DOI:10.32604/biocell.2022.020635

| BIOCELL DOI:10.32604/biocell.2022.020635 |  |
| Article |
The expression and function of miR-376a-3p/DLX axis in gastric cancer cells
Department of General Surgery, The First Medical Centre, Chinese PLA General Hospital, Beijing, 100853, China
*Address correspondence to: Chaojun Zhang, zcjdlyxzx@163.com; Lin Chen, chenlinbj@sina.com
Received: 04 December 2021; Accepted: 20 January 2022
Abstract: Gastric cancer (GC) was referred to a malignant tumor of the digestive tract originating from the epithelium of gastric mucosa. Transcription factor DLX5 was verified as an oncogene in various types of tumors, while miR-376a-3p was speculated as a tumor suppressor. Based on the bioinformatics database, we hypothesized that miR-376a participated in the regulation of GC development by targeting DLX5. Compared with adjacent tissue, a significant increase of DLX5 expression was determined in GC tissues, but the expression level is significantly reduced in miR-376a. Similar expression signature of DLX5 and miR-376a was also determined between 4 GC cells (HGC, SGC, MGC, and AGS cell lines) and GES cell line. The level of DLX5 was notably reduced in HGC and MGC cell lines after miR-376a-3p overexpression, and increased after miR-376a-3p inhibition. Then, the inhibition role of miR-376a-3p on DLX5 was further proved by dual-luciferase reporter assay. Gain-of-function experiments showed that upregulation of miR-376a-3p in GC cells could inhibit the ability of epithelial-mesenchymal transition, proliferation, and invasion, and enhance the GC cell apoptosis level. However, these roles of miR-376a-3p could be abolished by DLX5 overexpression. This study confirmed that reduction of miR-376a-3p expression level in GC cells would lead to the increase in cell growth and invasion, indicating that upregulation of miR-376a-3p might have a potential therapeutic role on GC.
Keywords: Gastric cancer; DLX; miR-376a-3p
Gastric cancer (GC) defines as a malignant tumor of the digestive tract originating from the epithelium of gastric mucosa, and is characterized by high incidence, insidious onset, and prone to metastasis (Seeneevassen et al., 2021). Although the conversion surgery following chemotherapy has greatly improved the prognosis and survival, the recurrence and mortality of patients with advanced GC are still high (Biagioni et al., 2019; Necula et al., 2019). The progression of GC is a is caused by a series of cell behaviors involving proliferation, migration, and epithelial-mesenchymal transition of GC cells, which is affected by genetic and environmental factors, and multiple signal pathways (Ashrafizadeh et al., 2020). The mechanism exploration of GC development could provide new therapeutic strategies for GC.
Distal-less homeobox 5 (DLX5) is a member of homeobox transcription factor gene family related to distal-less gene of Drosophila (Tan and Testa, 2021). In humans, DLX5 is convergently transcribed and share regulatory elements and expression patterns with other DLX members (Depew et al., 2002; Proudfoot et al., 2016). It is reported that the proliferation of tumor cells was promoted by DLX5 in lymphomas and lung cancers through transcriptionally regulating MYC (Xu and Testa, 2009). A recent study revealed that DLX5 is essential for the development of squamous cell carcinomas by regulate thousands of cis-regulatory elements, and silencing of DLX5 could potently inhibit viability of squamous cell carcinomas (Huang et al., 2021). However, it is still ill-defined how DLX5 regulates the pathogenesis of GC.
Increasing evidence has proved that microRNA (miRNA) is involved in multiple cellular processes by inhibiting the expression of target genes. Disordered expression of miRNA is also closely related to the progression of various diseases. miR-376a-3p, a member of miR-376 cluster, was identified as a potential predictor of pathological response of esophageal squamous cell carcinomas to neoadjuvant chemoradiotherapy (Wen et al., 2016). A recent report manifested that restoration of miR-376a-3p expression could counteract the phenotype of bone-derived stromal cell giant cell tumor (Fellenberg et al., 2016), indicating miR-376a-3p might be a tumor suppressor miRNA. It has been identified that overexpression of miR-376a-3p could alleviate the development of glioma (Chen et al., 2020b). However, the role of miR-376a-3p in biological behavior of GC cells has not yet been discovered.
In this article, we discussed the expression profile of DLX5 and miR-376a-3p in GC cells and tissues, the mechanism by which miR-376a-3p regulated the expression of DLX5 in GC, and the inhibitory effect of miR-376a-3p on GC cell proliferation and invasion. We believed that our results would provide valuable therapeutic targets for GC treatment.
GC tissue samples and adjacent normal tissue samples were gathered from GC patients that had undergone surgery at Department of General Surgery, the 6th Medical Center of PLA General Hospital from January 2020 to March 2021, and were cryopreserved in liquid nitrogen until further experiments. The study was approved by the Medical Ethics Committee in PLA General Hospital, and performed in accordance to the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to enrollment.
HGC, SGC, MGC, and AGS cell lines and gastric mucosal cells (GES) were got from National Collection of Authenticated Cell Culture (Shanghai, China). After resuscitation, cells were passaged and grown in M199 medium containing 10% FBS (Gibco, Grand Island, USA), 100 units/mL penicillin antibiotic, and 100 µg/mL streptomycin antibiotic (Thermo Fisher Scientific, Waltham, USA).
miR-376a-3p mimic, miR-376a-3p inhibitor control were ordered from GENEWIZ (Jiangsu, China). Co-transfection of indicated constructs into cells by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Cells were collected and detected after 48 h transfection.
Total RNA was extracted from tissues or cells with RNAiso Plus reagent (Takara, Dalian, China). The extracted RNA samples were examined on a Nanodrop spectrophotometer (Thermo Fisher Scientific) for quality and quantity evaluation. The protein samples were separated from cells or tissues by using RIPA buffer plus PMSF (Beyotime, Shanghai, China). The concentration of protein samples was determined by bicinchoninic acid assay.
Dual-luciferase reporter assay
The wild-type or mutant 3’ UTR of DLX5 was synthesized and subcloned into luciferase reporter plasmid (WT-DLX5 or MUT-DLX5). The WT-DLX5 (or MUT-DLX5) plasmid, renilla plasmid, and miR-376a-3p mimic (or negative control) were co-transfected into HEK293T cells by using Lipofectamine 3000 (Thermo Fisher Scientific). Cells were harvested at 48 h after transfection. Luciferase activities were detected with dual luciferase reporter assay kit (Beyotime, Shanghai, China). The experiment was repeated for 3 times.
Quantitative real-time PCR (qRT-PCR)
Equal amounts of RNA (200 ng) were used as template to synthesize cDNA with PrimeScript RT reagent Kit (Takara). qRT-PCR assay was carried out in a Real Time System III (Takara) using SYBR Green. ACTB was set as reference gene for mRNA detection, while U6 was set for miRNA detection. The data were evaluated based on 2−ΔΔCt method.
Equal amounts (30 μg) of protein samples were electrophoresed by SDS-Polyacrylamide gel electrophoresis (10%). After transferring, the PVDF membrane was blocked by BSA solution (1%) at room temperature for 2 h. Then, the diluted primary antibodies (1:1000 dilution for anti-DLX5, Abcam, Cambridge, UK; 1:2000 dilution for anti-ACTB, Proteintech, Wuhan, China) were added, respectively, and incubated with the membrane at 4°C overnight. After washing by TBS-T buffer for 5 times, the corresponding secondary antibody was added for 1-h incubation at room temperature. The immune band was developed with ECL chemiluminescence substrate (Boster, Wuhan, China) on ChemiDoc MP system (Bio-Rad, Hercules, USA). The reference gene ACTB was set as loading control.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assay was used to detected GC cell viability. Briefly, the indicated number of GC cells in each well were reseeded in 96-well plates and maintained in complete medium overnight. At the following day, cells were transfected with indicated plasmids. 10 μL of CCK-8 solution (Beyotime, Shanghai, China) was mixed with cells in each well under 37°C incubator with 5% CO2 for 2 h. Microplate spectrophotometer (Biotec, Basel, Switzerland) was subsequently applied to test the absorbance of 450 nm per well. The derived data represented cell viability.
Invasion assay was performed with invasion chambers (Millipore, Eschborn, Germany) plus Matrigel (BD Biosciences, San Jose, USA). Briefly, the chamber inserts were coated by Matrigel (20 mg/mL), and dried overnight. The upper chambers placed serum-free M199 medium containing GC cells (5 × 103), while the lower compartments placed M199 medium plus 20% FBS. Crystal violet (0.1%) was used to stain the migrated GC cells after 48 h. Counting of the invaded cells were conducted under 5 random fields by ImageJ software.
Flow cytometry assay for apoptosis
Flow cytometry assay was used to examine the apoptosis of GC cells with an Annexin V-FITC kit (Beyotime). Annexin V-FITC and propidium iodide-stained GC cells were measured within 30 min by FACS Canto II flow cytometer (BD Biosciences).
Difference analysis of statistical data was made by SPSS version 22.0. The difference was analyzed by independent sample t-test for 2 groups, and analyzed by one-way ANOVA for multiple groups. A statistically P-value of less than 0.05 was indicated to be significant.
Negative expression correlation of miR-376a-3p and DLX5 in GC
To investigate the correlation between miR-376a-3p expression and DLX5 level in GC, the relative expression levels of miR-376a-3p and DLX5 was determined in multiple GC cell lines. Analysis of qRT-PCR assay (Fig. 1A) confirmed that the mRNA level of DLX5 in GC cell lines (HGC, SGC, MGC, and AGS) was obviously higher than that in gastric mucosal cells (GES). The elevated protein expression of DLX5 in the 4 GC cell lines (Fig. 1B) was then approved in western blot detection. In contrast, the mRNA level of miR-376a-3p in the 4 GC groups (HGC, SGC, MGC, and AGS) was obviously lower than that in the GES group (Fig. 1C). The negative expression correlation between miR-376a-3p and DLX5 was more obvious in HGC and MGC cell lines. Then, the levels of miR-376a-3p and DLX5 were determined in tissues from normal and GC groups (n = 20). Compared with the normal group (Fig. 1F), the expression levels of DLX5 mRNA and protein were notably increased in tumor group (Figs. 1D and 1E), accompanied by the decreased expression level of miR-376a-3p in tumor group.
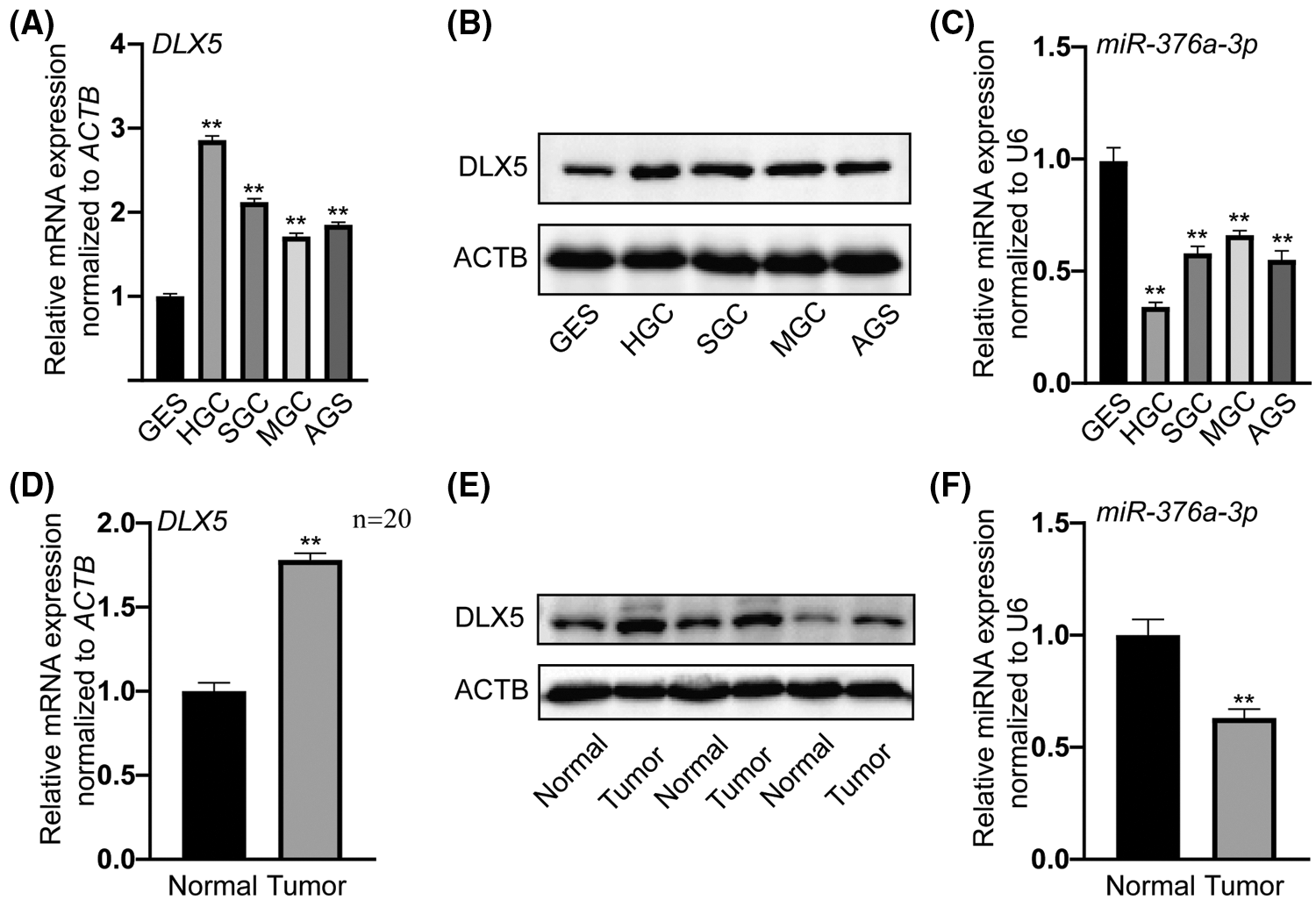
Figure 1: Negative expression correlation of miR-376a-3p and DLX5 in GC, all the cells were seeded in a 12-well plate and harvested after 2-day cultivation. A, qRT-PCR analysis of DLX5 between 4 GC cell lines (HGC, SGC, MGC, and AGS) and GES cell line. **P < 0.01. B, Western blot results of DLX5 protein between GC cell lines and GES cell line. ACTB was the loading control. C, qRT-PCR detection of miR-376a-3p between GC and GES cell lines. **P < 0.01. D, qRT-PCR analysis of DLX5 in tissues from normal and tumor groups. **P < 0.01. E, Western blot results of DLX5 protein in tissues from normal and tumor groups. ACTB was the loading control. F, qRT-PCR analysis of miR-376a-3p in tissues from normal and tumor groups. **P < 0.01.
The inhibitory effect of miR-376a-3p in DLX5 expression
To clarify whether DLX5 was a direct target of miR-376a-3p in GC, the gain-of function experiment was firstly performed in HGC and MGC cell lines. Analysis of qRT-PCR assay confirmed the elevated expression level of miR-376a-3p in HGC and MGC cell lines after transfection of miR-376a-3p mimic (Fig. 2A). Analysis of western blot assay demonstrated that the expression level of DLX5 protein was significantly decreased in HGC and MGC cell lines overexpressing miR-376a-3p (Fig. 2B). Then, loss-of-function experiment confirmed that the protein expression of DLX5 were significantly increased in HGC and MGC cell lines with miR-376a-3p inhibition (Figs. 2C and 2D). Subsequently, one miR-376a-3p binding site with high affinity was identified in the 3’ untranslated region (3’UTR) of DLX5 mRNA through bioinformatics algorithm analysis. Results of dual-luciferase reporter assay (Fig. 2E) confirmed that the was remarkably reduced after transfection of miR-376a-3p mimic in wild-type DLX5 (WT-DLX5) group. However, the luciferase activity did not notably change in the mutant (MUT-DLX5) groups with or without miR-376a-3p overexpression.
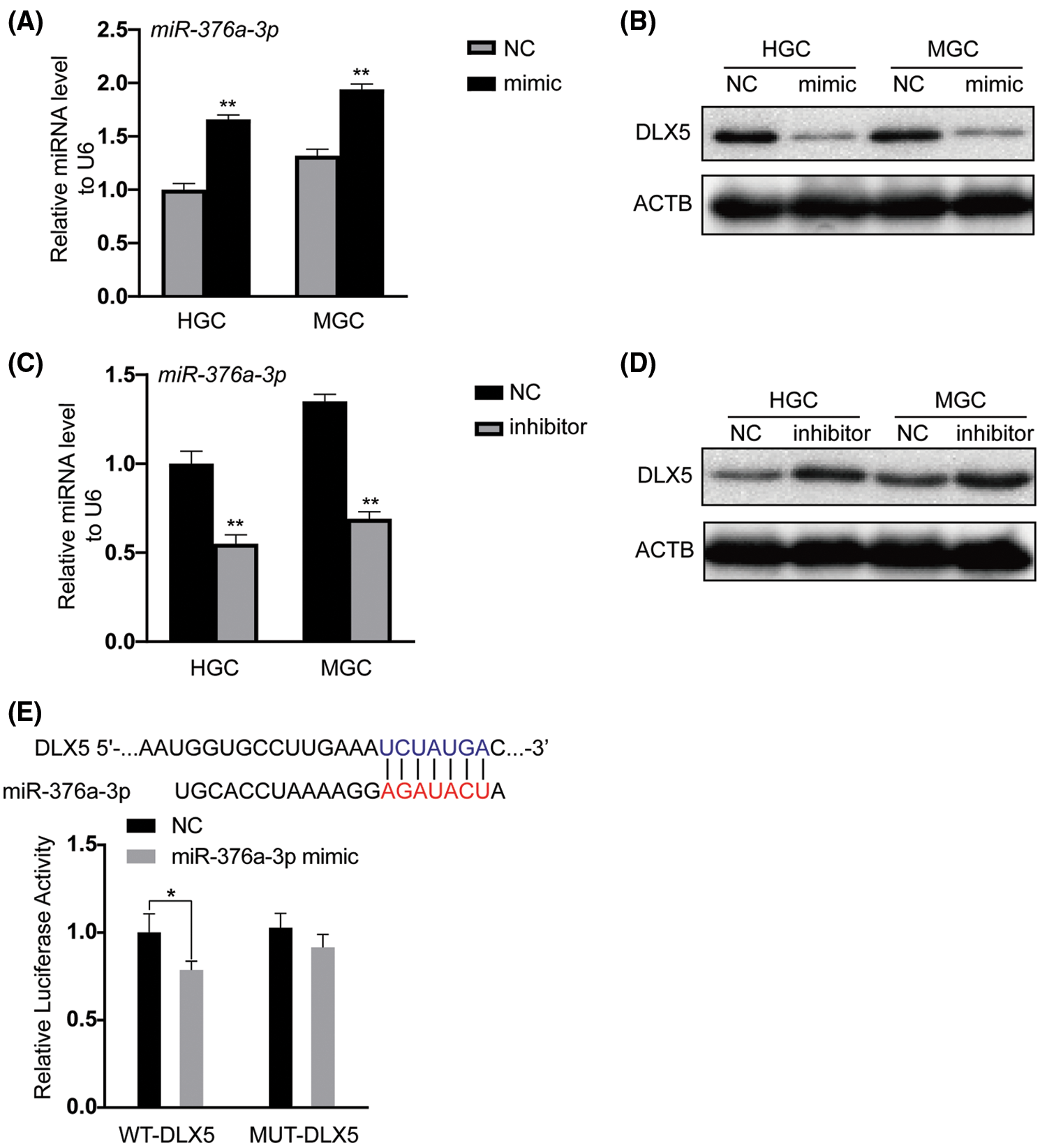
Figure 2: The inhibitory role of miR-376a-3p in DLX5 expression. A, qRT-PCR analysis of miR-376a-3p in HGC and MGC cell lines after mimic transfection. **P < 0.01. B, Analysis of DLX5 protein expression in HGC and MGC cell lines after transfection of mimic through western blot detection. ACTB was the loading control. C, qRT-PCR results of miR-376a-3p in HGC and MGC cell lines after transfection of inhibitor. **P < 0.01. D, Expression of DLX5 protein in HGC and MGC cell lines after transfection of inhibitor. ACTB was the loading control. E, Dual luciferase verification of the direct action between miR-376a-3p and DLX5. *P < 0.05.
The effect of miR-376a-3p/DLX5 in EMT and proliferation of GC cells
Epithelial to mesenchymal transition (EMT) is well-known as an important process during the development of various tumors. Result of western blot assay (Fig. 3A) showed that the expression of mesenchymal markers (claudin-1, vimentin, and N-cadherin) was down-regulated in HGC and MGC cell lines transfected with miR-376a-3p mimic, while the level of epithelial marker (E-cadherin) was up-regulated. However, overexpression of DLX5 could abolish the inhibitory role of miR-376a-3p in GC cell EMT, demonstrated by the decreased expression of mesenchymal markers and the increased expression of E-cadherin. Then, CCK-8 assay was used to confirm the effect of miR-376a-3p/DLX5 in GC cell growth. Both in HGC and MGC cell lines, the growth vitality of GC cells was significantly reduced in miR-376a-3p overexpression group, but was obviously alleviated in miR-376a-3p/DLX5 co-overexpression group (Figs. 3B and 3C).
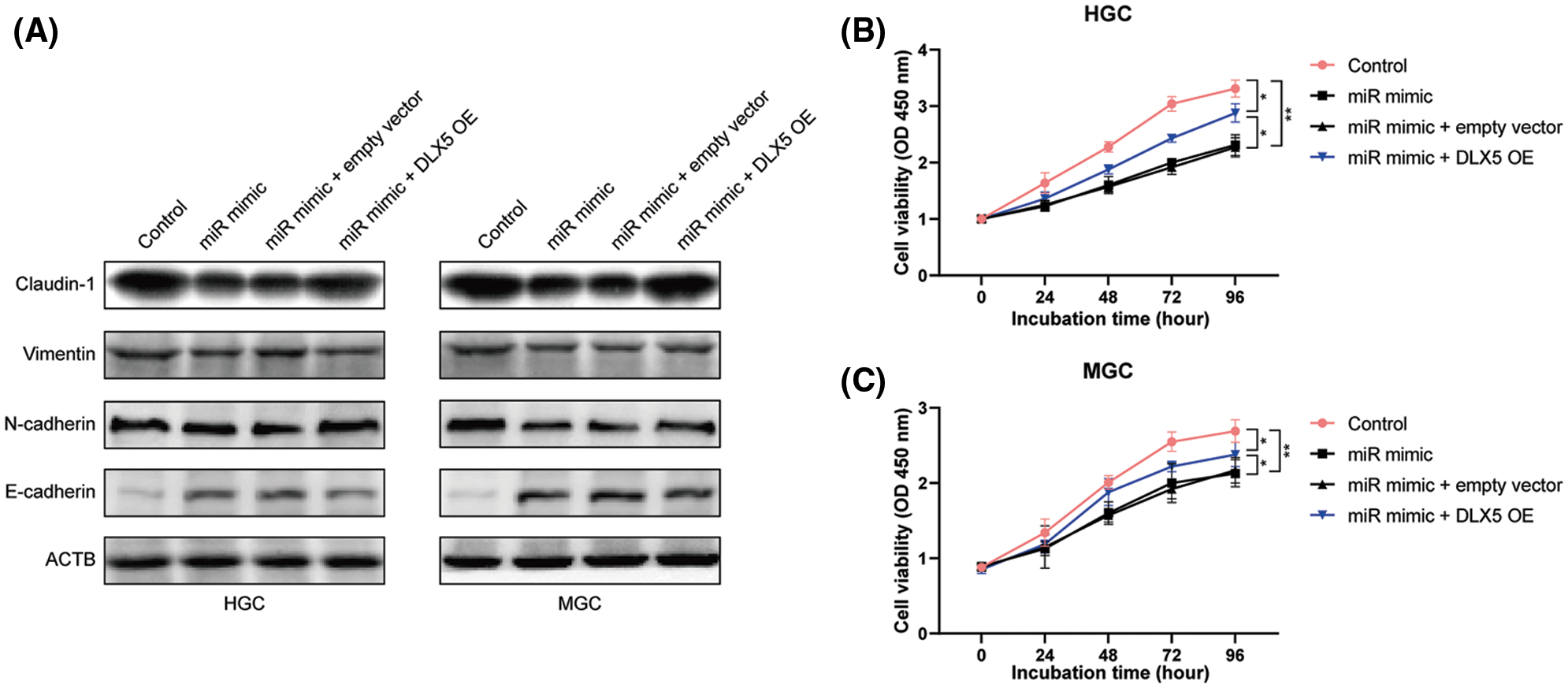
Figure 3: The effect of miR-376a-3p/DLX5 in EMT and proliferation of GC cells. A, Western blot results of EMT markers in HGC and MGC cell lines after overexpression of miR-376a and/or DLX5. B–C, CCK-8 results of HGC and MGC cell lines after overexpression of miR-376a and/or DLX5.
The inhibitory effect of miR-376a-3p/DLX5 in GC cell invasion
Trans-well invasion assay was implemented to examine the roles of miR-376a-3p/DLX5 in GC cell invasion. Compared with control group in HGC cell line, the number of transmembrane cells was significantly reduced in miR-376a-3p overexpression group. The anti-invasive effect of miR-376a-3p in GC cells could be partially offset by overexpression of DLX5. The number of transmembrane cells in miR-376a-3p/DLX5 co-overexpression group was significantly more than that in miR-376a-3p overexpression group (Figs. 4A and 4B). Although the invasion ability of MGC cell line was weaker than that of HGC cell line, the roles of miR-376a-3p/DLX5 in invasion of GC cells could be also tested and verified in MGC cell line (Figs. 4A and 4B).
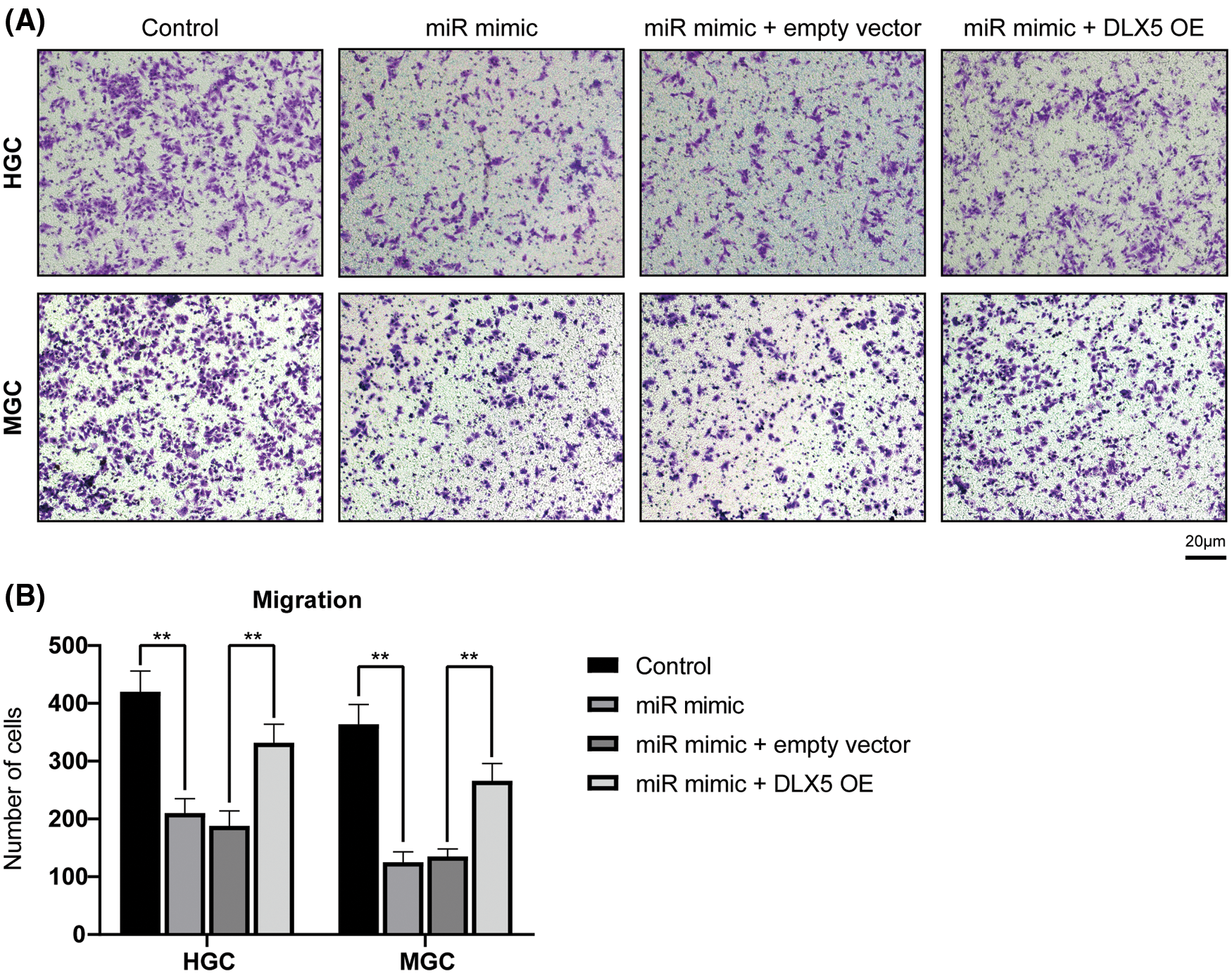
Figure 4: The functions of miR-376a-3p/DLX5 in GC cell invasion. A, Trans-well invasion results of HGC and MGC cell lines after overexpression of miR-376a and/or DLX5. Scar bar: 20 um. B, Counting results of invaded cells in GC cells after overexpression of miR-376a and/or DLX5. Upregulation of DLX5 could abolish the anti-invasive role of miR-376a-3p on GC cells. **P < 0.01.
The promotion effect of miR-376a-3p/DLX5 in GC cell apoptosis ability
The roles of miR-376a-3p/DLX5 in apoptosis level of GC cells was investigated through flow cytometry detection. The results confirmed the promotion effect of miR-376a-3p overexpression in GC cell apoptosis. The apoptotic rates of GC cells was significantly elevated after miR-376a-3p overexpression both in HGC cell line and MGC cell lines. The pro-apoptotic effect of miR-376a-3p could be partially abolished by DLX5 overexpression. The apoptosis rate of GC cells in the miR-376a-3p/DLX5 co-overexpression group was notably less than that in the miR-376a-3p-sole-overexpression group (Figs. 5A and 5B).
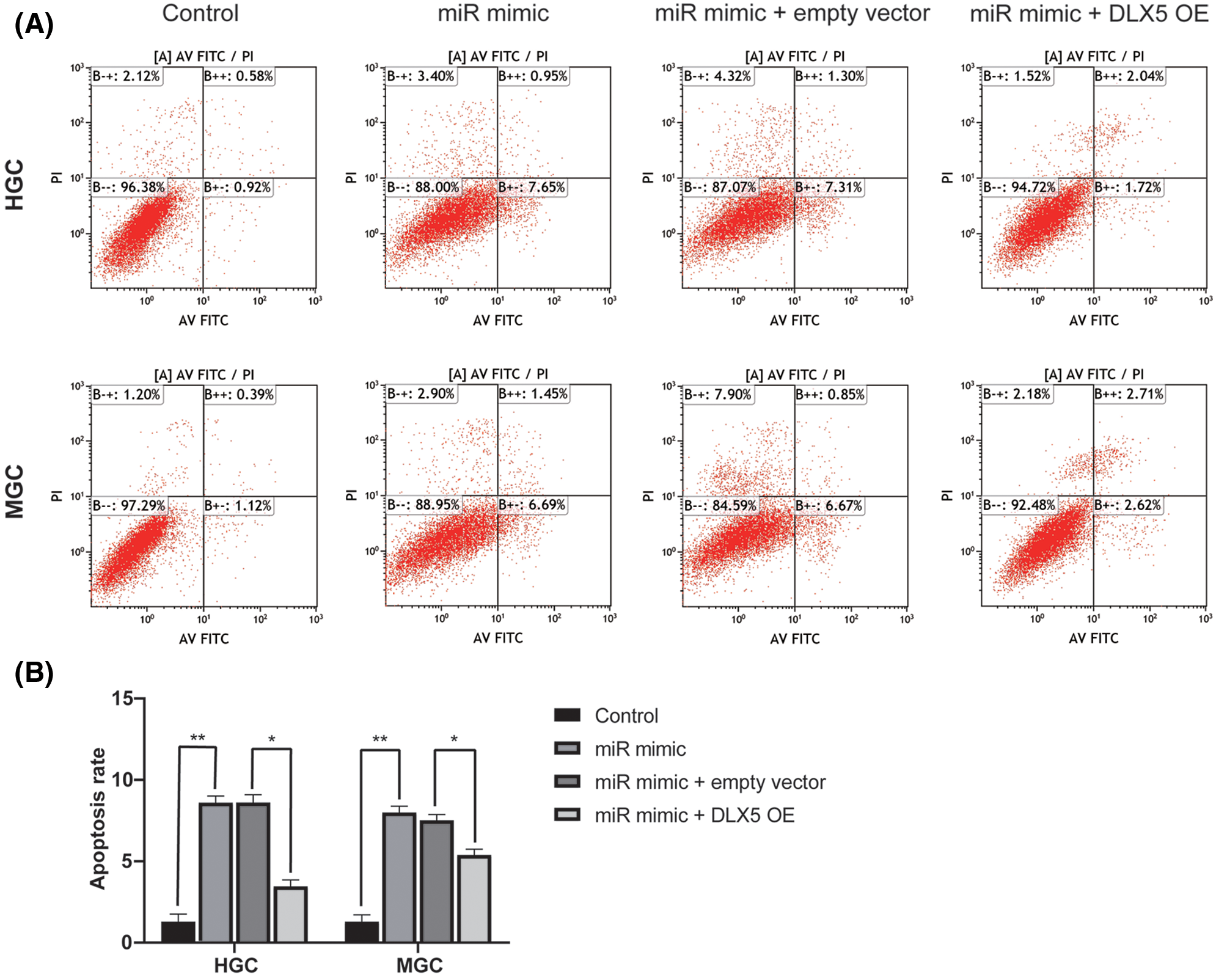
Figure 5: The roles of miR-376a-3p/DLX5 in GC cell apoptosis. A, Flow cytometry results of HGC and MGC cell apoptosis after overexpression of miR-376a and/or DLX5. B, Analysis results of the apoptotic proportion in HGC and MGC cell after overexpression of miR-376a and/or DLX5. *P < 0.05, **P < 0.01.
In the present study, the expression levels of miR-376a-3p and DLX5 were determined in GC tissues and cell lines. DLX5 was proved to be a novel target of miR-376a-3p in GC. Increased levels of miR-376a-3p could reduce the ability to proliferate and invade of GC cells by targeting DLX5. The apoptosis level of GC cells could be increased after miR-376a-3p overexpression. The present result suggested that upregulation of miR-376a-3p in GC patients might have a potential therapeutic role.
Increasing evidence has linked miRNA expression profile with the development of diseases, and some miRNAs have been proved as predictive markers of tumor staging or prognosis (Rajasegaran et al., 2021). The abnormal miRNA expression levels are tightly correlated to the pathogenesis, chemotherapy sensitivity, and prognosis of GC (Ouyang et al., 2021). Recently, a 4-miRNA expression signature (miR-200b, miR-103a, miR-199, and miR-152) was identified as a predictor for GC chemoresistance, which outperformed those clinicopathological factors (Cheng et al., 2021). A novel signature composed of 8 genome instability-related miRNAs was also constructed to stratify GC patients into distinct risk groups with different survival outcomes and immunotherapy efficacy (Liu et al., 2021). In this study, the reduced expression of miR-376a-3p was identified both in GC cell lines and tissues, indicating that miR-376a-3p might be a potential supplement to the current miRNA expression signature for GC diagnosis, treatment, or prognosis.
The etiological mechanism leading to GC has not been fully clarified. Recent reports have confirmed that a range of miRNAs are participated in regulation of GC development (Ouyang et al., 2021). Recently, miR-25, widely expressed in multiple tumors, was identified as a regulator of the growth and apoptosis of GC cells (Yang et al., 2021). Defect of miR-497 could contribute to tumorigenesis and progression of GC, and overexpression of miR-497 might be a promising strategy in preventing the development of GC (Zhang et al., 2021a). Furthermore, miR-199b-5p and miR-486-5p were also proved to function in the repression of GC tumorigenesis and progression (Chen et al., 2021; Dai et al., 2021). Chen et al. (2020a) found that miR-217 had a low level expression in gastric tumor tissues of 40 patients with GC, and a lower expression in the gastric tumor tissues of the patients with GC metastasis. Moreover, miR-217 markedly suppressed the metastasis and invasion of gastric cancer cell line in vitro. Furthermore, miR-217 inhibited the expression of PTPN14 by directly targeting its 3’UTR. Moreover, the down-regulation of PTPN14 reduced the metastasis and invasion, whereas up-regulation of PTPN14 led to the enhanced metastases and invasion of GC cells. Our results revealed the regulatory role of miR-376a-3p in inhibiting GC cell growth and apoptosis, indicating that overexpression of miR-376a-3p might be a feasible strategy for GC treatment.
As a member of DLX gene family, transcription factor DLX5 has been verified as an oncogene in a variety of tumors, including squamous cell carcinoma or osteosarcoma (Huang et al., 2021; Zhang et al., 2021b). Transcriptional activation of DLX5 could regulate thousands of enhancers and promoters, which converge on activating cancer-promoting pathways (Tan and Testa, 2021). MiRNAs targeting DLX5 have also investigated in various pathological processes. miR-141 and miR-200a were found to modulate pre-osteoblast differentiation through the translational repression of DLX5 (Itoh et al., 2009). miR-214 was also found to inhibit myogenic differentiation of mesenchymal stem cells via targeting DLX5 (Qadir et al., 2014). However, the function and mechanism of miRNA-DLX5 on tumorigenesis and progression have not been completely investigated. The present study found that miR-376a-3p could reduce the expression level of DLX5 in GC cells, and inhibit the ability of EMT, proliferation, and invasion. The results have broadened the regulatory network of DLX5 in GC. Additionally, the abnormally expressed DLX2 has been reported in various human hematological malignancies and solid tumors, including acute lymphoblastic leukemia, acute myeloid leukemia, etc. (Morini et al., 2010; Sunwoo et al., 2008; Yilmaz et al., 2011). A recent study showed that DlX2 was expressed in transforming growth factor β (TGF-β) and it plays a key role in transforming its tumor suppressive function into tumor promoting function (Sunwoo et al., 2008). In addition, abnormal TGF-β Expression is involved in tumor progression, metastasis, angiogenesis and poor survival of GC. Tang et al. (2013) demonstrated that increased expression of DLX2 may correlate with the advanced stage of gastric adenocarcinoma, and it may contribute to tumor development.
Overall, our findings identified the role of miR-376a-3p as a suppressor of GC, which was mediated through DLX5 expression inhibiting. This study highlighted the post-transcriptional regulation of miR376a-3p on DLX5 expression in GC cells, and provided novel molecular mechanisms on GC development.
Availability of Data and Materials: All the data could be accessed via reasonable request to the corresponding author.
Author Contribution: Conceptualization, supervision, project administration, writing–review & editing: Lin Chen; formal analysis, investigation, writing–original draft, validation: Yan Zhang; supervision, project administration, funding acquisition: Chao-jun Zhang; methodology, software: Zhen Cao; methodology, resources: Zhan-wei Zhao. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: The study was approved by the Ethics Committee of Chinese PLA General Hospital (No. 2020054; date: December 30, 2019).
Funding Statement: This work was supported by the Nature Science Foundation of China (81972320).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ashrafizadeh M, Zarrabi A, Orouei S, Zarrin V, Rahmani Moghadam E et al. (2020). STAT3 pathway in gastric cancer: Signaling, therapeutic targeting and future prospects. Biology 9: 126. DOI 10.3390/biology9060126. [Google Scholar] [CrossRef]
Biagioni A, Skalamera I, Peri S, Schiavone N, Cianchi F et al. (2019). Update on gastric cancer treatments and gene therapies. Cancer and Metastasis Reviews 38: 537–548. DOI 10.1007/s10555-019-09803-7. [Google Scholar] [CrossRef]
Chen G, Yang Z, Feng M, Wang Z (2020a). microRNA-217 suppressed epithelial-to-mesenchymal transition through targeting PTPN14 in gastric cancer. Bioscience Reports 40: BSR20193176. DOI 10.1042/BSR20193176. [Google Scholar] [CrossRef]
Chen L, Jing SY, Liu N, Han S, Yang YK et al. (2020b). MiR-376a-3p alleviates the development of glioma through negatively regulating KLF15. European Review for Medical and Pharmacological Sciences 24: 11666–11674. [Google Scholar]
Chen S, Wu H, Zhu L, Jiang M, Wei S et al. (2021). MiR-199b-5p promotes gastric cancer progression by regulating HHIP expression. Frontiers in Oncology 11: 728393. DOI 10.3389/fonc.2021.728393. [Google Scholar] [CrossRef]
Cheng X, Wei H, Zhang S, Zhang F (2021). Predictive and prognostic value of an microRNA signature for gastric carcinoma undergoing adjuvant chemotherapy. DNA and Cell Biology 40: 1428–1444. DOI 10.1089/dna.2021.0377. [Google Scholar] [CrossRef]
Dai ZT, Xiang Y, Zhang XY, Zong QB, Wu QF et al. (2021). Regulation of follistatin-like 3 expression by miR-486-5p modulates gastric cancer cell proliferation, migration and tumor progression. Aging 13: 20302–20318. DOI 10.18632/aging.203412. [Google Scholar] [CrossRef]
Depew MJ, Lufkin T, Rubenstein JL (2002). Specification of jaw subdivisions by Dlx genes. Science 298: 381–385. DOI 10.1126/science.1075703. [Google Scholar] [CrossRef]
Fellenberg J, Sahr H, Kunz P, Zhao Z, Liu L et al. (2016). Restoration of miR-127-3p and miR-376a-3p counteracts the neoplastic phenotype of giant cell tumor of bone derived stromal cells by targeting COA1, GLE1 and PDIA6. Cancer Letters 371: 134–141. DOI 10.1016/j.canlet.2015.10.039. [Google Scholar] [CrossRef]
Huang Y, Yang Q, Zheng Y, Lin L, Xu X et al. (2021). Activation of bivalent factor DLX5 cooperates with master regulator TP63 to promote squamous cell carcinoma. Nucleic Acids Research 49: 9246–9263. DOI 10.1093/nar/gkab679. [Google Scholar] [CrossRef]
Itoh T, Nozawa Y, Akao Y (2009). MicroRNA-141 and -200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. Journal of Biological Chemistry 284: 19272–19279. DOI 10.1074/jbc.M109.014001. [Google Scholar] [CrossRef]
Liu Y, Cheng L, Huang W, Cheng X, Peng W et al. (2021). Genome instability-related miRNAs predict survival, immune landscape, and immunotherapy responses in gastric cancer. Journal of Immunology Research 2021: 2048833. DOI 10.1155/2021/2048833. [Google Scholar] [CrossRef]
Morini M, Astigiano S, Gitton Y, Emionite L, Mirisola V et al. (2010). Mutually exclusive expression of DLX2 and DLX5/6 is associated with the metastatic potential of the human breast cancer cell line MDA-MB-231. BMC Cancer 10: 649. DOI 10.1186/1471-2407-10-649. [Google Scholar] [CrossRef]
Necula L, Matei L, Dragu D, Neagu AI, Mambet C et al. (2019). Recent advances in gastric cancer early diagnosis. World Journal of Gastroenterology 25: 2029–2044. DOI 10.3748/wjg.v25.i17.2029. [Google Scholar] [CrossRef]
Ouyang J, Xie Z, Lei X, Tang G, Gan R et al. (2021). Clinical crosstalk between microRNAs and gastric cancer (Review). International Journal of Oncology 58: 7. DOI 10.3892/ijo.2021.5187. [Google Scholar] [CrossRef]
Proudfoot A, Axelrod HL, Geralt M, Fletterick RJ, Yumoto F et al. (2016). Dlx5 homeodomain: DNA complex: Structure, binding and effect of mutations related to split hand and foot malformation syndrome. Journal of Molecular Biology 428: 1130–1141. DOI 10.1016/j.jmb.2016.01.023. [Google Scholar] [CrossRef]
Qadir AS, Woo KM, Ryoo HM, Yi T, Song SU et al. (2014). MiR-124 inhibits myogenic differentiation of mesenchymal stem cells via targeting Dlx5. Journal of Cellular Biochemistry 115: 1572–1581. DOI 10.1002/jcb.24821. [Google Scholar] [CrossRef]
Rajasegaran Y, Azlan A, Rosli AA, Yik MY, Kang Zi K et al. (2021). Footprints of microRNAs in cancer biology. Biomedicines 9: 1494. DOI 10.3390/biomedicines9101494. [Google Scholar] [CrossRef]
Seeneevassen L, Bessede E, Megraud F, Lehours P, Dubus P et al. (2021). Gastric cancer: Advances in carcinogenesis research and new therapeutic strategies. International Journal of Molecular Sciences 22: 3418. DOI 10.3390/ijms22073418. [Google Scholar] [CrossRef]
Sunwoo JB, Kim S, Yang L, Naik T, Higuchi DA et al. (2008). Distal-less homeobox transcription factors regulate development and maturation of natural killer cells. PNAS 105: 10877–10882. DOI 10.1073/pnas.0805205105. [Google Scholar] [CrossRef]
Tan Y, Testa JR (2021). DLX genes: Roles in development and cancer. Cancers 13: 3005. DOI 10.3390/cancers13123005. [Google Scholar] [CrossRef]
Tang P, Huang H, Chang J, Zhao GF, Lu ML et al. (2013). Increased expression of DLX2 correlates with advanced stage of gastric adenocarcinoma. World Journal of Gastroenterology 19: 2697–2703. DOI 10.3748/wjg.v19.i17.2697. [Google Scholar] [CrossRef]
Wen J, Luo K, Liu H, Liu S, Lin G et al. (2016). MiRNA expression analysis of pretreatment biopsies predicts the pathological response of esophageal squamous cell carcinomas to neoadjuvant chemoradiotherapy. Annals of Surgery 263: 942–948. DOI 10.1097/SLA.0000000000001489. [Google Scholar] [CrossRef]
Xu J, Testa JR (2009). DLX5 (distal-less homeobox 5) promotes tumor cell proliferation by transcriptionally regulating MYC. Journal of Biological Chemistry 284: 20593–20601. DOI 10.1074/jbc.M109.021477. [Google Scholar] [CrossRef]
Yang L, Li L, Chang P, Wei M, Chen J et al. (2021). miR-25 regulates gastric cancer cell growth and apoptosis by targeting EGR2. Frontiers in Genetics 12: 690196. DOI 10.3389/fgene.2021.690196. [Google Scholar] [CrossRef]
Yilmaz M, Maass D, Tiwari N, Waldmeier L, Schmidt P et al. (2011). Transcription factor Dlx2 protects from TGFbeta-induced cell-cycle arrest and apoptosis. EMBO Journal 30: 4489–4499. DOI 10.1038/emboj.2011.319. [Google Scholar] [CrossRef]
Zhang L, Yao L, Zhou W, Tian J, Ruan B et al. (2021a). miR-497 defect contributes to gastric cancer tumorigenesis and progression via regulating CDC42/ITGB1/FAK/PXN/AKT signaling. Molecular Therapy-Nucleic Acids 25: 567–577. DOI 10.1016/j.omtn.2021.07.025. [Google Scholar] [CrossRef]
Zhang X, Bian H, Wei W, Wang Q, Chen J et al. (2021b). DLX5 promotes osteosarcoma progression via activation of the NOTCH signaling pathway. American Journal of Cancer Research 11: 3354–3374. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |