DOI:10.32604/biocell.2022.019301

| BIOCELL DOI:10.32604/biocell.2022.019301 |  |
| Article |
Secondary antiviral metabolites from fungi with special reference to coronaviruses
1Department of Chemistry, Faculty of Science and Art, King Khalid University, Mohail, Assir, Saudi Arabia
2Department of Chemistry, Faculty of Science, Al-Azhar University, Cairo, Egypt
3Botany and Microbiology Department, Faculty of Science, Damanhour University, El-Behera, Egypt
4Microbial Activity Unit, Department of Microbiology, Soils, Water and Environment Research Institute, Agricultural Research Center, Giza, Egypt
5Life Sciences Department, College of Science and Art, King Khalid University, Mohail, Aseer, Saudi Arabia
6Unit of Food Bacteriology, Central Laboratory of Food Hygiene, Ministry of Health, Sharkia, Egypt
7Department of Biology, College of Science, King Khalid University, Abha, Saudi Arabia
8Zoology Department, Faculty of Science, Damanhour University, El-Behera, Egypt
9Department of Business Administration, Community College, King Khalid University, Abha, Saudi Arabia
10Department of Agricultural Economics, Faculty of Agriculture, Tanta University, Tanta, Egypt
11Department of Botany, Faculty of Agriculture, Menoufia University, Sheben El-Kom, Menoufia, Egypt
12Botany and Microbiology Department, Faculty of Science, South Valley University, Qena, Egypt
*Address correspondence to: Wesameldin Saber, wesameldin.saber@arc.sci.eg
Received: 15 September 2021; Accepted: 26 October 2021
Abstract: Profound inspection of the life forms on the earth teaches how to be the complexity of interrelationships among the various systems. Because of the emergence of novel viruses all the time and the inadequate of vaccines and antivirals, viral contagions are amongst the most causative diseases affecting people worldwide. Fungi exemplify a massive source of bioactive molecules as, many fungal secondary metabolities like Oxoglyantrypine, Carneic acid F, Scedapin C, Asteltoxin E, Phomanolide, Norquinadoline A and Quinadoline B have antiviral activity. This review deals with how secondary metabolites of fungi can help in the war against viruses in general and especially Coronaviruses moreover several pieces of literature pointed out that many clusters of fungi in different biotopes are waiting to be exploited.
Keywords: COVID-19; SARS-CoV-2; Antibiotics; Virus; Fungi
Biological sciences have accomplished enormous discoveries in numerous arenas. Microorganisms are one of them, despite this, viruses as unique creatures, however, still have several aspects to be discovered. One of the main mystery aspects is how to stop infectious viruses. Recently, unknown pneumonia was reported and rapidly spread around the world, leading to a unique pandemic. The newly emerged coronavirus disease 2019 (COVID-19) rapidly outspread as a challenge worldwide and now is one of the biggest infectious pandemics in human society (Spinelli and Pellino, 2020). The causative agent was soon revealed and identified by the World Health Organization as a 2019 novel coronavirus. The virus causes severe acute respiratory syndrome and causes direct tissue damage; furthermore, it can lead to extrapulmonary manifestations by affecting the endothelium, evoking thrombosis, dysregulating the immune responses, and causing the incompatibility of the pathways related to angiotensin-converting enzyme 2, with additional complications on the other organs (Ebrahimi et al., 2021; Gupta et al., 2020).
Antibiotics have been playing a vital role in the near past of human history by preventing the outbreak of various killing pandemics and fighting several diseases, such as urinary tract infections, strep throat, whooping cough, and life-threatening illnesses like sepsis generated by other microorganisms. On the other side, antibiotics can, unfortunately, have serious side effects and lead to antibiotic resistance, where the microorganism cannot be stopped by the antibiotic, representing a grave global problem. Therefore, the golden advice is not to consume an antibiotic unless needed. The matter here is that viruses, like the common cold, the flu, or the recently appeared COVID-19, cannot be inactive by antibiotics. That, in turn, urged to search for efficient alternatives antiviral drugs to deal with viral diseases (Murphy et al., 2012). Another effective and promising approach is the utilization of fungal secondary metabolites (FSMs). FsMs are small organic molecules (less than 4 kDa) not involved in cell growth and development but have a pivotal role in self-defense, niche adaptation, and communication with the environment. These compounds were reported to have various uses not only in medicines and recreational drugs as antiviral properties but also in vast applications worldwide as flavorings and pigments (Murphy et al., 2012).
Viruses; our friends and enemies
Viruses are the smallest microscopic obligate intracellular parasites, generally much smaller than bacteria. There is a controversial disagreement between biologists; many consider viruses to be non-living as they lack a cellular structure (only protein coating surrounding genetic material) and cannot metabolize by themselves, rather they lack the ability to thrive and duplicate outside a host cell, requiring a host cell to replicate and create new units. Some other scientists hold that viruses can be considered organisms because they have genomic material (DNA or RNA) and can employ the metabolism of their host to make copies of themselves to regenerate. Nanoparticles are another name that could be applied to viruses (Marintcheva, 2018; Murphy et al., 2012). Harmonically, they can be considered as living nanoparticles based on their unique way of reproduction, which is performed through three main steps; (1) the initiation of cell infection by the viral genome, followed by (2) the replication and expression of the viral genome, and finally (3) the release of progeny from infected cells. This unique mechanism argues for special treatment that differs from those applied to other microorganisms. Accordingly, their treatment is somehow different (Dimmock et al., 2015; Marintcheva, 2018). Viruses are a genuine challenge to humankind. They have a reputation for being the cause of contagion and millions of human deaths all over the world. This widespread disease and death, e.g., Ebola, Swine Influenza, Avian Influenza, Dengue fever, and currently, COVID-19, have, no doubt, bolstered such reputation (Srinivasa Rao and Vazquez, 2020; Xia et al., 2020). That is why finding out alternative antiviral medications represents one of the utmost important goals on the humankind level. On the far side, several benefits of viruses have been stated. For several pieces of evidence, it turns out that the virus does not necessarily mean diseases or pandemics. They are not bad at all, and some viruses might be good and required for the smooth continuation of life on Earth. Next are some examples.
Phage therapy, the next generation of drugs, involves the use of phages to treat fungal and bacterial contagions and could be a viable strategy to combat drug-resistant strains. For instance, phage treatment was applied to the aortic graft infected with Pseudomonas aeruginosa the patient had unrelated surgery without any side effects (Chan et al., 2018). In many cases, it is possible to prompt viruses to do the job thanks to their unique ability to integrate into DNA, viruses can be used to inject genes into cells, which can reverse some genetic diseases. For example, some viruses were used to cure hemophilia, a blood disorder that prevents clotting (Nienhuis et al., 2017). Recently, experiments have been successful in the lab on using oncolytic viruses to treat cancer cells and dissolve tumors (Ma et al., 2020). Viruses might be crucial to the development of healthy organs. Thanks to experiments on the human microbiome, it has become obvious that virome (the set of all viruses, both eukaryotic and prokaryotic, in a given niche), is unique to each individual, and it was clear that the microbiome health is mediated by virome in the body organs such as intestines and has a lot of important roles in the intestine and immune system development (Neuman and Koren, 2017; Trastoy et al., 2020). For another group of living organisms, i.e., plants, viruses can be quite devastating from the agricultural point of view, but on the other side, if it is utilized wisely, virus infection improves drought tolerance and abiotic stress of plants, which correlates with increased osmoprotectants and the levels of antioxidants in diseased plants such as Beta vulgaris (Xu et al., 2008). Other viruses can cause desirable effects in their hosts, e.g., Abutilon mosaic virus enhances the aesthetics of some ornamental plants such as Abutilon spp. and Hibiscus spp. (Valverde et al., 2012). However, these desirable effects are now carried out under controlled conditions.
Antivirals; challenges and difficulties
Antivirals are a class of compounds with an antimicrobial nature, either secreted by a living cell (animals, plants, fungi, bacteria, etc.) or chemically synthesized, that hinders viral duplication through interference with one or more of the viral life cycle phases (cell attachment, cell penetration, viral uncoating, viral genome replication, maturation, and viral progeny release). Antivirals represent an important therapeutic tool that complements the action of vaccine therapies in curing and hindering viral contagions (Stiver, 2003). Anyhow, there are numerous precautions and limitations that restrict the absolute application of antivirals. The curing process of viral infections has verified more obstacles because viruses are quite tiny and obligate intracellular parasites, and very specific to the host, even tissue and cell. Other viral infections are contagious with a high capability to be transmitted from one host to another such contagiousness extends for varying periods depending on the virus kind. The ease of viral transmission is another challenge. Viruses can spread through touch, saliva, air, sexual contact, sharing contaminated needles, insect vectors (mosquitoes and ticks), and finally, food and water (Dimmock et al., 2015; El-Hersh et al., 2013; Marintcheva, 2018).
Difficulties arise, also, when attempts are made to deal with viruses, because of the unique structural system, which does not react to the ordinary drugs (used to manage bacterial and fungal diseases) such as the ordinary antibiotics that cannot treat viral infections, fortunately, most viral illnesses could be managed by the immune system and can effectively heal the illness within 7 to 10 days (Murphy et al., 2012). However, the response degree of the immune system varies from one person to another based on several factors such as health conditions and age (Linden et al., 2015). The ordinary strategy of viral treatment depends mainly on the hindering of the viral life cycle via induction of immune system response through vaccination (immunizations), utilizing the weakened virus (live or killed state), or proteins or toxins from the virus. This strategy aids the immune system to mature self-protection from a disease. Since the enlargement of the vaccines strategy, they have drastically reduced viral diseases such as polio, measles, and chickenpox (Murphy et al., 2012). Besides, vaccines can protect humans from being infected with several viruses such as the flu, hepatitis A, hepatitis B, human papillomavirus, and others (Adams et al., 2004; El-Hersh et al., 2013; Stiver, 2003). However, the virus of human immunodeficiency (HIV), is still, another challenge to the immune system, which is easily attacked by the virus, leading to the development of acquired immunodeficiency syndrome (AIDS), till now, there is no radical medication curing HIV. Alternatively, people suffering from AIDS could use potent antiretroviral therapy to transform HIV infection into a chronic disease, such that people dealing with HIV/AIDS as a chronic disease, and have just a near-normal life expectancy close to the general population (Back and Marzolini, 2020).
For some viral diseases, such as herpes simplex virus infections (Adams et al., 2004), HCV (El-Hersh et al., 2013), and influenza (Stiver, 2003), antiviral medications have become available. But the use of antiviral prescriptions has been correlated with the expansion of drug-resistant viruses. Unfortunately, in some cases, the virus still survives in the nonactive form in the patients, as in the situation of HIV (Back and Marzolini, 2020). Anti-replication agents, which unravel virus and hinder viral cycles, guide the triggering of immune system response are other weapons against viruses. Such drugs can be obtained by modification of existing sources, their combinatory use, or the rediscovery of old drugs with new functions, the problem here is that several of these anti-replications remain unclear (Linden et al., 2015; Said and Abdelwahab, 2013; Vigant et al., 2015).
Moreover, the problems of targeting the viral proteins and/or cellular factors are the high rate at which viruses produce mutant resistant strains, and the viruses may deviate the cellular factors from their original pathway and still cause an effective infection, also, targeting cellular factors might have an antagonistic impact on the normal functioning of the host cells (Vigant et al., 2015). Furthermore, the mechanisms of non-enveloped viruses to break the host cell membrane barrier are less well known, which forms an additional challenge in developing strategies against these viruses (Linden et al., 2015; Said and Abdelwahab, 2013). Finally, human antivirals, including drugs and vaccines, struggle with viral infections by targeting the virus-specific factors, through the strategic dogma; one-drug for one virus (Vigant et al., 2015). Consequently, only a handful of synthetic antivirals have made it past the clinical phase, especially when considering the precautions, which are required through clinical and pre-clinical trials on the antiviral drug. Of the most vital precautions, the drug should not cause any cytotoxicity, minimal side effects to the host cells, and the antiviral should be able to completely constrain the virus infection since, partial inhibition leads to the generation of drug-resistant mutants (Vigant et al., 2015). Owing to these restrictions, besides the continual discoveries of new viruses, recently, the scientific communities have approved another paradigm based on one drug for multiple viruses, through targeting an essential viral function, shared in a wide-ranging of viruses i.e., the small molecules broad-spectrum antivirals (Bekerman and Einav, 2015; Bösl et al., 2019; Ianevski et al., 2019). The idea is to suppress and shut down the similar pathways and host factors that different viruses employ to replicate themselves inside the host cell (Bösl et al., 2019; Vigant et al., 2015). Hence, many questions have arisen. Are there any alternatives? If FSMs are the answer, do their effectiveness and efficacy comparable with these of ordinary antivirals? Next, these topics will be highlighted.
The necessity to find out alternative medication to viruses’ infection has been arisen with the outbreak of viral diseases, as in the situation of the most recent and obvious pandemic; COVID-19, no effective drug works with such newly borne virus till now. The necessity of FSMs is in line with such an aim. Interestingly, the kingdom of fungi is representing the most miscellaneous group of microbial species, inhabiting extreme environments such as deep-sea sediments and mangrove ecosystems, moreover, the recent estimates predicting fungal species that only 3%–8% of existing fungal species are discovered and described (Hawksworth and Lücking, 2017). The United States Food and Drug Administration registered about 40% of modern drugs and 49% of new chemical products, all are based on natural products or their derivatives (Brewer, 2000). Of them, many ascomycetous species share a large proportion and have been revealed to have antiviral and other biological behaviors (Deshmukh et al., 2017; Kumaresan and Suryanarayanan, 2001). Therefore, the fungal kingdom is a promising group of organisms that smoothly create a vast range of natural organic metabolic complexes.
The natural organic metabolites of fungi are categorized into primary (e.g., lignin derivatives, polysaccharides, proteins) and secondary metabolites. The latter category is usually low-molecular-weight metabolites that often have potent physiological activities; hence they are also called specialized metabolites, secondary products, or natural products (Keller et al., 2005; Kumaresan and Suryanarayanan, 2001). Opposite to primary metabolites, the secondary ones share the enigmatic properties of cellular dispensability that means, the absenteeism of secondary metabolites does not result in an immediate threat to the microorganism, simply, because these compounds are not intended metabolites, rather, they are formed naturally during the ordinary life cycle of the microorganism, particularly as specific classes of related compounds secreted in a specific stage in a restricted period of the life cycle. Further, they are often restricted to a narrow set of species within a phylogenetic group (Calvo et al., 2002; Yu and Keller, 2005). That is to say, only an individual or a minor group of organisms produces their own metabolite. Obviously, as mentioned earlier, microorganisms can grow without synthesizing these metabolites, since they are not directly involved in the normal growth, development, or reproduction but, in the long-term, may cause impairment of the organism’s survivability, or fecundity, or perhaps no significant change at all (Calvo et al., 2002; Keller et al., 2005). Natural sources, like fungi, have been found to be grander to combinatory chemistry for discovering novel pharmaceutical drugs that have the potential to be industrialized into new medication products. More, the biotope ecosystem varies greatly in the kind and quality of the secreted FSMs. In comparison for soil fungi, fungal endophytes, for instance, were found to have FSMs with higher and diverse biological activities. It has become commonplace to distinguish the diverse structural groups of FSMs, e.g., terpenoids, steroids, xanthones, chinones, phenols, isocumarines, benzopyranones, tetralones, cytochalasines, and enniatines (Schulz et al., 2002).
Among the fungal natural products, pigments are the most recognizable ingredients, which are typically brown and black pigments (melanins), giving color to spores, appressoria, sclerotia, sexual bodies, and other developmental organelles (Yu and Keller, 2005). The pigments are functioning as plant and animal virulence factors (Kimura and Tsuge, 1993; Yu and Keller, 2005) or that they are required for general survival, presumably as UV protectants (Lee and Adams, 1994), antigrowth deterrents (Scheu and Simmerling, 2004), or reactive oxygen species scavengers (Coccia et al., 2001). Nutritionally, the biosynthesis of FSMs depends on the growth conditions of each strain. Researchers have been applying different modifications in nutrients and physicochemical factors during fermentation processes to optimize the biosynthesis of the bioactive compounds. Fermentation processes are currently modeled and analyzed by the mathematical optimization approach, e.g., response surface methodology, which enables enhanced production of various metabolites (Al-Askar et al., 2018; Jakubiec-Krzesniak et al., 2018).
On the ecosystem level, extreme habitats such as tropical forest soil, caves, deserts, and Antarctic ecosystems are recognized as valuable sources of novel microbial metabolites of pharmacological importance (Jakubiec-Krzesniak et al., 2018; Sacramento et al., 2004). Genetically, the secretion of microbial secondary metabolites is regulated, employing gene clusters-based premise, such inherent criteria spurred efforts toward identifying the genetic factor involved in the biosynthesis process. The studies of known FSMs-biosynthetic genes reported that such genes are clustered in fungal genomes and have considerable bearing on the regulation of the secretion process. Consequently, several genome sequencings detected the genes responsible for the production of FSMs and encoded by biosynthetic gene clusters (Jakubiec-Krzesniak et al., 2018; Yu and Keller, 2005). The production of FSMs is driven via various pathways, their synthesis starts once the active fungal growth ceases (El-Hawary et al., 2017).
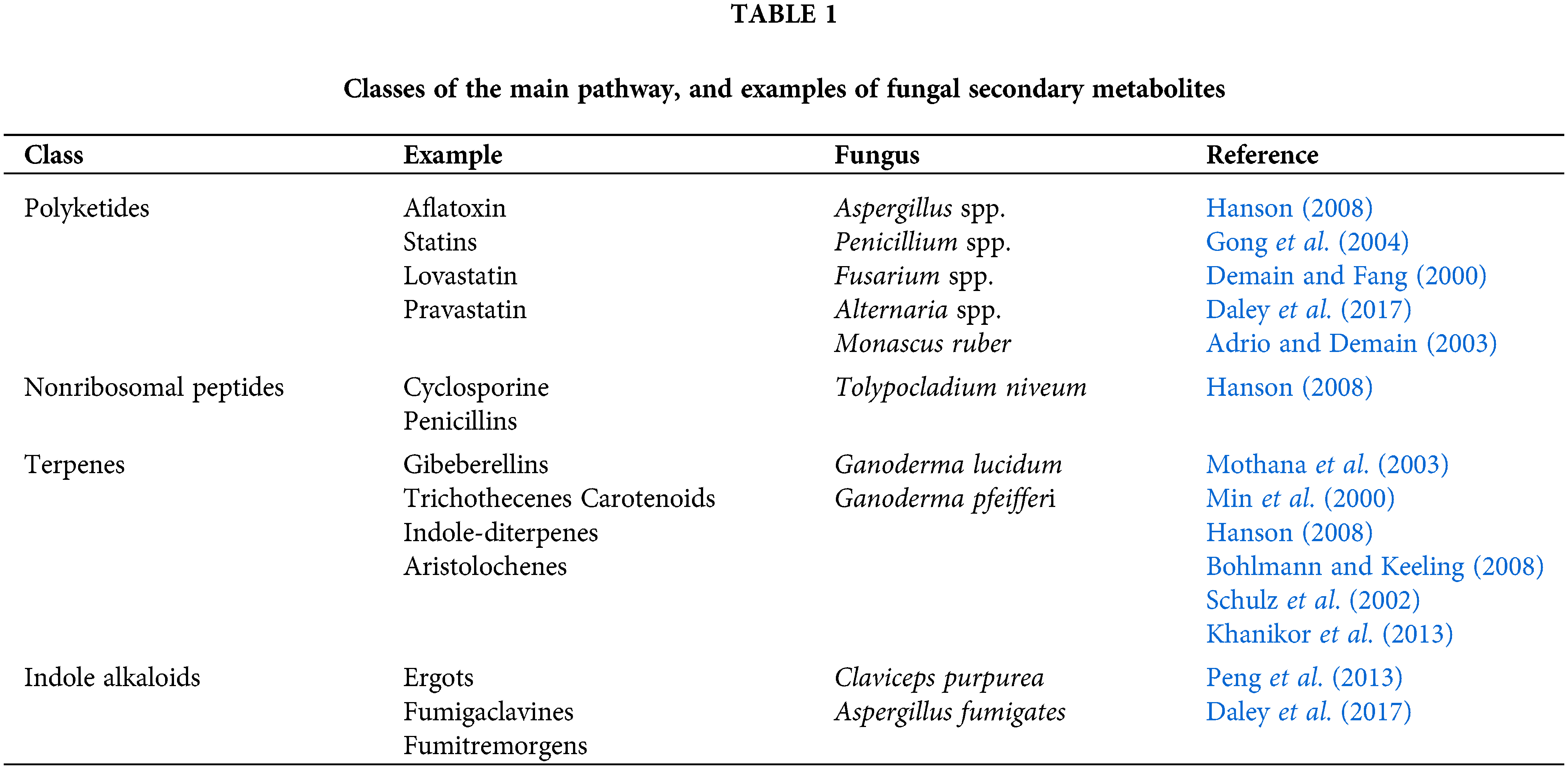
Table 1 shows four classes according to biochemical construction or biosynthetic origin. Polyketides represent an important category of secondary metabolites with great structural diversity from simple aromatics to highly modified complex architectures, such as macrolides, polyphenols, polyethers, polyenes, and enediynes (Luo et al., 2018). A promising therapeutic drug for cancers and hypoglycemia, as well as anti-influenza A virus infection, thus improving pulmonary function, without cytotoxicity. Furthermore, polyketides were reported as a potent inhibition of HIV replication (Herrmann et al., 2020). Nonribosomal peptides can act as anti-Zika virus activities by inhibiting RNA replication and nonstructural protein 5 production (Yuan et al., 2021). Terpenes are used as antivirus through maximizing the drug efficacy against different types of viruses, including the drug-resistant ones. Novel classes of terpenes can also target various sites in both the host and the virus, such as host transport machinery and/or viral polymerase (Al-Salihi and Alberti, 2021). The therapeutic indole alkaloids have displayed antiviral potential against RNA viruses, including coxsackievirus A-21, equine rhinovirus, and influenza A virus. For example, the antiviral activity of arbitol against influenza virus was found to improve hemagglutinin stability, and it inhibited low-pH-induced transformation of hemagglutinin to its fusogenic state, preventing infection at the viral fusion level (Mitra et al., 2021).
Some important advantages characterize the natural FSMs over the chemically created antivirals, i.e., lower healing dose, lower probability of developing antiviral-resistant strains of viruses, and the multi-activity against several viruses. For example, the antiviral activity of fungal coumarin and its derivatives has been detected against a broad range of viruses such as influenza viruses, HIV, coxsackievirus A16 (CVA16), dengue virus, Enterovirus 71 (EV71), and chikungunya virus (Hassan et al., 2016; Peng et al., 2013). There is another advantage regarding the mechanism of action of AFSMs, i.e., the targeting of several steps in the virus life cycle. For instance, the anti-HIV targets several steps in the virus life cycle, including virus-host cell attachment, cell membrane fusion, integration, and assembly, besides the conventional targets like inhibition of the reverse transcriptase, protease, and integrase (Moore and Stevenson, 2000). Another example of the wide spectrum bioactivity of FSMs comes from the endophytic fungi, which has a huge number of bioactive compounds of pharmaceutical importance viz., antitumor, antibiotic, neuroprotective, antioxidant, anti-inflammatory, antiviral, and immunomodulatory agents, etc. (Agrawal et al., 2019; Deshmukh et al., 2017). Several investigations obtained promising antiviral compounds from diverse taxa of fungi; these FSMs proved to have potent activity against a wide array of viruses, confirming hope and relying on using such compounds soon. Table 2 explores some of these fungi and their FSMs.

A prospective view of FSMs vs. SARS-CoV-2
Coronaviruses belong to Nidovirales order, which contains three families (Coronaviridae, Arteriviridae, and Roniviridae), all are enveloped and measure 65–125 nm in diameter, Coronaviridae family contains two subfamilies, of which the Coronavirinae subfamily is further separated into 4 subgroups (alpha, beta, gamma, and delta coronaviruses). Phenotypically, they named coronaviruses because of the crown- or wreath-like spikes on the outer surface of the virus. Genetically, the nucleic material of coronaviruses contains a non-segmented, single-stranded, and positive-sense RNA, representing the biggest presently known RNA virus genomes, ranging from 26 to 32 kbs in the case of the Coronaviridae and Roniviridae, in contrast to Arteriviridae members, that have tinier genome (13 to 16 kbp) (de Groot et al., 2011; Enjuanes et al., 2008; Shereen et al., 2020). Genomic assessment disclosed that the emerged respiratory coronavirus 2 (SARS-CoV-2) is phylogenetically associated with severe acute respiratory syndrome-like (SARS-like) bat viruses. The symptoms of the novel viral disease, COVID-19, include cough, fever, shortness of breath, and pneumonia (Desforges et al., 2019).
COVID-19 is a significant challenge for global public health, especially because of the ease and readily transmissibility, as well as, the asymptomatic infectivity periods. The novel virus was announced in December 2019, and its outbreak has been associated with a global pandemic, early, in 2020, followed by major quarantines to prevent further spread (Shereen et al., 2020; Srinivasa Rao and Vazquez, 2020). Besides being highly transmissible, the virus has a higher affinity to attach to the human Angiotensin Converting Enzyme-2 receptor, surpassing the presently known SARS-CoV and MERS-CoV infections (Zhou et al., 2020), which makes the novel SARS-CoV-2 infection even more dangerous. Additionally, few broad-range antiviral medications have been assessed against COVID-19 in clinical trials, little of them, such as the vaccine against SARS-CoV, can be partially effective (Shereen et al., 2020). Consequently, till now, there is no clinically approved antiviral prescription or vaccine available to be used against COVID-19. These were the bad news, next are the good ones. The findings indicate that 90% of naturally occurring antibiotics and compounds including FSMs may possess antiviral capabilities (Linnakoski et al., 2018), because of that the FSMs may be a gifted option.
Depending on the viral construction, SARS-CoV-2 is an enveloped virus, this feature can facilitate the antiviral activity of the FSMs. Since the existence of the envelope makes the virus sensitive to hot conditions and the acidic ecosystem (de Groot et al., 2011; Enjuanes et al., 2008). Fungi are already known to generate hot temperature and modify the medium to acidic conditions by their metabolites, the resultant FSMs, under these conditions, are expected to have some of the acids features of the growth medium, leading to perform better against enveloped viruses. This means that SARS-CoV-2 is expected not to be longer remain active in such conditions. Contrarily to the non-enveloped viruses, enveloped viruses cannot tolerate the environmental conditions encountered inside the gastrointestinal tract, such as bile salts, which show detergent-like action, thus can breakdown these viruses (de Groot et al., 2011; Enjuanes et al., 2008; Neuman and Koren, 2017). FSMs can do a similar action since many fungi have a lipolytic system that can catalyze the degradation of the lipid viral envelop like the same action occurs when treatment with lipid solvents (Linnakoski et al., 2018). This mechanism is supposed to apply to the enveloped; SARS-CoV-2.
In comparison to vaccines, FSMs exemplify the ideal choice of antiviral for the treatment of COVID-19. Simply, because the development of vaccines against the newly emerging virus serotypes is a challenging and complicated process. Also, vaccination cannot help if the infection is already existing in the biological system (Linnakoski et al., 2018). Fungi, actually, produce several effective molecules that could also be utilized as antivirals. Many novel bioactive natural products possessing antiviral activities have already been identified as promising (Cheung et al., 2014; Linnakoski et al., 2018). So, expectations nominate FSMs, as natural products, to play a critical task in the COVID-19 treatment. FSMs have several antiviral mechanisms, for instance, viruses can be directly attacked outside cells in order to irreversibly terminate the viral particles before their attachment to cellular receptors. Another, FSMs can inhibit the receptor binding to prevent virus infection, this strategy offers a nice possibility to prevent the viral infection process (Cagno et al., 2018). RNA-based viruses, such as SARS-CoV-2, start their translation and transcription usually in the cytoplasm. Several antivirals of FSMs can target this infection route, acting as protectants to prevent the progress of a larger number of viral infections (Linnakoski et al., 2018).
To the authors’ knowledge no experimental investigation was carried out to test the antiviral activity of fungal secondary metabolites, but, recently, in silico experiment using the computational methods, a sequence of blind and targeted molecular dockings were performed to test the inhibitory effect of endophytic fungi secondary metabolites against the COVID-19 RNA-dependent RNA polymerase. Of 99 compounds, the most five potent on the viral enzyme were predicted, and were further evaluated by both molecular dynamics simulation, and, the pharmacokinetics using the SwissADME server. Molecular docking showed that 18-methoxy cytochalasin J, (22E,24R)-stigmasta-5,7,22-trien-3-β-ol, beauvericin, dankasterone B, and pyrrocidine A compounds had the highest binding energy. The findings of molecular dynamics and SwissADME demonstrated that 18-methoxy cytochalasin J and pyrrocidine A had better effects than others in terms of protein instability, strong complex formation, and pharmacokinetic properties (Ebrahimi et al., 2021).
Summing up, the current knowledge of fungi as fabricators of antiviral compounds was discussed. It is probably only a matter of time before some FSMs will be clinically tested and/or approved against SARS-CoV-2. Therefore, it is significant to investigate vast groups of fungal species, as merely a small number of the known fungi have been investigated for antiviral activity. Finally, as fungi are a rich source of bioactive agents, more detailed knowledge on the antivirals from fungal metabolites is crucial, so that to develop an effective drug in order to efficiently combat SARS-CoV-2 in the near future. Also, the know-how of the bioactivity of FSMs, and their detailed targets in virus’ cells must be planned to expand in the coming era.
Author Contribution: MS, ME, WS, YM and AM conceived the work and wrote the first draft. SN, AE and MM critically reviewed the final draft. All authors contributed to drafting this review article, revised the manuscript, and approved submission.
Funding Statement: The authors are thankful to the Institute of Research and Consulting Studies at King Khalid University for supporting this research through Grant No. # 6–93–S–2020.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Adams O, Besken K, Oberdörfer C, Mackenzie CR, Takikawa O et al. (2004). Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. Journal of Virology 78: 2632–2636. [Google Scholar]
Adrio JL, Demain AL (2003). Fungal biotechnology. International Microbiology 6: 191–199. [Google Scholar]
Agrawal S, Deshmukh SK, Barrow CJ (2019). Marine fungi as a potential source of future cosmeceuticals. In: Satyanarayana T, Deshmukh S and Deshpande M (eds.Advancing Frontiers in Mycology & Mycotechnology. Singapore: Springer. [Google Scholar]
Al-Askar A, Saber W, Ghoneem KM, Rashad Y (2018). Oxalic acid as the main molecule produced by Trichoderma asperellum MG323528 fermented on corn stover based medium. Biotechnology 17: 95–103. [Google Scholar]
Al-Salihi SA, Alberti F (2021). Naturally occurring terpenes: A promising class of organic molecules to address influenza pandemics. Natural Products and Bioprospecting 11: 405–419. [Google Scholar]
Back D, Marzolini C (2020). The challenge of HIV treatment in an era of polypharmacy. Journal of the International AIDS Society 23: e25449. [Google Scholar]
Bashyal BP, Wellensiek BP, Ramakrishnan R, Faeth SH, Ahmad N et al. (2014). Altertoxins with potent anti-HIV activity from Alternaria tenuissima QUE1Se, a fungal endophyte of Quercus emoryi. Bioorganic and Medicinal Chemistry 22: 6112–6116. [Google Scholar]
Bekerman E, Einav S (2015). Infectious disease. Combating emerging viral threats. Science 348: 282–283. [Google Scholar]
Bohlmann J, Keeling CI (2008). Terpenoid biomaterials. Plant Journal 54: 656–669. [Google Scholar]
Bösl K, Ianevski A, Than TT, Andersen PI, Kuivanen S et al. (2019). Common nodes of virus-host interaction revealed through an integrated network analysis. Frontiers in Immunology 10: 2186. [Google Scholar]
Brewer S (2000). Relationship between Natural Products and Synthetic Chemistry in the Discovery Process. Cambridge, United Kingdom: The Royal Society of Chemistry. [Google Scholar]
Cagno V, Andreozzi P, D’alicarnasso M, Jacob Silva P, Mueller M et al. (2018). Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nature Materials 17: 195–203. [Google Scholar]
Calvo AM, Wilson RA, Bok JW, Keller NP (2002). Relationship between secondary metabolism and fungal development. Microbiology and Molecular Biology Reviews 66: 447–459. [Google Scholar]
Cardozo FT, Camelini CM, Mascarello A, Rossi MJ, Nunes RJ et al. (2011). Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antiviral Research 92: 108–114. [Google Scholar]
Coccia R, Foppoli C, Blarzino C, de Marco C, Rosei MA (2001). Interaction of enkephalin derivatives with reactive oxygen species. Biochimica et Biophysica Acta 1525: 43–49. [Google Scholar]
Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA et al. (2018). Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evolution, Medicine, and Public Health 2018: 60–66. [Google Scholar]
Cheung RC, Wong JH, Pan WL, Chan YS, Yin CM et al. (2014). Antifungal and antiviral products of marine organisms. Applied Microbiology and Biotechnology 98: 3475–3494. [Google Scholar]
Daley D, Brown K, Badal S (2017). Fungal metabolites. In: Pharmacognosy. Elsevier. [Google Scholar]
de Groot R, Cowley J, Enjuanes L, Faaberg K, Perlman S et al. (2011). Order nidovirales. In: King AMQ, Adams MJ, Carstens EB and Lefkowitz EJ (eds.Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses. London: Elsevier. [Google Scholar]
Demain AL, Fang A (2000). The natural functions of secondary metabolites. In: A Fiechter (ed.History of Modern Biotechnology I. Advances in Biochemical Engineering/Biotechnology. Berlin, Heidelberg: Springer. [Google Scholar]
Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L et al. (2019). Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses 12: 14. [Google Scholar]
Deshmukh SK, Prakash V, Ranjan N (2017). Marine fungi: A source of potential anticancer compounds. Frontiers in Microbiology 8: 2536. [Google Scholar]
Dimmock NJ, Easton AJ, Leppard KN (2015). Introduction to Modern Virology. John Wiley & Sons. [Google Scholar]
Ebrahimi KS, Ansari M, Moghaddam MSH, Ebrahimi Z, Shahlaei M et al. (2021). In silico investigation on the inhibitory effect of fungal secondary metabolites on RNA dependent RNA polymerase of SARS-CoV-II: A docking and molecular dynamic simulation study. Computers in Biology and Medicine 135: 104613. [Google Scholar]
El-Hawary SS, Sayed AM, Rateb ME, Bakeer W, Abouzid SF et al. (2017). Secondary metabolites from fungal endophytes of Solanum nigrum. Natural Product Research 31: 2568–2571. [Google Scholar]
El-Hersh MS, El-Fadaly HA, Saber WI, El-Deeb AM (2013). Human diseases prosecution among viral infection and food toxins: A review. International Journal of Pharmacology 9: 390–404. [Google Scholar]
Enjuanes L, Gorbalenya A, de Groot R, Cowley J, Ziebuhr J et al. (2008). The Nidovirales. Oxford, UK: Elsevier Ltd. [Google Scholar]
Estoppey D, Lee CM, Janoschke M, Lee BH, Wan KF et al. (2017). The natural product cavinafungin selectively interferes with Zika and dengue virus replication by inhibition of the host signal peptidase. Cell Reports 19: 451–460. [Google Scholar]
Faccin LC, Benati F, Rincão VP, Mantovani MS, Soares SA et al. (2007). Antiviral activity of aqueous and ethanol extracts and of an isolated polysaccharide from Agaricus brasiliensis against poliovirus type 1. Letters in Applied Microbiology 45: 24–28. [Google Scholar]
Gao H, Guo W, Wang Q, Zhang L, Zhu M et al. (2013). Aspulvinones from a mangrove rhizosphere soil-derived fungus Aspergillus terreus Gwq-48 with anti-influenza A viral (H1N1) activity. Bioorganic & Medicinal Chemistry Letters 23: 1776–1778. [Google Scholar]
Gong Y, Hounsa A, Egal S, Turner PC, Sutcliffe AE et al. (2004). Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environmental Health Perspectives 112: 1334–1338. [Google Scholar]
Guo YW, Liu XJ, Yuan J, Li HJ, Mahmud T et al. (2020). L-tryptophan induces a marine-derived Fusarium sp. to produce indole alkaloids with activity against the Zika virus. Journal of Natural Products 83: 3372–3380. [Google Scholar]
Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S et al. (2020). Extrapulmonary manifestations of COVID-19. Nature Medicine 26: 1017–1032. [Google Scholar]
Hanson JR (2008). Chemistry of Fungi. Cambridge, UK: Royal Society of Chemistry. [Google Scholar]
Hassan MZ, Osman H, Ali MA, Ahsan MJ (2016). Therapeutic potential of coumarins as antiviral agents. European Journal of Medical Chemistry 123: 236–255. [Google Scholar]
Hawas UW, Al-Farawati R, Abou El-Kassem LT, Turki AJ (2016). Different culture metabolites of the Red Sea fungus Fusarium equiseti optimize the inhibition of hepatitis C virus NS3/4A protease (HCV PR). Marine Drugs 14: 190. [Google Scholar]
Hawksworth DL, Lücking R (2017). Fungal diversity revisited: 2.2 to 3.8 million species. Microbiology Spectrum 5. [Google Scholar]
Herrmann A, Roesner M, Werner T, Hauck SM, Koch A et al. (2020). Potent inhibition of HIV replication in primary human cells by novel synthetic polyketides inspired by Aureothin. Scientific Reports 10: 1326. [Google Scholar]
Huang LH, Xu MY, Li HJ, Li JQ, Chen YX et al. (2017). Amino acid-directed strategy for inducing the marine-derived fungus Scedosporium apiospermum F41-1 to maximize alkaloid diversity. Organic Letters 19: 4888–4891. [Google Scholar]
Ianevski A, Andersen PI, Merits A, Bjørås M, Kainov D (2019). Expanding the activity spectrum of antiviral agents. Drug Discovery Today 24: 1224–1228. [Google Scholar]
Jakubiec-Krzesniak K, Rajnisz-Mateusiak A, Guspiel A, Ziemska J, Solecka J (2018). Secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties. Polish Journal of Microbiology 67: 259. [Google Scholar]
Keller NP, Turner G, Bennett JW (2005). Fungal secondary metabolism-from biochemistry to genomics. Nature Reviews. Microbiology 3: 937–947. [Google Scholar]
Khanikor B, Parida P, Yadav R, Bora D (2013). Comparative mode of action of some terpene compounds against octopamine receptor and acetyl cholinesterase of mosquito and human system by the help of homology modeling and docking studies. Journal of Applied Pharmaceutical Science 3: 6. [Google Scholar]
Kimura N, Tsuge T (1993). Gene cluster involved in melanin biosynthesis of the filamentous fungus Alternaria alternata. Journal of Bacteriology 175: 4427–4435. [Google Scholar]
Kumaresan V, Suryanarayanan TS (2001). Occurrence and distribution of endophytic fungi in a mangrove community. Mycological Research 105: 1388–1391. [Google Scholar]
Lee BN, Adams TH (1994). Overexpression of flbA, an early regulator of Aspergillus asexual sporulation, leads to activation of brlA and premature initiation of development. Molecular Microbiology 14: 323–334. [Google Scholar]
Li HL, Xu R, Li XM, Yang SQ, Meng LH et al. (2018). Simpterpenoid A, a meroterpenoid with a highly functionalized cyclohexadiene moiety featuring gem-propane-1,2-dione and methylformate groups, from the mangrove-derived Penicillium simplicissimum MA-332. Organic Letters 20: 1465–1468. [Google Scholar]
Li ZY, Yao XP, Liu B, Reheman HN, Yang G et al. (2015). Auricularia auricular-judae polysaccharide attenuates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress and inflammation. Biomedical Reports 3: 478–482. [Google Scholar]
Linden LVD, Wolthers KC, van Kuppeveld FJ (2015). Replication and inhibitors of enteroviruses and parechoviruses. Viruses 7: 4529–4562. [Google Scholar]
Linnakoski R, Reshamwala D, Veteli P, Cortina-Escribano M, Vanhanen H et al. (2018). Antiviral agents from fungi: Diversity, mechanisms and potential applications. Frontiers in Microbiology 9: 2325. [Google Scholar]
Liu SS, Jiang JX, Huang R, Wang YT, Jiang BG et al. (2019). A new antiviral 14-nordrimane sesquiterpenoid from an endophytic fungus Phoma sp. Phytochemistry Letters 29: 75–78. [Google Scholar]
Luo X, Yang J, Chen F, Lin X, Chen C et al. (2018). Structurally diverse polyketides from the mangrove-derived fungus Diaporthe sp. SCSIO 41011 with their anti-influenza A virus activities. Frontiers in Chemistry 6: 282. [Google Scholar]
Ma J, Ramachandran M, Jin C, Quijano-Rubio C, Martikainen M et al. (2020). Characterization of virus-mediated immunogenic cancer cell death and the consequences for oncolytic virus-based immunotherapy of cancer. Cell Death & Disease 11: 48. [Google Scholar]
Ma X, Li L, Zhu T, Ba M, Li G et al. (2013). Phenylspirodrimanes with anti-HIV activity from the sponge-derived fungus Stachybotrys chartarum MXH-X73. Journal of Natural Products 76: 2298–2306. [Google Scholar]
Marintcheva B (2018). Introduction to viral structure, diversity and biology. Harnessing the Power of Viruses, 1–26. [Google Scholar]
Min BS, Gao JJ, Nakamura N, Hattori M (2000). Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against meth-A and LLC tumor cells. Chemical & Pharmaceutical Bulletin 48: 1026–1033. [Google Scholar]
Mitra S, Prova SR, Sultana SA, Das R, Nainu F et al. (2021). Therapeutic potential of indole alkaloids in respiratory diseases: A comprehensive review. Phytomedicine 90: 153649. [Google Scholar]
Mizerska-Dudka M, Jaszek M, Błachowicz A, Rejczak TP, Matuszewska A et al. (2015). Fungus Cerrena unicolor as an effective source of new antiviral, immunomodulatory, and anticancer compounds. International Journal of Biological Macromolecules 79: 459–468. [Google Scholar]
Moore JP, Stevenson M (2000). New targets for inhibitors of HIV-1 replication. Nature Reviews. Molecular Cell Biology 1: 40–49. [Google Scholar]
Mothana RA, Awadh Ali NA, Jansen R, Wegner U, Mentel R et al. (2003). Antiviral lanostanoid triterpenes from the fungus Ganoderma pfeifferi. Fitoterapia 74: 177–180. [Google Scholar]
Murphy FA, Fauquet CM, Bishop DH, Ghabrial SA, Jarvis AW et al. (2012). Virus Taxonomy: Classification and Nomenclature of Viruses. Sixth Report of the International Committee on Taxonomy of Viruses. Wien: Springer Science & Business Media. [Google Scholar]
Neuman H, Koren O (2017). The gut microbiota: A possible factor influencing systemic lupus erythematosus. Current Opinion in Rheumatology 29: 374–377. [Google Scholar]
Nienhuis AW, Nathwani AC, Davidoff AM (2017). Gene therapy for hemophilia. Molecular Therapy 25: 1163–1167. [Google Scholar]
Ohta Y, Lee JB, Hayashi K, Fujita A, Park DK et al. (2007). In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. Journal of Agricultural and Food Chemistry 55: 10194–10199. [Google Scholar]
Peng J, Lin T, Wang W, Xin Z, Zhu T et al. (2013). Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41. Journal of Natural Products 76: 1133–1140. [Google Scholar]
Peyrat LA, Eparvier V, Eydoux C, Guillemot JC, Litaudon M et al. (2020). Carneic acids from an endophytic Phomopsis sp. as dengue virus polymerase inhibitors. Journal of Natural Products 83: 2330–2336. [Google Scholar]
Qin C, Lin X, Lu X, Wan J, Zhou X et al. (2015). Sesquiterpenoids and xanthones derivatives produced by sponge-derived fungus Stachybotry sp. HH1 ZSDS1F1-2. Journal of Antibiotics 68: 121–125. [Google Scholar]
Ren L, Perera C, Hemar Y (2012). Antitumor activity of mushroom polysaccharides: A review. Food & Function 3: 1118–1130. [Google Scholar]
Sacramento DR, Coelho RRR, Wigg MD, Linhares LFDTL, Dos Santos MGM et al. (2004). Antimicrobial and antiviral activities of an actinomycete (Streptomyces sp.) isolated from a Brazilian tropical forest soil. World Journal of Microbiology Biotechnology 20: 225–229. [Google Scholar]
Said ZN, Abdelwahab KS (2013). Antiviral replication agents. Viral Replication, 127–144. [Google Scholar]
Scheu S, Simmerling F (2004). Growth and reproduction of fungal feeding Collembola as affected by fungal species, melanin and mixed diets. Oecologia 139: 347–353. [Google Scholar]
Schulz B, Boyle C, Draeger S, Römmert AK, Krohn K (2002). Endophytic fungi: A source of novel biologically active secondary metabolites. Mycological Research 106: 996–1004. DOI 10.1017/S0953756202006342. [Google Scholar] [CrossRef]
Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R (2020). COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research 24: 91–98. DOI 10.1016/j.jare.2020.03.005. [Google Scholar] [CrossRef]
Spinelli A, Pellino G (2020). COVID-19 pandemic: Perspectives on an unfolding crisis. Journal of British Surgery 107: 785–787. DOI 10.1002/bjs.11627. [Google Scholar] [CrossRef]
Srinivasa Rao ASR, Vazquez JA (2020). Identification of COVID-19 can be quicker through artificial intelligence framework using a mobile phone-based survey when cities and towns are under quarantine. Infection Control and Hospital Epidemiology 41: 826–830. DOI 10.1017/ice.2020.61. [Google Scholar] [CrossRef]
Stiver G (2003). The treatment of influenza with antiviral drugs. Canadian Medical Association Journal 168: 49–56. [Google Scholar]
Sun JF, Lin X, Zhou XF, Wan J, Zhang T et al. (2014). Pestalols A-E, new alkenyl phenol and benzaldehyde derivatives from endophytic fungus Pestalotiopsis sp. AcBC2 isolated from the Chinese mangrove plant Aegiceras corniculatum. Journal of Antibiotics 67: 451–457. [Google Scholar]
Tan QW, Ouyang MA, Shen S, Li W (2012). Bioactive metabolites from a marine-derived strain of the fungus Neosartorya fischeri. Natural Product Research 26: 1402–1407. [Google Scholar]
Tian YQ, Lin XP, Wang Z, Zhou XF, Qin XC et al. (2016). Asteltoxins with antiviral activities from the marine sponge-derived fungus Aspergillus sp. SCSIO XWS02F40. Molecules 21: 34. [Google Scholar]
Trastoy B, Du JJ, Klontz EH, Li C, Cifuente JO et al. (2020). Structural basis of mammalian high-mannose N-glycan processing by human gut Bacteroides. Nature Communications 11: 899. [Google Scholar]
Valverde RA, Sabanadzovic S, Hammond J (2012). Viruses that enhance the aesthetics of some ornamental plants: Beauty or beast? Plant Disease 96: 600–611. [Google Scholar]
Vigant F, Santos NC, Lee B (2015). Broad-spectrum antivirals against viral fusion. Nature Reviews Microbiology 13: 426–437. DOI 10.1038/nrmicro3475. [Google Scholar] [CrossRef]
Wang Q, Li H, Chen T, Han J (2012). Yield, polysaccharides content and antioxidant properties of Pleurotus abalonus and Pleurotus geesteranus produced on asparagus straw as substrate. Scientia Horticulturae 134: 222–226. [Google Scholar]
Wang JF, Liang R, Liao SR, Yang B, Tu ZC et al. (2017). Vaccinols J-S, ten new salicyloid derivatives from the marine mangrove-derived endophytic fungus Pestalotiopsis vaccinii. Fitoterapia 120: 164–170. [Google Scholar]
Wang J, Wei X, Lu X, Xu F, Wan J et al. (2014). Eight new polyketide metabolites from the fungus Pestalotiopsis vaccinii endogenous with the mangrove plant Kandelia candel (L.) Druce. Tetrahedron 70: 9695–9701. [Google Scholar]
Wang XY, Zhang DD, Yin JY, Nie SP, Xie MY (2019). Recent developments in Hericium erinaceus polysaccharides: extraction, purification, structural characteristics and biological activities. Critical Reviews in Food Science Nutrition 59: S96–S115. [Google Scholar]
Xia Y, Wu Q, Wang H, Zhang S, Jiang Y et al. (2020). Global, regional and national burden of gout, 1990–2017: A systematic analysis of the Global Burden of Disease Study. Rheumatology 59: 1529–1538. [Google Scholar]
Xie J, Wu YY, Zhang TY, Zhang MY, Zhu WW et al. (2017). New and bioactive natural products from an endophyte of Panax notoginseng. RSC Advances 7: 38100–38109. [Google Scholar]
Xu P, Chen F, Mannas JP, Feldman T, Sumner LW, Roossinck MJ (2008). Virus infection improves drought tolerance. New Phytologist 180: 911–921. [Google Scholar]
Yang ZJ, Zhang YF, Wu K, Xu YX, Meng XG et al. (2020). New azaphilones, phomopsones AC with biological activities from an endophytic fungus Phomopsis sp. CGMCC No. 5416. Fitoterapia 145: 104573. [Google Scholar]
Yu JH, Keller N (2005). Regulation of secondary metabolism in filamentous fungi. Annual Review of Phytopathology 43: 437–458. [Google Scholar]
Yu G, Zhou G, Zhu M, Wang W, Zhu T et al. (2016). Neosartoryadins A and B, fumiquinazoline alkaloids from a mangrove-derived fungus Neosartorya udagawae HDN13-313. Organic Letters 18: 244–247. [Google Scholar]
Yuan B, Wu Z, Ji W, Liu D, Guo X et al. (2021). Discovery of cyclohexadepsipeptides with anti-Zika virus activities and biosynthesis of the nonproteinogenic building block (3S)-methyl-l-proline. Journal of Biological Chemistry 297: 100822. [Google Scholar]
Zhang SP, Huang R, Li FF, Wei HX, Fang XW et al. (2016). Antiviral anthraquinones and azaphilones produced by an endophytic fungus Nigrospora sp. from Aconitum carmichaeli. Fitoterapia 112: 85–89. [Google Scholar]
Zhang M, Li N, Chen R, Zou J, Wang C, Dai J (2014). Two terpenoids and a polyketide from the endophytic fungus Trichoderma sp. Xy24 isolated from mangrove plant Xylocarpus granatum. Journal of Chinese Pharmaceutical Sciences 23: 421–424. [Google Scholar]
Zhang M, Zhang L, Wang Y, Cheung PC (2003). Chain conformation of sulfated derivatives of beta-glucan from sclerotia of Pleurotus tuber-regium. Carbohydrate Research 338: 2863–2870. [Google Scholar]
Zhao Y, Liu D, Proksch P, Zhou D, Lin W (2018). Truncateols OV, further isoprenylated cyclohexanols from the sponge-associated fungus Truncatella angustata with antiviral activities. Phytochemistry 155: 61–68. [Google Scholar]
Zheng CJ, Shao CL, Guo ZY, Chen JF, Deng DS et al. (2012). Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. Journal of Natural Products 75: 189–197. [Google Scholar]
Zhou P, Yang XL, Wang XG, Hu B, Zhang L et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |