 Open Access
Open Access
Anisodine hydrobromide alleviates oxidative stress caused by hypoxia/reoxygenation in human cerebral microvascular endothelial cells predominantly via inhibition of muscarinic acetylcholine receptor 4
1 Institute of Biomedical Engineering, West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu, 610041, China
2 Department of Biomedical Engineering, The City College of the City University of New York, New York, 10031, USA
* Corresponding Author: YE ZENG. Email:
# Co-first authors
BIOCELL 2023, 47(10), 2255-2263. https://doi.org/10.32604/biocell.2023.030880
Received 29 April 2023; Accepted 31 July 2023; Issue published 08 November 2023
Abstract
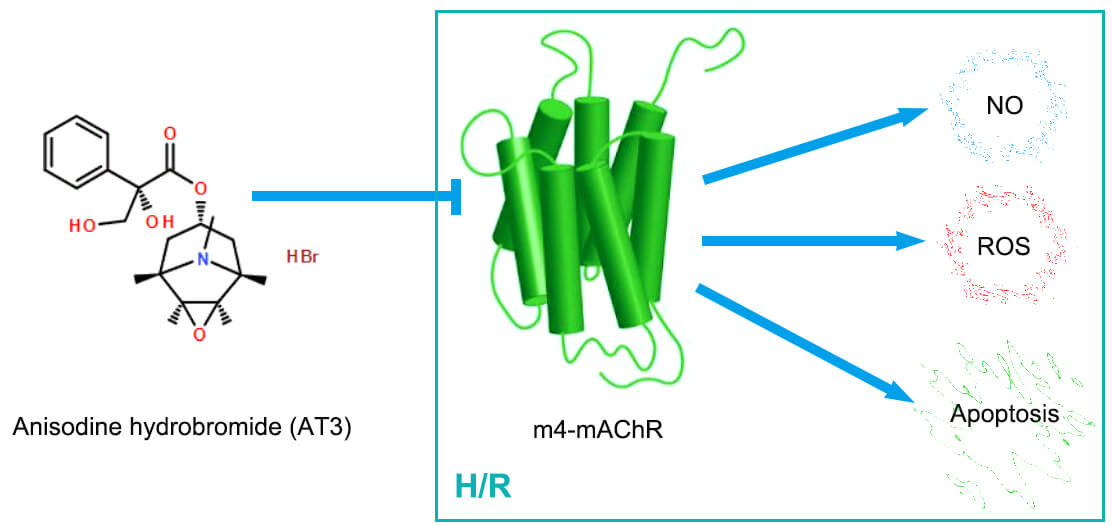
Background: Anisodine hydrobromide (AT3), an anti-cholinergic agent, could be delivered to the brain across the blood-brain barrier and has been used clinically for the treatment of cerebral ischemia/reperfusion injury. Endothelial dysfunction can be caused by hypoxia/reoxygenation (H/R) via oxidative stress and metabolic alterations. The present study investigated whether AT3 regulates the production of nitric oxide (NO) and reactive oxygen species (ROS), and the HIF-1α pathway via regulation of muscarinic acetylcholine receptors (mAChRs) in brain microvascular endothelial cells after H/R exposure. Methods: Under H/R conditions, hCMEC/D3 cerebral microvascular endothelial cells were treated with AT3. Specific inhibitors of M2- and M4- mAChRs were used to explore the mechanism by which AT3 influences oxidative stress in endothelial cells. Then, mAChRs expression was detected by western blotting and NO production was detected by Greiss reaction. The intracellular ROS level was measured using DCFH-DA probes. The expression of hypoxia-inducible transcription factor 1α (HIF-1α) was also detected. Results: While H/R induced the expression of M2- and M4-mAChRs, AT3 suppressed the H/R-upregulated M2- and M4-mAChRs. H/R also induced the production of NO, ROS, and apoptosis. AT3 and M4-mAChR inhibitors inhibited the H/R-induced production of NO and ROS and apoptosis. HIF-1α was induced by H/R, but was suppressed by AT3. Conclusion: Thus, the in vitro evidence shows that AT3 protects against H/R injury in cerebral microvascular endothelial cells via inhibition of HIF-1α, NO and ROS, predominantly through the downregulation of M4-mAChR. The findings offer novel understandings regarding AT3-mediated attenuation of endothelial cell apoptosis and cerebral ischemia/reperfusion injury.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools