DOI:10.32604/biocell.2022.019807

| BIOCELL DOI:10.32604/biocell.2022.019807 |  |
| Article |
Effect of Lycopus lucidus Turcz. supplementation on gut microflora and short chain fatty acid composition in Crj: CD-1 mice
1Ocean Science and Technology School, Korea Maritime & Ocean University, Busan, Korea
2Division of Convergence on Marine Science, Korea Maritime & Ocean University, Busan, Korea
*Address correspondence to: Sun Young Lim, sylim@kmou.ac.kr
Received: 16 October 2021; Accepted: 31 December 2021
Abstract: We investigated the diversity and composition of microflora in feces of Lycopus lucidus Turcz.-fed mice. In addition, we evaluated the production of major cytokines (Interleukin-6 and -10) which are related to inflammation and fatty acid composition of several tissues. 16S ribosomal DNA sequencing-based microbiome taxonomic profiling analysis was performed utilizing the EzBioCloud data base. Male mice fed on L. lucidus showed a significantly reduced number of lactic acid bacteria and coliform in the feces compared with the control group (p < 0.05). 16S rDNA sequencing analysis of fecal samples showed that L. lucidus supplementation decreased the community of harmful microflora (Enterobacteriaceae including Escherichia coli and Bacteroides sp.) in feces compared with the control group (p < 0.05). There were no significant differences in mRNA expression of cytokine IL-6 and IL-10 between the control and L. lucidus fed groups. The fecal fatty acid composition in the L. lucidus group had percentages of 4:0, 6:0, 8:0 and 10:0 in the intestine but those short chain fatty acids were not detected in the control group. Our results showed that L. lucidus supplementation influenced gut environment by decreasing harmful microflora and increased the percentages of several short fatty acids.
Keywords: Lycopus lucidus Turcz, Fatty acid composition; Intestinal microflora; 16S rDNA sequencing; Cytokines
Lycopus lucidus Turcz. (L. lucidus) is belong to A Lamiaceae family herb and belongs a common plant in East Asia including Korea and China. Roots of L. lucidus are consumed routinely in salad and fermented foods as a functional food (Lu et al., 2015) and have been traditionally used for healing menstrual disorder and inflammation in Asia (Liu et al., 2019; Fan et al., 2020). Bioactive polysaccharides, terpenes, flavonoids, and phenolic compounds, which are rich in roots of L. lucidus, are highly associated with antimicrobial (Yu et al., 2011), antioxidant (Lu et al., 2015; Song et al., 2016; Yang, 2017; Lee and Lim, 2018), anti-inflammation (Park, 2019; Min et al., 2021; Zhang et al., 2021; Kim et al., 2021a) and also anticancer properties (Yu et al., 2011; Park et al., 2013; Kim et al., 2018). Flavonoids are classified as a major class of secondary metabolites and the most-studied group of polyphenols in relation to antioxidant and anti-inflammatory activities (Panche et al., 2017). In the intestine, flavonoids may influence the composition of the microbiota and also may be metabolized by the resident microbiota. The resulting end-products may have a certain bioactivity, which will act differently from those of the initial compounds (Braune and Blaut, 2016). Espley et al. (2014) reported that the intake of a high-flavonoid apple decreased some inflammation markers and modulated gut microbiota when fed to healthy mice. Duenas et al. (2015) suggested that the intake of polyphenols can increase beneficial strains of Bifidobacterium and Lactobacillus, in the gut while reducing pathogenic strains such as Clostridium perfringens and C. bistolyticum. The frequent consumption of polyphenols could modify gastrointestinal environment with beneficial microorganisms. It has been also widely reported that the gastrointestinal microbiota composition is associated with improving human intestinal health (Holscher, 2017). Many studies suggest that disturbed gut microflora composition may affect the function of mucosal immune system, resulting in intestinal inflammation (Tung et al., 2011; Goldsmith and Sartor, 2014; Li et al., 2017).
One of our previous studies analyzed the total flavonoid content of aceton+methylene chloride extract from L. lucidus roots and showed a content of 233.2 mg/g (mg rutin equivalent/g) (Lee and Lim, 2018). The extract from L. lucidus roots had a scavenging effect towards 1,1-diphenyl-2picryhydrazyl (DPPH) and 2-2’-azino-bis(3-rthlbenz-thiazoline-6-sulfunic acid) (ABTs) radicals and decreased cellular reactive oxygen species production induced by H2O2. Woo and Piao (2004) isolated two flavonoids, luteolin and luteolin-7-beta-D-glucuronide methyl ester from root of L. lucidus and these flavonoids exhibited antioxidative activity. According to the current literature and our previous studies, L. lucidus contains polysaccharides and flavonoids as active compounds. However, the effect of L. lucidus supplementation on fecal microflora and cytokine expression has not yet been investigated. Thus, in this study, the changes in diversity and composition of microbiota in feces were analyzed in L. lucidus-supplemented mice. In addition, we evaluated the production of major cytokines (IL-6 and -10) related with inflammation and fatty acid composition in several tissues.
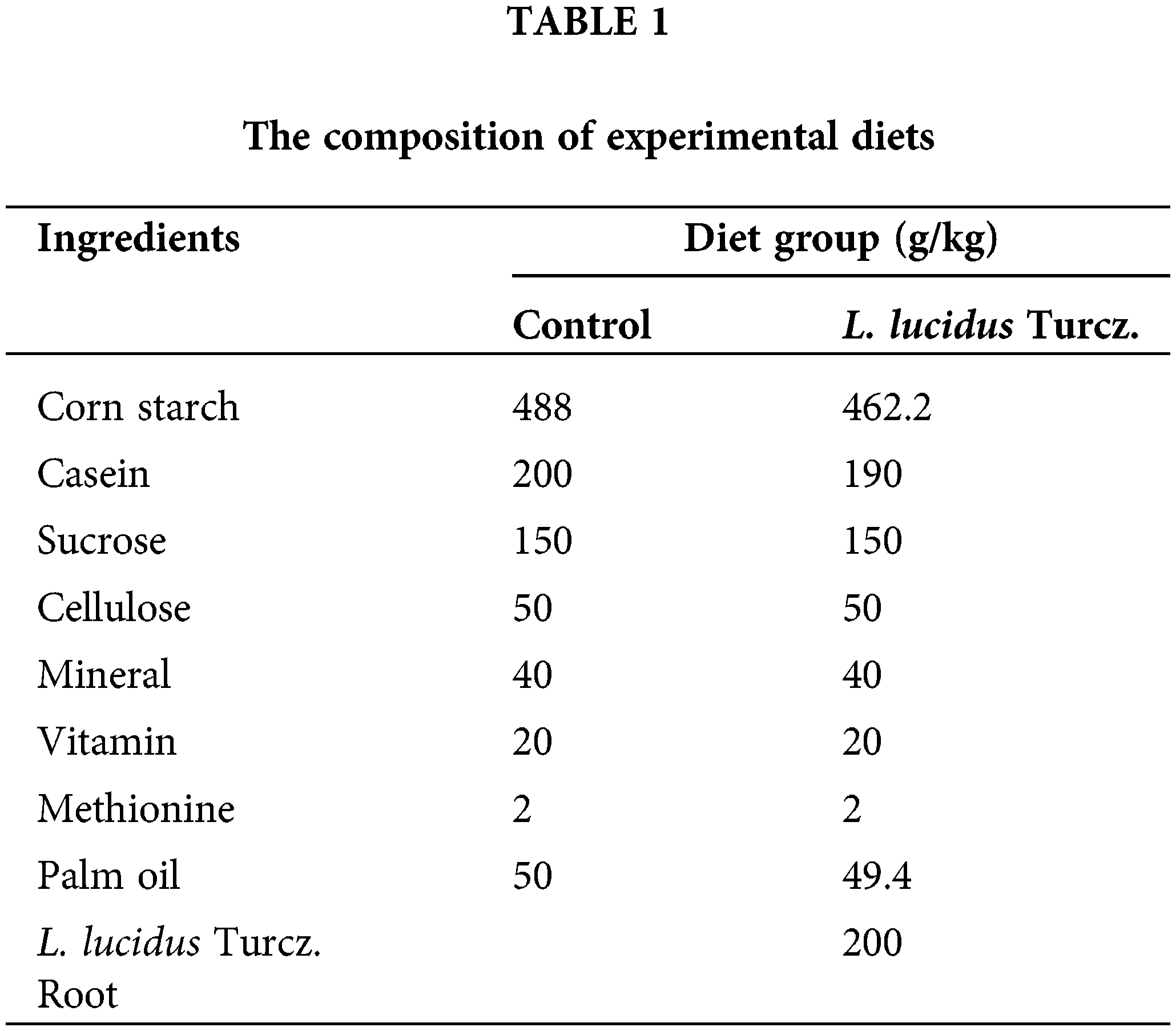
Dried L. lucidus roots were obtained from Misan Inc. (Daegu, Korea). Dried L. lucidus roots were extracted with boiled water at 100°C for 2 h. The extract was centrifuged at 1000 g for 20 min at 4°C and filtered with Whatman No. 3 filter paper. The resulting supernatant was lyophilized to produce a powder. Male Crj: CD-1 mice four weeks of age were obtained from Samtako Inc. (Osan, Korea). As soon as mice arrived at the laboratory, they were kept in habituation for one week. Then twenty-six mice were randomly divided into two groups of thirteen: The first group was the control group that was fed on 5% palm oil (Herbnoori Inc., Daegu, Korea) as a fat source (Control) (Table 1). The second group was fed on diet with 20% powder of L. lucidus roots. L. lucidus root extract contained 5% protein, 0.3% fat and 12.9% carbohydrate. The diet composition was adjusted according to the amounts of nutrients contained in L. lucidus root extract. The composition of diets followed the AIN-93M (Reeves et al., 1993). Customized diets were stored at –4°C, and fresh supplies were given to the mice once every two days. Body weights were measured once a week. Mice were maintained at our thermo-hygrostat facility under conventional conditions of controlled temperature (23 ± 1°C), relative humidity (65 ± 5%) and illumination (12-hours light:dark cycle). Two mice per cage were housed and were allowed to free access to food and water and maintained on these diets for 8 weeks. At the end experiments, the mice were sacrificed by decapitation. Intestine and mesenteric lymph node were removed and stored at –70°C.
Measurements of microorganism using the 3M petrifilmTM plate method
Feces were collected from three mice per each group before feeding. Microflora analysis of mouse feces followed recommendations from manufacturer 3M PetrifilmTM (Aerobic, Lactic Acid Bacteria, and Coliform Count plates). 1 g of each stool sample was weighed and diluted in 9 ml of phosphate buffer saline (PBS) in a sterile test tube. Subsequently they were homogenized in a Vortex shaker at 10−1 dilution, from which the other dilutions were made up to 10−3. Then, 1 ml of the 10−3 of each sample was inoculated into a 3M PetrifilmTM, respectively. Counting was performed after 24 and 48 hours of incubation. For total microbial counts, all colonies staining in various shades of red were counted. For ascertaining the number of coliform and lactic acid bacteria colonies, only red colonies with one or more gas-associated bubbles (within 1 colony diameter) were counted (Park et al., 2001). The microbial counts were calculated as colony-forming units (CFU) per milliliter sample according to the equation:
CFU per ml = (number of colonies x dilution factor of plate)/aliquot plated.
The results will be expressed as log (CFU/ml).
DNA extraction and analysis of 16S rDNA gene sequences
To investigate the change of intestinal microbial composition, we extracted DNA from feces (n = 1 per group) that collected right before the sacrifice using DNeasy Blood & Tissue Kit (Qiagen, CA, USA). DNA samples were prepared according to sequencing company’s guideline. Next generation sequencing (NGS) microbiome taxonomic profiling analysis was performed by ChunLab Inc. (Seoul, Korea) using EzBioCloud data base (Yoon et al., 2017).
RNA preparation and quantitative polymerase chain reaction (q-PCR)
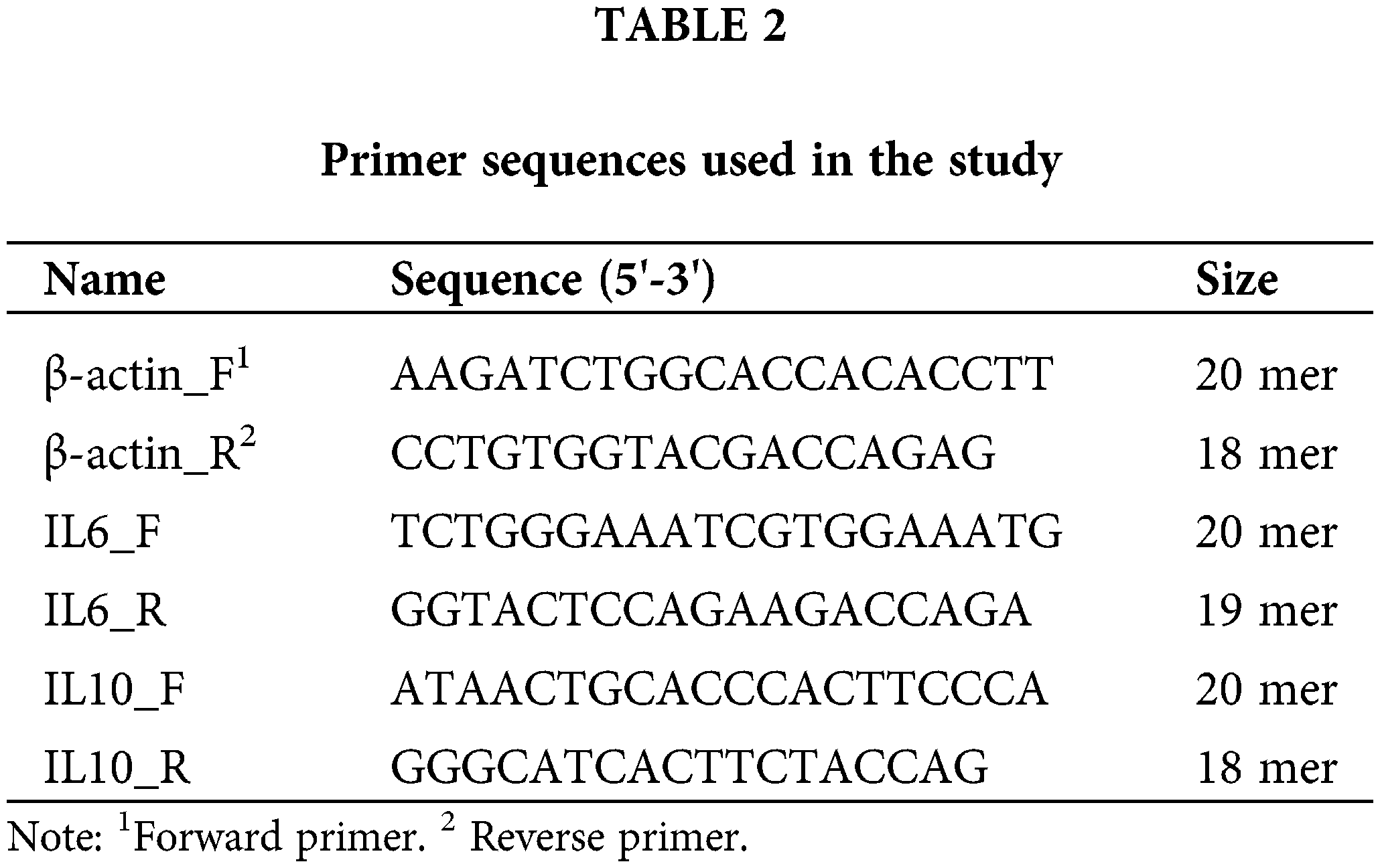
The total RNA from homogenized mesenteric lymph node tissues was isolated with the use of a Minibest Universal RNA Extraction Kit (Takara, Kusatsu, Japan) and performed according to the manufacturer’s protocol. The total RNA concentration has set to 476 ng and then synthesized using Prime Script 1st Strand cDNA Synthesis Kit (Takara, Kusatsu, Japan). qPCR was performed on cDNA samples using the SYBR™ Green PCR Master Mix (Applied Systems, IL, USA) and performed according to the manufacturer’s protocol. Primers used were at Table 2. The thermal cycle conditions were as follows: 50°C for 4 min and 95°C for 15 min of initial denaturation followed by 50 cycles at 95°C for 10 sec, 50°C for 20 sec, 72°C for 30 sec. This was then followed by a melt curve analysis, in which the temperature was 95°C for 20 sec, 60°C for 40 sec, 95°C for 15 sec. Analysis used the sequence detection software supplied with the instrument (StepOne Real-Time PCR System, applied biosystems, IL, USA). The relative quantitation value is expressed as 2− DcT, where DcT is the difference between the mean CT value of duplicates of the sample and of the β-actin control (Kim et al., 2010).
Measurement of fatty acid composition
After the experiment, the mice were then decapitated. Intestine and mesenteric lymph node were removed and stored at –80°C. The lipids extracted from the tissues were prepared (Kim et al., 2021b). The lipid extracts were then transmethylated with 14% BF3-methanol at 100°C for 60 min using a modified version of the method employed by Morrison and Smith (Morrison and Smith, 1964) that involved the addition of hexane. Fatty acid methyl esters were then analyzed by gas liquid chromatography (Varian, CA, USA) as previously described (Salem et al., 1996).
All results were expressed as means ± the standard error of the mean (SEM), with statistical significance determined by t-test using the SIGMASTAT statistical program package (Jandel Co., Erkrath, Germany).
At the start of the study, the body weight of the control and L. lucidus. supplemented mice groups did not differ significantly. The body weight increased gradually during the 8-week period, and that of the control and L. lucidus-supplemented mice was 43.9 ± 1.31 and 42.8 ± 1.40 g, respectively (Fig. 1). The daily food intake of the control and L. lucidus-supplemented mice was 7.0 ± 0.5 and 6.8 ± 0.00 g, respectively. The food efficiency rate (FER) of control and L. lucidus groups was 5.3 and 5.0, respectively.
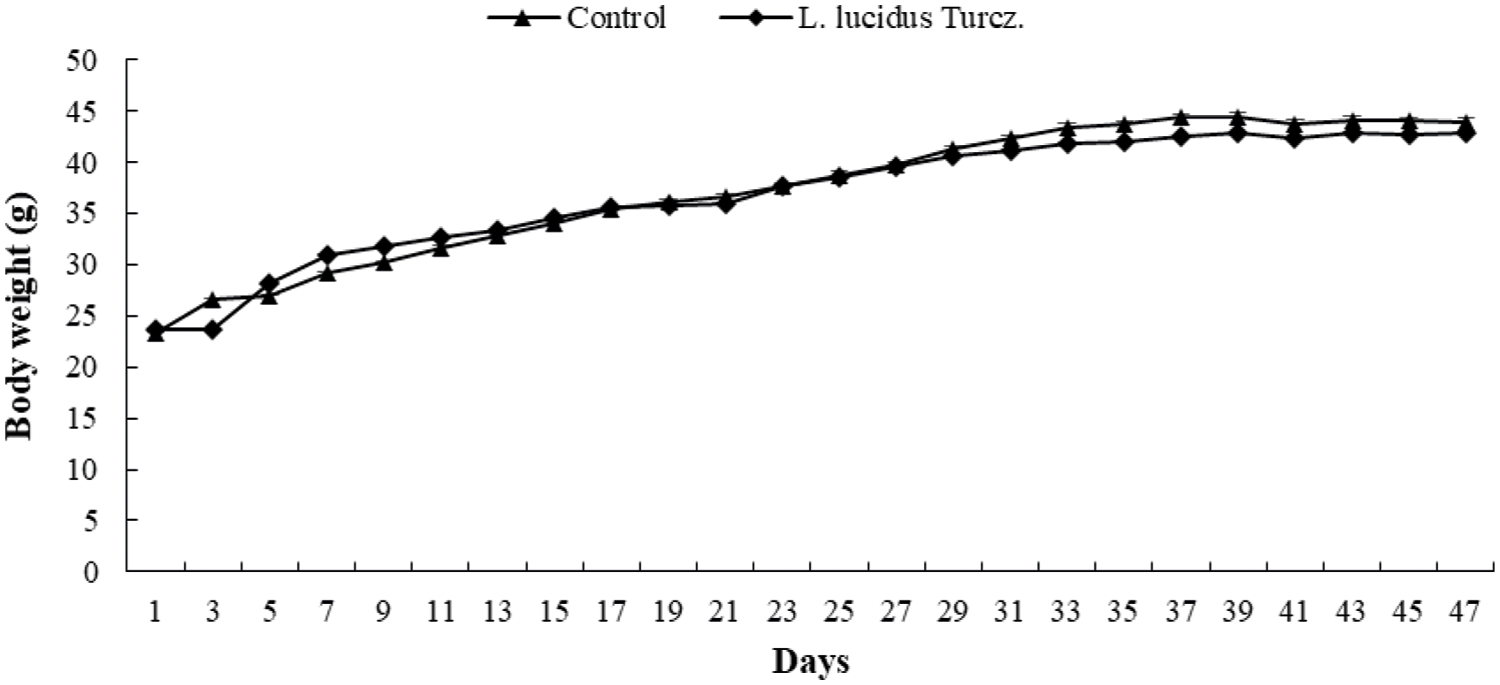
Figure 1: Effect of supplementation with L. lucidus Turcz. on body weight.
Effect of L. lucidus supplementation on fecal microorganism composition
Fig. 2 shows the community of fecal microbiota using 3M petrifilm assay. Supplementation with L. lucidus significantly reduced the counts of coliform and lactic acid bacteria in the feces compared with that in the control (p < 0.05). 16S rDNA sequencing data showed that supplementation with L. lucidus decreased greatly the community of harmful microflora (Enterobacteriaceae including E. coli, and Bacteroides sp.) in feces compared with that in the control (Figs. 3 and 4).
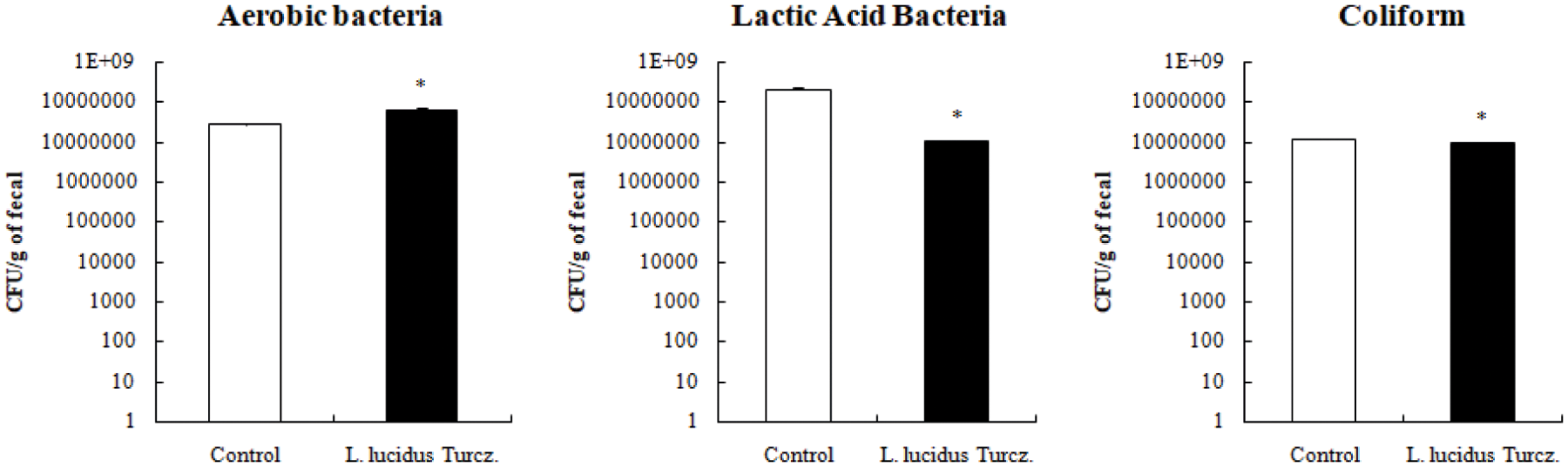
Figure 2: Effect of supplementation with L. lucidus Turcz. on bacterial composition of fecal samples using 3M petrifilm assay. The values were represented as the mean ± SEM and *significantly different between the control and L. lucidus Turcz. groups at p < 0.05.
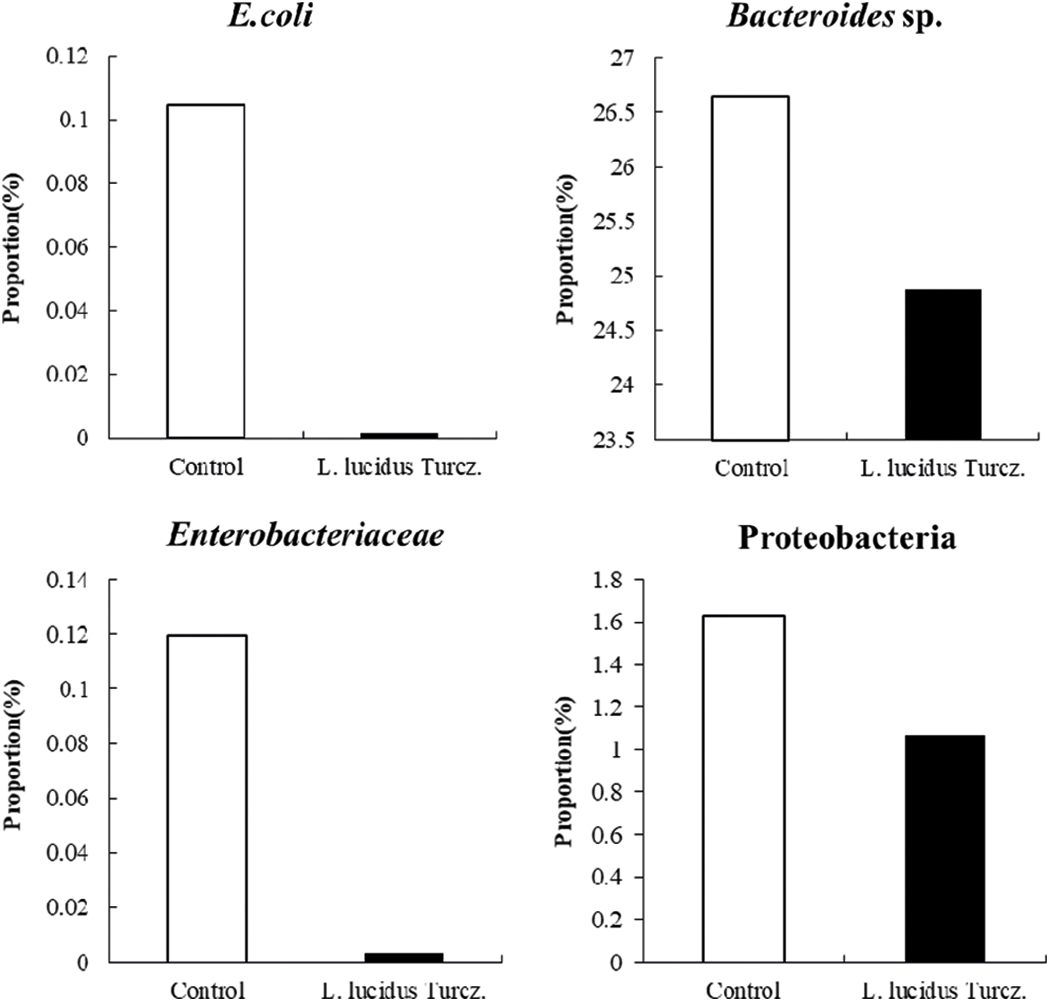
Figure 3: Effect of supplementation with S L. lucidus Turcz. on harmful bacterial community composition of fecal samples analyzed by 16S rDNA sequencing.
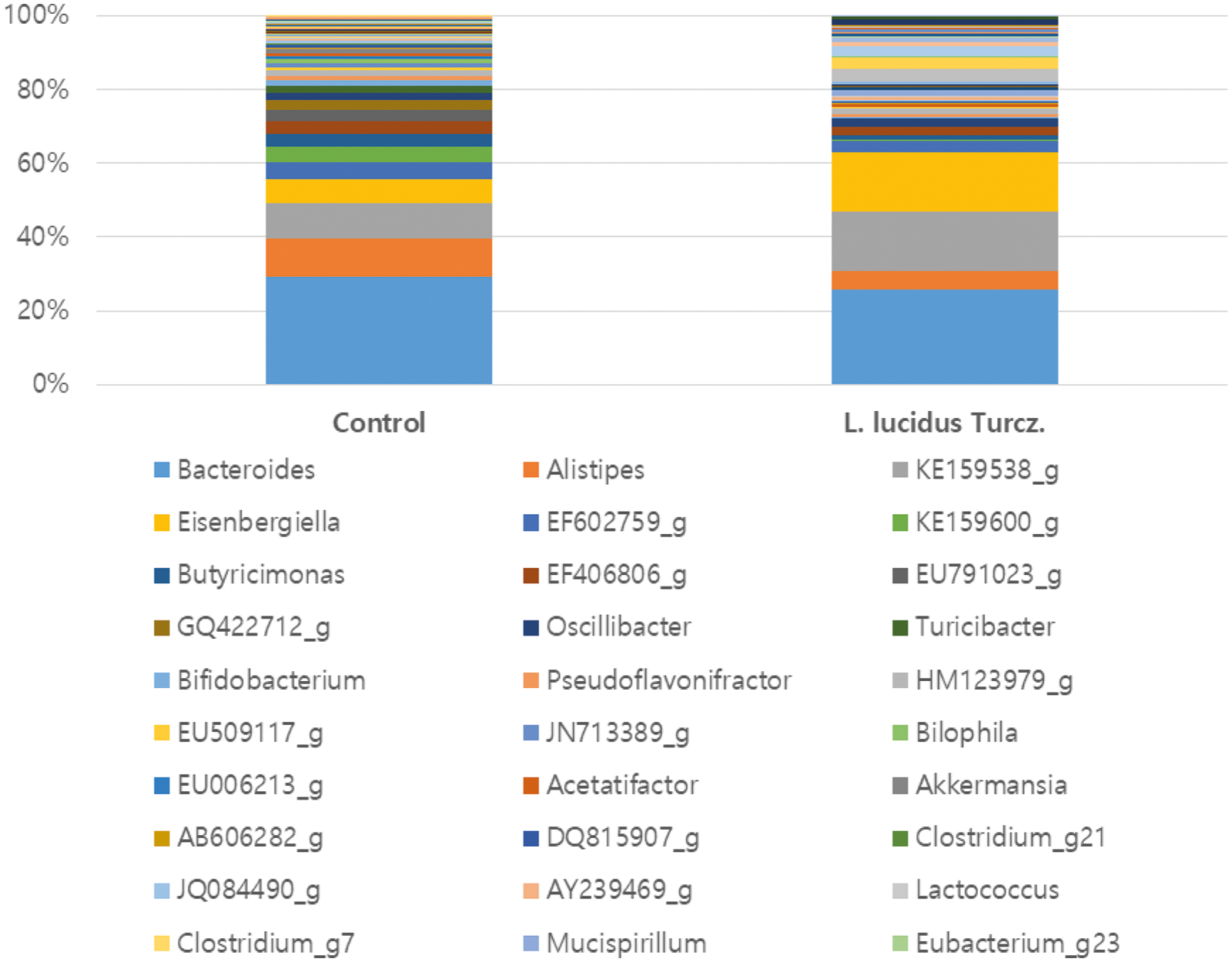
Figure 4: The column chart of genus classification of fecal samples analyzed by 16S rDNA sequencing.
Effect of L. lucidus supplementation on cytokine expression in mesenteric lymph node
The changes in cytokine expression patterns in stimulated mesenteric lymph node are shown in Fig. 5. Mice supplemented with L. lucidus had a higher mRNA expression of IL-6 and a lower mRNA expression of IL-10 compared with that in the control but the differences were not significant.
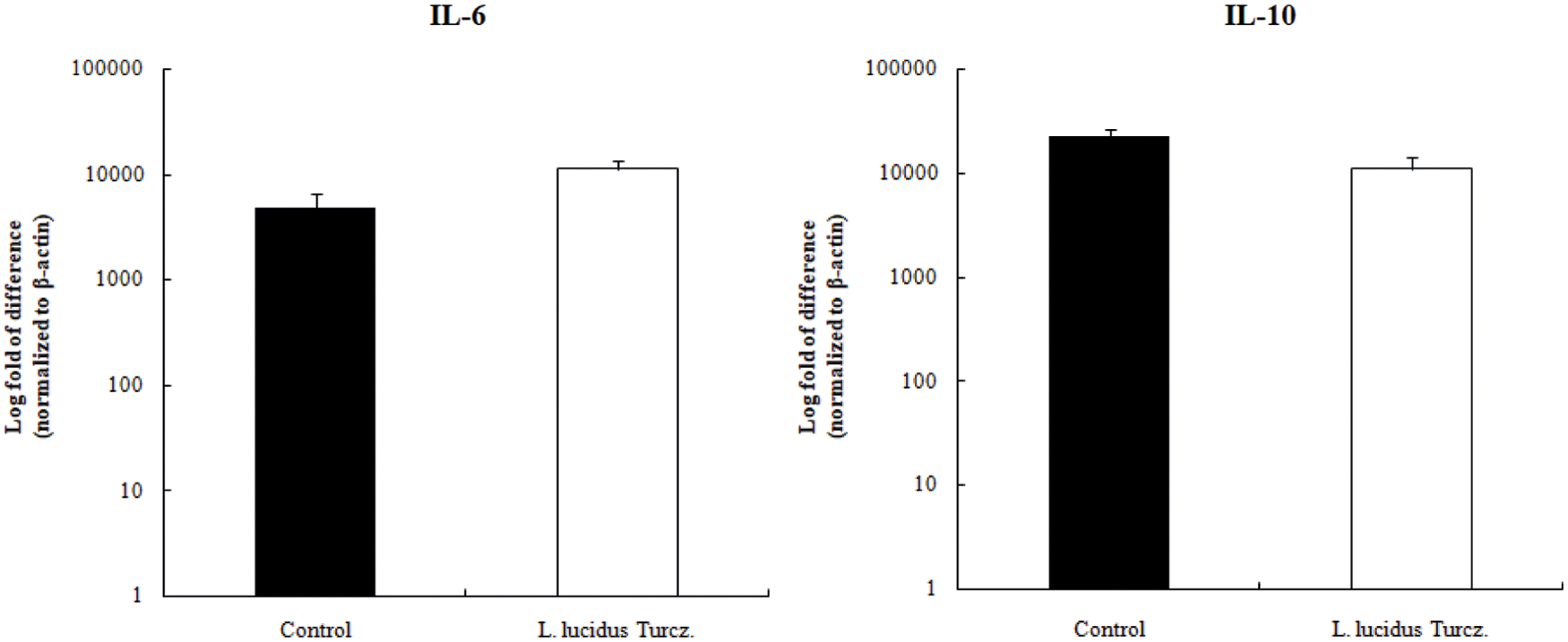
Figure 5: Effect of supplementation with L. lucidus Turcz. on cytokine expression in mesenteric lymph node analyzed by quantitative RT-PCR.
Effect of L. lucidus supplementation on fatty acid composition in intestine and feces
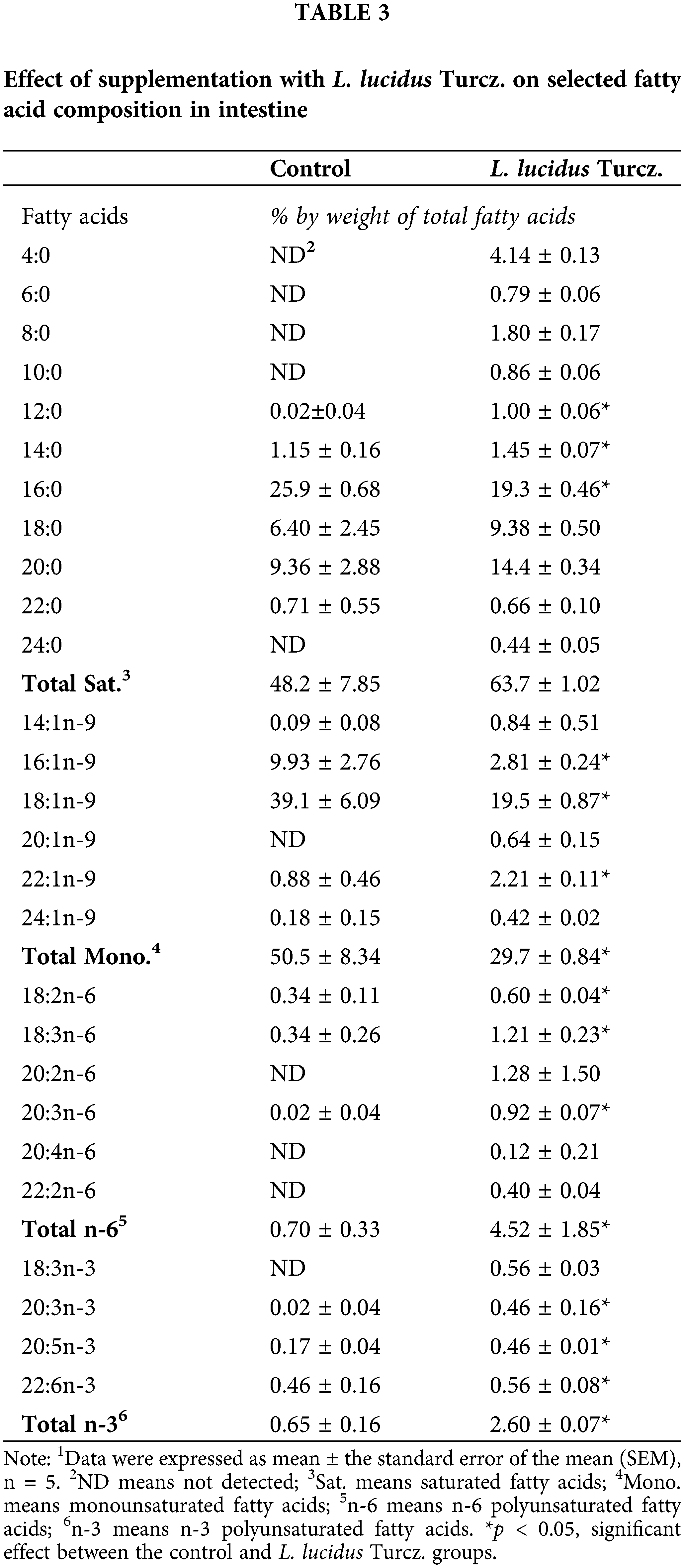
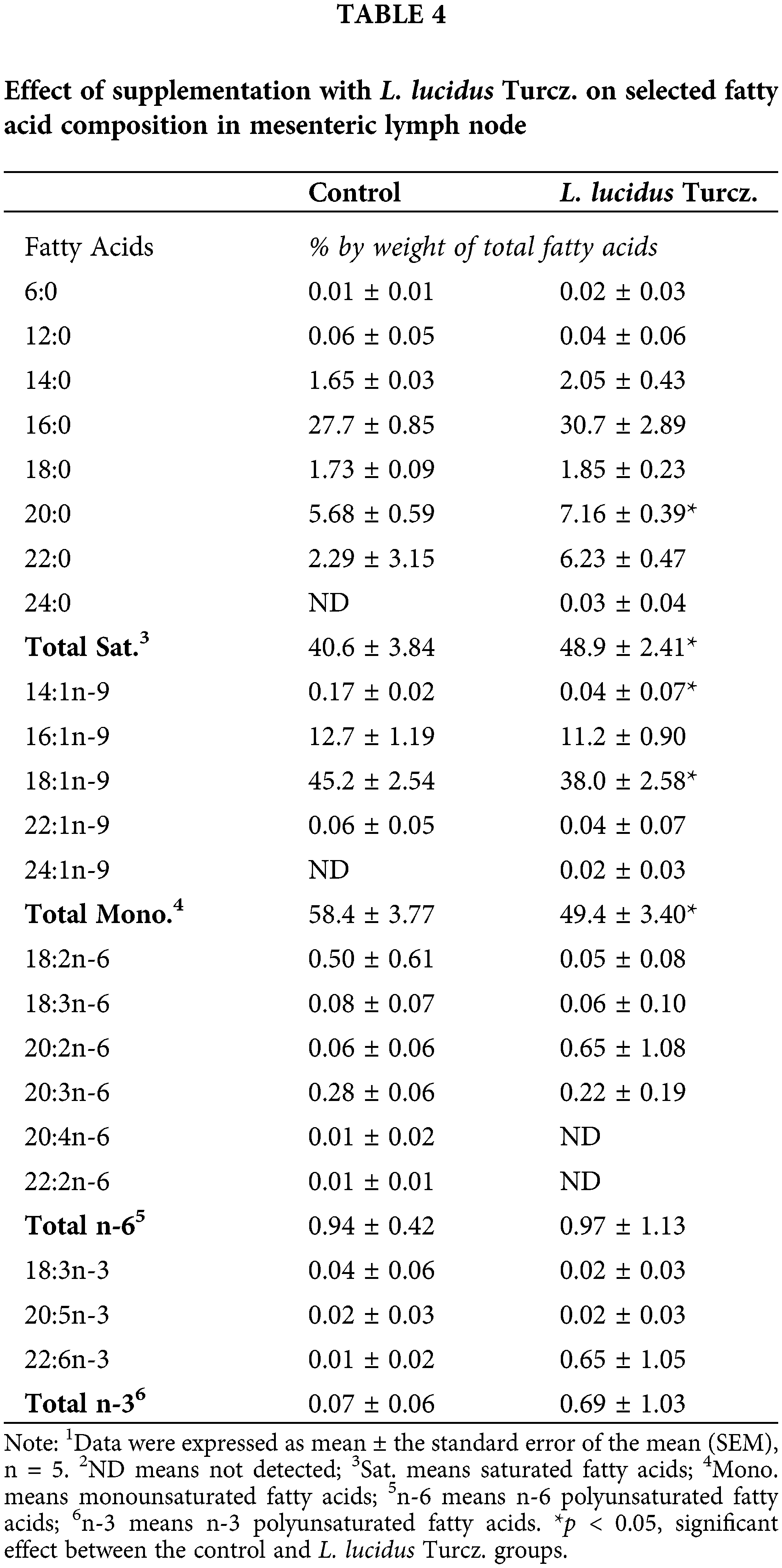
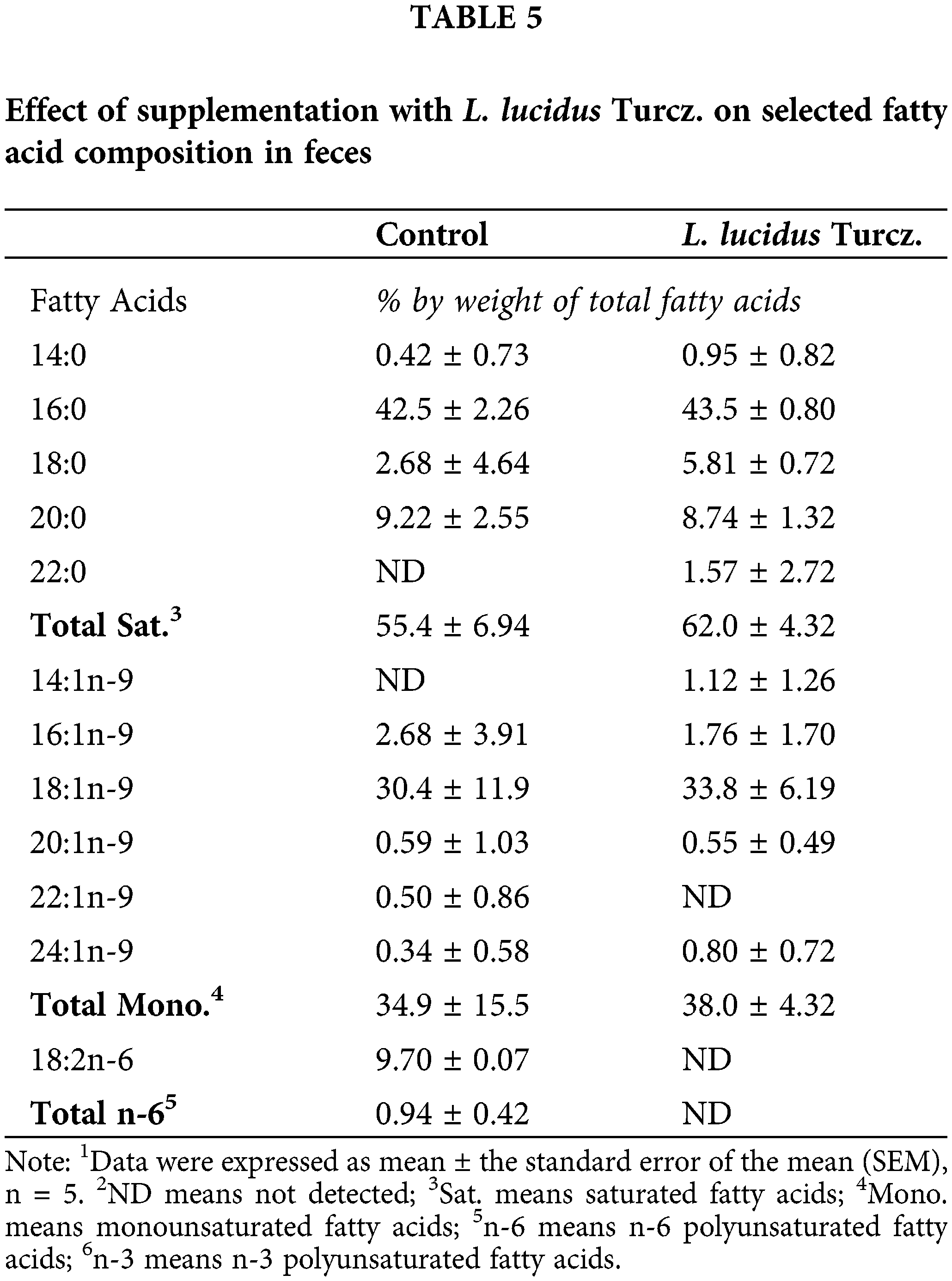
There were marked differences in fatty acid composition of intestine between the control and L. lucidus groups (Table 3). The L. lucidus group had short chain fatty acids (SCFAs) including 4:0, 6:0, 8:0 and 10:0 but there was no detection of these SCFAs in the control group. In addition, the L. lucidus group showed higher total saturated, n-6 and n-3 polyunsaturated fatty acids and lower total monounsaturated (p < 0.05). Among n-3 fatty acids, the percentages of 20:3n-3, 20:5n-3 and 22:6n-3 were significantly higher in the L. lucidus group than those of the control (p < 0.05). In the mesenteric lymph node, the percentage of total saturated was found to be significantly higher in the L. lucidus group, while the percentage total monounsaturated was lower compared with that in the control (p < 0.05) (Table 4). There was no difference in the percentage of 6:0 between two experimental groups. No differences were uncovered between the two groups in terms of the percentages of total saturated, monounsaturated, n-6 and n-3 polyunsaturated fatty acids found in feces (Table 5).
Healthy gastrointestinal microflora are characterized by high numbers and diversity of bacteria (Vandeputter et al., 2016), which interact with mucosal epithelium and are responsible for normal substance metabolism, immune response, and intestinal angiogenesis (Candela et al., 2014). In the current study, we found that supplementation with L. lucidus decreased the harmful microbiota in feces. Based on sequencing of 16S rDNA gene of gut microbiota, the four major phyla in the feces were identified to be Bacteroidetes, Verrucomicrobia, Firmicutes and Proteobacteria, which are consistent with our current results. Lactic acid bacteria are known probiotics with many health benefits, including improvement of normal microflora, inhibition of infectious diseases, reduction of serum cholesterol, and alleviation of intestinal bowel disease symptoms (Maldonado Galdeano et al., 2007). However, in the present study, supplementation with L. lucidus did not increase the population of lactic acid bacteria. There were few studies on relationship between L. lucidus intake and gut microorganisms. Xi et al. (2020) studied stachyose, a oligosaccharide from Chinese artichoke (Stachys sieboldii Miq.) tuber and found that its consumption increased the proliferation of Bifidobacterium and lactobacilli and decreased enteric bacilli.
Overproduction of cytokines are associated with lethal diseases since cytokines can act not only locally to amplify the cellular immune response, but also systemically to change behavior, metabolism, and neuroendocrine secretions (Johnson, 1997). T cells and macrophages secrete IL-6 to stimulate the immune response, particularly in tissue damages leading to numerous types of inflammatory processes (McCurry et al., 1993). IL-10 has potent anti-inflammatory properties and inhibits the Th1 cytokines such as tumor necrosis factor (TNF)-α and interferon (IFN)-γ (Mosmann, 1994). Bogert et al. (2014) suggested that some pathogenic bacteria including Streptococcus and Veillonella increased levels of cytokines IL-8, IL-6, IL-10, and TNF-α. Costa et al. (2014) reported that treatment of betulinic acid from L. lucidus led to increased IL-10 and reduced TNF-α expression but no alteration in IL-6 production. However, in the present study, we failed to prove the modulating effect of L. lucidus on cytokine expression of IL-6 and IL-10. A limitation of this study is that it could not explain the causal factor; therefore, the possibility that the resident microbes influenced IL-6 and IL-10 levels remains. Moreover, IL-6 is a pleiotropic property with pro and anti-inflammatory cytokine (Costa et al., 2014). Soares et al. (1998) suggested that vasoactive sand fly peptide inhibited inflammatory action through increased IL-6 levels.
Under the activation by prebiotics including oligosaccharides, bacteria produce a large amount of SCFAs, which reduce intestinal pH value, prevent the growth of harmful bacteria and promote intestinal peristalsis to accelerate the excretion of pathogenic bacteria and toxins (Bogert et al., 2014). Yang et al. (2010) isolated water-soluble polysaccharides from L. lucidus and identified that its polysaccharides were composed of galactose, followed by galacturonic acid, and nine monosaccharides. Lin et al. (2012) also separated L. lucidus polysaccharides and reported their anti-oxidation and anti-aging effects in aged mice induced by D-galactose. An increase in the amount of some beneficial gut microbiota can produce high SCFA and low ammonia contents compared to other feed ingredients (Velazquez et al., 2000; Topping and Clifton, 2001). Total SCFAs are produced in large intestine through gut microbiota fermentation of plant-derived complex oligosaccharides that have escaped digestion and absorption in small intestine (Blachier et al., 2007). Acetate, propionate, and butyrate are the most abundant SCFAs in gastrointestinal tract, whereas formate, valerate, caproate, etc., make up the remaining (Den Besten et al., 2013). Zhou et al. (2014) reported that supplementation with soybean oligosaccharides increased the concentrations of propionate, butyrate, and total SCFA in the ileum and colon contents as well as those of acetate and valerate. It has been known that SCFAs play an important role in colon epithelium renewal as a fuel for colon epithelial cells (Cotter and Hill, 2003). Therefore, we evaluated the fatty acid composition in intestine and feces. As SCFAs, fatty acids of 4:0, 6:0, 8:0 and 10:0 were increased in intestine of mice with L. lucidus. Thus, administration with L. lucidus would affect gut environment producing SCFAs in our system.
The human intestine is a complex ecosystem of more than 1,000 microbial species with a population of up to 104, providing a good habit for these microorganisms (Sender et al., 2016). Intestinal microorganisms are an important bridge between diet and human health and plays a vital role in maintaining the homeostasis of the human body diversity of the species, the stability of the flora structure and the balance of micro ecology (Uchiyama et al., 2019). The human body lacks carbohydrate active enzymes, meaning that most polysaccharides cannot be directly digested and absorbed by the stomach and intestine, and thus pass through to the colon (Chen et al., 2018). Polysaccharides are fermented by specific intestinal microorganism to produce SCFAs and other metabolites. SCFAs are easily absorbed and exert beneficial physiological effects on the host such as the prevention of type-2 diabetes, inflammatory bowel diseases (IBD) and colon cancer (Song et al., 2021). Natural polysaccharides can also suppress excessive inflammatory responses by improving the intestinal microbiota composition, strengthening intestinal barrier function, and reducing pro-inflammatory mediators (Tang et al., 2019). Thus, our hypothesis is that supplementation of L. lucidus, which contains polysaccharides, would improve gut microbiota composition, which may lead to increased percentages of SCFAs and decreased pro-inflammatory cytokines. We found that supplementation with L. lucidus greatly decreased the community of harmful microbiota including E. coli and Bacteroides sp. in feces. There were no significant differences in mRNA expression of IL-6 and IL-10 in mesenteric lymph node after supplementation with L. lucidus. Fatty acid composition analysis showed that supplementation with L. lucidus resulted in increased percentages of SCFAs (4:0, 6:0, 8:0 and 10:0). Many studies have reported an increase in the population of potentially harmful Enterobacteriaceae family in IBD patients, which may lead to reduced SCFAs and dysbiosis condition in the gut (Lupp et al., 2007; Morgan et al., 2012; Mukhopadhya et al., 2012; Lavelle et al., 2015). The loss of beneficial microbes that produce anti-inflammatory molecules provides a favorable environment for the expansion of pathogens (Baldelli et al., 2021). Because the associated mechanisms by which L. lucidus intake influences gut microbiota composition were not investigated in this study, the next step will be to conduct an in-depth study on the mechanism related to improvement in the gut environment.
The present study is first report to clarify the effect of L. lucidus supplementation on 16S rDNA gene sequence of fecal microflora. Public health and dietary guidance recommendations focus on disease prevention and health maintenance. In this context, we suggest that consistent intake of L. lucidus might be associated with maintaining healthy gut function.
Ethics Approval: The experimental protocol was approved by Gyeongsang National University (Approval No. GNU-171116-M0051). Ethical approval for this study was obtained from Korea Maritime & Ocean University–Institutional Animal Care and USE Committee (KMOU-IACUC).
Availability of Data and Materials: The datasets generated or analyzed during this current study are available from the corresponding authors on reasonable request.
Authors’ Contribution: Study conception, design and manuscript preparation: Sun Young Lim; data collection, analysis and interpretation of results: Eun Na. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This research was supported by Basic Science Research Program of the National Research Foundation of Korea (NRF) in the Ministry of Science, ICT and Future Planning (NRF-2017R1A2B4005915) supported this research.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Baldelli V, Scaldaferri F, Putignani L, Del Chierico F (2021). The role of Enterobacteriaceae in gut microbiota dysbiosis in inflammatory bowel diseases. Microorganisms 9. DOI 10.3390/microorganisms9040697. [Google Scholar] [CrossRef]
Blachier F, Mariotti F, Huneau JF, Tome D (2007). Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 33: 547–562. [Google Scholar]
Bogert BVD, Meijerink M, Zoetendal EG, Wells JM, Kleerebezem M (2014). Immunomodulatory Properties of Streptococcus and Veillonella Isolates from the human small intestine microbiota. PLoS One 9: e114277. DOI 10.1371/journal.pone.0114277. [Google Scholar] [CrossRef]
Braune A, Blaut M (2016). Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 7: 216–234. [Google Scholar]
Candela M, Turroni S, Biagi E, Carbonero F, Rampelli S et al. (2014). Inflammation and colorectal cancer, when microbiota-host mutualism breaks. World Journal of Gastroenterology 20: 908–922. [Google Scholar]
Chen G, Xie M, Wan P, Chen D, Ye H et al. (2018). Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fushuan brick tea. Food Chemistry 244: 331–339. [Google Scholar]
Costa JFO, Barbosa-Filho JM, Maia GLA, Guimaraes ET, Meira CS et al. (2014). Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. International Immunopharmacology 23: 469–474. [Google Scholar]
Cotter PD, Hill C (2003). Surviving the acid test: Response of gram-positive bacteria to low pH. Microbiology and Molecular Biology Reviews 67: 429–453. [Google Scholar]
Den Besten G, van Eunen K, Groen AK, Venema K, Reijingoud DJ et al. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research 54: 2325–2340. [Google Scholar]
Duenas M, Munoz-Gonzalez I, Cueva C, Jimenez-Giron A, Sanchez-Patan F et al. (2015). A survey of modulation of gut microbiota by dietary polyphenols. Biomed Research International 2015. DOI 10.1155/2015/850902. [Google Scholar] [CrossRef]
Espley RV, Burts CA, Laing WA, Martell S, Smith H et al. (2014). Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. Journal of Nutrition 144: 146–154. [Google Scholar]
Fan TT, Cheng BL, Fang XM, Chen YC, Su F (2020). Application of Chinese medicine in the management of critical conditions: A review on Sepsis. American Journal of Clinical Medicine 48: 1315–1330. [Google Scholar]
Goldsmith JR, Sartor B (2014). The role of diet on intestinal microbiota metabolism: Downstream impacts on host immune function and health, and therapeutic implications. Journal of Gastroenterology 49: 7855–7798. [Google Scholar]
Holscher H (2017). Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8: 172–184. [Google Scholar]
Johnson RW (1997). Inhibition of growth by pro-inflammatory cytokines: An integrated view. Journal of Animal Science 75: 1244–1255. [Google Scholar]
Kim H, Hong JY, Jeon WJ, Lee J, Baek SH et al. (2021a). Lycopus lucidus Turcz exerts neuroprotective effects against H2O2-induced neuroinflammation by inhibiting NLRP3 inflammasome activation in cortical neurons. Journal of Inflammation Research 14: 1759–1773. [Google Scholar]
Kim H, Jayapala HPS, Jo WH, Nam HS, Lim SY (2021b). A comparative study on the chemical characteristics and antioxidant effects of sea mustards sourced from different areas in Taejongdae. Journal of Life Science 31: 559–567. [Google Scholar]
Kim JY, Cho MK, Choi SH, Lee KH, Ahn SC et al. (2010). Inhibition of dextran sulfate sodium (DSS)-induced intestinal inflammation via enhanced IL-10 and TGF-β production by galectin-9 homologues isolated from intestinal parasites. Molecular and Biochemical Parasitology 174: 53–61. [Google Scholar]
Kim KY, Oh TW, Ma JY, Park KI (2018). Ethanol extract of Lycopus lucidus Turcz. ex Benth inhibits metastasis by downregulation of Runx-2 in mouse colon cancer cells. Evidence-Based Complementary and Alterative Medicine 2018. DOI 10.115/2018/9513290. [Google Scholar] [CrossRef]
Lavelle A, Lennon G, O’Sulivan O, Docherty N, Balfe A et al. (2015). Spatial variation of the colonic microbiota in patients with ulcerative colitis control volunteers. Gut 64: 1553–1561. [Google Scholar]
Lee JW, Lim SY (2018). Comparison of flavonoid content and antioxidant effect of extrcts from Stachys sieboldii Miq. and Lycopus lucidus Turcz. Journal of Life Science 28: 841–848. [Google Scholar]
Li HL, Lu L, Wang XS, Qin LY, Wang P et al. (2017). Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Frontiers Cellular Infection Microbial 7: 1490. DOI 10.3389/fcimb.2017.00455. [Google Scholar] [CrossRef]
Lin C, Zuo S, Xiong W, Chen G (2012). Antioxidation effects of Lycopus lucidus polysaccharides on aged induced by D-gallactose. Medicinal Plant 3: 53–55. [Google Scholar]
Liu J, Bhuvanagiri S, Qu X (2019). The protective effects of Lycopus lucidus Turcz. in diabetic retinopathy and its possible mechanisms. Artificial Cells, Nanomedicine, and Biotechnology 47: 2900–2908. [Google Scholar]
Lu Y, Huang J, Li Y, Ma T, Sang P et al. (2015). Variation in nutritional compositions, antioxidant activity and microstructure of Lycopus lucidus Turcz. root at different harvest times. Food Chemistry 183: 91–100. [Google Scholar]
Lupp C, Robertson ML, Wickham ME, Sekinov I, Champion OL et al. (2007). Host-mediated inflammation disrupts the intestinal mmicrobiota and promotes the overgrowth of Enterobacteriaceae. Cell Host & Microbe 2: 119–129. [Google Scholar]
Maldonado Galdeano C, de Moreno de LeBlianc A, Vinderola G, Bibas Bonet ME, Perdigon G (2007). Proposed model mechanisms of immunomodulation induced by probiotic bacteria. Clinical and Vaccine Immunology 14: 485–492. [Google Scholar]
McCurry KR, Campbell DAJr, Scales WE, Warren JS, Remick DG (1993). Tumor necrosis factor, interleukin 6 and the acute phase response following hepatic ischemia/reperfusion. Journal of Surgical Research 55: 49–54. DOI 10.1006/jsre.1993.1107. [Google Scholar] [CrossRef]
Min GY, Kim EY, Hong S, Kim JH, Kim M et al. (2021). Lycopus lucidus Turcz ameliorates DNCB-induced atopic dermatitis in BALB/c mice. Molecular Medicine Reports 827. DOI 10.3892/mmr.2021.12467. [Google Scholar] [CrossRef]
Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL et al. (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biology 13: R79. DOI 10.1186/gb-2012-13-9-r79. [Google Scholar] [CrossRef]
Morrison WR, Smith LM (1964). Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. Journal of Lipid Research 5: 600–608. DOI 10.1016/S0022-2275(20)40190-7. [Google Scholar] [CrossRef]
Mosmann TR (1994). Properties and functions of interleukin-10. Advances in Immunology 56: 1–26. DOI 10.1016/S0065-2776(08)60449-6. [Google Scholar] [CrossRef]
Mukhopadhya I, Hansen R, El-Omar EM, Hold GL (2012). IBD-what role do proteobacteia play? Nature Reviews Gastroenterology Hepatology 9: 219–230. DOI 10.1038/nrgastro.2012.14. [Google Scholar] [CrossRef]
Panche AN, Diwan AD, Channdra SR (2017). Flavonoids: An overview. Journal of Nutritional Science 8: 172–184. [Google Scholar]
Park HJ (2019). Isolation of constituent inhibiting nitric oxide formation from Lycopus lucidus in LPS-induced macrophage cells. Korean Journal of Plant Research 32: 264–269. [Google Scholar]
Park HJ, Jin S, Oh YM, yun SG, Lee JY et al. (2013). Induction of G1 arrest by methanol extract of Lycopus lucidus in human lung adenocarcinoma A549 cell. Journal of Life Science 23: 1109–1117. [Google Scholar]
Park YH, Seo KS, Ahn JS, Yoo HS, Kim SP (2001). Evaluation of the Petrifilm plate method for the enumeration of aerobic microorganisms and coliforms in retailed meat samples. Journal of Food Protection 64: 1841–1843. [Google Scholar]
Reeves PC, Nielson FH, Fahey GCJr (1993). AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. Journal of Nutrition 123: 1939–1951. [Google Scholar]
Salem N, Reyzer M, Karanian J (1996). Losses of arachidonic acid in rat liver after alcohol inhalation. Lipids 31: S153–S156. [Google Scholar]
Sender R, Fuchs S, Milo R (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biology 14: e1002533. DOI 10.1371/journal.pbio.1002533. [Google Scholar] [CrossRef]
Soares MB, Titus RG, Shoemaker CB, David JR, Bozza M (1998). The vasoactive peptide maxadilan from sand fly sliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. Journal of Immunology 160: 811–1816. [Google Scholar]
Song YJ, Chang JP, Yoo JH (2016). Antioxidant activities of water extracts from different parts of Lycopus lucidus Turcz. ex Benth. Korean Journal of Herbaology 31: 21–28. [Google Scholar]
Song Q, Wang Y, Huang L, Shen M, Yu Y et al. (2021). Review of the relationships among polysaccharides, gut microbiota, and human health. Food Research International 140. DOI 10.1016/j.fodres.2020.109858. [Google Scholar] [CrossRef]
Tang C, Ding R, Sun J, Liu J, Kan J, Jin C (2019). The impacts of natural polysaccharides on intestinal microbiota and immune responses–A review. Food Function 10: 2290–2312. [Google Scholar]
Topping DL, Clifton PM (2001). Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiology Review 81: 1031–1064. [Google Scholar]
Tung D, Cheung PH, Tudor G, Booth C, Saha S (2011). In vivo effects of immunomodulators in a murine model of fluorouracil-induced mucositis. Current Therapeutic Research 72: 262–272. [Google Scholar]
Uchiyama K, Naito Y, Takagi T (2019). Intestinal microbiome as a novel therapeutic target for local and systemic inflammation. Pharmacol Therapeutics 199: 164–172. [Google Scholar]
Vandeputter D, Falony G, Vieira-Silva S, Tiro RY, Joosens M et al. (2016). Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65: 57–62. [Google Scholar]
Velazquez M, Davies C, Marett R, Slavin JL, Feirtag JM (2000). Effect of oligosaccharides and fibre substitutes on short-chain fatty acid production by human faecal microflora. Anaerobe 6: 87–92. [Google Scholar]
Woo ER, Piao MS (2004). Antioxidant constituents from Lycopus lucidus. Archives of Pharmacology Research 27: 173–176. [Google Scholar]
Xi M, Yao Q, Ge W, Chen Y, Cao B et al. (2020). Effects of Stachyose on intestinal microbiota and immunity in mice infected with enterotoxigenic Escherichia coli. Journal of Functional Foods 64: 103689. DOI 10.1016/j.jff.2019.103689. [Google Scholar] [CrossRef]
Yang MO (2017). Antioxidant properties of hot water extract of Lycopus lucidus Turcz. tubers. Korean Journal of Community Living Science 28: 103–113. [Google Scholar]
Yang X, Lv Y, Tian L, Zhao Y (2010). Composition and systemic immune activity of the polysaccharides from an herbal tea (Lycopus lucidus Turcz). Journal of Agriculture and Food Chemistry 58: 6075–6080. [Google Scholar]
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y et al. (2017). Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. International Journal of Systematic Evolutionary Microbiology 67: 1613–1617. [Google Scholar]
Yu JQ, Lei JC, Zhang XQ, Yu HD, Tian DZ et al. (2011). Anticancer, antioxidant and antimicrobial activities of the essential oil of Lycopus lucidus Turcz. var. hirtus Regel. Food Chemistry 126: 1593–1598. [Google Scholar]
Zhang W, Hu Y, He J, Guo D, Zhao J et al. (2021). Structural characterization and immunomodulatory activity of a novel polysaccharide from Lycopi herba. Frontiers Pharmacology 25. DOI 10.3389/fphar.2021.69995. [Google Scholar] [CrossRef]
Zhou XL, Kong XF, Lian GQ, Blachier F, Geng MM et al. (2014). Dietary supplementation with soybean oligosaccharides increase short-chain fatty acids but decreases protein-derived catabolites in the intestinal luminal content of weaned Huanjiang mini-piglets. Nutrition Research 34: 780–788. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |