DOI:10.32604/biocell.2022.020181

| BIOCELL DOI:10.32604/biocell.2022.020181 |  |
| Viewpoint |
Cardiac stromal cells on stage: From dull filler to specialized actors
1Department of Medical Surgical Sciences and Biotechnologies, Sapienza University, Latina, 04100, Italy
2Institute of Biochemistry and Cell Biology, National Council of Research (IBBC-CNR), Monterotondo, 00015, Italy
3Mediterranea Cardiocentro, Napoli, 80122, Italy
*Address correspondence to: Isotta Chimenti, isotta.chimenti@uniroma1.it
Received: 09 November 2021; Accepted: 13 December 2021
Abstract: Cardiac stromal cells have faced through the years a significant evolution in their definitions concerning their phenotypes, markers, and functions. They are surging to key roles in physiopathology, becoming important targets to be exploited for cardiac repair. In this perspective, we briefly discuss their role in novel therapeutic strategies for enhancing cardiac repair and regeneration.
Keywords: Cardiac fibroblasts; Cardiac stroma; Cardiac repair; Cardiac remodeling
A New Profile for the Old Stroma
The stromal compartment has been long considered as an unspecialized space filled with extracellular matrix and support cells. These were basically all classified as fibroblasts, whose only duty was to produce and remodel the extra-cellular matrix (ECM), both under homeostatic and pathological conditions (Borg and Baudino, 2011; Ceausu et al., 2021). This has been the vision for the stroma of many organs and tissues, including the heart, and cardiac fibroblasts have long been considered as naïve cells with the only ability to respond to injury by producing scar tissue. Now we understand that cardiac stromal cells (CSCs) present many different phenotypes and profiles, involved in advanced and complex functions, such as vascular support and crosstalk with the immune system (Forte et al., 2018; Plikus et al., 2021). In fact, a striking change of perspective has arisen from single cell sequencing studies on whole dissociated organs, where transcriptomic evidence has defined the identity of many different cardiac stromal populations, some even able to go against fibrotic progression (Farbehi et al., 2019; Forte et al., 2020). This is creating a new identikit far from the dull ECM-producing cell type, which creates fibrosis in response to any kind of injury, paradoxically going against the interests of its own microenvironment.
Recent evidence suggests a co-causative role of fibroblasts electrical coupling to cardiomyocytes in the pathogenesis of arrhythmias (Giacomelli et al., 2020), as a sort of revelation against the dominant and much more noble functional role that has always been attributed to cardiomyocytes. Thus, if you somehow take a direct role in the organ’s function par excellence, are you really “stromal”? It seems to be somehow a game changer even in the classical definition of roles for parenchymal vs. stromal cells; even more so, if you consider that now stromal cells can be directly reprogrammed into parenchymal cells, i.e., into induced cardiomyocytes (iCMs) (Tani et al., 2018), therefore further challenging their functional and ontogenetic gaps.
Research in cardiac physiopathology and therapy in the last 20 years has evidenced remarkable potential in targeting and/or exploiting CSC abilities, in spite of everything else. Many different strategies have been explored that can fit in two main approaches (Fig. 1): 1) reducing the impact of the classical fibrotic function of activated stromal cells (i.e., fibroblasts and myofibroblasts) by either positively selecting beneficial phenotypes, or conversely by depleting the pro-fibrotic fraction; or 2) partially resetting the ontogenetic program and converting the stroma into parenchyma (in their more classical meanings).
Supporting Balanced Repair Mechanisms
Myocardial healing after injury requires an ideal balance among inflammatory and repair signals (Forte et al., 2018). In the typical condition of a cardiovascular patient (e.g., with advanced age, co-morbidities, multiple risk factors) this balance is strongly pushed towards excessive inflammatory signaling, cell death, and activation of fibroblasts towards ECM deposition, with consequent extensive scar tissue formation and progressive functional impairment of the organ (Schirone et al., 2017). Multiple studies have highlighted how ex vivo selection and subsequent transplantation into the injured organ of specific resident subpopulations of the heart (described by different authors with multiple terms, such as mesenchymal/stromal, primitive/progenitor, or non-activated cells) can exert positive conditioning on tissue repair by multiple mechanisms, including anti-apoptotic, pro-angiogenic, anti-inflammatory, and anti-fibrotic effects (de Couto et al., 2015; Pagano et al., 2018; Tseliou et al., 2015; Cencioni et al., 2017). Isolating, expanding, and re-infusing endogenous cell populations boosted in this beneficial phenotype has been the strategy of cardiac cell therapy protocols, that have yielded, at least to some extent, beneficial effects, including positive myocardial conditioning and reduction of scar tissue at both pre-clinical and clinical level (Fernandez-Aviles et al., 2017; Malliaras et al., 2014; Ostovaneh et al., 2021; Zwetsloot et al., 2016).
As an alternative to actively increasing the number of pro-repair cells by transplantation, many groups have worked on depleting stromal populations with enhanced pro-fibrotic drive in situ. This negative selection can be achieved by pharmacological targeting of fibrotic cells (e.g., by inhibitors of the renin-angiotensin-aldosterone system, or of β-adrenergic signaling, both implicated in cardiac fibrosis progression) (Chimenti et al., 2016; Fang et al., 2017; Kong et al., 2014), or by the use of monoclonal antibodies against specific pro-fibrotic cytokine pathways, such as TGF-β1 (Warisara et al., 2020). Alternatively, novel immune-therapy based strategies have been shown to be feasible in the heart, such as the generation of chimeric antigen receptor (CAR)-T cells against activated pro-fibrotic fibroblasts (Haig et al., 2019). Overall, providing an advantage to the stromal players that drive a balanced and cardioprotective healing process, or conversely penalizing cell populations that sustain a fibrotic override, both represent efficient (and possibly synergistic) strategies to improve tissue healing after acute injury, or in chronic disease conditions.
Converting Stromal Cells into (Classical) Parenchymal Cells
The exploitation of induced pluripotency as an effective way of producing differentiated cells starting from adult somatic cells has been considered for cardiac cell therapy applications, possibly allowing the easy “refill” of lost cardiomyocytes. The risk of cumulative somatic and acquired genetic aberrations, though, represents a serious obstacle to its large-scale application (Ben-David and Benvenisty, 2011). Instead, the discovery of direct cardiac reprogramming (Ieda et al., 2010; Tani et al., 2018) has provided many advantages compared to fully induced pluripotency, particularly for the risk of tumor formation. Therefore, its applicability appears much more feasible. This strategy is adaptable also to direct in situ and in vivo strategies, where the targeted delivery of reprogramming molecules by carriers of different nature may convert CSCs (possibly belonging to the activated profibrotic pool) into new cardiomyocytes, i.e., iCMs (Fig. 1). In this scenario, CSCs signify convenient targets already in situ and integrated with the tissue to be repaired and regenerated. Importantly, cardiac fibroblasts have been shown to be much more prone to induced reprogramming towards mature iCMs compared to heterologous cell sources (Ieda et al., 2010), thus making CSCs essential protagonists of this approach. Ideally, a strategy targeting specifically pro-fibrotic stromal cells, directly reprogramming them towards iCMs, would fulfill both aims of potentiating a constructive healing response while refilling the lost cardiomyocyte pool.
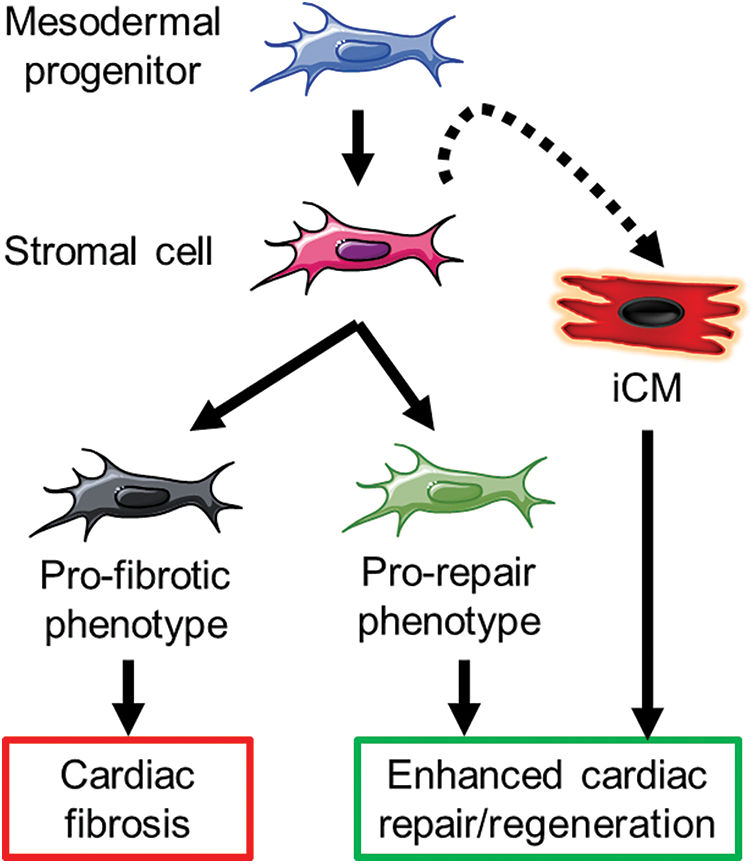
Figure 1: An overview of possible mechanisms to be exploited by targeting cardiac stromal cells for enhanced cardiac repair. iCM: induced cardiomyocyte.
In conclusion, the functional definition of CSCs has been profoundly changed through the years, with exciting novel approaches for exploiting them in cardiac repair strategies. Future developments in targeting or potentiating their tasks may further change their role as specialized actors in cardiac physiopathology.
Acknowledgement: Funders are thanked for supporting the work of this writing contributors.
Availability of Data and Materials: No original data is included in this Viewpoint.
Author Contribution: All authors contributed to conception and drafting of the manuscript, and approved its final version.
Ethics Approval: No ethical approvals are required for this manuscript.
Funding Statement: IC is supported by Grant # RG11916B85CDBF76 from Sapienza University. VP is supported by Grant # AR120172B8B543B3 from Sapienza University. FP is supported by Grant # A0375-2020-36621 from Regione Lazio (POR-FESR 2014-2021).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ben-David U, Benvenisty N (2011). The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer 11: 268–277. DOI 10.1038/nrc3034. [Google Scholar] [CrossRef]
Borg TK, Baudino TA (2011). Dynamic interactions between the cellular components of the heart and the extracellular matrix. Pflugers Archive–European Journal of Physiology 462: 69–74. DOI 10.1007/s00424-011-0940-7. [Google Scholar] [CrossRef]
Ceausu Z, Socea B, Costache M, Predescu D, Serban D et al. (2021). Fibroblast involvement in cardiac remodeling and repair under ischemic conditions. Experimental and Therapeutic Medicine 21: 269. DOI 10.3892/etm.2021.9700. [Google Scholar] [CrossRef]
Cencioni C, Atlante S, Savoia M, Martelli F, Farsetti A et al. (2017). The double life of cardiac mesenchymal cells: Epimetabolic sensors and therapeutic assets for heart regeneration. Pharmacological Therapy 171: 43–55. DOI 10.1016/j.pharmthera.2016.10.005. [Google Scholar] [CrossRef]
Chimenti I, Pagano F, Cavarretta E, Angelini F, Peruzzi M et al. (2016). Beta-blockers treatment of cardiac surgery patients enhances isolation and improves phenotype of cardiosphere-derived cells. Scientific Reports 6: 36774. DOI 10.1038/srep36774. [Google Scholar] [CrossRef]
de Couto G, Liu W, Tseliou E, Sun B, Makkar N et al. (2015). Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. Journal of Clinical Investigation 125: 3147–3162. DOI 10.1172/JCI81321. [Google Scholar] [CrossRef]
Fang L, Murphy AJ, Dart AM (2017). A clinical perspective of anti-fibrotic therapies for cardiovascular disease. Frontiers in Pharmacology 8: 186. DOI 10.3389/fphar.2017.00186. [Google Scholar] [CrossRef]
Farbehi N, Patrick R, Dorison A, Xaymardan M, Janbandhu V et al. (2019). Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife 8: e43882. DOI 10.7554/eLife.43882.061. [Google Scholar] [CrossRef]
Fernandez-Aviles F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R et al. (2017). Global position paper on cardiovascular regenerative medicine. European Heart Journal 38: 2532–2546. [Google Scholar]
Forte E, Furtado MB, Rosenthal N (2018). The interstitium in cardiac repair: Role of the immune-stromal cell interplay. Nature Reviews Cardiology 15: 601–616. DOI 10.1038/s41569-018-0077-x. [Google Scholar] [CrossRef]
Forte E, Skelly DA, Chen M, Daigle S, Morelli KA et al. (2020). Dynamic interstitial cell response during myocardial infarction predicts resilience to rupture in genetically diverse mice. Cell Reports 30: 3149–3163. DOI 10.1016/j.celrep.2020.02.008. [Google Scholar] [CrossRef]
Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X et al. (2020). Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell 26: 862–879.e811. DOI 10.1016/j.stem.2020.05.004. [Google Scholar] [CrossRef]
Haig A, Toru K, Joel GR, Aidan SH, Michael SL et al. (2019). Targeting cardiac fibrosis with engineered T cells. Nature 573: 430–433. DOI 10.1038/s41586-019-1546-z. [Google Scholar] [CrossRef]
Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y et al. (2010). Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142: 375–386. DOI 10.1016/j.cell.2010.07.002. [Google Scholar] [CrossRef]
Kong P, Christia P, Frangogiannis NG (2014). The pathogenesis of cardiac fibrosis. Cellular and Molecular Life Sciences 71: 549–574. DOI 10.1007/s00018-013-1349-6. [Google Scholar] [CrossRef]
Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E et al. (2014). Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). Journal of the American College of Cardiology 63: 110–122. DOI 10.1016/j.jacc.2013.08.724. [Google Scholar] [CrossRef]
Ostovaneh MR, Makkar RR, Ambale-Venkatesh B, Ascheim D, Chakravarty T et al. (2021). Effect of cardiosphere-derived cells on segmental myocardial function after myocardial infarction: ALLSTAR randomised clinical trial. Open Heart 8: e001614. DOI 10.1136/openhrt-2021-001614. [Google Scholar] [CrossRef]
Pagano F, Picchio V, Angelini F, Iaccarino A, Peruzzi M et al. (2018). The biological mechanisms of action of cardiac progenitor cell therapy. Current Cardiology Reports 20: 84. DOI 10.1007/s11886-018-1031-6. [Google Scholar] [CrossRef]
Plikus MV, Wang X, Sinha S, Forte E, Thompson SM et al. (2021). Fibroblasts: Origins, definitions, and functions in health and disease. Cell 184: 3852–3872. DOI 10.1016/j.cell.2021.06.024. [Google Scholar] [CrossRef]
Schirone L, Forte M, Palmerio S, Yee D, Nocella C et al. (2017). A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxidative Medicine and Cellular Longevity 2017: 3920195. DOI 10.1155/2017/3920195. [Google Scholar] [CrossRef]
Tani H, Sadahiro T, Ieda M (2018). Direct cardiac reprogramming: A novel approach for heart regeneration. International Journal of Molecular Science 19: 2629. DOI 10.3390/ijms19092629. [Google Scholar] [CrossRef]
Tseliou E, Fouad J, Reich H, Slipczuk L, de Couto G et al. (2015). Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. Journal of the American College of Cardiology 66: 599–611. DOI 10.1016/j.jacc.2015.05.068. [Google Scholar] [CrossRef]
Warisara P, Theerut L, Supachoke M, Hitoshi K (2020). Therapeutic targets for the treatment of cardiac fibrosis and cancer: Focusing on TGF-β Signaling. Frontiers in Cardiovascular Medicine 7: 34. [Google Scholar]
Zwetsloot PP, Vegh AM, Jansen of Lorkeers SJ, van Hout GP, Currie GL et al. (2016). Cardiac stem cell treatment in myocardial infarction: A systematic review and meta-analysis of preclinical studies. Circulation Research 118: 1223–1232. DOI 10.1161/CIRCRESAHA.115.307676. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |