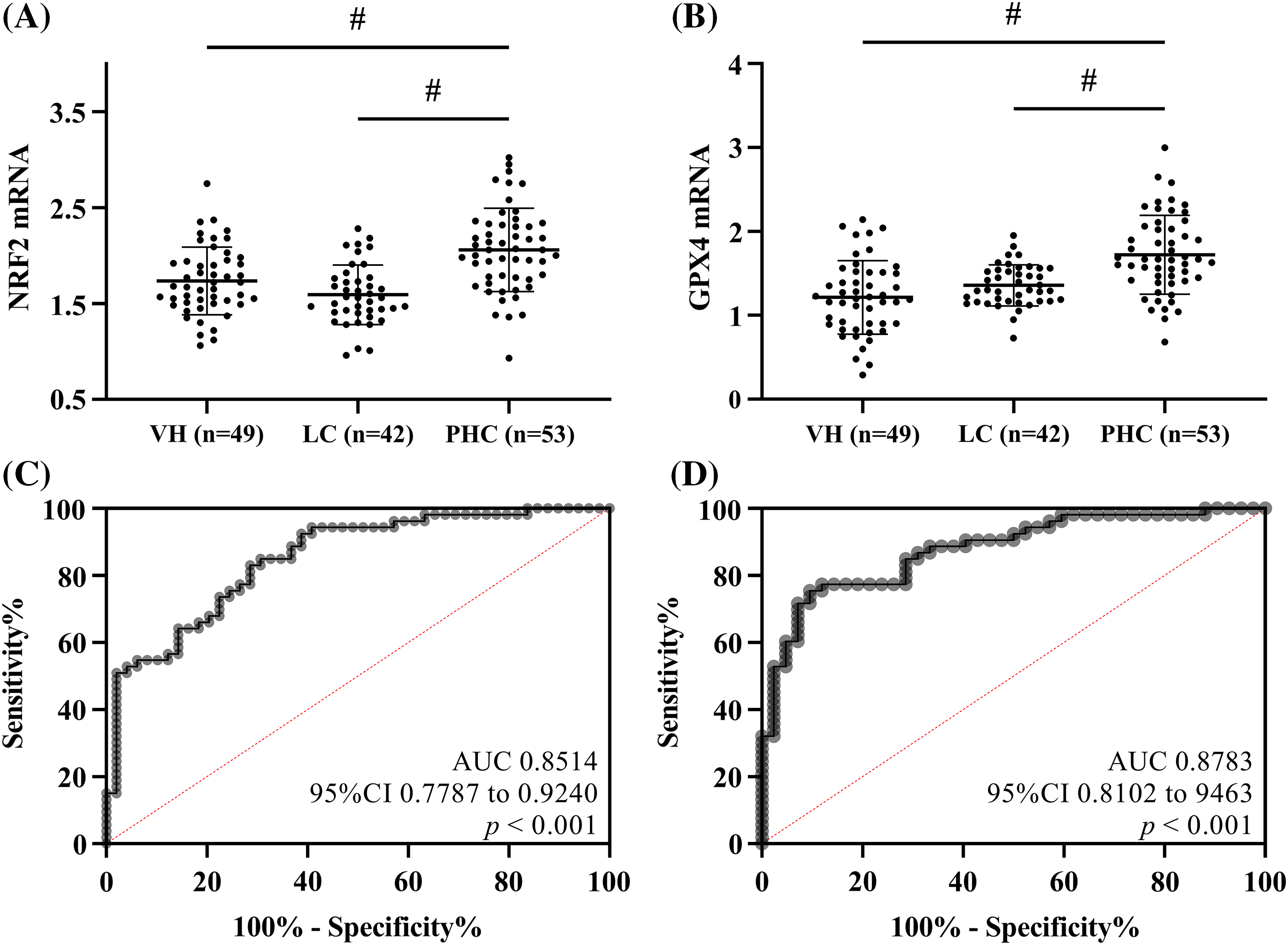
Figure 1: Clinical significance of nuclear factor erythroid 2-related factor 2 (NRF2) and glutathione peroxidase 4 (GPX4) expression in patients with different conditions. (A) Comparison of NRF2 mRNA. (B) Comparison of GPX4 mRNA. NRF2 and GPX4 mRNA were higher in PHC patients than in VH patients and LC patients. (C) Receiver operating characteristic curve (ROC) of NRF2 in combination with GPX4 for diagnosing primary hepatic carcinoma in patients with viral hepatitis. (D) ROC of NRF2 in combination with GPX4 for diagnosing PHC in patients with liver cirrhosis. # indicates p < 0.05.
Prognostic significance of nuclear factor erythroid 2-related factor 2 and glutathione peroxidase 4
Fifty patients were successfully followed up in the prognostic monitoring phase. Among them, 11 patients died, with an overall mortality rate of 22.00%. According to the median of NRF2 and GPX4 mRNA expression (2.06 and 1.72), the patients were categorized into the high NRF2 expression (NRF2 ≥ 2.06, n = 27) and low NRF2 expression (NRF2 < 2.06, n = 26) groups, and high GPX4 expression (GPX4 ≥ 1.72, n = 22), and low GPX 4 expression (GPX4 < 1.72, n = 31) groups, respectively. The survival curve showed that the prognostic mortality in high NRF-2 and GPX-4 expression groups was significantly higher than in low NRF-2 and GPX-4 expression groups (p < 0.05), depicted in Figs. 2A and 2B.
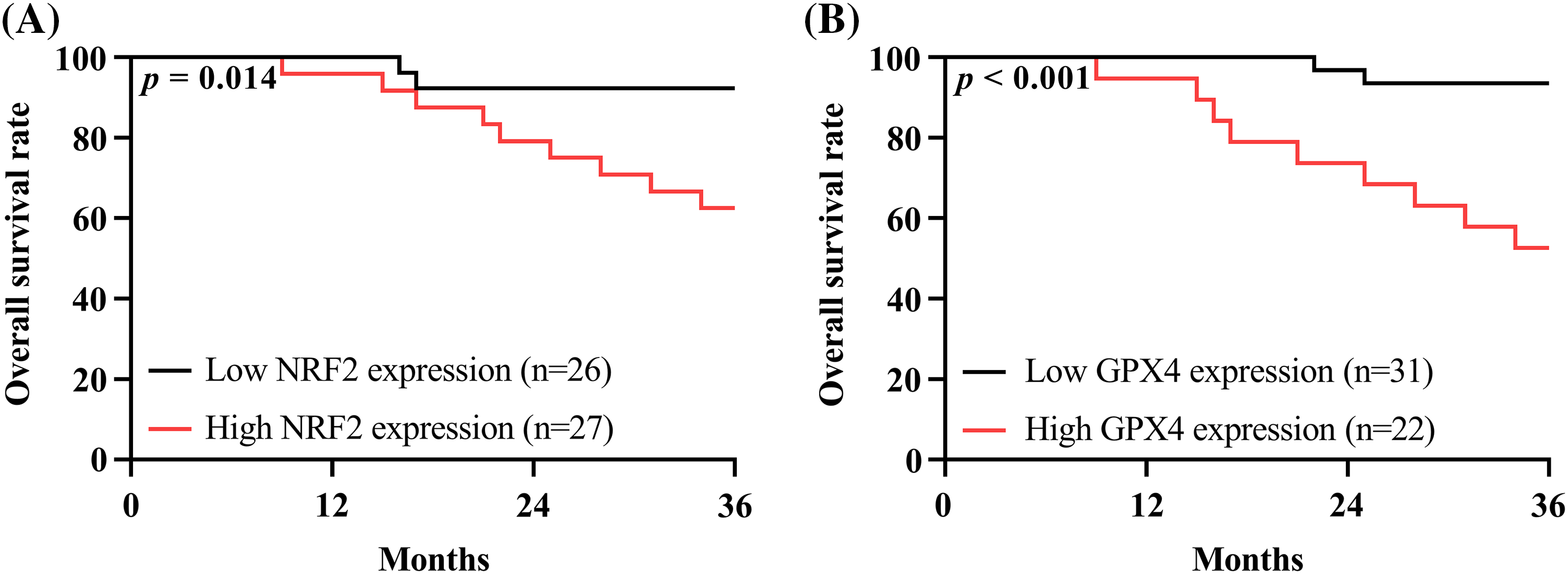
Figure 2: Prognostic significance of nuclear factor erythroid 2-related factor 2 (NRF2) and glutathione peroxidase 4 (GPX4). (A) Prognostic survival curves for high NRF2 expression group and low NRF2 expression group. (B) Prognostic survival curves for high GPX4 expression group and low GPX 4 expression group. The prognostic mortality in high NRF-2 and GPX-4 expression groups was significantly higher than in low NRF-2 and GPX-4 expression groups.
Expression of nuclear factor erythroid 2-related factor 2 and glutathione peroxidase 4in PHC cells
NRF2 and GPX4 protein expression levels in HepG2 were respectively (0.87 ± 0.05) and (0.88 ± 0.03), significantly higher than that in THLE-2 (p < 0.05), depicted in Fig. 3A. After transfection, NRF2 and GPX4 mRNA expression were the highest in the el-NRF2 and el-GPX4 groups, followed by the NC-NRF2 and NC-GPX4 groups, and the lowest in si-NRF2 and si-GPX4 groups (p < 0.05), suggesting successful transfection, depicted in Fig. 3B.
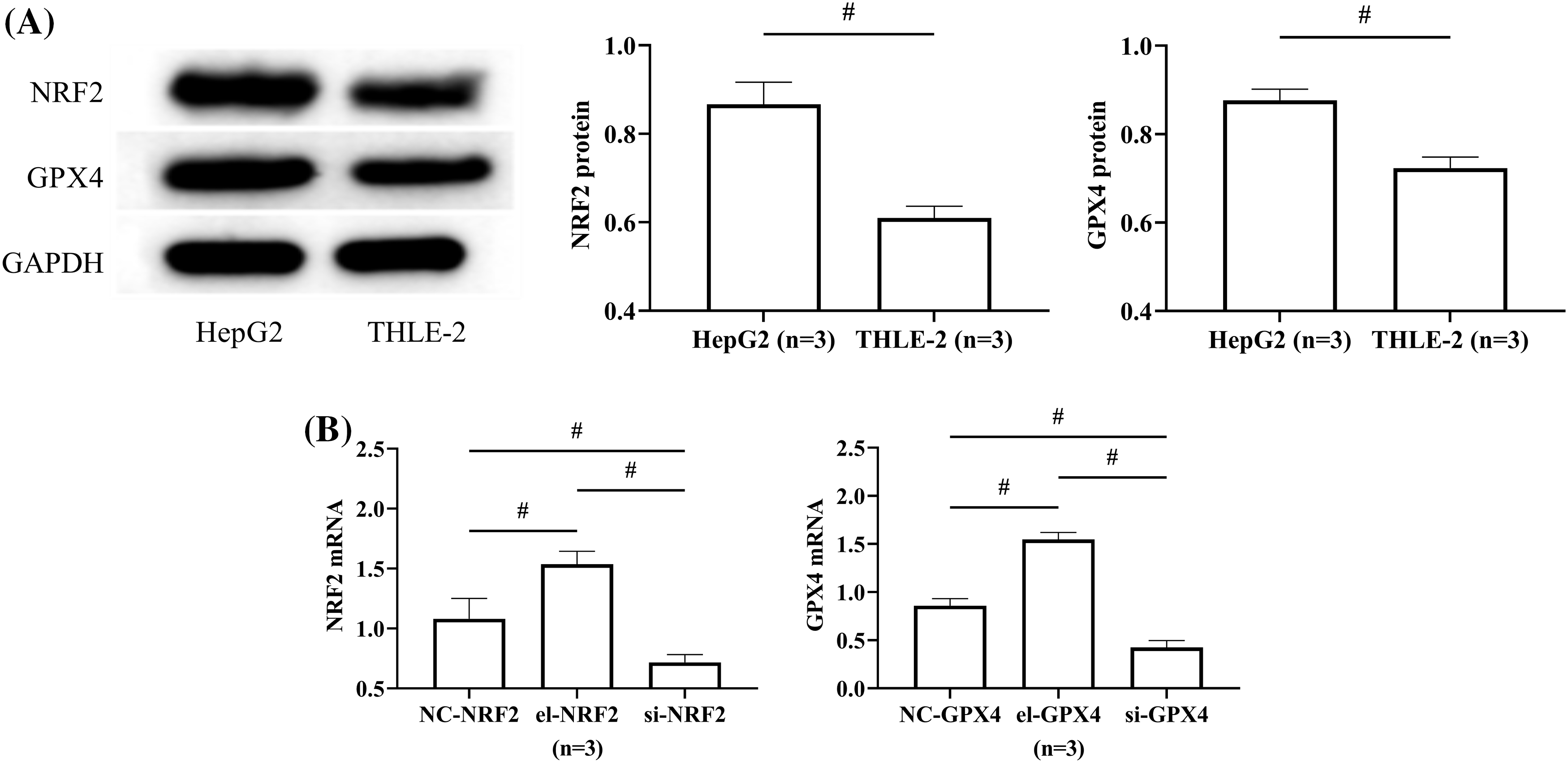
Figure 3: Expression of nuclear factor erythroid 2-related factor 2 (NRF2) and glutathione peroxidase 4 (GPX4) in PHC cells. (A) Comparison of NRF2 and GPX4 protein expression in HepG2 and THLE-2. (B) The expression of NRF2 and GPX4 was measured to verify the transfection success rate. # indicates p < 0.05. NRF2 and GPX4 protein expression was higher in HepG2 than in THLE-2.
Effect of nuclear factor erythroid 2-related factor 2 on HepG2 activity
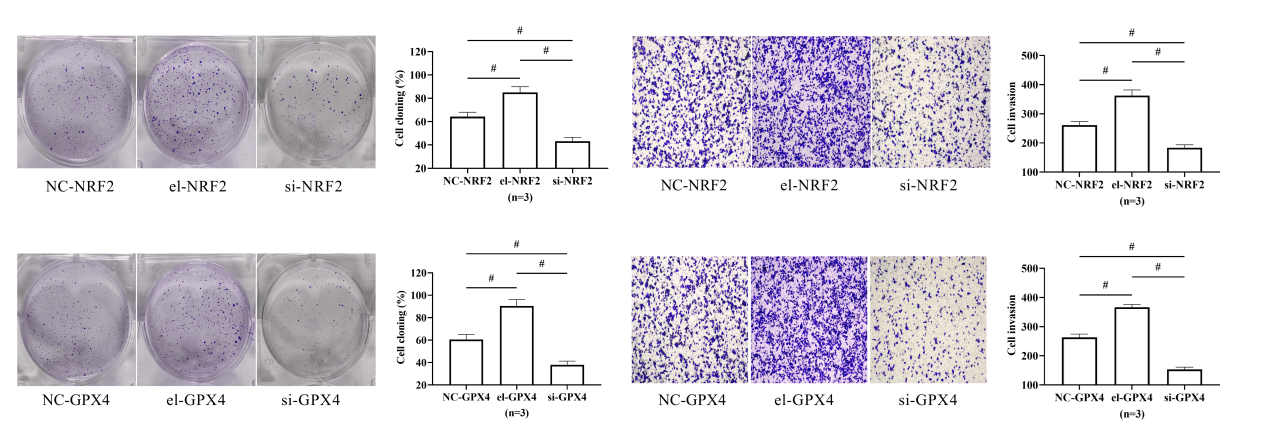
The results of cell cloning and Transwell experiments showed that the cell cloning rate and the number of invading cells were the highest in the el-NRF2 group, followed by the NC-NRF2 group, and the lowest in the si-NRF2 group (p < 0.05), depicted in Figs. 4A and 4B.
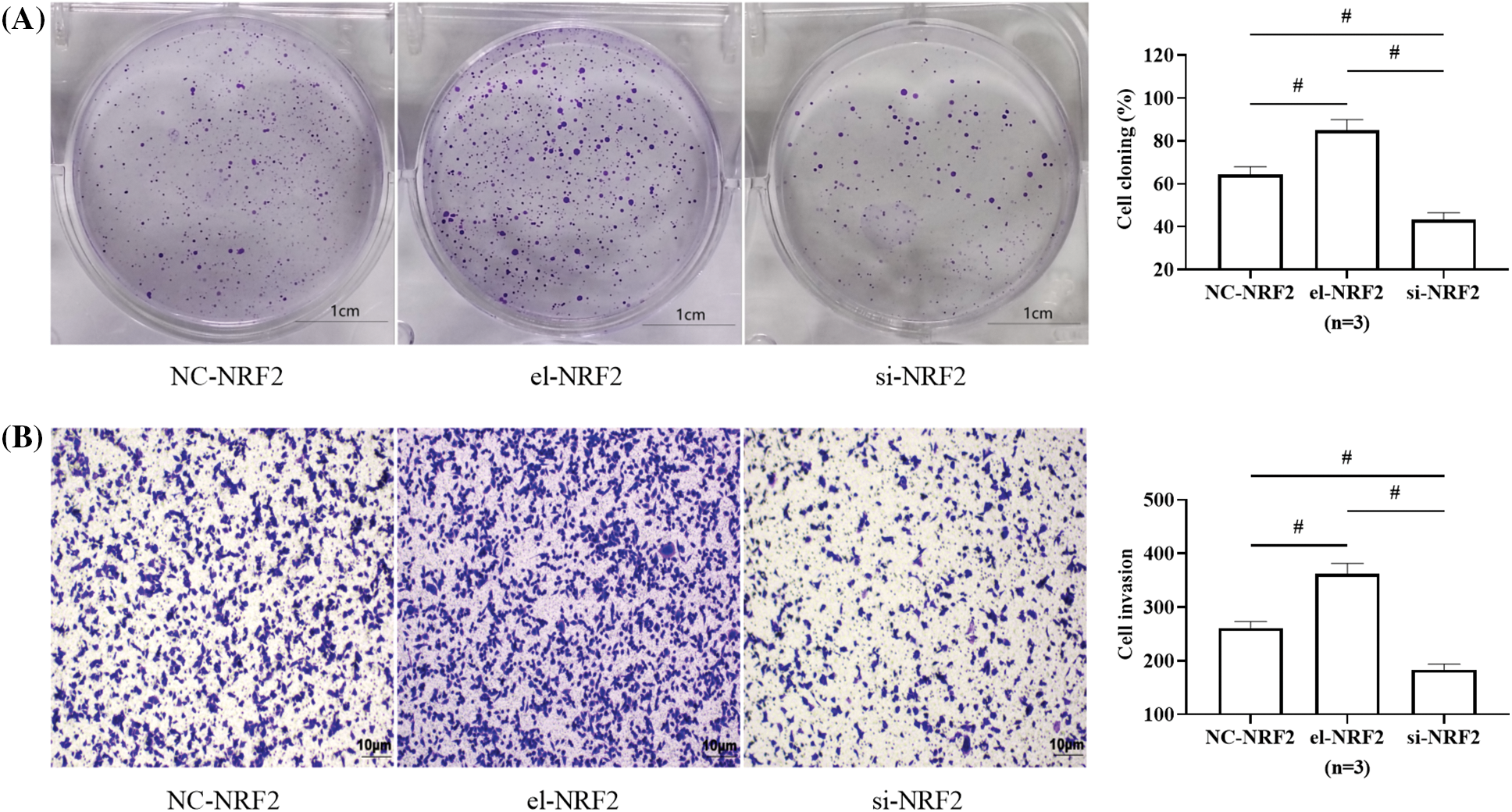
Figure 4: Effect of nuclear factor erythroid 2-related factor 2 (NRF2) on HepG2 activity. (A) Effect of NRF2 on HepG2 clonogenic ability. (B) Effect of NRF2 on HepG2 invasive ability. # indicates p < 0.05. Elevation of NRF2 expression in HepG2 was followed by enhanced cloning and invasive ability of the cells, whereas silencing of NRF2 expression decreased cellular activity.
Effect of glutathione peroxidase 4 (GPX4) on HepG2 activity
Similarly, the cell cloning rate and the number of invading cells were the highest in the el-GPX4 group and the lowest in the si-GPX4 group (p < 0.05), indicating that GPX4 expression elevation promoted the activity of HepG2, and GPX4 silence achieved the opposite results, depicted in Figs. 5A and 5B.
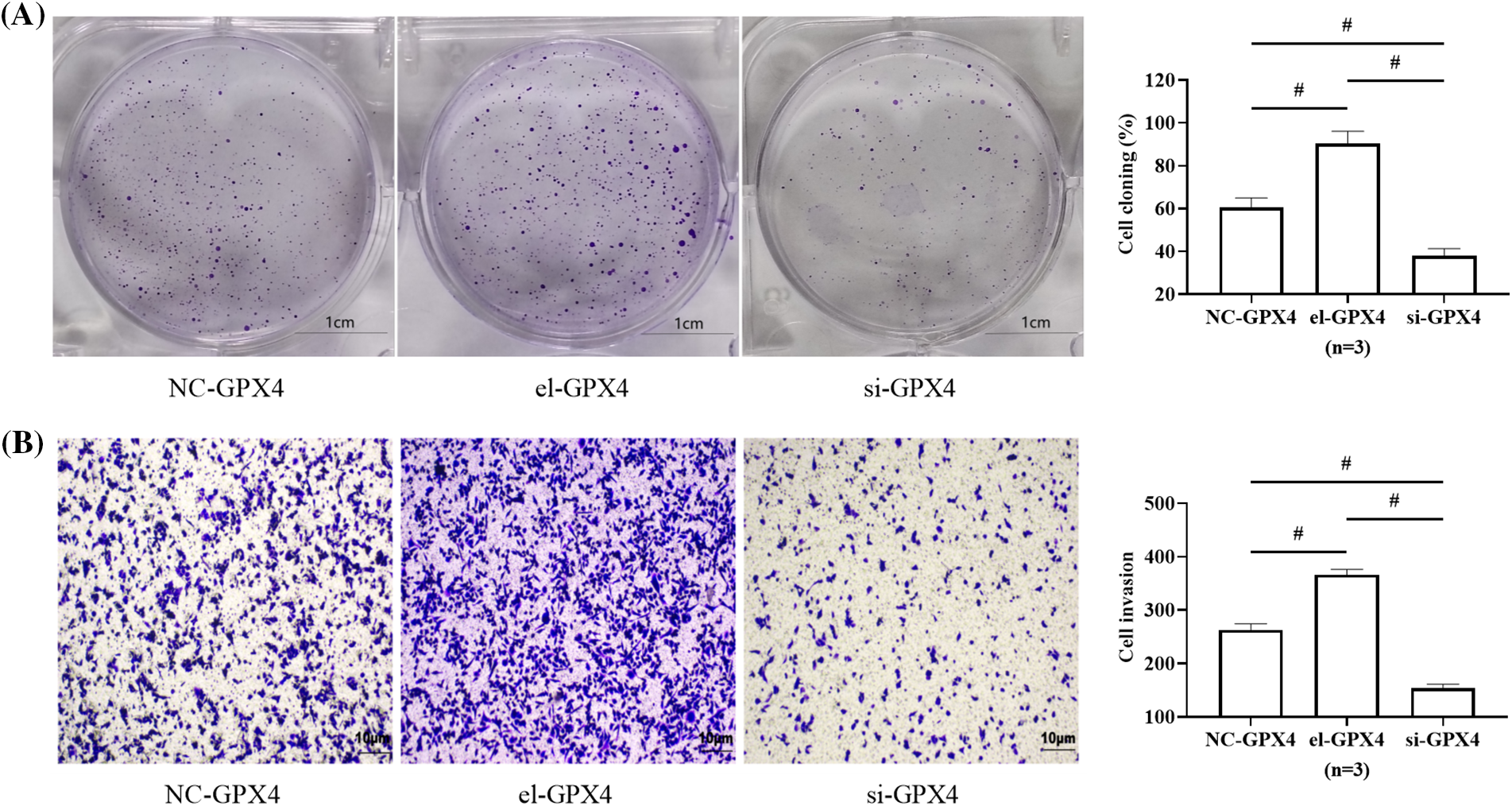
Figure 5: Effect of glutathione peroxidase 4 (GPX4) on HepG2 activity. (A) Effect of GPX4 on HepG2 clonogenic ability. (B) Effect of GPX4 on HepG2 invasive ability. # indicates p < 0.05. Elevation of GPX4 expression in HepG2 was followed by enhanced cloning and invasive ability of the cells, whereas silencing of GPX4 expression decreased cellular activity.
Finding new diagnostic and treatment options for PHC will improve the early diagnosis rate and provide patients with middle- and advanced-stage PHC with a better prognosis. In the present study, we found that NRF2 and GPX4 are highly expressed in PHC and have an excellent diagnostic evaluation for the occurrence and prognosis of PHC. This can provide new references and guidance for the future diagnosis and treatment of PHC.
PHC is generally caused by the progression of VH or LC (Samejo et al., 2022; Shin et al., 2023; Donne and Lujambio, 2023), so patients with PHC, LC, and VH were first enrolled for analysis. The levels of NRF2 and GPX4 in the peripheral blood of patients with PHC were significantly higher than in patients with LC and VH, suggesting that the two may be closely related to PHC occurrence and progression. The findings are consistent with the results of previous studies (He et al., 2020; Ulasov et al., 2022), demonstrating the results of the assay of NRF2 and GPX4 expression levels in the present study. In addition, according to cell experiments, both NRF2 and GPX4 expression was higher in HepG2 than in THLE-2, which further confirmed that the two were highly expressed in PHC. In addition, it was found that the two indicators combined are excellent in diagnosing PHC in patients with LC and VH, indicating that NRF2 and GPX4 might be used for early clinical detection of PHC in the future, thus improving the early detection rate. The results of prognostic follow-up showed that the mortality rate was higher in PHC patients with high expression of NRF2 and GPX4, also indicating the correlation of elevated NRF2 and GPX4 with an increased risk of death in patients with PHC. Such further proves the important role of NRF2 and GPX4 in assessing the progression of PHC, which is of great significance for a more objective and rapid understanding of the pathological development of PHC in the future.
Thus, it is evident that highly expressed NRF2 acts as a pro-oncogene in tumor diseases. To confirm the effects of NRF2 on PHC, NRF2 aberrant expression vectors were transfected into PHC cells. The results showed that the cloning and invasion capabilities were elevated in the el-NRF2 group while reduced in the si-NRF2 group, indicating that elevated NRF2 expression promotes PHC cell activity, and silenced NRF2 expression inhibits PHC growth. The results verify the view of the above studies and confirm that NRF2 promotes PHC development.
GPX4 is one of the most important defense factors against intracellular ferroptosis and the only antioxidant enzyme for lipid peroxide reduction; using reduced glutathione as the electron donor, it directly reduces PLOOH to hydroxylated phospholipids, leading to loss of activity of peroxides, thus inhibiting ferroptosis (Jia et al., 2020). Since GPX4 acts as a central inhibitor of ferroptosis in cells, its relationship with tumor ferroptosis has been widely discussed. Lu et al. (2021) demonstrated that the application of Kruppel like factor 2 to inhibit GPX4 expression activates ferroptosis and inhibits cell migration and invasion of clear cell renal cell carcinoma. Sha et al. (2021) observed enhanced sensitivity of triple-negative breast cancer cells to chemotherapeutic agents by inhibiting the GPX4-activated ferroptosis. This study also found that elevated GPX4 expression promoted PHC activity, and silenced GPX4 expression achieved contrary results, again validating the regulatory role of GPX4 on PHC progression. The mechanism may also be related to the inhibition of cell ferroptosis. The reduction of PHC cell activity by silencing either NRF2 or GPX4 indicated that molecular therapeutic pathways targeting the expression of NRF2 and GPX4 may become a new option for PHC treatment in the future.
However, NRF2 and GPX4 expression in PHC tissues was not determined in the current study, and only one PHC cell line was used in in vitro experiments, both of which may lead to a lack of comprehensiveness and representativeness of results obtained. Subcutaneous tumorigenicity assay in live animals is also needed to confirm the effect of abnormally expressed NRF2 and GPX4 on actual tumor growth. Besides, more studies are required to confirm the mechanism of their effects on PHC, which will be the focus of our subsequent studies.
In summary, NRF2 and GPX4 were highly expressed in PHC, and the two combined showed excellent diagnostic effects for PHC in patients with LC and VH. In addition, elevated NRF2 and GPX4 expression suggested an increased risk of death in the prognosis of PHC patients, proving their significance as a reference for the assessment of PHC in the future. In vitro experiments showed elevated expression of NRF2 and GPX4, which promoted the proliferation and invasion of PHC cells, while silencing of NRF2 and GPX4 expressions inhibited the growth of PHC, suggesting that highly expressed NRF2 and GPX4 act as oncogenes; further, targeted molecular immunotherapy by silencing the expression of NRF2 and GPX4 may be a new research direction for PHC treatment in the future.
Acknowledgement: None.
Funding Statement: This study did not receive any funding from any agency or an institution.
Author Contributions: Study conception and design: Jie Duan and Aidong Gu; data collection: Wei Chen and Changhao Chen; analysis and interpretation of results: Fangnan Song; draft manuscript preparation: Faxi Chen, Fangfang Jiang and Huiwen Xing. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data in this article can be obtained from the corresponding author under reasonable circumstances.
Ethics Approval: The study protocol was approved by the Ethics Committee of The Second Hospital of Nanjing (Approval No. N2021036).
Conflicts of Interest: The research was conducted with no commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bellezza I, Giambanco I, Minelli A, Donato R (2018). Nrf2-Keap1 signaling in oxidative and reductive stress. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1865: 721–733. [Google Scholar] [PubMed]
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B et al. (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575: 688–692. [Google Scholar] [PubMed]
Capelletti MM, Manceau H, Puy H, Peoc’h K (2020). Ferroptosis in liver diseases: An overview. International Journal of Molecular Sciences 21: 4908. [Google Scholar] [PubMed]
Chen J, Li X, Ge C, Min J, Wang F (2022). The multifaceted role of ferroptosis in liver disease. Cell Death and Differentiation 29: 467–480. [Google Scholar] [PubMed]
Donne R, Lujambio A (2023). The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology 77: 1773–1796. [Google Scholar] [PubMed]
Gilles H, Garbutt T, Landrum J (2022). Hepatocellular Carcinoma. Critical Care Nursing Clinics of North America 34: 289–301. [Google Scholar] [PubMed]
He F, Ru X, Wen T (2020). NRF2: A transcription factor for stress response and beyond. International Journal of Molecular Sciences 21: 4777. [Google Scholar] [PubMed]
Jia M, Qin D, Zhao C, Chai L, Yu Z et al. (2020). Redox homeostasis maintained by GPX4 facilitates STING activation. Nature Immunology 21: 727–735. [Google Scholar] [PubMed]
Kasai S, Shimizu S, Tatara Y, Mimura J, Itoh K (2020). Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 10: 320. [Google Scholar] [PubMed]
Lee DH, Park JS, Lee YS, Han J, Lee DK, Kwon SW, Han DH, Lee YH, Bae SH (2020). SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy 16: 1949–1973. [Google Scholar] [PubMed]
Lu Y, Qin H, Jiang B, Lu W, Hao J, Cao W, Du L, Chen W, Zhao X, Guo H (2021). KLF2 inhibits cancer cell migration and invasion by regulating ferroptosis through GPX4 in clear cell renal cell carcinoma. Cancer Letters 522: 1–13. [Google Scholar] [PubMed]
Lu Y, Yang A, Quan C, Pan Y, Zhang H et al. (2022). A single-cell atlas of the multicellular ecosystem of primary and metastatic hepatocellular carcinoma. Nature Immunology 13: 4594. [Google Scholar]
Nagaraju GP, Dariya B, Kasa P, Peela S, El-Rayes BF (2022). Epigenetics in hepatocellular carcinoma. Seminars in Cancer Biology 86: 622–632. [Google Scholar] [PubMed]
Sajadimajd S, Khazaei M (2018). Oxidative stress and cancer: The role of Nrf2. Current Cancer Drug Targets 18: 538–557. [Google Scholar] [PubMed]
Samejo KH, Mandhwani R, Majid Z, Mubarak M, Luck NH (2022). Primary hepatic sarcomatoid carcinoma presenting with dysphagia: A rare presentation. Journal of College of Physicians and Surgeons Pakistan 32: S159–S161. [Google Scholar]
Sha R, Xu Y, Yuan C, Sheng X, Wu Z et al. (2021). Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. eBioMedicine 71: 103560. [Google Scholar] [PubMed]
Shin WY, Lee KY, Kim KD (2023). Mixed primary hepatocellular carcinoma and hepatic neuroendocrine carcinoma: Case report and literature review. Medicina 59: 418. [Google Scholar] [PubMed]
Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS (2022). Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sciences 291: 120111. [Google Scholar] [PubMed]
Yang W, Wang Y, Zhang C, Huang Y, Yu J, Shi L, Zhang P, Yin Y, Li R, Tao K (2022). Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation. Frontiers in Pharmacology 13: 865689. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools