DOI:10.32604/biocell.2022.020383

| BIOCELL DOI:10.32604/biocell.2022.020383 |  |
| Article |
Boldine provides protective effect against nephrotoxicity induced by cisplatin in Wistar rats: Role of oxidative stress, inflammation and caspase-3
1Department of Pharmacology, Faculty of Pharmacy, Izmir Katip Celebi University, Izmir, 35620, Turkey
2Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Cumhuriyet University, Sivas, 58140, Turkey
3Department of Physiology, Faculty of Veterinary Medicine, Cumhuriyet University, Sivas, 58140, Turkey
4Department of Physiology, Faculty of Medicine, Izmir Katip Celebi University, Izmir, 35620, Turkey
5Department of Pathology, Faculty of Veterinary Medicine, Cumhuriyet University, Sivas, 58140, Turkey
*Address correspondence to: Nergiz Hacer Turgut, nergiz.turgut@ikcu.edu.tr
Received: 20 November 2021; Accepted: 27 January 2022
Abstract: Side effects of cisplatin, especially dose-dependent nephrotoxicity, are major factors limiting its use in cancer. Boldine ((S)-2, 9-dihydroxy-1, 10-dimethoxy-aporphine) is a natural alkaloid known for its strong antioxidant activity present in leaves/bark of boldo tree (Peumus boldus Molina), a native tree in Chile. Here, we aimed to investigate the nephroprotective effect of boldine and its underlying mechanisms on cisplatin-induced rat renal injury. Thirty Wistar albino rats divided into 5 groups (Control, Cis, Bold.40, Cis + Bold.20, Cis + Bold.40 groups) were used. Rats received boldine (20 or 40 mg/kg/day), or vehicle (saline) intraperitoneal for 14 days and a single dose cisplatin (7 mg/kg, ip) was applied on the 10th day to induce nephrotoxicity. Rats and kidney tissue were weighed to determine kidney index. Blood urea nitrojen (BUN) and creatinine levels, the amount of thiobarbituric acid reactive substances (TBARS, an index of lipid peroxidation), superoxide dismutase (SOD), glutathione peroxidase (GPx) enzyme activities and tumor necrosis factor alpha (TNF-α) levels were measured and histopathologic examination was performed. Inducible nitric oxide synthase (iNOS) and caspase-3 expressions were detected immunohistochemically. Nephrotoxicity induced by cisplatin was apparent by elevated levels of BUN, creatinine, kidney index, TBARS and TNF-α, and decreased body weight, SOD and GPx enzyme levels. Pretreatment with boldine protected the renal function at both boldine doses by fixing the renal damage markers, oxidative stress, caspase-3 and iNOS expression. Histopathological findings supported biochemical findings. Taken together these findings indicate that boldine has promising protective effect against cisplatin nephrotoxicity by improving oxidative stress, inflammation, histopathological alterations and by alleviating caspase 3 expression.
Keywords: Boldine; Caspase-3; Cisplatin nephrotoxicity; Inflammation; Oxidative stress
Cisplatin (cis-diamminedichloroplatinum-II), is a chemotherapeutic agent used against variety of cancers such as solid tumors, osteosarcoma, hematological malignancies, lymphoma, pulmonary, gastric, obstetric, urogenital, breast and neck cancers (Ghosh, 2019). Besides important anticancer activity, it also has severe adverse effects such as nephrotoxicity, ototoxicity, neurotoxicity and hepatotoxicity, and these side effects limit the use of cisplatin (Dos Santos et al., 2012). Nephrotoxicity is the most prevalent, severe and dose limiting side effect of cisplatin treatment, and despite preventive measures, one third of patients experience renal damage. Due to its strong renal affinity, cisplatin usually accumulates in renal proximal tubule cells by damaging these cells with cellular necrosis, microvillus decrease and replacement of lysosomes. The concentration of cisplatin in these cells is almost five times the serum concentration which attributes to its dose limiting nephrotoxicity (Manohar and Leung, 2018). Although the molecular mechanism of cisplatin nephrotoxicity is not fully known, recent studies reveal that it is a multi-factor process involving apoptosis, necrosis, ischemia inflammation, oxidative stress and mitochondrial dysfunction (Karasawa and Steyger, 2015; Yilmaz et al., 2004).
Oxidative stress is one of the main mechanisms of acute kidney injury. Cisplatin induced nephrotoxicity is closely associated with enhanced production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), increasing lipid peroxidation and decreased antioxidant enzyme activities. It has been shown that cisplatin accumulates in the mitochondria, inducing oxidative stress and causing mitochondrial ROS that cause mitochondrial degradation (Ma et al., 2017). One of the main sources of ROS is reduced nicotinamide adenine dinucleotide phosphate (NADPH). NADPH Oxidase 4 (Nox4), an NADPH oxidase, present in kidney tissues has been investigated for its role in kidney diseases (Meng et al., 2018). Inflammation has also been reported to play an important role in cisplatin nephrotoxicity (Qin et al., 2019). Oxidative stress leads to inflammatory response. Produced ROS and RNS stimulate Nuclear Factor kappa B (NF-κB) pathway generating cytokines, enzymes and other mediators of inflammation including, tumor necrosis factor (TNF-α), interleukin-6 (IL-6), interleukin-1 beta (IL-1β), cyclooxygenase-2 (COX-2), transforming growth factor-β (TGF-β), and inducible nitric oxide synthase (iNOS) (Kim et al., 2018a; Ueki et al., 2013). In vivo nephrotoxic dose of cisplatin leads to increase of necrosis and apoptosis in the kidney. Caspases, family of cysteine proteases, act as key mediators of apoptosis (Kim et al., 2018b). Cisplatin treated renal epithelial cells activates caspase which has an important role in the apoptosis execution stage (Lee et al., 2001).
Plant derived compounds have an important role in modern medicine. It is considered that the active ingredients in natural products have a potential therapeutic effect that controls the pathogenesis of chronic diseases caused by oxidative stress (Amato et al., 2019; Mattioli et al., 2018). Boldine ((S)-2, 9-dihydroxy-1, 10-dimethoxy-aporphine) is an aporphine alkaloid that is abundant in the leaf/bark of boldo (Peumus boldus Molina), a common tree native to central and southern Chile. Boldine is also one of the largest alkaloids in the bark of a Phoebe grandis tree located in the northern part of Malaysia. Dried boldo leaves are reported to contain 0.25–0.54% or 0.4–0.5% alkaloids, of which approximately 12 to 19% are boldine. Boldo bark is a rich source of alkaloids, and about 75% represents boldine (O’Brien et al., 2006). Many pharmacological activities have been attributed to boldine, such as cytoprotective, antipyretic, anti-inflammatory and antiplatelet. In addition, boldine is known for its strong antioxidant activity due to its ability to scavenge highly reactive free radicals. This alkaloid has been suggested to be useful in the treatment of some diseases including free radicals such as atherosclerosis, ischemia-reperfusion, hypertension and inflammatory diseases (Lau et al., 2015; Muthna et al., 2013). Recently it has been suggested that boldine improves kidney damage by avoiding the increase in TGF-β (Gómez and Velarde, 2018). In a clinical study done by Gotteland et al. (1995), twelve healthy volunteers treated daily with 2.5 g of a dry boldo extract (ethanol 60% V/V) containing 0.12% of boldine and 0.4% of total alkaloids during two successive periods of four days showed prolongation of oro cecal transit time compared to placebo. The Committee on Herbal Medicinal Products (HMPC) has concluded that boldo leaf preparations which are available as herbal tea can be used for the relief of symptoms of dyspepsia and mild spasms of the gastrointestinal tract (Thumann et al., 2019).
Many agents have been tried to protect from cisplatin-induced nephrotoxicity, however efficient methods for clinical use have not been determined yet. Despite side effects, cisplatin is still preferred in chemotherapy for its low cost and efficacy. Therefore, additional applications for the prevention of cisplatin-induced nephrotoxicity without compromising the potency are needed. The protective effect of boldine on cisplatin- induced nephrotoxicity has not been elucidated. Due to the high antioxidant and anti-inflammatory effect of boldine, we consider it worthwhile to investigate the protective effect of this active ingredient in the cisplatin-induced renal injury model. The aim of this study is to investigate the possible protective effect of boldine and its underlying mechanisms on cisplatin-induced renal injury in Wistar rats.
Boldine and cisplatin were purchased respectively from Sigma-Aldrich (St. Louis, MO, USA) and Kocak Pharmaceutical and Chemical Industry Inc. (Istanbul, Turkey). The kits, superoxide dismutase (SOD), glutathione peroxidase (GPx) were obtained from Cayman Chemical Company (USA) and TNF-α from Shanghai Sunred Biological Technology Co., Ltd. (Shanghai, China). Caspase-3 and iNOS antibody were obtained from Santa Cruz Biotechnology (USA). The purity of chemical reagents was at least analytical grade.
Thirty adult male Wistar albino rats with an average body weight of 200–220 g were used in the present study. Rats were kept under normal conditions (12-h light/dark cycle, 24 ± 2°C, 35–60% humidity), fed with standard pellet chow and water ad libitum. Experiments were performed in accordance with the Guideline on the Care and Use of Laboratory Animals (EU Directive 2010/63/EU for animal experiments). This study was carried out with the permission of Local Ethics Committee of the Animal Experiments of Cumhuriyet University (HADYEK, No. 65202830-050.04.04-222, Sivas, Turkey).
Induction of nephrotoxicity and treatment protocols
Thirty Wistar albino rats were randomly divided into five groups, 6 rats in each group (Control, Cis, Bold.40, Cis + Bold.20, Cis + Bold.40; Cis: cisplatin, Bold.: boldine). Rats received boldine (20 or 40 mg/kg/day), or vehicle (saline) intraperitoneal for 14 days and a single dose cisplatin (7 mg/kg, ip) was applied on the 10th day to induce nephrotoxicity. The same procedure was performed for the control group and alone boldine group with the exception that saline was substituted for cisplatin. Blood samples were collected via cardiac puncture from the heart of rats 96 h after the last administration under anesthesia (xylazine + ketamine, 3 mg/kg + 90 mg/kg, i.p.). Blood samples were transferred to gel tubes and serum were separated by centrifuging 15 min at 3000 rpm. Serum samples were placed in Eppendorf tube and stored at –80°C for further analysis. After cervical dislocation, kidneys were removed by autopsy procedures. The kidneys were trimmed from the extraneous tissue, rinsed and weighed. While one kidney was separated and fixed in 10% formaldehyde solution for histopathologic analysis, the second kidney was flash frozen in liquid nitrogen and stored at −80°C for further analysis. Kidney tissues were homogenized in ice-cold phosphate-buffered saline (PBS, 50 mM, pH 7.4), then centrifuged for 10 min at the speed of 3000 rpm at 4°C for supernatant collection. Cisplatin dose and application route was based on previous studies (Sahu et al., 2011; Sohn et al., 2011). Again the selected doses of boldine 20 mg/kg/day and 40 mg/kg/day and route were based on previous literature (Asencio et al., 1999; Lau et al., 2012; Lau et al., 2013; Paydar et al., 2014).
Assessment of body weight and kidney index
The body weight was measured at the beginning before drug treatment and at the end of the experiment prior to euthanasia. No mortality was observed in animals treated with cisplatin and/or boldine during the study. Kidney index (relative kidney weight) was calculated and statistically analyzed using the formula: (kidney weight/total body weight) × 100 (Atessahin et al., 2005; Yu et al., 2021).
Determination of BUN and creatinine
Blood urea nitrojen (BUN) and creatinine levels in serum was determined with the auto analyzer device (Mindray BS-200 Fully Automatic Clinical Chemistry Analyzer, Mindray Bio-Medical Electronics, Ltd., China) using a standard commercial kit (Mindray CREA kit, Mindray Bio-Medical Electronics Ltd., China). The working principle of the kit is based on the reaction of Jaffe (Toora and Rajagopal, 2002).
Determination of lipid peroxidation
Thiobarbituric acid reactive substances (TBARS), an index of lipid peroxidation was assessed spectrophotometrically by the method described by Ohkawa et al. (1979) based on the reaction between lipid peroxidation breakdown products mainly malondialdehyde (MDA) and thiobarbituric acid. When thiobarbituric acid reacts with MDA, a colored complex [MDA-(TBA) 2 complex] forms. The absorbance values were measured by spectrophotometer (Shimadzu V-1700, Japan) at 532 nm. Thiobarbituric acid reactive substances were expressed as MDA equivalents “nmol/mg protein” for kidney tissue. Standard solutions of 1, 1, 3, 3-tetraethoxypropane (TEP) were used for comparison.
Activity of superoxide dismutase
Superoxide dismutase activity was determined in kidney homogenate by the Enzyme-Linked ImmunoSorbent Assay (ELISA) reader (Thermo Scientific Multiskan GO Microplate Spectrophotometer, USA) using a commercially available standard enzymatic kit (Cayman Chemical Company, USA). Analyzes were performed according to the manufacturer’s instructions. Absorbance was recorded at 440–460 nm wavelength. SOD was expressed as “U/mg protein” for kidney tissue. The SOD test measures three types of SOD (CU/Zn, Mn, and Fe SOD). One SOD unit is defined as the amount of enzyme required to show 50% dismutation of the superoxide radical.
Activity of glutathione peroxidase
Glutathione peroxidase activity was determined in kidney homogenate by the ELISA reader (Thermo Scientific Multiskan GO Microplate Spectrophotometer, USA) using a commercially available standard enzymatic kit (Cayman Chemical Company, USA). Analyzes were performed according to the manufacturer’s instructions. Absorbance was recorded every minute at 340 nm using a plate reader to obtain at least 5 time points. GPx activity levels were expressed as “nmol/min/mg protein” for kidney tissue. One unit GPx is defined as the amount of enzyme that catalyzes the oxidation of 1 nmol NADPH at 25°C per minute.
Tumor necrosis factor alpha levels were detected in kidney tissue homogenate using a commercially available kit (Sunred Biological Technology Company, China). Analyzes were performed according to the manufacturer’s instructions. The absorbance values were recorded at 450 nm wavelength using an ELISA reader (Thermo Scientific Multiskan GO Microplate Spectrophotometer, USA). TNF-α levels were expressed as pg/mg protein.
Total protein content was assessed by the method of Bradford (1976).
Histopathological examinations
Kidney tissues (1–2 cm) obtained from rat were placed in standard pathology cassettes. They were kept for fixation in 10% formaldehyde for about a week. Following this period, the tissues were passed through serial alcohol and xylol and embedded in paraffin. Sections of paraffin blocked tissues (5 μm thick) were mounted on a slide and stained with hematoxylin-eosin (H x E). Sections of all stained groups were examined under a light microscope (Nikon, YS 100). At least 10 sites were examined for each kidney slide and the severity of the changes; were determined by an experienced pathologist unaware of the treatment groups In microscope examination the findings were scored as: (-) none, (+) mild, (++) moderate, (+++) severe (Koc et al., 2020).
For immunohistochemical (IHC) staining, the kidney tissues taken were fixed in 10% formaldehyde and then passed through alcohol series and embedded in paraffin. Sections (5 μm thick) were taken from the paraffin blocks. After all tissue sections were deparaffinized in xylene, they were rehydrated in graded alcohols. For IHC staining, the tissues were then antigen retrieval at 600 Watt for 20 min and the sections were cooled for 20 min. Endogenous peroxidase activity was treated with 3% hydrogen peroxide solution in methanol for 15 min. The sections were washed 3 times in phosphate buffer saline (PBS) for 5 min each. After all sections were treated with block solution for 10 min, anti-iNOS (1/250 dilution, Santa-Cruz) and caspase-3 (1/100 dilution, Santa-Cruz) antibodies were dropped on them without washing and left for 60 min incubation at room temperature. Following the washing, biotin labeled secondary antibody was dropped onto the sections and kept for 30 min, and then washed 3 times with PBS for 5 min. Then it was incubated with streptavidin peroxidase enzyme (Histostain-Plus Kits, California, USA) for 30 min. At the end of this period, the tissues were washed 3 times with PBS for 5 min. Finally, sections were stained with chromogen 3-amino-9-ethylcarbazole (AEC) (Zymed AEC RED substrate kit, USA) under the microscope for 10 min in a controlled manner. Counter staining was done with Gill’s hematoxylin. Sections were sealed with water-based adhesive (Shandon Immu-mount) (Buyuklu et al., 2014).
SPSS software version 25 (SPSS Inc. Chicago IL, USA) and GraphPad Prism software version 6 (GraphPad Software, USA) was used for statistical analyses. The normality of data distribution was tested by using the Shapiro Wilks test. One-way analysis of variance (ANOVA) was used for comparisons between groups since data were distributed normally for biochemical parameters. BUN, creatinine levels, antioxidant enzyme activities (SOD and GPx), lipid peroxidation (TBARS) levels, proinflammatory cytokine (TNF-α) levels were presented as mean ± standard error and statistically analyzed by using ANOVA, followed by Tukey, post hoc test. p < 0.05 was assumed statistically significant. Non-parametric, Kruskall-Wallis test was used to evaluate the histopatological statistical significance of differences among groups. Mann-Whitney U-test with Bonferroni correction was used for post hoc multiple comparisons. Statistical significance was considered at p < 0.05.
A single dose of cisplatin (7 mg/kg) resulted in notable weight loss compared to the control group (p < 0.001). We observed a 27.88% weight loss in cisplatin group. Weight loss was significantly prevented by boldine pretreatment at both doses of 20 and 40 mg/kg (p < 0.001) (Fig. 1a). In addition cisplatin showed significant increase in kidney index by 41.27% as compared to control group (p < 0.001). Boldine pretreatment significantly inhibited the increase in kidney index (p < 0.01). The reduction in kidney index was 19.05% in Cis + Bold.20 and 20.63% in Cis + Bold.40 groups (Fig. 1b). Treatment with boldine alone did not show any obvious alteration in these parameters (Fig. 1).
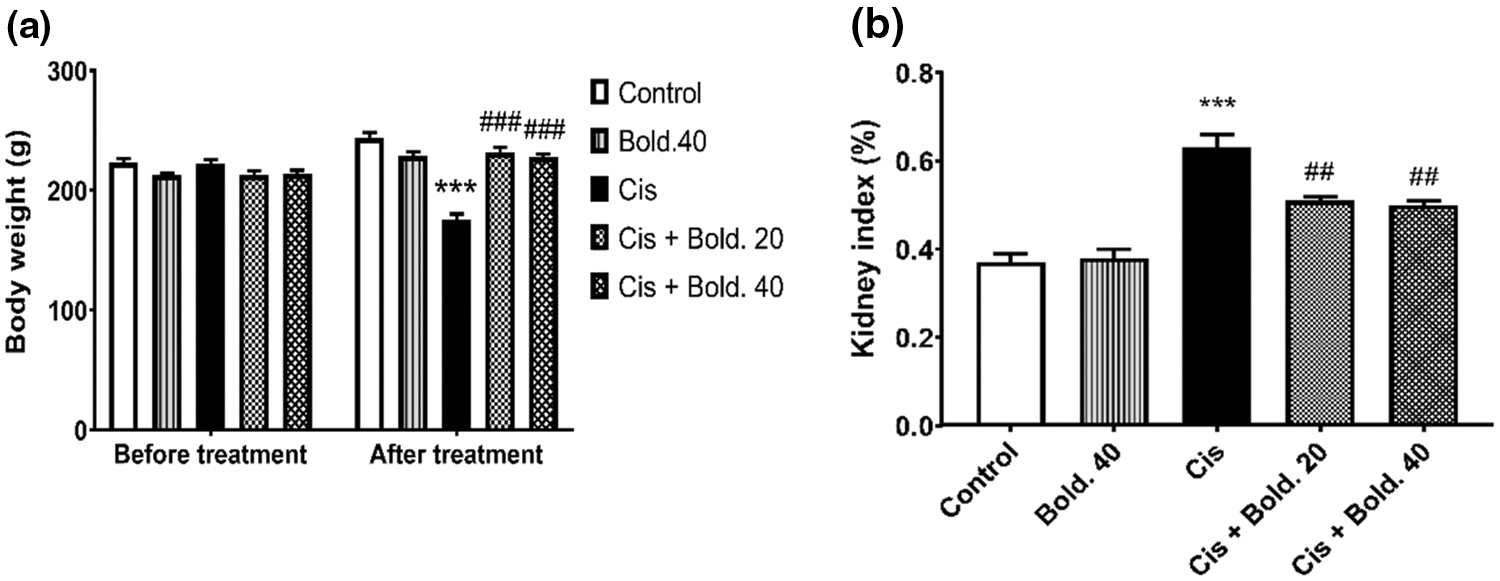
Figure 1: Body weight, kidney index. a) Effect of boldine on body weight in cisplatin-induced nephrotoxic rat. b) Effect of boldine on kidney index (relative kidney weight) in cisplatin- induced rat nephrotoxicity. Data are expressed as mean ± S.D. (n = 6 in each group). Cis: Cisplatin, Bold.: Boldine. ***p < 0.001, compared with the control group; ##p< 0.01, ###p < 0.001 compared with the cisplatin group.
BUN and serum creatinine levels
The effects of cisplatin, boldine and Cis + Bold. combination on renal function markers (BUN and creatinine) are shown in Fig. 2. A single injection of cisplatin resulted in a significant increase in BUN and serum creatinine levels compared to control values, indicating the induction of severe nephrotoxicity (p < 0.001). However treatment with boldine at both doses in cisplatin treated animals decreased BUN and creatinine levels (p < 0.001) when compared to cisplatin group. Boldine administration did not alter BUN and creatinine levels in cisplatin untreated rats (Fig. 2).
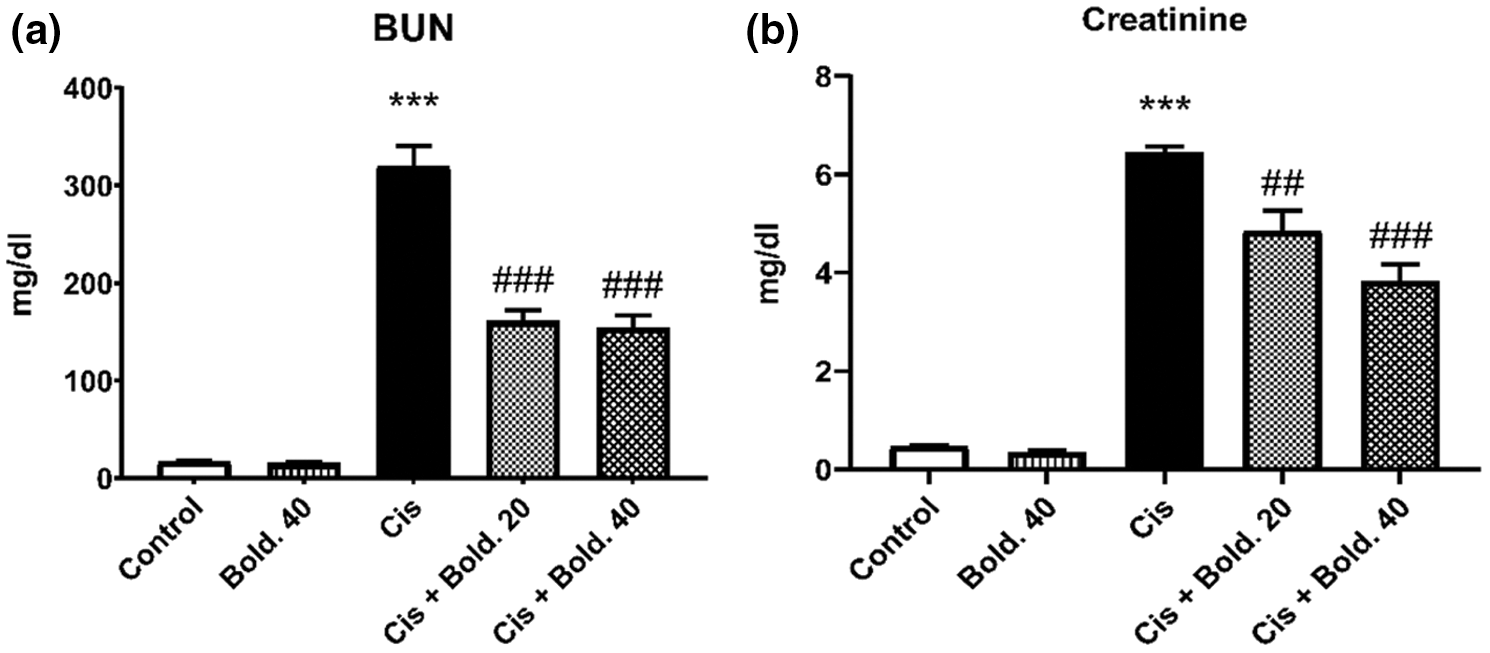
Figure 2: Blood urea nitrogen (BUN), creatinine levels. a) Effect of boldine on serum BUN levels in cisplatin-induced nephrotoxic rat. b) Effect of boldine on serum creatinine levels in cisplatin-induced rat nephrotoxicity. Data are expressed as mean ± S.D. (n = 6 in each group). Cis: Cisplatin, Bold.: Boldine. ***p < 0.001, compared with the control group; ##p < 0.01, ###p < 0.001 compared with the cisplatin group.
For lipid peroxidation significant differences were obtained between groups after ANOVA analysis. After a single injection of cisplatin, TBARS level increased in kidney tissue (p < 0.001) compared to control values. Boldine administration to cisplatin treated animals reduced TBARS level in kidney tissue dose dependently (20 and 40 mg/kg) (p < 0.01 and p < 0.001, respectively) (Fig. 3a). There were no notable differences in the level of TBARS between control and boldine alone groups.
Effect on enzymatic antioxidants
Superoxide dismutase activity and glutathione peroxidase activity
As shown in Fig. 3, the mean level of SOD activity in cisplatin alone group significantly decreased when compared to control group in kidney tissue (p < 0.001). Boldine administration at 40 mg/kg significantly increased SOD activity in cisplatin treated rat (p < 0.05); whereas 20 mg/kg did not cause a change in SOD levels (Fig. 3b). Similarly, significant differences in GPx activity were observed in kidney tissue between control and cisplatin groups, there was a significant decrease in GPx activity in cisplatin treated rat (p < 0.05). In kidney tissue boldine 40 mg/kg reversed the decrease in GPx activity to a significant increase (p < 0.05) and 20 mg/kg did not cause a change in GPx levels (Fig. 3c). Boldine administration did not change SOD and GPx levels in cisplatin untreated rats (Fig. 3).
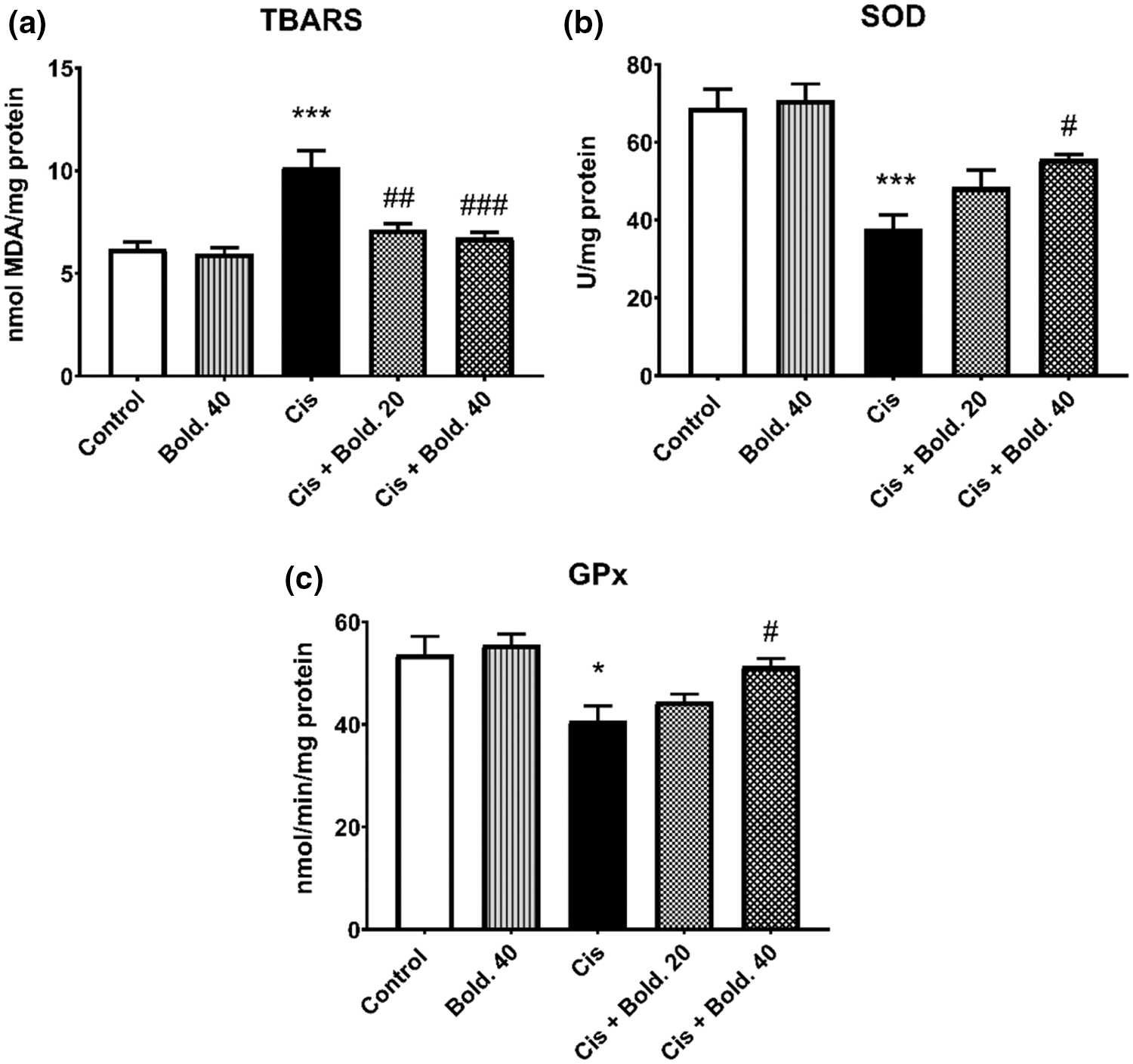
Figure 3: Thiobarbituric acid reactive substances (TBARS) level, superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity. a) Effect of boldine on oxidative stress marker TBARS in cisplatin-induced nephrotoxic rat. b) Effect of boldine on SOD activity in cisplatin induced rat nephrotoxicity. c) Effect of boldine on GPx activity in cisplatin-induced rat nephrotoxicity. Data are expressed as mean ± S.D. (n = 6 in each group). Cis: Cisplatin, Bold.: Boldine. *p < 0.05, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the cisplatin group.
Cisplatin administration elevated TNF-α levels compared to control group (p < 0.001). After pretreatment with two different doses of Boldine (20 and 40 mg/kg), the levels of TNF-α in kidney tissue significantly decreased compared to cisplatin group (p < 0.01) (Fig. 4). Alone boldine application induced no significant change in TNF-α levels in comparison with control levels.
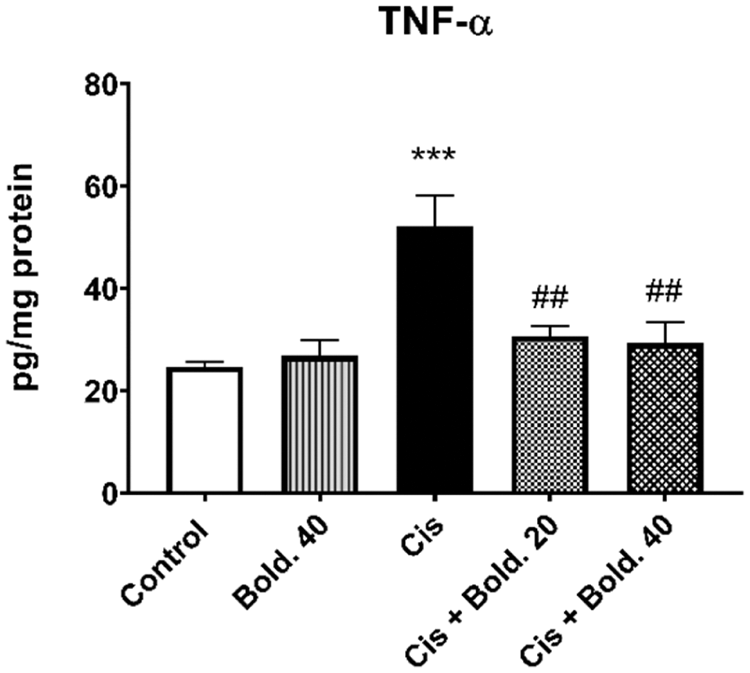
Figure 4: Effect of boldine on the levels of inflammatory cytokine tumor necrosis factor alpha (TNF-α) in cisplatin-induced nephrotoxic rat. Data are expressed as mean ± S.D. (n = 6 in each group). Cis: Cisplatin, Bold.: Boldine. ***p < 0.001, compared with the control group; ##p < 0.01, compared with the cisplatin group.
No histopathological changes were found in the kidney sections of the control group (Fig. 5a). However, there were significant histopathological changes in the rat kidneys treated with cisplatin. Severe hyperemia in interstitium vessels, mild degeneration in tubular epithelium and moderate hyaline cylinder accumulation in renal tubule lumens were observed in the cisplatin given group (Figs. 5b–5d). On the other hand, significant improvement was observed in the groups treated with boldine. In the Cis + Bold.20, when compared with the cisplatin group, it was observed that the degenerative changes in the kidney were eliminated, and the hyaline cylinders in the tubular lumens regressed from moderate to mild and the hyperemia in the intermediate tissue from severe to moderate (Fig. 5e). In the Cis + Bold.40 group, when compared to the cisplatin group in the renal tubular lumens, it was found that the hyaline cylinder formation, the degeneration in the tubules disappeared and the intensive hyperemia in the intermediate tissue regressed to mild severity (Fig. 5f). In only boldine given group no histopathological findings were found. Histopathologically, different regions were evaluated semi-quantitatively at 40 magnification. Scoring of findings are shown in Table 1.
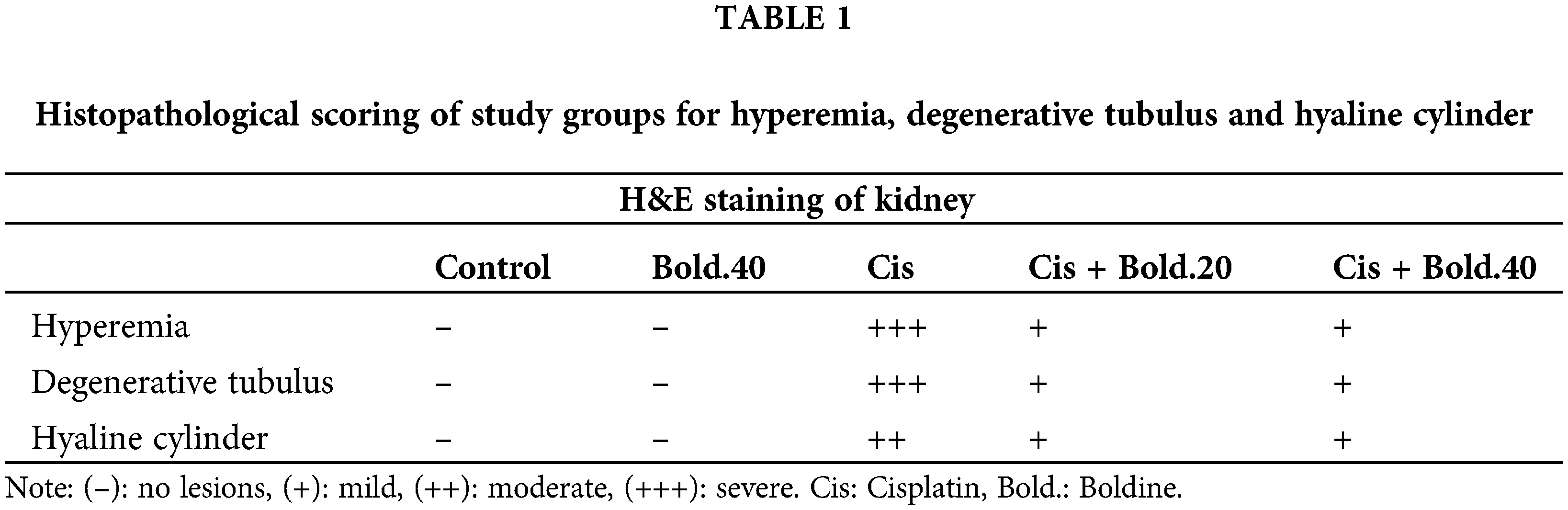
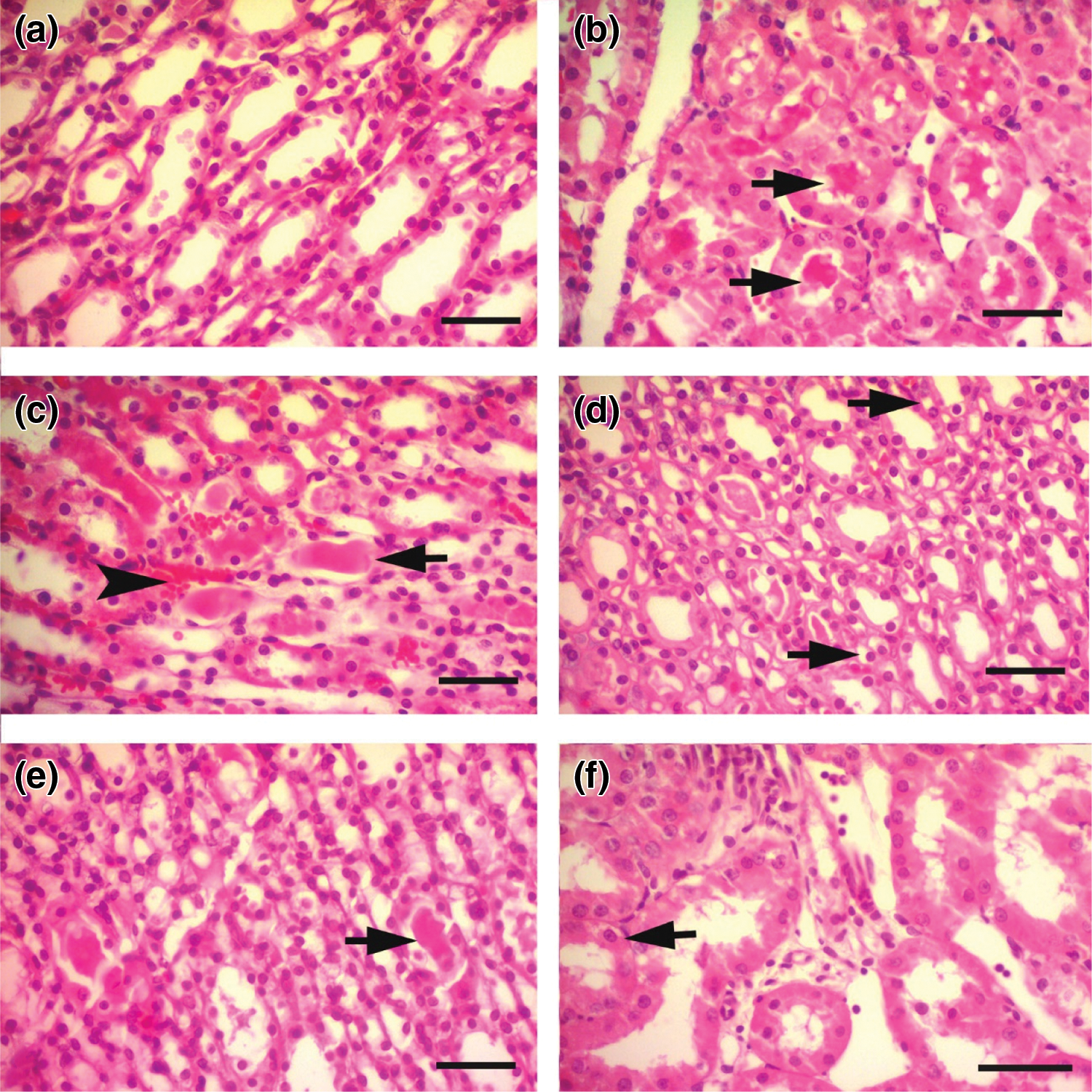
Figure 5: Section of kidney tissue. a) Control group, no histopathological lesions in the kidney sections HXE, Bar = 25 μm. b) Cis group, moderate hyaline cylinder accumulation in renal tubule lumens (arrows) HXE, Bar = 25 μm. c) Cis group, hyaline cylinder unit in renal tubular lumens (arrow) and hyperemia (arrowhead) HXE, Bar = 25 μm. d) Cis group, degeneration in renal tubular epithelium (arrows) HXE, Bar = 25 μm. e) Cis + Bold.20 group, light intensity hyaline cylinder accumulation in renal tubule lumens (arrow) HXE, Bar = 25 μm. f) Cis + Bold.40 group, mild degenerative changes in renal tubular epithelium (arrow) HE X Bar: 25 μm. Cis: Cisplatin, Bold.: Boldine.
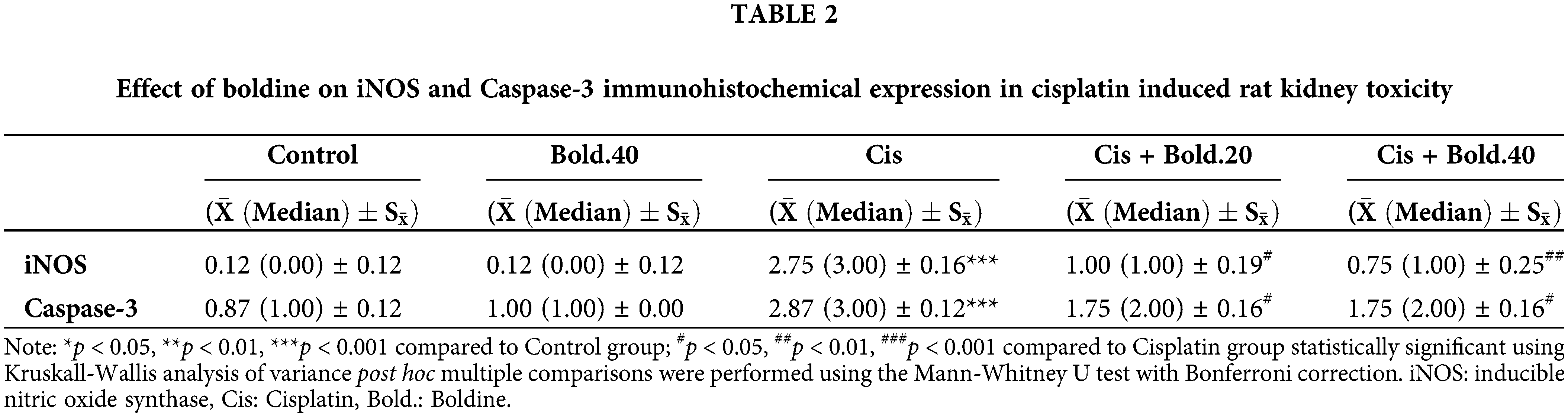
In IHC staining, iNOS showed no expression in the control tissues (Fig. 6a), while in the cisplatin group tissues, intense expression of iNOS was determined (Fig. 6b). This difference between the control and cisplatin group was found to be statistically significant (p < 0.001). iNOS staining was observed to be mild compared to the cisplatin groups in the Cis + Bold.20 and Cis + Bold.40 groups (Figs. 6c and 6d). The difference between the cisplatin and Cis + Bold.20 and Cis + Bold.40 groups was found to be statistically significant (p < 0.05 and p < 0.01, respectively). The IHC staining performed in alone boldine group was similar to the control group and it was observed that there was no statistically significant difference between the control groups.
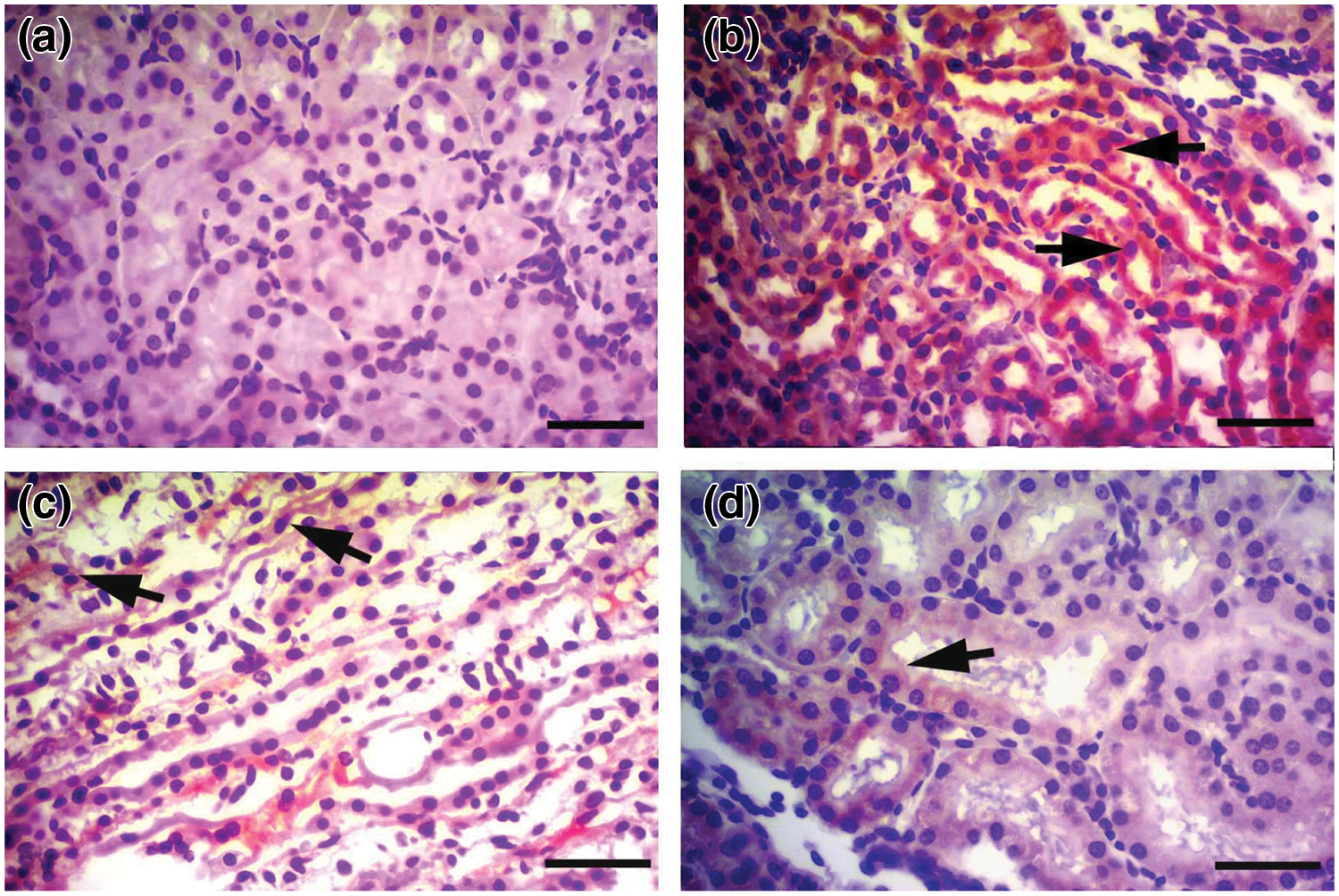
Figure 6: Inducible nitric oxide synthase (iNOS) immunostaining. a) Control group, immune negative iNOS staining in the renal tubular epithelium, IHC X Bar = 25 μm. b) Cisplatin group, intense iNOS immune positivity (arrows) in cisplatin group renal tubular epithelium, IHC X Bar = 25 μm. c) Cis + B20 group, mild iNOS immune positivity (arrow) in renal tubular epithelium, IHC X Bar = 25 μm. d) Cis + B40 group, mild iNOS immune positivity (arrow) in renal tubular epithelium, IHC X Bar = 25 μm. Cis: Cisplatin, Bold.: Boldine.
IHC staining with caspase-3 antibody showed only mild expression in control tissues (Fig. 7a), whereas intense expression of caspase-3 was determined in the cisplatin group tissues (Fig. 7b). This difference between the control and cisplatin group was found to be statistically significant (p < 0.001). It was observed that caspase-3 IHC staining intensity in the Cis + Bold.20 and Cis + Bold.40 groups was mild compared to the cisplatin group (Figs. 7c and 7d). The difference between the cisplatin and boldine treatment groups was found to be statistically significant (p < 0.05). The IHC staining performed in alone boldine group was similar to the control group and it was observed that there was no statistically significant difference between the control groups.
Figure 7: Caspase-3 immunostaining. a) Control group, mild caspase-3 staining in the renal tubular epithelium, IHC X Bar = 25 μm. b) Cisplatin group, intense caspase-3 immune positivity (arrows) in cisplatin group renal tubular epithelium, IHC X Bar = 25 μm. c) Cis + B20 group, moderate caspase-3 immune positivity (arrow) in renal tubular epithelium, IHC X Bar = 25 μm. d) Cis + B40 group, moderate caspase-3 immune positivity (arrow) in renal tubular epithelium, IHC X Bar = 25 μm. Cis: Cisplatin, Bold.: Boldine.
The reactivity and percent density of cells stained with caspase-3 and iNOS were evaluated at ×200 magnification using 100 square grid lenses in 10 adjacent areas forming a total area of 0.050 mm. Scoring for the density of stained cells was made as follows: (0) not stained, (1) stained area < 10%, (2) stained area = 10–30%, (3) stained area > 30%. Statistical analysis was performed for numerical values obtained (Table 2).
The use of cisplatin as a therapeutic agent is limited by its nephrotoxicity as 25–35% of patients experience a significant decrease of kidney function after following a single dose of cisplatin therapy (Manohar and Leung, 2018). In this study, we investigated the possible protective effect of boldine against cisplatin-induced rat nephrotoxicity. Induction of cisplatin induced nephrotoxicity is considered a fast process that reacts with proteins found in the renal tubules. Since the kidney damage produces within 1 h of cisplatin application, agents used for protection should be present in the kidney tissue before the damage occurs (Pabla and Dong, 2008). For this reason in the present study, rats received boldine for 14 days and cisplatin was applied a single dose one hour after boldine administration on the 10th day.
Studies mostly performed on isolated cells and different animal models state that boldine exhibits low toxicity and relatively high doses are needed for toxic or lethal effects (Gerhardt et al., 2013; Heidari and Reza, 2019; Konrath et al., 2008). For mice, when administered intraperitoneally, LD50 was estimated to be 250 mg/kg (Lévy-Appert-Collin and Lévy, 1977). It was observed that acute administration of boldine (500 mg/kg, p.o.) to rats in early pregnancy led to no fetal malformation. However with doses of 800 mg/kg of boldine, a weak but significant miscarriage and teratogenic effect was observed (Almeida et al., 2000). In our study rats received boldine (20 or 40 mg/kg/day) for 14 days. Boldine doses were similar to the doses used by earlier studies in literature which investigated therapeutic efficacy (Asencio et al., 1999; Lau et al., 2012; Lau et al., 2013; Paydar et al., 2014). There were no signs of toxicity and mortality over a period of 14 days in boldine treated groups indicating the safety of boldine. No physical changes were observed in the eyes, fur, skin, mucous membranes, sleep and behavioral patterns of the rats. Again with boldine (40 mg/kg) alone application, there was no significant change in body weight, kidney index and also BUN, creatinine, TBARS, SOD, GPx, TNF-α levels compared to control. The IHC staining performed in alone boldine group was similar to the control group and it was observed that there was no statistically significant difference between the control groups. Histopathological findings for alone boldine group supported biochemical findings.
The most common and two most serious health problems in cancer patients undergoing chemotherapy, especially those using cisplatin, are decreased food intake and weight loss (Karasawa and Steyger, 2015). In our study, rats treated with cisplatin showed a significant reduction in body weight associated with an increase in kidney weight and relative kidney index which is known as the ratio of kidney weight to body weight and is a predictor of kidney damage. Cisplatin-induced body weight loss may be due to toxicity of alimentary tract and/or a reduction in food consumption (Atessahin et al., 2005). As cisplatin can cause renal inflammation, the increase in kidney index may be due to the edema of renal parenchyma. Kidney with impaired function has a greater weight due to edema (Kim et al., 2015). Treatment with boldine prevented weight loss. The increase in kidney weight was prevented by boldine, which is attributable to its anti-inflammatory effect. Boldine treatment, which improves the decrease in body weight and the increase in kidney index, confirms the protective effect of boldine against cisplatin nephrotoxicity.
In preclinical studies BUN and creatinine, serum markers are recommended for the evaluation of kidney cell damage as they are considered as more sensitive and specific indicators of renal damage (Mehta et al., 2007). Renal damage in many patients treated with cisplatin, is determined with increased BUN and serum creatinine levels and decreased renal blood flow, hypocalcemia, hypomagnesemia, and proteinuria. It has been determined that a single injection of cisplatin (7 mg/kg body weight) in rat can cause marked kidney damage characterized by elevated BUN and serum creatinine levels and increased morphological changes in the renal tubules (Yilmaz et al., 2004). Cisplatin injection produces mainly proximal tubular necrosis which causes obstruction, fluid leakage and/or bulk formation (Dos Santos et al., 2012). In the present study, BUN and serum creatinine values were significantly increased in cisplatin treated group when compared with control rats. Our results indicated that boldine showed amelioration in BUN and serum creatinine levels. This may be related to the direct effect of boldine on free radicals to prevent kidney cellular damage by protecting membrane integrity. The decline of BUN and creatinine levels to near-normal levels indicated the regeneration of the kidney cell with improvement in nephrotoxicity.
It is well known that oxidative stress is one of the mechanisms involved in cisplatin induced cell damage. Measurement of lipid peroxidation as TBARS is a suitable method for monitoring oxidative damage in tissues. Studies in the literature have shown that cisplatin causes oxidative stress in kidney tissue and shows a significant increase in TBARS level, which indicates the level of lipid peroxidation (Borrego et al., 2004; Hagar et al., 2015). The present study showed that cisplatin causes increase in TBARS, which is consistent with the results of the cisplatin kidney toxicity studies in literature (Borrego et al., 2004; Elberry et al., 2013; Hagar et al., 2015). Boldine has been demonstrated for its strong antioxidant activity due to its ability to scavenge highly reactive free radicals in several studies (Lau et al., 2015; Muthna et al., 2013; O’Brien et al., 2006) Moreover it has been shown that boldine reduces kidney damage by decreasing oxidative stress in hypertensive 2K1C rats (Gómez and Velarde, 2018). In our study, cisplatin-induced TBARS production decreased with boldine administration in kidney tissue suggesting that boldine is reducing oxidative stress and decreasing the amount of species that can react with thiobarbituric acid in the kidney tissue of cisplatin treated rats.
Hydroxyl radicals, superoxide anion and hydrogen peroxide are produced under normal cellular conditions and are detoxified by endogenous antioxidants such as, catalase (CAT) glutathione (GSH) and SOD. However, excessive ROS deposition causes an antioxidant state imbalance and leads to lipid peroxidation and GSH depletion. A significant decrease in the activities of all major antioxidant enzymes, including GPx, CAT and SOD has been observed in the renal cortex and medulla with cisplatin treatment (Sahu et al., 2011; Sohn et al., 2011). It has been shown that cisplatin administration causes zinc and copper loss in renal tissues and the decrease in SOD activity in kidney may be due to loss of zinc and copper (Tanaka-Kagawa et al., 1999). Similar to these studies, a statistically significant decrease in SOD and GPx levels was observed with cisplatin administration in our study. The pharmacological properties of boldine appear to be useful where peroxidation products and/or free radicals act as mediators of intracellular damage. Boldine’s therapeutic and protective roles have been studied over oxidative stress in different models (Hernández-Salinas et al., 2013; Lau et al., 2015; Subramaniam et al., 2019). Boldine treatment (40 mg/kg daily) for 14 days reversed the altered enzyme activities in cisplatin-treated rat kidney tissue, but no significant change in enzyme activities was observed with boldine administration at a dose of 20 mg/kg.
In addition to oxidative stress, recent evidence has shown that cytokine activity and inflammation play an important role in the pathogenesis of kidney damage caused by cisplatin and that oxidative stress can induce inflammation. Inflammatory cell infiltration in damaged renal tissue may be an important process in cisplatin-induced nephrotoxicity. After 24–72 h of cisplatin application, infiltration of macrophages into kidney tissue increases (Meng et al., 2018; Ueki et al., 2013). The anti-inflammatory effects of boldine have been previously reported (Hernández-Salinas et al., 2013; Li et al., 2020). Also, boldine has been shown to ameliorate kidney damage in 2K1C rats by preventing angiotensin converting enzyme 1(ACE-1) and TGF-β increase (Gómez and Velarde, 2018). To investigate the anti-inflammatory mechanism underlying the protective effect of boldine, we measured the levels of TNF-α, a well-known critical mediator of inflammatory disorders that stimulates neutrophils and macrophages, promoting cytokine generation and inflammatory reaction, resulting in cellular necrosis or apoptosis. We demonstrated that cisplatin-induced proinflammatory cytokine TNF-α was reduced by boldine administration. This effect exhibited by boldine may contribute to suppression of inflammation associated with cisplatin-induced loss of kidney function.
iNOS is the main mediator of inflammation in different cell types and it takes place in the production of nitric oxide (NO). Superoxide anion reacts with excess NO and forms peroxynitrite radical which leads to protein nitration and organ damage. Also, excess NO increases sensitivity to ROS by decreasing intracellular GSH (Yui et al., 2016). Alteration in the renal level of NO, is a proposed mechanism for cisplatin-induced nephrotoxicity (Coats and Jain, 2017). It has been reported that the effect of cisplatin on NO is likely to occur through modulation of the iNOS and endothelial nitric oxide synthase (eNOS) expression levels (Chirino and Pedraza-Chaverri, 2009; Sattarinezhad et al., 2017). In our study the effect of boldine against cisplatin-induced inflammation was evaluated by the expression of iNOS in the kidney tissue of rat by IHC staining. The results showed that positive iNOS expression in renal tissue significantly increased in rats treated with cisplatin. The pathophysiological role of iNOS expression in this study supported findings demonstrating selective iNOS inhibition reduces cisplatin-induced nephrotoxicity (Chirino and Pedraza-Chaverri, 2009; Pan et al., 2009). Incontrast, pretreatment with boldine significantly suppressed the expression of iNOS. The suppression of iNOS suggests that the protective effect of boldine may be closely related to its inhibition on cisplatin-induced NO production.
Cisplatin application shifts the balance between proapoptotic and antiapoptotic proteins to the proapoptotic pathway. Proapoptotic effects caused by inducing the activation of caspases is another mechanism underlying the cisplatin nephrotoxicity (Helmy et al., 2018; Ma et al., 2019). Caspases are intracellular cysteine proteases that are required for the execution of apoptosis. Caspase-3, known as the main apoptosis mediator, is an important molecule for cisplatin-induced renal cell apoptosis. Caspase-3 activates the other caspase enzymes and leads to mitochondrial fragmentation and eventually apoptosis (Julien and Wells, 2017). Therefore in renal tubular cells, the activation of caspase is thought to be a main cellular mechanism for the induction of apoptosis in cisplatin-induced nephrotoxicity (Helmy et al., 2018). Additionally, both oxidative stress and inflammation have been associated with caspase dependent renal cellular apoptosis. ROS generation by cisplatin leads to accumulation of free radicals and depletion of intracellular antioxidants which leads to large pores in cell membrane, apoptosis and DNA damage in cisplatin-induced nephrotoxicity (Badawy et al., 2019; Deniz et al., 2020). The present study showed that the cisplatin treated group had elevated caspase-3 expression compared to the control group. This data shows that in the activation of caspase-3, oxidative stress has an important role. Antioxidants by reducing the activation of caspase-3, show protective effects against apoptosis in cisplatin-induced kidney damage (Badawy et al., 2019; Sahu et al. 2011). Treatment with boldine (20 and 40 mg/kg) significantly attenuated caspase-3 immunopositive staining. These results show that boldine affects caspase-3 signaling pathway by suppressing caspase-3 expression.
Typical histopathological changes in cisplatin-induced renal toxicity can be observed as tubular epithelial degeneration, tubular dilatation, cytoplasmic vacuolization, pyknotic nuclei and proteinaceous casts, among others (Perše and Večerić-Haler, 2018; Yuluğ et al., 2019). In the present study degenerated tubular structures, hemorrhage, hyaline cylinder formation, and interstitial congestion were seen with cisplatin application. Histopathological examination revealed the protective effect of boldine. Boldine administration significantly improved hyperemia in interstitium vessels, hyaline cylinder accumulation in renal tubule lumens and degeneration in tubular epithelium. Histopathological findings support the biochemical findings.
Similar effects were observed with both 20 and 40 mg/kg boldine doses for body weight, kidney index, BUN and creatinine levels, TBARS, TNF-α levels, histopathological scoring and for iNOS and Caspase-3 immunohistochemical expression however, the protective effect was more pronounced for most parameters with the 40 mg/kg dose. Boldine treatment with 40 mg/kg altered enzyme activities in cisplatin-treated rat kidney tissue, but no significant change in enzyme activities was observed with at a dose of 20 mg/kg. These results show that the effect of aporphine alkaloid on antioxidant enzyme activities becomes significant with increasing dose. These results suggest that normalization of enzyme activities through ROS scavenging actions may have a significant contribution for the protective efficacy created by 40 mg/kg boldine treatment.
The development of therapies designed to prevent the harmful effects of oxidative stress, inflammation and apoptosis may hinder the progression of cisplatin-induced kidney damage. The use of antioxidant natural substances against these side effects is of interest. For the first time in our study; we provided persuasive evidence for the nephroprotective efficacy of boldine, a pharmacologically active natural aporphine alkaloid found in the leaf/bark of boldo tree against cisplatin-induced renal injury in rats. We concluded that this nephroprotective effect of boldine may be mediated by anti-inflammatory, antioxidant effects and alleviation of caspase-3 expression. We think that boldine may represent a promising new protective strategy against cisplatin induced nephrotoxicity and the use of boldine as an adjunct agent, may contribute to cisplatin used cancer chemotherapy. Future in-depth studies that may relate to these results will explore additional mechanisms and improve our understanding of nephroprotection provided by boldine. Advanced analysis for apoptosis will be needed to explore in future research in order to strengthen the apoptotic approach. Again further extensive clinical research is necessary to determine the potential of boldine for its use in humans.
Availability of Data and Materials: The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Nergiz Hacer Turgut, Huseyin Gungor, Alper Mumin Erdogan; data collection: Huseyin Gungor, Mehmet Ekici, Mehmet Onder Karayigit, Alper Mumin Erdogan; analysis and interpretation of results: Nergiz Hacer Turgut, Haki Kara; draft manuscript preparation: Nergiz Hacer Turgut. All authors read and approved the manuscript.
Ethics Approval: Animal Ethics Committee’s Consent (Cumhuriyet University, HADYEK, No. 65202830-050.04.04-222, Sivas, Turkey).
Funding Statement: This work was supported by Cumhuriyet University Scientific Research Projects Coordination Unit (CUBAP, Sivas, Turkey, Grant No. V-087).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Almeida ER, Melo AM, Xavier H (2000). Toxicological evaluation of the hydro-alcohol extract of the dry leaves of Peumus boldus and boldine in rats. Phytotherapy Research 14: 99–102. [Google Scholar]
Amato A, Terzo S, Mulè F (2019). Natural compounds as beneficial antioxidant agents in neurodegenerative disorders: A focus on Alzheimer’s disease. Antioxidants 8: 608. [Google Scholar]
Asencio M, Delaquerrière B, Cassels BK, Speisky H, Comoy E, Protais P (1999). Biochemical and behavioral effects of boldine and glaucine on dopamine systems. Pharmacology Biochemistry Behavior 62: 7–13. [Google Scholar]
Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A (2005). Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology 212: 116–123. [Google Scholar]
Badawy AM, El-Naga RN, Gad AM, Tadros MG, Fawzy HM (2019). Wogonin pre-treatment attenuates cisplatin-induced nephrotoxicity in rats: Impact on PPAR-γ, inflammation, apoptosis and Wnt/β-catenin pathway. Chemico-Biological Interactions 308: 137–146. [Google Scholar]
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. DOI 10.1016/0003-2697(76)90527-3. [Google Scholar] [CrossRef]
Borrego A, Zamora ZB, González R, Romay C, Menéndez S, Hernández F, Montero T, Rojas E (2004). Protection by ozone preconditioning is mediated by the antioxidant system in cisplatin-induced nephrotoxicity in rats. Mediators Inflammation 13: 13–19. DOI 10.1080/09629350410001664806. [Google Scholar] [CrossRef]
Buyuklu M, Kandemir FM, Ozkaraca M, Set T, Bakirci EM, Topal E (2014). Protective effect of curcumin against contrast induced nephropathy in rat kidney: What is happening to oxidative stress, inflammation, autophagy and apoptosis? European Review for Medical and Pharmacological Sciences 18: 461–470. [Google Scholar]
Chirino YI, Pedraza-Chaverri J (2009). Role of oxidative and nitrosative stress in cisplatin induced nephrotoxicity. Experimental and Toxicologic Pathology 61: 223–242. DOI 10.1016/j.etp.2008.09.003. [Google Scholar] [CrossRef]
Coats A, Jain S (2017). Protective effects of nebivolol from oxidative stress to prevent hypertension-related target organ damage. Journal of Human Hypertension 31: 376–381. DOI 10.1038/jhh.2017.8. [Google Scholar] [CrossRef]
Deniz GY, Laloglu E, Altun S, Yigit N, Gezer A (2020). Antioxidant and anti-apoptotic effects of vitexilactone on cisplatin-induced nephrotoxicity in rats. Biotechnic and Histochemistry 95: 381–388. DOI 10.1080/10520295.2019.1703220. [Google Scholar] [CrossRef]
Dos Santos NA, Carvalho Rodrigues MA, Martins NM, Dos Santos AC (2012). Cisplatin induced nephrotoxicity and targets of nephroprotection: An update. Archives of Toxicology 86: 1233–1250. DOI 10.1007/s00204-012-0821-7. [Google Scholar] [CrossRef]
Sattarinezhad E, Panjehshahin MR, Torabinezhad S, Kamali-Sarvestani E, Farjadian S, Pirsalami F, Moezi L (2017). Protective effect of edaravone against cyclosporine-induced chronic nephropathy through antioxidant and nitric oxide modulating pathways in rats. Iranian Journal of Medical Sciences 42: 170–178. [Google Scholar]
Elberry AA, Refaie SM, Kamel M, Ali T, Darwish H, Ashour O (2013). Oxytocin ameliorates cisplatin-induced nephrotoxicity in Wistar rats. Annals of Saudi Medicine 33: 57–62. DOI 10.5144/0256-4947.2013.57. [Google Scholar] [CrossRef]
Gerhardt D, Bertola G, Bernardi A, Simões Pires EN, Frozza RL, Edelweiss MIA, Battastini AMO, Salbego CG (2013). Boldine attenuates cancer cell growth in an experimental model of glioma in vivo. Journal of Cancer Science and Therapy 5: 194–199. DOI 10.4172/1948-5956.1000206. [Google Scholar] [CrossRef]
Gómez GI, Velarde V (2018). Boldine improves kidney damage in the Goldblatt 2K1C model avoiding the increase in TGF-β. International Journal of Molecular Sciences 19: 1864. DOI 10.3390/ijms19071864. [Google Scholar] [CrossRef]
Gotteland M, Espinoza J, Cassels B, Speisky H (1995). Effect of a dry boldo extract on oro-cecal intestinal transit in healthy volunteers. Revista Medica de Chile 123: 955–960. [Google Scholar]
Ghosh S (2019). Cisplatin: The first metal based anticancer drug. Bioorganic Chemistry 88: 102925. DOI 10.1016/j.bioorg.2019.102925. [Google Scholar] [CrossRef]
Hagar H, El Medany A, Salam R, El Medany G, Nayal OA (2015). Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Experimental and Toxicologic Pathology 67: 133–141. DOI 10.1016/j.etp.2014.11.001. [Google Scholar] [CrossRef]
Heidari R, Reza M (2019). Boldine supplementation regulates mitochondrial function and oxidative stress in a rat model of hepatotoxicity. Pharmaceutical Sciences 25: 1–10. DOI 10.15171/PS.2019.1. [Google Scholar] [CrossRef]
Helmy MM, Hashim AA, Mouneir SM (2018). Zileuton alleviates cute cisplatin nephrotoxicity: Inhibition of lipoxygenase pathway favorably modulates the renaloxidative/inflammatory/caspase-3 axis. Prostaglandins and Other Lipid Mediators 135: 1–10. DOI 10.1016/j.prostaglandins.2018.01.001. [Google Scholar] [CrossRef]
Hernández-Salinas R, Vielma AZ, Arismendi MN, Boric MP, Sáez JC et al. (2013). Boldine prevents renal alterations in diabetic rats. Journal of Diabetes Research 2013: 593672. [Google Scholar]
Julien O, Wells JA (2017). Caspases and their substrates. Cell Death and Differentiation 24: 1380–1389. DOI 10.1038/cdd.2017.44. [Google Scholar] [CrossRef]
Karasawa T, Steyger PS (2015). An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicology Letters 237: 219–227. DOI 10.1016/j.toxlet.2015.06.012. [Google Scholar] [CrossRef]
Kim ES, Lee JS, Akram M, Kim KA, Shin YJ, Yu JH, Bae ON (2015). Protective activity of Dendropanax morbifera against cisplatin-induced acute kidney injury. Kidney and Blood Pressure Research 40: 1–12. DOI 10.1159/000368466. [Google Scholar] [CrossRef]
Kim IH, Kwon MJ, Jung JH, Nam TJ (2018a). Protein extracted from Porphyra yezoensis prevents cisplatin-induced nephrotoxicity by downregulating the MAPK and NF-κB pathways. International Journal of Molecular Medicine 41: 511–520. [Google Scholar]
Kim JY, Park JH, Kim K, Jo J, Leem J et al. (2018b). Pharmacological inhibition of caspase-1 ameliorates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation in mice. Mediators of Inflammation 2018: 1–10. DOI 10.1155/2018/6571676. [Google Scholar] [CrossRef]
Koc F, Tekeli MY, Kanbur M, Karayigit MÖ, Liman BC (2020). The effects of chrysin on lipopolysaccharide-induced sepsis in rats. Journal of Food Biochemistry 44: e13359. DOI 10.1111/jfbc.13359. [Google Scholar] [CrossRef]
Konrath EL, Santin K, Nassif M, Latini A, Henriques A, Salbego C (2008). Antioxidant and prooxidant properties of boldine on hippocampal slices exposed to oxygen-glucose deprivation in vitro. NeuroToxicology 29: 1136–1140. DOI 10.1016/j.neuro.2008.05.008. [Google Scholar] [CrossRef]
Lau YS, Machha A, Achike FI, Murugan D, Mustafa MR (2012). The aporphine alkaloid boldine improves endothelial function in spontaneously hypertensive rats. Experimental Biology and Medicine 237: 93–98. DOI 10.1258/ebm.2011.011145. [Google Scholar] [CrossRef]
Lau YS, Tian XY, Huang Y, Murugan D, Achike FI, Mustafa MR (2013). Boldine protects endothelial function in hyperglycemia-induced oxidative stress through an antioxidant mechanism. Biochemical Pharmacology 85: 367–375. DOI 10.1016/j.bcp.2012.11.010. [Google Scholar] [CrossRef]
Lau YS, Ling WC, Murugan D, Mustafa MR (2015). Boldine ameliorates vascular oxidative stress and endothelial dysfunction: Therapeutic implication for hypertension and diabetes. Journal of Cardiovascular Pharmacology 65: 522–531. DOI 10.1097/FJC.0000000000000185. [Google Scholar] [CrossRef]
Lee RH, Song JM, Park MY, Kang SK, Kim YK, Jung JS (2001). Cisplatin-induced apoptosis by translocation of endogenous Bax in mouse collecting duct cells. Biochemical Pharmacology 62: 1013–1023. [Google Scholar]
Lévy-Appert-Collin MC, Lévy J (1977). Galenic preparations from Peumus boldus leave (Monimiacea) (author’s transl). Journal de Pharmacie de Belgique 32: 13–22. [Google Scholar]
Li W, Veeraraghavan VP, Ma W (2020). Effects of boldine on antioxidants and allied inflammatory markers in mouse models of asthma. Journal of Environmental Pathology, Toxicology and Oncology 39: 225–234. DOI 10.1615/JEnvironPatholToxicolOncol.2020034039. [Google Scholar] [CrossRef]
Ma N, Wei W, Fan X, Ci X (2019). Farrerol attenuates cisplatin-induced nephrotoxicity by inhibiting the reactive oxygen species-mediated oxidation, inflammation, and apoptotic signaling pathways. Frontiers in Physiology 10: 1419. DOI 10.3389/fphys.2019.01419. [Google Scholar] [CrossRef]
Ma P, Xiao H, Yu C, Liu J, Cheng Z et al. (2017). Enhanced cisplatin chemotherapy by ironoxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Letters 17: 928–937. DOI 10.1021/acs.nanolett.6b04269. [Google Scholar] [CrossRef]
Manohar S, Leung N (2018). Cisplatin nephrotoxicity: A review of the literature. Journal of Nephrology 31: 15–25. DOI 10.1007/s40620-017-0392-z. [Google Scholar] [CrossRef]
Mattioli R, Mosca L, Sánchez-Lamar A, Tempera I, Hausmann R (2018). Natural bioactive compounds acting against oxidative stress in chronic, degenerative, and infectious diseases. Oxidative Medicine and Cellular Longevity 2018: 3894381. DOI 10.1155/2018/3894381. [Google Scholar] [CrossRef]
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A (2007). Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Critical Care 11: R31. DOI 10.1186/cc5713. [Google Scholar] [CrossRef]
Meng XM, Ren GL, Gao L, Yang Q, Li HD, Wu WF, Huang C, Zhang L, Lv XW, Li J (2018). NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Laboratory Investigation 98: 63–78. DOI 10.1038/labinvest.2017.120. [Google Scholar] [CrossRef]
Muthna D, Cmielova J, Tomsik P, Rezacova M (2013). Boldine and related aporphines: From antioxidant to antiproliferative properties. Natural Product Communications 8: 1797–1800. DOI 10.1177/1934578X1300801235. [Google Scholar] [CrossRef]
O’Brien P, Carrasco-Pozo C, Speisky H (2006). Boldine and its antioxidant or health-promoting properties. Chemico-Biological Interactions 159: 1–17. DOI 10.1016/j.cbi.2005.09.002. [Google Scholar] [CrossRef]
Ohkawa H, Ohishi N, Yagi K (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95: 351–358. DOI 10.1016/0003-2697(79)90738-3. [Google Scholar] [CrossRef]
Pabla N, Dong Z (2008). Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney International 73: 994–1007. DOI 10.1038/sj.ki.5002786. [Google Scholar] [CrossRef]
Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, Haskó G, Pacher P (2009). Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. Journal of Pharmacology and Experimental Therapeutics 328: 708–714. DOI 10.1124/jpet.108.147181. [Google Scholar] [CrossRef]
Paydar M, Kamalidehghan B, Wong YL, Wong WF, Looi CY, Mustafa MR (2014). Evaluation of cytotoxic and chemotherapeutic properties of boldine in breast cancer using in vitro and in vivo models. Drug Design Development and Therapy 8: 719–733. [Google Scholar]
Perše M, Večerić-Haler Ž (2018). Cisplatin-induced rodent model of kidney injury: Characteristics and challenges. Biomed Research International 2018: 1462802. DOI 10.1155/2018/1462802. [Google Scholar] [CrossRef]
Qin X, Meghana K, Sowjanya NL, Sushma KR, Krishna CG et al. (2019). Embelin attenuates cisplatin-induced nephrotoxicity: Involving inhibition of oxidative stress and inflammation in addition with activation of Nrf-2/Ho-1 pathway. Biofactors 45: 471–478. DOI 10.1002/biof.1502. [Google Scholar] [CrossRef]
Sahu BD, Rentam KK, Putcha UK, Kuncha M, Vegi GM, Sistla R (2011). Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food and Chemical Toxicology 49: 3090–3097. DOI 10.1016/j.fct.2011.08.018. [Google Scholar] [CrossRef]
Sohn SI, Rim HK, Kim YH, Choi JH, Park JH, Park HJ, Choi JW, Kim SD, Jeong SY, Lee KT (2011). The ameliorative effect of 23-hydroxytormentic acid isolated from Rubus coreanus on cisplatin-induced nephrotoxicity in rats. Biological and Pharmaceutical Bulletin 34: 1508–1513. DOI 10.1248/bpb.34.1508. [Google Scholar] [CrossRef]
Subramaniam N, Kannan PKA, Thiruvengadam D (2019). Hepatoprotective effect of boldine against diethylnitrosamine-induced hepatocarcinogenesis in wistar rats. Journal of Biochemical and Molecular Toxicology 33: e22404. DOI 10.1002/jbt.22404. [Google Scholar] [CrossRef]
Tanaka-Kagawa T, Kitahara J, Seko Y, Toyoda H, Imura N, Naganuma A (1999). Reduced sensitivity of HeLa cells to cis-platinum by simultaneous overexpression of copper, zincsuperoxide dismutase and catalase. Biochemical Pharmacology 57: 545–548. DOI 10.1016/S0006-2952(98)00328-1. [Google Scholar] [CrossRef]
Thumann TA, Pferschy-Wenzig EM, Moissl-Eichinger C, Bauer R (2019). The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for gastrointestinal disorders. Journal of Ethnopharmacology 245: 112153. DOI 10.1016/j.jep.2019.112153. [Google Scholar] [CrossRef]
Toora BD, Rajagopal G (2002). Measurement of creatinine by Jaffe’s reaction—determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian Journal of Experimental Biology 40: 352–354. [Google Scholar]
Ueki M, Ueno M, Morishita J, Maekawa N (2013). Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. Journal of Bioscience and Bioengineerinig 115: 547–551. DOI 10.1016/j.jbiosc.2012.11.007. [Google Scholar] [CrossRef]
Yilmaz HR, Iraz M, Sogut S, Ozyurt H, Yildirim Z, Akyol O, Gergerlioglu S (2004). The effects of erdosteine on the activities of some metabolic enzymes during cisplatin-induced nephrotoxicity in rats. Pharmacological Research 50: 287–290. DOI 10.1016/j.phrs.2004.03.003. [Google Scholar] [CrossRef]
Yu C, Dong H, Wang Q, Bai J, Li YN, Zhao JJ, Li JZ (2021). Danshensu attenuates cisplatin-induced nephrotoxicity through activation of Nrf2 pathway and inhibition of NF-kappaB. Biomedicine & Pharmacotherapy 142: 111995. DOI 10.1016/j.biopha.2021.111995. [Google Scholar] [CrossRef]
Yui K, Kawasaki Y, Yamada H, Ogawa S (2016). Oxidative stress and nitric oxide in autism spectrum disorder and other neuropsychiatric disorders. CNS & Neurologic Disordorders-Drug Targets 15: 587–596. DOI 10.2174/1871527315666160413121751. [Google Scholar] [CrossRef]
Yuluğ E, Türedi S, Yıldırım Ö., Yenilmez E, Aliyazıcıoğlu Y, Demir S, Özer-Yaman S, Menteşe A (2019). Biochemical and morphological evaluation of the effects of propolis on cisplatin induced kidney damage in rats. Biotechnic & Histochemistry 94: 204–213. DOI 10.1080/10520295.2018.1543895. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |