DOI:10.32604/biocell.2022.020407

| BIOCELL DOI:10.32604/biocell.2022.020407 |  |
| Review |
Efficacy of oral consumption of curcumin/Curcuma longa for symptom improvement in inflammatory bowel disease: A systematic review of animal models and a meta-analysis of randomized clinical trials
1Programa de Pós-Graduação em Nutrição (PPGNUT), Universidade Federal de Alagoas (UFAL), Maceió, AL 57072-970, Brazil
2Instituto de Química e Biotecnologia (IQB), Universidade Federal de Alagoas (UFAL), Maceió, AL 57072-970, Brazil
3Programa de Pós-Graduação em Ciências da Saúde (PPGCS), Universidade Federal de Alagoas (UFAL), Maceió, AL 57072-970, Brazil
4Programa de Pós-Graduação da Rede Nordeste de Biotecnologia (RENORBIO), Universidade Federal de Alagoas (UFAL), Maceió, AL 57072-970, Brazil
5Programa de Pós-Graduação em Ciências Médicas (PPGCM), Universidade Federal de Alagoas (UFAL), Maceió, AL 57072-970, Brazil
*Address correspondence to: Fabiana Andréa Moura, fabiana.moura@fanut.ufal.br
Received: 22 November 2021; Accepted: 11 February 2022
Abstract: The roots of the vegetal Curcuma due to its high content of polyphenols, has been used successfully in several clinical situations. This review assessed the effect of curcumin/Curcuma longa on symptoms and metabolic changes in inflammatory bowel disease (IBD). A systematic review of animal models and randomized clinical trials (RCTs) was conducted by searching the following electronic databases: PubMed, CENTRAL, LILACS, Science Direct, and ClinicalTrials.gov. From 997 found records, 62 were included. More than 90% of the animal studies reported an improvement in macroscopic, histologic and/or functional activity; 80% identified decreased oxidative and/or inflammatory biomarkers in animals treated with curcumin. Among the RCTs, intention-to-treat analysis showed that oral curcumin was effective in inducing clinical remission (n = 281, RR: 3.15 CI 95% [1.22–8.10] p = 0.0017; i² = 72.2%, p = 0.006) and clinical response (n = 259, RR: 1.60 CI 95% [1.09–2.35] p = 0.0017; i² = 59.7%, p = 0.042) but not endoscopic remission (n = 161, RR: 2.91 CI 95% [0.58–14.58] p = 0.195; i² = 72.7%, p = 0.026). These results confirm that oral supplementation with curcumin/Curcuma longa has beneficial actions in animal colitis and, when associated with drug therapy, is effective in the treatment of patients with IBD.
Keywords: Ulcerative colitis; Crohn’s disease; Oxidative stress; Curcuma; Turmeric
Curcuma or turmeric is a yellowish powder, known as a constituent of the curry condiment, extracted from Curcuma longa rhizome, and used as a religious item and as a medicine in both condiment and natural dye forms (Kotha and Luthria, 2019). For medicinal purposes, it has been used for at least 2500 years in traditional Chinese and Indian medicine in a range of clinical conditions. Its beneficial action on health is due to the presence of active polyphenols, called curcuminoids - demethoxycurcumin, bisdemethoxycurcumin, and especially curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], which has high antioxidant and anti-inflammatory power (Sueth-Santiago et al., 2015). Other properties are also attributed to curcumin, such as antimicrobial, antifungal, hypoglycemic, antiproliferative, anticarcinogenic, and with healing properties, through interaction with various gene transcription factors, enzymes, inflammatory cytokines, proteins, growth factors, and receptors (Jurenka, 2009; Stanić, 2017).
The antioxidant mechanism attributed to curcumin depends directly on the presence of two structural subunits, the phenolic hydroxyls, and the central methylene group. It may involve one or more of the following mechanisms: elimination or neutralization of reactive species (Lucas et al., 2021); inhibition of oxidative enzymes; interaction with oxygen-reduced species, making them less available for oxidative reactions; interaction with the oxidative cascade, and inhibition of its propagation; chelation, or deactivation of oxidative properties of metal ions, such as iron (Sueth-Santiago et al., 2015; Kumar et al., 2016).
Additionally, curcumin and other curcuminoids (to a lesser extent) present anti-inflammatory activity, regulating the expression of genes that encode proinflammatory interleukins, cytokines, and growth factors; reducing the levels of several reactive oxygen and nitrogen species (RONS); and inhibiting enzymes that produce RONS, such as nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), lipoxygenase (LOX), and xanthine oxidase (XO), thus suppressing nuclear factor B (NF-kB) activation (Kumar et al., 2016).
In general, oral consumption of curcumin is safe because no toxicity was observed in humans or animal models (Soleimani et al., 2018). However, in some animal models, the consumption of high doses can induce liver injury (Qiu et al., 2016). In a study conducted by Qiu et al. (2016), the overdose of oral curcumin in rats, (100 mg/90 days), each day, resulted in imbalance in animals by increased overexpression of interleukin (IL)-6 and reduction of superoxide dismutase (SOD) in liver tissues. Another study, that tested curcumin-loaded nanocomplexes, demonstrated changes in the liver function markers aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in high doses in chronic toxicity test (0,8 g/kg/day in mice - equivalent to 0.225 g/kg b.w. of curcumin-and 1,61 g/Kg/day in hamsters-0.45 g/kg b.w. of curcumin-for six months) (Jantawong et al., 2021).
Curcumin has a low bioavailability due to its poor aqueous solubility, instability at intestinal pH, and low intestinal permeability (Esatbeyoglu et al., 2012), which makes the clinical use of curcumin a challenge. Despite its low bioavailability (approximately 1%), curcumin and its metabolites appears to be pharmacologically effective, since it has shown effects, in several clinical conditions, such as cancer (Martínez et al., 2019), neurological disorders (Yavarpour-Bali et al., 2019), cardiovascular diseases (Oliver et al., 2016), metabolic disorders (Mohammadi et al., 2018; Panahi et al., 2018), autoimmune diseases (Momtazi-Borojeni et al., 2018), and inflammatory bowel disease (IBD) (Cunha Neto et al., 2019).
The IBD is a complex and multifactorial disease mediated by immunological components of the gastrointestinal tract and characterized by recurrent inflammation, with ulcerative colitis (UC) and Crohn’s disease (CD) being its main forms (Actis et al., 2019; Martins et al., 2021). IBD symptoms include pain, vomiting, diarrhea, weight loss, and fever and greatly impact the quality of life of the patients. Chronically, UC and CD increase the risk of surgical procedures, toxic megacolon, and colorectal cancer (CRC) (Moura et al., 2015). Several drug classes, such as aminosalicylates, corticosteroids, immunomodulators, and biological therapy are utilized to minimize these effects. However, adverse effects resulting from a prolonged use of these medications, disease recurrence, and medicine dependency are observed in many patients (Sairenji et al., 2017).
Due to the crosslink between redox imbalance, inflammatory activity, and immune deficiency in IBD, research suggests that the use of therapeutic strategies with antioxidant and anti-inflammatory substances may be a promising unconventional treatment alternative. Among these, the use of Curcuma longa and/or its curcuminoids, especially curcumin, is highlighted. In this context, the aim of this systematic review is to identify the effects of Curcuma longa, curcumin, or other curcuminoids on symptoms and metabolic changes in patients and animal models of IBD.
This systematic review was registered on the International Prospective Register of Systematic Reviews (PROSPERO) platform, with registration numbers CRD42020164513 and CRD42020168827 for the review with human studies and studies with animal models, respectively.
Search strategy and selection of studies
The search was conducted until January 2021 in the following databases: MEDLINE (via PubMed), Cochrane Controlled Register of Trials (CENTRAL), Literatura Latino-Americano e do Caribe em Ciências da Saúde (LILACS), Science Direct, and Clinical Trials. The following keywords were used: “inflammatory bowel disease”, “ulcerative colitis”, “colitis”, “Crohn’s disease”, “curcumin”, “curcuma”, “turmeric”, and “Indian saffron”. Boolean operators “OR” and “AND” were used. The full search strategy for all the databases is reported in the Supplementary Material. All records retrieved had their titles and abstracts evaluated. In addition, no year of publication filter was used. Then, we evaluated titles for the removal of duplicate records. A complete search strategy is shown in Suppl. Box 1.
Eligibility of animal models’ research
Studies with experimental models (rats or mice) of UC or CD were included, without restriction for the inducing agent or the inflammation model (acute or chronic) and in which Curcuma longa/curcuminoids were administered orally (diet or gavage) for treatment. The results of the research should include at least one of the following aspects: macroscopic, anatomical and/or histological evaluation of the colon; body weight; disease activity index; and serological and/or tissue analysis of biomarkers of nitroxidative stress and inflammation. Studies carried out exclusively in vitro, with the use a mixture of turmeric, and/or curcuminoids with other substances as an intervention, were excluded.
Studies were divided according to Curcuma longa/curcumin and/or other curcuminoids supplementation doses: ≤0.005 mg/kg day/0.01 mmol/kg day; 5–50 mg/kg day/0.01–0.15 mmol/kg day; 60–200 mg/kg day/0.25–0.50 mmol/kg day; >200 mg/kg day/0.54 mmol/kg day; ≤2% (w/w) of the diet; and >2% (w/w) of the diet. In studies that have tested more than one dose we chose to categorize it in the subgroup of higher dosages.
Eligibility of clinical research
Randomized clinical trials with participants of both sexes, aged 18 years or older, diagnosed with UC or DC, and treated with oral Curcuma longa/curcuminoids isolated or combined with drugs, were included. There was no restriction on the severity of the disease (mild, moderate, or severe) or the intestinal lesion location (proximal or distal). Studies were excluded if they evaluated pregnant or lactating women or participants with other associated comorbidities, such as diabetes and hepatic, kidney, and autoimmune diseases. Finally, records in languages other than English, Portuguese and Spanish were excluded.
The following data were extracted from the studies:
• Animal model research: sex, species, age (week), and/or body weight (b.w.); experimental model of IBD; supplement presentation; doses, time of supplementation; administration via; groups of treatment; nitroxidative stress and inflammation effects; other results. The studies with multiple doses of supplementation were allocated, according to the highest dose.
• RCT: IBD clinical situation; number of randomized individuals (n)/age (years)/and sex; supplement presentation/doses, and time of supplementation; treatment association; clinical, histological, and image parameters, nitroxidative stress/inflammation/serum and fecal biomarkers effects.
• Meta-analysis: RCTs included in the meta-analysis needed to present data on clinical remission (primary outcome) and endoscopic remission (secondary outcome). Outcomes that assessed disease activity based on one or more of the following parameters: clinical manifestations, histological patterns of intestinal lesions, inflammation/nitroxidative stress biomarkers, and serological or fecal markers were also included. The percentage of patients in the intervention and control groups who achieved remission and/or clinical response at the end of the experiment were statistically analyzed, using tools that assign scores based on the intensity and frequency of symptomatic manifestations. Other analyzed outcomes included the percentage of patients in the intervention and control groups who achieved remission as assessed by endoscopic examinations at the end of the test period. Trials were classified as ITT (intention-to-treat) or PP (per protocol). Missing data were requested to the authors of the studies by e-mail, when applicable.
Assessment of the risk of bias and quality of evidence
For animal model research, the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) was applied. This tool assesses the risk of bias, according to ten domains: bias in sequence generation, bias due to baseline characteristics, bias according to allocation concealment, bias due to random housing, bias in blinding of trial caregivers and researchers, bias in random outcome assessment, bias in blinding of outcome assessors, bias due to incomplete outcome data, bias in selective outcome reporting, and overall bias. Each category was assessed as having low, high, or uncertain risk of bias. Then, each study received an overall rating of risk of bias.
For RCTs, the risk of bias was assessed using the Cochrane Collaboration tool, which uses six domains: sequence generation, allocation concealment, blinding of participants and researchers, blinding of outcome assessors, missing data, and selective outcome report. Each category was defined as low, high risk or uncertain risk of bias, and introduced, in each study. The evidence quality was also assessed by the method proposed by the Grading of Recommendations Assessment, Developing and Evaluation (GRADE). This method classifies the evidence of each outcome in the meta-analysis into four categories: high, moderate, low, or very low. Five criteria were evaluated: study limitations (risk of bias), inconsistency of the results (heterogeneity), indirect evidence, inaccuracy, and publication of bias, which generated a score to allow the final classification.
As all the meta-analyzed variables were categorized, the relative risk (RR) between groups for each variable was calculated for each study. Studies weights were assigned, according to the inverse variance method, and calculations were based on a random-effects model. An alpha value of 0.05 was adopted.
Statistical heterogeneity among the studies was tested using the Cochran Q test, and inconsistency was assessed using I2 statistics. Whenever a result showed heterogeneity, it was explored by repeating the analysis with the removal of one study at a time to assess whether a particular study explained the heterogeneity. All analysis were conducted using the RevMan 5.3 program (The Nordic Cochrane Centre, The Cochrane Collaboration, Denmark).
From the nine hundred and ninety-seven unique screened records, sixty-two were included in the qualitative synthesis. Of these, fifty-four (87.1%) studies were in animal models, and eight (12.9%) were randomized controlled trials (RCTs). Five (62.5%) RCTs were included in the meta-analysis for the following outcomes: clinical remission and clinical response, and three RCTs (37,5%) were included for endoscopic remission. Fig. 1 shows the flow diagram of study selection.
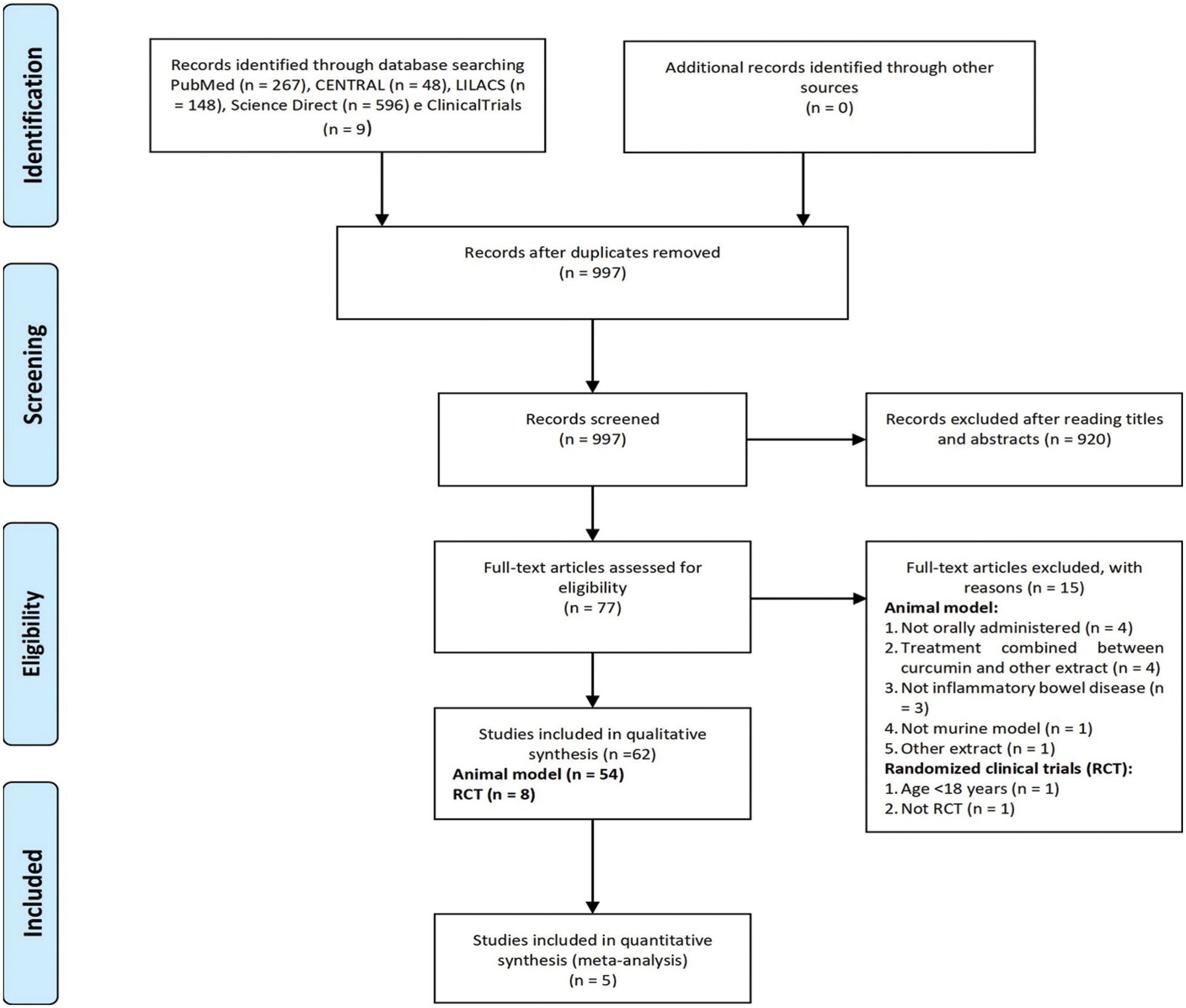
Figure 1: Flow diagram of study selection.
Animal model research: study characteristics
Among the fifty-four studies (Table 1) that evaluated oral Curcuma longa or curcumin (extract or modified formulations) in experimental colitis, twenty-seven used as inducing agent, the dextran sulfate sodium (DSS) (50%), thirteen used 2,4,6-trinitrobenzenesulfonic acid (TNBS) (24.1%), seven used acetic acid (13%), four used genetic modification (7.4%), and three used dinitrobenzene sulfonic acid (DNB) (5.6%). Curcumin was the main form of supplementation used in the studies (n = 52; 96.3%). Same studies used modified curcumin to improve its bioavailability (n = 20; 37%): nanoparticles/nanocarriers in seven (35%), microparticles/microspheres in six (30%), polymers complexed with other substances in two (10%), and other forms in three (15%). In the studies in which these modified forms were compared to curcumin (n = 12; 60%), all showed more beneficial results in oxidative stress/inflammation markers, general and histological parameters, or in both.
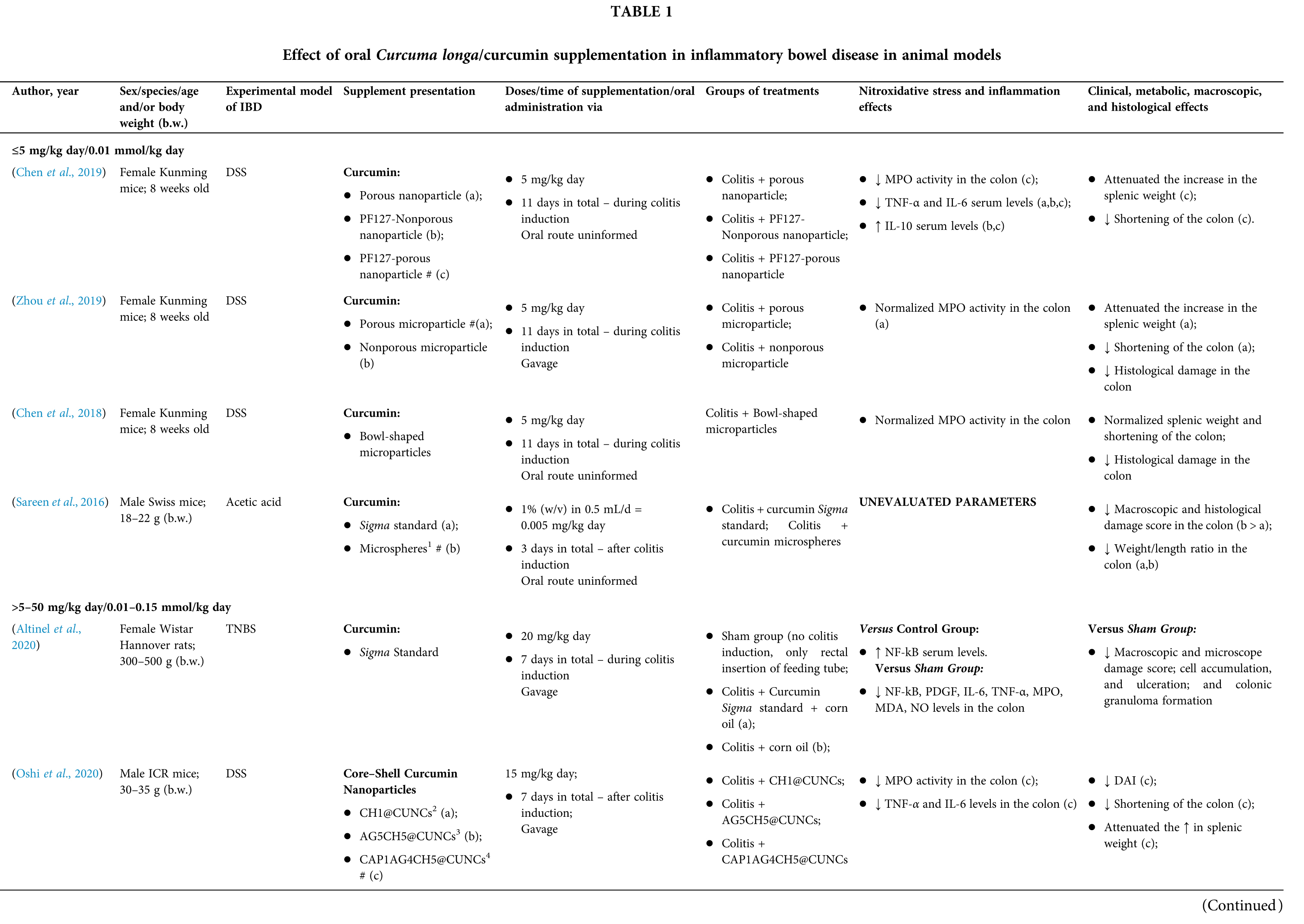
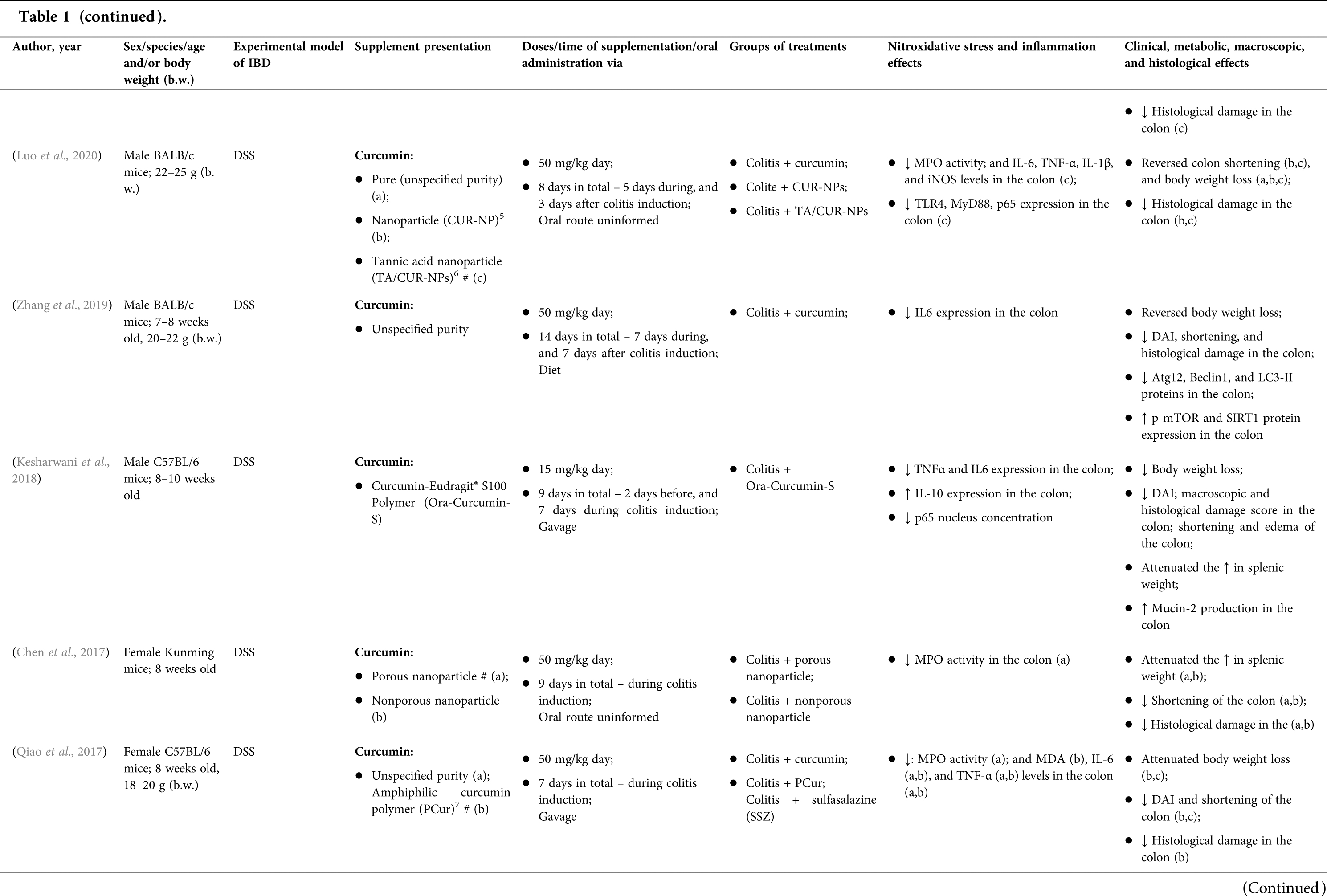

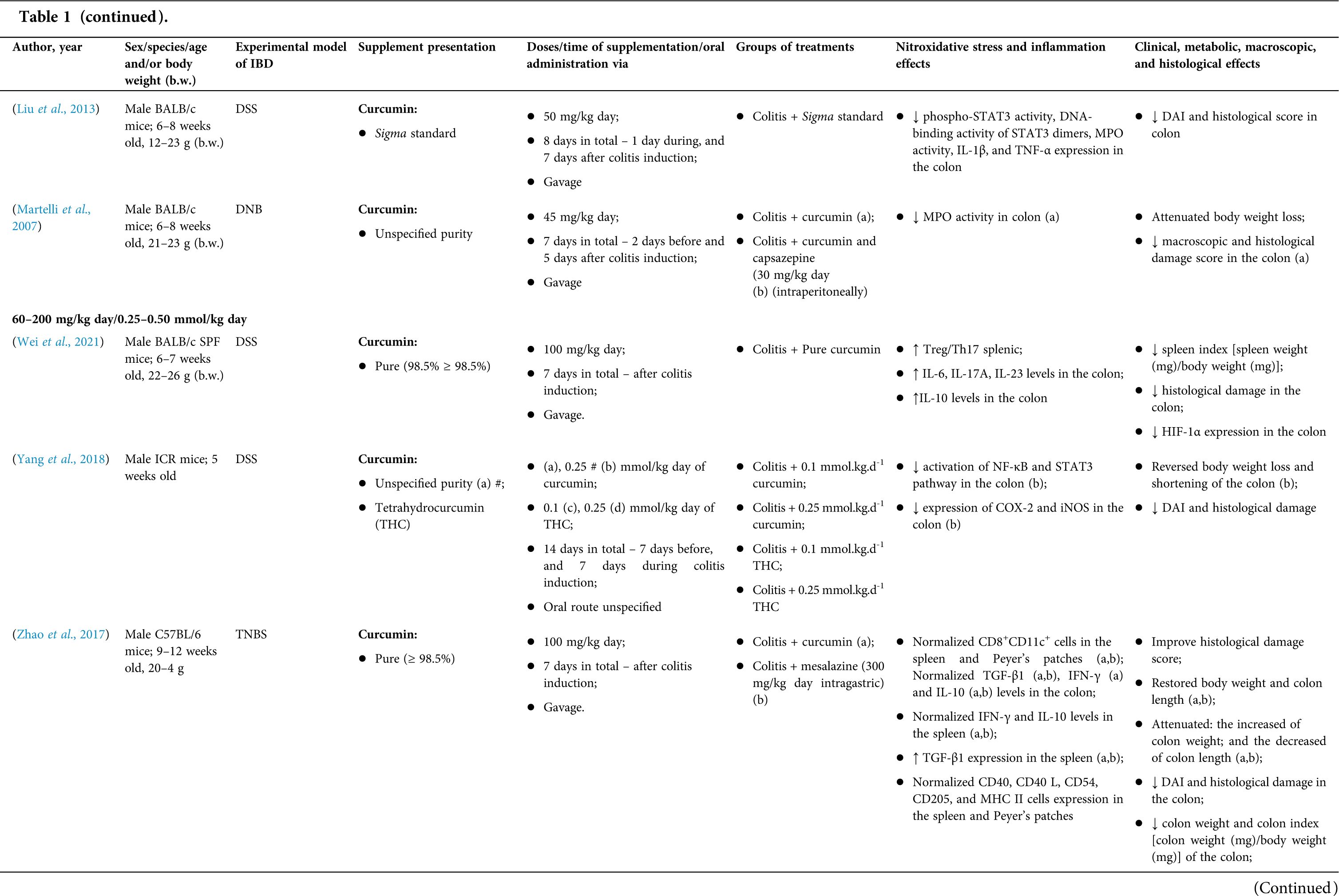
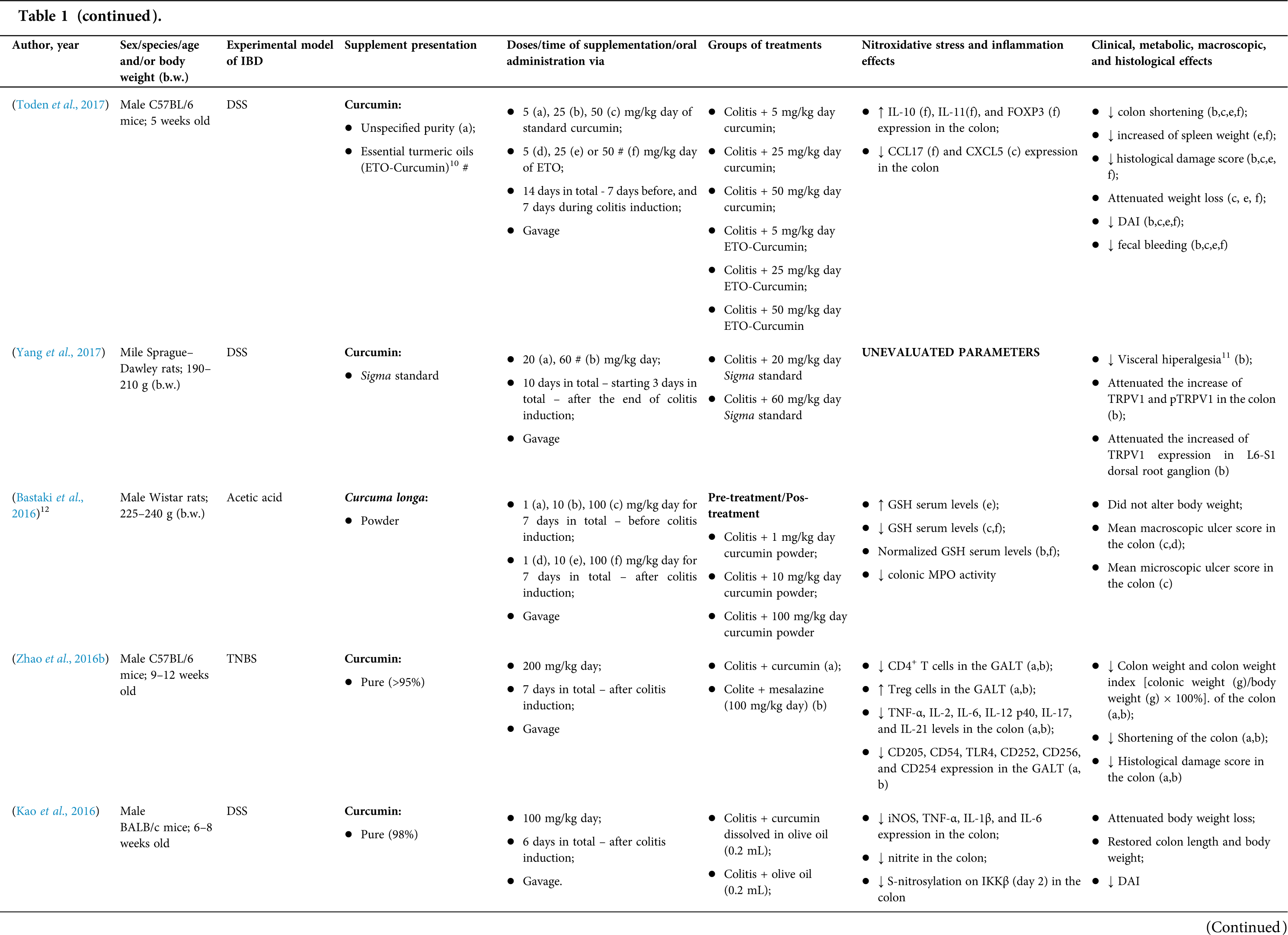
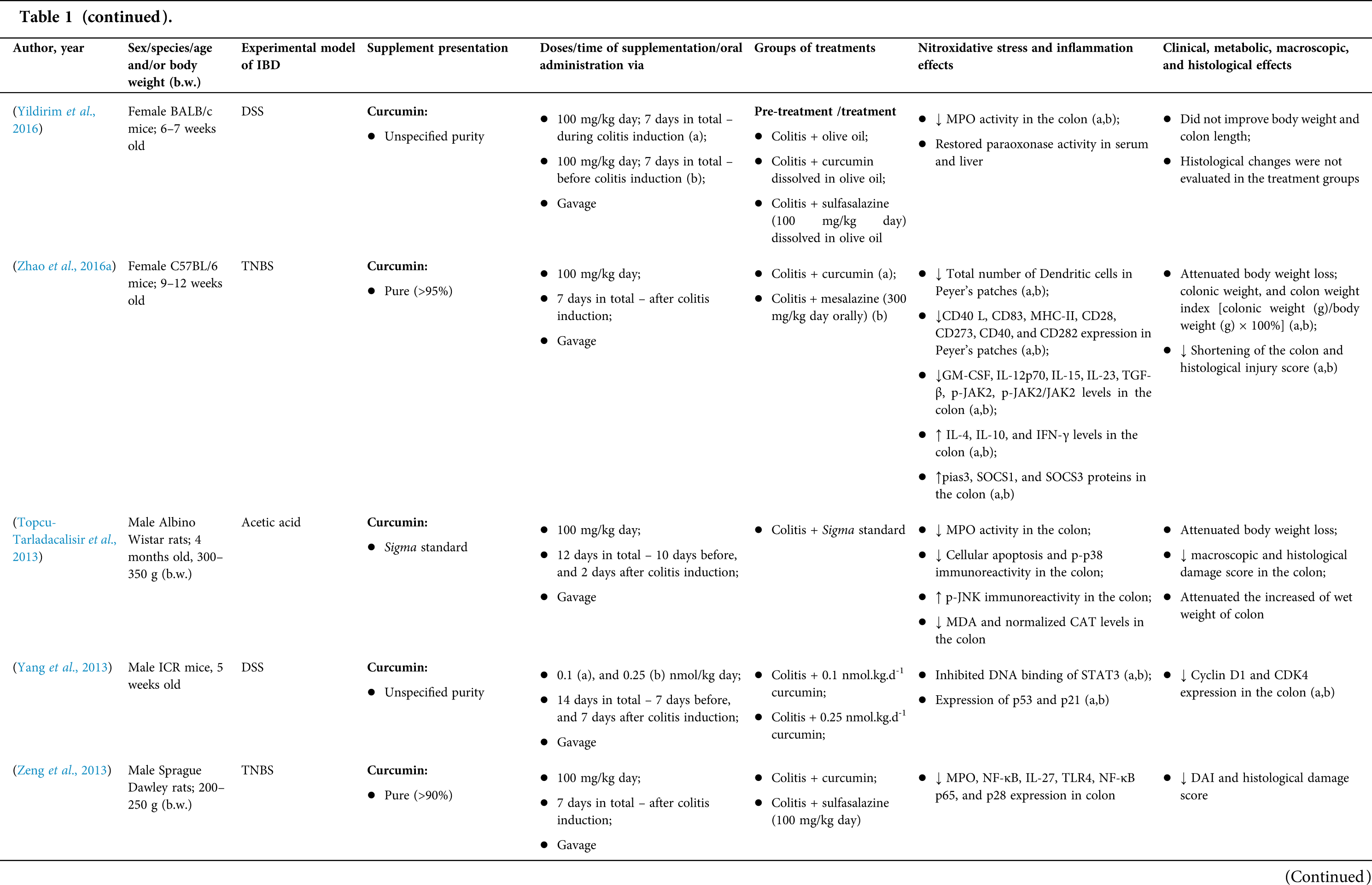
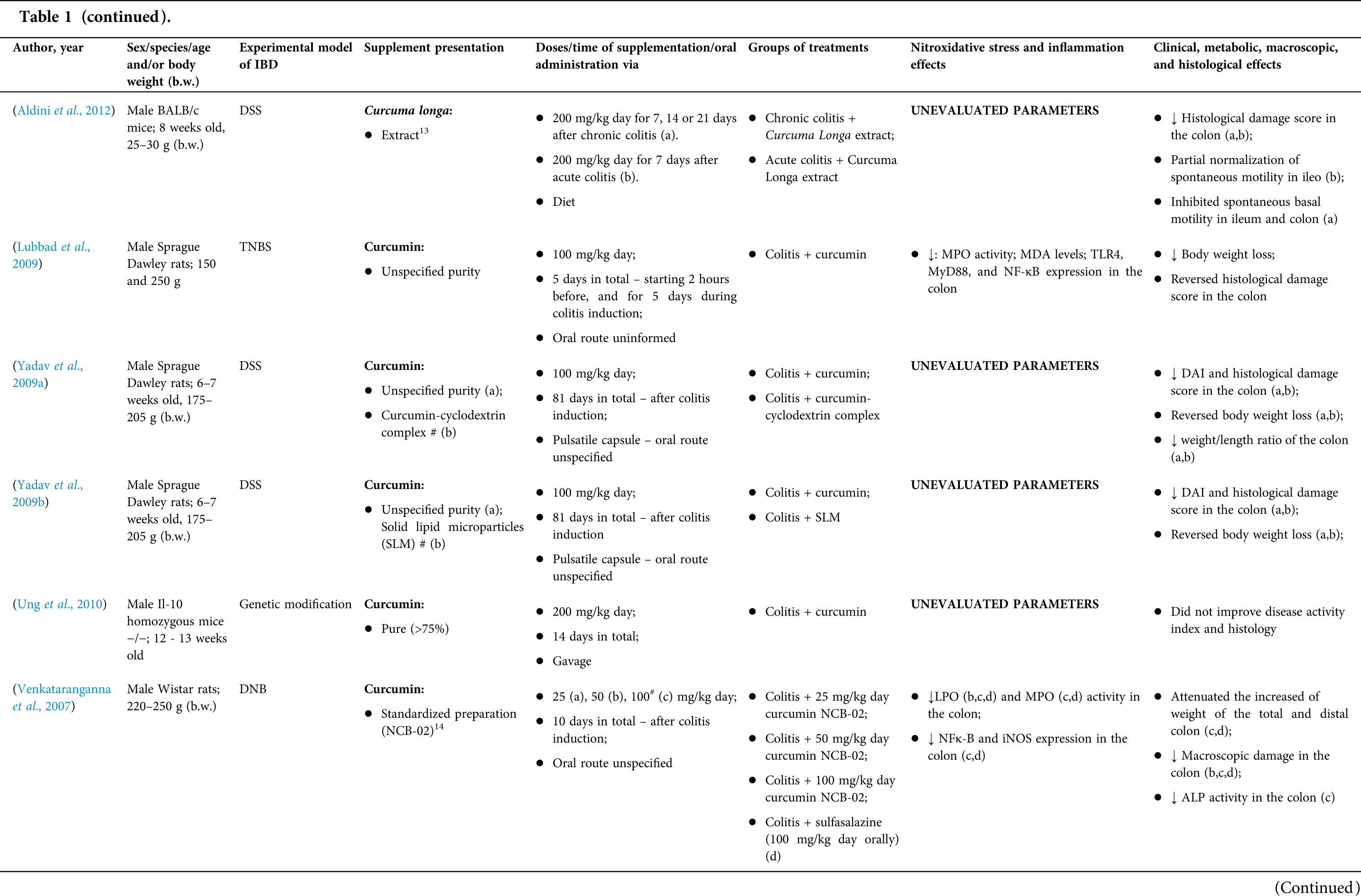

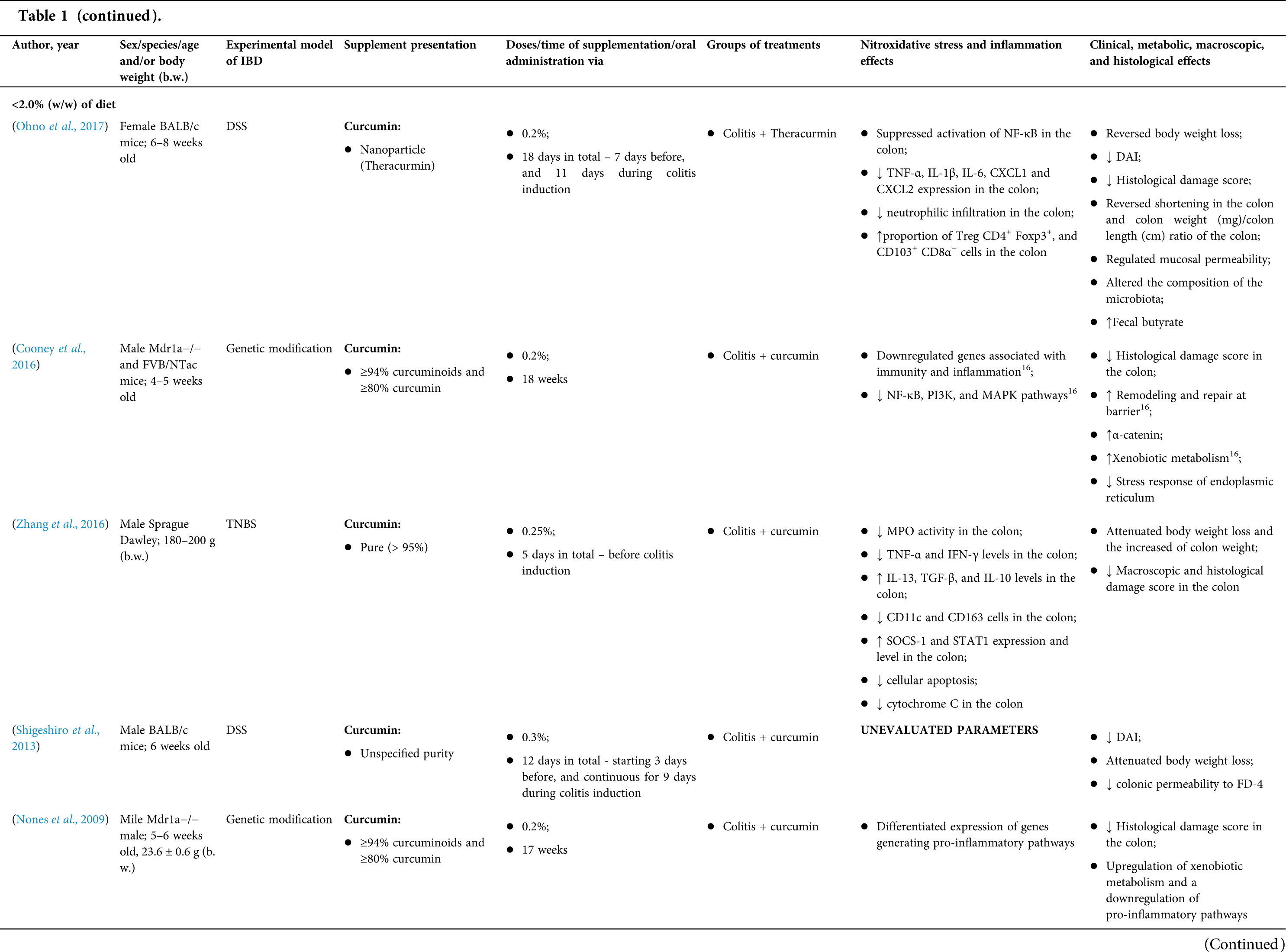
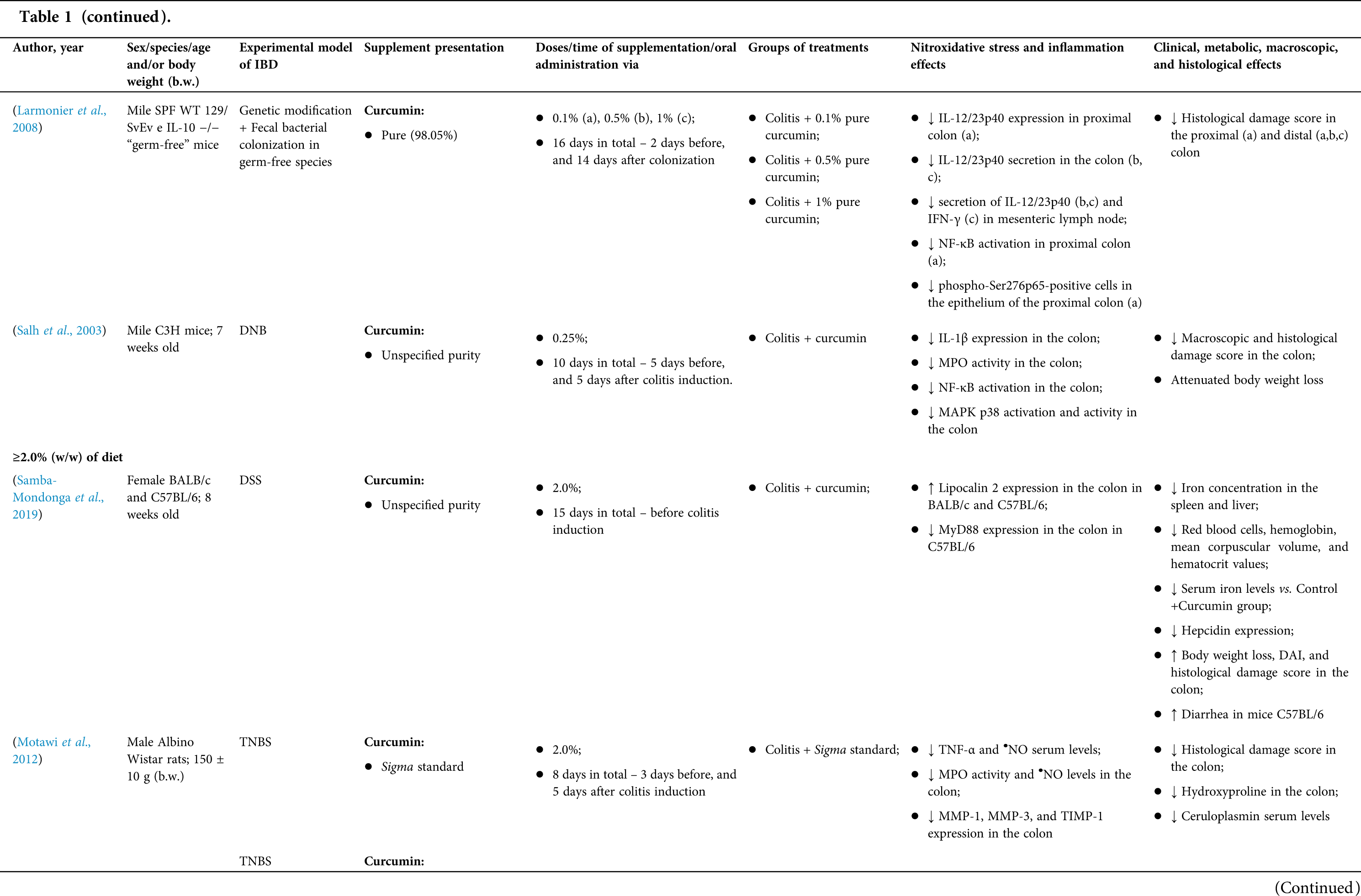
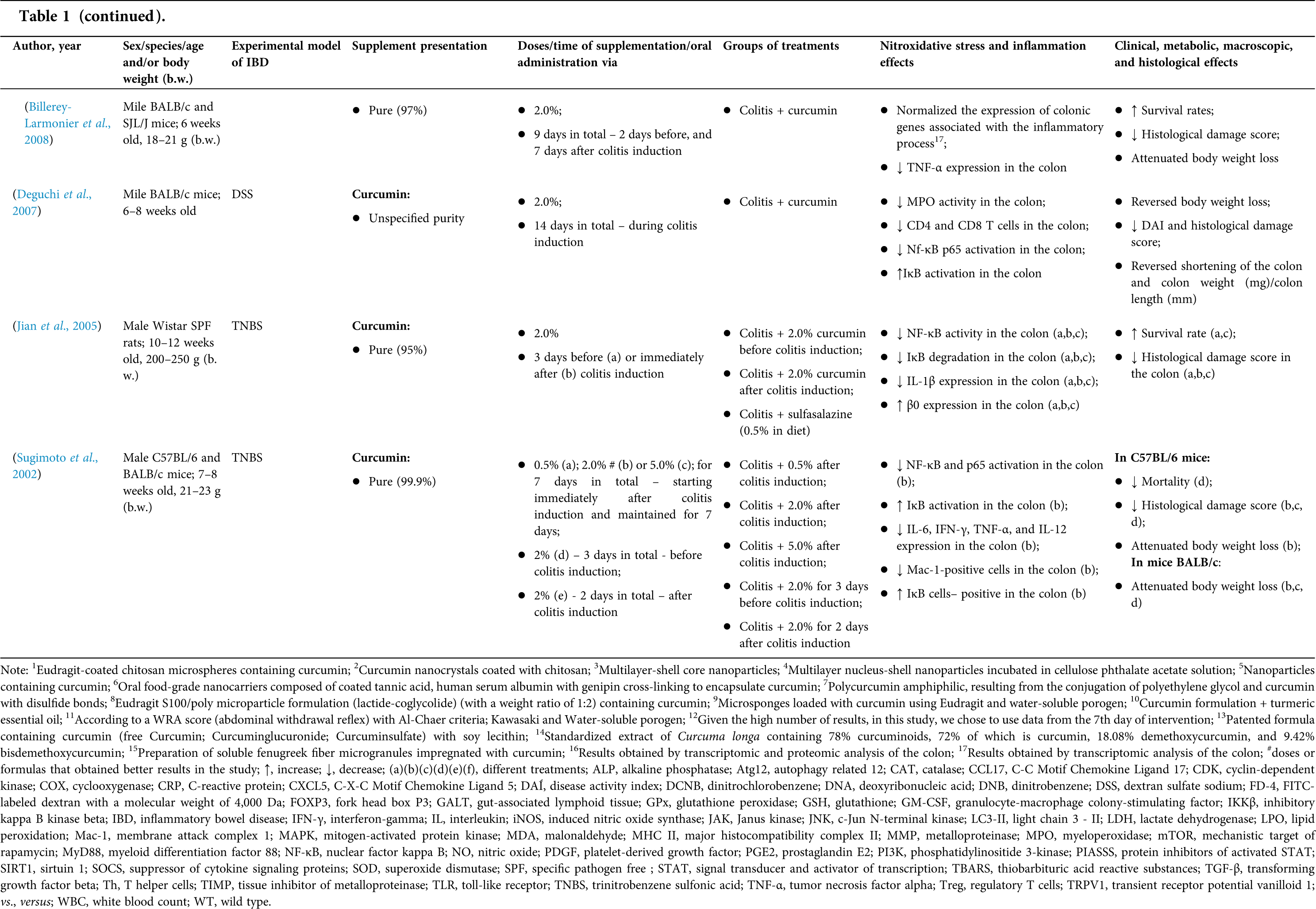
However, even in cases where pure curcumin was used, the majority of studies confirmed its antioxidant and anti-inflammatory roles as well as its effectiveness in improving clinical, metabolic, macroscopic, and histological parameters. Only two studies showed no beneficial effects or negative action of curcumin. In this latter study, a worsening of all biochemical parameters of anemia was observed, in addition to the worsening of clinical and histological signs of IBD.
There was a great variability in the included studies of the curcumin doses (0.005 mg/kg day to 500 mg/kg day), supplementation period (three days until eighteen weeks), and the moment when supplementation was performed (before, during or after colitis induction, or in two different moments of the disease).
The most used doses of curcumin (modified or not)/Curcuma longa were 100 mg/kg day, followed by 50 mg/kg day, and 15 mg/kg day. Among those who were supplemented in the diet (w/w), six had ≤0.3% (w/w) and five ≥2%. Multiple doses were used in twelve (22.2%) studies. In these cases, only one reported better results at the lowest dose (2 mg/kg day vs. 15 mg/kg day), using curcumin nanocarriers; in most of them, the highest dose led to better results. These results indicate that the action of curcumin may be dose-dependent, which would be related to its low toxicity and bioavailability.
The effect of oral curcumin/Curcuma longa supplementation on inflammatory and nitroxidative stress biomarkers was not evaluated in eight (14.8%) studies. Among those who analyzed these parameters (n = 46; 85.2%), forty-five (45, 97.8%) observed some beneficial effects. The main inflammatory biomarkers studied were the enzyme myeloperoxidase (MPO); the cytokines tumor necrosis factor alpha (TNF-α), the IL-6, IL-10, IL-1β and interferon gamma (INF-γ); the nuclear factors, NF-κB and signal transducer and activator of transcription 3 (STAT3); and the membrane receptor toll-like receptor 4 (TLR-4).
Concerning redox imbalance biomarkers, the reactive species-producing enzymes iNOS and COX-2; the enzymatic and nonenzymatic defense biomarkers catalase (CAT), SOD, glutathione peroxidase (GPx), and reduced glutathione (GSH); the cell membrane damage biomarkers thiobarbituric acid reactive substances (TBARS)/malondialdehyde (MDA); and the reactive species nitric oxide (•NO)/nitrite are highlighted.
The risk of bias assessment, according to SYRCLE, is shown in Suppl. Table 1. In general, there was a high risk of bias in the domains of “random sequence generation”, “allocation concealment”, “blinding”, and “incomplete outcome data”. Another consideration refers to the high number of studies whose main objective was to produce and test formulations containing curcumin. In such publications, the manuscript, specifically, on animal experimentation tends to be less detailed, which can result in the assessment of risks as “high” or “unclear” bias in several domains.
Randomized controlled trials: study characteristics
In the systematic review, eight RCTs (Table 2) were identified. Six (75%) included, in its supplementation protocol, only individuals with UC and two (25%), only patients with CD. Most of them (n = 6; 75%) chose to study mild or moderate IBD, while Bommelaer et al. (2020) analyzed CD surgical cases, and Kumar et al. (2020) comprised UC active forms. In all RCTs, individuals of both sexes were included.
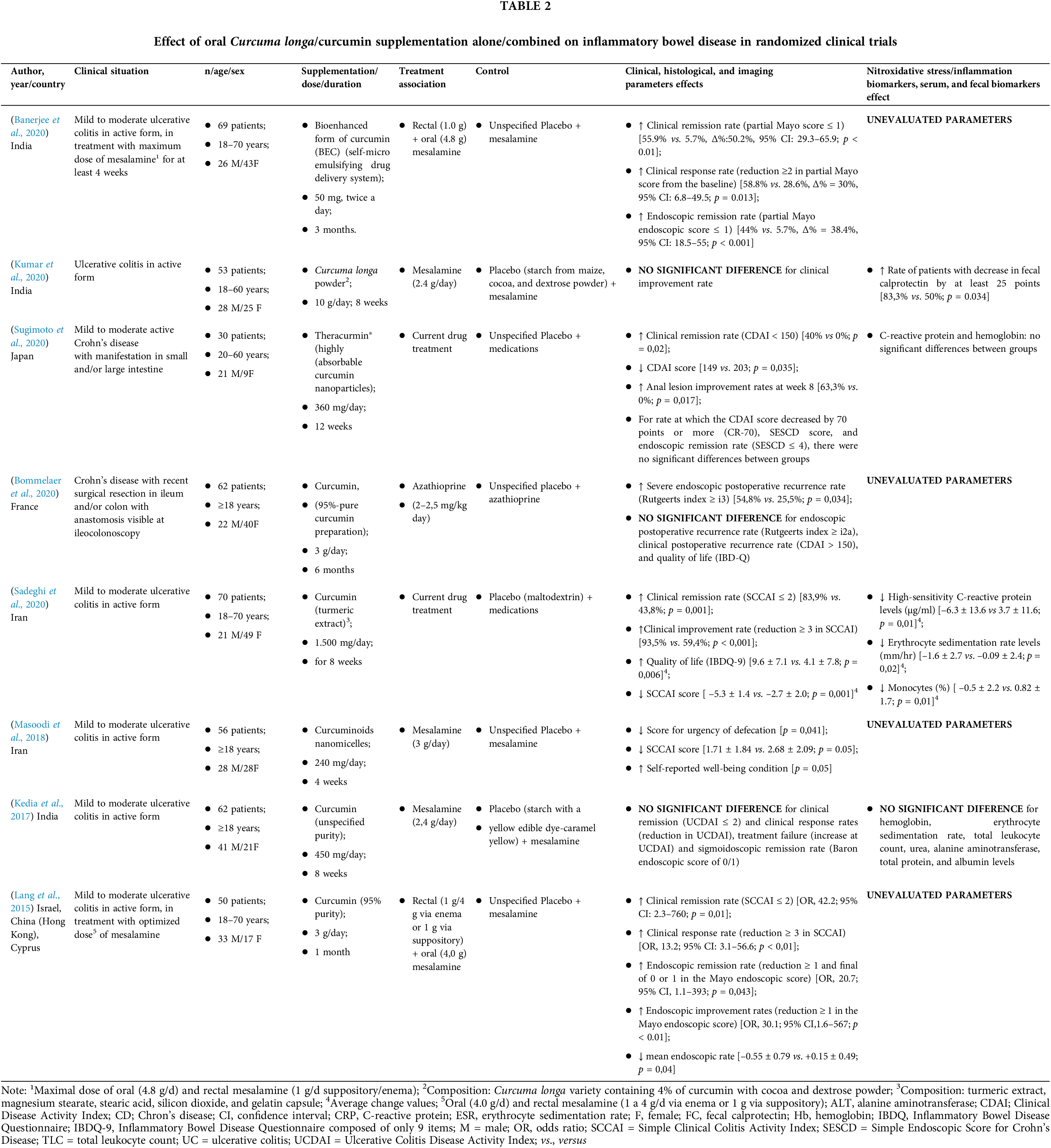
In three RCTs (3, 37.5%), modified curcumin/curcuminoids were utilized to increase its bioavailability; in other three RCTs (37.5%), pure curcumin was used; and in two RCTs (25%), Curcuma longa extract was evaluated. Doses and supplementation period showed a high variation between RCTs. The doses used ranged from 100 mg/day to 10 g/day, while the supplementation period varied from one month to six months. All included RCTs used an association of oral supplementation with drug therapy, especially mesalamine, either in the oral or oral + rectal route.
Finally, positive results in clinical, histological, and imaging parameters in the curcumin/Curcuma longa oral supplementation group versus the placebo group were observed in five (5, 62.5%) RCTs. The better effects were observed in the clinical remission rate, clinical response rate, endoscopic remission rate, and quality of life. Bommelaer et al. (2020), that tested 3 g/day, were the only to observe negative effects related to an increase in the severe endoscopic postoperative recurrence rate (54,8% vs. 25,5%; p = 0,034), while Kumar et al. (2020)–who used Curcuma longa (10 g/day for 8 weeks)–and Kedia et al. (2017)–who tested curcumin at a dose of 240 mg/day for 4 weeks–both in patients with UC– did not find any effect caused by oral supplementation.
Unlike the animal models included in this study, no nitroxidative stress/cytokines/nuclear factors were analyzed in the RCTs included in the present systematic review. In fact, half of them studied the effect of curcumin/Curcuma longa on serological or fecal parameters, and only two (25%) found positive results: decreased fecal calprotectin, C-reactive protein (CRP), erythrocyte sedimentation, and monocytes.
The risk of bias at the primary outcome level was assessed using the Cochrane collaboration tool. It is included in the Supplementary information. In general, all studies assessed risks were classified as “low” or “unclear” bias (Suppl. Table 2).
According to Fig. 2, oral supplementation with Curcuma longa extract or curcumin (modified or not) led to higher rates of clinical remission than placebo in both intention-to-treat (ITT) analysis (n = 281, RR: 3.15; CI 95% [1.22–8.10] p = 0.0017; i² = 72.2%, p = 0.006, Fig. 2A), and per-protocol (PP) analysis (n = 239, RR: 3.35; CI 95% [1.39–8.06] p = 0.007; i² = 71.7%, p = 0.006, Fig. 2B). High heterogeneity was observed in the analysis performed. When we removed the studies by Banerjee et al. (2020) and Lang et al. (2015), the heterogeneity of clinical remission in the ITT category was reversed (n = 162, RR: 1.70 ic95% [1.16–2.49] p = 0.006; i² = 0.0%, p = 0.38.
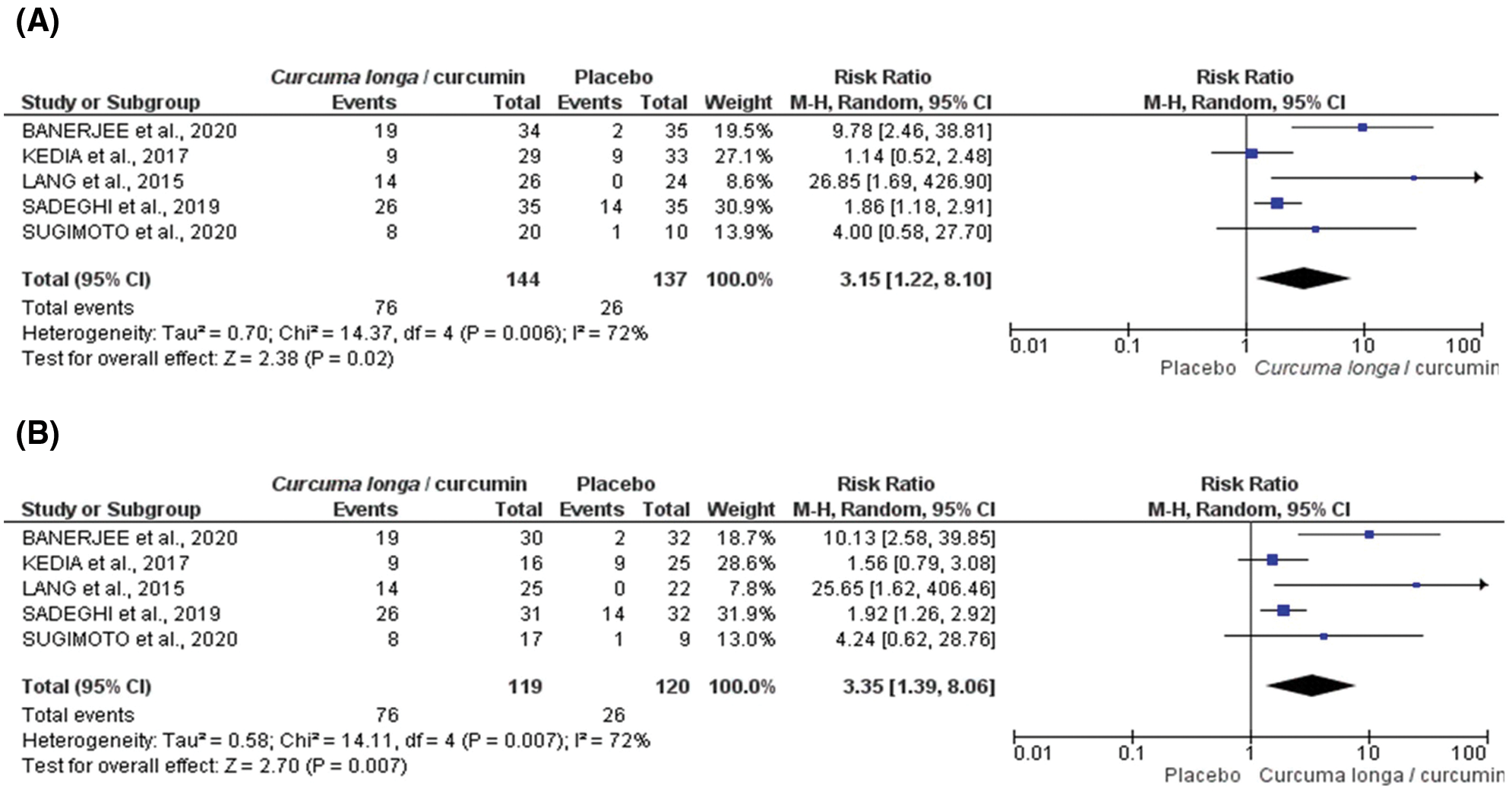
Figure 2: Forest plot for clinical remission induced by Curcuma longa extract or curcumin associated with drug therapy according to randomized clinical trial included in meta-analysis: ITT (intention-to-treat) (A) or PP (per protocol) (B).
For clinical response, PP analysis (n = 259, RR: 1.60 CI 95% [1.09–2.35] p = 0.0017; i² = 59.7%, p = 0.042, Fig. 3A), but not ITT analysis (n = 304, RR: 1.51 CI95% [0.94–2.41] p = 0.086; i² = 67.7%, p = 0.015, Fig. 3B), indicates a protective effect of the Curcuma longa extract and curcumin.
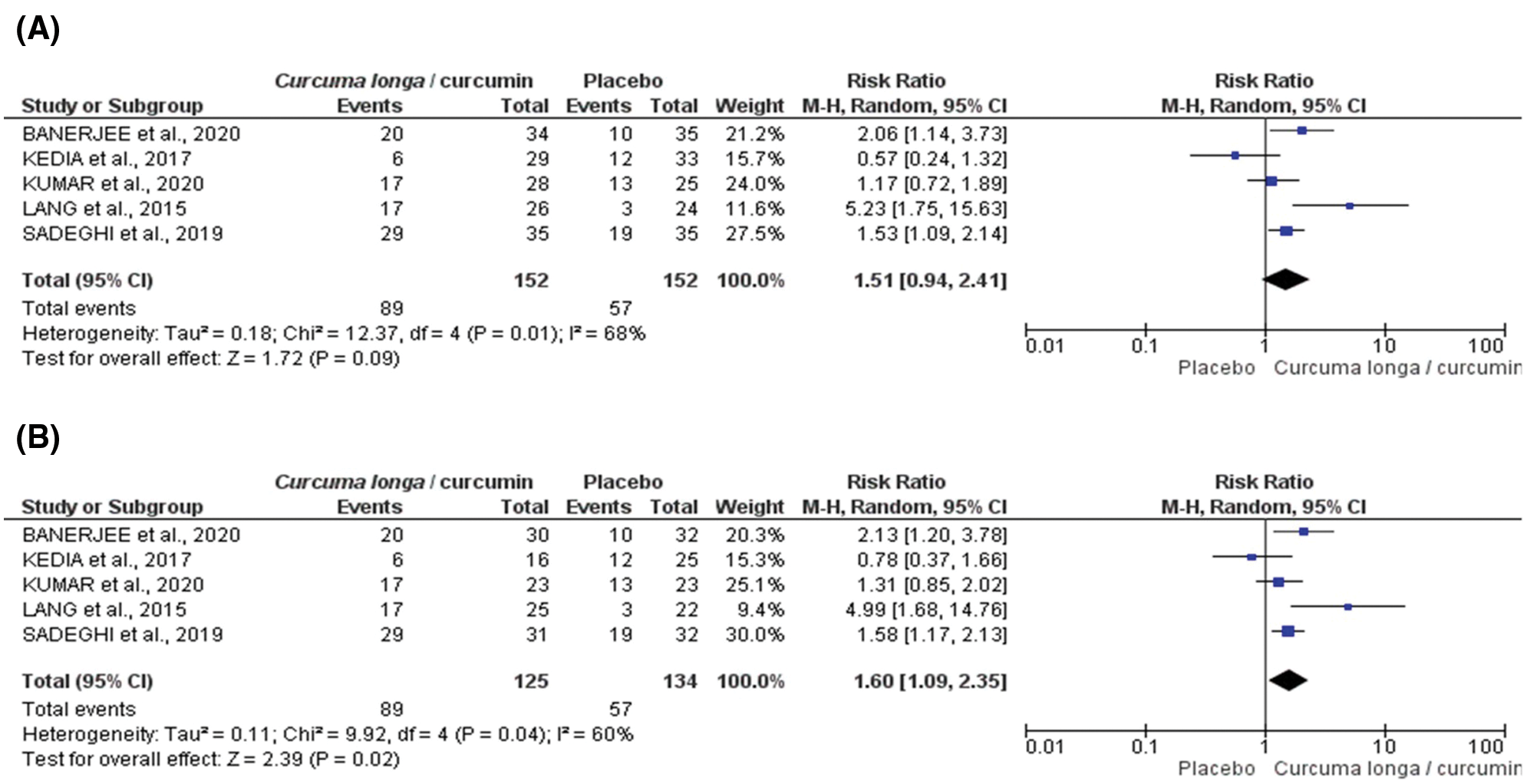
Figure 3: Forest plot for clinical response induced by Curcuma longa or curcumin associated with drug therapy according to randomized clinical trial included in meta-analysis: ITT (intention-to-treat) (A) or PP (per protocol) (B).
For endoscopic remission, both ITT (n = 161, RR: 2.91 CI 95% [0.58–14.58] p = 0.195; i² = 72.7%, p = 0.026, Fig. 4A), and PP analysis (n = 129, RR: 3.34 CI 95% [0.76–14.68] p = 0.11; i² = 69.7%, p = 0.033, Fig. 4B) did not demonstrate any superior efficacy of curcumin/Curcuma longa extract compared to placebo.
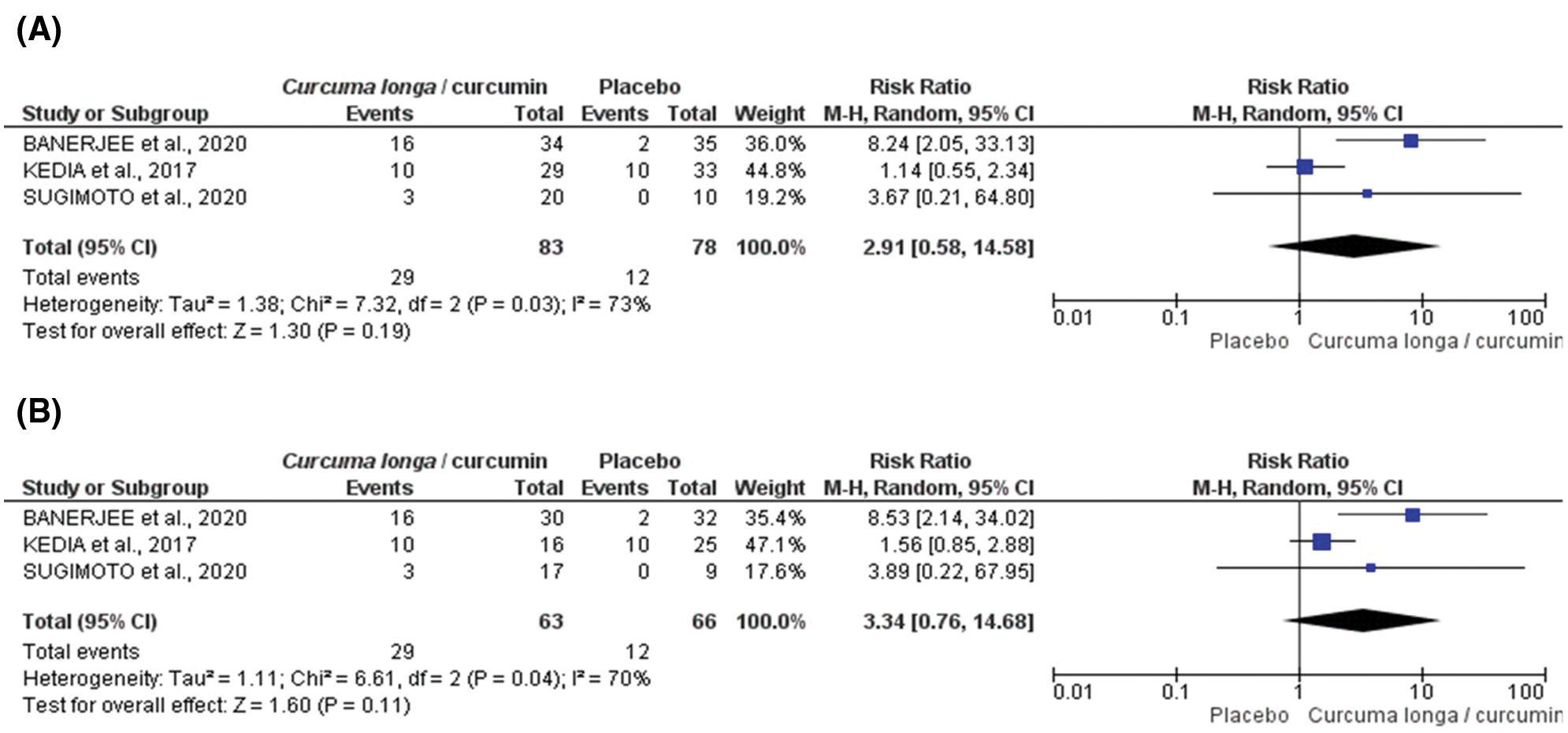
Figure 4: Forest plot for endoscopic remission induced by Curcuma longa or curcumin associated with drug therapy according to randomized clinical trial included in meta-analysis: ITT (intention-to-treat) (A) or PP (per protocol) (B).
A summary of the findings according to the GRADE assessment is shown in Table 3. In general, the quality of evidence ranged from low to very low, especially due to inconsistency and/or imprecision.
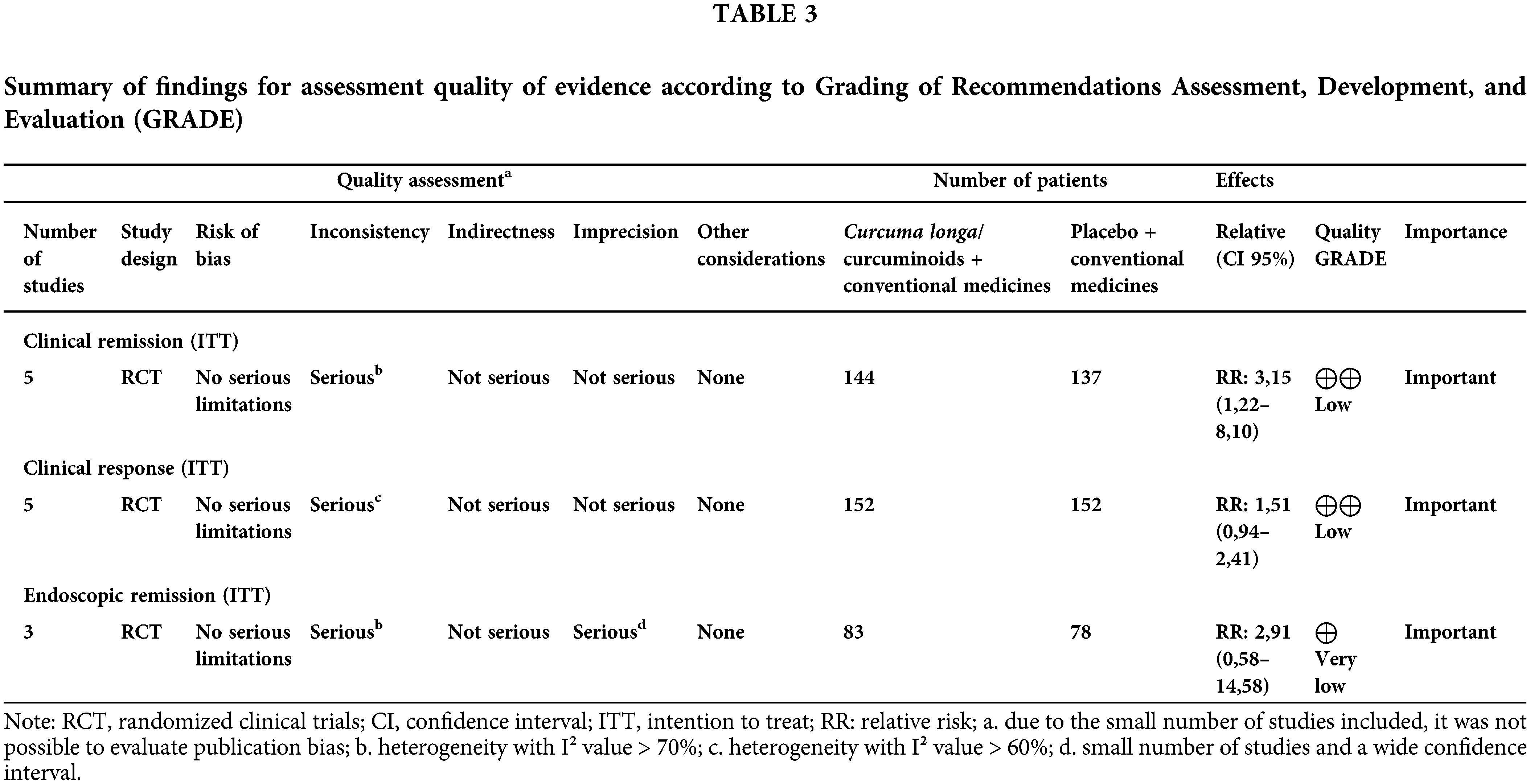
The results found in this systematic review with meta-analysis generally indicate that the therapeutic supplementation of curcumin/Curcuma longa has essential effects on animal-induced colitis and IBD in humans. These effects were evidenced by the improvement of clinical markers–animal and human models–and biological features–animal models–and given these results, together with the safety in consumption, the discussion presented should stimulate the interest of professionals who deal with this disease daily clinical practices.
According to the present systematic review, animal studies consistently explore the effects of turmeric, attenuating nitroxidative and inflammatory responses on the characteristics of IBD. Studies also show that the antioxidant action of supplementation occurs at the molecular level by inhibiting the formation of ERONs, increasing the endogenous antioxidant response, and reducing cell damage (Esatbeyoglu et al., 2012; Maiti and Dunbar, 2018).
Chronic intestinal inflammation is notably mediated by proinflammatory immune responses stimulated by antigens of different nature (Jian et al., 2005). In this context, immune cells such as macrophages and lymphocytes act as important agents of the expression of proinflammatory cytokines (Duque and Descoteaux, 2014). Cytokines lead to the main proinflammatory signals, characteristic of colitis. The IL-1, IL-1β, and IL-18 families can be synthesized by intestinal epithelial cells or phagocytes (Duque and Descoteaux, 2014). In response to pathogens, IL-1β can stimulate T-cell differentiation in Th17 cells and the production of the cytokine IFN-γ (Friedrich et al., 2019). In experimental models, the absence of IL-1β and IL-18, either due to genetic deficiency or signaling inhibition, is associated with colitis improvement (Dinarello et al., 2013).
TNF-α, produced by phagocytes, participates in intestinal inflammation, exerting multiple intestinal cell effects (Sands and Kaplan, 2007). This cytokine’s high levels may alter the intestinal barrier’s functionality and integrity since stimulation of apoptosis occurs in epithelial cells. IL-6 is produced by phagocytes, epithelial cells, and mesenchymal cells. In the latter two, it influences the healing process, as it participates in the recruitment of polymorphonuclear leukocytes and macrophages (Mudter and Neurath, 2007). It also acts to prevent apoptosis of type T cells. On the other hand, IL-10, one of the main cytokines, is produced by innate and adaptive immune cells such as dendritic cells, macrophages, mast cells, natural killer cells, and Treg cells, among other cell types (Moore et al., 2001).
In IBD, the reduction in IL-10 production has already been associated with more severe clinical cases (Engelhardt and Grimbacher, 2014). IL-10 may trigger different signaling pathways related to anti-inflammatory activity and has already been considered a potential therapeutic target in IBD (Katsanos and Papadakis, 2017). Thus, by associating with higher levels of anti-inflammatory cytokines and decreased pro-inflammatory mediators, curcumin seems to exert a protective effect against inflammation.
Another line of evidence identified in the results of this systematic review indicates that curcumin can inhibit or reduce NF-κB activation. NF-κB is a factor that plays a central role in regulating the transcription of proinflammatory cytokine genes. Physiologically, the NF-κB transcription factor family in mammals consists of five proteins, p65 (RelA), RelB, c-Rel, p105/p50 (NF-κB1), and p100/52 (NF-κB2) that associate with each other to form distinct transcriptionally active homo and heterodimeric complexes (Oeckinghaus and Ghosh, 2009). By inhibiting the activation of transcription factors, curcumin interferes with the start-up of the process and the subsequent cascade of characteristic reactions of the inflammatory process by negatively regulating the suppression of multiple proinflammatory genes (Katsanos and Papadakis, 2017).
Some stimuli that culminate in NF-κB activation pass through cellular receptors that recognize pathogen-associated molecular patterns (PAMPs), especially TLR-4 (Ni et al., 2015). The stimulation of the TLR-4 isoform and the polymorphism of these receptors were identified as important mechanisms in the inflammatory stimulus in IBD (De Jager et al., 2007; Fukata and Abreu, 2008). The TLR-4-MyD88-NF-κB signaling pathway is activated when TLR-4 activates myeloid differentiation factor 88 (MyD88), which stimulates other molecules that act together for NF-κB activation with consequent expression of proinflammatory genes (Lubbad et al., 2009; Luo et al., 2020). Through these results, it seems that curcumin presents a mechanism of action: inhibition of the TLR-4-MyD88-NF-κB signaling pathway.
Intestinal tissue in IBD is histologically characterized by infiltration and accumulation of immune system cells in the mucous region, such as neutrophils and monocytes (Gui et al., 2018). In a complex tissue environment and loaded with proinflammatory molecules, these immune cells are probably recruited by chemotaxis mechanisms (Harbord et al., 2006). Neutrophils and monocytes release the MPO enzyme, whose activity may be able to indirectly reflect colonic inflammation (Chami et al., 2018). MPO may also be associated with the oxidative mechanisms of the inflamed colon, since this enzyme generates reactive species of oxygen (ROS), including hypochlorous acid (HOCl), through halogenation or peroxidase cycles (Myzak and Carr, 2002). The great number of experimental studies that observed a decrease in MPO activity, independent of dose, supplementation time, or moment of colitis, seem to indicate that curcuminoids can attenuate the infiltration of immune cells and reduce changes in the mucous region generated by intestinal inflammation in animals (Zeng et al., 2013).
Redox imbalance is related to inflammation and is characterized by an imbalance between oxidants and antioxidants, with a predominance of oxidation reactions (Vasconcelos et al., 2007). The gastrointestinal tract, which commonly interacts with food metabolites, microorganisms, and its own immune system, is a region that favored pro-oxidant molecules (Moura et al., 2015). Research conducted with animals and humans demonstrated the presence of redox imbalance in the disease by increasing the levels of markers of oxidative damage and reducing the level of antioxidant systems present in the blood, saliva, or colonic tissue. Lipid peroxidation, protein denaturation, and deoxyribonucleic acid (DNA) mutation are examples of damage caused by nitroxidative stress and interfere with the integrity of the intestinal mucosal barrier (Wang et al., 2016). Additionally, the evolution of IBD symptoms such as ulcer, toxic megacolon, and colorectal cancer has been related to the action of RONs (Moura et al., 2015; Moura et al., 2020).
The process of inflammation characteristic of IBD may incur gene transcription of some enzymes implicated in the endogenous generation process of reactive species. Peroxidases such as xanthine oxidase (XO), lipoxygenases (LPO), MPO, iNOS, COX, and the NADPH oxidase complex (NOX) (Balmus et al., 2016a; Balmus et al., 2016b) COX-2 are directly involved in ulceration formation and may be associated with inflammatory processes in the colon region and precancerous changes in the gastrointestinal tract (Binion et al., 2008; Camacho-Barquero et al., 2007; Mccarty, 2012). The results of the experimental studies in this systematic review demonstrate that oral curcumin supplementation can inhibit these enzymes directly or by blocking NF-κB.
In this systematic review, damage to the lipid membrane was the most evaluated cell damage and showed the best results after curcumin supplementation. Lipid peroxidation results in the formation of products such as TBARS and MDA (Niki, 2013). In this context, it has been shown that MDA is increased in IBD in animals and humans in different biological environments, such as saliva (Jahanshahi et al., 2004; Rezaie et al., 2006; Szeląg et al., 2012) and blood (Boehm et al., 2012; Alzoghaibi et al., 2007). Another finding of the study points to the existence of a positive correlation of MDA with Crohn’s Disease Activity Index (CDAI) and C-reactive protein (CRP) and a negative correlation with antioxidant defense (Szczeklik et al., 2018), composed of enzymatic and nonenzymatic biomarkers. The positive effects of curcumin/Curcuma longa on this cell damage marker suggest that this polyphenol/extract may act, protecting membrane integrity.
Although few studies, pointed out in this review, have evaluated the antioxidant defense system, represented by enzymes SOD, CAT, and GPx, and nonenzymatic system GSH, several studies on humans confirm that these biomarkers are altered in IBD (Guan and Lan, 2018; Szczeklik et al., 2018). SOD–represented by its three main isoforms SOD1 (Cu/ZnSOD) present in the cytosol, SOD2 (MnSOD) located in mitochondria, and SOD3 (Cu/ZnSOD) present in extracellular medium–catalyzes the conversion from anion radical superoxide (O2•-) to oxygen (O2); in sequence CAT–present in peroxisomes–and GPx–found in cytosol–convert O2 in hydrogen peroxide (H2O2). In turn, the GSH system (reduced glutathione–GSH–, oxidized (GSSG) and glutathione reductase–GR), a nonenzymatic system defense, also responds against oxidative damage, and their levels are altered in CD or UC (Pinto et al., 2013; Sido et al., 1998). The improvement or normalization of the levels of antioxidant defense in animals that received oral curcumin/Curcuma longa indicates that they may directly stimulate enzymatic end nonenzymatic synthesis/activity.
In the inflamed colon, the presence of macrophages and neutrophils that are stimulated by proinflammatory cytokines, as well as the recognition of bacterial lipopolysaccharides (LPS) by TRL-4, and the action of IFN-γ are associated with super expression of iNOS and consequent production of •NO (Camacho-Barquero et al., 2007; Motawi et al., 2012), which appears to be associated with a more severe inflammatory response and tissue injury, in experimental colitis (Motawi et al., 2012). On the other hand, •NO may interact with O2•- and generate peroxynitrate (-OONO) that can react with DNA (Cooke et al., 2003). In this context, the decrease in •NO levels observed in some animal studies (Altinel et al., 2020; Camacho-Barquero et al., 2007; Kao et al., 2016; Motawi et al., 2012; Venkataranganna et al., 2007; Ukil et al., 2003) may reflect the scavenging action of curcumin/Curcuma longa or their role in inhibiting iNOS activity. The alteration of the microbiome stands out as a factor involved in the pathogenesis of IBD (Guan, 2019). It is hypothesized that curcumin may interfere in the microbiome, either through the action of the compound and/or metabolites or the action of products resulting from the microbial metabolism of the substance itself. Regulatory effects can be attributed to modulation in the intestinal biome’s amount, diversity, and composition (Scazzocchio et al., 2020). This relationship between curcumin and microbiota can be confirmed in the study by Ohno et al. (2017), which when evaluating mice with DSS-induced colitis, observed that a diet consumption containing 2.0% (w/w) of curcumin for 18 days was able to stimulate the proliferation of bacteria Clostridium cluster IV and XIVa, which was accompanied by increased levels of fecal butyrate. Unfortunately, human studies have not yet tested this hypothesis, leaving this gap in the beneficial actions of curcumin in the health of patients with IBD.
Together, these beneficial actions reveal the protective role of curcuminoids/Curcuma longa against inflammation, redox imbalance, and dysbiosis and suggest the necessity of evaluating these biomarkers in human studies.
RCT: Systematic review and meta-analysis
The IBD is not a disease with an established cure; therefore, the treatment adopted in current protocols is nonspecific and aims to minimize symptoms, improve quality of life, achieve remission, and decrease complications of the disease (Abraham et al., 2017). In addition, IBD pathogenic mechanisms remain unclear, and global epidemiologic data suggest an important crosslink with socioeconomic development (Molodecky et al., 2012). Furthermore, IBD has a low mortality rate and is usually associated with complications such as colorectal cancer (CRC), infections and surgical complications. Its clinical manifestations, such as diarrhea, weight loss, and low digestive bleeding, have a great impact on the quality of life of its patients (Moura et al., 2015). Together, these data confirm IBD as an important global public health problem.
Conventional IBD therapy involves, in general, the use of sulfasalazine, corticosteroids, immunosuppressive agents, such as azathioprine, mercaptopurine or methotrexate (Lamb et al., 2019). In addition, is used the biological therapy, represented especially by the anti-TNF, anti-integrin, and anti-IL 12/23 drugs (Hindryckx et al., 2018). On the other hand, the adverse effects associated with the prolonged use of these drugs and the high rate of relapses significantly limit their use (Xu et al., 2004). In this context, the estimate indicates that 10%–30% of patients with UC and 38%–70% of patients with CD, with complications, will undergo some surgical intervention (Palacio et al., 2021) and the long-term collateral effects of drug therapy, together with the high cost of surgical management, leads the scientific community to investigate alternative treatments for IBD.
In the present meta-analysis, even with the variation in the rates of assessment of disease severity among RCTs, curcumin/Curcuma longa oral supplementation combined with conventional drug therapy was able to induce clinical remission and response in adult IBD patients. These outcomes were assessed by the score of different tools, such as Crohn’s Disease Activity Index (CDAI) (Sugimoto et al., 2020), Simple Clinical Colitis Activity Index (SCCAI) (Lang et al., 2015; Masoodi et al., 2018; Sadeghi et al., 2020) and Ulcerative Colitis Disease Activity Index (Mayo/UCDAI) (Banerjee et al., 2020; Kedia et al., 2017; Kumar et al., 2020), which measure disease activity through the severity of the presented symptoms (Table 4).
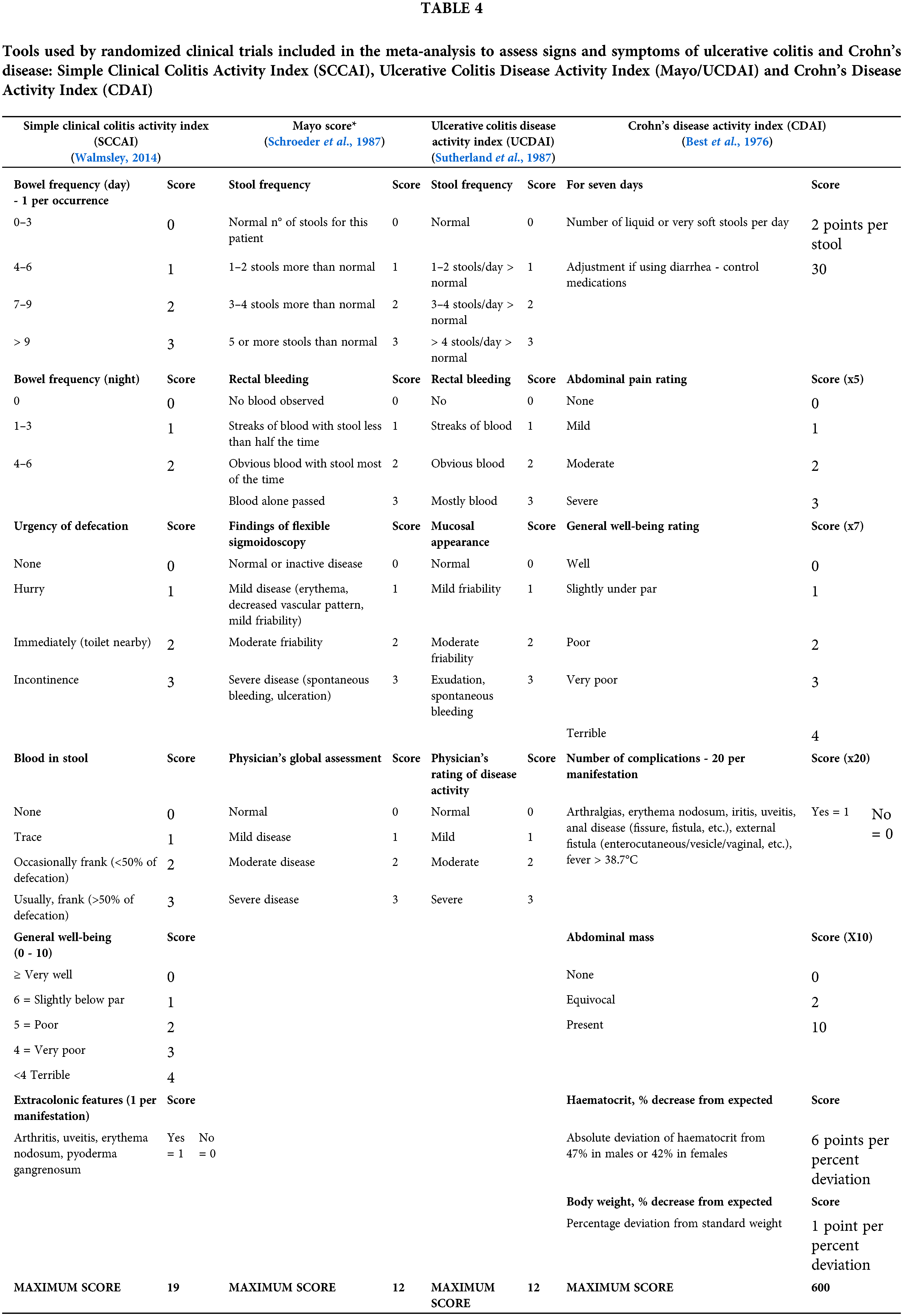
The SCCAI includes assessing six questions, including the number of nighttime evacuations and fecal urgency. This index was proposed by Walmsley et al. (1998) and has been used by several authors (Lang et al., 2015; Sadeghi et al., 2020; Schroeder et al., 1987; Walmsley, 2014). The Mayo Clinic Score and UCDAI are similar tools composed of four questions, and in addition to including clinical symptoms, they also evaluate endoscopic changes. Such tools are the most adopted in the methodology for assessing disease activity in RCTs (Bewtra et al., 2014). Although the CDAI is one of the tools commonly used in CD and evaluates the severity of several signs and symptoms, such as weight loss and anemia, it is considered an insufficient form since the questions included in the tool can be influenced by subjectivity, in addition to presenting little agreement with histological altars identified by the Global Histologic Activity Score (GHAS) (Tajra et al., 2019).
Despite the different methods used, clinical activity is an important parameter to assess IBD. The intensity with which symptoms manifest in the patient and endoscopic and histological changes can predict whether the disease is active. However, few tools have been validated to categorize the severity of the disease (Peyrin-Biroulet et al., 2016). Patients with active disease have a worse quality of life, increasing between 70% and 80% chances of having a recurrent episode in one year (Sairenji et al., 2017). In addition, IBD clinical activity can be an independent risk factor for extraintestinal manifestations, such as acute arterial disease, ischemic heart, and cerebrovascular arterial disease (Le Gall et al., 2018) and anemia (Parra et al., 2020).
Endoscopic evaluation is known to be recommended for diagnosis, monitoring and therapeutic evaluation of patients with IBD. Although endoscopic remission is not statistically evidenced in this meta-analysis, it is important to highlight those other methods are emerging as an alternative for the accompaniment of these patients, such as fecal markers (such as calprotectin), because they have lower cost and impact for these individuals, facilitating their performance, including in places of difficult access to imaging methods (common in poor or developing countries). In this context, we strongly suggest that the new human trials include these biomarkers aiming at an expanded view of the effects of curcumin (and other antioxidants) on the intestinal health of patients with IBD.
In this context, identifying therapies that reduce clinical activity, and consequently, lead and keep the patient in the remission phase is one of the main objectives of clinical research in IBD.
This study presents to the scientific community two-point of view about oral supplementation of curcumin/Curcuma longa: the impact of this polyphenol/extract on animal models of UC, and a systematic review and meta-analysis of RCTs. In both, beneficial effects were observed. However, RCTs still do not carry out analysis on the nitroxidative and anti-inflammatory impacts of curcumin, even with several confirmations observed both in vitro and in animal models. Additionally, the curcumin administration led to different effects in animal experiments and in clinical trials. Probably, these differences occurred because colitis in animal models is induced by external factor. Since IBD is a multifactorial disease, its induction cannot mimic the various risk factors involved, and for that, it is not possible to have the same results in animals and humans. However, the results in animals serve as a basis for conducting human trials.
Another unanswered question is whether curcumin changes its beneficial effects in symptomatic or asymptomatic colitis. Although animal studies show different models of colitis, in randomized studies, curcumin/curcuminoids were mostly tested in the acute phase of the disease. Likewise, studies are still insufficient to determine whether curcuma/curcuminoids is more effective among patients with CD or UC.
Given this information, new clinical studies’ objectives should confirm whether curcumin shows similar results in humans, which would justify its positive result on the clinical improvement found in this meta-analysis.
Future studies should evaluate the effect of Curcuma longa/curcuminoids on nitroxidative stress and its crosslink with inflammation and disease activity and remission.
Some questions, as listed below, should direct research efforts.
Which is the better moment to initiate supplementation: the symptomatic or the asymptomatic phase?
What are the better doses and period of treatment?
Does modified curcumin (nanoparticles, microparticles, combined, and other formulations) show beneficial effects vs. pure curcumin in humans?
In addition to these issues, it is necessary to be established reproducible models that ensure the evaluation of this bioavailability in different IBD scenarios.
However, despite these still unanswered questions, the analysis of the present data, allow to suggest that the oral prescription of Curcuma longa and/or curcumin, when associated with drug therapy, is safe and effective in the treatment of patients with IBD.
Author Contribution: Fabiana Andréa Moura designed the study; Nassib Bezerra Bueno analyzed data and performed statistical analysis; Marla de Cerqueira Alves, Monise Oliveira Santos and Orlando Roberto Pimentel de Araújo wrote the paper; Fabiana Andréa Moura and Nassib Bezerra Bueno had primary responsibility for the final content; Marília Oliveira Fonseca Goulart critically revised the paper for intellectual content and provided final approval of the manuscript; all authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq–Brazil) [Grant No. 435704/2018-4]; and Fundação de Amparo à Pesquisa do Estado de Alagoas (FAPEAL)/PPSUS/Ministério da Saúde (MS) [Grant No. 60030-000876/2016].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abraham BP, Ahmed T, Ali T (2017). Inflammatory bowel disease: Pathophysiology and current therapeutic approaches. Handbook of Experimental Pharmacology 239: 115–146. DOI 10.1007/978-3-319-56360-2. [Google Scholar] [CrossRef]
Actis GC, Pellicano R, Fagoonee S, Ribaldone DG (2019). History of inflammatory bowel diseases. Journal of Clinical Medicine 8: 1970. DOI 10.3390/jcm8111970. [Google Scholar] [CrossRef]
Aldini R, Budriesi R, Roda G, Micucci M, Ioan P et al. (2012). Curcuma longa extract exerts a myorelaxant effect on the ileum and colon in a mouse experimental colitis model, independent of the anti-inflammatory effect. PLoS One 7: e44650. DOI 10.1371/journal.pone.0044650. [Google Scholar] [CrossRef]
Altinel Y, Yalçın Ş, Ercan G, Yavuz E, Erçetin C et al. (2020). The efficacy of curcumin on PDGF expression and NF-Kappa B pathway: TNBS-Induced colitis. Ulusal Travma ve Acil Cerrahi Dergisi = Turkish Journal of Trauma & Emergency Surgery: TJTES 26: 663–670. DOI 10.14744/tjtes.2019.45570. [Google Scholar] [CrossRef]
Alzoghaibi MA, Al IA, Al-jebreen AM (2007). Lipid peroxides in patients with inflammatory bowel disease. Saudi Journal of Gastroenterology: Official Journal of the Saudi Gastroenterology Association 13: 187–190. DOI 10.4103/1319-3767.36750. [Google Scholar] [CrossRef]
Balmus IM, Ciobica A, Trifan A, Stanciu C (2016a). The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: Clinical aspects and animal models. Saudi Journal of Gastroenterology 22: 3–17. DOI 10.4103/1319-3767.173753. [Google Scholar] [CrossRef]
Balmus IM, Ciobica A, Antioch I, Dobrin R, Timofte D (2016b). Oxidative stress implications in the affective disorders: Main biomarkers, animal models relevance, genetic perspectives, and antioxidant approaches. Oxidative Medicine and Cellular Longevity 3975101: 1–25. DOI 10.1155/2016/3975101. [Google Scholar] [CrossRef]
Banerjee R, Pal P, Penmetsa A, Kathi P, Girish G et al. (2020). Novel bioenhanced curcumin with mesalamine for induction of clinical and endoscopic remission in mild-to-moderate ulcerative colitis: A randomized double-blind placebo-controlled pilot study. Journal of Clinical Gastroenterology 55: 702–708. DOI 10.1097/MCG.0000000000001416. [Google Scholar] [CrossRef]
Bastaki SMA, Al Ahmed MM, Al Zaabi A, Amir N, Adeghate E (2016). Effect of turmeric on colon histology, body weight, ulcer, IL-23, MPO and glutathione in acetic-acid-induced inflammatory bowel disease in rats. BMC Complementary and Alternative Medicine 16: 72. DOI 10.1186/s12906-016-1057-5. [Google Scholar] [CrossRef]
Beloqui A, Coco R, Memvanga PB, Ucakar B, Des Rieux A et al. (2014). PH-Sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. International Journal of Pharmaceutics 473: 203–212. DOI 10.1016/j.ijpharm.2014.07.009. [Google Scholar] [CrossRef]
Beloqui A, Memvanga PB, Coco R, Reimondez-Troitiño S, Alhouayek M et al. (2016). A comparative study of curcumin-loaded lipid-based nanocarriers in the treatment of inflammatory bowel disease. Colloids and Surfaces B: Biointerfaces 143: 327–335. DOI 10.1016/j.colsurfb.2016.03.038. [Google Scholar] [CrossRef]
Best WR, Becktel JM, Singleton JW, Kern FJr (1976). Development of a crohn’s disease activity index. Gastroenterology 70: 439–444. DOI 10.1016/S0016-5085(76)80163-1. [Google Scholar] [CrossRef]
Bewtra M, Brensinger CM, Tomov VT, Hoang TB, Sokach CE et al. (2014). An optimized patient-reported ulcerative colitis disease activity measure derived from the mayo score and the simple clinical colitis activity index. Inflammatory Bowel Diseases 20: 1. DOI 10.1097/MIB.0000000000000053. [Google Scholar] [CrossRef]
Billerey-Larmonier C, Uno JK, Larmonier N, Midura AJ, Timmermann B et al. (2008). Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflammatory Bowel Diseases 14: 780–793. DOI 10.1002/ibd.20348. [Google Scholar] [CrossRef]
Binion DG, Otterson MF, Rafiee P (2008). Curcumin inhibits VEGF-Mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57: 1509–1517. DOI 10.1136/gut.2008.152496. [Google Scholar] [CrossRef]
Boehm D, Krzystek-Korpacka M, Neubauer K, Matusiewicz M, Paradowski L et al. (2012). Lipid peroxidation markers in crohn’s disease: The associations and diagnostic value. Clinical Chemistry and Laboratory Medicine 50: 1359–1366. DOI 10.1515/cclm-2011-0817. [Google Scholar] [CrossRef]
Bommelaer G, Laharie D, Nancey S, Hebuterne X, Roblin X et al. (2020). Oral curcumin no more effective than placebo in preventing recurrence of crohn’s disease after surgery in a randomized controlled trial. Clinical Gastroenterology and Hepatology 18: 1553–1560.e1. DOI 10.1016/j.cgh.2019.08.041. [Google Scholar] [CrossRef]
Camacho-Barquero L, Villegas I, Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S et al. (2007). Curcumin, a Curcuma longa constituent, acts on MAPK P38 pathway modulating COX-2 and INOS expression in chronic experimental colitis. International Immunopharmacology 7: 333–342. DOI 10.1016/j.intimp.2006.11.006. [Google Scholar] [CrossRef]
Chami B, Martin NJJ, Dennis JM, Witting PK (2018). Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Archives of Biochemistry and Biophysics 645: 61–71. DOI 10.1016/j.abb.2018.03.012. [Google Scholar] [CrossRef]
Chen Q, Gou S, Huang Y, Zhou X, Li Q et al. (2018). Facile fabrication of bowl-shaped microparticles for oral curcumin delivery to ulcerative colitis tissue. Colloids and Surfaces B: Biointerfaces 169: 92–98. DOI 10.1016/j.colsurfb.2018.05.012. [Google Scholar] [CrossRef]
Chen Q, Gou S, Ma P, Song H, Zhou X et al. (2019). Oral administration of colitis tissue-accumulating porous nanoparticles for ulcerative colitis therapy. International Journal of Pharmaceutics 557: 135–144. DOI 10.1016/j.ijpharm.2018.12.046. [Google Scholar] [CrossRef]
Chen Q, Si X, Ma L, Ma P, Hou M et al. (2017). Oral delivery of curcumin via porous polymeric nanoparticles for effective ulcerative colitis therapy. Journal of Materials Chemistry 5: 5881–5891. DOI 10.1039/C7TB00328E. [Google Scholar] [CrossRef]
Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003). Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB Journal 17: 1195–1214. DOI 10.1096/fj.02-0752rev. [Google Scholar] [CrossRef]
Cooney JM, Barnett MP, Dommels YE, Brewster D, Butts CA et al. (2016). A combined omics approach to evaluate the effects of dietary curcumin on colon inflammation in the Mdr1a-/- Mouse model of inflammatory bowel disease. Journal of Nutritional Biochemistry 27: 181–192. DOI 10.1016/j.jnutbio.2015.08.030. [Google Scholar] [CrossRef]
Cunha Neto F, Marton LT, de Marqui SV, Lima TA, Barbalho SM (2019). Curcuminoids from curcuma longa: New adjuvants for the treatment of crohn’s disease and ulcerative colitis? Critical Reviews in Food Science and Nutrition 59: 2136–2143. DOI 10.1080/10408398.2018.1456403. [Google Scholar] [CrossRef]
de Jager PL, Franchimont D, Waliszewska A, Bitton A, Cohen A et al. (2007). The role of the Toll receptor pathway in susceptibility to inflammatory bowel diseases. Genes and Immunity 8: 387–397. DOI 10.1038/sj.gene.6364398. [Google Scholar] [CrossRef]
Deguchi Y, Andoh A, Inatomi O, Yagi Y, Bamba S et al. (2007). Curcumin prevents the development of dextran sulfate sodium (DSS)-Induced experimental colitis. Digestive Diseases and Sciences 52: 2993–2998. DOI 10.1007/s10620-006-9138-9. [Google Scholar] [CrossRef]
Dinarello CA, Novick D, Kim S, Kaplanski G (2013). Interleukin-18 and IL-18 binding protein. Frontiers in Immunology 8: 289. DOI 10.3389/fimmu.2013.00289. [Google Scholar] [CrossRef]
Duque AG, Descoteaux A (2014). Macrophage cytokines: Involvement in immunity and infectious diseases. Frontiers in Immunology 5: 491. DOI 10.3389/fimmu.2014.00491. [Google Scholar] [CrossRef]
Engelhardt KR, Grimbacher B (2014). IL-10 in humans: Lessons from the gut, IL-10/IL-10 receptor deficiencies, and IL-10 polymorphisms. Current Topics in Microbiology and Immunology 380: 1–18. DOI 10.1007/978-3-662-43492-5. [Google Scholar] [CrossRef]
Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE et al. (2012). Curcumin-from molecule to biological function. Angewandte Chemie-International Edition 51: 5308–5332. DOI 10.1002/anie.201107724. [Google Scholar] [CrossRef]
Friedrich M, Pohin M, Powrie F Cytokine (2019). Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 50: 992–1006. DOI 10.1016/j.immuni.2019.03.017. [Google Scholar] [CrossRef]
Fukata M, Abreu MT (2008). Role of toll-like receptors in gastrointestinal malignancies. Oncogene 27: 234–243. DOI 10.1038/sj.onc.1210908. [Google Scholar] [CrossRef]
Gopu B, Dileep R, Rani MU, Kumar CS, Kumar MV, Reddy AG (2015). Protective role of curcumin and flunixin against acetic acid-induced inflammatory bowel disease via modulating inflammatory mediators and cytokine profile in rats. Journal of Environmental Pathology, Toxicology and Oncology 34: 309–320. DOI 10.1615/JEnvironPatholToxicolOncol.2015013049. [Google Scholar] [CrossRef]
Guan G, Lan S (2018). Implications of antioxidant systems in inflammatory bowel disease. Bowel Disease. BioMed Research International 1290179: 1–7. DOI 10.1155/2018/1290179. [Google Scholar] [CrossRef]
Guan Q (2019). A comprehensive review and update on the pathogenesis of inflammatory bowel disease. Journal of Immunology Research 7247238: 1–16. DOI 10.1155/2019/7247238. [Google Scholar] [CrossRef]
Gui X, Li J, Ueno A, Iacucci M, Qian J et al. (2018). Histopathological features of inflammatory bowel disease are associated with different CD4+T cell subsets in colonic mucosal lamina propria. Journal of Crohn’s and Colitis 12: 1448–1458. DOI 10.1093/ecco-jcc/jjy116. [Google Scholar] [CrossRef]
Harbord MW, Marks DJ, Forbes A, Bloom SL, Day RM et al. (2006). Impaired neutrophil chemotaxis in Crohn’s disease relates to reduced production of chemokines and can be augmented by granulocyte-colony stimulating factor. Alimentary Pharmacology & Therapeutics 24: 651–660. DOI 10.1111/j.1365-2036.2006.03016.x. [Google Scholar] [CrossRef]
Hindryckx P, Casteele NV, Novak G, Khanna R, D’Haens G et al. (2018). The expanding therapeutic armamentarium for inflammatory bowel disease: How to choose the right drug[s] for our patients? Journal of Crohn’s & Colitis 12: 105–119. DOI 10.1093/ecco-jcc/jjx117. [Google Scholar] [CrossRef]
Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE et al. (2004). Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Digestive Diseases and Sciences 49: 1752–1757. DOI 10.1007/s10620-004-9564-5. [Google Scholar] [CrossRef]
Jantawong C, Priprem A, Intuyod K, Pairojkul C, Pinlaor P et al. (2021). Curcumin-loaded nanocomplexes: Acute and chronic toxicity studies in mice and hamsters. Toxicology Reports 8: 1346–1357. DOI 10.1016/j.toxrep.2021.06.021. [Google Scholar] [CrossRef]
Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC et al. (2005). Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World Journal of Gastroenterology 11: 1747–1752. DOI 10.3748/wjg.v11.i12.1747. [Google Scholar] [CrossRef]
Jurenka JS (2009). Anti-Inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Alternative Medicine Review 14: 141–153. [Google Scholar]
Kao NJ, Hu JY, Wu CS, Kong ZL (2016). Curcumin represses the activity of inhibitor-κb kinase in dextran sulfate sodium-induced colitis by S-Nitrosylation. International Immunopharmacology 38: 1–7. DOI 10.1016/j.intimp.2016.05.015. [Google Scholar] [CrossRef]
Katsanos KH, Papadakis KA (2017). Inflammatory bowel disease: Updates on molecular targets for biologics, 11: 455–463 DOI 10.5009/gnl16308. [Google Scholar] [CrossRef]
Kedia S, Bhatia V, Thareja S, Garg S, Mouli VP et al. (2017). Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: Results from a randomized double blind placebo controlled trial. World Journal of Gastrointestinal Pharmacology and Therapeutics 8: 147–154. DOI 10.4292/wjgpt.v8.i2.147. [Google Scholar] [CrossRef]
Kesharwani SS, Ahmad R, Bakkari MA, Rajput MKS, Dachineni R et al. (2018). Site-Directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBDFormulation development, characterization and pharmacological evaluation. Journal of Controlled Release 290: 165–179. DOI 10.1016/j.jconrel.2018.08.004. [Google Scholar] [CrossRef]
Kotha RR, Luthria DL (2019). Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 24: 2930. DOI 10.3390/molecules24162930. [Google Scholar] [CrossRef]
Kumar A, Singh R, Yadav A, Giri DD, Singh PK et al. (2016). Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech 6: 60. DOI 10.1007/s13205-016-0393-y. [Google Scholar] [CrossRef]
Kumar S, Dutta U, Shah J, Singh P, Vaishnavi C et al. (2020). Impact of curcuma longa on disease activity in patients with ulcerative colitis: A double-blind randomized trial (Data no published). [Google Scholar]
Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ et al. (2019). IBD guidelines e delphi consensus group, British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68: s1–s106. DOI 10.1136/gutjnl-2019-318484. [Google Scholar] [CrossRef]
Lang A, Salomon N, Wu JCY, Kopylov U, Lahat A et al. (2015). Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clinical Gastroenterology and Hepatology 13: 1444-9.e1. DOI 10.1016/j.cgh.2015.02.019. [Google Scholar] [CrossRef]
Larmonier CB, Uno JK, Lee KM, Karrasch T, Laubitz D et al. (2008). Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. American Journal of Physiology. Gastrointestinal and Liver Physiology 295: G1079–91. DOI 10.1152/ajpgi.90365.2008. [Google Scholar] [CrossRef]
Le Gall G, Kirchgesner J, Bejaoui M, Landman C, Nion-Larmurier I et al. (2018). Clinical activity is an independent risk factor of ischemic heart and cerebrovascular arterial disease in patients with inflammatory bowel disease. Edited by Emiko Mizoguchi. PLoS One 13: e0201991. DOI 10.1371/journal.pone.0201991. [Google Scholar] [CrossRef]
Liu L, Liu YL, Liu GX, Chen X, Yang K et al. (2013). Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking stat3 signaling pathway. International Immunopharmacology 17: 314–320. DOI 10.1016/j.intimp.2013.06.020. [Google Scholar] [CrossRef]
Lubbad A, Oriowo MA, Khan I (2009). Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Molecular and Cellular Biochemistry 322: 127–135. DOI 10.1007/s11010-008-9949-4. [Google Scholar] [CrossRef]
Lucas M, Freitas M, Xavier JA, Moura FA, Goulart MOF et al. (2021). The scavenging effect of curcumin, piperine and their combination against physiological relevant reactive pro-oxidant species using in vitro non-cellular and cellular models. Chemical Papers 75: 5269–5277. DOI 10.1007/s11696-021-01710-y. [Google Scholar] [CrossRef]
Luo R, Lin M, Zhang C, Shi J, Zhang S et al. (2020). Genipin-crosslinked human serum albumin coating using a tannic acid layer for enhanced oral administration of curcumin in the treatment of ulcerative colitis. Food Chemistry 330: 127241. DOI 10.1016/j.foodchem.2020.127241. [Google Scholar] [CrossRef]
Maiti P, Dunbar GL (2018). Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. International Journal of Molecular Sciences 19: 1637. DOI 10.3390/ijms19061637. [Google Scholar] [CrossRef]
Martelli L, Ragazzi E, di Mario F, Martelli M, Castagliuolo I et al. (2007). A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society 19: 668–674. DOI 10.1111/j.1365-2982.2007.00928.x. [Google Scholar] [CrossRef]
Martínez N, Herrera M, Frías L, Provencio M, Pérez-Carrión R et al. (2019). A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: Results of a pilot study. Clinical and Translational Oncology 21: 489–498. DOI 10.1007/s12094-018-1950-0. [Google Scholar] [CrossRef]
Martins ASP, Campos SBG, Goulart MOF, Moura FA (2021). Extraintestinal manifestations of inflammatory bowel disease, nitroxidative stress and dysbiosis: What is the link between them? BIOCELL 45: 461–481. DOI 10.32604/biocell.2021.014332. [Google Scholar] [CrossRef]
Masoodi M, Mahdiabadi MA, Mokhtare M, Agah S, Kashani AHF et al. (2018). The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients’ self-reported well-being: A randomized double-blind controlled trial. Journal of Cellular Biochemistry 119: 9552–9559. DOI 10.1002/jcb.27273. [Google Scholar] [CrossRef]
Mccarty MF (2012). Minimizing the cancer-promotional activity of Cox-2 as a central strategy in cancer prevention. Medical Hypotheses 78: 45–57. DOI 10.1016/j.mehy.2011.09.039. [Google Scholar] [CrossRef]
Mohammadi E, Tamaddoni A, Qujeq D, Nasseri E, Zayeri F et al. (2018). An investigation of the effects of curcumin on iron overload, hepcidin level, and liver function in β-thalassemia major patients: A double-blind randomized controlled clinical trial. Phytotherapy Research 32: 1828–1835. DOI 10.1002/ptr.6118. [Google Scholar] [CrossRef]
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M et al. (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142: 46–54.e42. DOI 10.1053/j.gastro.2011.10.001. [Google Scholar] [CrossRef]
Momtazi-Borojeni AA, Haftcheshmeh SM, Esmaeili SA, Johnston TP, Abdollahi E, Sahebkar A (2018). Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmunity Reviews 17: 125–135. DOI 10.1016/j.autrev.2017.11.016. [Google Scholar] [CrossRef]
Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A (2001). Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology 19: 683–765. DOI 10.1146/annurev.immunol.19.1.683. [Google Scholar] [CrossRef]
Motawi TK, Rizk SM, Shehata AH (2012). Effects of curcumin and ginkgo biloba on matrix metalloproteinases gene expression and other biomarkers of inflammatory bowel disease. Journal of Physiology and Biochemistry 68: 529–539. DOI 10.1007/s13105-012-0168-9. [Google Scholar] [CrossRef]
Moura FA, de Andrade KQ, Dos Santos JCF, Araújo ORP, Goulart MOF (2015). Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biology 6: 617–639. DOI 10.1016/j.redox.2015.10.006. [Google Scholar] [CrossRef]
Moura FA, Goulart MOF, Campos SBG, Martins ASP (2020). The close interplay of nitro-oxidative stress, advanced glycation end products and inflammation in inflammatory bowel diseases. Current Medicinal Chemistry 27: 2059–2076. DOI 10.2174/0929867325666180904115633. [Google Scholar] [CrossRef]
Mudter J, Neurath MF (2007). IL-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflammatory Bowel Diseases 13: 1016–1023. DOI 10.1002/ibd.20148. [Google Scholar] [CrossRef]
Myzak MC, Carr AC (2002). Myeloperoxidase-Dependent caspase-3 activation and apoptosis in HL-60 cells: Protection by the antioxidants ascorbate and (Dihydro) lipoic acid. Redox Report: Communications in Free Radical Research 7: 47–53. DOI 10.1179/135100002125000181. [Google Scholar] [CrossRef]
Nones K, Dommels YE, Martell S, Butts C, McNabb WC et al. (2009). The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (Mdr1a-/-) mice, a model of inflammatory bowel diseases. British Journal of Nutrition 101: 169–181. DOI 10.1017/S0007114508009847. [Google Scholar] [CrossRef]
Ni H, Jin W, Zhu T, Wang J, Yuan B et al. (2015). Curcumin modulates TLR4/NF-κB inflammatory signaling pathway following traumatic spinal cord injury in rats. Journal of Spinal Cord Medicine 38: 199–206. DOI 10.1179/2045772313Y.0000000179. [Google Scholar] [CrossRef]
Niki E (2013). Biomarkers of lipid peroxidation in clinical material. Biochimica et Biophysica Acta 1840: 809–817. DOI 10.1016/j.bbagen.2013.03.020. [Google Scholar] [CrossRef]
Oeckinghaus A, Ghosh S (2009). The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor perspectives in biology 1: a000034. DOI 10.1101/cshperspect.a000034. [Google Scholar] [CrossRef]
Ohno M, Nishida A, Sugitani Y, Nishino K, Inatomi O et al. (2017). Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS One 12: e0185999. DOI 10.1371/journal.pone.0185999. [Google Scholar] [CrossRef]
Oliver JM, Stoner L, Rowlands DS, Caldwell AR, Sanders E et al. (2016). Novel form of curcumin improves endothelial function in young, healthy individuals: A double-blind placebo controlled study. Journal of Nutrition and Metabolism 1089653: 1–6. DOI 10.1155/2016/1089653. [Google Scholar] [CrossRef]
Oshi MA, Lee J, Naeem M, Hasan N, Kim J et al. (2020). Curcumin nanocrystal/PH-responsive polyelectrolyte multilayer core-shell nanoparticles for inflammation-targeted alleviation of ulcerative colitis. Biomacromolecules 21: 3571–3581. DOI 10.1021/acs.biomac.0c00589. [Google Scholar] [CrossRef]
Palacio F, de Souza L, Moreira J, Luiz RR, de Souza H, Zaltman C (2021). Hospitalization and surgery rates in patients with inflammatory bowel disease in Brazil: A time-trend analysis. BMC Gastroenterology 21: 192. DOI 10.1186/s12876-021-01781-x. [Google Scholar] [CrossRef]
Panahi Y, Khalili N, Sahebi E, Namazi S, Simental-Mendía LE et al. (2018). Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled trial. Drug Research 68: 403–409. DOI 10.1055/s-0044-101752. [Google Scholar] [CrossRef]
Parra RS, Feitosa MR, Ferreira SDC, Rocha JJRD, Troncon LEA et al. (2020). Anemia and iron deficiency an inflammatory bowel disease patients an a referral center in Brazil: Prevalence and risk factors. Arquivos de Gastroenterologia 57: 272–277. DOI 10.1590/s0004-2803.202000000-51. [Google Scholar] [CrossRef]
Peyrin-Biroulet L, Panés J, Sandborn WJ, Vermeire S, Danese S et al. (2016). Defining disease severity in inflammatory bowel diseases: Current and future directions. Clinical Gastroenterology and Hepatology 14: 348–354.e17. DOI 10.1016/j.cgh.2015.06.001. [Google Scholar] [CrossRef]
Pinto MA, Lopes MS, Bastos ST, Reigada CL, Dantas RF et al. (2013). Does active crohn’s disease have decreased intestinal antioxidant capacity? Journal of Crohn’s and Colitis 7: e358–66. DOI 10.1016/j.crohns.2013.02.010. [Google Scholar] [CrossRef]
Qiao H, Fang D, Chen J, Sun Y, Kang C et al. (2017). Orally delivered polycurcumin responsive to bacterial reduction for targeted therapy of inflammatory bowel disease. Drug Delivery 24: 233–242. DOI 10.1080/10717544.2016.1245367. [Google Scholar] [CrossRef]
Qiu P, Man S, Li J, Liu J, Zhang L et al. (2016). overdose intake of curcumin initiates the unbalanced state of bodies. Journal of Agricultural and Food Chemistry 64: 2765–2771. DOI 10.1021/acs.jafc.6b00053. [Google Scholar] [CrossRef]
Rezaie A, Ghorbani F, Eshghtork A, Zamani MJ, Dehghan G et al. (2006). Alterations in salivary antioxidants, nitric oxide, and transforming growth factor beta-1 in relation to disease activity in crohn’s disease patients. Annals of the New York Academy of Sciences 1091: 110–122. DOI 10.1196/annals.1378.060. [Google Scholar] [CrossRef]
Rotrekl D, Šalamúnová P, Paráková L, Baďo O, Saloň I et al. (2021). Composites of yeast glucan particles and curcumin lead to improvement of dextran sulfate sodium-induced acute bowel inflammation in rats. Carbohydrate Polymers 252: 117142. DOI 10.1016/j.carbpol.2020.117142. [Google Scholar] [CrossRef]
Sadeghi N, Mansoori A, Shayesteh A, Hashemi SJ (2020). The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytotherapy Research 34: 1123–1133. DOI 10.1002/ptr.6581. [Google Scholar] [CrossRef]
Sairenji T, Collins KL, Evans DV (2017). An update on inflammatory bowel disease. Primary Care-Clinics in Office Practice 44: 673–692. DOI 10.1016/j.pop.2017.07.010. [Google Scholar] [CrossRef]
Salh B, Assi K, Templeman V, Parhar K, Owen D et al. (2003). Curcumin attenuates DNB-induced murine colitis. american journal of physiology. Gastrointestinal and Liver Physiology 285: G235–43. DOI 10.1152/ajpgi.00449.2002. [Google Scholar] [CrossRef]
Samba-Mondonga M, Constante M, Fragoso G, Calvé A, Santos MM (2019). Curcumin induces mild anemia in a DSS-induced colitis mouse model maintained on an iron-sufficient diet. PLoS One 14: e0208677. DOI 10.1371/journal.pone.0208677. [Google Scholar] [CrossRef]
Sands BE, Kaplan GG (2007). The role of TNFα in ulcerative colitis. Journal of Clinical Pharmacology 47: 930–941. DOI 10.1177/0091270007301623. [Google Scholar] [CrossRef]
Sareen R, Jain N, Rajkumari A, Dhar KL (2016). PH triggered delivery of curcumin from eudragit-coated chitosan microspheres for inflammatory bowel disease: Characterization and pharmacodynamic evaluation. Drug Delivery 23: 55–62. DOI 10.3109/10717544.2014.903534. [Google Scholar] [CrossRef]
Sareen R, Nath K, Jain N, Dhar KL (2014). Curcumin loaded microsponges for colon targeting in inflammatory bowel disease: Fabrication, optimization, and in vitro and pharmacodynamic evaluation. BioMed Research International 340701: 1–7. DOI 10.1155/2014/340701. [Google Scholar] [CrossRef]
Scazzocchio B, Minghetti L, D’Archivio M (2020). Interaction between gut microbiota and curcumin: A new key of understanding for the health effects of curcumin. Nutrients 12: 2499. DOI 10.3390/nu12092499. [Google Scholar] [CrossRef]
Schroeder KW, Tremaine WJ, Ilstrup DM (1987). Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. New England Journal of Medicine 317: 1625–1629. DOI 10.1056/NEJM198712243172603. [Google Scholar] [CrossRef]
Sheethal S, Ratheesh M, Jose SP, Asha S, Krishnakumar IM et al. (2020). Anti-ulcerative effect of curcumin-galactomannoside complex on acetic acid-induced experimental model by inhibiting inflammation and oxidative stress. Inflammation 43: 1411–1422. DOI 10.1007/s10753-020-01218-9. [Google Scholar] [CrossRef]
Shigeshiro M, Tanabe S, Suzuki T (2013). Dietary polyphenols modulate intestinal barrier defects and inflammation in a murine model of colitis. Journal of Functional Foods 5: 949–955. DOI 10.1016/j.jff.2013.02.008. [Google Scholar] [CrossRef]
Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C et al. (1998). Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut 42: 485–492. DOI 10.1136/gut.42.4.485. [Google Scholar] [CrossRef]
Soleimani V, Sahebkar A, Hosseinzadeh H (2018). Turmeric (Curcuma Longa) and its major constituent (Curcumin) as nontoxic and safe substances: Review. Phytotherapy Research 32: 985–995. DOI 10.1002/ptr.6054. [Google Scholar] [CrossRef]
Stanić Z (2017). Curcumin, a compound from natural sources, a true scientific challenge–A review. Plant Foods for Human Nutrition 72: 1–12. DOI 10.1007/s11130-016-0590-1. [Google Scholar] [CrossRef]
Sueth-Santiago V, Mendes-Silva GP, Decoté-Ricardo D, de Lima MEF (2015). Curcumina, o Pó Dourado Do Açafrão-Da-Terra: Introspecções sobre química e atividades biológicas. Quimica Nova 38: 538–552. DOI 10.5935/0100-4042.20150035. [Google Scholar] [CrossRef]
Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M et al. (2002). Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 123: 1912–1922. DOI 10.1053/gast.2002.37050. [Google Scholar] [CrossRef]
Sugimoto K, Ikeya K, Bamba S, Andoh A, Yamasaki H et al. (2020). Highly bioavailable curcumin derivative ameliorates crohn’s disease symptoms: A randomized, double-blind, multicenter study. Journal of Crohn’s and Colitis 14: 1693–1701. DOI 10.1093/ecco-jcc/jjaa097. [Google Scholar] [CrossRef]
Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N et al. (1987). 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology 92: 1894–1898. DOI 10.1016/0016-5085(87)90621-4. [Google Scholar] [CrossRef]
Szczeklik K, Krzyściak W, Cibor D, Domagała-Rodacka R, Pytko-Polończyk J et al. (2018). Markers of lipid peroxidation and antioxidant status in the serum and saliva of patients with active crohn disease. Polish Archives of Internal Medicine 128: 362–370. DOI 10.20452/pamw.4273. [Google Scholar] [CrossRef]
Szeląg M, Mikulski D, Molski M (2012). Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites. Journal of Molecular Modeling 18: 2907–2916. DOI 10.1007/s00894-011-1306-y. [Google Scholar] [CrossRef]
Tajra JB, Calegaro JU, de Paula AP, Bachour D, Silveira D et al. (2019). Correlation and concordance measures between clinical, endoscopic and histological scores activity in Crohn’s disease under treatment. Scandinavian Journal of Gastroenterology 54: 441–445. DOI 10.1080/00365521.2019.1596305. [Google Scholar] [CrossRef]
Toden S, Theiss AL, Wang X, Goel A (2017). Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Scientific Reports 7: 814. DOI 10.1038/s41598-017-00812-6. [Google Scholar] [CrossRef]
Topcu-Tarladacalisir Y, Akpolat M, Uz YH, Kizilay G, Sapmaz-Metin M et al. (2013). Effects of curcumin on apoptosis and oxidoinflammatory regulation in a rat model of acetic acid-induced colitis: The roles of c-Jun N-Terminal kinase and P38 mitogen-activated protein kinase. Journal of Medicinal Food 16: 296–305. DOI 10.1089/jmf.2012.2550. [Google Scholar] [CrossRef]
Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR et al. (2003). Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. British Journal of Pharmacology 139: 209–218. DOI 10.1038/sj.bjp.0705241. [Google Scholar] [CrossRef]
Ung VY, Foshaug RR, MacFarlane SM, Churchill TA, Doyle JS et al. (2010). Oral administration of curcumin emulsified in carboxymethyl cellulose has a potent anti-inflammatory effect in the IL-10 gene-deficient mouse model of IBD. Digestive Diseases and Sciences 55: 1272–1277. DOI 10.1007/s10620-009-0843-z. [Google Scholar] [CrossRef]
Vasconcelos SML, Goulart MOF, de Moura JB, Manfredini V, Benfato MS et al. (2007). Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: Principais métodos analíticos para sua determinação. Química Nova 30: 1323–1338. DOI 10.1590/S0100-40422007000500046. [Google Scholar] [CrossRef]
Venkataranganna MV, Rafiq M, Gopumadhavan S, Peer G, Babu UV et al. (2007). NCB-02 (standardized curcumin preparation) protects dinitrochlorobenzene-induced colitis through down-regulation of NFkappa-B and INOS. World Journal of Gastroenterology 13: 1103–1107. DOI 10.3748/wjg.v13.i7.1103. [Google Scholar] [CrossRef]
Walmsley RS (2014). Comment on an optimized patient-reported ulcerative colitis disease activity measure derived from the mayo score and the simple clinical colitis activity index. Inflammatory Bowel Diseases 20: E25–E26. DOI 10.1097/MIB.0000000000000248. [Google Scholar] [CrossRef]
Walmsley RS, Ayres RCS, Pounder RE, Allan RN (1998). A simple clinical colitis activity index. Gut 43: 29–32. DOI 10.1136/gut.43.1.29. [Google Scholar] [CrossRef]
Wang Z, Li S, Cao Y, Tian X, Zeng R et al. (2016). Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxidative Medicine and Cellular Longevity 9875298: 1–15. DOI 10.1155/2016/9875298. [Google Scholar] [CrossRef]
Wei C, Wang JY, Xiong F, Wu BH, Luo MH et al. (2021). Curcumin ameliorates DSS‐induced colitis in mice by regulating the Treg/Th17 signaling pathway. Molecular Medicine Reports 23: 34. DOI 10.3892/mmr.2020.11672. [Google Scholar] [CrossRef]
Xiao B, Si X, Zhang M, Merlin D (2015). Oral administration of ph-sensitive curcumin-loaded microparticles for ulcerative colitis therapy. Colloids and Surfaces B: Biointerfaces 135: 379–385. DOI 10.1016/j.colsurfb.2015.07.081. [Google Scholar] [CrossRef]
Xu CT, Meng SY, Pan BR (2004). Drug therapy for ulcerative colitis. World Journal of Gastroenterology 10: 2311. DOI 10.3748/wjg.v10.i16.2311. [Google Scholar] [CrossRef]
Yadav VR, Suresh S, Devi K, Yadav S (2009a). Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech 10: 752–762. DOI 10.1208/s12249-009-9264-8. [Google Scholar] [CrossRef]
Yadav VR, Suresh S, Devi K, Yadav S (2009b). Novel formulation of solid lipid microparticles of curcumin for anti-angiogenic and anti-inflammatory activity for optimization of therapy of inflammatory bowel disease. Journal of Pharmacy and Pharmacology 61: 311–321. DOI 10.1211/jpp.61.03.0005. [Google Scholar] [CrossRef]
Yang JY, Zhong X, Kim SJ, Kim DH, Kim HS et al. (2018). Comparative effects of curcumin and tetrahydrocurcumin on dextran sulfate sodium-induced colitis and inflammatory signaling in mice. Journal of Cancer Prevention 23: 18–24. DOI 10.15430/JCP.2018.23.1.18. [Google Scholar] [CrossRef]
Yang JY, Zhong X, Yum HW, Lee HJ, Kundu JK et al. (2013). Curcumin inhibits STAT3 signaling in the colon of dextran sulfate sodium-treated mice. Journal of Cancer Prevention 18: 186–191. DOI 10.15430/JCP.2013.18.2.186. [Google Scholar] [CrossRef]
Yang M, Wang J, Yang C, Han H, Rong W, Zhang G (2017). Oral administration of curcumin attenuates visceral hyperalgesia through inhibiting phosphorylation of TRPV1 in rat model of ulcerative colitis. Molecular Pain 13: 1744806917726416. DOI 10.1177/1744806917726416. [Google Scholar] [CrossRef]
Yavarpour-Bali H, Ghasemi-Kasman M, Pirzadeh M (2019). Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. International Journal of Nanomedicine 14: 4449–4460. DOI 10.2147/IJN. [Google Scholar] [CrossRef]
Yildirim H, Sunay FB, Sinan S, Köçkar F (2016). In vivo effects of curcumin on the paraoxonase, carbonic anhydrase, glucose-6-phosphate dehydrogenase and β-Glucosidase enzyme activities in dextran sulphate sodium-induced ulcerative colitis mice. Journal of Enzyme Inhibition and Medicinal Chemistry 31: 1583–1590. DOI 10.3109/14756366.2016.1158173. [Google Scholar] [CrossRef]
Zeng Z, Zhan L, Liao H, Chen L, Lv X (2013). Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27 expression via the TLR4/NF-κB signaling pathway. Planta Medica 79: 102–109. DOI 10.1055/s-00000058. [Google Scholar] [CrossRef]
Zhang L, Xue H, Zhao G, Qiao C, Sun X et al. (2019). Curcumin and resveratrol suppress dextran sulfate sodium‐induced colitis in mice. Molecular Medicine Reports 19: 3053–3060. DOI 10.3892/mmr.2019.9974. [Google Scholar] [CrossRef]
Zhang X, Wu J, Ye B, Wang Q, Xie X et al. (2016). Protective effect of curcumin on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway. BMC Complementary and Alternative Medicine 16: 299. DOI 10.1186/s12906-016-1273-z. [Google Scholar] [CrossRef]
Zhao HM, Han F, Xu R, Huang XY, Cheng SM et al. (2017). Therapeutic effect of curcumin on experimental colitis mediated by inhibiting CD8(+)CD11c(+) cells. World Journal of Gastroenterology 23: 1804–1815. DOI 10.3748/wjg.v23.i10.1804. [Google Scholar] [CrossRef]
Zhao HM, Xu R, Huang XY, Cheng SM, Huang MF et al. (2016a). Curcumin suppressed activation of dendritic cells via JAK/STAT/SOCS signal in mice with experimental colitis. Frontiers in Pharmacology 7: 455. DOI 10.3389/fphar.2016.00455. [Google Scholar] [CrossRef]
Zhao HM, Xu R, Huang XY, Cheng SM, Huang MF et al. (2016b). Curcumin improves regulatory T cells in gut-associated lymphoid tissue of colitis mice. World Journal of Gastroenterology 22: 5374–5383. DOI 10.3748/wjg.v22.i23.5374. [Google Scholar] [CrossRef]
Zhou X, Chen Q, Ma Y, Huang Y, Gou S, Xiao B (2019). Porous polymeric microparticles as an oral drug platform for effective ulcerative colitis treatment. Journal of Pharmaceutical Sciences 108: 2238–2242. DOI 10.1016/j.xphs.2019.02.001. [Google Scholar] [CrossRef]
Supplementary Materials

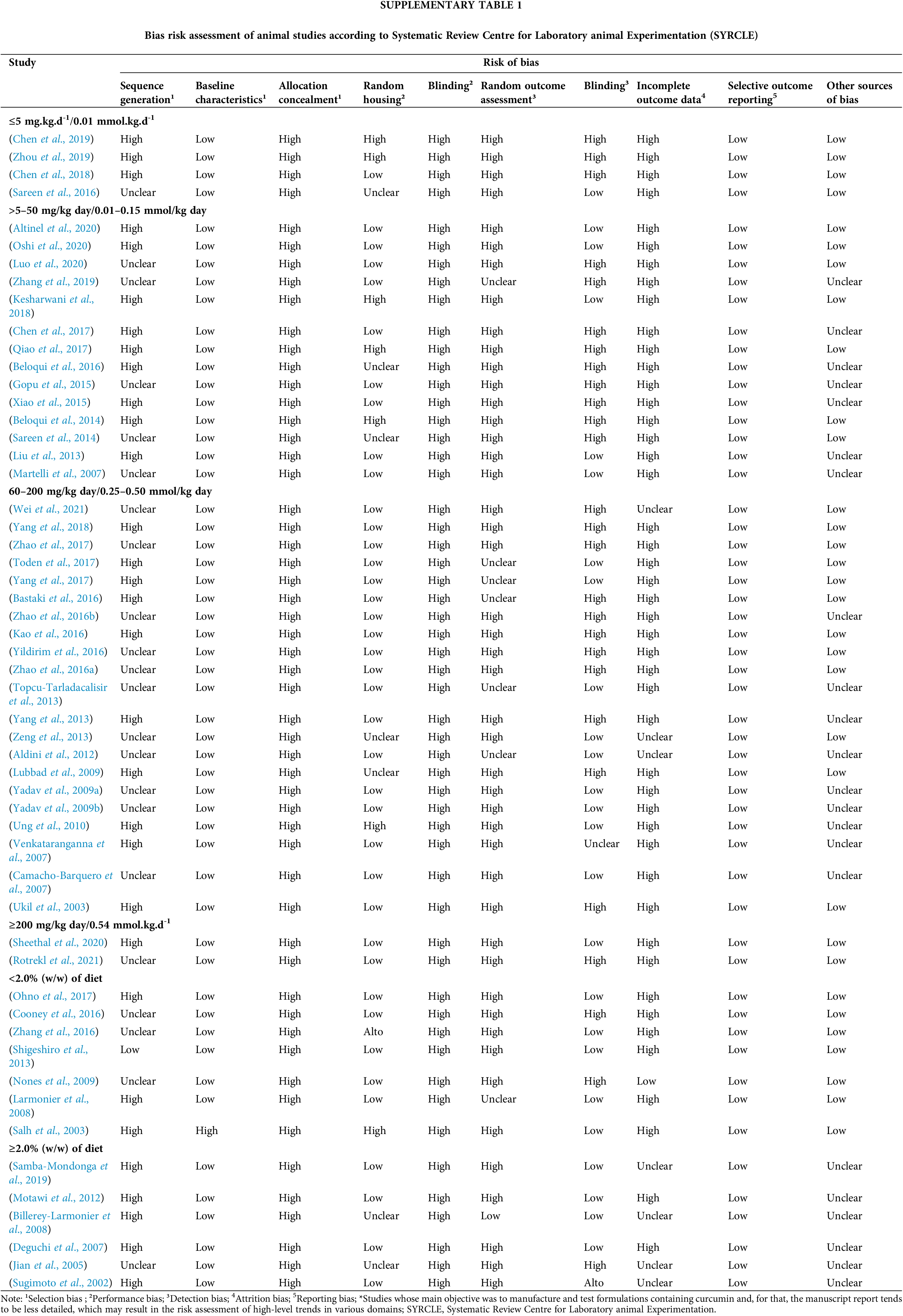
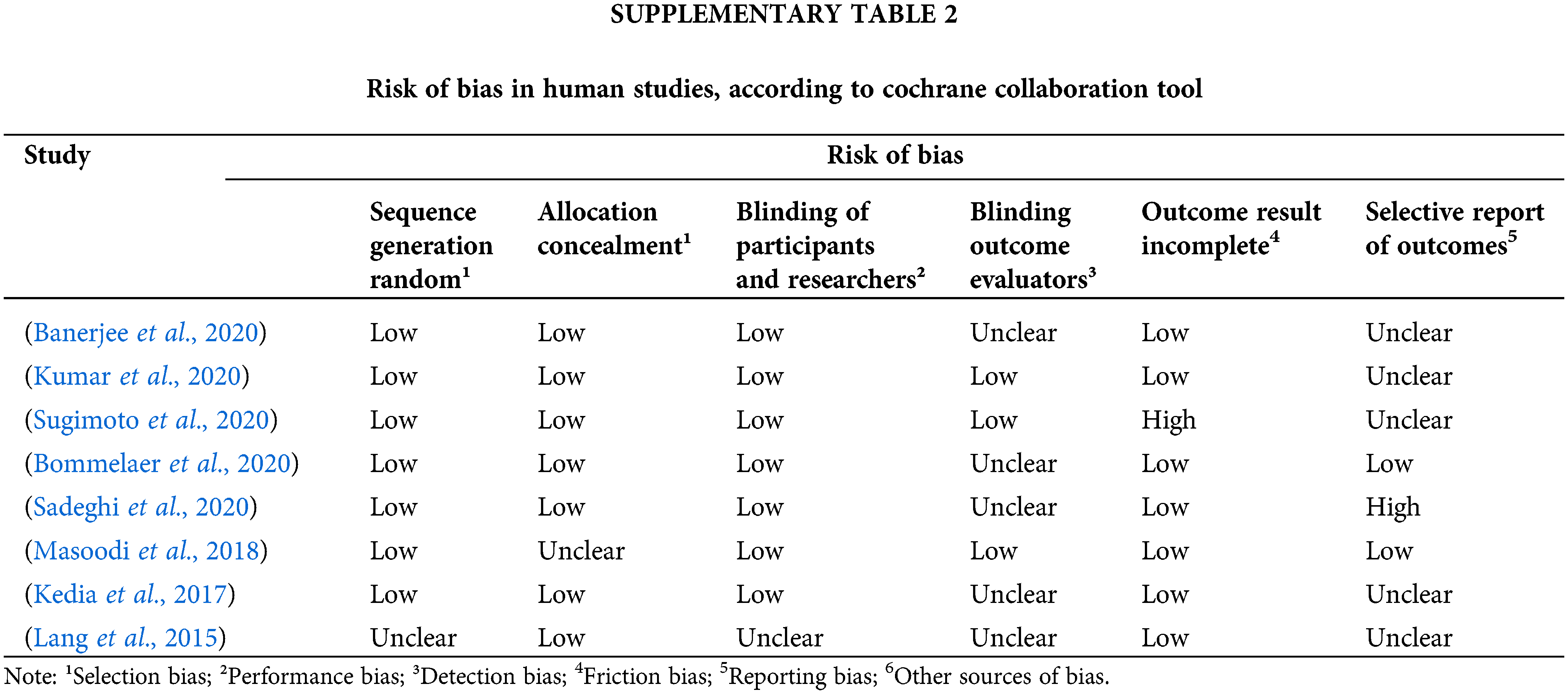
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |