DOI:10.32604/biocell.2022.020570

| BIOCELL DOI:10.32604/biocell.2022.020570 |  |
| Review |
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR): A critical overview on the most promising applications of molecular scissors in oral medicine
1Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari Aldo Moro, Bari, 74124, Italy
2Department of Interdisciplinary Medicine, University of Bari Aldo Moro, Bari, 74124, Italy
3Marrelli Health-Tecnologica Research Institute, Biomedical Section, Stem Cells and Medical Genetics Units, Crotone, 88900, Italy
4Department of Neurosciences, Reproductive and Odontostomatological Sciences, University of Naples Federico II, Naples, 80131, Italy
*Address correspondence to: Marco Tatullo, marco.tatullo@uniba.it
Received: 01 December 2021; Accepted: 27 January 2022
Abstract: The scientific community is continuously working to translate the novel biomedical techniques into effective medical treatments. CRISPR-Cas9 system (Clustered Regularly Interspaced Short Palindromic Repeats-9), commonly known as the “molecular scissor”, represents a recently developed biotechnology able to improve the quality and the efficacy of traditional treatments, related to several human diseases, such as chronic diseases, neurodegenerative pathologies and, interestingly, oral diseases. Of course, dental medicine has notably increased the use of biotechnologies to ensure modern and conservative approaches: in this landscape, the use of CRISPR-Cas9 system may speed and personalize the traditional therapies, ensuring a good predictability of clinical results. The aim of this critical overview is to provide evidence on CRISPR efficacy, taking into specific account its applications in oral medicine.
Keywords: Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR); Dentistry; Stem cells
The scientific community has increasingly focused attention towards the last innovations in medicine. Over the years, scientific knowledge has been ever more created in specific sectors, such as the human genetics, also thank to the breakthrough boosted by the modern technologies working on genome editing. In fact, through artificial nucleases such as zinc-finger (ZNF) and activators of the nucleases of the effector of the transcription (TALENs), researchers can modify defective genes without any pharmacological support (Zhang et al., 2019; Huang et al., 2020; Yu et al., 2021a, 2021b).
The last scientific research has focused on CRISPR-Cas9 system (Clustered Regularly Interspaced Short Palindromic Repeats-9), most commonly known as “molecular scissors” (Bao et al., 2019). CRISPR is a versatile approach that allows physicians to edit nearly several loci in the human genome; this ability is currently investigated to develop innovative therapies against a large number of diseases. This system confers resistance to the phages containing sequences similar to the ones showed in CRISPR system; CRISPR loci typically consist of short fragments of DNA of viral origin, identically repeated (CRISPR-RNA), trans-activating the crRNA (trans-crRNA) and a series of genes coding for CAS endonucleases (Gong et al., 2021; Liu et al., 2021). These endonucleases using a leading strand of RNA (crRNA) and cutting DNA in specific loci, allow the insertion of the sequence where it is necessary (Jiang and Doudna, 2017).
Currently, there in a notable increase of research focused to the development of this system in the treatment human diseases, such as HIV/AIDS (Xiao et al., 2019), Malaria, Epstein Barr Viruses (Rodriguez-Rodriguez et al., 2019), chronic granulomatous diseases (de Ravin et al., 2017), heart diseases (Gifford et al., 2019), Alzheimer’s disease (Kurochkin et al., 2018), and recently also a possible application in the diagnosis of SARS-CoV-2 related syndromes (Esbin et al., 2020) and specific dental diseases (Divaris, 2019; Gong et al., 2020).
On the other hand, it has been observed a correlation between patient with diabetes, inflammatory bowel disease, obesity, and oral diseases (Le Bars et al., 2017).
The aim of this critical overview is to provide evidence on CRISPR efficacy, taking into specific account its applications in oral medicine.
Oral tissues are generally contaminated by bacteria able to promote, or worsen, several oral pathologies (Curtis et al., 2020). Dental decays, periodontitis and other gingival infections are often co-caused by bacterial flora hosted in dental plaque: many oral bacteria have been demonstrated to be linked to several systemic infections (Agbo-Godeau, 2019).
CRISPR loci are frequently located on sites residing in human oral microbiota (Toyomane et al., 2021); such loci may be a trigger to modulate the overall behaviour of oral microbiota (Akram et al., 2020). The comparison between healthy patients and those affected by periodontitis has revealed that CRISPR loci found in healthy patients are able to identify a bacterial community characterized by a resistance to the bacteriophages better than the one present in patients affected by periodontitis (Gong et al., 2019; Steier et al., 2019). Cas9 was detected in gingival epithelial cells, gingival fibroblasts, and inflammatory infiltration cells (Steier et al., 2019). The modulation of local inflammation seems to be concretely guided by the association of superoxide dismutase 2 and BIRC3; moreover, some recent trials have suggested an increase of Sod2, Birc3, Casp3 e Casp9 in the gingiva of subjects with periodontitis (Yoon et al., 2018; Deschner et al., 2021). The use of CD40 as a biological target has demonstrated how this system inhibits the development of local inflammation after injection of antibodies of anti-CD40 in the inflammatory bowel diseases (Anka Idrissi et al., 2021; Wang et al., 2020a). Similarly, recent studies on periodontitis have highlighted a pivotal pathogenetic role of the oxidative stress reactive oxygen species (ROS); ROS are produced by bacteria (Oveisi et al., 2019; Sulijaya et al., 2019; Zeng et al., 2019) and a key factor for oxidative stress is the regulation of Nerf2, which has been reported to have a protective role in many oral pathologies. Furthermore, Keap1 allows the translocation of Nerf2 in the cell core, where it activates the transcription of genes with antioxidant function. Therefore, Nrf2 activity is critical for the balance of redox homeostasis of the cell (Shaw and Chattopadhyay, 2020). Moreover, Nrf2 activation seems to reduce the activation of the Nlp3 inflammasome, by suppressing the production of ROS (Liu et al., 2017; Saha et al., 2020).
CRISPR also allows the deletion of NLRP3 inflammasome by stopping its activation during the transition process from epithelium to mesenchymal in the fibrosis (Alyaseer et al., 2020). As regards craniofacial abnormalities, instead, in zebrafish, CRISPR technique has been used to inhibit WNT-associated gene lrp5 (Hao et al., 2019).
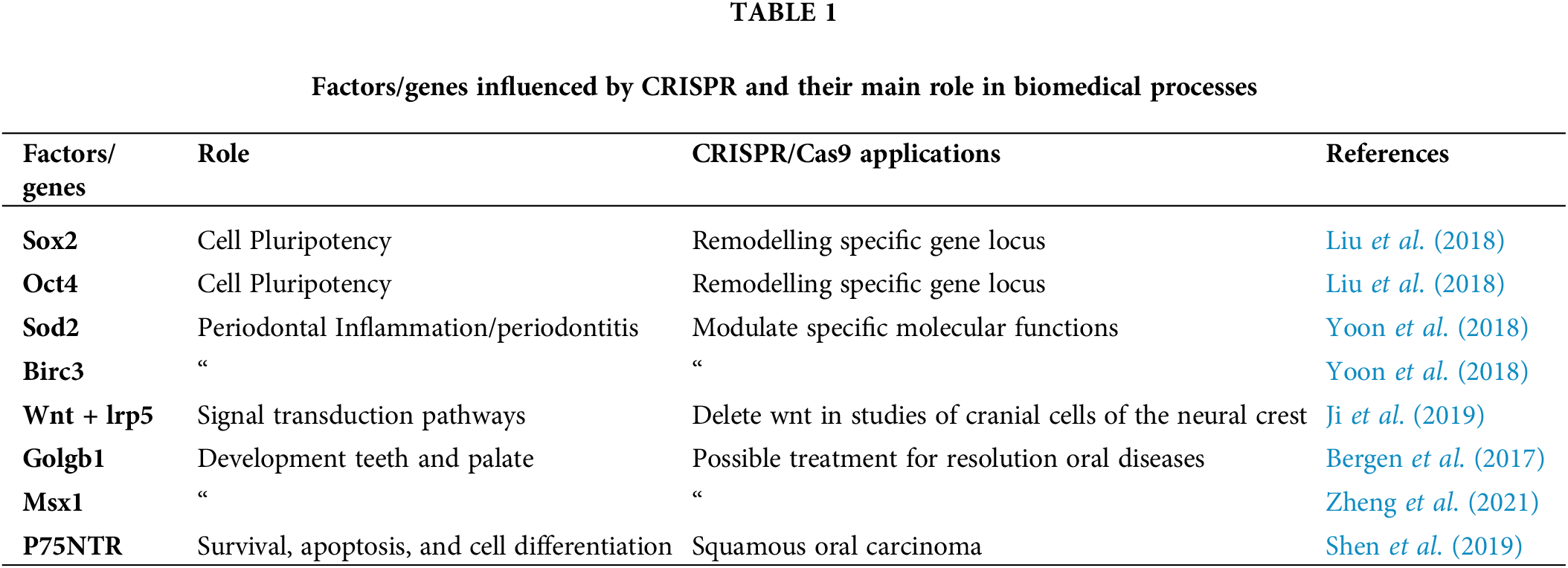
In the studies on craniofacial development, CRISPR-based genome modification system accelerated the formation of animal models thanks to the rapid manipulation of specific genes (Wu et al., 2019). Knockout mice generated by CRISPR/Cas9 have showed the importance of Golgb1 and MSX 1 in the development of teeth, palate, and normal cilia function in zebrafish (Zheng et al., 2021; Bergen et al., 2017). In fact, mutations of the transcriptional factor MSX1 cause cranio-facial malformations and agenesia of teeth (Goto et al., 2016).
Genome-editing with CRISPR-Cas9 represents the most important starting point for the resolution of the previously untreatable diseases (Zhan et al., 2019). An example is the identification of various therapeutic targets, such as p75NTR and its receptor, in the treatment of the oesophageal squamous cell carcinoma and tooth morphogenesis (Shen et al., 2019; Zhao et al., 2019). The receptor p75NTR carries out different functions impacting cell survival, apoptosis, differentiation (Meier et al., 2019) it is expressed in the neural crest cell population (Wislet et al., 2018), mouse alveolar bone cells (Wang et al., 2020b) and human oesophageal keratinocyte stem cells (Daltoe et al., 2020). Another intriguing use of this system is within the protocol that allows to study the human epithelial cells exosomes released from the oral mucosa infected by EV71 (Enterovirus 71) (Wang et al., 2020c). Thus, the well-recognized genome editing ability of the CRISPR-Cas system has triggered significant advances in CRISPR diagnostics and potential treatments related to oral medicine.
CRISPR/Cas9 and modified oral-derived stem cells
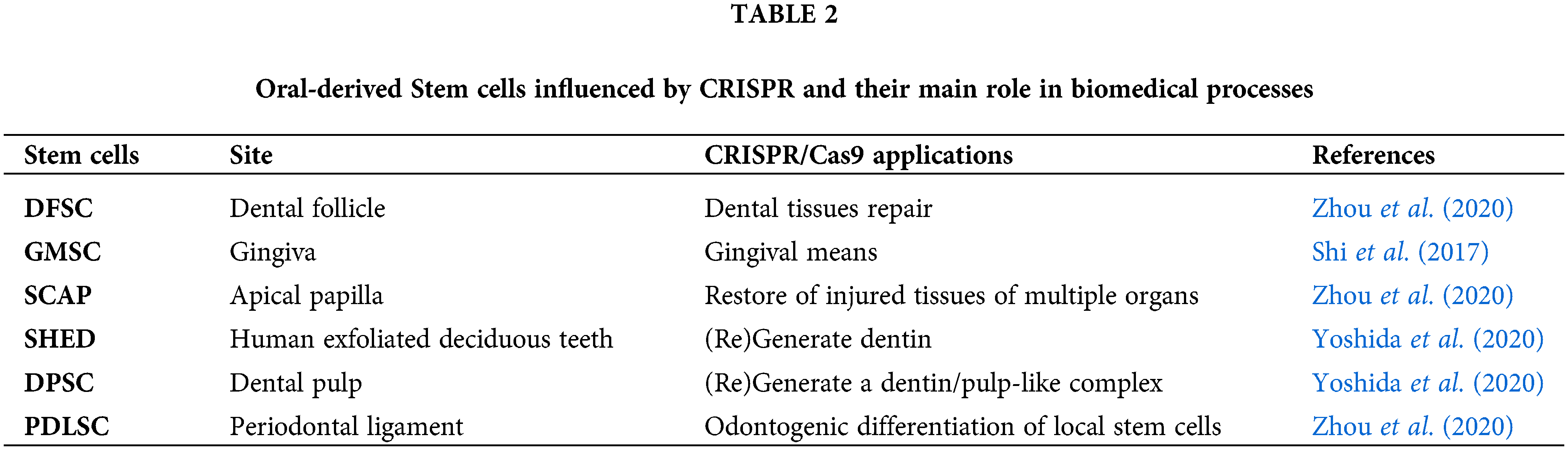
There are different aspects of stem cells that can be strongly influenced by CRISPR (Table 1). The changes promoted by CRISPR-Cas9 on mesenchymal stem cells can be useful for the correction of several pathological defects. Stem cells have been isolated from different human tissues, including the dental tissues (Berebichez-Fridman and Montero-Olvera, 2018; Granz and Gorji, 2020; Yoshida et al., 2020): DPSC (dental pulp stem cells), PDLSC (periodontal ligament stem cells), SHED (mesenchymal stem cells from human exfoliated deciduous teeth), GMSC (mesenchymal gingival stem cells), DFSC (dental follicle stem cells), and SCAP (apical papilla stem cells) have active role in the healing of bone tissues, such as the post-extractive sockets; moreover, PDLSC and GMSC have demonstrated osteogenic and regenerative potential in vitro (Yoshida et al., 2020). On the other hand, GMSC have been investigated and successfully used to promote regeneration in subjects with severe periodontal defects (Table 2) (Zhou et al., 2020; Liu et al., 2019; Shi et al., 2017). In recent studies, it has been observed that stem cells modified by CRISPRS may support a better and faster tissue regeneration (Sürün et al., 2020; Ben Jehuda et al., 2018): the biological pathways potentially involved in this biological behaviour is related to the increase of the expression of Oct4, Sox2, and Klf7 (Liu et al., 2018; Corbineau et al., 2017; Yang et al., 2020). To date, researchers are working on the potential application of genome editing to the treatment of several systemic disorders (Frangoul et al., 2021). As an example, the sickle cell anaemia has already taken advantage from other techniques, such as Zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) two chimeric nucleases linked to a non-specific DNA cleavage domain (Demirci et al., 2019). In the patients with Fanconi anaemia, somatic cells have been genetically reprogrammed towards oral-derived induced-Pluripotent Stem Cells (iPSCs) with CRISPR/Cas9 system to treat patients with gene-therapy; also, the Diamond-Blackfan anaemia has shown interesting results from studies based on the inactivation of the p53 gene into modifies pre-erythrocytes obtained from such iPSCs (Kapralova et al., 2020). Recently, CRISPR-Cas9 system has been used to achieve efficient treatments based on gene silencing and insertion of gene sequences in oral-derived MSCs (Komor et al., 2017). Interestingly, significant results have been achieved with the use of CRISPR-Cas9 system in the therapy of diabetes mellitus associated with Wolfram syndrome, a rare and untreated disease associated with a defect of WFS1 gene (Maxwell et al., 2020).
Other strategies are related the production of oral-derived stem cells carrying a correction of faulty gene expressed on LDL receptors that cause homozygous familial hypercholesterolemia (Okada et al., 2019). Finally, it has been obtained a significant slowdown of the aging process, and a doubling in life expectancy, in mice-models suffering by progeria, treated with iPSCs modified with CRISPS-Cas9 technique (Santiago-Fernández et al., 2019).
In the last decades, a consistent scientific literature has widely debated about a novel way to approach translational medicine. Recently, several biological pathways have been investigated because of their impact on important clinical aspects. Interestingly, scientific researchers have discovered a number of biochemical and genetic mechanisms belonging to ancestral pathways able to deeply modulate the biology of complex organisms, such as the pathway that makes the bacteria able to deactivate their ability to damage the host organism (Gong et al., 2019; Chen et al., 2019). In this landscape, it was recently identified the biological strategy able to turn-off CRISPR system: it was called “anti-CRISPR” mechanism. In detail, the phages are able to produce the anti-CRISPR proteins named ACR: those proteins are able to stop the molecular scissor Cas, avoiding that Cas will be able to cut and modify the human genome (Marino et al., 2020). The function of anti-CRISPR mechanisms was investigated and fully demonstrated, basically, administering AcrII4 to human cells, obtaining that the anti-CRISPR mechanism was enabled and working (Kim et al., 2018). Currently, the biological strategies introduced by the recent discovery of the anti-CRISPR system has been turned into studies aimed to understand the so called “off-target systemic effects” (Shin et al., 2017). In a study on this matter, it was described an anti-CRISPR pathway that blocks the genomic editing in every organ of the body, unless in the liver; in fact, in the hepatocytes it has been found a tissue-specific micro-RNA (microRNA-122), still not completely understood (Lee et al., 2019).
It is even more evident that the anti-CRISPR strategy may represent a safeguard mechanism, highly used by simple organisms like the bacteria, which may have pathogenetic role, still not fully under-stood, in complex metabolisms also involving the human tissues. The anti-CRISPR mechanisms may have an overall function to promote inflammation in several acute conditions; in fact, they are often recognized as “non-self” by the human immune system, and this specific condition would result in a severe inflammatory response involving several organs (Liu et al., 2020).
In conclusion, CRISPR and anti-CRISPR are two specular mechanisms that offer the potential control of human genome. Unfortunately, both can result in collateral severe consequences that make them not safe to manage. Researchers are closely working toward the identification of biological molecules able to turn-off Cas9 in a safe procedure, in order not to activate the immune response (Hille et al., 2018). One the major challenges is to clarify the mechanisms to by-pass the immune system, thus making CRISPR and anti-CRISPR able to work in human organism; currently, the investigations are based on the molecules involved in the activation of these mechanisms, and on their physiological effects (Fu et al., 2021). Many enzymes have been certainly associated to CRISPR: one interesting example is the Cpf1 enzyme that is similar to Cas9; it has a number of deactivated forms that can be useful for the study of Cas13 transcriptional effects (Safari et al., 2019) and RNA adenosine deaminase transfer. To fully understand these mechanisms, we need to fully understand the role of genome editing not only related to the potential advantages, but more importantly to the hypothetical damages; in fact, several enzymes can work on DNA, also converting the AT couple to GC in the target position inside the genome without breaking the double-stranded of DNA (Gaudelli et al., 2017). Nevertheless, also this system may have some issues; in fact, genome editing efficiency may be influenced by several factors, also able to create mutations elsewhere in the genome, known as ‘off-target’ modifications.
We strive to safely manage these biological scissors, and the complete understanding of their use will certainly change the way of performing the future medicine.
In this overview, we have tried to clarify the latest breakthrough on CRISPR, as it represents a valuable tool in the field of genetics and epigenetics. The impact of CRISPR and anti-CRISPR systems can improve the understanding of several oral pathology and may impact the way to make diagnosis and therapy of all the main diseases. Unfortunately, such systems allow to directly manipulate the human genome, for example eliminating a gene responsible for a specific pathology, even if we do not fully know the collateral impact of these modifications; nevertheless, there are many unanswered questions on the ethical implications regarding embryonic stem cell lines/germ lines. Undoubtedly, CRISPR-Cas system may be involved in more further strategies, rather than the only immunological tasks. Promising insights could be carried out from studies aimed at a deeper understanding of this tool on several impacting oral diseases, such as the periodontitis, targeting the research on why P. gingivalis plays a pivotal role in that class of diseases. Also, pharmacological target may be studied for several inflammatory pathologies affecting the oral mucosa; in fact, cutting-edge CRISPR/Cas9-based technology may transform the field of oral pathology research by efficiently introducing genetic alterations, so to investigate the main genes function in experimental models of different oral pathologies. The hope is to develop more consistent knowledge in the translational applications of genome editing applied to oral pathology and, more in general, to medical sciences.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: MT, LL, RMM, AV, AT, SR; data collection: LL, RMM, AV, AT. Analysis and interpretation of results: MT, SR; draft manuscript preparation: MT, AV, SR. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Agbo-Godeau S (2019). Pathologies bénignes de la muqueuse buccale [Benign pathologies of the oral mucosa]. Revue du Praticien 69: 850–855. [Google Scholar]
Akram F, Ikram Ul, Ahmed Z, Khan H, Ali MS (2020). CRISPR-Cas9, a promising therapeutic tool for cancer therapy: A review. Protein and Peptide Letters 27: 931–944. DOI 10.2174/0929866527666200407112432. [Google Scholar] [CrossRef]
Alyaseer AA, de Lima MH, Braga TT (2020). The role of NLRP3 inflammasome activation in the epithelial to mesenchymal transition process during the fibrosis. Frontiers in Immunology 11: 883. DOI 10.3389/fimmu.2020.00883. [Google Scholar] [CrossRef]
Anka Idrissi D, Senhaji N, Aouiss A, Khalki L, Tijani Y et al. (2021). IL−1 and CD40/CD40L platelet complex: Elements of induction of Crohn’s disease and new therapeutic targets. Archives of Pharmacal Research 44: 117–132. DOI 10.1007/s12272-020-01296-1. [Google Scholar] [CrossRef]
Bao A, Burritt DJ, Chen H, Zhou X, Cao D et al. (2019). The CRISPR/Cas9 system and its applications in crop genome editing. Critical Reviews Biotechnology 39: 321–336. DOI 10.1080/07388551.2018.1554621. [Google Scholar] [CrossRef]
Ben Jehuda R, Shemer Y, Binah O (2018). Genome editing in induced pluripotent stem cells using CRISPR/Cas9. Stem Cell Reviews and Reports 14: 323–336. DOI 10.1007/s12015-018-9811-3. [Google Scholar] [CrossRef]
Berebichez-Fridman R, Montero-Olvera PR (2018). Sources and clinical applications of mesenchymal stem cells: State-of-the-art review. Sultan Qaboos University Medical Journal 18: e264–e277. DOI 10.18295/squmj.2018.18.03.002. [Google Scholar] [CrossRef]
Bergen DJ, Stevenson NL, Skinner RE, Stephens DJ, Hammond CL (2017). The Golgi matrix protein giantin is required for normal cilia function in zebrafish. Biology Open 6: 1180–1189. [Google Scholar]
Chen K, Wang Y, Zhang R, Zhang H, Gao C (2019). CRISPR/Cas genome editing e precision plant breeding in agriculture. Annual Review of Plant Biology 70: 667–697. DOI 10.1146/annurev-arplant-050718-100049. [Google Scholar] [CrossRef]
Corbineau S, Lassalle B, Givelet M, Souissi-Sarahoui I, Firlej V et al. (2017). Spermatogonial stem cells and progenitors are refractory to reprogramming to pluripotency by the transcription factors Oct3/4, c-Myc, Sox2 and Klf4. Oncotarget 8: 10050–10063. DOI 10.18632/oncotarget.14327. [Google Scholar] [CrossRef]
Curtis MA, Diaz PI, van Dyke TE (2020). The role of the microbiota in periodontal disease. Periodontology 2000 83: 14–25. DOI 10.1111/prd.12296. [Google Scholar] [CrossRef]
Daltoe FP, Oliveira NA, Peron CN, Sharpe PT, Mantesso A (2020). Phenotype changes of oral epithelial stem cells after in vitro culture. Brazilian Oral Research 34: e033. DOI 10.1590/1807-3107bor-2020.vol34.0033. [Google Scholar] [CrossRef]
De Ravin SS, Li L, Wu X, Choi U, Allen C et al. (2017). CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granuloma-tous disease. Science Translational Medicine 9: eaah3480. DOI 10.1126/scitranslmed.aah3480. [Google Scholar] [CrossRef]
Demirci S, Leonard A, Haro-Mora JJ, Uchida N, Tisdale JF (2019). CRISPR/Cas9 for sickle cell disease: Applica-tions, future possibilities, and challenges. Advances in Experimental Medicine and Biology 1144: 37–52. DOI 10.1007/978-3-030-17589-4. [Google Scholar] [CrossRef]
Deschner B, Nogueira AV, Memmert S, Nokhbehsaim M, Augusto Cirelli J et al. (2021). Regulation of anti-apoptotic SOD2 and BIRC3 in periodontal cells and tissues. International Journal of Molecular Sciences 22: 591. DOI 10.3390/ijms22020591. [Google Scholar] [CrossRef]
Divaris K (2019). The era of the genome and dental medicine. Journal of Dental Research 98: 949–955. DOI 10.1177/0022034519845674. [Google Scholar] [CrossRef]
Esbin MN, Whitney ON, Chong S, Maurer A, Darzacq X et al. (2020). Overcoming the bottleneck to wide-spread testing: A rapid review of nucleic acid testing approaches for COVID-19 detection. RNA 26: 771–783. DOI 10.1261/rna.076232.120. [Google Scholar] [CrossRef]
Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J et al. (2021). CRISPR-Cas9 gene editing for sickle cell disease and β-Thalassemia. New England Journal of Medicine 384: 252–260. DOI 10.1056/NEJMoa2031054. [Google Scholar] [CrossRef]
Fu Y, Zhu Z, Meng G, Zhang R, Zhang Y (2021). A CRISPR-Cas9 based shuffle system for endogenous histone H3 and H4 combinatorial mutagenesis. Scientific Reports 11: 3298. DOI 10.1038/s41598-021-82774-4. [Google Scholar] [CrossRef]
Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH et al. (2017). Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551: 464–471. DOI 10.1038/nature24644. [Google Scholar] [CrossRef]
Gifford CA, Ranade SS, Samarakoon R, Salunga HT, de Soysa TY et al. (2019). Oligogenic inheritance of a human heart disease involving a genetic modifier. Science 364: 865–870. DOI 10.1126/science.aat5056. [Google Scholar] [CrossRef]
Gong H, Wu Y, Zeng R, Zeng Y, Liu X et al. (2021). CRISPR/Cas12a-mediated liposome-amplified strategy for the photoelectrochemical detection of nucleic acid. Chemical Communications 57: 8977–8980. DOI 10.1039/D1CC03743A. [Google Scholar] [CrossRef]
Gong T, Lu M, Zhou X, Zhang A, Tang B et al. (2019). CRISPR-Cas systems in streptococci. Current Issues in Molecular Biology 32: 1–38. [Google Scholar]
Gong T, Zeng J, Tang B, Zhou X, Li Y (2020). CRISPR-Cas systems in oral microbiome: From immune defense to physiological regulation. Molecular Oral Microbiology 35: 41–48. DOI 10.1111/omi.12279. [Google Scholar] [CrossRef]
Goto N, Fujimoto K, Fujii S, Ida-Yonemochi H, Ohshima H et al. (2016). Role of MSX1 in osteogenic differentiation of human dental pulp stem cells. Stem Cells International 2016: 8035759. DOI 10.1155/2016/8035759. [Google Scholar] [CrossRef]
Granz CL, Gorji A (2020). Dental stem cells: The role of biomaterials and scaffolds in developing novel therapeutic strategies. World Journal of Stem Cells 12: 897–921. DOI 10.4252/wjsc.v12.i9.897. [Google Scholar] [CrossRef]
Hao Y, Tang S, Yuan Y, Liu R, Chen Q (2019). Roles of FGF8 subfamily in embryogenesis and oral–maxillofacial diseases (Review). International Journal of Oncology 54: 797–806. DOI 10.3892/ijo.2019.4677. [Google Scholar] [CrossRef]
Hille F, Richter H, Wong SP, Bratovič M, Ressel S et al. (2018). The biology of CRISPR-Cas: Backward and forward. Cell 172: 1239–1259. DOI 10.1016/j.cell.2017.11.032. [Google Scholar] [CrossRef]
Huang L, Chen J, Yu Z, Tang D (2020). Self-powered temperature sensor with seebeck effect transduction for photothermal-thermoelectric coupled immunoassay. Analytical Chemistry 92: 2809–2814. DOI 10.1021/acs.analchem.9b05218. [Google Scholar] [CrossRef]
Ji Y, Hao H, Reynolds K, McMahon M, Zhou CJ (2019). Wnt signaling in neural crest ontogenesis and oncogenesis. Cells 8: 1173. DOI 10.3390/cells8101173. [Google Scholar] [CrossRef]
Jiang F, Doudna JA (2017). CRISPR-Cas9 structures and mechanisms. Annual Review of Biophysics 46: 505–529. DOI 10.1146/annurev-biophys-062215-010822. [Google Scholar] [CrossRef]
Kapralova K, Jahoda O, Koralkova P, Gursky J, Lanikova L et al. (2020). Ox-idative DNA damage, inflammatory signature, and altered erythrocytes properties in diamond-blackfan anemia. International Journal of Molecular Sciences 21: 9652. DOI 10.3390/ijms21249652. [Google Scholar] [CrossRef]
Kim I, Jeong M, Ka D, Han M, Kim NK et al. (2018). Solution structure and dynamics of anti-CRISPR AcrIIA4, the Cas9 inhibitor. Scientific Reports 8: 3883. DOI 10.1038/s41598-018-22177-0. [Google Scholar] [CrossRef]
Komor AC, Badran AH, Liu DR (2017). CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell 168: 20–36. DOI 10.1016/j.cell.2016.10.044. [Google Scholar] [CrossRef]
Kurochkin IV, Guarnera E, Berezovsky IN (2018). Insulin-degrading enzyme in the fight against Alzheimer’s disease. Trends in Pharmacological Sciences 39: 49–58. DOI 10.1016/j.tips.2017.10.008. [Google Scholar] [CrossRef]
Le Bars P, Matamoros S, Montassier E, Le Vacon F, Potel G et al. (2017). The oral cavity microbiota: Between health, oral disease, and cancers of the aerodigestive tract. Canadian Journal of Microbiology 63: 475–492. DOI 10.1139/cjm-2016-0603. [Google Scholar] [CrossRef]
Lee J, Mou H, Ibraheim R, Liang SQ, Liu P et al. (2019). Tissue-restricted genome editing in vivo specified by microRNA-repressible anti-CRISPR proteins. RNA 25: 1421–1431. DOI 10.1261/rna.071704.119. [Google Scholar] [CrossRef]
Liu J, Chen B, Bao J, Zhang Y, Lei L et al. (2019). Macrophage polarization in periodontal ligament stem cells en-hanced periodontal regeneration. International Journal of Stem cell Research & Therapy 10: 320. DOI 10.1186/s13287-019-1409-4. [Google Scholar] [CrossRef]
Liu J, Wan Q, Zeng R, Tang D (2021). An ultrasensitive homogeneous electrochemical biosensor based on CRISPR-Cas12a. Analytical Methods 13: 3227–3232. DOI 10.1039/D1AY00725D. [Google Scholar] [CrossRef]
Liu P, Chen M, Liu Y, Qi LS, Ding S (2018). CRISPR-Based chromatin remodeling of the endogenous Oct4 or Sox2 locus enables reprogramming to pluripotency. Cell Stem Cell 22: 252–261. DOI 10.1016/j.stem.2017.12.001. [Google Scholar] [CrossRef]
Liu Q, Zhang H, Huang X (2020). Anti-CRISPR proteins targeting the CRISPR-Cas system enrich the toolkit for genetic engineering. Biochemische Zeitschrift, European Journal of Biochemistry 287: 626–644. DOI 10.1111/febs.15139. [Google Scholar] [CrossRef]
Liu X, Zhang X, Ding Y, Zhou W, Tao L et al. (2017). Nuclear factor E2-related factor-2 nega-tively regulates NLRP3 inflammasome activity by inhibiting reactive oxygen species-induced NLRP3 priming. Antioxidants & Redox Signaling 26: 28–43. DOI 10.1089/ars.2015.6615. [Google Scholar] [CrossRef]
Marino ND, Pinilla-Redondo R, Csörgő B, Bondy-Denomy J (2020). Anti-CRISPR protein applications: Natural brakes for CRISPR-Cas technologies. Nature Methods 17: 471–479. DOI 10.1038/s41592-020-0771-6. [Google Scholar] [CrossRef]
Maxwell KG, Augsornworawat P, Velazco-Cruz L, Kim MH, Asada R et al. (2020). Gene-edited human stem cell-derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Science Translational Medicine 12: eaax9106. DOI 10.1126/scitranslmed.aax9106. [Google Scholar] [CrossRef]
Meier S, Alfonsi F, Kurniawan ND, Milne MR, Kasherman MA et al. (2019). The p75 neurotrophin receptor is required for the survival of neuronal progenitors and normal formation of the basal forebrain, striatum, thalamus and neocortex. Development 146: 181933. DOI 10.1242/dev.181933. [Google Scholar] [CrossRef]
Okada H, Nakanishi C, Yoshida S, Shimojima M, Yokawa J et al. (2019). Function and immunogenicity of gene-corrected iPSC-derived hepatocyte-like cells in restoring low density lipoprotein uptake in homozygous familial hy-percholesterolemia. Scientific Reports 9: 4695. DOI 10.1038/s41598-019-41056-w. [Google Scholar] [CrossRef]
Oveisi M, Shifman H, Fine N, Sun C, Glogauer N et al. (2019). Novel assay to character-ize neutrophil responses to oral biofilms. Infection and Immunity 87: e00790–18. [Google Scholar]
Rodriguez-Rodriguez DR, Ramirez-Solis R, Garza-Elizondo MA, Garza-Rodriguez MD, Barrera-Saldana HA (2019). Genome editing: A perspective on the application of CRISPR/Cas9 to study human diseases. International Journal of Molecular Medicine 43: 1559–1574. [Google Scholar]
Safari F, Zare K, Negahdaripour M, Barekati-Mowahed M, Ghasemi Y (2019). CRISPR Cpf1 proteins: Structure, func-tion and implications for genome editing. Cell and Bioscience 9: 36. DOI 10.1186/s13578-019-0298-7. [Google Scholar] [CrossRef]
Saha S, Buttari B, Panieri E, Profumo E, Saso L (2020). An overview of Nrf2 signaling pathway and its role in in-flammation. Molecules 25: 5474. DOI 10.3390/molecules25225474. [Google Scholar] [CrossRef]
Santiago-Fernández O, Osorio FG, Quesada V, Rodríguez F, Basso S et al. (2019). Development of a CRISPR/Cas9-based therapy for Hutchinson-Gilford progeria syndrome. Nature Medicine 25: 423–426. DOI 10.1038/s41591-018-0338-6. [Google Scholar] [CrossRef]
Shaw P, Chattopadhyay A (2020). Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. Journal of Cellular Physiology 235: 3119–3130. DOI 10.1002/jcp.29219. [Google Scholar] [CrossRef]
Shen DJ, Jiang YH, Tao KY (2019). Expression and clinical significance of p75NTR in esophageal squamous cell car-cinoma. Mathematical Biosciences and Engineering 16: 8060–8068. DOI 10.3934/mbe.2019405. [Google Scholar] [CrossRef]
Shi Q, Qian Z, Liu D, Sun J, Wang X et al. (2017). GMSC-Derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Frontiers in Physiology 8: 904. DOI 10.3389/fphys.2017.00904. [Google Scholar] [CrossRef]
Shin J, Jiang F, Liu JJ, Bray NL, Rauch BJ et al. (2017). Disabling Cas9 by an anti-CRISPR DNA mimic. Science Advances 3: e1701620. DOI 10.1126/sciadv.1701620. [Google Scholar] [CrossRef]
Steier L, de Oliveira SD, de Figueiredo JA (2019). Bacteriophages in dentistry-state of the art and perspectives. Dentistry Journal 7: 6. DOI 10.3390/dj7010006. [Google Scholar] [CrossRef]
Sulijaya B, Takahashi N, Yamazaki K (2019). Host modulation therapy using anti-inflammatory and antioxidant agents in periodontitis: A review to a clinical translation. Archives of Oral Biology 105: 72–80. DOI 10.1016/j.archoralbio.2019.07.002. [Google Scholar] [CrossRef]
Sürün D, Schneider A, Mircetic J, Neumann K, Lansing F et al. (2020). Efficient generation and correction of mutations in human iPS cells utilizing mRNAs of CRISPR base editors and prime editors. Genes 11: 511. DOI 10.3390/genes11050511. [Google Scholar] [CrossRef]
Toyomane K, Yokota R, Watanabe K, Akutsu T, Asahi A et al. (2021). Evaluation of CRISPR diversity in the human skin microbiome for personal identification. mSystems 6: e01255–20. DOI 10.1128/mSystems.01255-20. [Google Scholar] [CrossRef]
Wang R, Graham S, Sun N, McCarthy D, Peng R et al. (2020a). CRISPR/Cas9-targeting of CD40 in hematopoietic stem cells limits immune activation mediated by anti-CD40. PLoS One 15: e0228221. DOI 10.1371/journal.pone.0228221. [Google Scholar] [CrossRef]
Wang Y, Yang K, Li G, Liu R, Liu J et al. (2020b). Mice exhibit an al-veolar bone loss phenotype and inhibited PI3K/Akt/β-catenin pathway. Cell Proliferation 53: e12800. [Google Scholar]
Wang Y, Zhang S, Song W, Zhang W, Li J et al. (2020c). Exosomes from EV71-infected oral epithelial cells can transfer miR-30a to promote EV71 infection. Oral Diseases 26: 778–788. DOI 10.1111/odi.13283. [Google Scholar] [CrossRef]
Wislet S, Vandervelden G, Rogister B (2018). From neural crest development to cancer and vice versa: How p75NTR and (Pro)neurotrophins could act on cell migration and invasion? Frontiers in Molecular Neuroscience 11: 244. DOI 10.3389/fnmol.2018.00244. [Google Scholar] [CrossRef]
Wu N, Liu B, Du H, Zhao S, Li Y et al. (2019). Deciphering Disorders Involving Scoliosis and COmorbidities (DISCO) study, The progress of CRISPR/Cas9-mediated gene editing in generating mouse/zebrafish models of human skeletal diseases. Computational and Structural Biotechnology Journal 17: 954–962. DOI 10.1016/j.csbj.2019.06.006. [Google Scholar] [CrossRef]
Xiao Q, Guo D, Chen S (2019). Application of CRISPR/Cas9-Based gene editing in HIV-1/AIDS therapy. Frontiers in Cellular and Infection Microbiology 9: 69. DOI 10.3389/fcimb.2019.00069. [Google Scholar] [CrossRef]
Yang J, Xie K, Wang Z, Li C (2020). Elevated KLF7 levels may serve as a prognostic signature and might contribute to progression of squamous carcinoma. FEBS Open Bio 10: 1577–1586. DOI 10.1002/2211-5463.12912. [Google Scholar] [CrossRef]
Yoon Y, Kim TJ, Lee JM, Kim DY (2018). SOD2 is upregulated in periodontitis to reduce further inflammation progression. Oral Diseases 24: 1572–1580. DOI 10.1111/odi.12933. [Google Scholar] [CrossRef]
Yoshida S, Tomokiyo A, Hasegawa D, Hamano S, Sugii H et al. (2020). Insight into the role of dental pulp stem cells in regenerative therapy. Biology 9: 160. DOI 10.3390/biology9070160. [Google Scholar] [CrossRef]
Yu Z, Cai G, Liu X, Tang D (2021a). Pressure-based biosensor integrated with a flexible pressure sensor and an electrochromic device for visual detection. Analytical Chemistry 93: 2916–2925. DOI 10.1021/acs.analchem.0c04501. [Google Scholar] [CrossRef]
Yu Z, Gong H, Li Y, Xu J, Zhang J et al. (2021b). Chemiluminescence-derived self-powered photoelectrochemical immunoassay for detecting a low-abundance disease-related protein. Analytical Chemistry 93: 13389–13397. DOI 10.1021/acs.analchem.1c03344. [Google Scholar] [CrossRef]
Zeng MY, Miralda I, Armstrong CL, Uriarte SM, Bagaitkar J (2019). The roles of NADPH oxidase in modulating neutrophil effector responses. Molecular Oral Microbiology 34: 27–38. DOI 10.1111/omi.12252. [Google Scholar] [CrossRef]
Zhan T, Rindtorff N, Betge J, Ebert MP, Boutros M (2019). CRISPR/Cas9 for cancer research and therapy. Seminars in Cancer Biology 55: 106–119. DOI 10.1016/j.semcancer.2018.04.001. [Google Scholar] [CrossRef]
Zhang HX, Zhang Y, Yin H (2019). Genome editing with mRNA encoding ZFN, TALEN, and Cas9. Molecular Therapy 27: 735–746. DOI 10.1016/j.ymthe.2019.01.014. [Google Scholar] [CrossRef]
Zhao M, Wen X, Li G, Ju Y, Wang Y et al. (2019). The spatiotemporal expression and mineralization regulation of p75 neurotrophin receptor in the early tooth development. Cell Proliferation 52: e12523. DOI 10.1111/cpr.12523. [Google Scholar] [CrossRef]
Zheng J, Yu M, Liu H, Cai T, Feng H et al. (2021). Novel MSX1 variants identified in families with non-syndromic oligodontia. International Journal of Oral Science 13: 2. DOI 10.1038/s41368-020-00106-0. [Google Scholar] [CrossRef]
Zhou LL, Liu W, Wu YM, Sun WL, Dörfer CE et al. (2020). Oral mesenchymal stem/progenitor cells: The immunomodulatory masters. Stem Cells International 2020: 1327405. DOI 10.1155/2020/1327405. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |