 Open Access
Open Access
VIEWPOINT
Future of the current anticoronaviral agents: A viewpoint on the validation for the next COVIDs and pandemics
Drug Discovery & Clinical Research Department, Dikernis General Hospital (DGH), Dikernis, 35744, Egypt
* Corresponding Author: AMGAD M. RABIE. Email: Array
BIOCELL 2023, 47(10), 2133-2139. https://doi.org/10.32604/biocell.2023.030057
Received 20 March 2023; Accepted 27 May 2023; Issue published 08 November 2023
Abstract
Despite the global decline in the severity of the coronavirus disease 2019 (COVID-19) cases, the disease still represents a major concern to the relevant scientific and medical communities. The primary concern of drug scientists, virologists, and other concerned specialists in this respect is to find ready-to-use suitable and potent anticoronaviral therapies that are broadly effective against the different species/strains of the coronaviruses in general, not only against the current and previous coronaviruses (e.g., the recently-appeared severe acute respiratory syndrome coronavirus 2 “SARS-CoV-2”), i.e., effective antiviral agents for treatment and/or prophylaxis of any coronaviral infections, including those of the coming ones from the next species and strains (if any). As an expert in this field, I tried, in this up-to-date perspective “viewpoint” article, to evaluate the suitability and applicability of using the currently-available anticoronaviral agents for the next coronavirus diseases (COVIDs) and coronaviral pandemics, highlighting the most important general guidelines that should be considered in the next pandemics from the therapeutic points of view.Graphic Abstract
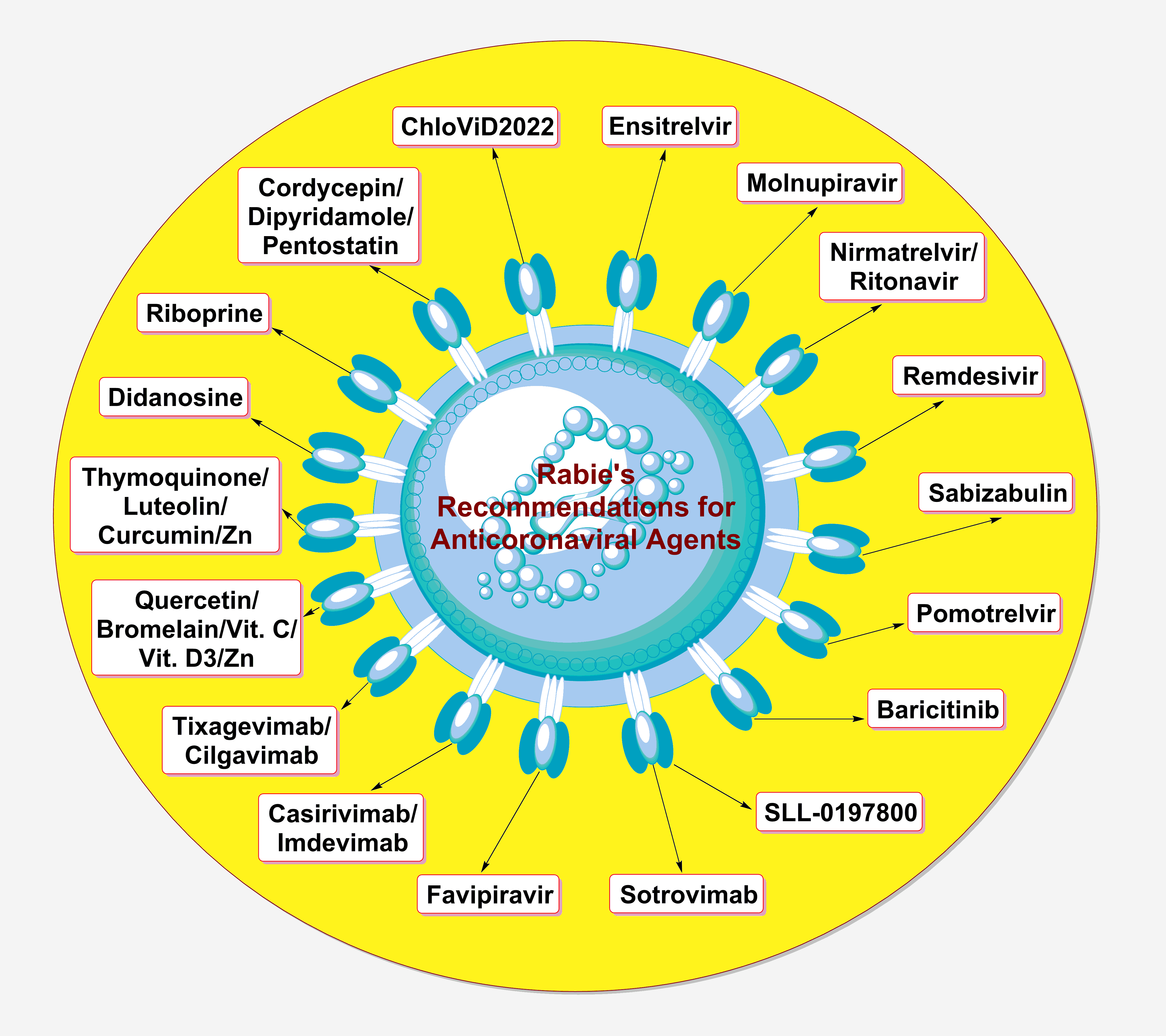
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools