DOI:10.32604/biocell.2022.018265

| BIOCELL DOI:10.32604/biocell.2022.018265 |  |
| Article |
Transplantation of BMP-7 gene-transfected bone marrow mesenchymal stem cells for the treatment of spinal cord injury in rats
1Department of Orthopedics, The First Affiliated Hospital of Bengbu Medical College, Bengbu, 233000, China
2Department of Orthopedics, The Second Affiliated Hospital of Medical College of Shihezi University, Urumqi, 832000, China
3Department of Spinal Surgery, The Second Affiliated Hospital of Medical College of Shihezi University, Urumqi, 832000, China
*Address correspondence to: Kuanxin Li, ligu19790315@126.com
Received: 10 August 2021; Accepted: 11 July 2021
Abstract: Background: Spinal cord injury (SCI) is a serious traumatic disease of the central nervous system, and there is currently no effective treatment for SCI because of its complicated pathophysiology. Bone marrow mesenchymal stem cells (BMSCs) have multidirectional differentiation abilities. Our study aims to explore the effects of bone morphogenetic protein 7 (BMP-7)-modified BMSCs transplantation on the repair of SCI in rats. Methods: In this study, a rat spinal cord injury model was established with the modified Allen method. Then, BMSCs transfected with the BMP7 gene were transplanted to treat the spinal cord injury in rats. Forty Sprague-Dawley rats were randomly divided into the sham operation group (sham group), spinal cord injury group (model group), BMSC treatment group (BMSC group) and LV-BMP7-BMSC treatment group (LV-BMP7-BMSC group). The Basso, Beattie, and Bresnahan (BBB) score was used to evaluate the recovery of hindlimb function in the rats. The levels of neurofilament protein NF-200 (NF-200) and glial fibrillary acidic protein (GFAP) were detected by immunofluorescence, RT-PCR and Western blotting. Results: At 14 d, 21 d, and 28 d after treatment, the BBB score of the rats in the LV-BMP7-BMSC group was higher than that of the rats in the model group and BMSC group. The results showed that NF-200 was expressed at the local spinal cord injury site. Compared with that of the sham group, the NF-200 expression level of the BMSC group and LV-BMP7-BMSC group was increased (P < 0.05). The results showed that the mRNA expression levels of NF-200 in the spinal cord tissue of the BMSC group and LV-BMP7-BMSC group were increased compared with those of the sham group (P < 0.05). The western blotting results further confirmed the PCR results. Conclusion: BMP-7 gene-modified BMSC transplantation can promote the repair of spinal cord functions after SCI in rats.
Keywords: Bone morphogenetic protein 7; Bone marrow mesenchymal stem cells; Spinal cord injury
Abbreviations
| SCI | Spinal cord injury |
| BMSCs | Bone marrow mesenchymal stem cells |
| BMP-7 | Bone morphogenetic protein 7 |
| BBB | Basso, Beattie, and Bresnahan |
| NF-200 | Neurofilament protein NF-200 |
| GFAP | Glial fibrillary acidic protein |
Spinal cord injury (SCI) is a serious traumatic disease of the central nervous system. SCI is clinically characterized by high morbidity, disability and mortality rates, and SCI not only seriously affects families and causes serious damage to the body but also results in heavy economic and psychological burdens on society (North et al., 2015). Surgical decompression and internal fixation can only reduce the edema of the injured portion of the spinal cord and restore the stability of the spine. Neurons, which are permanent cells, are difficult to repair after damage (Celik et al., 2014). Stem cell-based therapy has been considered a potential treatment for treating spinal cord injury. Bone marrow mesenchymal stem cells (BMSCs) possess the advantages of easy collection, rapid proliferation, and persistent self-renewal capacity, and there are no ethical issues associated with their use (Chen et al., 2014b). BMSCs are stem cells that possess self-renewal and multiple differentiation abilities. BMSCs can differentiate into bone, cartilage, and cardiomyocytes under appropriate induction conditions. Recent studies have shown that BMSCs also have the ability to differentiate across germ layers and can differentiate into neurons from the outer germ layer (Kim et al., 2017). Bone morphogenetic protein 7 (BMP-7) is a multifunctional differentiation factor of the transforming growth factor β superfamily. Previous studies have shown that BMP-7 is closely related to the repair of the nervous system. Kusakawa reported that BMP-7 was observed in astrocytes, gray nerve fibers, and dopamine-producing cells in the central nervous system by immunohistochemistry (Kusakawa et al., 2017). BMP7 expression was observed in both neurons and noradrenergic neurons, which indicates that BMP-7 plays an important role in the development of the central nervous system. Setoguchi T found that BMP-7 mRNA expression was significantly upregulated after spinal cord injury (Setoguchi et al., 2001). It is speculated that BMP-7 plays a protective role in the early stage of spinal cord injury. Therefore, in this study, Allen’s method was used to establish a rat spinal cord injury model. The effects of BMSCs transfected with the BMP7 gene on neurological recovery after spinal cord injury in rats were observed, and the possible mechanism was explored.
SPF-grade male SD rats were provided by the Experimental Animal Center of The First Affiliated Hospital of Bengbu Medical College (license number: FAHBMC (new) 2019-005). The rats were housed in a standard environment with a normal day/night light schedule, relative humidity of 60 +/− 10%, temperature of 25 +/− 1°C, and free access to food and water. All the experimental procedures met the requirements of the ethical use of experimental animals.
BMP-7 antibody was purchased from American R&D Company (Minneapolis, MN, USA). DMEM was purchased from American GIBCO Company (USA), and fetal bovine serum was purchased from Hangzhou Siqingqing Biological Engineering Materials Co., Ltd. (China). A TRIzol kit, one-step reverse transcription PCR kit and real-time PCR kit were all obtained from TAKARA (Otsu, Shiga, Japan). MgSO4 solution and 10% chloral hydrate were obtained from Shanghai Rongbai Biotechnology Co., Ltd. (China). Penicillin was purchased from Shanghai Yubo Biotechnology Co., Ltd. (China). NF200 and GFAP monoclonal antibodies were purchased from Abcam (Cambridge, MA); goat anti-rabbit IgG and goat anti-mouse IgG secondary antibodies were purchased from Beijing Boaosen Biotechnology Co., Ltd. (China). The BCA protein detection kit was purchased from Wako Corporation, Japan.
The fluorescence inverted phase contrast microscope was purchased from Carl Zeiss (Germany); Allences’s beater was provided by the Orthopaedics Center of the First Affiliated Hospital of Medical College of Shihezi University; the animal surgical instruments were obtained from Shenzhen Ruiward Company (China); ELISAs were obtained from Thermo Scientific Company (China); the CO2 incubator was obtained from Beijing Altai Technology Development Co., Ltd. (China); the real-time quantitative PCR instrument was obtained from Roche (Switzerland); the nucleic acid and protein quantifier was obtained from American Yingjie Life Technology Co., Ltd. (USA); and the transfer membrane instrument was obtained from American Pierce.
All the protocols and procedures were approved by the Animal Experiment Ethic Committee of Jilin University (Changchun, China; Approval No. 20180143). SD rats weighing 180–220 g were fully anaesthetized by intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 g), and the skin was disinfected with iodophor and alcohol. The skin was dissected under a microscope to expose the T9 or T10 spinal cord segments. A modified Allen’s device was used to strike the spinal cord with a traumatic force of 10 g × 10 cm. Edema and hemorrhage appeared in the impacted part, and both lower limbs were paralyzed after the rat’s lower limbs and body retracted with a flutter-like movement. The SCI model was determined to have been successfully established when the tail exhibited spastic swings.
Isolation and identification of BMSCs
Bone marrow mesenchymal stem cells were collected from the tibia and fibula of SD rats. The cells were cultured in DMEM + 10% fetal bovine serum + 1% penicillin-streptomycin at 37°C, 95% humidity, and 5% CO2.
For MSC characterization, cells in the third passage were digested using 0.25% trypsin and then incubated with CD90-PE, CD29-APC and CD74-FITC and CD45-FITC antibodies at room temperature for 30 min.
For differentiation into adipocytes, bone marrow mesenchymal stem cells were treated with adipogenesis induction medium (94.498% DMEM + 4% FBS + 1% penicillin-streptomycin + 0.4% dexamethasone + 0.1% insulin + 0.002% indomethacin) for 23 d. After 28 d of adipogenic induction, cell morphology was observed under an inverted phase contrast microscope, and oil droplets were observed by oil red O staining.
To investigate the osteogenic differentiation capacity of bone marrow mesenchymal stem cells, the cells were cultured with osteogenesis induction medium (10% FBS, 10 mmol/L β-glyceryl phosphate, 1 × 10−7 mol/L dexamethasone, 50 mg/L L-ascorbic acid) for 28 d. Then, the cells were stained with 2% Alizarin Red solution (Sigma-Aldrich) and photographed under a phase-contrast inverted microscope (Nikon, Tokyo, Japan).
BMSC transfection with BMP-7 lentiviral expression vectors
Bone marrow mesenchymal stem cells from the third passage and in the logarithmic growth phase were seeded into a 25-cm2 culture flask at a concentration of 5 × 107 cells/mL and routinely cultured at 37°C in 5% CO2 for 2 d. LV-BMP7-GFP (lentivirus titer of 1 × 107 TU/mL) was used to infect the cells for 12 h, and then, the medium was replaced with complete medium (DMEM + 10% fetal bovine serum + 1% penicillin-streptomycin) for culture. The transfection efficiency was determined by evaluating the BMP-7 levels using flow cytometry analysis after 48 h.
Forty Sprague-Dawley rats (weighing 220–250 g) were randomly divided into the sham operation group (sham group), spinal cord injury group (model group), BMSC treatment group (BMSC group) and LV-BMP7-BMSC treatment group (LV-BMP7-BMSC group), with 10 rats in each group. In the BMSC and LV-BMP7-BMSC groups, after establishing the spinal cord injury model, BMSCs (2 × 105 cells) or LV-BMP7-BMSCs (2 × 105 cells) were injected into the injured site. After the surgery, the incision was sutured layer by layer.
At 6 h, 1 d, 3 d, 7 d, 14 d, 21 d, and 28 d after treatment, hind limb BBB scores were calculated to evaluate the recovery of hind limb function.
The spinal cord tissues of the rats were harvested, fixed with 4% paraformaldehyde for 24 h, and then dehydrated, embedded, and sliced conventionally. Then, the slices were incubated in an oven at 65°C for 45 min, dehydrated normally, and incubated in 3% H2O2 for 10 min at room temperature to eliminate the endogenous peroxidase activity. Then, the sections were boiled in 0.01 mol/L citric acid solution for 10 min to repair the antigens, and the sections were cooled and incubated in blocking solution with 5% goat serum for 30 min. Next, the sections were incubated with primary antibodies (mouse monoclonal antibodies against NF200 and GFAP; diluted 1:200) at 4°C overnight followed by incubation at room temperature for 45 min. Then, the secondary antibodies (sheep anti-mouse IgG) were added to the sections and incubated at room temperature for 1 h. ImageJ software was used for fluorescence intensity analysis.
Rat spinal cord tissues were harvested, and RNA was extracted from the tissues by the TRIzol method. The total reaction volume was 20 μl, the reaction was conducted at 42°C for 60 min, and the reaction was stopped by incubation at 70°C for 5 min. First-strand cDNA was synthesized by reverse transcription. One microliter of the abovementioned cDNA produced by reverse transcription was used as a reaction template, and the total reaction volume was 20 μl. The primer sequences were: NF-200 Forward: 5′-GATGGCATTGGACATTGAGA-3′, Reverse: 5′-GAGAGTAGCCGCTGGTTATG- 3′, product size of 296 bp, annealing temperature of 58°C; GFAP Forward: 5′-GCAGGTGAGGAAGAAATGG-3′, Reverse: 5′-AAGGTTAGCAGAGGTGACAAG-3′, product size of 296 bp, annealing temperature of 58°C; rat actin Forward: 5′-GAGAGGGAAATCGTGCGTGAC-3′, Reverse: 5′-CATCTGCTGGAAGGTGGACA-3′, product size of 452 bp, annealing temperature of 57°C. The reaction conditions were predenaturation at 95°C for 5 min, followed by 35 cycles of annealing at the indicated temperatures for 30 s and incubation at 72°C for 40 s, and the final incubation was 72°C for 10 min. The mRNA expression of NF200 and GFAP in each group was calculated.
Rat spinal cord specimens were harvested and cut into small tissue pieces with scissors. Then, all the tissues were placed on a Bio Masher tissue cell homogenizer and dissolved in RIPA buffer. Protein samples were extracted with routine procedures and stored at –80°C. The protein concentration of the spinal cord tissue samples after treatment was calculated according to the BCA method in order to determine the amount of protein to be loaded into each well. After incubation with conventional primary antibodies and secondary antibodies, images and photographs were obtained with the Fluor ChemTM 8000 system. After the gray values of the tissue proteins were quantified, these values were compared with the gray value of the GAPDH protein. The data were semiquantitatively analyzed using ImageJ software.
SPSS 19.0 statistical software was used for statistical analysis. The measurement data are expressed as the mean ± standard deviation. The comparison of means between multiple groups was performed by analysis of variance followed by q test. A P value < 0.05 was considered to indicate statistically significant differences.
Rat BMSC isolation and culture
Rat bone marrow mesenchymal stem cells were cultured for 14 d, and inverted phase contrast microscopy revealed that the cells were fusiform and polygonal in shape, aggregated and mixed, and arranged in the same direction. The cells were tightly arranged and gradually fused into a monolayer (Fig. 1).
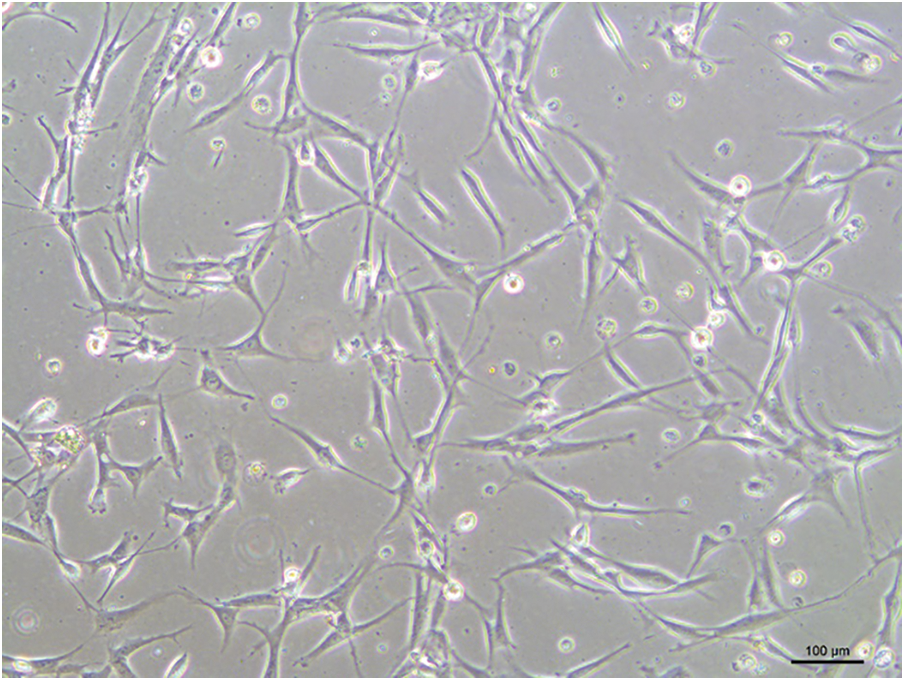
Figure 1: Morphological observation of rat BMSCs.
Rat BMSC differentiation induction
Surface antigens of P3 generation of BMSCs were analysed by flow cytometry, as shown in Fig. 2; BMSCs express CD45 and CD90 with positive rates of 1.1% and 98.7%, ADSCs cells express CD74 and CD29 with positive rates of about 0.5% and 98.5% respectively, which is similar to the surface antigen profile of BMSCs.
Bone marrow mesenchymal stem cells in the third generation overexpressed CD45 and CD90, with positive rates of 1.1% and 98.7%, respectively (Fig. 2).
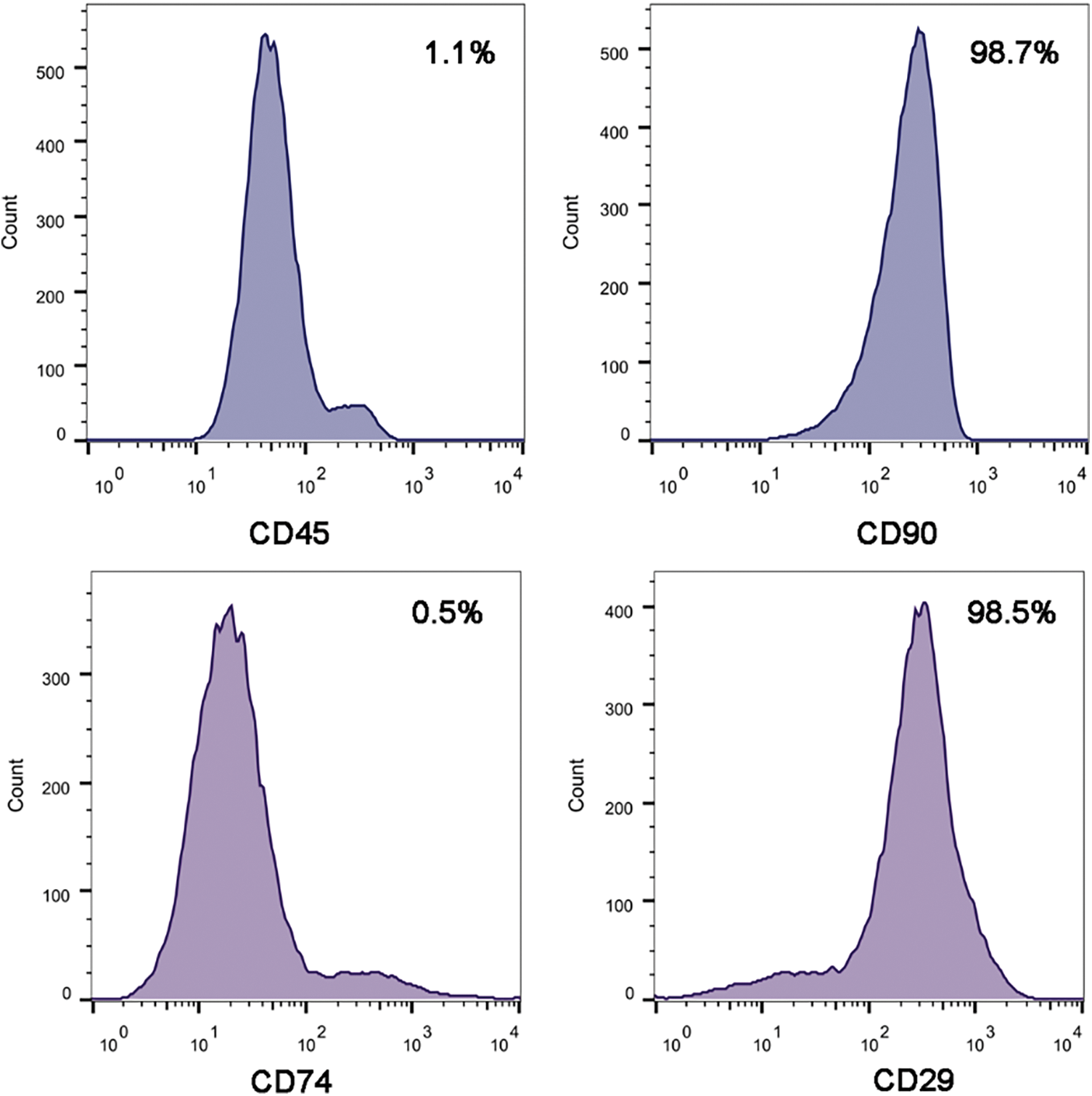
Figure 2: The expression of cell surface markers (CD45, CD90, CD74 and CD29) present on bone marrow mesenchymal stem cells using flow cytometry.
After BMSCs were induced to undergo osteogenesis, opaque mass-like mineral nodules appeared, which were stained with alizarin red, and the extracellular matrix mineral nodules were observed to be strongly positive and appeared to be orange-red or dark-red, as shown in Fig. 3A. Oil red O staining was then performed to assess the adipogenic differentiation of the BMSCs. Microscopic observation showed that the lipid droplets stained positive and were orange-red, as shown in Fig. 3B.
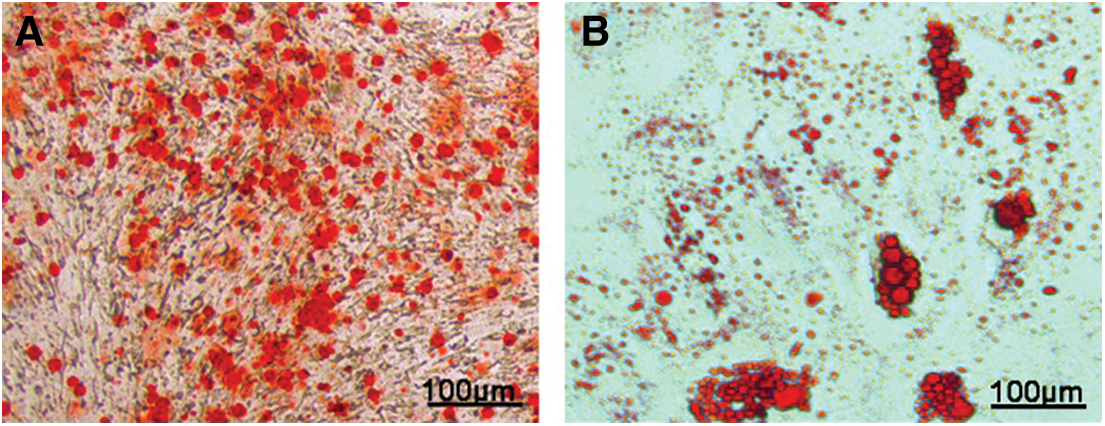
Figure 3: A, Alizarin red staining and B, Oil red O staining of bone marrow mesenchymal stem cells under osteogenic and adipogenic induction conditions.
After 48 h of transfection, viral transfection was visualized by immunofluorescence analysis of GFP expression. The immunofluorescence results showed that GFP positive staining appeared green (Supplement S1 A). Transfection efficacy was assessed by flow cytometry. The results suggested that the transfection efficacy was nearly 60.6% (Supplement S1 B).
After treatment, the BBB score of the rats in the model group, BMSC group, and LV-BMP7-BMSC group gradually increased. At 14 d, 21 d, and 28 d after treatment, the BBB score of the rats in the LV-BMP7-BMSC group was higher than that of rats in the model group and BMSC group (P < 0.05, Fig. 4), which indicated that the LV-BMP7-BMSC group exhibited better recovery of hindlimb function.
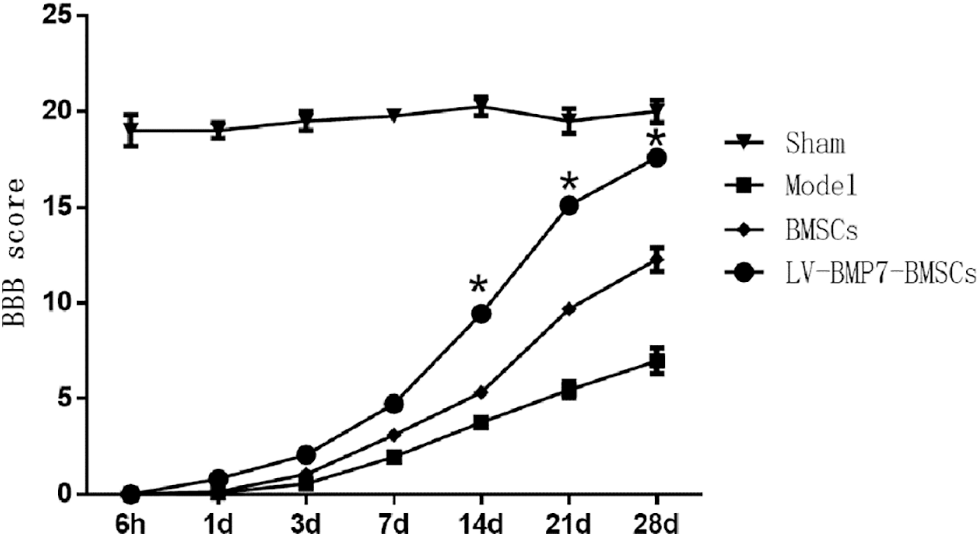
Figure 4: BBB score after spinal cord injury in rats, *P < 0.05, compared with the sham and model groups.
After 28 d of treatment, immunochemical experiments were conducted to detect the protein expression of NF-200 and GFAP. The results showed that NF-200 was expressed at the local spinal cord injury site. Compared with that of the sham group, the NF-200 expression levels of the BMSC group and LV-BMP7-BMSC group were increased (P < 0.05). Compared with that of the model group and BMSC group, the expression level of NF-200 of the LV-BMP7-BMSC group was increased (P < 0.05, Fig. 5). After treatment, the rats in each group exhibited GFAP-positive cells. Compared with that in the sham group, the expression of GFAP in the other groups increased after spinal cord injury (P < 0.05, Fig. 5). There was no significant difference in the expression of GFAP among the model group, BMSC group, and LV-BMP7-BMSC group (P > 0.05, Fig. 5).
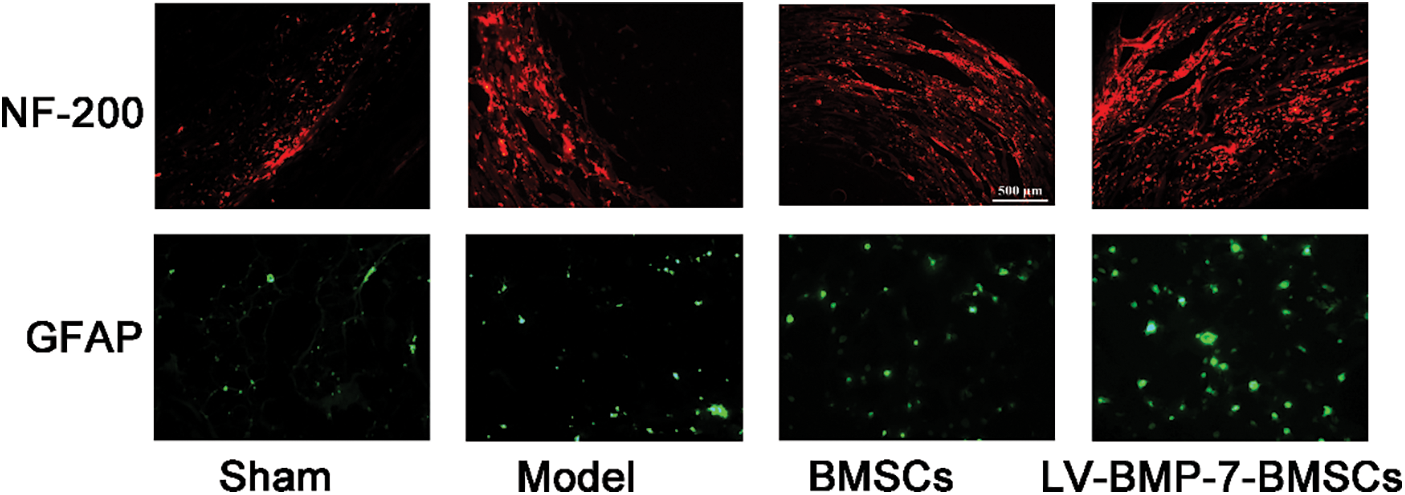
Figure 5: mmunofluorescence staining of NF-200 and GFAP in each group.
After 28 d of treatment, qRT-PCR was conducted to determine the relative mRNA expression levels of NF-200 and GFAP. The results showed that the mRNA expression levels of NF-200 in the spinal cord tissue of the BMSC group and LV-BMP7-BMSC group were increased compared with those of the sham group (P < 0.05, Fig. 6A). Compared with that in the model group and BMSC group, the mRNA expression level of NF-200 in the LV-BMP7-BMSC group was increased (P < 0.05, Fig. 6A). Compared with that in the sham group, the mRNA expression of GFAP in the other groups was increased after spinal cord injury (P < 0.05, Fig. 6B). There was no significant difference in the mRNA expression of GFAP among the model group, BMSC group, and LV-BMP7-BMSC group (P > 0.05, Fig. 6B).
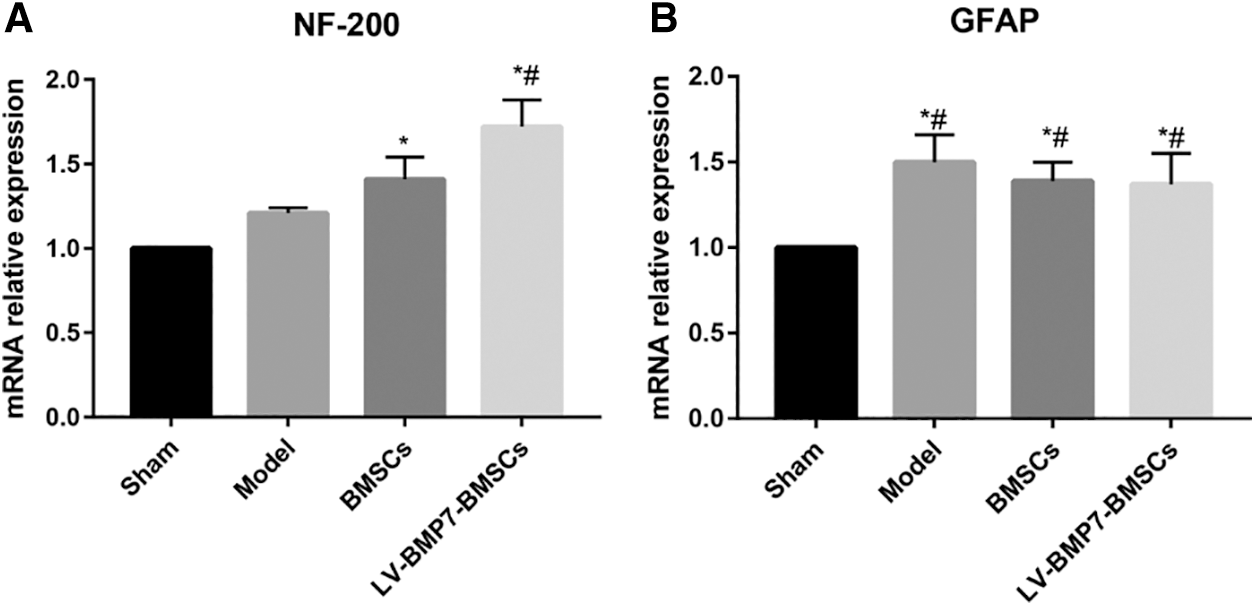
Figure 6: qRT-PCR results of NF-200 and GFAP expression. *P < 0.05, compared with the sham group; #P < 0.05, compared with the model group and BMSC group.
After 28 d of treatment, western blotting was conducted to determine the protein expression of NF-200 and GFAP. The results showed that the expression of NF-200 in the spinal cord tissues of the BMSC group and LV-BMP7-BMSC group was increased compared with that of the sham group (P < 0.05, Fig. 7). Compared with that in the model group and BMSC group, the expression level of NF-200 in the LV-BMP7-BMSC group was increased (P < 0.05).
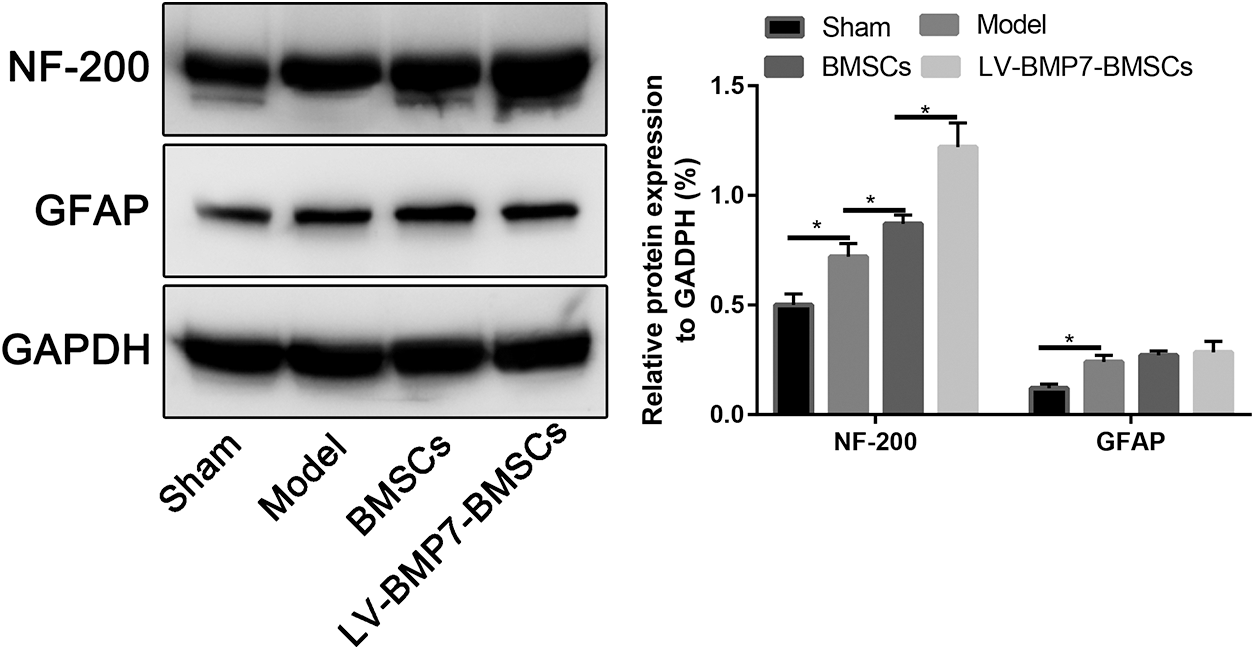
Figure 7: Western blotting results of NF-200 and GFAP expression.
Compared with that in the sham group, the expression of GFAP in the other groups was increased after spinal cord injury (P < 0.05); there was no significant difference in the expression of GFAP among the model group, BMSC group, and LV-BMP7-BMSC group (P > 0.05, Fig. 7).
In this study, a BMP-7 overexpression plasmid was successfully constructed. Furthermore, we found that transplantation of BMP-7 gene-modified BMSCs can promote the repair of nerve function after SCI in rats.
Spinal cord injury (SCI) is a major disorder that causes sensory, motor, and autonomic dysfunction in the parts of the body that are served by the spinal cord and are located below the level of injury. SCI mainly includes two pathological processes: primary injury and secondary injury. Primary injury is direct mechanical damage to spinal cord tissue and includes the demyelination and necrosis of neurons and axons; secondary injury is caused by a variety of pathophysiological mechanisms, including local hemorrhage, ischemia, edema, ionic imbalance, free radical stress and inflammatory responses (Kramer et al., 2016 and Wilems et al., 2015). Secondary injury can exacerbate spinal cord injury, causing damage to myelin sheaths and death in nerve cells in adjacent spinal cord tissues that were not affected by the primary injury (Ghada, 2017). In the treatment of severe spinal cord injury, the objectives of surgery are to restore the stability of the spine and to create favorable conditions for the recovery of damaged nerves. However, the problem of nerve cell loss is unlikely to be solved (Lawless et al., 2017). The current hotspot in the field of spinal cord injury research focuses on using cytokines or stem cell transplantation to improve the spinal cord injury microenvironment, which is not conducive to nerve cell repair, or supplementation with injured neurons to achieve the goal of healing the spinal cord injury (van Lieshout and Alt, 2016). To implement these strategies, it is necessary to explore the influencing factors that are beneficial for promoting the differentiation of stem cells into neurons.
Bone mesenchymal stem cells are current research hotspots for the use of stem cell transplantation in the treatment of spinal cord injury. These cells have the potential to differentiate into neural cells across the germ layer, can be easily acquired, and are not associated with any ethical controversy. Therefore, bone marrow mesenchymal stem cells are ideal seed cells for stem cell transplantation for the treatment of spinal cord injury. Bone morphogenetic protein 7 (BMP7) is a member of the BMP family. BMP7 was first described as a protein with osteogenic ability. At present, an increasing number of studies have shown that BMP7 performs a neuroprotective function in the early stage of spinal cord injury. Wang found that BMP7 can inhibit TNF-α-induced oligodendrocyte apoptosis during spinal cord injury. BMP7 can reduce the occurrence of demyelination and has a protective effect on neural function (Wang et al., 2016). In early studies conducted by this research group, spinal cord injury was treated by injecting BMP-7 into the spinal cord of rats. According to the BBB score and electrophysiological test results, the hind limb function of these rats was significantly better than that of the control-treated rats. Moreover, BMP7 can promote neural functional recovery (Chen et al., 2014a). Previously, we also found that BMP7 promotes the neuronal differentiation of BMSCs in vitro (Zhang et al., 2021). BMP-7 could enhance MAP-2 and Nfh expression, and these two proteins contribute to maintaining the stability of neuronal structures. BMP-7 has been shown to act as a trophic factor in all regeneration processes, including neural regeneration.
Therefore, this study was designed to explore the role and underlying mechanism of the transplantation of BMP7 gene-transfected bone marrow mesenchymal stem cells for the treatment of spinal cord injury in rats.
A rat spinal cord injury model was established with the Allen method. After treatment, the LV-BMP7-BMSC group had significantly better BBB scores than the other groups. The results indicate that transplantation of BMSCs transfected with the BMP7 gene can restore nerve function in rats with spinal cord injury. To further study the specific mechanism, immunohistochemistry, qRT-PCR, and western blotting were conducted to detect the expression of marker proteins by nerve cells at the site of spinal cord injury.
Neurofilament protein 200 (NF200) is a specific marker of neuronal cells. NF200 is mainly expressed in the cytoplasm and axons of neuronal cells (Peng et al., 2017). The level of NF200 expression reflects the number and function of neuronal cells. Glial fibrillary acidic protein (GFAP) is a specific protein and skeletal component of astrocytes, and its expression level can reflect the degree of changes in astrocyte proliferation and necrosis (Lam et al., 2014 and Jong et al., 2016). The results of this study showed that NF200 expression in the injury site was significantly increased in the LV-BMP7-BMSC group. The results indicate that after the transplantation of BMP7-BMSCs for the treatment of spinal cord injury, the number and function of the neurons in the spinal cord were increased. The results of this study showed that there was no significant difference in the expression levels of GFAP among the groups, indicating that transfection of BMSCs with the BMP-7 gene for the treatment of spinal cord injury had no significant effect on the number and activity of astrocytes in the injured area. Transplantation of BMP-7 gene-transfected BMSCs for the treatment of spinal cord injury plays a role in promoting neural function recovery, which may be related several mechanisms. (1) BMSCs transfected with the lentiviruses carrying the BMP-7 gene are injected into the spinal arachnoid cavity. The sustained expression of BMP-7 promotes the differentiation of BMSCs into neurons, which can supplement the number of neurons in the local injury site and promote the growth of axons, which is manifested by the increased expression of NF200 (Qiang et al., 2017; Han et al., 2015 and Gámez et al., 2013). (2) BMSCs transfected with lentivirus carrying the BMP-7 gene are injected into the arachnoid cavity. Then, BMP-7 is continuously secreted, which improves the local microenvironment of central nervous system injury by improving local blood circulation, and BMP-7 plays a role in nerve function repair (Guan et al., 2013). Therefore, this study confirms that transplantation of BMSCs transfected with the BMP-7 gene for the treatment of spinal cord injury in rats can promote the repair of injured spinal cord neurons and restore neural function to a certain extent; however, whether the neurons differentiated by BMSCs perform a beneficial function and the specific mechanism underlying this repair is not yet known. Clearly, further exploration is needed.
The major limitation of this study was the lack of research on possible downstream signaling pathways. To further explore the downstream signaling pathways related to the transplantation of BMP-7 gene-transfected BMSCs, RNA sequencing technology will be used for comprehensive analysis. Another limitation of this study is that the optimal BMSC number was not determined.
In summary, BMSCs transfected with lentiviruses carrying the BMP-7 gene and injected into the arachnoid cavity promote nerve functional recovery in rats after spinal cord injury. These findings provide an experimental basis for current clinical therapy.
Author Contribution: XYW, WZ, and LG designed the study and conducted the experiments. KXL and XYW performed the statistically analyses. XYW, WZ, and LG wrote the draft. KXL edited and approved the final manuscript. All the authors read and approved the final manuscript.
Availability of Data and Materials: All the data pertaining to the present study were included in figures in the manuscript, and the authors are pleased to share the raw data upon reasonable request.
Ethics Approval: All the protocols and procedures were approved by the Animal Experiment Ethic Committee of Jilin University (Changchun, China; Approval No. 20180143).
Funding Statement: This study was supported by the China Natural Science Foundation, No. 81560216.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Celik B, Ones K, Celik EC, Bugdayci DS, Paker N et al. (2014). The effects of using the internet on the health-related quality of life in people with spinal cord injury: A controlled study. Spinal Cord 52: 388–391. DOI 10.1038/sc.2014.7. [Google Scholar] [CrossRef]
Chen C, Bai G, Jin H, Lei K, Li K (2014a). Local injection of bone morphogenetic protein 7 promotes neuronal regeneration and motor function recovery after acute spinal cord injury. Neural Regeneration Research 13: 1054–1060. DOI 10.4103/1673-5374.233449. [Google Scholar] [CrossRef]
Chen J, Zhang Z, Liu J, Zhou R, Zheng X et al. (2014b). Acellular spinal cord scaffold seeded with bone marrow stromal cells protects tissue and promotes functional recovery in spinal cord-injured rats. Journal of Neuroscience Research 92: 307–317. DOI 10.1002/jnr.23311. [Google Scholar] [CrossRef]
Gámez B, Edgardo RC, Francesc V (2013). BMP signaling in telencephalic neural cell specification and maturation. Frontiers in Cellular Neuroscience 7: 87. [Google Scholar]
Ghada A. Abdel-Hamid (2017). Histological, immunohistochemical and ultrastructural study of secondary compressed spinal cord injury in a rat model. Folia Histochemica Et Cytobiologica, 2017 55: 11–20. [Google Scholar]
Guan J, Li H, Lv T, Chen D, Yuan Y et al. (2013). Bone morphogenetic protein-7 (BMP-7) mediates ischemic preconditioning-induced ischemic tolerance via attenuating apoptosis in rat brain. Biochemical and Biophysical Research Communications 441: 560–566. DOI 10.1016/j.bbrc.2013.10.121. [Google Scholar] [CrossRef]
Han D, Wu C, Xiong Q, Zhou L, Tian Y et al. (2015). Anti-inflammatory mechanism of bone marrow mesenchymal stem cell transplantation in rat model of spinal cord injury. Cell Biochemistry and Biophysics 71: 1341–1347. DOI 10.1007/s12013-014-0354-1. [Google Scholar] [CrossRef]
Jong JY, Hwang CH, Hong HN (2016). A model of glial scarring analogous to the environment of a traumatically injured spinal cord using kainate. Annals of Rehabilitation Medicine 40: 757. DOI 10.5535/arm.2016.40.5.757. [Google Scholar] [CrossRef]
Kim Y, Seo T, Kim C, Ji E (2017). Treadmill exercise with bone marrow stromal cells transplantation potentiates recovery of locomotor function after spinal cord injury in rats. Journal of Exercise Rehabilitation 13: 273–278. DOI 10.12965/jer.1735014.507. [Google Scholar] [CrossRef]
Kramer JLK, Minhas NK, Jutzeler CR, Erskine ELKS, Liu L et al. (2016). Neuropathic pain following traumatic spinal cord injury: Models, measurement and mechanisms. Journal of Neuroscience Research 95: 1295–1306. DOI 10.1002/jnr.23881. [Google Scholar] [CrossRef]
Kusakawa Y, Mikawa S, Sato K (2017). BMP7 expression in the adult rat brain. IBRO Reports 3: 72–86. DOI 10.1016/j.ibror.2017.06.002. [Google Scholar] [CrossRef]
Lam SK, Sayama C, Harris DA, Briceño V, Luerssen TG et al. (2014). Nationwide practice patterns in the use of recombinant human bone morphogenetic protein-2 in pediatric spine surgery as a function of patient-, hospital-, and procedure-related factors. Journal of Neurosurgery: Pediatrics 14: 476–485. [Google Scholar]
Lawless MH, Lytle EJ, Mcglynn AF, Engler JA (2017). Surgical management of penetrating spinal cord injury primarily due to shrapnel and its effect on neurological outcome: A literature review and meta-analysis. Journal of Neurosurgery Spine 28: 1–9. [Google Scholar]
North HA, Pan L, Mcguire TL, Brooker S, Kessler JA (2015). β1-Integrin alters ependymal stem cell BMP receptor localization and attenuates astrogliosis after spinal cord injury. Journal of Neuroscience 35: 3725–3733. DOI 10.1523/JNEUROSCI.4546-14.2015. [Google Scholar] [CrossRef]
Peng RJ, Bing J, Ding XP, Huang H, Liao Y et al. (2017). Effect of TNF-α Inhibition on bone marrow-derived mesenchymal stem cells in neurological function recovery after spinal cord injury via the Wnt signaling pathway in a Rat Model. Cellular Physiology and Biochemistry 42: 743–752. DOI 10.1159/000477891. [Google Scholar] [CrossRef]
Qiang F, Liu Yi, Liu Xiu, Zhang Q, Chen L et al. (2017). Engrafted peripheral blood-derived mesenchymal stem cells promote locomotive recovery in adult rats after spinal cord injury. American Journal of Translational Research 9: 3950–3966. [Google Scholar]
Setoguchi T, Yone K, Matsuoka E, Takenouchi H, Nakashima K et al. (2001). Traumatic injury-induced BMP7 expression in the adult rat spinal cord. Brain Research 921: 219–225. DOI 10.1016/S0006-8993(01)03123-7. [Google Scholar] [CrossRef]
van Lieshout EMM, Alt V (2016). Bone graft substitutes and bone morphogenetic proteins for osteoporotic fractures: What is the evidence? Injury 47: S43–S46. DOI 10.1016/S0020-1383(16)30011-0. [Google Scholar] [CrossRef]
Wang X, Xu JM, Wang YP, Yang L, Li Z (2016). Protective effects of BMP-7 against tumor necrosis factor α-induced oligodendrocyte apoptosis. International Journal of Developmental Neuroscience 53: 10–17. DOI 10.1016/j.ijdevneu.2016.04.011. [Google Scholar] [CrossRef]
Wilems TS, Jennifer P, Nisha I, Sakiyama-Elbert SE (2015). Combination therapy of stem cell derived neural progenitors and drug delivery of anti-inhibitory molecules for spinal cord injury. Acta Biomaterialia 28: 23–32. DOI 10.1016/j.actbio.2015.09.018. [Google Scholar] [CrossRef]
Zhang H, Zhang W, Bai G, Gao L, Li K (2021). Bone morphogenetic protein-7 (BMP-7) promotes neuronal differentiation of bone marrow mesenchymal stem cells (BMSCs) in vitro. BioMed Research International 2021: 7239783–7239789. DOI 10.1155/2021/7239783. [Google Scholar] [CrossRef]
Supplement
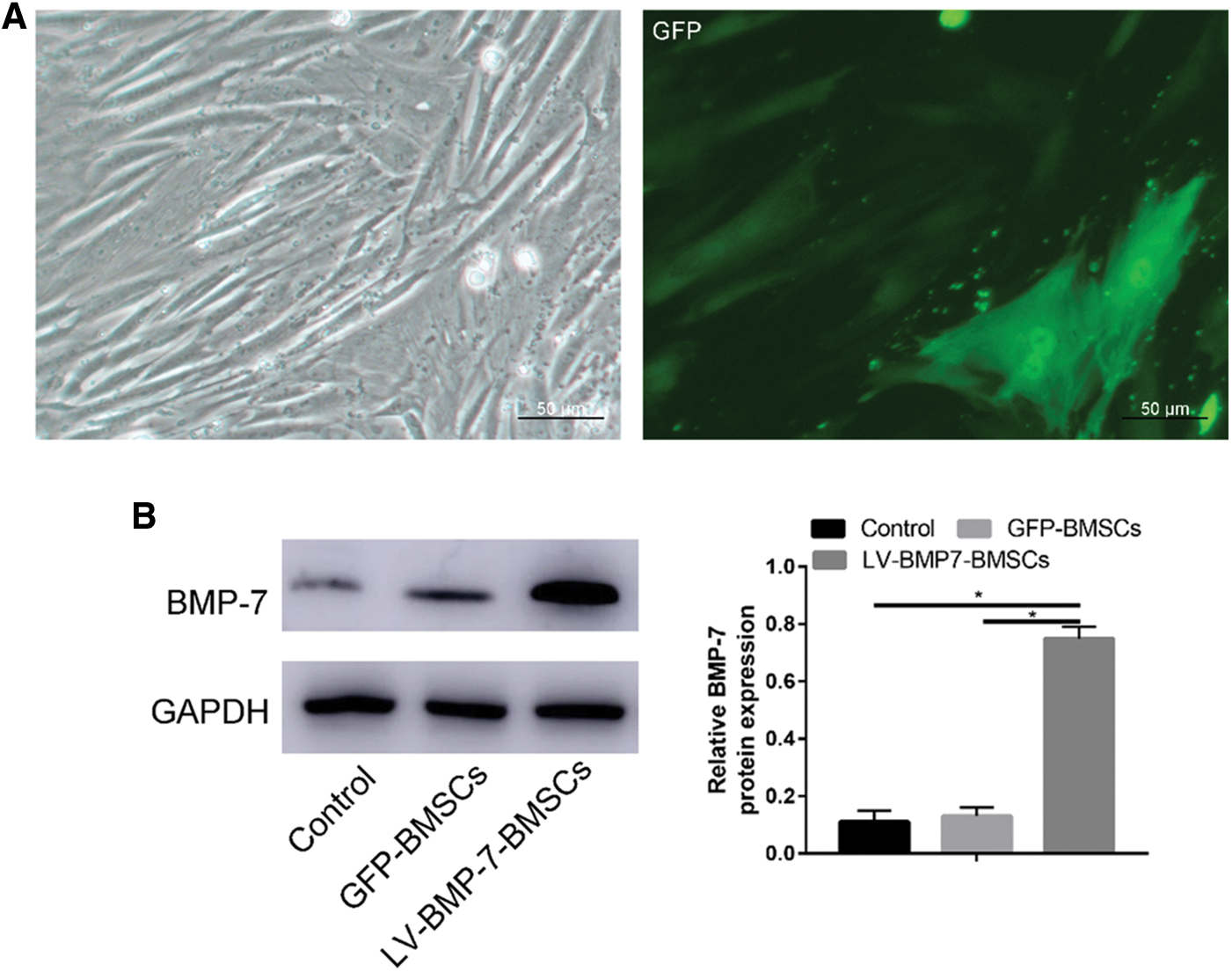
Supplement S1: A, Light microscopy and immunofluorescence results of transfected BMSCs; B, Flow cytometry assay to evaluate transfection efficacy of GFP-BMP-7-BMSC.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |