 Open Access
Open Access
REVIEW
Cancer-associated fibroblasts of colorectal cancer: Translational prospects in liquid biopsy and targeted therapy
1 Human Genome Centre, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150, Malaysia
2 Department of Internal Medicine, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150, Malaysia
3 Department of Surgery, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150, Malaysia
4 Department of Pathology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150, Malaysia
* Corresponding Author: MARAHAINI MUSA. Email:
(This article belongs to the Special Issue: Frontiers in cancer: tumor microenvironment)
BIOCELL 2023, 47(10), 2233-2244. https://doi.org/10.32604/biocell.2023.030541
Received 12 April 2023; Accepted 01 June 2023; Issue published 08 November 2023
Abstract
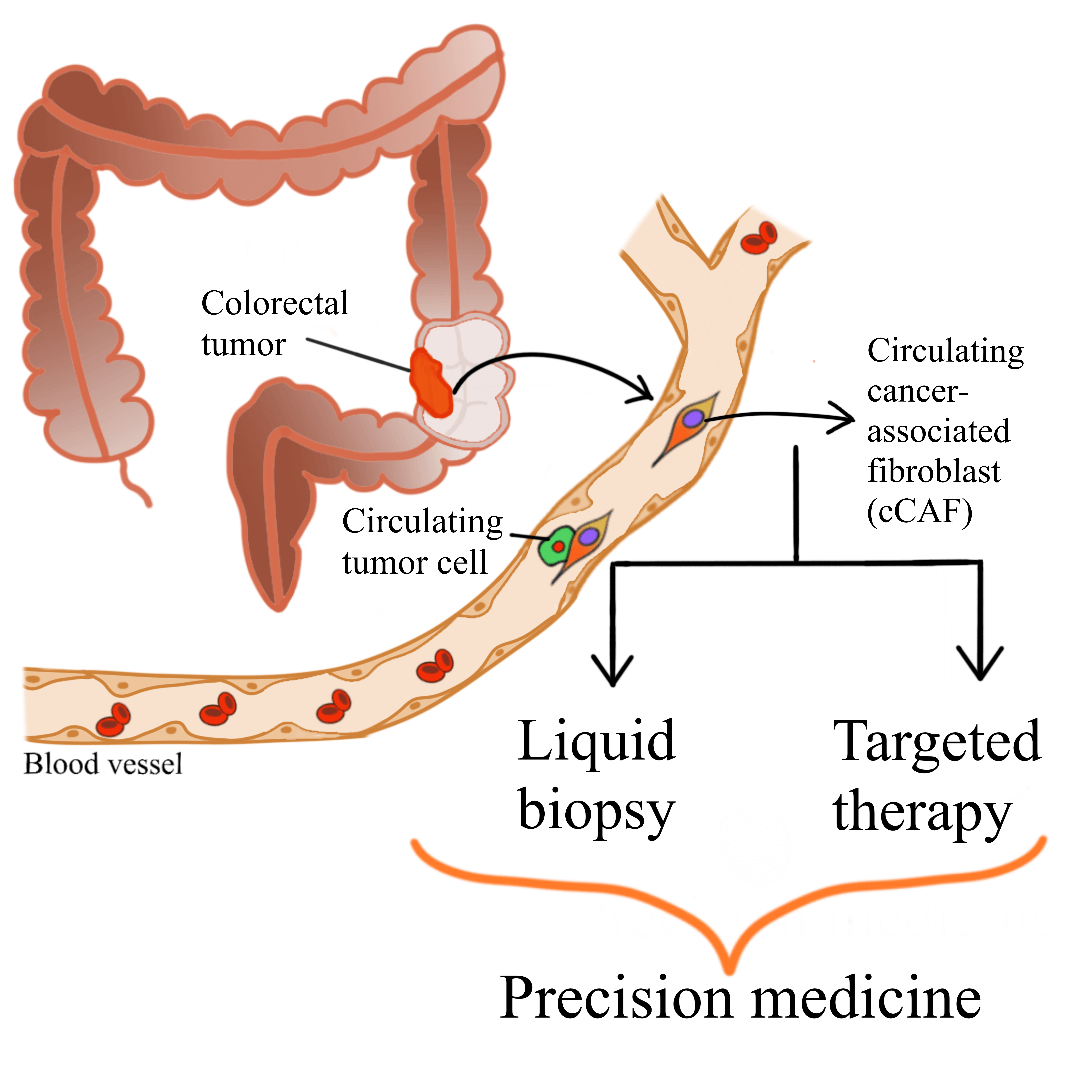
Colorectal cancer (CRC) is a major global health concern. Accumulation of cancer-associated fibroblasts (CAFs) in CRC is associated with poor prognosis and disease recurrence. CAFs are the main cellular component of the tumor microenvironment. CAF-tumor cell interplay, which is facilitated by various secretomes, drives colorectal carcinogenesis. The complexity of CAF populations contributes to the heterogeneity of CRC and influences patient survival and treatment response. Due to their significant roles in colorectal carcinogenesis, different clinical applications utilizing or targeting CAFs have been suggested. Circulating CAFs (cCAFs) which can be detected in blood samples, have been proposed to help in determining patient prognosis and enables the detection of cancer through liquid biopsy. Liquid biopsy is gaining traction as it is non-invasive, allows frequent and easy sampling, and shows concordance to tissue biopsy analysis. In addition, CAF-targeted therapy is currently being studied extensively to be used as one of the treatment avenues for CRC. Various mechanisms of CAF-targeted therapy have been reported, including blocking the signaling pathways involving CAFs and cancer cells, thus abolishing the CAF-tumor cell crosstalk and subsequently hindering tumorigenesis. These translational applications of cCAFs and utilization of CAFs as key targets for CRC therapy, although still in the early phases of development, will potentially improve CRC patient management in the future.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools