 Open Access
Open Access
HOXB8 contributed to oxaliplatin chemo-resistance in colon cancer cells by activating STAT3
1 Department of Colorectal Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, China
2 Cancer and Anticancer Drug Research Center, School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou, 325035, China
* Corresponding Authors: RI CUI. Email: ; SHAOTANG LI. Email:
# These authors contributed equally to this work
(This article belongs to the Special Issue: Frontiers in cancer: tumor microenvironment)
BIOCELL 2023, 47(10), 2245-2254. https://doi.org/10.32604/biocell.2023.030147
Received 24 March 2023; Accepted 28 June 2023; Issue published 08 November 2023
Abstract
Background: Homeobox B8 (HOXB8), a member of HOX family, plays a key role in the development of colorectal cancer (CRC). However, the function of HOXB8 in oxaliplatin (OXA) resistance in CRC is still unclear. This study investigated the role and precise molecular mechanism of HOXB8 in OXA-resistant CRC cells. Methods: The cell viability was measured by the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay, and the colony forming ability was determined by colony formation assay. The silencing RNA (siRNA) approach was used to knockdown HOXB8 in CRC cells while the lentiviral transfection system was used to establish stable HOXB8 overexpressing CRC cells. The protein and mRNA levels were evaluated by western blot and real-time reverse transcription-polymerase chain reaction. Results: HOXB8 expression was upregulated in OXA-resistant HCT116 cells (HCT116/OXA) compared to its level in the parent HCT116 cells. Knockdown of HOXB8 significantly inhibited CRC cell growth by suppressing the signal transducer and activator of transcription 3 (STAT3) pathway. HOXB8 knockdown also potentiated cytotoxicity of OXA in CRC cells. Inversely, HOXB8 overexpression attenuated OXA-induced growth inhibition of HCT116 cells and RKO cells by activating STAT3 signaling. HOXB8 knockdown effectively inhibited HCT116/OXA cell viability regardless of OXA treatment by suppressing STAT3 signaling. Conclusions: These results shed light on the important functions of HOXB8 in OXA-resistant CRC and suggested that targeting HOXB8 might be an effective therapeutic strategy for select OXA-resistant CRC patients.Keywords
Abbreviations
| ATP7A | ATPase copper transporting alpha |
| ANOVA | One-way analysis of variance |
| CAFs | Carcinoma-associated fibroblasts |
| CRC | Colorectal cancer |
| CSCO | Chinese Society of Clinical Oncology |
| ECL | Enhanced chemiluminescence |
| EMT | Epithelial-mesenchymal transition |
| FOLFOX4 | Fluorouracil, leucovorin, and oxaliplatin |
| HCT116/OXA | OXA-resistant HCT116 cells |
| HOXB8 | Homeobox B8 |
| HRP | Horseradish peroxidase |
| IC50 | Half-maximal inhibitory concentration |
| JAK | Janus kinase |
| LATS1 | Large tumor suppressor kinase 1 |
| LUAD | Lung adenocarcinoma |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| NBAT-1 | Neuroblastoma associated transcript 1 |
| ORR | Objective response rate |
| OS | Overall survival |
| OXA | Oxaliplatin |
| PBS | Phosphate-buffered saline |
| PFS | Progression-free survival |
| PVDF | Polyvinylidene difluoride |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RPMI | Roswell Park Memorial Institute |
| SD | Standard deviation |
| STAT3 | Signal transducer and activator of transcription 3 |
| SiRNA | Silencing RNA |
| TF | Transcription factor |
| TBST | Tween® 20 detergent |
| UHMK1 | U2AF homology motif kinase 1 |
| WWC3 | WW-and-C2-domain-containing protein family member 3 |
| YAP | Yes-associated protein |
| ZEBI | Zinc finger E-box binding homeobox I |
Colorectal cancer (CRC) is one of the most common malignant tumors in the world. The incidence and the mortality rate of CRC occupies the third position in European countries (Biller and Schrag, 2021). Further, the numbers of CRC cases are increasing every year due to an unhealthy lifestyle (Cardoso et al., 2021). Currently, surgical resection, chemotherapy, radiotherapy, targeted therapy, and immunotherapy have been often used for the treatment of CRC (Akgül et al., 2014; Ganesh et al., 2019). The prognosis and treatment strategy of CRC is closely related to the tumor stage. In recent years, oxaliplatin (OXA) has been widely used as a first-line chemotherapeutic agent in CRC treatment (Biswas et al., 2019). According to the Chinese Society of Clinical Oncology (CSCO) reports, OXA significantly improved the objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) of stage III–IV CRC after surgical resection (Deng, 2021). Mechanistically, OXA binds to DNA and forms platinum-DNA adducts to suppress DNA replication and cause DNA lesions (Yin et al., 2017). Although OXA has many therapeutic advantages in CRC, subsequent drug resistance impairs its further clinical application. The drug-resistant mechanisms of oxaliplatin include altered detoxifying antioxidant levels and increased nucleotide excision repair of the DNA lesions (Jensen et al., 2015). However, molecular mechanisms underlying OXA resistance are not fully understood in patients with recurrent CRC (Zhu et al., 2020). Therefore, dissecting and understanding underlying molecular mechanisms in OXA-resistant CRC cells are significant research avenues and can provide alternative therapeutic methods for OXA-resistant CRC patients.
Homeobox (HOX) genes comprise 61 amino acids, first discovered in Drosophila melanogaster (Qian et al., 1989). Evidence has shown that HOX genes regulated the transcriptional activators or repressors in various cancers (Bhatlekar et al., 2014). Homeobox B8 (HOXB8), a member of HOX family, modulates the proliferation, invasion, and metastasis of various cancer cells such as gastric cancer and osteosarcoma by regulating Wnt/β-catenin or epithelial-mesenchymal transition (EMT) (Stavnes et al., 2013; Ding et al., 2017; Guo et al., 2019). In addition, HOXB8 promoted the proliferation and metastasis of colorectal cancer cells by activating STAT3 (Wang et al., 2019). Knocking down HOXB8 increased chemotherapy sensitivity with fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) in colon cancer cells and suppressed CRC progression by regulating miR-196 (Shen et al., 2016). Furthermore, HOXB8 expression was upregulated in CRC tissues compared to those in adjacent normal tissues. This higher expression was related to prognosis and pathological parameters, suggesting HOXB8 might be a useful biomarker in CRC (Abuderman et al., 2020).
Signal transducers and activators of transcription 3 (STAT3) was highly activated in various cancers and involved in cancer progression, metastasis, and drug resistance. Additionally, such STAT3 hyperactivation facilitated CRC cell growth, invasion, and metastasis (Fu et al., 2016). Inversely, inhibition of STAT3 signaling suppressed CRC progression and metastasis (Shan et al., 2022). In another report, STAT3 hyperactivation could mediate drug resistance in the brain, thoracic and gastrointestinal tumors (Sadrkhanloo et al., 2022). Our previous study demonstrated that HOXB8 promoted the proliferation and metastasis of CRC cells by activating the EMT signaling pathway (Wang et al., 2019). Thus, we hypothesized that HOXB8 may be involved in the OXA-resistance of colon cancer cells by activating the STAT3 pathway.
The expression of HOXB8 and p-STAT3 was up-regulated in OXA-resistant CRC cells. Further, HOXB8 knockdown reduced p-STAT3 expression and sensitized colon cancer cells to OXA treatment. Inversely, HOXB8 overexpression had opposite effects in colon cancer cells. HOXB8 knockdown in OXA-resistant CRC cells significantly inhibited cell growth by suppressing p-STAT3 expression, suggesting that HOXB8 might be a potential therapeutic target for the treatment of OXA-resistant CRC.
We obtained RKO, HCT116 parental, and Oxaliplatin-resistant (HCT116/OXA) cell lines from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Eggenstein, Germany) with 10% fetal bovine serum (FBS; Gibco, Eggenstein, Germany) and 100 U/ml penicillin-streptomycin (Solarbio, Beijing, China) at 37°C and 5% CO2.
Oxaliplatin was purchased from TargetMol (Shanghai, China), stored at −80°C, and dissolved in phosphate-buffered saline (PBS) (Solarbio, Beijing, China). HOXB8 antibody was obtained from Biorbyt (San Francisco, California, USA). The antibodies against phospho-STAT3 (p-STAT3) and STAT3 were purchased from Cell Signaling Technology (Danvers, USA). A quantitative real-time polymerase chain reaction (qRT-PCR) kit was purchased from Takara (Shiga, Japan). TRIzol reagent and PCR primers were obtained from Invitrogen (Carlsbad, USA).
Cell proliferation was assessed by the MTT assay (Solarbio, Beijing, China). The cells (3–4 × 103 cells/100 μl per well) were seeded in a 96-well plate overnight and then exposed to different concentrations of OXA (0.5, 1, 2.5, 5, 10, 20, 40, 60, and 80 µM) for 48 h. Subsequently, the MTT reagent was added to each well and incubated in a 5% CO2 atmosphere in an incubator at 37°C. The culture medium was removed after 3 h, and 150 μL dimethyl sulfoxide (DMSO) was added into each well to dissolve the formazan. The absorbance (OD 490 nm) was measured using the SpectraMax iD3 Multi-Mode Microplate Reader (MD, USA).
The colony formation assay was done to assess the cell colony-forming ability. The cells (1500 cells per well) were incubated in a 6-well plate overnight. After treatment with various concentrations of OXA (0.1, 0.5, and 1 µM) for 24 h, the cells were continued to be cultured for 7–10 days at 37°C and 5% CO2 in an incubator. Finally, the colonies were fixed in 4% paraformaldehyde and stained with 0.1% crystal violet. Colonies were counted using Image J software.
small interfering RNA transfection
Negative control siRNA and siRNA against HOXB8 were synthesized from GenePharma (Shanghai, China). To knockdown HOXB8 in RKO, HCT116, and HCT116/OXA cell lines, HOXB8 siRNA was transfected into cells using LipofectamineTM 3000 (Invitrogen, USA) according to the manufacturer’s instructions. Transfection efficiency was evaluated by qRT-PCR and western blot analyses. The negative control siRNA and HOXB8 siRNA sequences have been listed as follows: siHOXB8-1 (sense: 5′-CCUAUUUAAUCCCUAUCUGTT-3′ and antisense: 5′-CAGAUAGGGAUUAAAUAGGTT-3′); siHOXB8-2 (sense: 5′-UCAACUCACUGUUCUCCAATT-3′ and antisense: 5′-UUGGAGAACAGUGAGUUGATT-3′); negative control (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense: 5′-ACGUGACACGUUCGGAGAATT-3′).
Establishment of HOXB8 overexpressing cell lines
The HOXB8 overexpressing vector and empty lentiviral vector were used to establish HOXB8 overexpressing colon cancer cells as described previously (Lin et al., 2015). The HOXB8 overexpressing vector, pVSV-G vector, and pGag/Pol vector were transfected into 293T cells to produce lentiviral particles using LipofectamineTM 3000 reagent (Invitrogen) according to the manufacturer’s instruction. After 48 h, the viruses were collected and the target cells were infected with 10 μg/mL Polybrene to establish stable HOXB8 overexpressing cell lines. The culture media was replaced after 8 h. The transfection efficiency was evaluated by western blot and qRT-PCR analyses.
Quantitative real-time polymerase chain reaction
Total RNA was isolated from cells using the TRIzol reagent. The RNA integrity and quantity were evaluated in the NanoDrop One Spectrophotometer (Thermo Fisher Scientific, USA). The total RNA was reverse transcribed into the cDNA using the PrimeScriptTM RT Master Mix (Takara, Shiga, Japan). Subsequently, RT-PCR was performed using the Mastercycler ep realplex Real-time PCR system (Eppendorf, Germany) with the TB Green Premix Ex TaqTM II reagent (Takara, Shiga, Japan). The relative expressions of the mRNA molecules were normalized using the 2−ΔΔCT method (Livak and Schmittgen, 2002). The primers used for qRT-PCR analysis have been listed as follows: HOXB8 (Forward: 5′-TAAGCGGCGATTCGAGGTAT-3′, Reverse: 5′-TGTTTCTCCAGCTCCTCCTG-3′); GAPDH (Forward: 5′-TCAAGGCTGAGAACGGGAAG-3′, Reverse: 5′-GACTCCACGACGTACTCAGC-3′).
The total protein was extracted from cells by using RIPA lysis buffer and the protein concentration was measured by using the Bradford method (Bio-Rad, Hercules, CA, USA). Equivalent amounts of proteins (80 μg) were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% skim milk for 1.5 h at room temperature and incubated with the primary antibodies HOXB8 (1:1000), p-STAT3 (1:1000), STAT3 (1:1000) overnight at 4°C. Subsequently the membranes were washed with Tris-buffered saline with 0.1% Tween® 20 detergent (TBST), and incubated with horseradish peroxidase (HRP)-labeled goat anti-mouse or anti-rabbit (1:4000) secondary antibodies at room temperature for 1 h. The enhanced chemiluminescence (ECL) developing solution (Bio-Rad, Hercules, CA, USA) was used to visualize the protein bands.
All data were presented as mean ± standard deviation (SD) from three independent assays. All results were analyzed using GraphPad Prism 8.0 and SPSS 21.0 software. The differences between different groups were assessed using the Student’s t-test or one-way analysis of variance (ANOVA). Statistical significance was fixed at a p-value < 0.05.
Oxaliplatin-resistant colorectal cancer cells showed elevated Homeobox 8 expression
In order to confirm that HCT116/OXA was indeed resistant to oxaliplatin, we performed MTT assays for both HCT116 parent and HCT116/OXA cells after treatment with various concentrations of oxaliplatin. The results showed that oxaliplatin inhibited HCT116 cell growth in a dose-dependent manner with a half-maximal inhibitory concentration (IC50) value of 6.45 µM. As expected, HCT116/OXA cells exhibited significant resistance to oxaliplatin (IC50 > 80 µM) (Fig. 1A). Colony formation assays further showed that treatment with 1.5 µM oxaliplatin markedly inhibited the colony-forming ability of HCT116 cells, however, it almost had no effect on HCT116/OXA cells (Figs. 1B and 1C). These results suggested that HCT116/OXA cells are specifically and significantly resistant to oxaliplatin. Accumulating evidence has suggested that both HOXB8 and STAT3 were involved in drug resistance documented in several cancers like osteosarcoma, colorectal cancer, and non-small lung cancer (Lu et al., 2021; Gao et al., 2022; Ochi et al., 2022). Hence, we checked and compared the expression of HOXB8 and p-STAT3 in both HCT116 and HCT116/OXA cells. The expression of HOXB8 and p-STAT3 was markedly upregulated in HCT116/OXA cells compared with those in HCT116 cells (Figs. 1D and 1E). This suggested that HOXB8 and p-STAT3 were involved in oxaliplatin resistance in colon cancer cells.
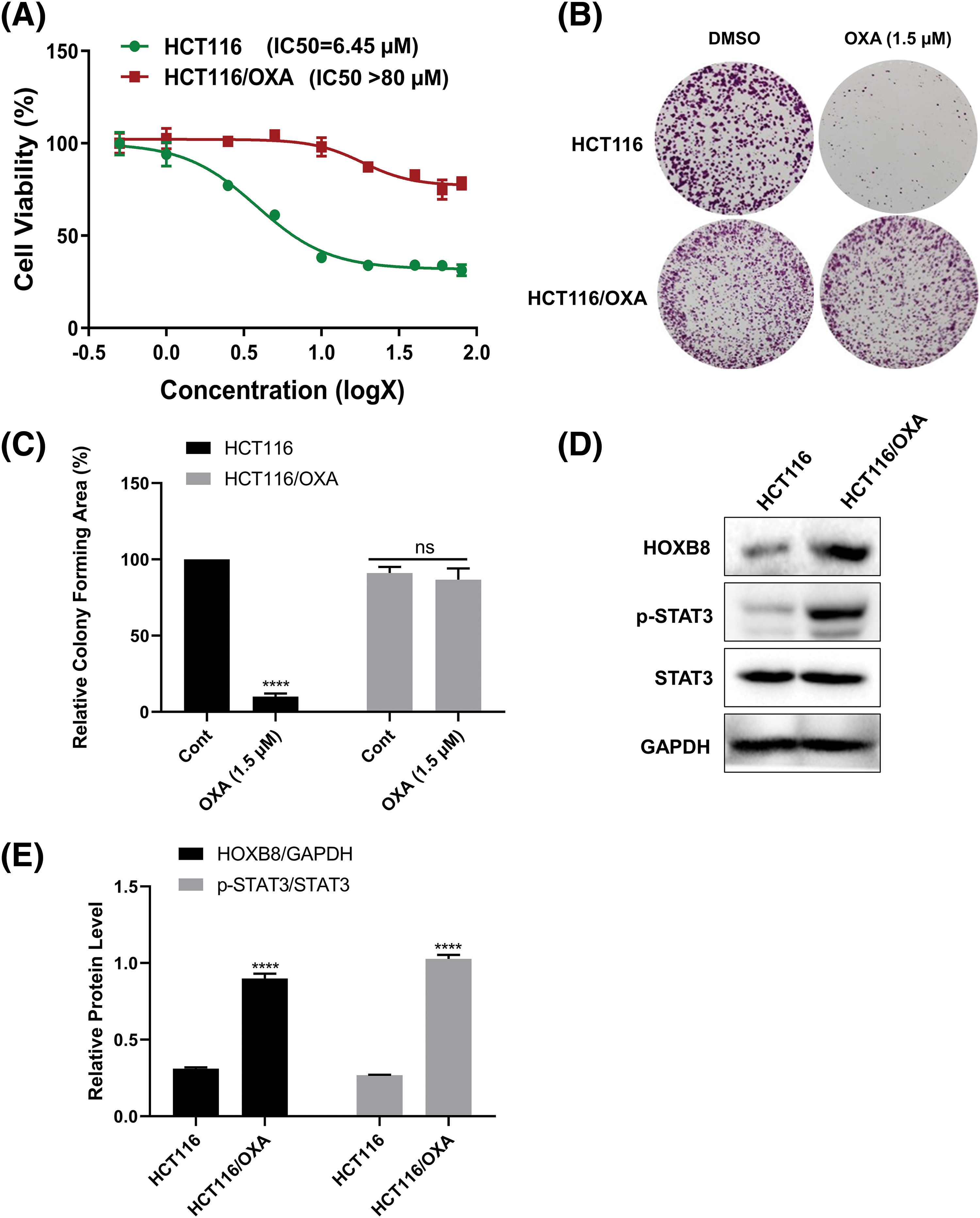
Figure 1: Homeobox 8 (HOXB8) expression is upregulated in oxaliplatin (OXA) resistant colorectal cancer (CRC) cells. HCT116/OXA and parent HCT116 cells were treated with range of OXA concentrations (0.5, 1, 2.5, 5, 10, 20, 40, 60, and 80 µM) for 48 h. Cell viability was assessed by the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. The red line indicates HCT116/OXA and the green line indicates relative cell viability of parent HCT116 at different concentrations of OXA compared to the control (A). The colony formation assay was done in HCT116/OXA and parent HCT116 cells after the treatment with 1.5 µM OXA (B, C). ****p < 0.0001 and ns (not significant). OXA (1.5 μM) vs. controls. The protein levels of HOXB8, phosphorylation signal transducer and activator of transcription 3 (p-STAT3), and signal transducer and activator of transcription 3 (STAT3) were evaluated by western blot analysis in HCT116/OXA and parent HCT116 cells (D, E). ****p < 0.0001, HCT116 vs. HCT116/OXA.
Knockdown of homeobox 8 enhanced the oxaliplatin sensitivity in HCT116 and RKO cells
To explore the effect of HOXB8 on oxaliplatin-mediated colon cancer cell growth inhibition, we knocked down HOXB8 by using two independent siRNAs in HCT116 and RKO cells. Both qRT-PCR and western blot analyses confirmed the knockdown efficiency. This was deduced as HOXB8 mRNA and protein levels were significantly decreased after transfection of HOXB8 siRNAs (Figs. 2A–2C). Further, HOXB8 knockdown markedly inhibited p-STAT3 expression in both HCT116 and RKO cells (Fig. 2C). HOXB8 knockdown inhibited colon cancer cell growth and potentiated oxaliplatin-mediated colon cancer cell growth inhibition (Figs. 2D and 2E). This suggested that the HOXB8-STAT3 axis is closely involved in oxaliplatin sensitivity in colon cancer cells.
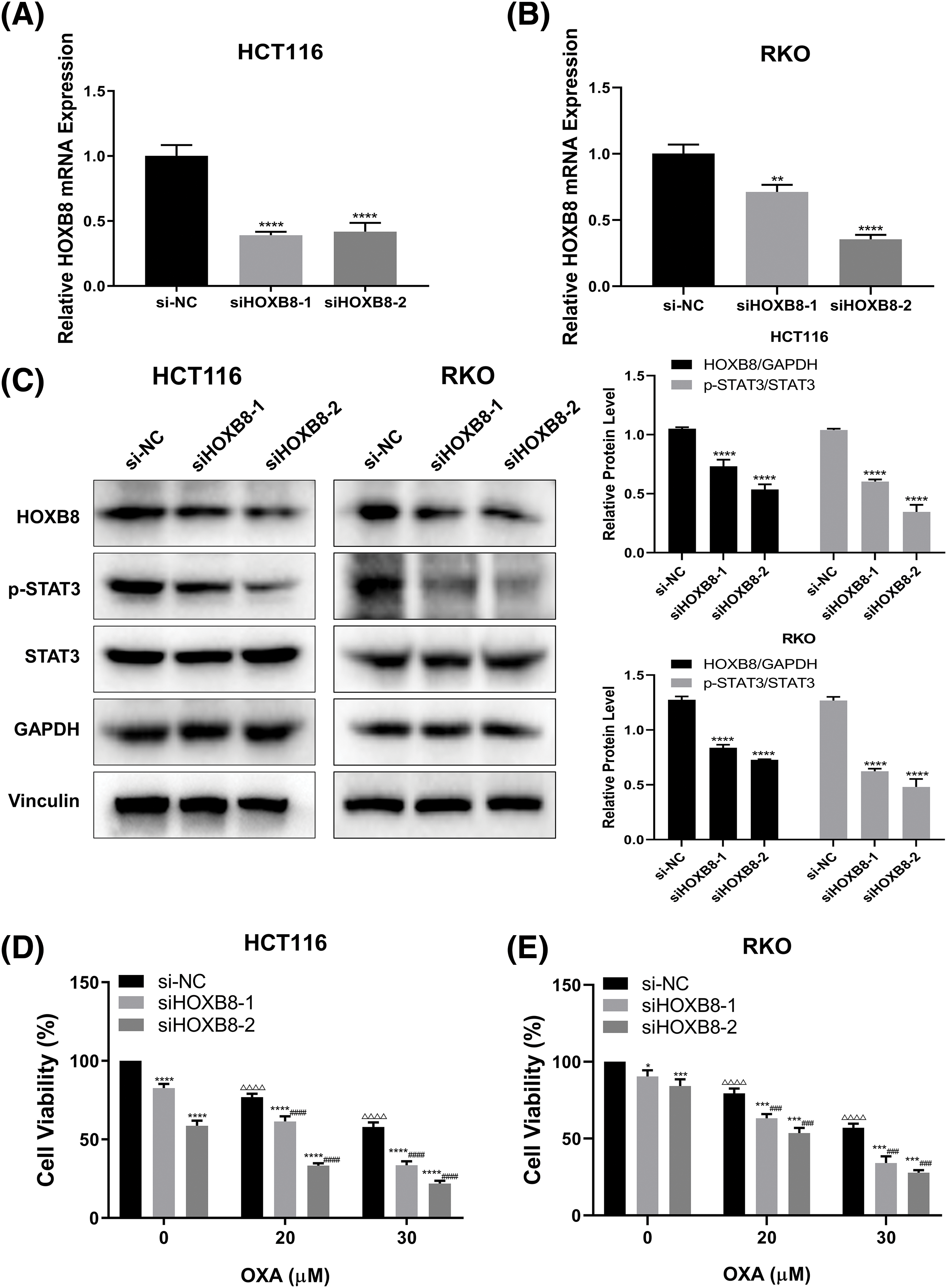
Figure 2: Homeobox 8 (HOXB8) knockdown potentiated oxaliplatin (OXA) sensitivity in both HCT116 cells and RKO cells by down-regulating signal transducer and activator of transcription (STAT3) signaling. Quantitative real-time polymerase chain reaction was used to measure HOXB8 mRNA expression after transfection of si-negative control (si-NC), siHOXB8-1, and siHOXB8-2 into HCT116 and RKO cells for 24 h (A, B). **p < 0.01, ****p < 0.0001, siHOXB8-1 or siHOXB8-2 vs. si-NC. After the siRNA-based silencing of HOXB8 in HCT116 and RKO cells for 24 h, the protein expressions of HOXB8, phosphorylation signal transducer and activator of transcription 3 (p-STAT3) and STAT3 were determined by western blotting (C). ****p < 0.0001, siHOXB8-1 or siHOXB8-2 vs. si-NC. HOXB8 siRNA were transfected into HCT116 (D) and RKO (E) cells. After 24 h, the cells were treated with different concentrations of OXA (20 and 30 μM). The relative cell viability was assessed by the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay (D, E). The results were presented as mean ± standard deviation (SD) (n = 3). *p < 0.05, ***p < 0.001, ****p < 0.0001, siHOXB8-1 or siHOXB8-2 vs. si-NC; ΔΔΔp < 0.001, ΔΔΔΔp < 0.0001, si-NC with 20 or 30 μM OXA vs. si-NC with 0 μM OXA; ###p < 0.001, ####p < 0.0001, siHOXB8-1 or siHOXB8-2 with OXA (20 or 30 μM) vs. siHOXB8-1 or siHOXB8-2 with 0 μM OXA.
Homeobox 8 overexpression reduced oxaliplatin sensitivity in colon cancer cells
To further investigate whether HOXB8 plays an important role in oxaliplatin resistance in colon cancer cells, we established stable HOXB8 overexpressing HCT116 (HCT116/HOXB8) and RKO (RKO/HOXB8) cells. We found that the expression of HOXB8 mRNA and protein was significantly upregulated in HCT116/HOXB8 and RKO/HOXB8 compared to corresponding control cells (Figs. 3A–3D). Further analysis showed that HOXB8 overexpression promoted colon cancer cell growth and significantly attenuated the growth inhibitory effect of oxaliplatin in both HCT116 and RKO cells (Figs. 3E and 3F). Similarly, HOXB8 overexpression increased the colony-forming ability of both HCT116 and RKO cells and impaired the inhibition of the colony-forming ability induced by oxaliplatin (Figs. 4A and 4B). As we hypothesized, HOXB8 overexpression significantly induced p-STAT3 expression (Figs. 4C and 4D), indicating the pivotal role of the HOXB8-STAT3 axis in oxaliplatin resistance in colon cancer cells.
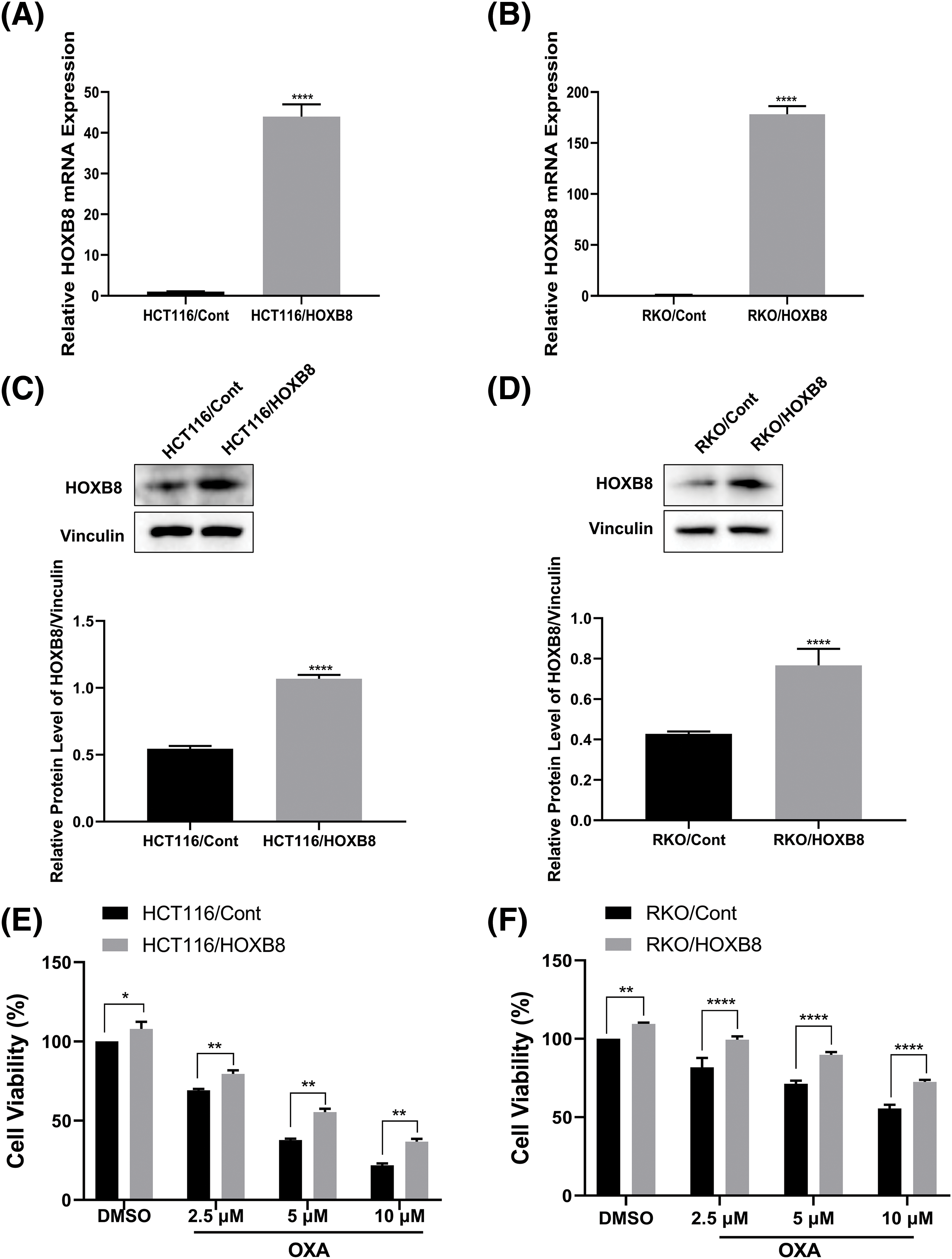
Figure 3: Homeobox 8 (HOXB8) overexpression reduced oxaliplatin (OXA) sensitivity in HCT116 and RKO cell lines. Quantitative real-time polymerase chain reaction (A, B) and western blot (C, D) analyses were used to investigate HOXB8 mRNA and protein levels, respectively after overexpressing HOXB8 in HCT116 and RKO cells (A–D). ****p < 0.0001, HOXB8 vs. control. HOXB8 overexpressing HCT116 and RKO cells, and control cells were treated with various concentrations of OXA (0, 2.5, 5, and 10 μM). The relative cell viability was detected by the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay (E, F). *p < 0.05, **p < 0.01, ****p < 0.0001, HOXB8 vs. control.
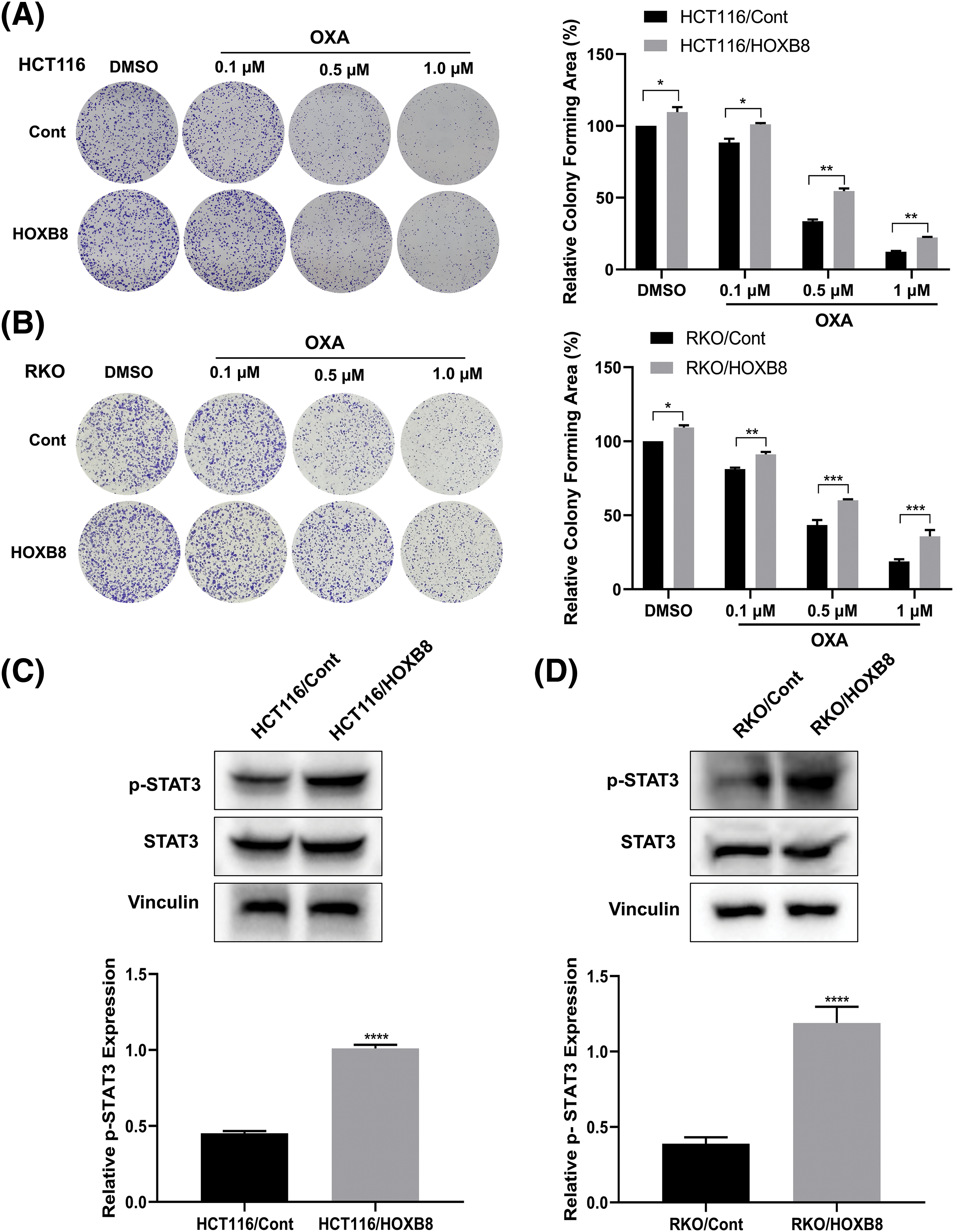
Figure 4: Homeobox 8 (HOXB8) overexpression enhanced oxaliplatin (OXA) resistance in HCT116 and RKO cells by activating signal transducer and activator of transcription (STAT3) signaling. HCT116 and RKO cells overexpressing HOXB8 were treated with various concentrations of OXA (0, 0.1, 0.5, and 1 μM). The colony-forming ability of the cells was evaluated and analyzed by Image J software (A, B). *p < 0.05, **p < 0.01, ***p < 0.001, HOXB8 vs. control. Western blotting analysis was used to assess the phosphorylation signal transducer and activator of transcription 3 (p-STAT3) and STAT3 protein expression in HOXB8 overexpressing HCT116 cells, RKO cells and control cells (C, D). Data were shown as mean ± standard deviation (SD) (n = 3). ****p < 0.0001, HOXB8 vs. control.
Homeobox 8 knockdown suppressed HCT116/OXA cell growth
We demonstrated in the previous sections that HOXB8 was involved in oxaliplatin resistance in colon cancer cells. To further confirm the critical function of HOXB8 in OXA-resistant colon cancer cells, we knocked down HOXB8 in HCT116/OXA cells. We observed downregulated levels of HOXB8 mRNA and protein by qRT-PCR and western blot analyses, respectively (Figs. 5A–5C). In lieu of our hypothesis, HOXB8 knockdown significantly reduced p-STAT3 expression in HCT116/OXA cells (Figs. 5B and 5C). While OXA treatment (60 µM) did not inhibit HCT116/OXA cell proliferation, HOXB8 knockdown markedly suppressed HCT116/OXA cell proliferation (Fig. 5D). Importantly, the growth inhibitory effect of HOXB8 knockdown is similar between HCT116 and HCT116/OXA cells, suggesting that targeting HOXB8 might be effective not only for CRC cells but also for OXA-resistant CRC cells.
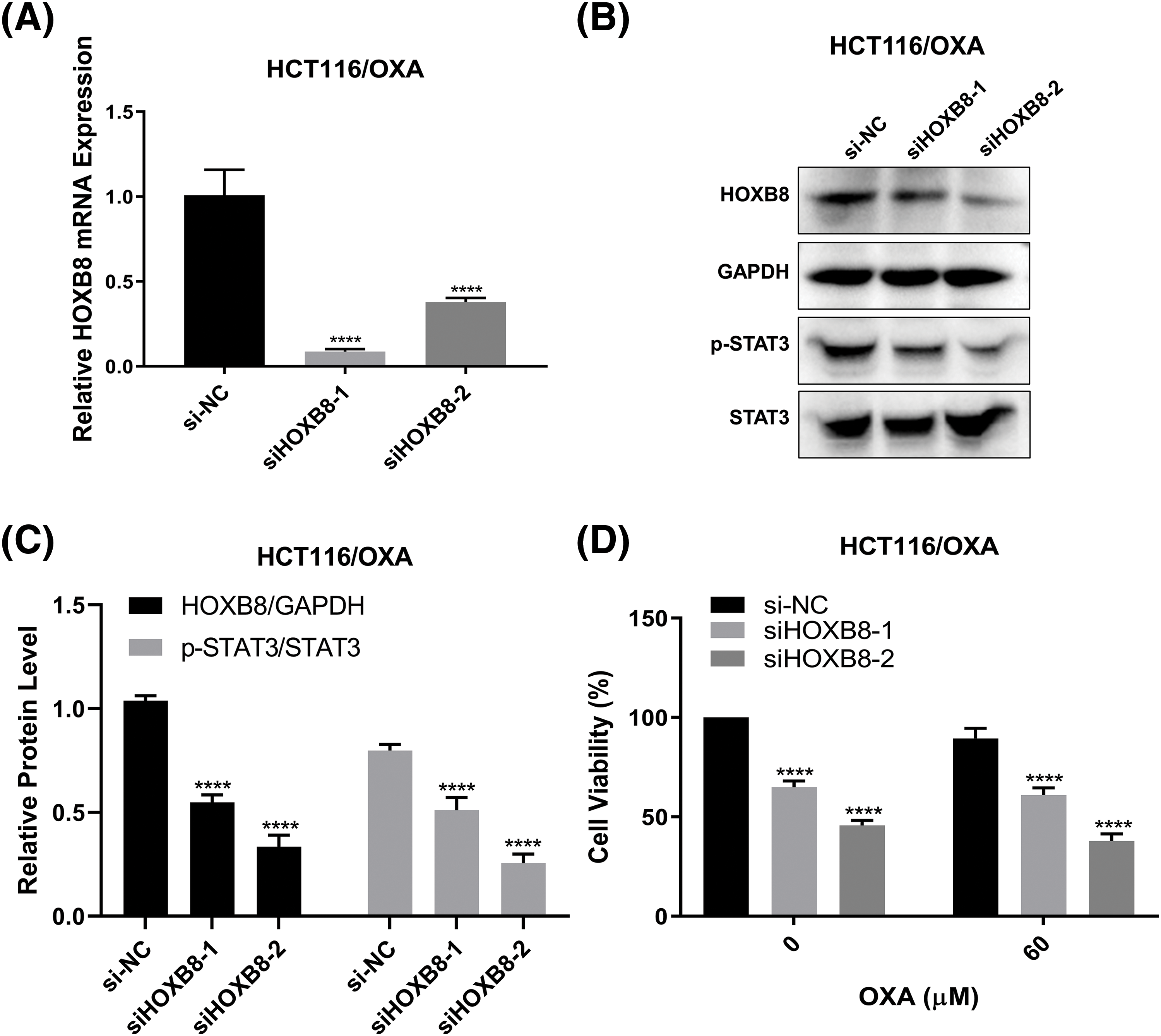
Figure 5: Silencing Homeobox 8 (HOXB8) repressed the cell proliferation of HCT116/OXA cells. HOXB8 mRNA expression was measured by quantitative real-time polymerase chain reaction in HOXB8 knockdown HCT116/OXA and control cells (A). ****p < 0.0001, siHOXB8-1 or siHOXB8-2 vs. si-negative control (si-NC). The protein expression levels of HOXB8, p-STAT3, and STAT3 were analyzed by western blotting after knocking down HOXB8 in HCT116/OXA and control cells (B, C). ****p < 0.0001, siHOXB8-1 or siHOXB8-2 vs. si-NC. HOXB8 knockdown HCT116/OXA cells and control cells were treated with 60 μM of OXA, and 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay was used to determine the cell viability (D). The data were presented as mean ± standard deviation (SD) (n = 3). ****p < 0.0001, siHOXB8-1 or siHOXB8-2 vs. si-NC.
Oxaliplatin, a third-generation platinum-based drug, is one of the most effective chemotherapeutic agents to treat CRC clinically (Park, 2014). The occurrence of OXA resistance is a complex process, in which several genes have been implicated to be involved in this process. For instance, Zinc finger E-box binding homeobox I (ZEBI) induced OXA resistance by promoting EMT (Guo et al., 2017). Additionally, lncRNA neuroblastoma associated transcript 1 (NBAT-1) enhanced the sensitivity of OXA by sponging miR-4504 and regulated the WW-and-C2-domain-containing protein family member 3 (WWC3)/large tumor suppressor kinase 1 (LATS1)/yes-associated protein (YAP) axis in CRC (Li and Li, 2022). Furthermore, knockdown of ATPase copper transporting alpha (ATP7A) overcame OXA resistance in CRC by increasing OXA-induced apoptosis (Zhou et al., 2022). Notwithstanding these reports, the underlying molecular mechanisms of OXA resistance in CRC are still vague. In this study, we found that HOXB8 was closely involved in OXA resistance in CRC cells by activating the STAT3 signaling pathway. An important finding was that the knockdown of HOXB8 could suppress the cell growth of OXA-resistant colon cancer cells by inhibiting STAT3 signaling, suggesting the crucial function of HOXB8 in OXA-resistant CRC cells.
To our knowledge, HOXB cluster genes were shown to play an oncogenic function in solid tumors (Padam et al., 2022). It has been reported that the HOXB cluster of genes, including HOXB8 was upregulated in lung adenocarcinoma (LUAD) and was associated with poor overall survival in LUAD (Yan et al., 2022). Additionally, a recent study reported that the HOXB8 gene is a master transcription factor (TF) of a core regulatory circuit that regulates the metastasis and chemoresistance of osteosarcoma (Lu et al., 2021). Further, HOXB8 knockdown suppressed cell proliferation and enhanced paclitaxel sensitivity in ovarian cancer cell lines (Li et al., 2019). These reports suggested that HOXB8 has an important role in drug resistance in cancer cells. Notwithstanding these reports, the exact role of HOXB8 in OXA-resistant CRC is still unclear. In our work, we found that HOXB8 expression was upregulated in OXA-resistant CRC cells. Further, knocking down HOXB8 increased oxaliplatin sensitivity, while overexpressing HOXB8 attenuated oxaliplatin sensitivity in CRC cells by activating the STAT3 pathway.
The aberrant activation of the STAT3 transcription factor in CRC to promote tumor growth is documented (Wang et al., 2021). Further, STAT3 overexpression facilitated CRC cell proliferation and inhibited cell apoptosis by suppressing the P53 pathway (Park and Park, 2022). Given these findings, targeting the STAT3 pathway has been considered as an ideal therapeutic strategy to overcome drug resistance in various human cancers. In another report, carcinoma-associated fibroblasts (CAFs) were reported to contribute to the resistance to OXA and 5-FU in CRC cells by activating the cytokine-mediated STAT3 pathway (Gonçalves-Ribeiro et al., 2016). Further, Gao et al. (2022) reported that U2AF homology motif kinase 1 (UHMK1) enhanced OXA resistance by regulating the Janus kinase (JAK)/STAT3 signaling pathway in CRC (Gao et al., 2022). Additionally, HOXB8 promoted malignancy in ovarian cancer by activating the STAT3 signaling pathway (Liu et al., 2022). Furthermore, HOXB8 facilitated CRC metastasis by promoting EMT through STAT3 activation (Wang et al., 2019). Given these observations, we hypothesized that the HOXB8-STAT3 axis might be involved in OXA resistance in CRC cells. As we expected, the expression of HOXB8 and p-STAT3 was increased in OXA-resistant CRC cells. Knocking down HOXB8 in OXA-resistant CRC cells suppressed p-STAT3 expression that inhibited the cell growth in OXA-resistant CRC cells.
In conclusion, our study revealed the critical functions of HOXB8 in OXA-resistant CRC cells and suggested that targeting HOXB8 might be an effective therapeutic strategy in select OXA-resistant CRC cells.
Acknowledgement: None.
Funding Statement: This work was supported by the Natural Science Foundation of Zhejiang Province (LZ22H160006), Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (LHDMY22H160002), and Wenzhou Municipal Science and Technology Bureau (Y20180085).
Author Contributions: The authors confirm their contribution to the paper as follows: Lianli Ni, Yun Yu, and Han Lin collected the data. Ri Cui and Shaotang Li conceived and designed the study. Lu Tao, Weishan Zhuge, and Yiwei Shen performed the analysis. All authors read and approved the final manuscript.
Availability of Data and Materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Abuderman A, Harb O, Gertallah L (2020). Prognostic and clinic-pathological significances of HOXB8, ILK and FAT4 expression in colorectal cancer. Contemporary Oncology 24: 183–192. https://doi.org/10.5114/wo.2020.100281 [Google Scholar] [PubMed] [CrossRef]
Akgül Ö, Çetinkaya E, Ersöz Ş, Tez M (2014). Role of surgery in colorectal cancer liver metastases. World Journal of Gastroenterology 20: 6113–6122. https://doi.org/10.3748/wjg.v20.i20.6113 [Google Scholar] [PubMed] [CrossRef]
Bhatlekar S, Fields J, Boman B (2014). HOX genes and their role in the development of human cancers. Journal of Molecular Medicine 92: 811–823. https://doi.org/10.1007/s00109-014-1181-y [Google Scholar] [PubMed] [CrossRef]
Biller L, Schrag D (2021). Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 325: 669–685. https://doi.org/10.1001/jama.2021.0106 [Google Scholar] [PubMed] [CrossRef]
Biswas R, Bugde P, He J, Merien F, Lu J, Liu DX, Myint K, Liu J, McKeage M, Li Y (2019). Transport-mediated oxaliplatin resistance associated with endogenous overexpression of MRP2 in Caco-2 and PANC-1 cells. Cancers 11: 1330. https://doi.org/10.3390/cancers11091330 [Google Scholar] [PubMed] [CrossRef]
Cardoso R, Guo F, Heisser T, Hackl M, Ihle P et al. (2021). Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. The Lancet Oncology 22: 1002–1013. https://doi.org/10.1016/S1470-2045(21)00199-6 [Google Scholar] [PubMed] [CrossRef]
Deng Y (2021). The “Chinese expert consensus on the clinical application of the chinese modified triplet combination with irinotecan (CPT-11oxaliplatin (LOHPcontinuous infusion 5-fluorouracil, and leucovorin for colorectal cancer”. Gastroenterology Report 9: 279–289. https://doi.org/10.1093/gastro/goab033 [Google Scholar] [PubMed] [CrossRef]
Ding W, Zhou M, Chen M, Qu C (2017). HOXB8 promotes tumor metastasis and the epithelial-mesenchymal transition via ZEB2 targets in gastric cancer. Journal of Cancer Research and Clinical Oncology 143: 385–397. https://doi.org/10.1007/s00432-016-2283-4 [Google Scholar] [PubMed] [CrossRef]
Fu Q, Jiang Y, Zhang D, Liu X, Guo J, Zhao J (2016). Valosin-containing protein (VCP) promotes the growth, invasion, and metastasis of colorectal cancer through activation of STAT3 signaling. Molecular and Cellular Biochemistry 418: 189–198. https://doi.org/10.1007/s11010-016-2746-6 [Google Scholar] [PubMed] [CrossRef]
Ganesh K, Stadler Z, Cercek A, Mendelsohn R, Shia J, Segal NH, DiazJr LA (2019). Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nature Reviews Gastroenterology & Hepatology 16: 361–375. https://doi.org/10.1038/s41575-019-0126-x [Google Scholar] [PubMed] [CrossRef]
Gao X, Bao W, Bai J, Fan K, Li L, Li Y (2022). UHMK1 aids colorectal cancer cell proliferation and chemoresistance through augmenting IL-6/STAT3 signaling. Cell Death & Disease 13: 424. https://doi.org/10.1038/s41419-022-04877-8 [Google Scholar] [PubMed] [CrossRef]
Gonçalves-Ribeiro S, Díaz-Maroto N, Berdiel-Acer M, Soriano A, Guardiola J et al. (2016). Carcinoma-associated fibroblasts affect sensitivity to oxaliplatin and 5FU in colorectal cancer cells. Oncotarget 7: 59766–59780. https://doi.org/10.18632/oncotarget.11121 [Google Scholar] [PubMed] [CrossRef]
Guo C, Ma J, Deng G, Qu Y, Yin L, Li Y, Han Y, Cai C, Shen H, Zeng S (2017). ZEB1 promotes oxaliplatin resistance through the induction of epithelial—mesenchymal transition in colon cancer cells. Journal of Cancer 8: 3555–3566. https://doi.org/10.7150/jca.20952 [Google Scholar] [PubMed] [CrossRef]
Guo J, Zhang T, Dou D (2019). Knockdown of HOXB8 inhibits tumor growth and metastasis by the inactivation of Wnt/β-catenin signaling pathway in osteosarcoma. European Journal of Pharmacology 854: 22–27. https://doi.org/10.1016/j.ejphar.2019.04.004 [Google Scholar] [PubMed] [CrossRef]
Jensen NF, Stenvang J, Beck MK, Hanáková B, Belling KC et al. (2015). Establishment and characterization of models of chemotherapy resistance in colorectal cancer: Towards a predictive signature of chemoresistance. Molecular Oncology 9: 1169–1185. https://doi.org/10.1016/j.molonc.2015.02.008 [Google Scholar] [PubMed] [CrossRef]
Li R, Gong L, Li P, Wang J, Bi L (2019). MicroRNA-128/homeobox B8 axis regulates ovarian cancer cell progression. Basic & Clinical Pharmacology & Toxicology 125: 499–507. https://doi.org/10.1111/bcpt.13288 [Google Scholar] [PubMed] [CrossRef]
Li C, Li X (2022). Antitumor activity of lncRNA NBAT-1 via inhibition of miR-4504 to target to WWC3 in oxaliplatin-resistant colorectal carcinoma. Journal of Healthcare Engineering 2022: 9121554. https://doi.org/10.1155/2022/9121554 [Google Scholar] [PubMed] [CrossRef]
Lin FY, Wu A, Zhang PL, Jiang L, Li ST (2015). Effect of HoxB8 gene on colon cancer cells growth and migration. Journal of Wenzhou Medical University 45: 785–790. [Google Scholar]
Liu L, Wang L, Li X (2022). The roles of HOXB8 through activating Wnt/β-catenin and STAT3 signaling pathways in the growth, migration and invasion of ovarian cancer cells. Cytotechnology 74: 77–87. https://doi.org/10.1007/s10616-021-00508-w [Google Scholar] [PubMed] [CrossRef]
Livak KJ, Schmittgen TD (2002). Analysis of relative gene expression data using real-time quantitative PCR. Methods 25: 402–408. https://doi.org/10.1006/meth.2001.1262 [Google Scholar] [PubMed] [CrossRef]
Lu B, Zou C, Yang M, He Y, He J et al. (2021). Pharmacological inhibition of core regulatory circuitry liquid-liquid phase separation suppresses metastasis and chemoresistance in osteosarcoma. Advanced Science 8: e2101895. https://doi.org/10.1002/advs.202101895 [Google Scholar] [PubMed] [CrossRef]
Ochi K, Suzawa K, Thu Y, Takatsu F, Tsudaka S et al. (2022). Drug repositioning of tranilast to sensitize a cancer therapy by targeting cancer-associated fibroblast. Cancer Science 113: 3428–3436. https://doi.org/10.1111/cas.15502 [Google Scholar] [PubMed] [CrossRef]
Padam K, Basavarajappa D, Shenoy U, Chakrabarty S, Kabekkodu S, Hunter K, Radhakrishnan R (2022). In silico interaction of HOX cluster-embedded microRNAs and long non-coding RNAs in oral cancer. Journal of Oral Pathology & Medicine 51: 18–29. https://doi.org/10.1111/jop.13225 [Google Scholar] [PubMed] [CrossRef]
Park DG (2014). Antichemosensitizing effect of resveratrol in cotreatment with oxaliplatin in HCT116 colon cancer cell. Annals of Surgical Treatment and Research 86: 68–75. https://doi.org/10.4174/astr.2014.86.2.68 [Google Scholar] [PubMed] [CrossRef]
Park HJ, Park SH (2022). Root bark of Morus Alba L. induced p53-independent apoptosis in human colorectal cancer cells by suppression of STAT3 activity. Nutrition and Cancer 74: 1837–1848. https://doi.org/10.1080/01635581.2021.1968444 [Google Scholar] [PubMed] [CrossRef]
Qian YQ, Billeter M, Otting G, Muller M, Gehring WJ, Wuthrich K (1989). The structure of the Antennapedia homeodomain determined by NMR spectroscopy in solution: Comparison with prokaryotic repressors. Cell 59: 573–580. https://doi.org/10.1016/0092-8674(89)90040-8 [Google Scholar] [PubMed] [CrossRef]
Sadrkhanloo M, Entezari M, Orouei S, Ghollasi M, Fathi N et al. (2022). STAT3-EMT axis in tumors: Modulation of cancer metastasis, stemness and therapy response. Pharmacological Research 182: 106311. https://doi.org/10.1016/j.phrs.2022.106311 [Google Scholar] [PubMed] [CrossRef]
Shan S, Niu J, Yin R, Shi J, Zhang L, Wu C, Li H, Li Z (2022). Peroxidase from foxtail millet bran exerts anti-colorectal cancer activity targeting cell-surface GRP78 to inactivate STAT3 pathway. Acta Pharmaceutica Sinica B 12: 1254–1270. https://doi.org/10.1016/j.apsb.2021.10.004 [Google Scholar] [PubMed] [CrossRef]
Shen S, Pan J, Lu X, Chi P (2016). Role of miR-196 and its target gene HoxB8 in the development and proliferation of human colorectal cancer and the impact of neoadjuvant chemotherapy with FOLFOX4 on their expression. Oncology Letters 12: 4041–4047. https://doi.org/10.3892/ol.2016.5210 [Google Scholar] [PubMed] [CrossRef]
Stavnes H, Holth A, Don T, Kærn J, Vaksman O, Reich R, Trope’ CG, Davidson B (2013). HOXB8 expression in ovarian serous carcinoma effusions is associated with shorter survival. Gynecologic Oncology 129: 358–363. https://doi.org/10.1016/j.ygyno.2013.02.021 [Google Scholar] [PubMed] [CrossRef]
Wang Y, Fu J, Yang L, Liang Z (2021). Long non‐coding RNA SNHG20 promotes colorectal cancer cell proliferation, migration and invasion via miR‐495/STAT3 axis. Molecular Medicine Reports 23: 31. https://doi.org/10.3892/mmr.2020.11669 [Google Scholar] [PubMed] [CrossRef]
Wang T, Lin F, Sun X, Jiang L, Mao R, Zhou S, Shang W, Bi R, Lu F, Li S (2019). HOXB8 enhances the proliferation and metastasis of colorectal cancer cells by promoting EMT via STAT3 activation. Cancer Cell International 19: 1–12. https://doi.org/10.1186/s12935-018-0717-6 [Google Scholar] [PubMed] [CrossRef]
Yan M, Yin X, Zhang L, Cui Y, Ma X (2022). High expression of HOXB3 predicts poor prognosis and correlates with tumor immunity in lung adenocarcinoma. Molecular Biology Reports 49: 2607–2618. https://doi.org/10.1007/s11033-021-07064-8 [Google Scholar] [PubMed] [CrossRef]
Yin X, Tang B, Li JH, Wang Y, Zhang L, Xie XY, Zhang BH, Qiu SJ, Wu WZ, Ren ZG (2017). ID1 promotes hepatocellular carcinoma proliferation and confers chemoresistance to oxaliplatin by activating pentose phosphate pathway. Journal of Experimental & Clinical Cancer Research 36: 166. https://doi.org/10.1186/s13046-017-0637-7 [Google Scholar] [PubMed] [CrossRef]
Zhou Y, Zhang Q, Wang M, Huang C, Yao X (2022). Effective delivery of siRNA-loaded nanoparticles for overcoming oxaliplatin resistance in colorectal cancer. Frontiers in Oncology 12: 827891. https://doi.org/10.3389/fonc.2022.827891 [Google Scholar] [PubMed] [CrossRef]
Zhu H, Shi Y, Jiao X, Yang G, Wang R, Yuan Y (2020). Synergistic antitumor effect of dual PI3K and mTOR inhibitor NVP-BEZ235 in combination with cisplatin on drug-resistant non-small cell lung cancer cell. Oncology Letters 20: 326. https://doi.org/10.3892/ol.2020.12189 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools