 Open Access
Open Access
ARTICLE
Chrysophanol inhibits the progression of gastric cancer by activating nod-like receptor protein-3
1
Department of Gastroenterology, The First Affiliated Hospital of Bengbu Medical College, Bengbu, 233004, China
2
Department of Orthopedics, The First Affiliated Hospital of Bengbu Medical College, Bengbu, 233004, China
* Corresponding Author: Min Deng,
BIOCELL 2023, 47(1), 175-186. https://doi.org/10.32604/biocell.2022.021359
Received 10 January 2022; Accepted 24 May 2022; Issue published 26 September 2022
Abstract
Aim: Gastric cancer (GC) is one of the most common malignant tumors. Chrysophanol has been reported to possess antitumor effects on a variety of cancers; however, its role in GC remains unclear. This study aimed to investigate the effects of chrysophanol on the proliferation, pyroptosis, migration, and invasion of GC cells. Methods: Human GC cell lines MKN 28 and AGS cells were treated with different concentrations of chrysophanol, then cell proliferation, migration, invasion and pyroptosis were determined by CCK-8, colony-forming assay, wound healing assay, Transwell assay, and flow cytometry. Cell migration and invasion were reassessed in these transfected cells following the transfection of nod-like receptor protein-3 (NLRP3) siRNA in MKN 28 and AGS cells. To examine the downstream signaling pathway of the NLRP3 signaling pathway, NLRP3, caspase-1, gasdermin-D, interleukin (IL)-1β, and IL-18 were detected by quantitative real-time-polymerase chain reaction or western blotting. Results: Chrysophanol inhibited the proliferation of GC cells, caused pyroptosis, inhibited cell migration and invasion, and increased the expression of NLRP3 inflammasomes in GC cells. Knockdown of NLRP3 inhibited the effects of chrysophanol on proliferation, pyroptosis, migration, and invasion of GC cells. Chrysophanol plays an anticancer role by enhancing NLRP3. Conclusions: Chrysophanol exerts anti-neoplastic effects in vitro in GC cells by modulating NLRP3, thus highlighting its therapeutic potential in GC.Keywords
Abbreviations
| AKT | protein kinase B |
| ASC | adaptor apoptosis-related speckle-like protein |
| Chrysophanol | 1,8-dihydroxy-3-methyl-9,10-anthraquinone |
| DAMPs | damage-related molecular patterns |
| ERK1/2 | extracellular signal-regulated kinase1/2 |
| GC | Gastric cancer |
| GSDMD | gasdermin-D |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor kappa-B |
| NLRP3 | Nod-1ike receptor protein 3 |
| PCD | programmed cell death |
| ROS | Reactive oxygen species |
Gastric cancer (GC) is one of the most common malignant tumors in the digestive system, ranking fifth in the global malignant tumor incidence and fourth in terms of fatality rates (Machlowska et al., 2020). Patients with GC are currently treated primarily through surgical operation, supplemented by radiotherapy and chemotherapy (Song et al., 2017). However, although many methods have been adopted to treat tumors (Liu et al., 2022), the median survival time of patients with advanced GC is rarely longer than 12 months, and patients with metastasis have a 5-year survival rate below 10% (Crew and Neugut, 2004). Despite the effective use of anticancer drugs such as trastuzumab (Shitara et al., 2020), paclitaxel (Shitara et al., 2018), and oxaliplatin (Al-Batran et al., 2016) in the treatment of clinically common GC patients, their drug resistance and side effects call us to further understand the molecular biological mechanisms of occurrence and growth of GC to develop targets for GC treatment.
In recent years, many drugs have been isolated from natural plants. Chinese medicine has been established as a common alternative and complementary treatment method for a wide variety of cancers, indicating that it has the potential to become a promising alternative treatment method in the future. Chrysophanols (1,8-dihydroxy-3-methyl-9,10-anthraquinone) are the main components of Rheum officinale, found in nature as anthraquinone compounds, which have a wide range of biological activities, such as antibiosis, anti-inflammatory (Lian et al., 2017; Meng et al., 2018) antitumor (Su et al., 2020).
According to our previous research, chrysophanol inhibits the activity of colorectal cancer cells by targeting the decorin protein (Deng et al., 2020). These results provide a reference for the study of the antitumor activity of chrysophanol. However, the exact mechanism of action of chrysophanol on GC cells is still unknown. Therefore, the purpose of this study was to investigate the anti-GC effects of chrysophanol and its specific molecular targets.
Pyroptosis is a type of programmed cell death (PCD) due to inflammation (Humphries et al., 2020). In contrast to other types of cell death, pyroptosis is associated with morphological changes such as swollen cells, large bubbles, pore formation in the plasma membrane, and changes in subcellular organelles (Wang et al., 2017). Nod-1ike receptor protein 3 (NLRP3) inflammasome is a cytoplasmic protein complex containing NLRP3, adaptor apoptosis-related speckle-like protein, and pro-caspase-1 (Fu et al., 2022; Ren et al., 2019). Activation of NLRP3 inflammasomes leads to the maturation and secretion of interleukin (IL)-1β and IL-18, which leads to cell damage and pyroptosis (Sharif et al., 2019). NLRP3 inflammasome plays a crucial role in the progression of various cancers such as lung cancer (Wang et al., 2016), colorectal cancer (Chung et al., 2019), and breast cancer (Quagliariello et al., 2020). Previous studies have shown that NLRP3 is highly expressed in GC (Ren et al., 2020). In addition, antitumor agents such as resveratrol (Zou et al., 2018) and parthenolide (Juliana et al., 2010) have been shown to trigger cell death via NLRP3 inflammasomes (He et al., 2018). The regulation of NLRP3 is, therefore, a promising therapeutic strategy. In this study, we examined the effect of chrysophanol on GC cell pyroptosis, as well as its regulation and mechanism on NLRP3 in that process, to provide a theoretical basis for its potential value in the treatment of GC.
Chrysophanol was purchased from MCE (Monmouth Junction, NJ, USA), RPMI 1640 medium and fetal bovine serum (FBS) were purchased from Gibco (CA, USA), and FAM-FLICA Caspase-1 kit was purchased from ImmunoChemistry Technologies (Bloomington, MN, USA), lactate dehydrogenase (LDH) kit was purchased from Jiancheng Biotechnology (Nanjing, China), NLRP3 (catalog number: 19771-1-AP), caspase-1 (Catalog number: 22915-1-AP), gasdermin D (GSDMD; catalog number: 20770-1-AP), IL-1β (catalog number: 16806-1-AP), IL-18 (catalog number: 10663-1-AP), and nuclear protein Ki67 (catalog number: 27309-1-AP) were purchased from ProteinTech (Wuhan, China). NLRP3 siRNA was purchased from Gemma Pharmaceutical Technology (Shanghai, China); enzyme-linked immunosorbent assay (ELISA) kit IL-1β (CSB-E08053h), IL-18 (CSB-E07450h) were purchased from CUSABIO (Wuhan, China); Radioimmunoprecipitation assay buffer (RIPA) cell lysate, phenylmethylsulfonyl fluoride (PMSF), BCA protein quantitative kit, Trizol, Cell counting kit-8 (CCK-8) were purchased from Beyotime Biotechnology (Shanghai, China), lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA), retro-transcription kit was purchased from TransGen Biotech (Beijing, China).
This study was approved by the Institutional Review Board of The First Affiliated Hospital of Bengbu Medical College. All the participants provided written informed consent. Thirty patients who underwent surgical resection were sampled for tumor tissue and adjacent tissue. All the clinical tissue samples were immediately stored at −80°C for further use.
Cell lines and culture conditions
The human GC cell lines MKN 28 and AGS were purchased from the Chinese Academy of Medical Sciences (CAS, Beijing, China). Cells were cultured in RPMI-1640 medium, supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin in 5% CO2 at 37°C. The culture medium was renewed every 2 days, and cells were passaged once they reached 70%–80% confluence. Cells were seeded at an appropriate density according to each experimental design.
Cells were plated uniformly in 96-well plates containing 5 × 103 cells per well. The cells were incubated overnight with chrysophanol at 0, 2, 5, and 10 μM for 24 and 48 h each. Ten microliters of each concentration was added to each well for 2 h in a microplate (Bio-Tek USA) to determine the absorbance at 450 nm. Three parallel holes were set in each treatment group. In addition to the negative control and blank zero hole, and the survival rate% = [OD (450) treatment group−OD (450) empty self-group]/[OD (450) control group−OD (450) empty self-group] 100%. The cell growth curve was drawn with time as the abscissa and cell survival rate as the ordinate. All experiments were conducted independently and repeated three times.
Cells were inoculated evenly in a 6-well plate with 500 cells per well. The cells were cultured in different concentrations of chrysophanol (0, 5, and 10 μM) for 2 weeks, during which the culture medium containing chrysophanol was changed once every 3 days for 2 weeks. The medium was discarded, and the cells were washed with phosphate-buffered saline (PBS) once, then fixed in 4% paraformaldehyde at room temperature for 20 min, and then stained with 0.1% crystal violet at room temperature for 30 min. After washing the cells thrice with PBS, the cells were observed and photographed under an inverted microscope. The cell colony number was counted by Image-J.
Cells were uniformly inoculated in a 6-well plate with 5 × 105 cells/well. The cells were treated with different concentrations of chrysophanol (0, 5, and 10 μM) for 24 h and then collected with reference to the FAM-FLICA Caspase-1 assay kit, which describes double-positive staining for activated caspase-1 and propidium iodide (PI) in cells to assess cell pyroptosis. All assays were performed independently and repeated three times.
Cells were uniformly cultured at 1 × 105 cells/well in a 24-well culture plate. The cells were treated with different concentrations of chrysophanol (0, 5, and 10) for 24 h. The absorbance value at 490 nm was measured by a microplate reader, and the release rate of LDH was calculated as rate% = (measured OD value − control OD value)/(standard OD value − blank OD value) × 100%.
Wound healing assay to detect migration
Cells were evenly inoculated in 6-well plates at 5 × 105 cells/well, and cultured to 70%–80% of the fusion, and were treated with different concentrations of chrysophanol (0, 5, and 10 μM) to scratch monolayer cells with disinfected pipet tips. The fragments were washed in PBS for 0 and 24 h to observe the migration of cells to the wound surface. Cell migration to the wound surface was observed under an inverted microscope and photographed. All experiments were performed independently and repeated three times.
The cells were suspended in 100 μL of RPMI 1640 containing 2% FBS and then inoculated with Matrigel (BD Biosciences, New Jersey, USA). The bottom chamber was filled with 500 μL RPMI 1640 supplemented with 10% FBS for 24 h. Then, the medium was discarded, washed once with PBS, and fixed with 4% paraformaldehyde at room temperature for 20 min, after which the cells in the chamber were wiped with a wet cotton swab and stained with 0.5% crystalline violet at room temperature for 30 min. After washing the cells thrice with PBS, they were dried and observed under an inverted microscope and photographed. The number of cells in each group was counted by Image-J, and all experiments were conducted independently three times.
Cells were lysed for 30 min using RIPA buffer containing 1% PMSF. The protein concentration was determined by BCA protein assay kit. The proteins were electroblotted to a polyvinylidene fluoride membrane (Millipore, Massachusetts, USA). The membrane was incubated with primary antibodies against NLRP3, cleaved caspase-1, IL-18, and GSDMD. β-actin was used as the internal reference. All experiments were carried out independently three times.
RNA extraction and quantitative real-time-polymerase chain reaction (qRT-PCR)
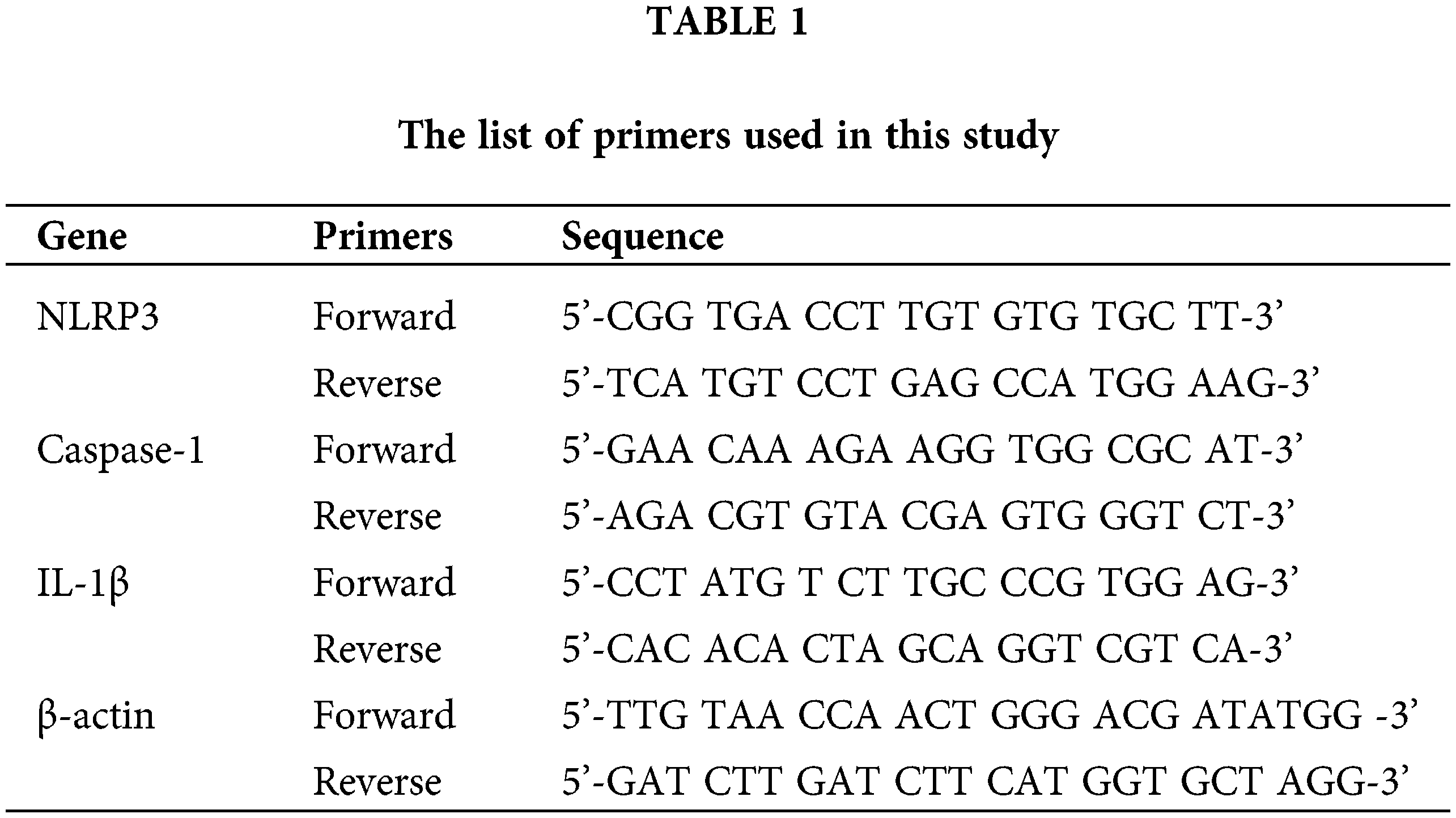
Cells were uniformly seeded into 6-well plates at 5 × 105 cells/well with different concentrations of chrysophanol (0, 5, and 10 μM) for 24 h. Total RNA from the culture cells was isolated using the TRIzol Reagent and reverse transcribed into cDNA using One-Step gDNA Removal and cDNA Synthesis SuperMix of TransGen Biotech kit. Target gene expression was analyzed by RT-PCR using Power SYBR Green PCR Master Mix, with β-actin as the internal control. The forward and reverse primers were synthesized by Sangon (Shanghai, China) according to previously published sequences. The relative expression of the target genes was calculated using the 2−ΔΔCT method (Table 1).
Nod-like receptor protein-3 small interfering RNA (siRNA) transfection
NLRP3 siRNA (sense: 5’-CCUACCUUCUCUAUCAGAUTT-3’; anti-sense: 5’ - AUCUGAUAGAGAAGGUAGGTT-3’), NLRP3 siRNA was purchased from GenePharma (Shanghai, China). NLRP3 siRNA (100 nM) or negative control siRNA was mixed with Lipofectamine 2000 to make the transformation mixture. The cells were incubated with the transfection mixture for 6 h and then changed with RPMI-1640 medium with 10% FBS. The cells were incubated for an additional 48 h before harvest.
Tissues fixed in 4% paraformaldehyde were embedded in paraffin and sliced into 4 μm thick sections. Then these sections were treated with 0.3% TritonX-100 for 30 min and immunostained with Ki67, NLRP3, and caspase-1 primary antibodies at 4°C overnight and then with universal secondary antibodies at 37°C for 60 min. Next, tissues were visualized using the 3-amino-9-ethylcarbazole after applying for 10 min. The tissues were washed with PBS three times, counterstained with hematoxylin, then dehydrated, and then a cover slip was placed according to the protocol.
Enzyme-linked immunosorbent assay
The supernatants of the cell culture medium were collected and detected using a human IL-1β and IL-18 ELISA kit according to the kit instructions.
NLRP3 expression was derived from the Oncomine database (http://www.oncomine.com). The Cancer Genome Atlas (TCGA) database the NLRP3 gene expression in GC was obtained from ULCAN online database (http://ualcan.path.uab.edu/). The survival curve of NLRP3 gene in GC was obtained from the UALCAN online database (http://ualcan.path.uab.edu/).
SPSS 25.0 software (SPSS Inc., USA) was used for the student’s t-test analysis of the experimental data. The results were expressed as the means ± standard deviation (SD) of three independent experiments. p < 0.05 was considered statistically significant.
Chrysophanol inhibits gastric cancer cell proliferation
The chemical structure of chrysophanol is shown in Fig. 1A. MKN 28 and AGS cells were treated with different concentrations of chrysophanol for 24 and 48 h. The CCK8 assay revealed markedly decreased viability of GC cells after treatment with 0. 5 and 10 μM chrysophanol (p < 0.05 or p < 0.01), suggesting that chrysophanol inhibits cell viability in a dose-dependent manner (Figs. 1B and 1C). The colony formation assay revealed that chrysophanol significantly reduced the rate of proliferation of MKN 28 and AGS cells (p < 0.01, Figs. 1D and 1E). Colony formation experiments indicated that chrysophanol treatment significantly reduced the number of human GC cell colonies. These results suggest that chrysophanol inhibits human GC cell proliferation in a dose-dependent manner.

Figure 1: Chrysophanol inhibits the proliferation of MKN 28 and AGS cells. (A) Chemical structure of chrysophanol; (B–C) Two human gastric cancer lines (MKN 28 and AGS) were treated with chrysophanol at different concentrations (0, 2, 5, 10 μM) for 24 and 48 h, each. Cell viability was measured by CCK8 assay; (D–E) Colony formation assay of MKN 28 and AGS cells treated with chrysophanol at different concentrations (0.5 and 10 μM) * p < 0.05; ** p < 0.01.
Chrysophanol up-regulates the expression of nod-1ike receptor protein 3 inflammasomes in gastric cancer cells
To explore the relationship between chrysophanol and the NLRP3 inflammasome, the expression level of NLRP3, cleaved caspase-1, and IL-1β were determined by qRT PCR following treatment with different concentrations of chrysophanol (0. 5 and 10 μM). As shown in Figs. 2A–2C, the expression level of NLRP3 inflammasome was significantly up-regulated after treatment with 5 and 10 μM chrysophanol in MKN 28 and AGS cells (p < 0.01). Pyroptosis is also known as gasdermin-mediated programmed necrosis (Gross et al., 2009); our results indicated that the expression of gasdermin-D (GSDMD) and IL-18 increased in MKN 28 and AGS cells after 5 and 10 μM chrysophanol treatment (p < 0.05, Figs. 2D–2G). These results indicate that chrysophanol and NLRP3 are positively regulated in GC cells.

Figure 2: Chrysophanol increases the expression of nod-1ike receptor protein 3 (NLRP3) inflammasome in MKN 28 and AGS cells. MKN 28 and AGS cells were treated with different concentrations of chrysophanol (0.5 and 10 μM). (A–C) The mRNA expression level of NLRP3, caspase-1, and IL-1β was detected by quantitative real-time-polymerase chain reaction. (D–G) The protein expression level of gasdermin D (GSDMD) and IL-18 was detected by western blotting. * p < 0.05; ** p < 0.01.
Chrysophanol could trigger pyroptosis in gastric cancer cells
Chrysophanol treatment induced morphological changes in GC cells, including decreased cell density, cell rounding, and cell floating (Fig. 3A). Compared with the control group, LDH release in 5 and 10 μM chrysophanol groups increased significantly in a dose-dependent manner (p < 0.01; Fig. 3B). Next, the assessment of cell pyroptosis by flow cytometry (Fig. 3C) revealed that chrysophanol significantly increased the activation percentage of caspase-1 cells in a dose-dependent manner. These results thus suggest that chrysophanol triggers downstream cell pyroptosis through activation of the NLRP3 inflammasome.

Figure 3: Chrysophanol promotes pyroptosis of MKN 28 and AGS cells. (A) MKN 28 and AGS were treated with chrysophanol at different concentrations (0.5 and 10 μM), cell morphology was observed and photographed under a 100x magnification microscope; (B) Lactate dehydrogenase (LDH) release rate was detected by LDH assay; (C) Cell pyroptosis was detected by Caspase-1/PI flow cytometry. * p < 0.05 ** p < 0.01.
Chrysophanol suppresses the migration and invasion of MKN 28 and AGS cells
To investigate the effect of chrysophanol on metastasis of GC cells, wound healing and Transwell invasion assays were performed. As compared to the control group, the migration rate of MKN 28 and AGS cells treated with 5 and 10 μM chrysophanol was significantly reduced (p < 0.01 or p < 0.001; Fig. 4A). Similarly, the invasion ability of 5 and 10 μM chrysophanol to GC cells was also lower than that of the control group (p < 0.05 or p < 0.001; Fig. 4B). Taken together, chrysophanol can significantly inhibit MKN 28 and AGS cell migration and invasion.

Figure 4: Chrysophanol suppresses the migration and invasion of MKN 28 and AGS cells. MKN 28 and AGS were treated with different concentrations of chrysophanol (0.5 and 10 μM), and then (A) cell migration was assessed by wound healing assay; (B) cell invasion was detected by Transwell assay. Magnification: ×100. Scale bar = 20 μM. * p < 0.05; **p < 0.01 and ***p < 0.001.
Chrysophanol induces pyroptosis by regulation of nod-1ike receptor protein-3
To investigate the possible roles of NLRP3 in GC, we searched the OncoMine database and found that the level of NLRP3 is significantly higher in GC cells (p < 0.05; Fig. 5A). The expression of NLRP3 in GC tissues was higher than in adjacent tissues in the TCGA database (p < 0.001; Fig. 5B). NLRP3 expression was significantly increased in MKN 28 and AGS cells compared to that in GES-1 cells (p < 0.01, p < 0.001; Figs. 5C–5E). The expression of NLRP3 and caspase-1 appeared to be low in paracarcinoma tissue, and was significantly elevated in human GC tissue, while the expression of Ki 67, which is associated with cell proliferation, was higher in cancer tissues than in paracancerous tissues (p < 0.001; Figs. 5F–5H). Moreover, NLRP3 expression correlated negatively with survival in Kaplan-Meier survival analysis (p < 0.05; Fig. 5I). To further explore the effect of NLRP3 on GC occurrence and development, NLRP3 siRNA and NC were transfected into MKN 28 and AGS cells. As a result of siRNA-mediated knockdown of NLRP3 gene, the expression level of NLRP3 was dramatically reduced compared to the control group (p < 0.01, or p < 0.001; Figs. 6A–6D). The results indicated a successful transfection of the NLRP3 siRNA and NC into MKN 28 and AGS cells.

Figure 5: Chrysophanol induces pyroptosis through the regulation of nod-1ike receptor protein-3 (NLRP3). The expression of NLRP3 in human GC tissues and adjacent tissues, (A) Data from OncoMine Database (normal = 19, GC = 79, http://www.oncomine.com); (B) Data from TCGA Database (http://ualcan.path.uab.edu/); (C) quantitative real-time-polymerase chain reaction analysis of NLRP3 in GES-1, MKN 28, and AGS cells; (D–E) The protein levels of NLRP3 in GES-1, MKN 28, and AGS was detected by western blotting; (F–H) IHC staining for NLRP3, caspase-1, and Ki 67; (I) Kaplan–Meier analysis of NLRP3 expression in the survival of GC patients (http://ualcan.path.uab.edu/). Magnification: ×200. Scale bar = 20 μM. *p < 0.05; **p < 0.01 and ***p < 0.001.

Figure 6: Detection of nod-1ike receptor protein 3 (NLRP3) expression and LDH release after transfection. (A–D) MKN 28 and AGS cells were transfected with NLRP3 siRNA and NC, and the expression level of NLRP3 siRNA in these transfected cells was detected by qRT-PCR and western blot; the transfected cells were treated with 10 μM chrysophanol for 24 h, then (E) the release of LDH was examined by LDH kit. *p < 0.05; **p < 0.01; ***p < 0.001.
Subsequently, the LDH assay was examined again. The results of Fig. 6E demonstrated that chrysophanol significantly increased the release of LDH compared with that in the control group (p < 0.01; Fig. 6E). NLRP3 siRNA, together with chrysophanol, reduced the release of LDH in MKN 28 cells compared with that in the chrysophanol (10 μM) group. These data suggest that NLRP3 siRNA could partially reverse pyroptosis in MKN 28 and AGS cells.
Chrysophanol exerted antitumor effect via the nod-like receptor protein-3 signaling pathway in gastric cancer cells
Wound healing and Transwell assays were used to examine the effects of NLRP3 on cell migration and invasion. The migration ability of MKN 28 cells and AGS cells was significantly decreased following chrysophanol treatment (p < 0.001; Fig. 7A). Similarly, the invasion ability of MKN 28 and AGS cells was decreased after treatment with chrysophanol compared to the control group (p < 0.001, Fig. 7B). However, these effects were reversed by NLRP3 siRNA suppression in MKN 28 and AGS (p < 0.001, Figs. 7A and 7B). The results indicated that NLRP3 siRNA partially reversed the inhibitory effects of chrysophanol on cell migration and invasion in GC cells.

Figure 7: Chrysophanol inhibited MKN 28 and AGS cell migration and invasion by regulation of nod-like receptor protein-3 (NLRP3). Cell migration was assessed by wound healing assay. (B) Cell invasion was assessed by Transwell assay; Magnification: ×100. Scale bar = 20 μM.*p < 0.05; **p < 0.01; ***p < 0.001.
To further investigate the underlying mechanism, downstream signaling pathways of NLRP3 were analyzed. According to qRT PCR assay results, the mRNA level of IL-1β in the chrysophanol group was higher than in the control group (p < 0.01; Fig. 8A). Western blot results displayed that the levels of cleaved caspase-1 and IL-18 protein significantly increased after treatment with chrysophanol compared to the control group in MKN 28 cells (p < 0.01; Fig. 8B). The results showed that chrysophanol increased caspase-1, IL-18, and IL-1β expression levels in MKN 28 cells (p < 0.01; Figs. 8A–8D). Nevertheless, this effect was partially reversed by chrysophanol + NLRP3 siRNA (p < 0.001; Figs. 8A–8D). According to the results, increased expression of IL-1β and IL-18 was observed in the supernatant of chrysophanol-treated MKN 28 and AGS cells (p < 0.001; Figs. 8E and 8F). The level of these inflammatory factors declined by NLRP3 siRNA treatment. These results indicated that chrysophanol may exert its antitumor effects through the NLRP3 signaling pathway in GC cells.

Figure 8: Chrysophanol exerts an antitumor effect via the nod-like receptor protein-3 (NLRP3) signaling pathway. (A) The mRNA levels of IL-1β were detected by quantitative real-time-polymerase chain reaction; (B–D) The protein levels of cleaved caspase-1 and IL-18 were detected by western blot; (E–F) Inflammatory factors IL-1β and IL-18 were detected by enzyme-linked immunosorbent assay. *p < 0.05, **p < 0.01, ***p < 0.001.
In recent years, many natural compounds have been found to have antitumor effects (Zhang et al., 2021), and the advantages of small side effects and low drug resistance make it a hot spot for researchers. Chrysophanol is an anthraquinone component isolated from rhubarb (Kuo et al., 2020). A number of preclinical studies have shown that similar to other rhubarb extracts, chrysophanol has anticancer effects in various types of tumors by targeting multiple signaling pathways. Thus, chrysophanol is potential cancer prevention or treatment agent. Chrysophanol was observed to inhibit ovarian cancer cell proliferation by activating the MAPK (mitogen-activated protein kinase) signaling pathway (Lim et al., 2018). Chrysophanol inhibits breast cancer cell growth via the NF-κB (nuclear factor kappa-B) signaling pathway (Ren et al., 2018). Chrysophanol induces apoptosis in choriocarcinoma mainly by inducing reactive oxygen species (ROS) production and AKT (protein kinase B) and ERK1/2 (extracellular signal-regulated kinase1/2) pathways (Lim et al., 2017). In addition, our previous study revealed that chrysophanol inhibits colorectal cancer activity by targeting decorin (Deng et al., 2020). In this study, chrysophanol was found to inhibit GC cell proliferation and induce pyroptosis.
Pyroptosis is a type of PCD caused by inflammasomes-mediated cell lysis, which causes rupture of the plasma membrane to release contents and proinflammatory mediators, further leading to cell death via both the classical caspase-1 dependent pathway and the non-classical caspase-4/5/11 dependent pathway (Shi et al., 2017). Some drugs have been shown to induce pyroptosis to inhibit tumor cell growth (Du et al., 2021). Induced pyroptosis may be a potential treatment for the tumors. We found in this study that chrysophanol promotes the pyroptosis of GC cells through the caspase-1 signaling pathway. GSDMD-mediated pyroptosis is one of the key mechanisms involved in GC pathogenesis and chemotherapy, including proliferation, immune response, and resistance to chemotherapy in GC (Wang et al., 2018). As a result of chrysophanol treatment, GSDMD expression and LDH were increased, suggesting that chrysophanol might play a role in pyroptosis through caspase-1 signaling to reduce cancer growth.
NLRP3 inflammasome plays a key role in activating the caspase-1 signaling pathway and is the most characteristic inflammasome (Ren et al., 2019). It plays an important role in the occurrence and development of various types of tumors (Guo et al., 2018). Breast cancer fibroblasts have been found to sense damage-related molecular patterns and respond by activating NLRP3 inflammasome pathways, leading to the secretion of pro-inflammatory signals and IL-1β. Activation of the NLRP3 inflammasome enhances the proliferation, invasion, and migration of A549 lung cancer cells (Ershaid et al., 2019). Polyphyllin VI induces pyroptosis in caspase-1-mediated non-small cell lung cancer cells via induction of ROS/NF-κB/NLRP3/GSDMD signaling axis (Teng et al., 2020). We found in this study that the antitumor effect of chrysophanol may be related to the up-regulation of NLRP3 expression. Chrysophanol induced pyroptosis in GC cells in a dose-dependent manner and increased the expression of NLRP3 and its downstream components, including caspase-1 and IL-1β in GC cell lines after treatment. The results suggest that chrysophanol may induce pyroptosis of GC cells through the NLRP3/caspase-1 signaling pathway.
The role of chrysophanol in the prevention of GC via the NLRP3 inflammasome was examined using NLRP3 siRNA. After treatment with chrysophanol, the levels of NLRP3 and its molecules downstream of this pathway, including caspase-1, IL-1β, and IL-18 were increased in GC cells when compared to the control group. The initial stage of tumor metastasis involves the migration and invasion of tumor cells into surrounding tissues and blood vessels, and is a major reason for a low prognosis and survival (Mahauad-Fernandez et al., 2018). In one study, resveratrol was found to inhibit renal carcinoma cell invasion and migration by regulating NLRP3 (Tian et al., 2020). In this study, we found that chrysophanol inhibited the invasion and migration of GC cells by regulating NLRP3 expression. In addition, the antitumor effects of chrysophanol were partially reversed when combined with NLRP3 siRNA treatment. These results suggest that chrysophanol might play an antitumor role by up-regulating NLRP3 expression.
In conclusion, chrysophanol inhibited the growth, migration, and invasion of GC cells and induced pyroptosis. In terms of molecular mechanisms, chrysophanol plays an antitumor role through up-regulation of the NLRP3 (Fig. 9). To our knowledge, this is the first report on the relationship between chrysophanol and NLRP3 in GC cells. Chrysophanol is expected to be a potential treatment for GC, and NLRP3 may be a potential molecular target. There is still much to learn about the mechanism of chrysophanol in NLRP3.

Figure 9: Schematic illustration of this study findings. Chrysophanol treatment activated nod-like receptor protein-3 inflammasome and induced cell pyroptosis.
However, our current study has some limitations. The pleiotropy of NLRP3 in tumorigenesis and whether ’the antitumor effect of chrysophanol on GC cells is due to other factors or non-canonical pyroptosis warrant further research. In addition, this study cannot exclude the possibility that other forms of cell death, such as apoptosis and necrosis, also play an important role in the antitumor pathophysiology due to chrysophanol. Moreover, chemotherapeutic drugs have also been found to contribute to immune cell infiltration in the tumor microenvironment, thereby increasing the antitumor immunity induced by chemotherapeutic drugs through direct interaction with NLRP3 and dephosphorylation of PTEN by bone marrow cells (Huang et al., 2020). It is reasonable to assume that chrysophanol plays a role in regulating the tumor microenvironment, but future studies are needed to verify these findings.
Acknowledgement: We thank the Natural Science Research Project of Anhui Province, the Graduate Research Innovation Project of Bengbu Medical College, Outstanding University Talents Support Project and Key science and technology project of Anhui Province Fund for providing support to conduct this study.
Availability of Data and Materials:The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contribution:BFH performed experiments and generated, analyzed, and interpreted data, and drafted the manuscript. LZ performed the statistical analysis. MD conceived of the idea, designed experiments, assisted in data analyses, and critical review of the manuscript, and obtained the most of funding for the study. XDC, XQK participated in the project design, THZ, MMY, WWZ participated in the experiment operation, ZZM, LG, MW directed the modification and submission of the article. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval:This article has been approved by the Medical Ethics Committee of Bengbu Medical College. Ethics Approval No. [2019] No. 050.
Funding Statement: This study was supported by the Natural Science Research Project of Anhui Province, Grant/Award No. [2008085MH282]; Graduate Research Innovation Project of Bengbu Medical College, Grant/Award No. [Byycx20022]. Key Science and Technology Project of Anhui Province Fund, Grant/Award No. [201904a07020022]. 2020 Outstanding University Talents Support Project, Grant/Award No. [gxyq2020023].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM et al. (2016). Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIOResults from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. The Lancet Oncology 17: 1697–1708. DOI 10.1016/S1470-2045(16)30531-9. [Google Scholar] [CrossRef]
Chung IC, Ouyang CN, Yuan SN, Lin HC, Huang KY et al. (2019). Pretreatment with a heat-killed probiotic modulates the NLRP3 inflammasome and attenuates colitis-associated colorectal cancer in mice. Nutrients 11: 511–516. DOI 10.3390/nu11030516. [Google Scholar] [CrossRef]
Deng M, Xue YJ, Xu LR, Wang QW, Wei J, Ke XQ, Wang JC, Chen XD (2020). Chrysophanol exhibits inhibitory activities against colorectal cancer by targeting decorin. Cell Biochemistry and Function 38: 47–57. DOI 10.1002/cbf.3445. [Google Scholar] [CrossRef]
Du TT, Gao J, Li PL, Wang YS, Qi QC, Liu XY, Li J, Wang CX, Du LT (2021). Pyroptosis, metabolism, and tumor immune microenvironment. Clinical and Translational Medicine 11: e492–e520. DOI 10.1002/ctm2.492. [Google Scholar] [CrossRef]
Ershaid N, Sharon Y, Doron H, Raz Y, Shani O et al. (2019). NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nature Communications 10: 4375–4389. DOI 10.1038/s41467-019-12370-8. [Google Scholar] [CrossRef]
Fu L, Jiang J, Chen X, Zhu F, Zhang H (2022). Downregulation of hsa_circ_0002198 inhibits keloid fibroblast activities in vitro by reducing NLRP3 inflammasome activity. BIOCELL 46: 1289–1297. DOI 10.32604/biocell.2022.016726. [Google Scholar] [CrossRef]
Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N et al. (2009). Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459: 433–436. DOI 10.1038/nature07965. [Google Scholar] [CrossRef]
Guo C, Fu R, Wang S, Huang Y, Li X, Zhou M, Zhao J, Yang N (2018). NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clinical and Experimental Immunology 194: 231–243. DOI 10.1111/cei.13167. [Google Scholar] [CrossRef]
He HB, Jiang H, Chen Y, Ye J, Wang AL et al. (2018). Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nature Communications 9: 2550–2561. DOI 10.1038/s41467-018-04947-6. [Google Scholar] [CrossRef]
Huang Y, Wang HY, Hao YZ, Lin HL, Dong MH et al. (2020). Myeloid PTEN promotes chemotherapy-induced NLRP3-inflammasome activation and antitumour immunity. Nature Cell Biology 22: 716–727. DOI 10.1038/s41556-020-0510-3. [Google Scholar] [CrossRef]
Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang BW et al. (2020). Succination inactivates gasdermin D and blocks pyroptosis. Science 369: 1633–1637. DOI 10.1126/science.abb9818. [Google Scholar] [CrossRef]
Juliana C, Fernandes-Alnemri T, Wu JH, Datta P, Solorzano L et al. (2010). Anti-inflammatory compounds parthenolide and bay 11-7082 are direct inhibitors of the inflammasome. Journal of Biological Chemistry 285: 9792–9802. DOI 10.1074/jbc.M109.082305. [Google Scholar] [CrossRef]
Crew KD, Neugut AI (2004). Epidemiology of upper gastrointestinal malignancies. Seminars in Oncology 31: 450–464. DOI 10.1053/j.seminoncol.2004.04.021. [Google Scholar] [CrossRef]
Kuo CY, Chiu V, Hsieh PC, Huang CY, Huang SJ et al. (2020). Chrysophanol attenuates hepatitis B virus X protein-induced hepatic stellate cell fibrosis by regulating endoplasmic reticulum stress and ferroptosis. Journal of Pharmacological Sciences 144: 172–182. DOI 10.1016/j.jphs.2020.07.014. [Google Scholar] [CrossRef]
Lian YG, Xia XJ, Zhao HY, Zhu YF (2017). The potential of chrysophanol in protecting against high fat-induced cardiac injury through Nrf2-regulated anti-inflammation, anti-oxidant and anti-fibrosis in Nrf2 knockout mice. Biomedicine & Pharmacotherapy 93: 1175–1189. DOI 10.1016/j.biopha.2017.05.148. [Google Scholar] [CrossRef]
Lim W, An Y, Yang CW, Bazer FW, Song G (2018). Chrysophanol induces cell death and inhibits invasiveness via mitochondrial calcium overload in ovarian cancer cells. Journal of Cellular Biochemistry 119: 10216–10227. DOI 10.1002/jcb.27363. [Google Scholar] [CrossRef]
Lim W, Yang CW, Bazer FW, Song G (2017). Chrysophanol induces apoptosis of choriocarcinoma through regulation of ROS and the AKT and ERK1/2 pathways. Journal of Cellular Physiology 232: 331–339. DOI 10.1002/jcp.25423. [Google Scholar] [CrossRef]
Liu P, Chen, Z, Ma X (2022). Arsenic trioxide inhibits the activity of SphK1 by decreasing the level of phosphatidylserine and phosphatidic acid in the human gastric cancer cell line MGC-803. BIOCELL 46: 737–743. DOI 10.32604/biocell.2021.015786. [Google Scholar] [CrossRef]
Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R (2020). Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. International Journal of Molecular Sciences 21: 4012–4031. DOI 10.3390/ijms21114012. [Google Scholar] [CrossRef]
Mahauad-Fernandez WD, Naushad W, Panzner TD, Bashir A, Lal G, Okeoma CM (2018). BST-2 promotes survival in circulation and pulmonary metastatic seeding of breast cancer cells. Scientific Reports 8: 17608–17621. DOI 10.1038/s41598-018-35710-y. [Google Scholar] [CrossRef]
Meng CT, Bai CM, Brown TD, Hood LE, Tian Q (2018). Human gut microbiota and gastrointestinal cancer. Genomics, Proteomics & Bioinformatics 16: 33–49. DOI 10.1016/j.gpb.2017.06.002. [Google Scholar] [CrossRef]
Quagliariello V, De Laurentiis M, Cocco S, Rea G, Bonelli A et al. (2020). NLRP3 as putative marker of ipilimumab-induced cardiotoxicity in the presence of hyperglycemia in estrogen-responsive and triple-negative breast cancer cells. International Journal of Molecular Sciences 21: 7802–7823. DOI 10.3390/ijms21207802. [Google Scholar] [CrossRef]
Ren GM, Zhang XY, Xiao Y, Zhang W, Wang Y et al. (2019). ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. The EMBO journal 38: e100371–e100376. DOI 10.15252/embj.2018100376. [Google Scholar] [CrossRef]
Ren L, Li ZP, Dai CM, Zhao DY, Wang YJ, Ma CY, Liu C (2018). Chrysophanol inhibits proliferation and induces apoptosis through NF-κB/cyclin D1 and NF-κB/Bcl-2 signaling cascade in breast cancer cell lines. Molecular Medicine Reports 17: 4376–4382. DOI 10.3892/mmr.2018.8443. [Google Scholar] [CrossRef]
Ren NS, Jiang T, Wang CB, Xie SL, Xing YW, Piao DX, Zhang TM, Zhu YK (2020). LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-223-3p/NLRP3 axis. Aging 12: 11025–11041. DOI 10.18632/aging.103314. [Google Scholar] [CrossRef]
Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L et al. (2019). Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570: 338–343. DOI 10.1038/s41586-019-1295-z. [Google Scholar] [CrossRef]
Shi JJ, Gao WQ, Shao F (2017). Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends in Biochemical Sciences 42: 245–254. DOI 10.1016/j.tibs.2016.10.004. [Google Scholar] [CrossRef]
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH et al. (2020). Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. The New England Journal Medicine 382: 2419–2430. DOI 10.1056/NEJMoa2004413. [Google Scholar] [CrossRef]
Shitara K, Özgüroğlu M, Bang Y, Di Bartolomeo M, Mandalà M et al. (2018). Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061A randomised, open-label, controlled, phase 3 trial. The Lancet 392: 123–133. DOI 10.1016/S0140-6736(18)31257-1. [Google Scholar] [CrossRef]
Song ZY, Wu YY, Yang JB, Yang DQ, Fang XD (2017). Progress in the treatment of advanced gastric cancer. Tumor Biology 39: 568834234–568834240. DOI 10.1177/1010428317714626. [Google Scholar] [CrossRef]
Su SY, Wu JS, Gao Y, Luo Y, Yang D, Wang P (2020). The pharmacological properties of chrysophanol, the recent advances. Biomedicine & Pharmacotherapy 125: 110002–110014. DOI 10.1016/j.biopha.2020.110002. [Google Scholar] [CrossRef]
Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R et al. (2020). Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer. Cancers 12: 193–219. DOI 10.3390/cancers12010193. [Google Scholar] [CrossRef]
Tian XX, Zhang SZ, Zhang Q, Kang LC, Ma CZ et al. (2020). Resveratrol inhibits tumor progression by down-regulation of NLRP3 in renal cell carcinoma. The Journal of Nutritional Biochemistry 85: 108489–108499. DOI 10.1016/j.jnutbio.2020.108489. [Google Scholar] [CrossRef]
Wang WJ, Chen D, Jiang MZ, Xu B, Li XW et al. (2018). Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. Journal of Digestive Diseases 19: 74–83. DOI 10.1111/1751-2980.12576. [Google Scholar] [CrossRef]
Wang YP, Gao WQ, Shi XY, Ding JJ, Liu W, He HB, Wang K, Shao F (2017). Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547: 99–103. DOI 10.1038/nature22393. [Google Scholar] [CrossRef]
Wang YL, Kong H, Zeng XN, Liu WR, Wang ZL, Yan XP, Wang H, Xie WP (2016). Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells. Oncology Reports 35: 2053–2064. DOI 10.3892/or.2016.4569. [Google Scholar] [CrossRef]
Zhang Y, Lou YN, Wang JB, Yu CG, Shen WJ (2021). Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Frontiers in Immunology 11: 609705–609717. DOI 10.3389/fimmu.2020.609705. [Google Scholar] [CrossRef]
Zou J, Yang Y, Yang Y, Liu XR (2018). Polydatin suppresses proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via NF-κB pathway. Biomedicine & Pharmacotherapy 108: 130–136. DOI 10.1016/j.biopha.2018.09.051. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools