DOI:10.32604/biocell.2022.020009

| BIOCELL DOI:10.32604/biocell.2022.020009 |  |
| Viewpoint |
SARS-CoV-2 induced myocarditis: Current knowledge about its molecular and pathophysiological mechanisms
1AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Cardiology Unit, Policlinico University Hospital of Bari, Bari, 70124, Italy
2Department of Precision Medicine, University of Campania Luigi Vanvitelli, Naples, 80138, Italy
3AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Clinical Pathology Unit, Policlinico University Hospital of Bari, Bari, 70124, Italy
4CNR Institute of Biomembranes, Bioenergetics and Molecular Biotechnologies (IBIOM), Bari, 70125, Italy
5Department Interdisciplinary of Medicine, University of Bari Aldo Moro, Bari, 70124, Italy
6School of Medicine, University of Bari Aldo Moro, Bari, 70124, Italy
7Department of Emergency and Urgency, National Poisoning Centre, Riuniti University Hospital of Foggia, Foggia, 71122, Italy
*Address correspondence to: Andrea Ballini, andrea.ballini@me.com; Mariarosaria Boccellino, mariarosaria.boccellino@unicampania.it
§Contributed equally as co-first authors
#These authors contributed equally to this work
Received: 29 October 2021; Accepted: 22 December 2021
Abstract: The existence of an inflammatory process in the heart muscle, related to a progressive worsening of myocardial function, different etiopathogenetic mechanisms concur and often overlap, thus making the diagnosis and the therapeutic approach complex. As the COVID-19 pandemic progresses, the effects of the disease on the organ systems and in particular on the cardiovascular system are becoming more and more profound. Cardiac involvement is a well-known event with a high percentage of findings in the heart’s magnetic field, even in asymptomatic areas. There are numerous uncertainties regarding their evolution, in the long and short term, due not only to a difficult to determine the varied clinical expression and the rarely performed intramyocardial biopsy which additionally presents diagnostic problems but also in part to different clinical prognosis. Today, the new SARS-CoV-2 virus that uses the angiotensin converting enzyme 2 (ACE2) which is present at high levels in myocardial cells as its entrance it can create even severe heart injury. The pathophysiology in all of these cases can involve multiple immune and non-immune mechanisms within organs and vessels and can be occur in the clinical phases. Possible mechanisms of direct and indirect myocardial infarction in patients with COVID-19 include additional lesion and oxygen-rich and generalized inflammation response with myocardial immune hyperactivity (myocarditis). Therefore, these can occur through the excessive release of cytokines, the presence of thrombocytopenia, endocrine damage, heart failure, arrhythmias and more. Patients can show average signs of myocardial damage, and some develop spontaneous cardiac complications, such as heart failure, arrhythmias and, rarely, rare cardiogenic disorders. Pathophysiology in all of these may involve multiple mechanisms within the cytokine cephalic membrane, endocrine damage and thrombogenicity. The diagnosis of this myocardial injuri is mainly based on the myocardial enzyme troponin. This viewpoint paper explains today’s knowledge on viral myocarditis, in particular that from SARS-CoV-2 infection, if there is a connection with other possible biomolecular pathogenetic factors that can influence its natural course. In fact, it is for this reason that the pathogenetic mechanisms are analyzed and described. At the same time, its possible interaction with other parameters that are documented risk factors for cardiovascular disease was examined. Although these biomolecular findings were mainly related to necrotic parts of the myocardium, it is important to recognize that myocardial damage early for a better approach and prognosis.
Keywords: Viruses; Infections; SARS-CoV-2; Pandemics; Myocarditis; Molecular diagnosis
The existence of an inflammatory process in the heart muscle, related to a progressive worsening of myocardial function, has been known for at least two centuries. Myocarditis is inflammation of the myocardium, and is diagnosed based on certain histological, immunological and immunohistochemical criteria. Indeed, myocarditis can be caused by infectious and non-infectious agents (as in the cases of certain intoxications and others) (Fig. 1) (Valiton et al., 2020; Basso et al., 2013; Pandey and Rajasurya, 2021; Olson, 2018; Lovreglio et al., 2006; Charitos et al., 2020; Pennisi et al., 2020).

Figure 1: Some non infectious Heart-induce damage factors. Credits: Original figure by I. A. Charitos.
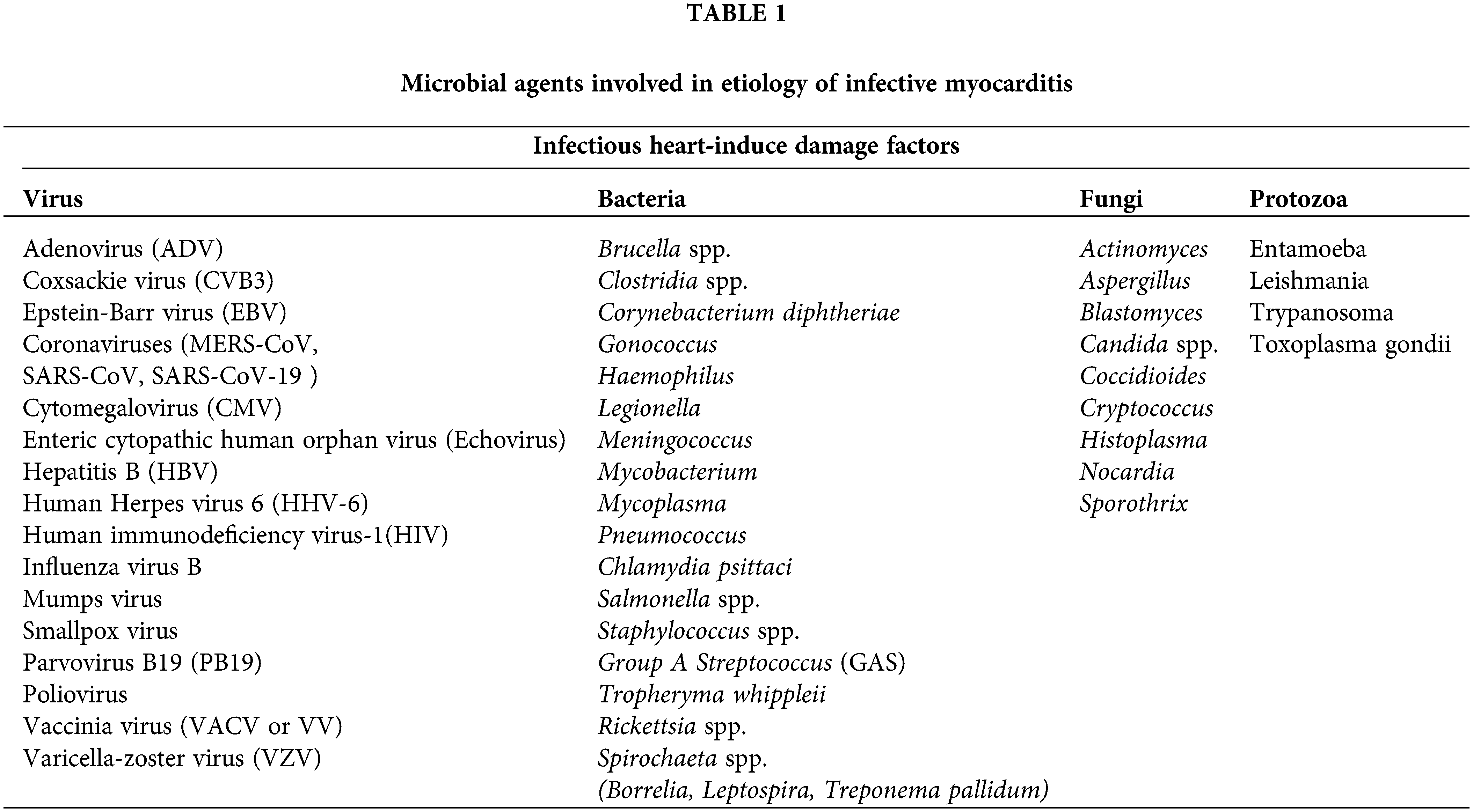
Inflammatory cell infiltrates of the myocardium can develop mainly of three different etiopathogenetic mechanisms: idiopathic, autoimmune, and infectious. Sometimes different etiopathogenetic mechanisms concur and overlap, thus making the diagnosis and the therapeutic approach complex. Thus, the etiology of myocarditis is, in fact, extremely heterogeneous, including infectious and non-infectious agents, immune mechanisms and genetic factors, which would justify a greater individual susceptibility to develop an autoimmune reaction capable of causing inflammatory infiltration of the myocardium (Krejci et al., 2016; Bracamonte-Baran and Čiháková, 2017). The infectious etiology can be supported by viral agents, including Enterovirus, Adenovirus (ADV), Coxsackievirus (CVB3), Cytomegalovirus (CMV), Epstein-Barr virus (EBV), Human immunodeficiency virus-1 (HIV-1). In addition, bacterial agents such as Borrelia or intracellular bacteria such as Rickettsia spp. but also fungi, protozoa and other infectious agents cant may be involved (Table 1) (Krejci et al., 2016; Ammirati et al., 2021).
SARS-CoV-2 infection by COVID-19 has reached pandemic proportions and is undoubtedly a major problem for health systems worldwide. Until today, 09 September 2021, we have 222.406.582 confirmed cases with 4.592.934 deaths and 5.352.927.296 vaccine doses have been administered (Santacroce et al., 2020a; WHO, 2021). As our knowledge of the natural history of the disease increases day by day, we find that very little is known about specific high-risk groups, such as patients with pre-existing cardiovascular disease, and in particular patients with heart failure (HF). Cardiac arrhythmias have been noted in the two previous pandemics SARS and MERS. In the first, tachyarrhythmias were the most common. Understandably, any such information acquires special weight, as it helps both prognostically and therapeutically. In addition, cardiac involvement in COVID-19 infection, contrary to popular belief, is much more common. Cases of cardiomyopathy and myocarditis have been described in patients with severe SARS-CoV-2 infection and all data indicate a worse prognosis in these cases (Carretta et al., 2021; Ho et al., 2020; Siripanthong et al., 2020; Alhogbani, 2016).
For the present study we have performed the most significant literature in the field of biomolecular mechanisms regarding viral myocarditis in general. In addition, targeted articles were sought that had for patients who were identified as suffering from COVID-19-induced acute myocarditis for both blood chemistry, instrumental and autopsy research. For this research we used the PubMed database, Elsevier Connect, Cochrane Library, Web of Science, Embase, conducting manual searches on Google Scholar, and using the bibliographies of the articles. In addition, articles that he considered to be similar content to those found were excluded, for a period of six months of research.
Acute viral myocarditis is a relatively rare disease and is an inflammatory reaction of the myocardium that is mobilized by infectious causes (viruses) and is diagnosed with histological, immunological and immunohistochemical criteria. In recent years it has also been defined with the term “inflammatory cardiomyopathy” to describe the close association between myocarditis and subsequent dilated cardiomyopathy. The pathophysiology of viral myocarditis presupposes the “synergy” of two factors: the viral spread in the myocardium and the subsequent immune response. Hence, the initial invasion of myocardial cells by the virus (acute phase) leads to the mobilization of the immune system, culminating in the production of antibodies against the virus which, however, cross-react with antigens of the myocardial cells and thus cause the final myocardial damage (subacute phase with clinical signs), leading to complete healing or the development of dilated cardiomyopathy (chronic phase) (Krejci et al., 2016; Carretta et al., 2021). So, the diagnosis of viral myocarditis can be based on clinical signs, histopathological examination, immuno-haematochemical tests, detection of genetic material of viruses through molecular techniques (PCR), presence of anti-heart autoantibodies, imaging techniques (ultrasound, etc.) and instrumental techniques (ECG, etc.) (Könemann et al., 2020; Valiton et al., 2020).
(a) Clinical signs and diagnosis
Cardiac involvement during a viral infection is usually favored both by the characteristics of the host (genetic, immunological factors, age), and by the virulence and different cardiac tropism of the infecting agent. Several studies conducted on mouse models of viral myocarditis highlighted the importance of genetic factors, in determining different degrees of susceptibility to infection and severity of histological damage, even in the face of infections caused by the same virus, probably related to particular haplotypes of the major histocompatibility complex (Basso et al., 2013; Kang and An, 2021). There are also some physiological conditions recognized as unfavorable, such as pregnancy or physical exercise. In several cases the causative agent remains unidentifiable and therefore is defined as “diopathic myocarditis”, which are however, in most cases, attributed to a viral infection investigated when the direct myo-cytolysis phase has now passed (Ammirati et al., 2021; Blauwet and Cooper, 2010).
To better understand the etiopathogenetic mechanisms of viral infection, it may be useful to consider all the myocarditis as an evolutionary process, in which three different chronologically distinct phases of histological damage are distinguished (Schultz et al., 2009). At the physical examination of the we can notice the presence of tachycardia or arrhythmia, reduction of the first, third and four heart sounds, systolic murmur at the level of the mitral/tricuspid valves, pericardial friction sound (mill wheel), and and moist rales in auscultation at the level of the pulmonary bases (Könemann et al., 2020; Padilla-Ortiz and Ibarra, 2018).
The variations of their evolution and prognosis, in the long and short term, are due, in part to clinical polymorphism of this disease and so, can make difficult disease recognition. Scientific interest has focused its attention above all on the need to identify and offer standardized diagnostic and prognostic criteria to clinical practice to improve the clinical management of patients with myocarditis (Dennert et al., 2008). Alongside completely asymptomatic or less symptomatic forms there may in fact be, at the onset, clinical pictures of severe chronic heart failure with possible evolution towards dilated cardiomyopathy, infarct-like chest pain. Furthermore the ECG may be normal but more commonly there are nonspecific changes, can be observed the presence of conduction delay and left bundle branch block (in 20% of cases), diffuse ST segment elevations, acute myocardial infarction with ST segment elevation (myocardial infarction pattern), sinus tachycardia (appears as the most common), several premature ventricular contractions, atrial or ventricular ectopic beats, hypokinetic arrhythmias (atrio-ventricular and sino-atrial blocks) and hyperkinetic both atrial (atrial fibrillation, paroxysmal supraventricular tachycardia) and ventricular (ventricular tachycardias and fibrillation) (Shauer et al., 2013; Santacroce et al., 2018).
In some cases, myocarditis can present as fulminant and be characterized by severe acute heart failure (requiring mechanical ventricular assistance), or by life-threatening arrhythmias, sustained ventricular tachycardias, ventricular fibrillation, which can clinically be characterized by sudden death. In viral forms, the anamnestic finding of a flu-like syndrome affecting the patient in the days or weeks preceding the onset of symptoms is frequent, but not constant (Tschöpe et al., 2021).
The knowledge of myocarditis expanded thanks to the endomyocardial biopsy in vivo diagnosis, and the consequent need to establish standard criteria for histological diagnosis. The introduction of endomyocardial biopsy and molecular techniques has certainly helped to better define the physio-pathological bases and identify the histological and immunohistochemical aspects of the inflammatory and autoimmune process, which, in the long term, can lead to dilated cardiomyopathy (probable causal correlation between viral myocarditis and dilated cardiomyopathy (Kearney et al., 2001). In addition, the identification of viral etiological agents, studied in animal models, aspect of the immune-mediated mechanism of histological damage occurs through molecular and immunohistochemical investigations (Tschöpe et al., 2019). Troponin is a valid index for detecting damage to the myocardium. It is notice that, troponin T (values > 0.1 ng/mL) had a sensitivity of 53%, a specificity of 94%, a positive predictive value of 93%, and a negative predictive value of 56%. Troponin I was also found to have a sensitive specificity of 34% and 89%. A recent onset of cardiac symptoms (<4 weeks) increases sensitivity to troponin and therefore it is recommended to measure troponin T or I as a routine when clinical myocarditis is suspected (Xu et al., 2013; Lopez-Jimenez et al., 1997; Roldán Torres et al., 2003). Furthermore, another index value together with Troponin would be N-terminal pro b-type natriuretic peptid (NT pro-BNP), which is a biomarker of heart failure, and its high value can be associated with high mortality (Carretta et al., 2021). Furthermore, certain methods on myocardial tissue samples now allow us to obtain a precise quantification of the viral content of endomyocardial samples, using Real time-PCR and the Southern blot hybridization. Thanks to these methods it is possible to characterize the replicative activity of the viral genome. The viral persistence of actively replicating viruses is associated with a higher mortality (Ammirati et al., 2021; Hoebeeck et al., 2007). Chest X-ray, may show a normal cardiothoracic index which is often within or increased, findings in the pulmonary parenchyma such as lung congestion and pulmonary edema (Blauwet and Cooper, 2010). In the ultrasound heart exam we can have a view of the anatomical structure (pericardial effusion, dilated heart or sin hypetrofic ventricle and more), functional (valves, etc.), hemodynamics alterations and presence of thrombosis. In detail, the ultrasound findings of myocarditis typically include partial kinetic disturbances, diffuse left ventricular systolic dysfunction, and partial wall thickness swelling due to edema. The last eardrum should be distinguished by a slight diffuse symmetrical increase in wall thickness with normal diastolic function observed in intensely lying individuals. (Carretta et al., 2021; Perera et al., 2014) On the other hand, the presence of diastolic dysfunction with reduced effort (<16%) should lead to suspicion of myocardial involvement (Schannwell et al., 2002). In patients with signs of myocarditis, they must not engage in excessive physical activity or training abstinence for the athletes should be at least six months and cardiac function follow-up is recommended after this period, which includes biomarkers (troponin, etc.), heart ultrasound, Holter dynamic ECG, electrocardiogram (also during physical stress), and Magnetic resonance imaging (MRI). With the of MRI can obtain for more detailed structural alterations and has a sensitivity value of 75%–100% with a specificity of 90%. It can be noted that there is a late signaling potential after gadolinium administration and shows at the epicardial level, and subepicardial and subendocardial increase of the uptake contrast predominant in the inferior and lateral wall of the heart (Nadjiri et al., 2019; Friedrich et al., 2009). Finally, scintigraphy radioisotope studies can be performed with gallium 67 (which can detect myocardial inflammation) or Indium-111 antimyosin antibody imaging which can detect a necrotic area of the myocardium (has a sensitivity of 83%, specificity of 53%) (Hung et al., 2007; Kindermann et al., 2012).
(b) The bio-molecular pathogenic mechanisms
The virus-induced myocardial damage occurs in three phases. At the beginning (including the first two weeks), the viral infection creates an initial histological damage, often insignificant, determined by the cytopathic effect direct virus, with processes of myo-cytolysis that may eventually assume a predominant role in fulminant myocarditis (Tschöpe et al., 2021; Billingham and Tazelaar, 1986).
The initial inflammatory response of the humoral type is fundamental in the resolution processes of the viral infection. It was shown that, high levels of immune response markers (such as anti-myocardial IgG), in this first phase, they would correlate with a better prognosis. In the second phase (from the second to the seventh week), a host immune reaction mechanism develops: under normal conditions, in this stage, the virus is completely eradicated and cured without any further damage. However, in some individuals the elimination of the virus is not complete and in this case the humoral response of the host results in a persistent inflammatory infiltration of the myocardium, with consequent immune-mediated damage (Tschöpe et al., 2021; Shauer et al., 2013; Maisch, 2019). The hypothesis of the immune-mediated mechanism was supported, in part, by the extreme difficulty in diagnosing an infection in progress (through the search for specific blood antibodies or viral isolation techniques in patients with clinical evidence of myocarditis. In part, the histological finding of the presence, in the weeks following the onset, of a mononuclear cell infiltrate also suggests the pre-eminent role of “cell-mediated” immunity (Schultz et al., 2009; Dennert et al., 2008; Liu et al., 1996).
The virus-induced myocardial damage is therefore determined by both direct and indirect mechanisms. Viral proteases directly change the interconnections between the cytoskeleton and the extracellular matrix, thus determining a reciprocal sliding of the myocytes and thus favoring a ventricular dilation mechanism (Meessen et al., 1975).
Immunological phenomena, on the other hand, can be responsible for indirect myocardial damage, through different mechanisms: production of proinflammatory cytokines, release of cytotoxic substances, such as reactive oxygen species and metal proteases, which determine a structural alteration of the myocardium, thus favoring the infiltration of other immunocompetent elements, thus exacerbating and amplifying the immune-mediated damage. Finally, in the third phase, a process of chronic inflammatory infiltration of the myocardium develops, associated with fibrosis, cardiac remodeling, hypertrophy, re-induction of the expression of fetal genes, progressive deterioration of ventricular function, with probable evolution towards forms of dilated cardiomyopathy (Fig. 2) (Shauer et al., 2013; Kong et al., 2014).

Figure 2: The natural course evolution of the viral myocarditis. The three phases of the natural history of the viral myocarditis. Viral myocarditis appears to be a multifactorial disease in which several immune mechanisms determine its development and outcome. Given its complex pathogenesis, predisposing factors such as sex and genetic background along with the type of virus trigger the onset of the disease. Therefore, it is more likely that other factors, both endogenous and environmental, are also involved in such a way as to provoke a specific autoimmune process that influences the development of viral infection in myocarditis. Cardiotropic viruses probably act as a primary and “auxiliary” stimulus to the subsequent processes of the immune system, but it varies according to the host. Thus, the pathophysiology of viral myocarditis probably involves the interaction of three factors, viral infection, genetic predisposition, and immune response. The initial invasion of myocardial cells by the virus (acute phase) leads to the mobilization of the immune system, eventually leading to the production of antibodies against the virus. However, these subsequently has a cross-react with myocardial cell antigens and thus cause eventual myocardial damage (subacute phase), leading either to complete healing or to the development of dilated cardiomyopathy (chronic phase). Credits: Original figure by I. A. Charitos.
Therefore, it is essential to consider the persistence of inflammatory infiltration and the important role played in the pathogenesis of virus-induced myocardial damage, by the activation of clones of T lymphocytes, both CD4+ and CD8+ (Thomas and Grisanti, 2020). The activated T lymphocytes, in fact, release a series of inflammatory cytokines (interferon gamma INFγ, tumor necrosis factor TNF, chemotactic factor macrophage and interleukins), determining the proliferation and differentiation of effector lymphocytes and B lymphocytes in plasma cells secreting antibodies. The activation of cell mediated immune response is therefore accompanied by a “humoral” immunological response, mediated by autoantibodies (Basso et al., 2013; Thomas and Grisanti, 2020).
The presence of autoantibodies is related to two main mechanisms: release of autoantigens, following cell damage and phenomena of molecular mimicry between myocardiocytes and non-self-antigens. Circulating autoantibodies directed against mitochondrial antigens, myosin epitopes, M2 receptors and ß1 adrenergic receptors have been identified in patients with myocarditis or idiopathic dilated cardiomyopathy (Basso et al., 2013; Wang and Han, 2020). The role of these autoantibodies as factors remains uncertain. causal in the pathogenesis of viral myocarditis, rather than as an “associated epiphenomenon”, without correlation with the severity of the myocardial inflammatory process. The presence of autoantibodies is more evident especially in the acute initial phase of myocarditis, while the antibody titer progressively decreases in the chronic phase of inflammation and consequent worsening of ventricular function. Furthermore, these autoantibodies have been detected in asymptomatic relatives, many years before echocardiographic abnormalities were found (Corsten et al., 2015). In the light of these latest data, the recent hypothesis of considering these antibodies as early markers of a possible future evolution in cardiomyopathy has emerged. The data on the real clinical importance of the identification of viral sequences with replicative potential also remain controversial: according to some authors, but the topic is controversial, the identification of enteroviral genome, with replication capacity, could have possible therapeutic consequences such as with Interferon (INF) and normal human immunoglobulin (NHIG) (Katsuragi et al., 1993; Calabrese and Thiene, 2003). Initially, attention was mainly focused on EVs since these represent the most common cause of myocarditis and in recent years, other viral genome sequences have also been identified in patients with pictures of chronic ventricular dysfunction, often referred to a previous myocarditis, or with de novo dysfunction, focusing attention on the possible role of the persistence of other viruses such as Human herpesvirus 6 (HHV6), Adenovirus (ADV) and Parvovirus B19 (PB19) (Valiton et al., 2020; Basso et al., 2013; Kühl et al., 2005). The relative frequency of these viruses in biopsies performed on different groups of patients in different studies, is extremely variable. Multiple factors can determine these differences: age, ethnic group, geographical implications in the epidemiology of viral infections, time elapsed between the onset of symptoms and the execution of the biopsy and number of biopsy samples subjected to PCR investigation. There is a lack of data regarding the natural history of certain infectious processes sustained by these viruses and the respective prevalence of viral persistence in these patient groups (Valiton et al., 2020; Maisch et al., 2005).
SARS-CoV-2 infection and heart-induce injury
a) Pathophysiological aspects
The most common clinical symptoms for SARS-CoV-2 infection are in the respiratory tract, but in some patients the symptoms of the cardiovascular system are prominent. These patients usually have a severe underlying heart problem and heart damage can be disproportionately greater than lung damage and manifest as acute coronary syndrome or acute myopericarditis or acute heart failure. Usually, patients with pre-existing cardiac insufficiency are likely to be at increased risk in prolonged hospitalization especially in the ICU and undergoing intubation and mechanical ventilation (Basu-Ray et al., 2021). To date, epidemiological data on cardiac involvement in the form of cardiomyopathy during SARS-CoV-2 infection are still evolving. These patients may have biomarkers of cardiac necrosis (troponins) and/or natriuretic peptides (BNP and NT-proBNP) elevated (have worse prognosis), reduced ScVO2 and cardiogenic shock (responsible for patient’s mortality). Therefore, in the case of elevated troponin levels, mortality increases in the absence of known heart disease, while with patients already suffering from heart it can rise. It was noted instead that patients with negative troponin had a mortality rate lower regardless of the presence or absence of pre-existing cardiovascular diseases (Khan et al., 2021). The mechanisms leading to an increase in troponin levels are associated with direct myocardial ischemia of the virus, and the abnormal inflammatory response of the myocytes against the virus with hypotension and/or myocardiocytes damage. Finally, it is important to note that evidence of cardiac involvement may be even in the absence of significant lung disease suggesting that pure cardiac involvement is low. In these cases, the increased severity/mortality is likely to be associated with the use of angiotensin converting enzyme inhibitors or angiotensin-II receptor blockers (Tersalvi et al., 2020; Babapoor-Farrokhran et al., 2020).
b) Bio-molecular dynamics
ACE2 plays a dominant role in the cardiovascular and immune systems. On the one hand it is involved in normal heart function and on the other hand in the development of certain pathologies such as hypertension and diabetes. This enzyme is the key mediator of the receptor for the action of several Coronaviruses, as it had also been for SARS-CoV-2. The virus besieges the alveolar epithelial (direct damage), causing respiratory symptoms. These symptoms appear to be more pronounced in patients with pre-existing heart problems and may be associated with overexpression of the ACE2 enzyme in these patients (Gheblawi et al., 2020; Santacroce et al., 2021).
This has led to skepticism about continuing treatment of chronic heart failure with renin-angiotensin system inhibitors in patients with SARS-CoV-2 infection (Perrotta et al., 2020). It has therefore become apparent that treatment with ACEs/ARB should be continued normally, as the benefit of continuing treatment is likely to be greater than the risk of SARS-CoV-2 infection. COVID-19 is directly and indirectly involved in cardiac damage and therefore the compromission of its electrophysiological function be mediated by three distinct mechanisms of action (Yehualashet and Belachew, 2020; Gathiram et al., 2021). The indirect damage it starts from the excessive systemic inflammatory hyperreaction (abnormal cytokine release) overcomes myocardial contractility and leads to cardiomyopathy (clinically also present) or deregulation of pre-existing hearth failure. However, this mechanism is also found in other inflammatory pathological conditions (such as septic patient), where the excessive release of interleukin IL-1β/β and TNFα leads to a direct suppressive effect on the myocardial fiber. Another cause of the indirect damage is that the ACE2 receptor-lead to microangiopathy through the endothelial dysfunction and tissue ischemia, resulting in an increased incidence of dysfunction and arrhythmogenesis (Fig. 3) (Trentadue et al., 2012; Iwasaki et al., 2021; Balzanelli et al., 2021; Charitos et al., 2019; Caramaschi et al., 2021).
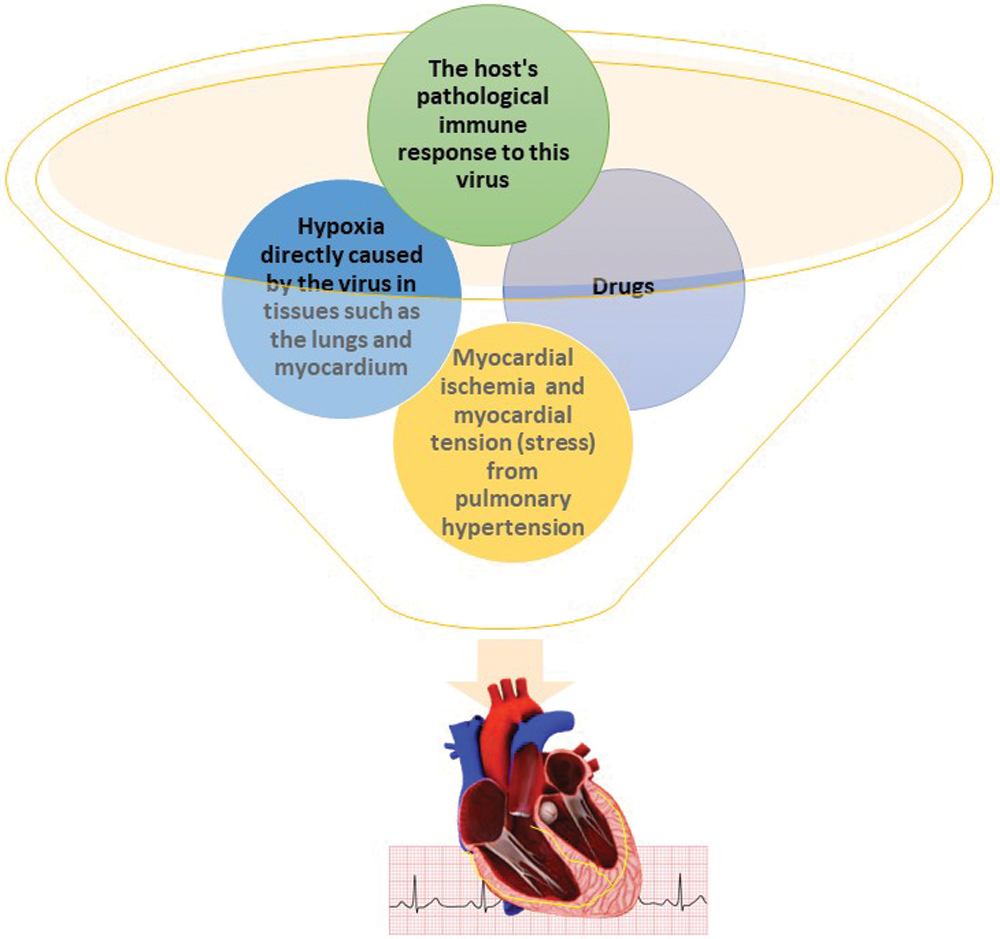
Figure 3: Hypothesis of the pathophysiological mechanisms of arrhythmogenesis in COVID-19 patients. Credits: Original figure by I. A. Charitos.
The most common arrhythmias notice are bradyarrhythmias, atrial fibrillation, atrial flutter, torsade de pointes ventricular tachycardia, ventricular fibrillation. Furthermore, drugs medications that are QTc prolonging, such as hydroxychloroquine or azithromycin, antiretroviral protease inhibitors (ritonavir, lopinavir), and other that can lead to ventricular arrhythmias and torsade de pointes. The direct damage that the virus carries out in the myocardium with SARS-CoV-2 direct myocardial infarction, resulting in acute immune response and onset of myocarditis. In addition, autopsy studies of patients who died from SARS-CoV-2 infection showed the presence of SARS-CoV-2 genetic material in the myocardium in nearly two-thirds of the deceased. Therefore, although the predominant injury is caused by widespread inflammation in the context of SARS-CoV-2 infection, direct damage to the heart by the virus itself is not excluded (Caramaschi et al., 2021, di Domenico et al., 2021a). Thus, the infection can deregulate heart failure in patients who are already present or exacerbate pre-existing mild cardiac dysfunction that was asymptomatic and thus lead to long-term complications. Due to myocardial expression of the SARS-CoV-2 ACE2 receptor leads to myocardial involvement. Indeed, for patients with heart failure but with preserved ejection fraction they are included in the vulnerable population, since in case of SARS-CoV-2 infection and development of SARS-CoV-2 infection they have a particularly increased risk of complications and mortality (Fig. 4) (Charitos et al., 2019; Dal Ferro et al., 2021; Lazzerini et al., 2020).

Figure 4: The pathogenesis damage of the healthy heart and the arrhythmic manifestations hypothesis from COVID-19. Credits: Original figure by I. A. Charitos.
The inflammation of the myocardium both from the storm of cytokines and the virus itself (through the intestinal edema which leads to fibrosis), causes an electrophysiological and structural alteration to the conduction system. This condition over time leads to an altered intercellular coupling, an irregular flow of Ca2+, the downregulation of the K+ channels so, causing anomalies of repolarization, conduction and action potential. This can lead to tachyarrhythmias (atrial fibrillation, atrial flutter, atrial tachycardia, sinusal and ventricular tachycardia) and/or bradyarrhythmia (atrioventricular nodal block, sinus bradycardia and sinus node dysfunction) (Carretta et al., 2021; di Domenico et al., 2021b).
The underlying mechanisms of heart failure with or not preserved ejection fraction (HFPEF), beyond systemic inflammation combined with low oxygen levels leads to tissue and endothelium lesions which aggravate thrombosis phenomena. However, it has been noted that both in myocardial cells and in monocyte-derived macrophages, ACE2 is overexpressed, and this has the effect of reducing the production of cytokines (mainly chemotactic protein-1) caused by Ang II (Santacroce et al., 2020b). Therefore, to this hypothesis we must also add the damage previously to the lungs or/and heart and the intake of some arhythmogenic drugs (such as antidepressants, certain antibiotics, diuretics to others). Finally, it can also be the hospitalization environment such as Intensive Care Unit (ICU) and mechanical ventilation as a concomitant (Santacroce et al., 2021; Carretta et al., 2021). In SARS-CoV-2 infection, reduced ACE2 expression in myocardial infarcts is likely to contribute to cardiac dysfunction in multi-organ failure and macrophage disturbance is involved in the cytokine storm. ACE/Ang II with simultaneous delay of the ACE2/Ang 1-7 axis (Caramaschi et al., 2021). In addition, hypokalemia was a consistent finding in patients with hypertension, which would support the theory of an imbalance between the hypertensive component of Ang II-Aldosterone, with a concomitant delay of the “hypotensive” component Ang II-NO10. Indeed, they have emerged to date as the most common comorbidities in people with SARS-CoV-2 infection (they are also more unfavorable prognostic factors for the outcome of the disease), the therapeutic use of converting enzyme inhibitors and receptor inhibitors angiotensin, hypertension, cardiovascular disease and diabetes mellitus. Thus, the likelihood of requiring intensive care and mechanical respiratory support, or the death of the patient with these comorbidities is sometimes higher (Liu et al., 2021; Freaney et al., 2020; Brojakowska et al., 2020; Cantore and Ballini, 2020; Schirinzi et al., 2020).
Understanding the pathophysiological mechanisms of viral myocarditis, and especially in the current pandemic from the SARS-CoV-2 virus, is extremely important for a timely and effective treatment of patients with or without pre-existing heart disease, to reduce the morbidity and mortality of this disease. Thus, we can avoid making diagnostic errors to avoid organ damage (such as myocardial infarction and heart failure). SARS-CoV-2 infection is triggered by binding of the SARS-CoV-2 virus head to angiotensin-2 converting enzyme (ACE2), a membrane-bound aminopeptidase of various cells, which is highly expressed in the heart and in the lungs. SARS-CoV-2 infection can cause damage to the myocardium directly or indirectly. Until now, the Published studies have shown myocardial infarction with increased biochemical markers in many patients. In addition, the study of heart function with ultrasound more often detects dilation and dysfunction of the right abdomen, as well as disturbances in the relaxation process (diastolic function) of the left ventricle. The increased risk of complications in patients with heart failure imposes the high suspicion rate of myocardial damage both in those with established heart disease and in those with risk factors for heart failure (diabetes, obesity, etc.). Early diagnosis using cardiac biomarkers, ECG, and ultrasound of the heart is important for locating arrhythmias, exacerbating pre-existing heart disease, and early diagnosis of myocardial infarction. The value of heart-focused diagnostic tests has both a prognostic role and a value in the long-term follow-up of any complications. In conclusion, SARS-CoV-2 infection can worsen and/or cause heart failure relapses through both direct and indirect heart damage. Due to the increased risk of fatal complications, it is imperative to be vigilant for early recognition of these, to take personal protective measures on the part of patients and regular communication with the doctor so as not to neglect the treatment of the underlying disease.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article (and it supplementary information files).
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: all the authors; data collection: all the authors; analysis and interpretation of results: all the authors; draft manuscript preparation: I.A.C., L.S., A.B., and M.B. Conceptualization and final validation: A.B., I.A.C. and L.S. Finally D.C. and M.D.D. equally contributed as co-first authors and I.A.C. and L.S. as co-last authors. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Alhogbani T (2016). Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Annals of Saudi Medicine 36: 78–80. DOI 10.5144/0256-4947.2016.78. [Google Scholar] [CrossRef]
Ammirati E, Veronese G, Bottiroli M, Wang DW, Cipriani M et al. (2021). Update on acute myocarditis. Trends in Cardiovascular Medicine 31: 370–379. DOI 10.1016/j.tcm.2020.05.008. [Google Scholar] [CrossRef]
Babapoor-Farrokhran S, Rasekhi RT, Gill D, Babapoor S, Amanullah A (2020). Arrhythmia in COVID-19. SN Comprehensive Clinical Medicine 4: 1–6. DOI 10.1007/s42399-020-00454-2. Epub ahead of print. [Google Scholar] [CrossRef]
Balzanelli MG, Distratis P, Aityan SK, Amatulli F, Catucci O et al. (2021). An alternative “Trojan Horse” hypothesis for COVID-19: Immune deficiency of IL-10 and SARS-CoV-2 biology. Endocrine, Metabolic & Immune Disorders. DOI 10.2174/1871530321666210127141945. Online ahead of print. [Google Scholar] [CrossRef]
Basso C, Calabrese F, Angelini A, Carturan E, Thiene G (2013). Classification and histological, immunohistochemical, and molecular diagnosis of inflammatory myocardial disease. Heart Failure Reviews 18: 673–681. DOI 10.1007/s10741-012-9355-6. [Google Scholar] [CrossRef]
Basu-Ray I, Almaddah NK, Adeboye A, Soos MP (2021). Cardiac Manifestations of Coronavirus (COVID-19). Treasure Island (FLStatPearls Publishing. [Google Scholar]
Blauwet LA, Cooper LT (2010). Myocarditis. Progress in Cardiovascular Diseases 52: 274–288. DOI 10.1016/j.pcad.2009.11.006. [Google Scholar] [CrossRef]
Bracamonte-Baran W, Čiháková D (2017). Cardiac autoimmunity: Myocarditis. Advances in Experimental Medicine and Biology 1003: 187–221. DOI 10.1007/978-3-319-57613-8. [Google Scholar] [CrossRef]
Billingham ME, Tazelaar HD (1986). The morphological progression of viral myocarditis. Postgraduate Medical Journal 62: 581–584. DOI 10.1136/pgmj.62.728.581. [Google Scholar] [CrossRef]
Brojakowska A, Narula J, Shimony R, Bander J (2020). Clinical implications of SARS-CoV-2 interaction with renin angiotensin system: JACC review topic of the week. Journal of the American College of Cardiology 75: 3085–3095. DOI 10.1016/j.jacc.2020.04.028. [Google Scholar] [CrossRef]
Calabrese F, Thiene G (2003). Myocarditis and inflammatory cardiomyopathy: Microbiological and molecular biological aspects. Cardiovascular Research Journal 60: 11–25. DOI 10.1016/S0008-6363(03)00475-9. [Google Scholar] [CrossRef]
Charitos IA, Topi S, Castellaneta F, D’Agostino D (2019). Current issues and perspectives in patients with possible sepsis at emergency departments. Antibiotics 8: 56. DOI 10.3390/antibiotics8020056. [Google Scholar] [CrossRef]
Charitos IA, Ballini A, Bottalico L, Cantore S, Passarelli PC et al. (2020). Special features of SARS-CoV-2 in daily practice. World Journal of Clinical Cases 26: 3920–3933. DOI 10.12998/wjcc.v8.i18.3920. [Google Scholar] [CrossRef]
Cantore S, Ballini A (2020). Coronavirus disease 2019 (COVID-19) pandemic burst and its relevant consequences in dental practice. Open Dentistry Journal 14: 111–112. DOI 10.2174/1874210602014010111. [Google Scholar] [CrossRef]
Caramaschi S, Kapp ME, Miller SE, Eisenberg R, Johnson J et al. (2021). Histopathological findings and clinicopathologic correlation in COVID-19: A systematic review. Modern Pathology 34: 1614–1633. DOI 10.1038/s41379-021-00814-w. [Google Scholar] [CrossRef]
Carretta DM, Silva AM, D’Agostino D, Topi S, Lovero R et al. (2021). Cardiac involvement in COVID-19 patients: A contemporary review. Infectious Disease Reports 13: 494–517. DOI 10.3390/idr13020048. [Google Scholar] [CrossRef]
Corsten MF, Heggermont W, Papageorgiou AP, Deckx S, Tijsma A et al. (2015). The microRNA-221/-222 cluster balances the antiviral and inflammatory response in viral myocarditis. European Heart Journal 36: 2909–2919. DOI 10.1093/eurheartj/ehv321. [Google Scholar] [CrossRef]
Dal Ferro M, Bussani R, Paldino A, Nuzzi V, Collesi C et al. (2021). SARS-CoV-2, myocardial injury and inflammation: Insights from a large clinical and autopsy study. Clinical Research in Cardiology 110: 1822–1831. DOI 10.1007/s00392-021-01910-2. [Google Scholar] [CrossRef]
Dennert R, Crijns HJ, Heymans S (2008). Acute viral myocarditis. European Society of Cardiology Journal 29: 2073–2082. DOI 10.1093/eurheartj/ehn296. [Google Scholar] [CrossRef]
di Domenico M, de Rosa A, Boccellino M (2021a). Detection of SARS-COV-2 proteins using an ELISA test. Diagnostics 11: 698. DOI 10.3390/diagnostics11040698. [Google Scholar] [CrossRef]
di Domenico M, de Rosa A, Di Gaudio F, Internicola P, Bettini C et al. (2021b). Diagnostic accuracy of a new antigen test for SARS-CoV-2 detection. International Journal of Environmental Research and Public Health 18: 6310. DOI 10.3390/ijerph18126310. [Google Scholar] [CrossRef]
Freaney PM, Shah SJ, Khan SS (2020). COVID-19 and heart failure with preserved ejection fraction. JAMA 324: 1499–1500. DOI 10.1001/jama.2020.17445. [Google Scholar] [CrossRef]
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P et al. (2009). International consensus group on cardiovascular magnetic resonance in myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC white paper. Journals of the American College of Cardiology 53: 1475–1487. DOI 10.1016/j.jacc.2009.02.007. [Google Scholar] [CrossRef]
Gathiram P, Mackraj I, Moodley J (2021). The renin-angiotensin system, hypertension, and SARS-CoV-2 infection: A review. Current Hypertension Reports 23: 17. DOI 10.1007/s11906-021-01134-9. [Google Scholar] [CrossRef]
Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC et al. (2020). Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circulation Research 126: 1456–1474. DOI 10.1161/CIRCRESAHA.120.317015. [Google Scholar] [CrossRef]
Hoebeeck J, Speleman F, Vandesompele J (2007). Real-time quantitative PCR as an alternative to Southern blot or fluorescence in situ hybridization for detection of gene copy number changes. Methods in Molecular Biology 353: 205–226. DOI 10.1385/1597452297. [Google Scholar] [CrossRef]
Ho JS, Sia CH, Chan MY, Lin W, Wong RC (2020). Coronavirus-induced myocarditis: A meta-summary of cases. Heart & Lung 49: 681–685. DOI 10.1016/j.hrtlng.2020.08.013. [Google Scholar] [CrossRef]
Hung MY, Hung MJ, Cheng CW (2007). Use of gallium 67 scintigraphy to differentiate acute myocarditis from acute myocardial infarction. Texas Heart Institute Journal 34: 305–309. [Google Scholar]
Iwasaki M, Saito J, Zhao H, Sakamoto A, Hirota K et al. (2021). Inflammation triggered by SARS-CoV-2 and ACE2 augment drives multiple organ failure of severe COVID-19: Molecular mechanisms and implications. Inflammation 44: 13–34. DOI 10.1007/s10753-020-01337-3. [Google Scholar] [CrossRef]
Kang M, An J (2021). Viral Myocarditis. Treasure Island (FLStatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459259/. [Google Scholar]
Katsuragi M, Yutani C, Mukai T, Arai Y, Imakita M et al. (1993). Detection of enteroviral genome and its significance in cardiomyopathy. Cardiology 83: 4–13. DOI 10.1159/000175941. [Google Scholar] [CrossRef]
Kearney MT, Cotton JM, Richardson PJ, Shah AM (2001). Viral myocarditis and dilated cardiomyopathy: Mechanisms, manifestations, and management. Postgraduate Medical Journal 77: 4–10. DOI 10.1136/pmj.77.903.4. [Google Scholar] [CrossRef]
Khan S, Rasool ST, Ahmed SI (2021). Role of cardiac biomarkers in COVID-19: What recent investigations tell us? Current Problems in Cardiology 46: 100842. DOI 10.1016/j.cpcardiol.2021.100842. [Google Scholar] [CrossRef]
Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M et al. (2012). Update on myocarditis. Journal of the American College of Cardiology 59: 779–792. DOI 10.1016/j.jacc.2011.09.074. [Google Scholar] [CrossRef]
Könemann S, Beug D, Dörr M, Felix SB (2020). Update myokarditis [Update Myocarditis]. Deutsche Medizinische Wochenschr 145: 166–170. DOI 10.1055/a-1000-6968. [Google Scholar] [CrossRef]
Kong P, Christia P, Frangogiannis NG (2014). The pathogenesis of cardiac fibrosis. Cellular and Molecular Life Science 71: 549–574. DOI 10.1007/s00018-013-1349-6. [Google Scholar] [CrossRef]
Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T et al. (2005). High prevalence of viral genomes and multiple viral infections in the myocardium of adults with. Circulation 111: 887–893. DOI 10.1161/01.CIR.0000155616.07901.35. [Google Scholar] [CrossRef]
Krejci J, Mlejnek D, Sochorova D, Nemec P (2016). Inflammatory cardiomyopathy: A current view on the pathophysiology, diagnosis, and treatment. BioMed Research International Publishes 2016: 4087632–4087611. DOI 10.1155/2016/4087632. [Google Scholar] [CrossRef]
Lazzerini PE, Boutjdir M, Capecchi PL (2020). COVID-19, arrhythmic risk, and inflammation: Mind the gap! Circulation 142: 7–9. DOI 10.1161/CIRCULATIONAHA.120.047293. [Google Scholar] [CrossRef]
Lopez-Jimenez F, Goldman L, Sacks DB, Thomas EJ, Johnson PA et al. (1997). Prognostic value of cardiac troponin T after noncardiac surgery: 6-month follow-up data. Journals of the American College of Cardiology 29: 1241–1245. DOI 10.1016/S0735-1097(97)82754-4. [Google Scholar] [CrossRef]
Lovreglio P, Bukvic N, Fustinoni S, Ballini A, Drago I et al. (2006). Lack of genotoxic effect in workers exposed to very low doses of 1,3-butadiene. Archives of Toxicology 80: 378–381. DOI 10.1007/s00204-005-0046-0. [Google Scholar] [CrossRef]
Liu P, Martino T, Opavsky MA, Penninger J (1996). Viral myocarditis: Balance between viral infection and immune response. Canadian Journal of Cardiology 12: 935–943. [Google Scholar]
Liu J, Deswal A, Khalid U (2021). COVID-19 myocarditis and long-term heart failure sequelae. Current Opinion in Cardiology 36: 234–240. DOI 10.1097/HCO.0000000000000832. [Google Scholar] [CrossRef]
Maisch B (2019). Cardio-immunology of myocarditis: Focus on immune mechanisms and treatment options. Frontiers in Cardiovascular Medicine 6: 48. DOI 10.3389/fcvm.2019.00048. [Google Scholar] [CrossRef]
Maisch B, Richter A, Sandmöller A, Portig I, Pankuweit S (2005). BMBF-heart failure network. inflammatory dilated cardiomyopathy (DCMI). Herz Kardiovaskulre Erkrankungen 30: 535–544. DOI 10.1007/s00059-005-2730-5. [Google Scholar] [CrossRef]
Meessen H, Müntefering H, Schmidt WA, Müller-Ruchholty ER, Keeker WR (1975). Virus-induced damage of the myocardial cell. Recent Advances in Studies on Cardiac Structure and Metabolism 6: 525–533. [Google Scholar]
Nadjiri J, Zaschka AL, Straeter AS, Sauter A, Englmaier M et al. (2019). Evaluation of a shortened cardiac MRI protocol for left ventricular examinations: Diagnostic performance of T1-mapping and myocardial function analysis. BMC Medical Imaging 19: 57. DOI 10.1186/s12880-019-0358-9. [Google Scholar] [CrossRef]
Olson KR (2018). Poisoning & Drug Overdose (7th Edition). McGraw-Hill Education. [Google Scholar]
Padilla-Ortiz AL, Ibarra D (2018). Lung and heart sounds analysis: State-of-the-art and future trends. Critical Reviews™ in Biomedical Engineering 46: 33–52. DOI 10.1615/CritRevBiomedEng.2018025112. [Google Scholar] [CrossRef]
Pandey S, Rajasurya V (2021). Nonviral Myocarditis. Treasure Island (FLStatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK536928/. [Google Scholar]
Pennisi L, Lepore A, Gagliano-Candela R, Santacroce L, Charitos IA (2020). A report on mushrooms poisonings in 2018 at the Apulian regional poison center: Mushrooms poisonings in 2018. Open Access Macedonian Journal of Medical Sciences 8: 616–622. [Google Scholar]
Perrotta F, Matera MG, Cazzola M, Bianco A (2020). Severe respiratory SARS-CoV2 infection: Does ACE2 receptor matter? Respiratory Medicine 168: 105996. DOI 10.1016/j.rmed.2020.105996. [Google Scholar] [CrossRef]
Perera P, Lobo V, Williams SR, Gharahbaghian L (2014). Cardiac echocardiography. Critical Care Clinics 30: 47–92. DOI 10.1016/j.ccc.2013.08.003. [Google Scholar] [CrossRef]
Roldán Torres I, Baello Monge P, Sevilla Toral B, Salvador Sanz A, Salim Martínez M et al. (2003). Valor pronóstico de la tropinina T en pacientes hospitalizados con angina o infarto sin elevación del segmento ST [Prognostic value of troponin T in hospitalized patients with angina or non-ST-segment elevation myocardial infarction]. Revista Española de Cardiología 56: 35–42. DOI 10.1016/S0300-8932(03)76819-5. [Google Scholar] [CrossRef]
Santacroce L, Charitos IA, Del Prete R (2020a). COVID-19 in Italy: An overview from the first case to date. Electronic Journal of General Medicine 17: em235. DOI 10.29333/ejgm/7926. [Google Scholar] [CrossRef]
Santacroce L, Charitos IA, Ballini A, Inchingolo F, Luperto P et al. (2020b). The human respiratory system and its microbiome at a glimpse 2020b. Biology 1: E318. DOI 10.3390/biology9100318. [Google Scholar] [CrossRef]
Santacroce L, Charitos IA, Carretta DM, de Nitto E, Lovero R (2021). The human coronaviruses (HCoVs) and the molecular mechanisms of SARS-CoV-2 infection. Journal of Molecular Medicine 99: 93–106. DOI 10.1007/s00109-020-02012-8. [Google Scholar] [CrossRef]
Santacroce L, D’agostino D, Charitos IA, Bottalico L, Ballini A (2018). A short review about electrophysiology and bioimpedance: History and perspectives. Indian Journal of Public Health Research & Development 9: 577–591. DOI 10.5958/0976-5506.2018.01521.8. [Google Scholar] [CrossRef]
Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE (2002). Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology 98: 33–39. DOI 10.1159/000064682. [Google Scholar] [CrossRef]
Schirinzi A, Cazzolla AP, Lovero R, Lo Muzio L, Testa NF et al. (2020). New insights in laboratory testing for COVID-19 patients: Looking for the role and predictive value of human epididymis secretory protein 4 (HE4) and the innate immunity of the oral cavity and respiratory tract. Microorganisms 8: 1718. DOI 10.3390/microorganisms8111718. [Google Scholar] [CrossRef]
Schultz JC, Hilliard AA, Cooper LTJr, Rihal CS (2009). Diagnosis and treatment of viral myocarditis. Mayo Clinic Proceedings 84: 1001–1009. DOI 10.1016/S0025-6196(11)60670-8. [Google Scholar] [CrossRef]
Shauer A, Gotsman I, Keren A, Zwas DR, Hellman Y et al. (2013). Acute viral myocarditis: Current concepts in diagnosis and treatment. Israel Medical Association Journal 15: 180–185. [Google Scholar]
Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LTJr, Chahal CAA (2020). Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 17: 1463–1471. DOI 10.1016/j.hrthm.2020.05.001. [Google Scholar] [CrossRef]
Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G et al. (2020). Elevated troponin in patients with coronavirus disease 2019: Possible mechanisms. Journal of Cardiac Failure 26: 470–475. DOI 10.1016/j.cardfail.2020.04.009. [Google Scholar] [CrossRef]
Thomas TP, Grisanti LA (2020). The dynamic interplay between cardiac inflammation and fibrosis. Frontiers in Physiology 11: 529075. DOI 10.3389/fphys.2020.529075. [Google Scholar] [CrossRef]
Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT et al. (2019). Management of myocarditis-related cardiomyopathy in adults. Circulation Research 124: 1568–1583. DOI 10.1161/CIRCRESAHA.118.313578. [Google Scholar] [CrossRef]
Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT et al. (2021). Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nature Reviews Cardiology 18: 169–193. DOI 10.1038/s41569-020-00435-x. [Google Scholar] [CrossRef]
Trentadue R, Fiore F, Massaro F, Papa F, Iuso A et al. (2012). Induction of mitochondrial dysfunction and oxidative stress in human fibroblast cultures exposed to serum from septic patients. Life Sciences 91: 237–243. DOI 10.1016/j.lfs.2012.06.041. [Google Scholar] [CrossRef]
Valiton V, Carballo D, Seebach JD, Meyer P (2020). La myocardite en 2020 [Myocarditis in 2020]. Revue Médicale Suisse 16: 1133–1139. [Google Scholar]
Wang J, Han B (2020). Dysregulated CD4+ T Cells and microRNAs in Myocarditis. Frontiers in Immunology 11: 539. DOI 10.3389/fimmu.2020.00539. [Google Scholar] [CrossRef]
WHO (2021). Coronavirus (COVID-19) dashboard overview. https://covid19.who.int/. [Google Scholar]
Xu RY, Zhu XF, Yang Y, Ye P (2013). High-sensitive cardiac troponin T. Journal of Geriatric Cardiology 10: 102–109. DOI 10.3969/j.issn.1671-5411.2013.01.015. [Google Scholar] [CrossRef]
Yehualashet AS, Belachew TF (2020). ACEIs and ARBs and their correlation with COVID-19: A Review. Infection and Drug Resistance 13: 3217–3224. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |