DOI:10.32604/biocell.2022.019304

| BIOCELL DOI:10.32604/biocell.2022.019304 |  |
| Article |
Claroideoglomus etunicatum improved the growth and saline–alkaline tolerance of Potentilla anserina by altering physiological and biochemical properties
College of Landscape Architecture, Northeast Forestry University, Harbin, 150040, China
*Address correspondence to: Chunxue Yang, senxiu99@163.com
Received: 15 September 2021; Accepted: 13 December 2021
Abstract: To investigate the effects of arbuscular mycorrhizal (AM) fungi on the growth and saline–alkaline tolerance of Potentilla anserina L., the seedlings were inoculated with Claroideoglomus etunicatum (W.N. Becker & Gerd.) C. Walker & A. Schüßler in pot cultivation. After 90 days of culture, saline–alkaline stress was induced with NaCl and NaHCO3 solution according to the main salt components in saline–alkaline soils. Based on the physiological response of P. anserina to the stress in the preliminary experiment, the solution concentrations of 0 mmol/L, 75 mmol/L, 150 mmol/L, 225 mmol/L and 300 mmol/L were treated with stress for 10 days, respectively. The mycorrhizal colonization rate, mycorrhizal dependence, chlorophyll content, malondialdehyde content, antioxidant enzyme activities, osmoregulation substances content and water status were measured. The results showed that with the increase of NaCl and NaHCO3 stress concentration, mycorrhizal colonization rate, colonization intensity, arbuscular abundance and vesicle abundance decreased, and reached the lowest value at 300 mmol/L. Strong mycorrhizal dependence was observed after the symbiosis with AM fungus, and the dependence was higher under NaHCO3 treatment. Under NaCl and NaHCO3 stress, inoculation with AM fungus could increase chlorophyll content, decrease malondialdehyde content, increase activities of superoxide dismutase, peroxidase and catalase, increase contents of proline, soluble sugar and soluble protein, increase tissue relative water content and decrease water saturation deficit. It was concluded that salt–alkali stress inhibited the colonization of AM fungus, but the mycorrhiza still played a positive role in maintaining the normal growth of plants under salt–alkali stress.
Keywords: Arbuscular mycorrhizal fungi; Colonization characteristics; Antioxidant system; Osmoregulation substances; Water status
Soil salinization is a worldwide environmental problem. Relevant data show that saline–alkaline land accounts for more than 7% of the world’s total land area (Evelin et al., 2009). Moreover, by 2050, more than 50% of cultivated land will be seriously salinized (Wang et al., 2003). Excessive salinity (especially NaCl) in these saline–alkaline soils is the main abiotic stress factor restricting plant growth and development (Asish and Anath, 2005), and the high pH of alkaline salts (mainly NaHCO3 and Na2CO3) will cause more serious damage to plant cells (Shi and Wang, 2005). Saline–alkaline stress leads to membrane peroxidation, increases membrane permeability, and exudes small molecular substances and ions in cells, resulting in increased osmotic potential and water outflow of plants, further leading to osmotic stress (Zhang et al., 2019; Balliu et al., 2015); In addition, the stability of cell membrane structure and some biological macromolecules (such as enzymes and proteins) will be destroyed, resulting in a series of physiological and biochemical metabolic disorders in plants (Dinneny, 2015). In order to reduce the influence of saline–alkaline stress on plant growth, many technical measures such as physical, chemical and biological methods have been adopted. Among them, the biological method using arbuscular mycorrhizal (AM) fungi to enhance the tolerance of plants is considered to be a green and efficient method, which plays an irreplaceable role in the ecosystem (Zhang et al., 2019; Deng et al., 2019). Therefore, it is necessary to screen and apply more mycorrhizal symbionts with salt–alkali tolerance.
AM fungi, one of the largest components of the soil microbial community, which account for more than 10% of the total biomass of soil microorganisms (Fitter et al., 2011). About 90% of vascular plants can be colonized by AM fungi and form a symbiotic system (Lanfranco and Yong, 2012), through which plants provide carbohydrates whereas the vast mycelium system of AM fungi improves plant absorption of water and mineral nutrients, and the two are mutually beneficial (Wang et al., 2018a). In addition, AM fungi naturally exist in the saline environment, which can affect the metabolic process of plants in different ways, promote plant growth and development, and improve the ability of plants to resist saline–alkaline stress (Becerra et al., 2014; Estrada et al., 2013; Parvin et al., 2020). An increasing number of studies have clearly demonstrated that Claroideoglomus etunicatum (W.N. Becker & Gerd.) C. Walker & A. Schüßler, a common AM fungus, is of great value to host plants (such as Prunus maritima, Trifolium repens, and Puccinellia distans) in saline habitats, including promoting the absorption of water and nutrition, the enhancement of photosynthesis and the activation of antioxidant system and so on (Zai et al., 2013; Huang et al., 2017; Dashtebani et al., 2014). More importantly, C. etunicatum can be isolated in saline soil, so symbiosis with plants to improve salinizationmay have great application potential (Yang et al., 2015; Wang and Liu, 2002).
Potentilla anserina L., a perennial herb of Rosaceae, has high ornamental value and can be used as a low maintenance ground cover (Yan et al., 2020). Besides, P. anserina can rapidly occupy patch grassland and secondary bare land through asexual propagation and it is also a halophyte with great development and utilization potential (Wang et al., 2015). This important salt–tolerant germplasm could be found in the lakeside saline–alkaline land of Qinghai Lake in China (salt concentration up to 2400 ds·cm−1) and in the saline–alkaline grassland of Songnen with pH up to 8.4, and be considered as an ideal candidate for maintaining the sustainability of saline soils (Su et al., 2014; Wang and Yang, 2020; Khan et al., 2009). Therefore, the combination of P. anserina and AM fungi may provide a new idea for the restoration of saline–alkaline land by mycorrhizal technology. However, related studies only reported the spore morphological diversity of AM fungi in the rhizosphere of P. anserina, and the mechanism of how AM fungi affect the plant growth of P. anserina was not reported (Wang and Yang, 2020). Based on this, the work simulated saline–alkaline stress with different concentrations of NaCl and NaHCO3 solutions, and took C. etunicatum as AM fungus inoculum to explore the mechanism of AM fungi on the growth and saline–alkaline tolerance of P. anserina from the aspects of physiological and biochemical characteristics, which provided a theoretical basis for the application of AM –P. anserina symbiotic system in saline–alkaline land greening. For this purpose, we put forward hypotheses: 1) saline–alkaline stress has negative effects on the colonization of AM fungi and P. anserina; 2) AM fungi may play an important role in promoting the growth, physiology and biochemistry of P. anserina under saline–alkaline stress.
The AM fungus (C. etunicatum) used in mycorrhizal treatment was provided by the Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry Sciences, China. The AM fungus inoculum consisted of hyphae and spores (around 18 spores/g). P. anserina was introduced from Songnen grassland in Zhaodong City, Heilongjiang Province, China (located at 125°54' E, 46°2' N) in 2017, and cultivated in the seedling cultivation base of Northeast Forestry University. The seedlings that germinated in the year of the experiment were collected, which with the same status and vigorous growth were selected and cultured in sterile water for 5 days after cleaning the residual soil of their roots for seedling adaptation and recovery. After that, the lateral roots and some main roots of the seedlings were cut off, leaving only about 3 cm long main roots to avoid the influence of the original rhizosphere soil and microorganisms. The roots of seedlings were washed with sterile water 3 times for experiment. The culture substrate (pH = 5.96, EC = 179 us·cm−1, 304.18 g·kg–1 of soil organic matter, 129.53 mg·kg−1 of alkali hydrolyzable nitrogen, 58.27 mg·kg−1 of available phosphorus, and 142.65 mg·kg−1 of available potassium) was vermiculite, river sand and peat soil, mixed evenly in the volume ratio of 1:1:3, and autoclaved (121°C, 2 h). The specification of pot for cultivating seedlings is 15 cm × 15 cm, sterilized under the same conditions.
Culture of mycorrhizal seedlings
The experiment design included AM fungal inoculation and saline–alkali stress. The treatment of AM fungi level was divided into inoculation treatment (AM group) and non–inoculation treatment (N–AM group). In the AM group, after 400 g substrate was added to the pot, 20 g inoculum was evenly mixed in 2 cm soil on the surface of each pot, while 20 g sterilized inoculum and filtrate were added to the N–AM group. As for stress treatment, according to the physiological response value of P. anserina to saline–alkaline stress obtained in the pre–experiment, 4 stress gradients of NaCl solution and NaHCO3 solution with concentrations of 75 mmol/L, 150 mmol/L, 225 mmol/L, and 300 mmol/L were set respectively. At the same time, distilled water (0 mmol/L) was used as the control treatment. There were 18 treatments in the present research, and each treatment was repeated 6 times. Therefore, 108 pots of plants were randomly arranged. The cultivation of mycorrhizal seedlings is carried out in the plant light culture laboratory. The cultural environment was: the average temperature in the day and night was 24°C, the air relative humidity was 60%–70%, the light intensity was 8000lx, the light time was 14 h a day, the distilled water was watered regularly to keep the soil water content at 55%–70% of the maximum field water capacity. When the seedlings were 90 days old, stress treatment began. In order to avoid the impact effect of salt–alkali, referring to the method of Yang et al. (2014), the NaCl solution and NaHCO3 solution were increased by a gradient of 50 mmol/L every day until the predetermined concentrations were reached. Then, each pot was irrigated with 100 ml of different stress solutions at the predetermined concentrations every day for 10 consecutive days. After that, the colonization indexes, growth indexes, as well as physiological and biochemical indexes were measured.
Observation of colonization characteristics
The fresh lateral roots of mycorrhizal seedlings were cleaned and cut into 1 cm segments, then soaked in FAA fixed solution (5 ml formalin, 5 ml glacial acetic acid, 90 ml 70% alcohol), labeled and stored at 4°C. The AM fungal colonization rate was measured as described by Philips and Hayman (1970). The root segments were washed 3–5 times until the pungent smell was not detected, after which they were incubated in a 10% KOH solution at 90°C for 60 min to make them soft and transparent, then decolorized in alkaline hydrogen peroxide for 15 min. After that, the roots were neutralized with 2% hydrochloric acid for 5–10 min, and then stained at 90°C for 30 min with glycerol lactate reagent containing 0.05% trypan-blue. The morphological structures of hypha, arbuscules and vesicles were examined under an optical microscope (OLYMPUS-DSX500). The colonization status was assessed and graded using the method of Trouvelot et al. (1986), after which the colonization rate (%), arbuscular abundance (%), vesicle abundance (%) and colonization intensity (%) were determined using MYCOCALC software.
Growth indexes and mycorrhizal dependency
The fresh weight (FW), dry weight (DW), and saturated fresh weight (SFW) of the whole seedlings were determined by the weighing method. The mycorrhizal dependency (MD) was calculated according to the formula (1) (Gong et al., 2000):
MD of P. anserina was divided into the following four levels:
(1) MD < 1: The host plants showed no dependency on AM fungi, that is, AM fungi played little role on plant individual biomass accumulation;
(2) 1 < MD < 2 (Grade I): The host plants depended on AM fungi to a certain extent, that is, AM fungi could promote the individual biomass accumulation of host plants;
(3) 2 < MD < 3 (Grade II): The host plants had moderate dependence on AM fungi;
(4) MD ≥ 3 (Grade III): The host plants had a strong dependence on AM fungi.
Physiological and biochemical indexes
After stress treatment, according to the method described by Wang (2006), the chlorophyll content was determined by the 95% ethanol extraction method and the content of malondialdehyde (MDA) was determined by the thiobarbituric acid method, the superoxide dismutase (SOD) activity was determined by the NBT method, the activity of peroxidase (POD) was determined by the guaiacol method, the activity of catalase (CAT) was determined by the permanganate titration, and the content of proline, soluble protein, and soluble sugar were determined by the acid ninhydrin method, Coomassie brilliant blue G–250 staining, and anthrone colorimetry method. Relative water content (RWC) and water saturation deficit (WSD) were measured according to the method described by Barr and Weatherley (1962) to analyze the water status of leaves, and the formulas are as follows:
SPSS (Statistical Product and Service Solutions) 22.0 was used to test the normality and homogeneity of the data, and to compare and analyze the data between each treatment. When variance was homogeneous, one-way ANOVA with Duncan’s multiple range test was used to analyze the average value and the significant difference between the treatments, and two-way ANOVA was used to analyze the effects of the stresses, AM fungi and their interactions on the growth and physiology of P. anserina. When the variance was not homogeneous, ARtool of the R Programming Language (version 3.6.3) was used for the non–parametric test. Spearman correlation coefficient was used to describe the correlation between mycorrhizal colonization indexes and the correlation between plant growth physiological parameters. When P < 0.05, the difference was statistically significant. The mean ± standard error (SE) was used to express the measurement results. Excel (2019) was used to make charts.
Mycorrhizal colonization characteristics
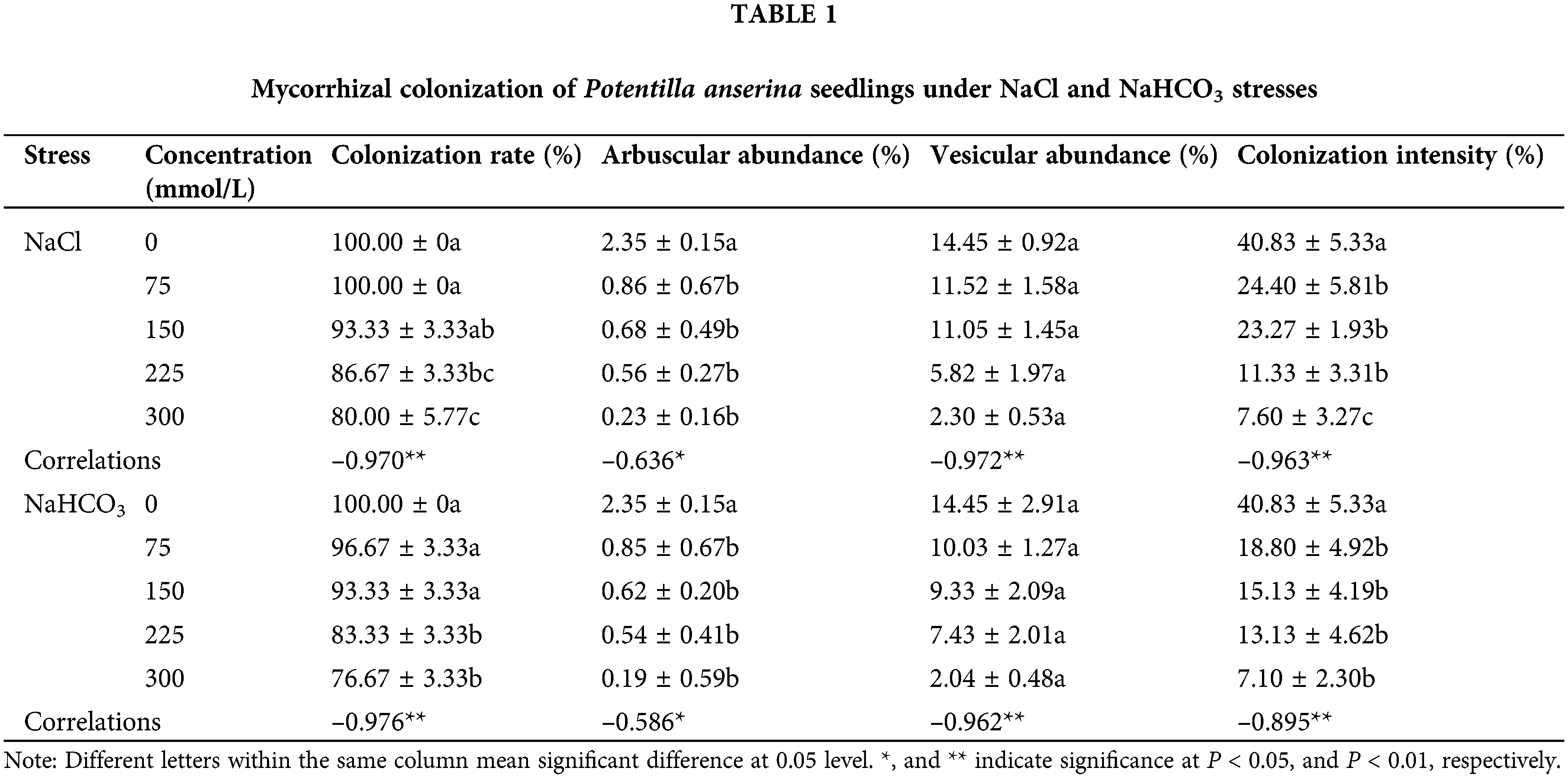
It was found that the roots were colonized to different degrees and formed an Arum–type mycorrhizal colonization. That is to say, after the external hyphae invaded into the root, the main hyphae (Fig. 1A) were formed between the cells in the root, and then the ends of some hyphae expanded to form elliptical (Figs. 1A and 1C), spherical (Fig. 1D), strip (Fig. 1D) vesicles and some endophytic hyphae in the root formed arbuscules (Fig. 1B). According to Table 1, hyphae were the most frequently observed structures in the stained root segments, vesicles were relatively less observed, and arbuscules were rarely observed.
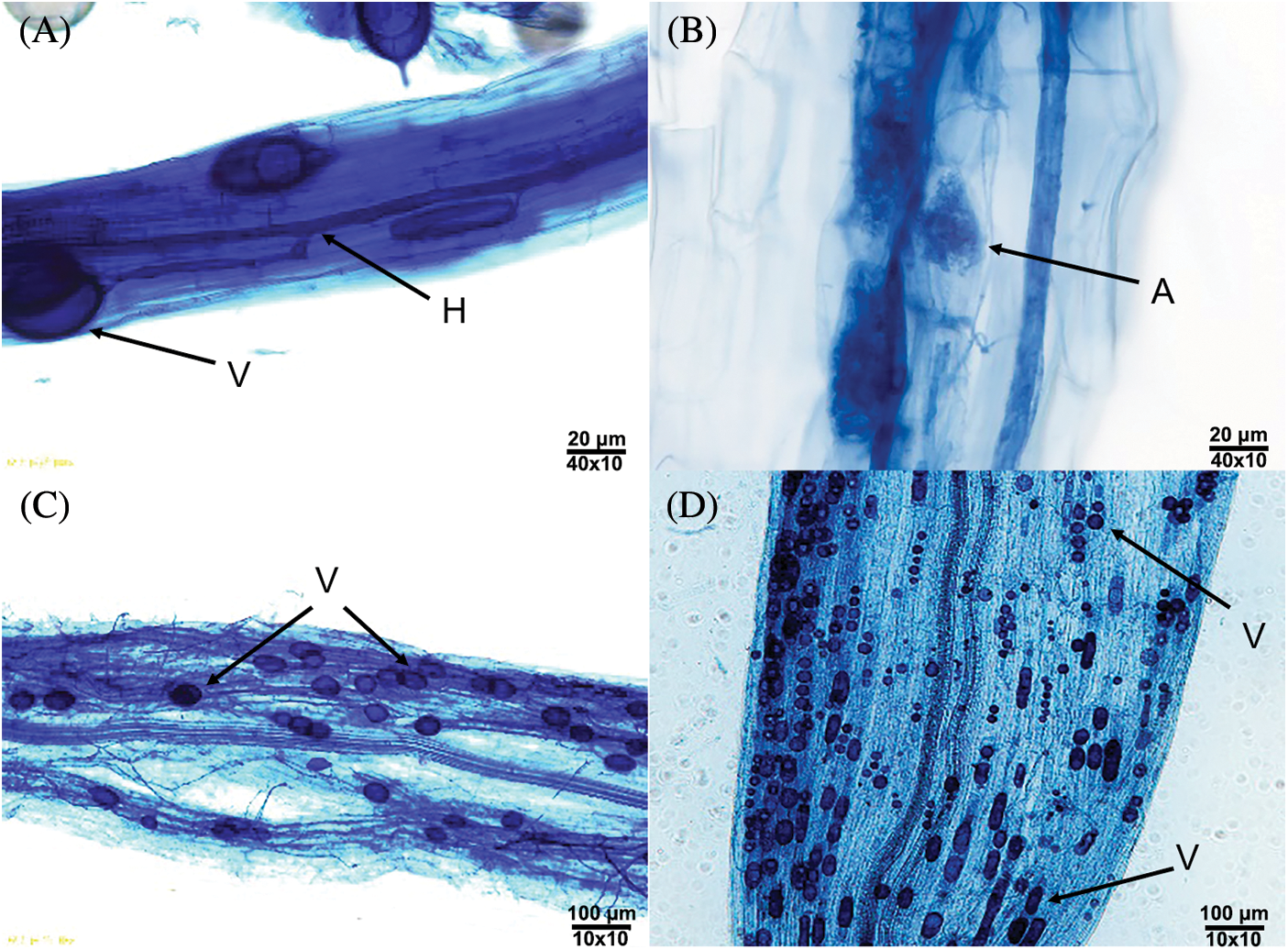
Figure 1: Mycorrhizal colonization of P. anserina seedlings (A–D). Note: H–Hypha, V–Vesicle, A–Arbuscule.
The colonization of AM fungi was inhibited by NaCl and NaHCO3 stresses, that is to say, colonization rate, arbuscular abundance, vesicular abundance, and colonization intensity showed a decreasing trend with the increase of stress, and reached the lowest value at 300 mmol/L (Table 1). Moreover, compared with the colonization rate under NaCl stress, the colonization rate under NaHCO3 treatment decreased to a greater extent. Correlation analysis showed that salt–alkali stress was significantly and negatively correlated with colonization rate, vesicle abundance and colonization intensity at P < 0.01 level, and with arbuscular abundance at P < 0.05 level (Table 1).
Plant growth and mycorrhizal dependence
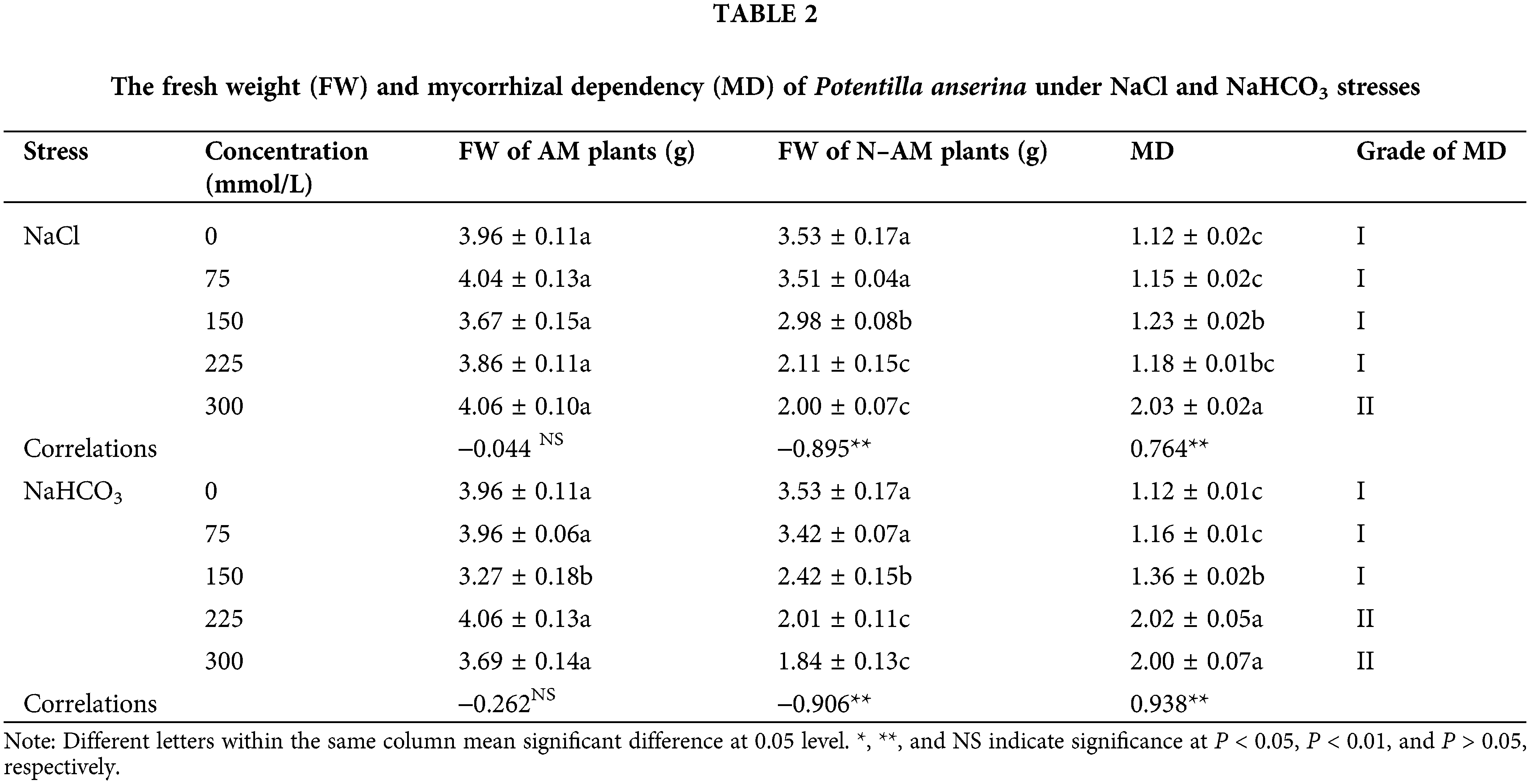
The MD of plants refers to the percentage of biomass increased by plants with the help of AM fungi, which can reflect the close relationship between plants and AM fungi. The higher the MD level is, the closer the relationship between plants and AM fungi is, and the greater the effect of AM fungi on plants is. The results (Table 2) showed that NaCl and NaHCO3 stresses had no significant effect on the FW of AM plants (P > 0.05), but had significant negative effect on the FW of N–AM plants (P < 0.01). There was a significant correlation between the stresses and MD (P < 0.01). Additionally, under the same stress treatment, the FW of AM plants was higher than that of N–AM. When the concentration of NaCl was 300 mmol/L, the FW of AM plants increased by 103% compared with that of N–AM plants, and the MD reached grade II. Under NaHCO3 stress, the FW increased by 102% and 100% at 225 mmol/L and 300 mmol/L, respectively, and the MD also reached grade II. Besides, the average value of MD was 1.6142 under NaHCO3 treatment and 1.3648 under NaCl treatment, indicating that P. anserina had a stronger dependence on AM fungi under NaHCO3.
Two–way ANOVA results showed that the chlorophyll content was significantly affected by AM fungi, the stresses and the interactions (P < 0.01; Table 3). With the increase of concentration, the chlorophyll content showed a decreasing trend under salt–alkali stress, and the reductions of the chlorophyll content in N–AM plants were higher than that in AM plants. For example, when NaCl concentration reached 300 mmol/L, compared to the control plants, the chlorophyll content of N–AM plants and AM plants decreased by 25.93% and 11.14%, respectively (Fig. 2A), which was more significant under the stress of the same concentration of NaHCO3, decreased by 44.87% and 20.81%, respectively (Fig. 2B). In addition, the chlorophyll content of AM plants was higher than that of N–AM plants except for the content at 75 mmol/L NaCl, which was 2.25% lower than the N–AM group.
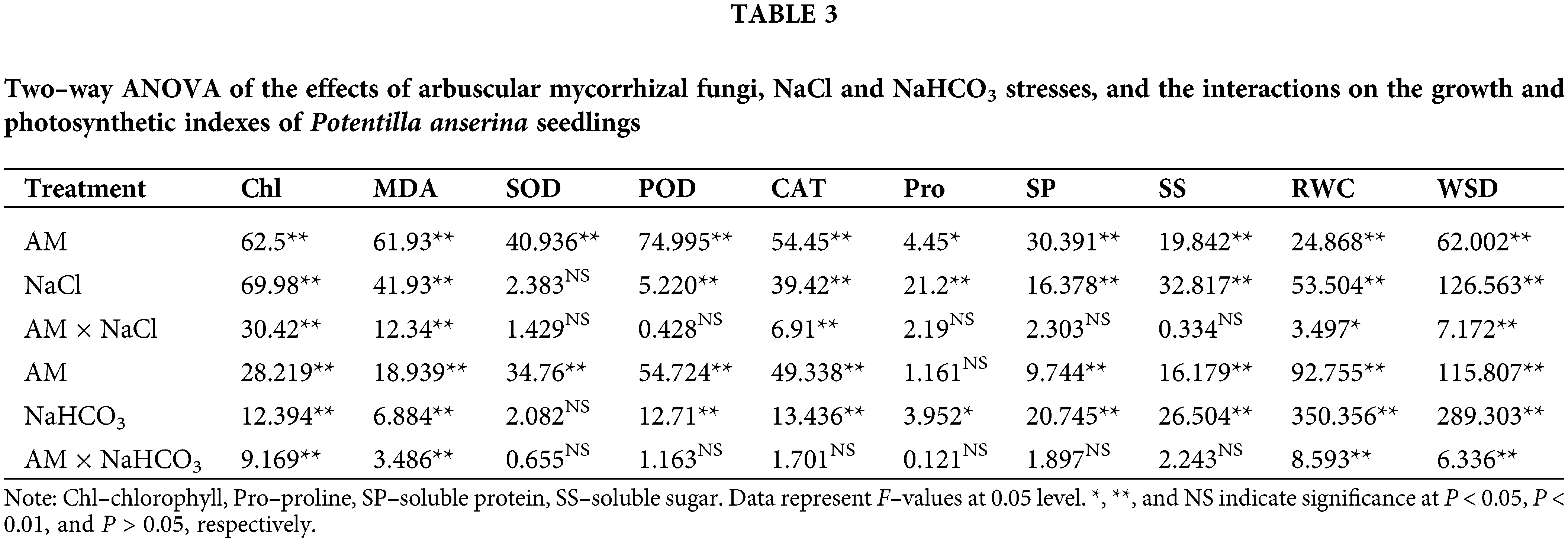
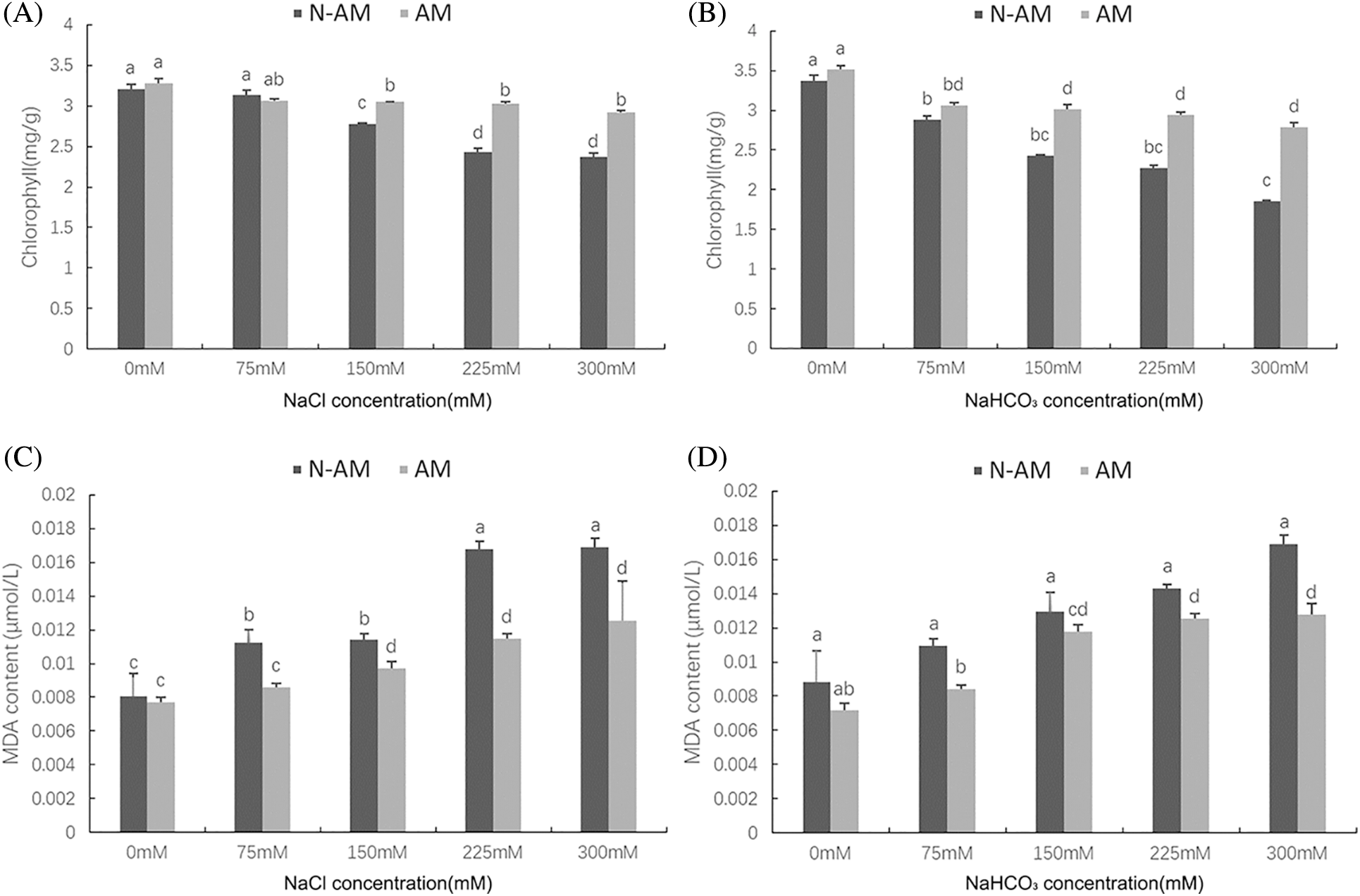
Figure 2: The effect of arbuscular mycorrhizal fungi on chlorophyll content (A, B) and MDA (C, D) content of P. anserina under NaCl and NaHCO3 stresses. Different small letters indicate significant difference in different treatments. The differences in each index were detected by one–way ANOVA at P < 0.05 level. Bars represent mean ± SE (n = 3).
Two-way ANOVA results showed that the MDA content was significantly affected by AM fungi, the stresses and the interactions (P < 0.01; Table 3). In the stress environment, the content of MDA of all plants increased with the increasing concentration, and mycorrhizal treatment resulted in a significant reduction compared to non–mycorrhizal treatment (Figs. 2C and 2D). For example, at 225 mmol/L NaCl and 300 mmol/L NaHCO3, the differences between AM groups and N–AM groups were more marked, with a decrease of 31.63% and 24.39%, respectively.
Compared with the N–AM seedlings, mycorrhizal treatment significantly (P < 0.01; Table 3; Fig. 3) raised the activities of SOD, POD, and CAT under NaCl and NaHCO3 stresses. At the same time, NaCl and NaHCO3 affected the POD and CAT activities of all plants to varying degrees (P < 0.01; Table 3), while SOD activity was not significantly affected by these two stresses (P > 0.05; Table 3). Additionally, NaCl and AM fungi had significant interaction effects on CAT (P < 0.01; Table 3).
With the increase of stress concentration, POD activity (Figs. 3C and 3D) and CAT (Figs. 3E and 3F) activity in both AM and N–AM plants showed a trend of first increasing and then decreasing, and reached the peak at 225 mmol/L. It was worth noting that the overall level of POD activity under NaHCO3 stress (Fig. 3D) was lower than that under NaCl stress (Fig. 3C).
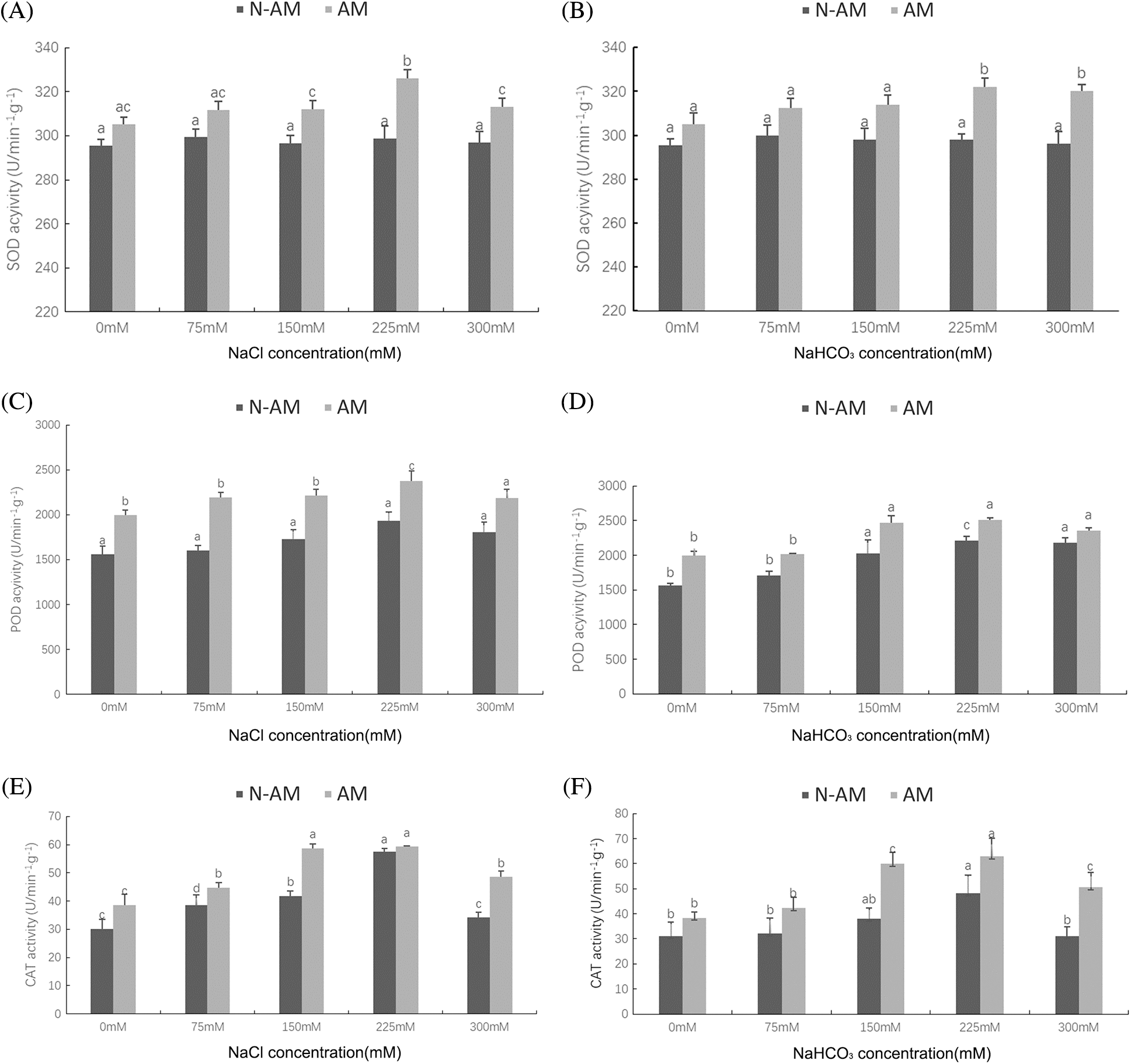
Figure 3: The effect of arbuscular mycorrhizal fungi on the activities of SOD (A, B), POD (C, D) CAT (E, F) of P. anserina under NaCl and NaHCO3 stresses. Different small letters indicate significant difference in different treatments. The differences in each index were detected by one–way ANOVA at P < 0.05 level. Bars represent mean ± SE (n = 3).
Two–way ANOVA results showed that the content of proline was affected by AM fungi under NaCl stress (P < 0.05; Table 3, Fig. 4A), which was not affected under NaHCO3 stress (P > 0.05; Table 3, Fig. 4B). For example, at the NaCl concentration of 150 mmol/L and 225 mmol/L, the proline content of inoculated plants was significantly increased by 10.59% and 8.39%, respectively, compared with the non–inoculated plants (P < 0.05; Fig. 4A).
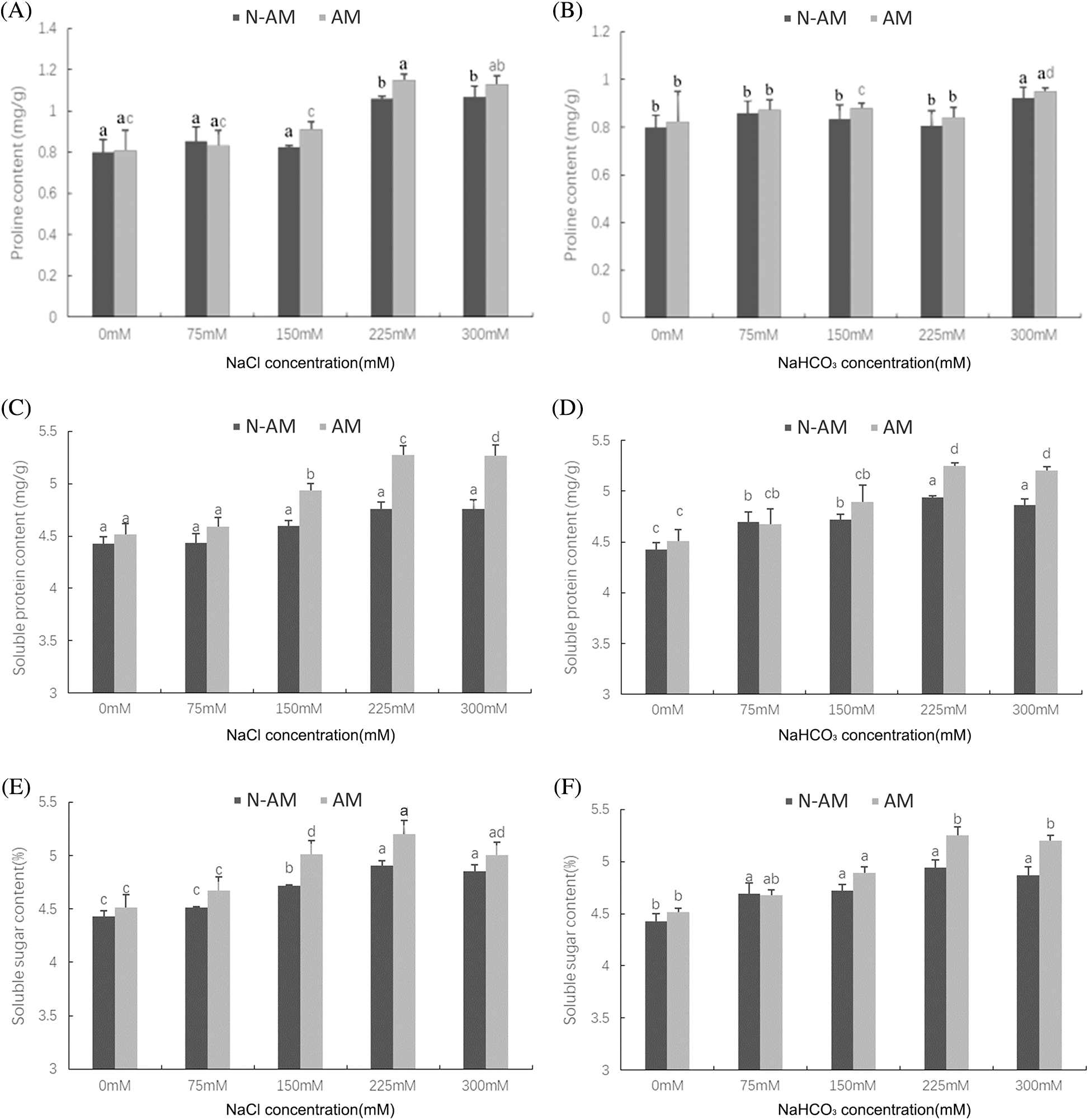
Figure 4: The effect of arbuscular mycorrhizal fungi on the contents of proline (A, B), soluble protein (C, D), and soluble sugar (E, F) of P. anserina under NaCl and NaHCO3 stresses. Different small letters indicate significant difference in different treatments. The differences in each index were detected by one–way ANOVA at P < 0.05 level. Bars represent mean ± SE (n = 3).
Unlike the proline, the contents of soluble protein and soluble sugar were affected by AM fungi, NaCl and NaHCO3 stresses (P < 0.01; Table 3), which were not affected by their interactions (P > 0.05; Table 3). For example, under 150 mmol/L, 225 mmol/L, and 300 mmol/L NaCl stress, AM fungi increased the content of soluble protein by 7.36%, 10.68% and 10.80% respectively compared to N–AM groups (P < 0.05; Fig. 4C). When the NaHCO3 concentration was 225 mmol/L and 300 mmol/L, AM fungi increased the content of soluble protein by 6.27% and 6.89%, respectively, compared to the N–AM groups (P < 0.05; Fig. 4D).
Two–way ANOVA results showed that the RWC and WSD were significantly affected by AM fungi, the NaCl and NaHCO3 stresses (P < 0.01; Table 3), which were significantly affected by the interaction between NaCl and AM fungi at P < 0.05 (Table 3), and significantly affected by the interaction between NaHCO3 and AM fungi at P < 0.01 (Table 3).
With the increasing stress concentration, the RWC (Figs. 5A and 5B) increased first and then decreased, while the WSD (Figs. 5C and 5D) decreased first and then increased. When NaCl and NaHCO3 concentration was 75 mmol/L, the RWC of N–AM and AM groups reached the highest value, and the WSD was the lowest value. In addition, under all concentrations of NaCl and NaHCO3 stresses, the RWC of AM groups was always higher than that of N–AM groups, and the WSD of AM groups was always lower than that of N–AM groups. For example, under 75 mmol/L NaCl and NaHCO3, the RWC in AM groups was 31.44% and 28.07% higher than that in N–AM groups, and the WSD was 17.47% and 15.56% lower, respectively.
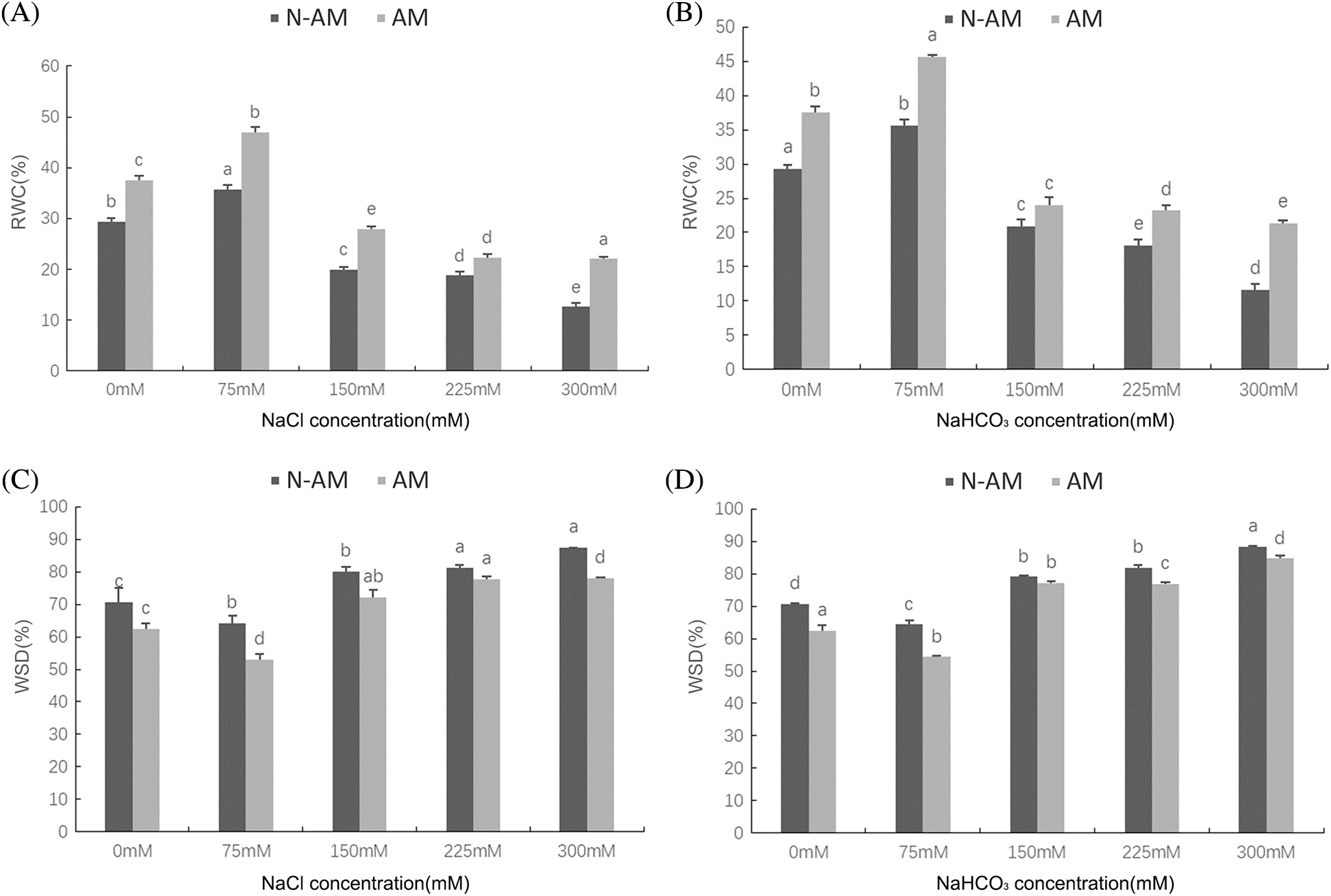
Figure 5: The effect of arbuscular mycorrhizal fungi on water the RWC (A, B) and WSD (C, D) of P. anserina under NaCl and NaHCO3 stresses. Different small letters indicate significant difference in different treatments. The differences in each index were detected by one–way ANOVA at P < 0.05 level. Bars represent mean ± SE (n = 3).
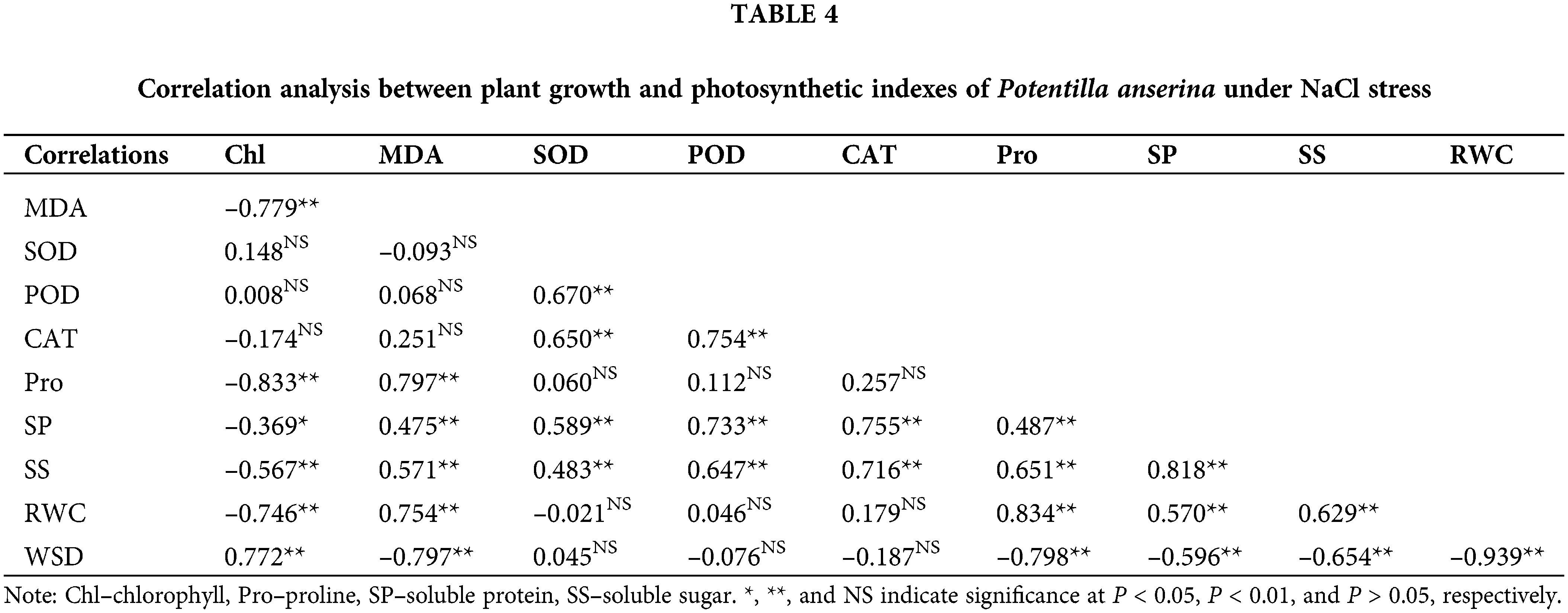
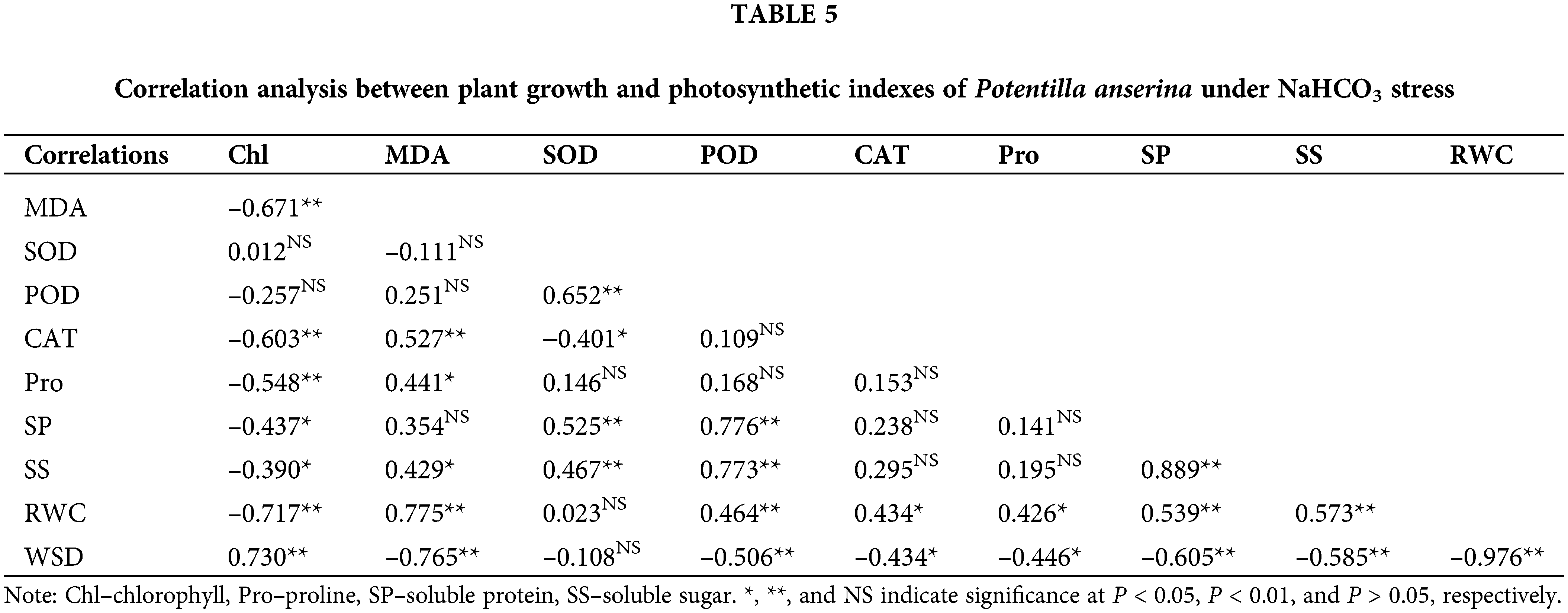
Correlation analysis among plant growth and photosynthetic indexes
Correlation analysis results (Tables 4 and 5) showed that the chlorophyll content had negative correlation with the content of MDA, osmoregulation substances as well as the RWC of NaCl and NaHCO3 stresses, and had significantly positive correlation with the WSD. Besides, the MDA content had obvious correlation with the water status and the content of osmoregulation substances except the soluble protein content of NaHCO3 stress. For the antioxidant enzymes, except CAT under NaHCO3 stress, others were not related to chlorophyll and MDA content. Moreover, the osmoregulation of P. anserina had significant positive correlation with the water status.
Effects of saline–alkaline stress on AM fungi colonization and seedling growth
In the current study, with the increase of salt and alkali stress intensity, the colonization of AM fungi in the rhizosphere decreased, which indicated that soil salinity not only caused stress to plants, but also directly hindered the growth of AM fungi, which resulted in the decreased colonization ability of AM fungi (Giri et al., 2007; López–Ráez, 2016; Bencherif et al., 2015). The effect of salt–alkali stress on mycorrhizal colonization was related to the inhibition of spore germination and hyphal growth on the one hand (Zhu et al., 2007). On the other hand, the decrease of chlorophyll content caused by saline alkali stress may reduce the accumulation of photosynthetic products and the distribution to roots, so as to reduce the nutrients required by AM fungi in roots, and finally inhibit the colonization of AM fungi (Deng et al., 2019). In addition, the colonization of mycorrhiza was reduced under alkali stress than under salt stress, which further indicated that the toxic effect of alkali stress with high pH was stronger, and the main reason might be that the root damage of plant was more serious under alkali stress with high pH. In addition to the reduced ability to absorb water and nutrients, a large number of harmful metabolites in plants secreted into the soil through roots, can also affect AM fungi colonization to a certain extent (Wang et al., 2018b).
Under the stress, compared with AM plants, FW of N–AM plants decreased, indicating that salinity inhibited plant growth, which might be due to the following reasons: 1) the saline–alkaline stress exacerbated membrane lipid peroxidation, damaged biofilm and its function in plants, slowed down or inhibited a series of physiological and biochemical reactions in plants, and finally weakened plant growth; 2) Membrane lipid peroxidation damaged chloroplast structure, led to the decrease of plant chlorophyll content and affected photosynthesis.
Interestingly, even if mycorrhizal stress affected mycorrhizal, AM fungi could still improve the FW of P. anserina under salt and alkali stress. This might be the fact that AM fungi to a certain extent, increased the absorption area of P. anserina root system, promoted the absorption of nutrients and water by host plants, and formed a certain defense mechanism against saline–alkaline stress (Augé, 2001). At the same time, AM fungi also increased the chlorophyll content of P. anserina. It was likely that under the action of AM fungi, the inhibitory effect of Na+ on the absorption of mineral elements such as Mg2+ by plants was weakened, which reduced the effect of stress on chlorophyll synthesis and was conducive to the accumulation of dry matter (Giri et al., 2003; Estrada et al., 2013). This further explained the mycorrhizal dependence of P. anserina under saline–alkaline stress.
Effects of AM fungi on antioxidant system of P. anserina under saline–alkaline stress
As with other abiotic stresses, when plants are subjected to saline–alkaline stress, the dynamic balance between the production and clearance of reactive oxygen species (ROS) will be destroyed, resulting in membrane lipid peroxidation and the production of a large amount of MDA, whose content can reflect the stress damage degree of plants (Zhang et al., 2008). At this time, the activity of antioxidant enzymes in plants will be increased, and directly react with ROS under saline–alkali stress, effectively preventing the accumulation of ROS (Pandey and Garg, 2017). The present study found that the colonization of AM fungi effectively increased the activities of SOD, POD and CAT and reduced the production of MDA under salt–alkali stress, which was consistent with some previous studies (Xu et al., 2017; Huang et al., 2011). In addition, it was also found that even without stress, the enzyme activities of plants inoculated with AM fungi was still higher than that of N–AM plants, which showed that the stimulation of SOD, POD and CAT activities of P. anserina came from AM fungi, which was consistent with He et al. (2007). Accordingly, mycorrhiza could stimulate the activity of antioxidant enzymes, reduce the degree of membrane lipid peroxidation and oxidative damage to some extent, which was an effective defense mechanism induced by AM fungi under a certain concentration of saline–alkaline stress, thus improving the saline–alkaline tolerance of P. anserina.
Effects of AM fungi on osmoregulation substances of P. anserina under saline–alkaline stress
Osmotic regulation is also one of the basic characteristics of plant adaptation to salt and alkali stress. Under stress, plant cells accumulate some substances, such as proline and soluble sugar, to regulate the osmotic potential of cells, maintain water balance and protect the activities of many enzymes in cells (Yang et al., 2006). A series of studies have previously reported that inoculation with AM fungi can promote the accumulation of osmoregulation substances in plants. For example, the experiment of Funneliformis mosseae colonizing alfalfa (Medicago sativa) has proved that AM fungi has a positive effect on the accumulation of proline, soluble sugar and soluble protein (Cai et al., 2019). In current study, saline–alkaline stress promoted the synthesis of proline, soluble sugar and soluble protein, indicating that P. anserina itself could improve endurance through the accumulation of osmoregulation substances and had the ability of self–regulation. Besides, mycorrhizal treatment generally increased the content of these substances, which provided direct evidence that AM fungi improved the salt and alkali resistance of seedlings by promoting the accumulation of osmoregulation substances. By contrast, Zhang et al. (2018) reported that inoculation with Funneliformis mosseae increased the content of soluble sugar and soluble protein of Ligustrum lucidum under salt stress, but decreased the content of proline. In addition, Rabie and Almadini (2005) suggested that the proline content of Vicia faba under salinity stress also decreased after inoculation with AM fungi. Therefore, it was concluded that proline might play only a relatively minor role in plant osmotic regulation, and the influence mechanism of AM fungi on proline accumulation in plants under salt stress was complex and needed further exploration (Carillo et al., 2008).
From the perspective of water status, the result of the study clearly showed that AM symbiosis improved plant water use, which was in agreement with the results of Sheng et al. (2008). The RWC of mycorrhizal P. anserina increased significantly and the WSD decreased significantly in the presence of salinity and alkalinity stress, which also reflected indirectly that the accumulation of osmoregulation substances in P. anserina could make the water potential of plant cells lower than that of the outside in high–salt environment, so as to keep the cells filled with water and maintain the water balance in plants. In addition, the enhancement of water use efficiency by colonization was also directly attributed to the huge hyphal system, that is, AM fungal hyphae contributed to plants absorbing the water in the soil pores that could not be absorbed by the normal root system (Augé, 2001), and the water transfer rate in hyphae was faster than that in roots, which was also conducive to plant water uptake (Garcia and Mendoza, 2007).
In short, the assumptions we put forward are valid. The results of our inoculation test showed that due to high pH, alkalinity stress had a stronger inhibitory effect on mycorrhizal colonization, but the positive effect of C. etunicatum on P. anserina was not weakened. The colonization of C. etunicatum could promote the growth of P. anserina and improve its saline–alkaline tolerance. The results of this study laid a foundation for further understanding the tolerance of AM fungi–P. anserina, and provided a theoretical basis for the application of the symbiont in saline–alkaline land greening. However, there are still many deficiencies in the current study, such as the effects of salt–alkali stress on the hyphal growth in the soil and the effects of AM fungi on the nutritional elements of P. anserina under salt–alkali stress, etc., which are worth further study.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Chunxue Yang; data collection: Yudan Wang; analysis and interpretation of results: Yajie Liu; draft manuscript preparation: Yunhui Zhou. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was supported by the National Natural Science Foundation of China (31601986), and Heilongjiang Postdoctoral Scientific Research Developmental Fund (LBH–Q16005).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Asish KP, Anath BD (2005). Salt tolerance and salinity effects on plants: A review. Ecotoxicology and Environmental Safety 60: 324–349. DOI 10.1016/j.ecoenv.2004.06.010. [Google Scholar] [CrossRef]
Augé RM (2001). Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11: 3–42. DOI 10.1007/s005720100097. [Google Scholar] [CrossRef]
Balliu A, Sallaku G, Rewald B (2015). AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 7: 15967–15981. DOI 10.3390/su71215799. [Google Scholar] [CrossRef]
Barr HD, Weatherley PE (1962). A re-examination of the relative turgidity technique for estimating water deficit in leaves. Australian Journal of Biological Science 15: 413–428. DOI 10.1071/BI9620413. [Google Scholar] [CrossRef]
Becerra A, Bartoloni N, Cofré N, Soteras F, Cabello M (2014). Arbuscular mycorrhizal fungi in saline soils: Vertical distribution at different soil depth. Brazilian Journal of Microbiology 45: 585–594. DOI 10.1590/S1517-83822014000200029. [Google Scholar] [CrossRef]
Bencherif K, Boutekrabt A, Fontaine J, Laruelle F, Dalpè Y, Sahraoui AL (2015). Impact of soil salinity on arbuscular mycorrhizal fungi biodiversity and microflora biomass associated with Tamarix articulata Vahll rhizosphere in arid and semi-arid Algerian areas. Science of the Total Environment 533: 488–494. DOI 10.1016/j.scitotenv.2015.07.007. [Google Scholar] [CrossRef]
Cai H, Dong L, Xing YM, Yang SQ, Song TT et al. (2019). Effect of arbuscular mycorrhizal fungi on alkali tolerance of alfalfa under alkaline stress. Journal of Northeast Agricultural University 50: 40–46. [Google Scholar]
Carillo P, Mastrolonardo G, Nacca F, Parisi D, Verlotta A et al. (2008). Nitrogen metabolism in durum wheat under salinity: Accumulation of proline and glycine betaine. Functional Plant Biology 35: 412–426. DOI 10.1071/FP08108. [Google Scholar] [CrossRef]
Dashtebani F, Hajiboland R, Aliasgharzad N (2014). Characterization of salt-tolerance mechanisms in mycorrhizal (Claroideoglomus etunicatum) halophytic grass, Puccinellia distans. Acta Physiologiae Plantarum 36: 1713–1726. DOI 10.1007/s11738-014–1546-4. [Google Scholar] [CrossRef]
Deng J, Li F, Gu LJ, Duan TY (2019). Effect of arbuscular mycorrhizal fungi on alfalfa seedling growth at different soil pH. Pratacultural Science 36: 2854–2862. [Google Scholar]
Dinneny JR (2015). Traversing organizational scales in plant salt-stress responses. Current Opinion in Plant Biology 23: 70–75. DOI 10.1016/j.pbi.2014.10.009. [Google Scholar] [CrossRef]
Estrada B, Aroca R, Maathuis JM, Barea JM, Ruiz-lozano JM (2013). Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell & Environment 36: 1771–1782. DOI 10.1111/pce.12082. [Google Scholar] [CrossRef]
Evelin H, Kapoor R, Giri B (2009). Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Annals of Botany 104: 1263–1280. DOI 10.1093/aob/mcp251. [Google Scholar] [CrossRef]
Fitter AH, Helgason T, Hodge A (2011). Nutritional exchanges in the arbuscular mycorrhizal symbiosis: Implications for sustainable agriculture. Fungal Biology Reviews 25: 68–72. DOI 10.1016/j.fbr.2011.01.002. [Google Scholar] [CrossRef]
Garcia IV, Mendoza RE (2007). Arbuscular mycorrhizal fungi and plant symbiosis in a saline-sodic soil. Mycorrhiza 17: 167–174. DOI 10.1007/s00572-006-0088-z. [Google Scholar] [CrossRef]
Giri B, Kapoor R, Mukerji KG (2003). Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biology and Fertility of Soils 38: 170–175. DOI 10.1007/s00374-003. [Google Scholar] [CrossRef]
Giri B, Kapoor R, Mukerji KG (2007). Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microbial Ecology 54: 753–760. DOI 10.1007/s00248-007–9239-9. [Google Scholar] [CrossRef]
Gong MQ, Wang FZ, Chen Y, Chen YL (2000). Mycorrhizal dependency and inoculant effects on the growth of Betula alnoides Seedlings. Forest Research 13: 8–14. [Google Scholar]
He ZQ, He CX, Zhang ZB (2007). Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Colloids and Surfaces B: Biointerfaces 59: 128–133. DOI 10.1016/j.colsurfb.2007.04.023. [Google Scholar] [CrossRef]
Huang SC, Chen F, Li LL, Yang CX (2017). Effect of AMF on growth and physio-biochemistry of Trifolium repens under stress of salt alkaline soil in Songnen Plain. Guizhou Agricultural Sciences 45: 61–67. [Google Scholar]
Huang Z, Zou ZR, He CX, Zhang ZB (2011). Physiiological and photosynthetic responses of melon (Cucumis melo L.) seedlings to three Glomus species under water deficit. Plant & Soil 339: 391–399. DOI 10.1007/s11104-010–0591-z. [Google Scholar] [CrossRef]
Khan MA, Ansari R, Ali H, Gul B, Nielsen BL (2009). Panicum turgidum, a potentially sustainable cattle feed alternative to maize for saline areas. Agriculture Ecosystems & Environment 129: 542–546. DOI 10.1016/j.agee.2008.10.014. [Google Scholar] [CrossRef]
Lanfranco L, Yong JPW (2012). Genetic and genomic glimpses of the elusive arbuscular mycorrhizal fungi. Current Opinion Plant Biology 15: 454–461. DOI 10.1016/j.pbi.2012.04.003. [Google Scholar] [CrossRef]
López–Ráez JA (2016). How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis? Planta 243: 1375–1385. DOI 10.1007/s00425-015–2435-9. [Google Scholar] [CrossRef]
Pandey R, Garg N (2017). High effectiveness of Rhizophagus irregularis is linked to superior modulation of antioxidant defense mechanisms in Cajanus cajan (L.) Millsp. genotypes grown under salinity stress. Mycorrhiza 27: 669–682. DOI 10.1007/s00572-017–0778-8. [Google Scholar] [CrossRef]
Parvin S, Geel MV, Yeasmin T, Verbruggen E, Honnay O (2020). Effects of single and multiple species inocula of arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 30: 431–444. DOI 10.1007/s00572-020–00957-9. [Google Scholar] [CrossRef]
Philips JM, Hayman DS (1970). Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society 55: 158–161. DOI 10.1016/S0007-1536(70)80110-3. [Google Scholar] [CrossRef]
Rabie GH, Almadini AM (2005). Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. African Journal of Biotechnology 4: 210–222. [Google Scholar]
Sheng M, Tang M, Chen H, Yang BW, Zhang FF, Huang YH (2008). Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18: 287–296. DOI 10.1007/s00572-008–0180-7. [Google Scholar] [CrossRef]
Shi DC, Wang DL (2005). Effects of various salt-alkaline mixed stresses on Aneurolepidium chinense (Trin.) Kitag. Plant and Soil 271: 15–26. [Google Scholar]
Su X, Liu YP, Zuo XL (2014). Microstructure of three typical halophytes at the Qinghai lakeside. Acta Agrestia Sinica 22: 194–198. [Google Scholar]
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986). Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de Methodes d’estimation ayant une Significantion Fonctionnelle. Mycorrhizae: Physiology and Genetics. Paris: INRA Press. [Google Scholar]
Wang FY, Liu RJ (2002). Arbuscular mycorrhizal fungi in saline-alkaline soils of Yellow River Delta. Mycosystema 21: 196–202. [Google Scholar]
Wang H, Fang Y, Liu RJ, Chen YL (2018a). Recent advances in the studies of nutrient transportation, metabolism, utilization and regulation in arbuscular mycorrhizas. Plant Physiology Communications 54: 1645–1658. DOI 10.13592/j.cnki.ppj.2018.0346. [Google Scholar] [CrossRef]
Wang TM, Zhang JN, Ping XY (2015). Investigation and application analysis on the wild ornamental plants germplasm in Guyuan Bashang Plateau Grassland. Northern Horticulture 24: 58–61. DOI 10.11937/bfyy.201524018. [Google Scholar] [CrossRef]
Wang WX, Vinocur B, Altman A (2003). Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 218: 1–14. DOI 10.1007/s00425-003-1105-5. [Google Scholar] [CrossRef]
Wang XK (2006). Principles and Techniques of Plant Physiological and Biochemical Experiments. China: higher education Press. [Google Scholar]
Wang YD, Yang CX (2020). Infection characteristics and diversity of AM fungi in rhizosphere of Potentilla anserina. Acta Agriculturae Boreali-Occidentalis Sinica 29: 254–265. DOI 10.7606/j.issn.1004-1389.2020.02.012. [Google Scholar] [CrossRef]
Wang YN, Tao S, Hua S, Yu XY, Yan XY et al. (2018b). Effects of arbuscular mycorrhizal fungi on the growth and physiological metabolism of Leymus chinensis under salt-alkali stress. Acta Ecologica Sinica 38: 2187–2194. [Google Scholar]
Xu WP, Xie XH, Huang Z, He M, Lai Y (2017). Physiological responses of melon (Cucumis melo L.) seedlings to Glomus under low light and salt stress. Acta Botanica Boreali-Occidentalia Sinica 37: 1781–1788. [Google Scholar]
Yan SB, Qian YQ, Zhang Y, Yan L, Dong L (2020). Effects of soil moisture content on morpho-physiological of four Potentilla. Pratacultural Science 37: 98–105. DOI 10.11829/j.issn.1001-0629.2019-0512. [Google Scholar] [CrossRef]
Yang CX, Chen F, Yue YN, Yan XF (2015). Diversity characteristics of arbuscular mycorrhizal fungi in the rhizosphere of twenty six species of plants in Songnen saline-alkaline grassland. Pratacultural Science 32: 2008–2020. [Google Scholar]
Yang HX, Xu M, Liu N, Guo SX (2014). Effects of arbuscular mycorrhizal fungi on salinity tolerance of two turfgrass. Pratacultural Science 31: 1261–1268. DOI 10.11829\\j.issn.1001-0629.2013-0031. [Google Scholar]
Yang LF, Zhu YL, Hu CM, Liu ZL, Zhang GW (2006). Effects of NaCl stress on the contents of the substances regulating membrane lipid oxidation and osmosis and photosynthetic characteristics of grafted cucumber. Acta Botanica Boreali-Occidentalia Sinica 26: 1195–1200. [Google Scholar]
Zai XM, Zhang HS, Qin P (2013). Growth and nutrient uptake of the beach plum (Prunus maritima) to inoculation with AM fungi under NaCl stress. South China Fruit 42: 25–28. [Google Scholar]
Zhang AD, Zheng YX, Huang DB (2018). Effects of arbuscular mycorrhizal fungi on salt tolerance of Ligustrum lucidum. Jiangsu Agricultural Science 46: 129–133. DOI 10.15889/j.issn.1002-1302.2018.19.034. [Google Scholar] [CrossRef]
Zhang JF, Xie JH, Tian L, Ji L, Chen DG et al. (2019). Advances in mycorrhizal fungi to improve salt tolerance of plants. Northern Horticulture 23: 146–152. DOI 10.11937/bfyy.20191224. [Google Scholar] [CrossRef]
Zhang Y, Zhao S, Li Y, Xie LD, Sheng KL (2008). Radiation effects on styrene-butadiene–styrene copolymer. Nuclear Instruments &. Methods in Physics Research Section B–Beam Interactions with Materials and Atoms 266: 3431–3436. DOI 10.1016/j.nimb.2008.04.018. [Google Scholar] [CrossRef]
Zhu HH, Yao Q, Sun XT, Hu YL (2007). Colonization, ALP activity and plant growth promotion of native and exotic arbuscular mycorrhizal fungi at low pH. Soil Biology and Biochemistry 39: 942–950. DOI 10.1016/j.soilbio.2006.11.006. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |