DOI:10.32604/biocell.2022.019993

| BIOCELL DOI:10.32604/biocell.2022.019993 |  |
| Viewpoint |
Inflammatory priming of mesenchymal stem cells: Focus on growth factors enhancement
Cell Physiology Laboratory, Institute of Biomedical Problems of Russian Academy of Sciences, Moscow, 123007, Russia
*Address correspondence to: Elena Andreeva, cellphysiology@imbp.ru; Ludmila Buravkova, buravkova@imbp.ru
Received: 28 October 2021; Accepted: 17 January 2022
Abstract: Multipotent mesenchymal stromal cells (MSCs) are actively involved in reparation and inflammation processes, providing damaged tissue reparation and suppressing immune cell responses in vivo. The effects are mostly due to the production of a wide range of paracrine factors, including growth factors and immunomodulatory mediators. To induce immunosuppressive activity, MSCs are primed by inflammatory cytokines, which results in an increased production of immunomodulatory molecules. However, stimulation of reparative properties is also necessary. This viewpoint manuscript highlights the possibilities of inflammatory priming to increase the production of growth factors by MSCs.
Keywords: Multipotent mesenchymal stromal cells; Priming; Growth factors
MSCs from various sources are considered as a promising tool for cell therapy and regenerative medicine. Their capability of multilineage differentiation and the production of a wide range of paracrine factors involved in inflammation and cell growth regulation makes it possible to use MSCs to stimulate the processes of damaged tissue regeneration and reparation. Another most therapeutically demanded MSC property is the ability to modulate the activity of immune cells and avoid their response (immune evasiveness) (Ankrum et al., 2014), allowing allogeneic application of MSCs. Therefore, MSCs are successfully used for the treatment of undesirable immune response in graft-versus-host disease reaction (GVHD), autoimmune diseases and both chronic and acute inflammation.
The immunosuppressive properties of MSCs were found not to be constitutively manifested, but to be stimulated by paracrine mediators typical of the inflammatory microenvironment, which became the basis for the development of in vitro preconditioning protocols to increase the MSC immunomodulatory potential by “priming” with inflammatory cytokines TNFa, IFNg, IL-1α, IL-1β, etc. (Gornostaeva et al., 2016; de Cássia Noronha et al., 2019; Micheli et al., 2021). Both in vitro and in vivo experimental models have demonstrated the enhancement of MSC immunosuppression after priming (Bartholomew et al., 2002; Crop et al., 2010; Wobma et al., 2018; Ragni et al., 2020).
In addition to immunosuppression, the ability of MSCs to stimulate the activity of endogenous cell sources in situ due to the secretion of growth factors is in high demand. Therefore, the increased levels of these mediators in inflammatory-activated MSCs would be a very significant bonus. However, mainly immunosuppression-related outcomes are usually evaluated after priming of MSCs with IFN-γ, TNF-α, and other inflammatory cytokines. Commonly, IDO, PGE2, TFG-β production and antiproliferative effects of above are measured (Noone et al., 2013; Guan et al., 2017; Redondo-Castro et al., 2017; Wobma et al., 2018).
We believe that such a narrowed view on the inflammatory priming results, in particular the underestimation of growth factor production, is not entirely justified. It is assumed that resident MSCs are both involved in reparation processes and inhibit tissue inflammatory responses (Nimiritsky et al., 2019; Girousse et al., 2021). From an applied point, the administration of MSCs in damage tissue should not only help suppress the excessive immune response, but also activate endogenous cell populations. It is reasonable that the inflammatory microenvironment should stimulate not only the immunomodulatory, but also the reparative MSC properties. Very few studies are available in this area. Screening the Pubmed database for the 2006-2021 period using the “priming”, “MSC”, and “growth factors” keywords, we found ten publications that had evaluated the production of growth factors after an inflammatory stimulation of MSCs (Table 1).
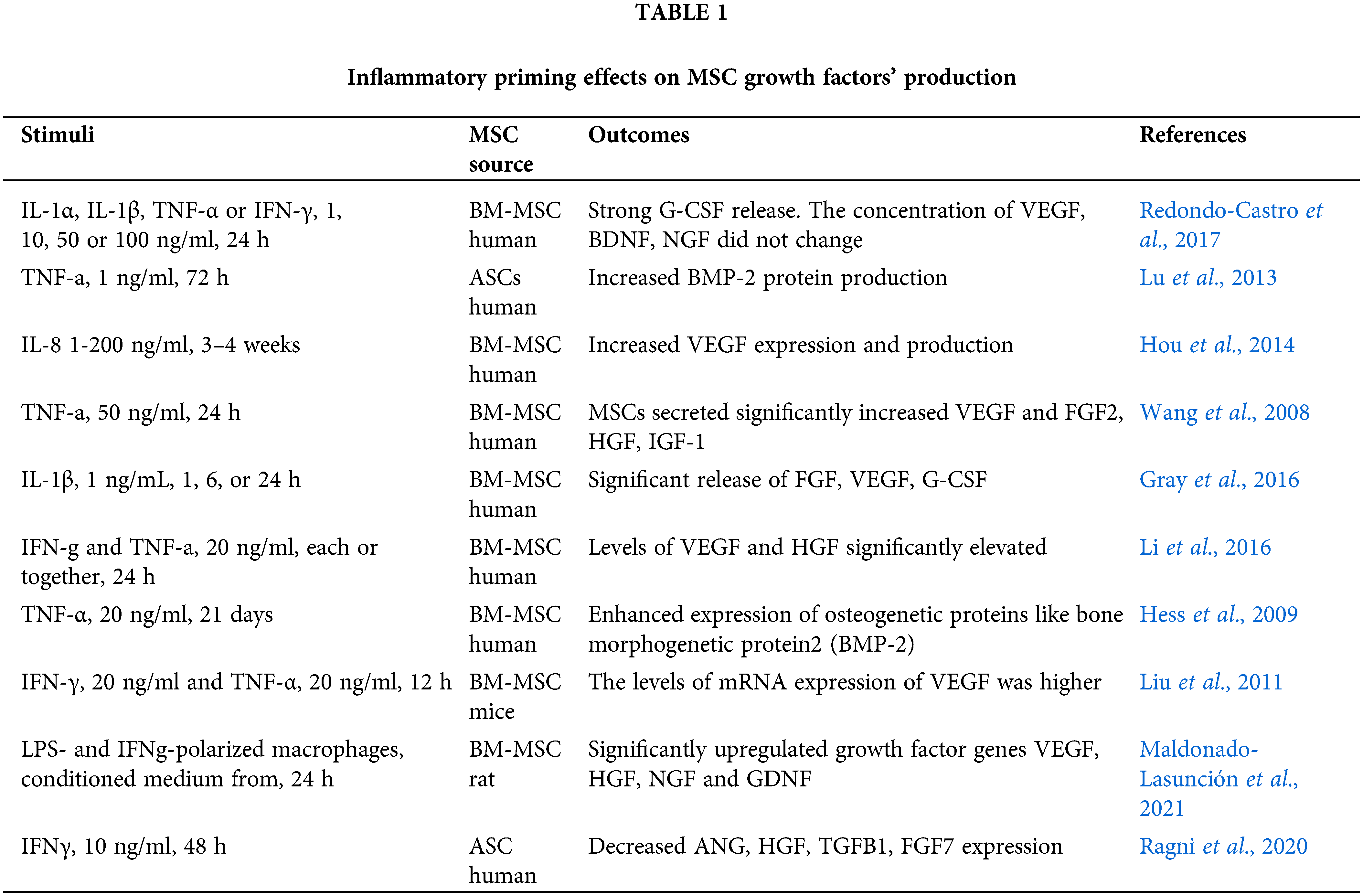
MSCs treated with IFN-γ, TNF-α, IL-1α, IL-1β, and IL-8 alone or in combination were found to increase the production of growth factors such as G-CSF, BMP-2, VEGF, FGF2, HGF, IGF-1, NGF, and GDNF (Wang et al., 2008; Hess et al., 2009; Liu et al., 2011; Lu et al., 2013; Hou et al., 2014; Li et al., 2016; Gray et al., 2016; Redondo-Castro et al., 2017). Also, the genes encoded growth factors were found to be upregulated when MSCs were primed with conditioned media from LPS- and IFN-γ-polarized macrophages (Maldonado-Lasunción et al., 2021). Meanwhile, Ragni et al. (2020) found a downregulation of ANG, HGF, TGFB1, and FGF7 after IFN-γ priming, and Redondo-Castro et al. (2017) did not detect any changes in the levels of VEGF, BDNF, and NGF in MSC stimulated with IL-1α, IL-1β, TNF-α, or IFN-γ). In above studies, the cytokines, concentrations and time intervals for MSC priming differed significantly, which could result in data heterogeneity. Despite the fewness and some inconsistency of data, it can be concluded that production of growth factors in MSCs may be stimulated with inflammatory priming, while the selection of optimal conditions is still to be done.
We have supposed that the stimulation of growth factor secretion would be more stable and pronounced in case of priming with inflammatory cocktail produced by activated immune cells directly interacted with MSCs.
Recently, we analyzed the growth factor levels in MSC monoculture and in coculture with PHA-stimulated allogeneic T cells (Fig. 1). A number of growth factors including epidermal growth factor (EGF), platelet-derived growth factor-AA and AB/BB (PDGF-AA, PDGF-AB/BB), fibroblast growth factor-2 (FGF-2), transforming growth factor-α (TGF-α), and vascular endothelial growth factor (VEGF) were identified in the conditioned medium from monocultured MSCs. These mediators play an important role in the implementation of MSC reparative potential. TGF-α treatment was shown to enhance MSC-mediated cardioprotection (Herrmann et al., 2010). MSC preconditioning with FGF-2 enhanced vascularization in vivo (Gorin et al., 2016). PDGF-AB/BB was found to be involved in resident cardiac MSC migration to the damage site (Windmolders et al., 2014). bEGF, VEGF and FGF accelerated wound closure, promoted angiogenesis, reepithelization and collagen deposition (Padeta et al., 2017; Tarcisia et al., 2018). TGFβ-1, VEGF, FGF, HGF, and PDGF reduced transepidermal water loss and accelerated healing (Zhou et al., 2013). These factors can also interact with one another, mutually enhancing their effects; e.g., FGF-2 increased HGF and VEGF secretion (Gorin et al., 2016) and TGF-α caused an increased production of VEGF in MSCs via MEK and PI3-K-dependent mechanisms (Wang et al., 2008).
Interaction with activated T cells, was followed by a significant increase in the levels of EGF, PDGF-AB/BB, FGF-2, and VEGF by MSCs (p < 0.05). TGF-a tended to increase and PDGF-AA production remained unchanged (Fig. 1). Therefore, we found that interaction with immune cells resulted in an increased production of a number of growth factors in MSCs, and hence contributing to the increase of reparative potential.
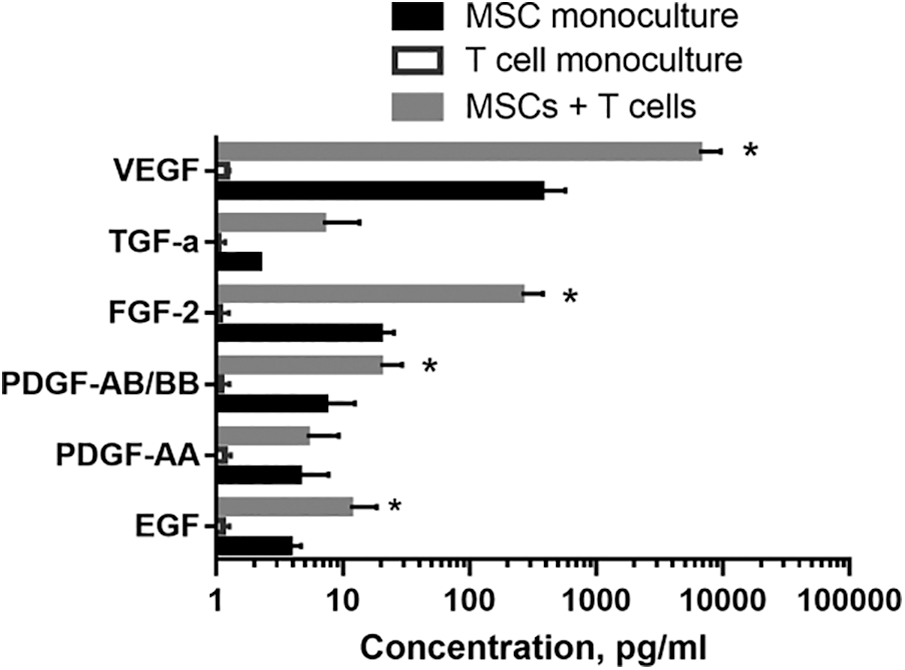
Figure 1: The effect of activated T cells on MSC growth factors’ production. MSCs were isolated from human adipose tissue and cultured as described elsewhere. T cells were magnetically separated (Miltenyi Biotec, Germany) from peripheral blood mononuclear cells of healthy volunteers and stimulated with 10 μg/mL phytohemagglutinin (PHA, Sigma, USA). MSCs of 2-4th passages were cocultured with activated T cells. After 72 h, conditioned medium was collected from MSC monocultures and MSC+T cell cocultures, and cytokine profiles were analyzed using a Cyto/Chemo MAG Premix 41 Milliplex kit. MSCs produced growth factors in a wide range (from 100 to 10,000 pg/mL). The logarithmic scale was used to present the results. The statistical analysis was performed using the GraphPad Prism 7 software package. Two-way ANOVA was used (with the subsequent application of Tukey’s post hoc test) to assess the reliability of differences. Differences were considered significant at the 0.05 level. The data are presented as mean ± SD (n = 6). * – significance level (p < 0.05) vs. MSC monoculture.
Thereby, at the inflammatory microenvironment created by activated immune cells, not only MSC immunosuppressive properties are enhanced, but their regenerative properties as well. The above phenomenon is in demand for the practical use of MSCs and requires a comprehensive study. The development of the MSC priming approach for the induction of both immunomodulatory and growth factors production will make it possible not only to optimally “tune” the MSC potential for therapeutic purposes, but also to obtain cell-free products for clinical use.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Ludmila Buravkova; data collection: Aleksandra Gornostaeva; analysis and interpretation of results: Elena Andreeva, Aleksandra Gornostaeva, Ludmila Buravkova; draft manuscript preparation: Aleksandra Gornostaeva, Elena Andreeva. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: All cell culture procedures were approved by the Biomedicine Ethics Committee of the Institute of Biomedical Problems, Russian Academy of Sciences (Permit #314/МCK/09/03/13).
Funding Statement: The work was supported by Basic Research Program of Institute of Biomedical Problems of RAS, theme 65.3.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ankrum JA, Ong JF, Karp JM (2014). Mesenchymal stem cells: Immune evasive, not immune privileged. Nature Biotechnology 32: 252–260. DOI 10.1038/nbt.2816. [Google Scholar] [CrossRef]
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K et al. (2002). Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology 30: 42–48. DOI 10.1016/S0301-472X(01)00769-X. [Google Scholar] [CrossRef]
Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Pescatori M et al. (2010). Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clinical and Experimental Immunology 162: 474–486. DOI 10.1111/j.1365-2249.2010.04256.x. [Google Scholar] [CrossRef]
de Cássia Noronha N, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM et al. (2019). Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Research & Therapy 10: 1–21. [Google Scholar]
Girousse A, Mathieu M, Sastourné-Arrey Q, Monferran S, Casteilla L et al. (2021). Endogenous mobilization of mesenchymal Stromal cells: A pathway for interorgan communication? Frontiers in Cell and Developmental Biology 8: 598520. DOI 10.3389/fcell.2020.598520. [Google Scholar] [CrossRef]
Gorin C, Rochefort GY, Bascetin R, Ying H, Lesieur J et al. (2016). Priming dental pulp stem cells with fibroblast growth factor-2 increases angiogenesis of implanted tissueengineered constructs through hepatocyte growth factor and vascular endothelial growth factor secretion. Stem Cells Translational Medicine 5: 392–404. DOI 10.5966/sctm.2015-0166. [Google Scholar] [CrossRef]
Gornostaeva A, Andreeva E, Buravkova L (2016). Factors governing the immunosuppressive effects of multipotent mesenchymal stromal cells in vitro. Cytotechnology 68: 565–577. DOI 10.1007/s10616-015-9906-5. [Google Scholar] [CrossRef]
Gray A, Schloss RS, Yarmush M (2016). Donor variability among anti-inflammatory pre-activated mesenchymal stromal cells. Technology (Singapore World Scientific) 4: 201–215. DOI 10.1142/S2339547816500084. [Google Scholar] [CrossRef]
Guan Q, Ezzati P, Spicer V, Krokhin O, Wall D et al. (2017). Interferon gamma induced compositional changes in human bone marrow derived mesenchymal stem/stromal cells. Clinical Proteomics 14: 26. DOI 10.1186/s12014-017-9161-1. [Google Scholar] [CrossRef]
Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J et al. (2010). Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock 33: 24–30. DOI 10.1097/SHK.0b013e3181b7d137. [Google Scholar] [CrossRef]
Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T (2009). TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone 45: 367–376. DOI 10.1016/j.bone.2009.04.252. [Google Scholar] [CrossRef]
Hou Y, Ryu CH, Jun JA, Kim SM, Jeong CH et al. (2014). IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biology International 38: 1050–1059. DOI 10.1002/cbin.10294. [Google Scholar] [CrossRef]
Li C, Li G, Liu M, Zhou T, Zhou H (2016). Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. Journal of Bioscience and Bioengineering 121: 213–219. DOI 10.1016/j.jbiosc.2015.05.017. [Google Scholar] [CrossRef]
Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX et al. (2011). Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. Journal of Biological Chemistry 286: 25007–25015. DOI 10.1074/jbc.M110.213108. [Google Scholar] [CrossRef]
Lu Z, Wang G, Dunstan CR, Chen Y, Lu WY et al. (2013). Activation and promotion of adipose stem cells by tumour necrosis factor-alpha preconditioning for bone regeneration. Journal of Cellular Physiology 228: 1737–1744. DOI 10.1002/jcp.24330. [Google Scholar] [CrossRef]
Maldonado-Lasunción I, Haggerty AE, Okuda A, Mihara T, de la Oliva N et al. (2021). The effect of inflammatory priming on the therapeutic potential of mesenchymal stromal cells for spinal cord repair. Cells 10: 1316. DOI 10.3390/cells10061316. [Google Scholar] [CrossRef]
Miceli V, Bulati M, Iannolo G, Zito G, Gallo A et al. (2021). Therapeutic properties of mesenchymal stromal/stem cells: The need of cell priming for cell-free therapies in regenerative medicine. International Journal of Molecular Sciences 22: 763. DOI 10.3390/ijms22020763. [Google Scholar] [CrossRef]
Nimiritsky PP, Eremichev RY, Alexandrushkina NA, Efimenko AY, Tkachuk VA et al. (2019). Unveiling mesenchymal stromal cells’ organizing function in regeneration. International Journal of Molecular Sciences 20: 823. DOI 10.3390/ijms20040823. [Google Scholar] [CrossRef]
Noone C, Kihm A, English K, O’Dea S, Mahon BP (2013). IFN-gamma stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells and Development 22: 3003–3014. DOI 10.1089/scd.2013.0028. [Google Scholar] [CrossRef]
Padeta I, Nugroho WS, Kusindarta DL, Fibrianto YH, Budipitojo T (2017). Mesenchymal stem cell-conditioned medium promote the recovery of skin burn wound. Asian Journal of Animal and Veterinary Advances 12: 132–141. DOI 10.3923/ajava.2017.132.141. [Google Scholar] [CrossRef]
Ragni E, Orfei CP, de Luca P, Mondadori C, Viganò M et al. (2020). Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: The example of joint disease. Stem Cell Research and Therapy 11: 1–19. DOI 10.1186/s13287-020-01677-9. [Google Scholar] [CrossRef]
Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S et al. (2017). Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Research and Therapy 8: 79. DOI 10.1186/s13287-017-0531-4. [Google Scholar] [CrossRef]
Tarcisia T, Damayanti L, Antarianto RD, Moenadjat Y, Pawitan JA (2018). Adipose derived stem cell conditioned medium effect on proliferation phase of wound healing in Sprague Dawley rat. Medical Journal of Indonesia 26: 239–245. DOI 10.13181/mji.v26i4.1755. [Google Scholar] [CrossRef]
Wang Y, Crisostomo PR, Wang M, Markel TA, Novotny NM et al. (2008). TGF-alpha increases human mesenchymal stem cell-secreted VEGF by MEK- and PI3-K- but not JNK- or ERK-dependent mechanisms. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 295: R1115–1123. DOI 10.1152/ajpregu.90383.2008. [Google Scholar] [CrossRef]
Windmolders S, De Boeck A, Koninckx R, Daniëls A, de Wever O et al. (2014). Mesenchymal stem cell secreted platelet derived growth factor exerts a pro-migratory effect on resident Cardiac Atrial appendage Stem Cells. Journal of Molecular and Cellular Cardiology 66: 177–188. DOI 10.1016/j.yjmcc.2013.11.016. [Google Scholar] [CrossRef]
Wobma HM, Tamargo MA, Goeta S, Brown LM, Duran-Struuck R et al. (2018). The influence of hypoxia and IFN-γ on the proteome and metabolome of therapeutic mesenchymal stem cells. Biomaterials 167: 226–234. DOI 10.1016/j.biomaterials.2018.03.027. [Google Scholar] [CrossRef]
Zhou BR, Xu Y, Guo SL, Xu Y, Wang Y et al. (2013). The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Research International 2013: 519126. DOI 10.1155/2013/519126. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |