DOI:10.32604/biocell.2022.020500

| BIOCELL DOI:10.32604/biocell.2022.020500 |  |
| Article |
Identification of a new Hazelnut disease in Liaoning Province: Hazelnut husk brown rot
1Liaoning Institute of Economic Forestry, Dalian, 116031, China
2Shenyang Agricultural University, Shenyang, 110866, China
3Southwest University, Chongqing, 400700, China
*Address correspondence to: Jun Sun, sunjun3200@163.com; Lijing Chen, chenlijing1997@126.com
Received: 29 November 2021; Accepted: 27 January 2022
Abstract: Hazelnut husk brown rot has been identified as a new disease in Liaoning Province in recent years. The objective of this study as to identify the pathogen. [Method] In this study, a standard sample of hazelnut husk brown rot was collected from Songmudao Base in Dalian City, Liaoning Province. The pathogen was identified by the studies of the morphology, pathogenicity, and analyses of ITS and LSU sequences. The pathogen was isolated and purified, which was confirmed by Koch’s postulates. The symptoms after inoculation were the same as those collected directly from a diseased tree, which showed that it was the pathogenic fungus. The cultural characteristics and conidia and the morphology of the pathogenic fungi were similar to those of Botrytis cinerea’s. The ITS sequences and LSU sequences were compared to the associated strain sequences in GenBank, with 100% identity to Botrytis cinerea (GenBank accession number: MN589848.1) and Botrytis cinerea (GenBank accession number: KU140653.1), respectively. The infection status of the pathogen on the hazelnut husks was also observed. The studies suggested that the pathogen leading to the hazelnut husk brown rot as a new disease in Liaoning Province was Botrytis cinerea.
Keywords: Hazelnut husk brown rot; Pathogen identification; Botrytis cinerea; Infection process
Hazel (Corylus heterophylla Fisch.) is a woody plant of the genus Corylus, also known as hazel, hazelnut and filberts. Hazelnut is a nut, which is red brown or golden brown, with colored stripes (Li, 2011). Hazelnuts are widely distributed in Northeast and North China and Shanxi, Gansu and other places. Hazelnuts are highly nutritious, widely used, and highly valuable (with the production of 3000 kg per hectare and the output value of RMB 90000 yuan per hectare); they are processed into a variety of chocolates, candies, ice cream, hazelnut powder and hazelnut milk senior nutrition as important raw materials (Jiang et al., 2005). Hazelnuts are anti-inflammatory, can act against cardiovascular disease and enhance sexual function, body care, longevity and limit the effects of aging (Chen, 2004).
China is one of the centers of origin of hazelnuts. Hazelnut is rich in plant resources, and Chinese citizens have a long history of eating these prized nuts. They are grown on approximately 1.3 million hm2. There is approximately 1.3 million hm2 Ping hazelnut (C. heterophylla) in China. Liaoning Province is the original research and development site of Ping’ou hybrid hazelnuts. Since 2010, the Ping’ou hybrid hazelnut industry has entered a period of rapid development. Now the national area cultivated is approximately 100,000 hm2, and the area cultivated in Liaoning Province is approximately 40,000 hm2 (Xie and Wang, 2019).
With the nutritional and medicinal value of hazelnuts gradually gaining attention, the area planted to this crop has undergone a rapid increase. Unfortunately, this growth has been followed by a serious occurrence of disease. In view of this, this study reported and systematically identified the new hazelnut husk brown rot disease in Liaoning Province to provide a further theoretical basis for the cultivation and management, in-depth research and prevention of this disease.
The study found that the Hazelnut husk brown rot has occurred seriously in Northeast China in recent years. It has been identified in Jinzhou County, Dalian City, Heishan County, Jinzhou City and Huanren County, Benxi City, and it has spread rapidly. In severe cases, 50%–60% of the husks of the whole tree are browned and decayed, which seriously affects the yield.
Few official reports have been made. Sezer et al. (Sezer and Dolar, 2012; 2015; 2017) have been engaged in hazelnut disease research in Turkey for many years. B. cinerea, Pestalotiopsis sp. and Colletotrichum fioriniae were detached from hazelnut clusters, leaves and twigs as the pathogenic fungus in several regions of Turkey from 2008 to 2010. But Hazelnut husk brown rot has not been reported in China. In view of this, the author identified the new disease of hazelnut, hazelnut husk brown rot.
The Hazelnut husk brown rot samples were collected from Songmudao Base (121°75'E,39°40'N) in Dalian City, Liaoning Province, China during July 2019 to July 2020. The hazel tree species was Ping’ou hybrid hazelnut “Yuzhui” in five orchards. The characteristics of the symptoms of the disease was recorded and followed up in the field.
Observation of the morphological characteristics of the pathogen: The pathogen was isolated and purified by conventional isolation methods (Fang, 1998). The diseased husks with obvious spots were taken directly as fresh samples. Next, a typical single diseased spot was selected, and the ill/healthy junctions were cut into 3–5 mm tissue pieces with sterile scissors or a scalpel. The pathogens were grown on potato dextrose agar (PDA) plates at 25°C for 5 days, and colony and conidial morphology were observed and measured after purification under an optical microscope.
Koch’s postulated to identify the disease
Following Koch’s rule, five-year-old growing hazelnut plants in the orchard were selected, and 20 hazelnut husks were inoculated with fungal plates. Ten stitches with a sterilized needle were made on the hazelnut husks. Plates that were 7 mm across were rubbed with a sterile spreader. The mycelial surfaces were covered toward where the hazelnut husks were pricked and incubated under humid conditions for 36 h. The pathogenic results were observed. A conventional tissue isolation method was used to separate the pathogen, and the pathogen was observed under a microscope after cultivation for identification.
Molecular identification by ITS and LSU gene sequencing
Denaturation: The mycelia in the PDA media were picked and denatured in a 50 μL kit (TaKaRa Lysis Buffer for Microorganism to Direct PCR, Code No. 9164, Dalian, China), and the supernatant was centrifuged to obtain the template. PCR amplification: The target fragment was amplified using a kit (TaKaRa Fungi Identification PCR Kit, Code No. RR178). The ITS amplified primers were ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC). The LSU amplified primers were LR0R (5’-ACCCGCTGAACTTAAGC-3’) and LR7 (5’-TACTACCACCAAGATCT-3’). The PCR product was purified using TaKaRa MiniBEST Agarose Gel DNA Extraction Kit v. 4.0 (Code No. 9762) cutting edge fragments for recovery. The DNA was also sequenced, and the results were aligned with those of the NCBI. These were completed by Takara Biotechnology Company.
SEM observation of the pathogen infection process
The harvested tissue was blocked and fixed: Targeted fresh tissues should be selected to minimize mechanical damage, such as pulling, contusion and extrusion. A sharp blade was used to cut and quickly harvest fresh tissue blocks within 1–3 min. The area of tissue block should be no more than 3 mm2. The washed tissue blocks were immediately fixed by electron microscopy fixative for 2 h at room temperature, and then transferred to 4°C for preservation and transportation. Post-fix: The tissue blocks were washed with 0.1 M phosphate buffer (PB) (pH 7.4) three times, 15 min each. The tissue blocks were then transferred into 1% OsO4 in 0.1 M PB (pH 7.4) for 1–2 h at room temperature. After that, the tissue blocks were washed in 0.1 M PB (pH 7.4) three times, 15 min each. The samples were dehydrated as follows: 30% ethanol for 15 min; 50% ethanol for 15 min; 70% ethanol for 15 min; 80% ethanol for 15 min; 90% ethanol for 15 min; 95% ethanol for 15 min; and two changes of 100% ethanol for 15 min. Finally, the blocks were treated with isoamyl acetate for 15 min. Drying: The samples were dried with a Critical Point Dryer. Conductive metal coating: The specimens were attached to metallic stubs using carbon stickers and sputter-coated with gold for 30 s. The specimens were observed and photographed with a scanning electron microscope.
Disease symptoms and pathogen morphology
The pathogen primarily harmed the husks of hazelnut trees, causing husk browning. High humidity caused many husks to turn brown and rot, seriously affecting hazelnut production. Hazelnut husk brown rot occurred on mature husks in early July. As the husks gradually developed, the pathogen first infected the middle of the husk. At first, they formed light brown oval disease spots. As the husks gradually grew larger, disease spots also gradually expanded to the edge of husks. The color of the diseased spots deepened, and the color on the edge of the spots became deeper than the center of the spots, which became dark brown. The disease spots gradually expanded into pieces. Subsequently, the pathogen gradually infected the nutshells, thus, hindering normal kernel growth and affecting the production of hazelnuts during July to August. When the humidity was high to 75%, a gray-black mildew layer occurred on the husks. When the conditions were suitable, the leaves around the sick husks were also affected, and the leaves became browned and withered.
The pathogens on PDA medium produced mycelia that were grey and white in the early stage and grey and flocculant after old ripening (Fig. 1). Sporophores were produced from hyphelia. The conidiophores were 280–550 × 12–24 μm, conidia oval, subspherical, 9–15 × 6.5–10 μm (Figs. 2A and 2B). The sexual spores were spherical, colorless, and approximately 3 μm in diameter. The nuclei were produced on the colonies after approximately 30 d of culture, which were black with an irregular shape, and 4~10 × 0.1~5 mm.
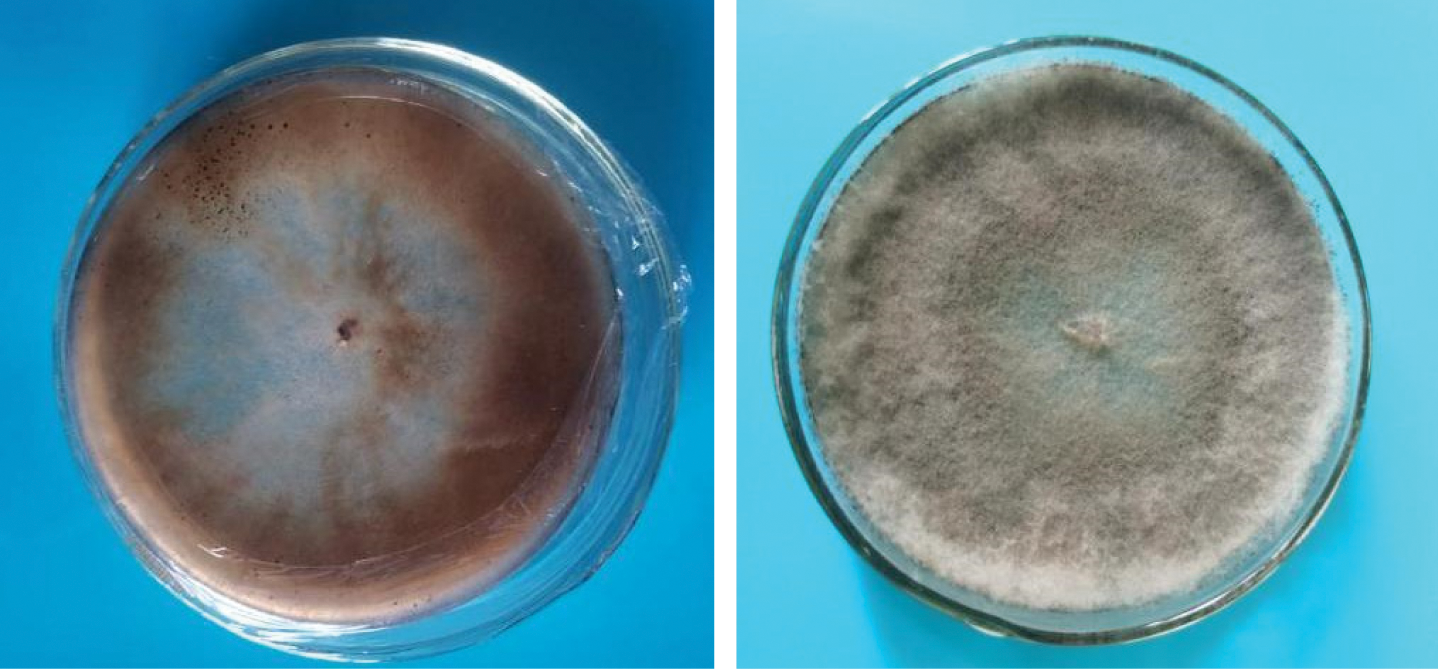
Figure 1: Culture characters of pathogenic fungus on PDA media.

Figure 2: Pathogenic fungal morphology. a. Conidiophore b. Conidia.
Compared with the symptoms under natural conditions, the color of the artificially inoculated hazelnut husks was slightly darker, and the speed of onset and plaque expansion took place more quickly. After inoculation, the symptoms were consistent with those under natural conditions in the field (Figs. 3A and 3B), and the pathogens obtained by further isolation were the same as the original pathogen.

Figure 3: The symptoms of Hazelnut Husk Brown Rot. a. Field disease symptoms of Hazelnut Husk Brown Rot; b. Typical symptom of Hazelnut Husk Brown Rot inoculated with the pathogen.
Molecular identification by ITS gene sequencing
PCR amplification was performed for the measured strains, and electrophoresis revealed fragments of approximately 490 bp in size (Fig. 4). Comparison of the ITS sequence to be tested with the ITS sequence of the relevant strain in GenBank indicated that the strain had 100% identity to Botrytis cinerea (GenBank accession number: MN589848.1) and had applied to GenBank for accession number (Botrytis cinerea, GenBank accession number: MW474926).
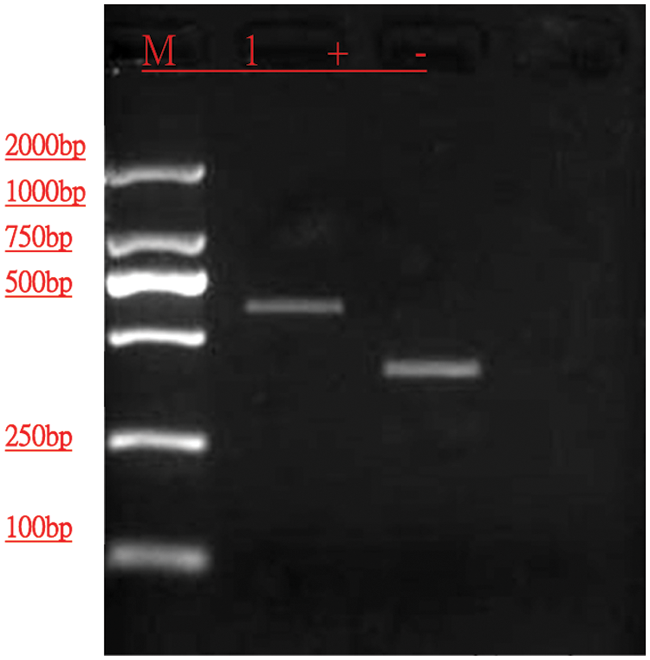
Figure 4: Detection profile of DNA template after ITS reaction.
Molecular identification by LSU gene sequencing
PCR amplification was performed for the measured strains, and electrophoresis revealed fragments of approximately 1311 bp in size (Fig. 5). Comparison of the LSU sequence to be tested with the LSU sequence of the relevant strain in GenBank indicated that the strain had 100% identity to Botrytis cinerea (GenBank accession number: KU140653.1) and had applied to GenBank for accession number (Botrytis cinerea, GenBank accession number: MW474943).

Figure 5: Detection profile of DNA template after LSU reaction.
The relevant data and results of identification showed that the isolated pathogen caused by hazelnut husk brown rot collected in this test was classified as Fungi Imperfecti, Hyphomycetes, Moniliales, Moniliaceae, Botrytis, Botrytis cinerea (Zhang, 2006).
SEM observation of the pathogen infection process
Scanning electron microscopy (SEM) observations of B. cinerea infecting hazelnut husks revealed that the conidiophores and mycelia grew lush, intricate, and tightly structured at 3 d after inoculation. The phenomenon of conidiophores and mycelia directly invading the epidermis of hazelnut husks were also observed. Conidia were also found to be all over the husks and had a trend of invading by Fig. 6.

Figure 6: SEM observation of B. cinerea infection process.
Species of Botrytis have a wide host range, can infect many plants and produce grey mildew (leading to their common name of gray mold in the USA), and many studies on Botrytis have been reported. To clarify the resistance to common fungicides to treat the B. cinerea known as kiwi grey mildew fungi, the types and frequency of fungicides used to prevent and control of grey mildew in Sichuan kiwi production were counted (Pei et al., 2021). To clarify the sensitivity level and resistance mechanism of B. cinerea to the succinate dehydrogenase inhibitor fungicide fluorimilecide in Shanghai, the resistance of 90 strains in five main strawberry producing areas in Shanghai was determined by their mycelial growth rate when subjected to the fungicide, and the sequence of succinate dehydrogenase (SHD) subunit was analyzed (Gao et al., 2021). Lily grey mildew and biological biology caused by B. cinerea were reported between 2017 and 2018 (Chen et al., 2017; Ma et al., 2018).
Few official reports have been made for hazelnut husk disease. The prevalence of B. cinerea causing disease on hazelnut clusters in different growing areas and determination of the reactions of some cultivars against pathogen were reported (Sezer and Dolar, 2012). As one of the pathogenic fungi of the disease after the hazelnut postharvest, Trichothecium roseum was determined as the inoculum sourees on the vegetative and reproductive structures of European hazelnut on different varieties and different hazelnut tissues in Chile (Guerrero et al., 2014). Pestalotiopsis sp. could cause disease on hazelnut husk in Turkey, which also infected hazelnut leaves and twigs with different severity (Sezer and Dolar, 2015). Hazelnut husk disease was found in several regions of Trabzon province, Turkey from 2008 to 2009. The disease symptoms were brown to black, round, or irregularly shaped sunken decayed spots at the base of the husk near the stalk. The pathogen Colletotrichum fioriniae was isolated and obtained. Pathogenic test results showed that the pathogenic fungus could infect hazelnut leaves, husk and stem (Sezer et al., 2017). However hazelnut husk disease caused by Botrytis sp. has not been reported in China. The author found the disease in Liaoning Province for the first time. Through a combination of ITS and LSU sequence analyses, we identified the pathogen that causes hazelnut husk brown rot in Liaoning Province by molecular and biological means. The method of identification was convenient, fast and accurate.
During repeated recycling in the trials, the pathogen was found to be highly susceptible to hazelnut husks through wounds. The trees were more susceptible to infection in warm, rainy and foggy environments, which facilitated the spread of the pathogen. The trial results indicate that the symptoms of inoculation were the same as those under natural conditions. This study on the new disease of hazelnut still merits study on the biological characteristics of the pathogen. The host range of the pathogen needs to be thoroughly studied, as do the characteristics of its infection process and its mechanism, to provide a systematic theoretical basis for disease control in production.
Availability of Data and Materials: The authors declare that all data is availability.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: S. data collection: S. X. analysis and interpretation of results: S. X. C. draft manuscript preparation: S. X. C. H. M. Q. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was financially supported by the Liaoning Provincial Natural Science Foundation of China (Grant No. 2021-MS-057).
Conflicts of Interest: The authors declare that they have no competing interests and there is no conflict of interest regarding the publication of this paper.
Chen J (2004). Hazelnut can fill gas and bright eyes. Afforestation 1: 45. [Google Scholar]
Chen LJ, Yin YY, Sun SK, Sun J (2017). First report of a gray mold on Lilium cernuum Komar. leaves caused by Botrytis cinerea in Liaoning province of China. Journal of Plant Pathology 99: 301. [Google Scholar]
Fang ZD (1998). Plant Pathology Research Methods. Beijing: China Agriculture Publishing. [Google Scholar]
Gao P, Gao SG, Cheng W, Zeng R, Xu LH et al. (2021). Sensitivity and resistance molecular mechanism of Botrytis cinerea to fluopyram in strawberry in Shanghai. Plant Protection 47: 215–220. [Google Scholar]
Guerrero JC, Curihuinca DN, Ferrada EQ, Perez SF (2014). Phytopathogens of hazelnut (Corylus avellana L.) in Southern Chile. Acta Horticulturae 1052: 269–274. [Google Scholar]
Jiang SQ, Ding SL, Zhang JW (2005). Preliminary discussion on the synthetical development and utilization of wild flat hazel. Special Economic Animals and Plants 10: 19–20. [Google Scholar]
Li XL (2011). Development and utilization of hazelnuts. Forestry Exploration Design 4: 114–115. [Google Scholar]
Ma S, Hu Y, Liu S, Sun J, Irfan M et al. (2018). Isolation, identification and the biological characterization of Botrytis cinerea. International Journal of Agriculture & Biology 20: 1033–1040. [Google Scholar]
Pei YG, Zhu YH, Sui LY, Tao QJ, Gong GS (2021). Detection of the resistance of Botrytis cinerea from kiwifruit to four fungicides in Sichuan. Plant Protection 47: 180–185. [Google Scholar]
Sezer A, Dolar FS (2012). Prevalence of Botrytis cinerea Pers. ex Fr. scausing disease on fruit clusters in hazelnut growing areas of Ordu, Giresun and Trabzon provinces and determination of the reactions of some cultivars against pathogen. Bitki Koruma Bülteni 52: 93–110. [Google Scholar]
Sezer A, Dolar FS (2015). Determination of Pestalotiopsis sp. causing disease on fruit clusters in hazelnut growing areas of Ordu, Giresun and Trabzon provinces in Turkey. Agriculture and Forestry 61: 183–188. [Google Scholar]
Sezer A, Dolar FS, Unal F (2017). First report of Colletotrichum fioriniae infection of hazelnut. Mycotaxon 132: 495–502. [Google Scholar]
Xie M, Wang DM (2019). Research on the development of economic forest industry in liaoning province (V)-Current situation, existing problems and development suggestions of Ping’ou hybrid hazelnuts industry in Liaoning Province. Journal of Liaoning Forestry Science and Technology 2: 8–40. [Google Scholar]
Zhang ZY (2006). Chinese Fungi Chronicles, vol. 26. Beijing: Science Publishing. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |