DOI:10.32604/biocell.2022.019291

| BIOCELL DOI:10.32604/biocell.2022.019291 |  |
| Review |
Plant growth-promoting rhizobacteria (PGPR) and its mechanisms against plant diseases for sustainable agriculture and better productivity
1School of Crop Protection, Central Agricultural University (Imphal), Umiam, India
2Department of Soil Science and Agricultural Chemistry, Agricultural College and Research Institute, Tamil Nadu Agricultural University, Koyingbatore, India
3Department of Crop physiology, Agricultural College and Research Institute, Tamil Nadu Agricultural University, Koyingbatore, India
4Departments of Pulses, Tamil Nadu Agricultural University, Coimbatore, Koyingbatore, India
5Department of Environmental Sciences, Horticultural College and Research Institute, Tamil Nadu Agricultural University, Koyingbatore, India
6Department of Plant pathology, Assam Agricultural University, Dhubri, India
7KVK-Tinsukia, Assam Agricultural University, Tinsukia, India
8Department of Plant Pathology, Central Agricultural University (Imphal), Pasighat, India
9Department of Plant Pathology, Dr Rajendra Prasad Central Agricultural University, Bihar, India
*Address correspondence to: Pranab Dutta, pranabdutta74@gmail.com; Gomathy Muthukrishnan, gomathymicro@gmail.com
Received: 14 September 2021; Accepted: 21 January 2022
Abstract: Plant growth-promoting rhizobacteria (PGPR) are specialized bacterial communities inhabiting the root rhizosphere and the secretion of root exudates helps to, regulate the microbial dynamics and their interactions with the plants. These bacteria viz., Agrobacterium, Arthobacter, Azospirillum, Bacillus, Burkholderia, Flavobacterium, Pseudomonas, Rhizobium, etc., play important role in plant growth promotion. In addition, such symbiotic associations of PGPRs in the rhizospheric region also confer protection against several diseases caused by bacterial, fungal and viral pathogens. The biocontrol mechanism utilized by PGPR includes direct and indirect mechanisms direct PGPR mechanisms include the production of antibiotic, siderophore, and hydrolytic enzymes, competition for space and nutrients, and quorum sensing whereas, indirect mechanisms include rhizomicrobiome regulation via. secretion of root exudates, phytostimulation through the release of phytohormones viz., auxin, cytokinin, gibberellic acid, 1-aminocyclopropane-1-carboxylate and induction of systemic resistance through expression of antioxidant defense enzymes viz., phenylalanine ammonia lyase (PAL), peroxidase (PO), polyphenyloxidases (PPO), superoxide dismutase (SOD), chitinase and β-glucanases. For the suppression of plant diseases potent bio inoculants can be developed by modulating the rhizomicrobiome through rhizospheric engineering. In addition, understandings of different strategies to improve PGPR strains, their competence, colonization efficiency, persistence and its future implications should also be taken into consideration.
Keywords: Plant growth-promoting rhizobacteria; Biocontrol; Plant diseases; PGPR mechanisms; Sustainable agriculture
Microbes dwelling in the soil ecosystem are always associated in close affinity with different types of plant systems, such association is commonly termed as phytomicrobiome and the associated plant is known as holobiont (Bulgarelli et al., 2015; Smith et al., 2017; Lyu et al., 2021). This communal relationship plant-microbe interaction not only regulates the microbial community comprised of bacteria, fungi, actinomycetes and other groups of microorganisms but also plays a vital role in soil biogeochemical cycling. Microbes render a wide range of services to the plants and in turn plants provide photosynthates and other metabolic compounds to the microbial community. Even though microbes exist in several environments including extreme conditions, they preferred to dwell in the soil as it is rich in nutrients. In the soil, the most dynamic region is the rhizosphere region where the nutrient turnover is versatile and favourable for the multiplication of microorganisms (Mahmud et al., 2021). The term rhizosphere denotes the narrow region of soil surrounding the root in which microbial population would be higher. It is a nutrient-rich zone where the presence of organic acids, amino acids, sugars, enzymes is abundant. These compounds are responsible for plant growth and they mobilize nutrients in soils, protect the plants from phytopathogens, improve the soil structure and soil health, remove toxic pollutants from soil, degrades xenobiotics compounds, etc. (Chen et al., 2020). Among different microbes, those flourishing in the rhizosphere region are commonly known as plant growth-promoting rhizobacteria (PGPR), are the real driving force behind enriching soil fertility and soil nutrients, thereby, cause wonders in the region as compared to the bulk soil (Glick, 2012; Basu et al., 2021). The major role of these PGPR can be categorized into three, i.e., a) To supply vital nutrients for plant growth b) produce plant growth-promoting substances c) to produce antimicrobial substances to control plant pathogens. This region acts as a storehouse of nutrients and contains a group of bacteria that might be symbiotic or non-symbiotic based on the host plant it gets to dwell, and the association of microorganisms present in the vicinity of the rhizosphere is famously called rhizomicrobiome. The root exudates analysis revealed that the composition varies according to the root system it belongs to, that determines and chooses the microbial community in the region and in return, enhance the plant growth and yield by 20%–30% (Nehra, 2011). Hence a bioformulation that contains efficient PGPR strains do wonders to efficiently control the plant pathogens and to enhance crop production. The present review article elucidates detailed mechanisms utilized by PGPRs in conferring plant protection against diseases and how these mechanisms relate to the improvement of yield in different crops. Further, understandings on different strategies to improve rhizomicrobiome, colonization of PGPR strains, their competence, persistence and its future implications were also discussed in this review.
Rhizosphere and plant growth-promoting rhizobacteria
The term Rhizosphere was coined for the first time in 1904 by the German plant pathologist and agronomist Lorenz Hiltner to describe the plant root interface (Hiltner, 1904). The rhizosphere is the zone surrounding plant roots influenced by the compounds i.e., exudates released by roots that regulate the rhizospheric soil and the microbial community prevailing in the region. The rhizospheric region is categorized into three different zones viz., rhizosphere, rhizoplane and root itself based on physical, chemical and biological properties of the roots. The richness of microbial population especially symbiotic and non-symbiotic bacteria in the rhizospheric region is governed by several factors such as species of plants, soil physiological status and species of microbes (Kundan et al., 2015). Some groups of microbes are always associated with the rhizosphere of plants, these groups may vary based on the nutrients that were released by plant roots (Bulgarelli et al., 2015; Hakim et al., 2021). The root exudates were changed based on the stages of plant growth, development and genotype of plants (Bouffaud et al., 2012; Cordovez et al., 2021). The plant growth and microbes are mutually affected by root releasing materials called root exudates (Zhang et al., 2017). The root exudates are also called rhizodeposits which include phenolics, carbohydrates, fatty acids, amino acids, organic acids, sterols, putrescine, vitamins and growth regulators (Uren, 2007).
The rhizospheric microbes attracted by plant root secretions exhibits symbiotic association following plant-root interactions enhancing plant growth and productivity, thereby termed as rhizospheric effect (Chai and Schachtman, 2022). Rhizobacteria secrete wide array of stimulants that facilitate water and nutrient uptake in plants, thereby directly assisting plants for nitrogen and phosphorus assimilation or altering hormone level and indirectly decreasing the population of pathogenic bacteria (Walker et al., 2003; Backer et al., 2018). Various studies showed that plant growth and productivity increased through the application of PGPR in stress and normal conditions. Up to date, many non-pathogenic rhizobacteria were identified which promote plant growth through the release of phytohormones such as auxins and cytokinin, production of siderophore, acting as a biocontrol agent and promotes the induced systemic resistance of the host plant (van Loon, 2007). The bacteria that exist in the extracellular of roots are called ePGPR which commonly include Arthrobacter, Azospirillum, Burkholderia, Chromobacterium, Flavobacterium, Pseudomonas, Agrobacterium, Azotobacter, Bacillus, Caulobacter, Erwinia, Micrococcus and Serratia etc. (Bhattacharyya and Jha, 2012). Some bacteria that persist in intracellular roots are called iPGPR such as Azorhizobium, Mesorhizobium, Rhizobium, Allorhizobium and Bradyrhizobium There were also report of actinomycetes found in the rhizosphere region benefitting the plant growth (Bhattacharyya and Jha, 2012; El-Tarabily, 2021).
Mechanisms utilized by PGPR to combat diseases
Antibiotics are low molecular weight toxin complex (<10 ppm) produced by one microorganism that are capable of inhibiting the growth of specific microorganisms especially pathogenic microbes, by interfering synthesis of the pathogen cell wall, cell membrane structures and biogenesis of initiation complexes of the ribosome (Bakker et al., 2013; Peterson and Kaur, 2018). Antibiotics are categorized into volatile antibiotics, i.e., alcohols, aldehydes, ketones, sulfides, hydrogen cyanides and non-volatile antibiotics, i.e., cyclic lipopeptide amino polyols, polyketides, phenylpyrrole, and heterocyclic nitrogenous compounds (Gouda et al., 2017). Antibiotic-producing microbes are directly applied in the agricultural field to fight against the pathogenic organisms around the plants or root surfaces. PGPR is the main antibiotic-producing microorganisms and their secretions serve as an alternative method to chemical fertilizers, meet primary, secondary nutrient requirements and protect the plants by smothering the development of target pathogen and opposing numerous phytopathogens. Bacillus and Pseudomonas produce antibacterial and anti-fungal ribosomal-origin agents such as subtilin, sublancin, TasA and subtilosin A and non-ribosomal peptide products namely, iturin, bacilysin, bacillaene, mycobacillin, Difficidin, chlorotetain, rhizocticins, lipopeptides, fengycin and surfactin (Sherathia et al., 2016). The nine gene clusters of B. amyloliquefaciens FZB42 are able to synthesis various bioactive peptides and polyketides. Some Pseudomonas species also produce antibiotics namely Ecomycins, Cepaciamide A, Rhamnolipids, Kanosamine, OomycinA, Aerugine, Zwittermycin-A, Phenazine-1-carboxylic acid (PCA), Azomycin, Viscosinamide, Pseudomonic acid, Butyrolactones, Pyrrolnitrin (Prn). These antibiotics also act as an antimicrobial, antiviral, insecticidal, antihelminthic, phytotoxic, antitumor and cytotoxic agents (Fernando et al., 2018). PGPR changes the root exudates through Arbuscular Mycorrhizal (AM) fungal colonization that degrades the toxins and pathogens inhabiting around the root (Vandana et al., 2021).
In this mechanism, microbes as well as their products like more than one antibiotic act as antagonistic effect on plant pathogens (Köhl et al., 2019). PGPR produces Polyketide’s type antibiotics Mupirocin, 2,4 Diacetylphloroglucinol and Pyoluteorin are highly active in the destruction of phytopathogens. There are six classes of antibiotics that involved in biocontrol of root related diseases pyoluteorin, phenazines, cyclic lipopeptides, phloroglucinols, hydrogen cyanide and pyrrolnitrin. Pseudomonas and Bacillus synthesize lipopeptide which actively controls competitive organisms like bacteria, virus, nematode, fungi and protozoans (Kenawy et al., 2019). Several antibiotics were derived from the PGPR strains that react on the pathogens by altering the mechanisms of bacterial cell wall synthesis (Backer et al., 2018). Bacillus species were reported to produce some antibiotics namely colistin, circulin and polymyxin, which are active against fungi, Gram-positive and Gram-negative bacteria that cause severe yield loss (Maksimov et al., 2011). B. cereus UW85 produces antibiotic kanosamine (aminoglycoside), (aminopolyol) and zwittermicin A that suppressed the alfalfa damping off and destroy oomycete pathogens. Pyocins derived from P. pyogenes, cloacins from Enterobacter cloacae, marcescins from Serratia marcescens, megacins from B. megaterium and bacteriocins produced by Gram negative bacteria are some important antibiotics produced by significant PGPR. These antibiotic mixes secreted by PGPRs conceal disease suppression of soil-borne phytopathogens in rhizospheric region by inducing fungistasis, lysis of fungal mycelia and inhibition of spore germination (Adhya et al., 2018). A study by Kulimushi et al. (2018) reported that antibiotics viz., phenazine, 2,4-diacetylphloroglucinol (DAPG) produced by Pseudomonas sp. showed antagonistic activity against Meloidogyne incognita and Fusarium oxysporum f. sp. nivetum by membrane disruption. Genes involved in bacilysin biosynthesis was found to be associated with antagonistic behaviour of Bacillus pumilus strains against Phytopthora infectans (Caulier et al., 2018). Cao et al. (2018) isolated Bacillus velezensis from the rhizosphere of banana that suppressed the pathogens such as Ralstonia solanacearum and Fusarium oxysporum due to the production of antibiotic compounds surfactin, iturin, and fengycin. These bioactive secondary metabolites are encoded by biosynthetic gene clusters (BGCs) and classified as ribosomally synthesized peptides, non-ribosomally synthesized peptides and polyketide synthases (Kenawy et al., 2019). PGPR strains such as Bacillus and Paenibacillus isolated from rhizosphere of tomato plants showed strong antagonistic activity against tomato bacterial, fungal and oomycetal pathogens and the compounds were found to be surfactin, fengycin, bacillibactin, petrobactin, lichenysin and bacillaene (Zhou et al., 2021). Wang et al. (2021) suggested that PGPR consortium proved to protect the crops from various diseases rather individual strains of PGPR. Plant growth-promoting rhizobacteria HN 6 decreased the abundance of Fusarium pathogen, increased the beneficial bacteria, shaped the rhizospheric microflora and promoted the growth of banana (Yang et al., 2021).
Biological control methods incorporating enzyme producing PGPRs are potential alternative to synthetic chemical methods, not only for efficient management of plant pathogens but also contribute to the establishment of pollution free environment. In host rhizosphere, a wide variety of PGPRs shows hyperparasitic activity against pathogens through secretion of several hydrolytic enzymes viz., proteases, lipases, cellulases, chitinases and β-1,3 glucanases, which disrupt cell wall of bacterial and fungal pathogens by acting on glycolytic linkages of prokaryote and eukaryote cell wall (Santoyo et al., 2021). These hydrolases are highly active, stable, substrate specific, low molecular weight compounds that act either directly or indirectly, inhibiting growth of pathogens and exotoxins reduce pathogen multiplication. The lytic enzymes like lysozyme are bactericidal, fungicidal and nematicidal in nature. Extracellular enzymes viz., chitinases, β-1,4-glucanases, proteases, cellulases and xylanases secreted by PGPRs Bacillus sp. BPR7, B. thuringiensis strain UM96, B. atrophaeus strain JZB120050 and B. subtilis strain RH5 inhibit mycelial growth of fungal pathogens viz., Fusarium oxysporum, F. solani, R. solani and Botrytis cinerea (Martinez-Absalon et al., 2014; Ni et al., 2018; Jamali and Sharma, 2020). Proteases act on peptide bonds of protein compounds of fungal cell into amino acids, thus, break down fungal mycelia and damage structural integrity. These proteases are also released by other strains of Bacillus, i.e., B. megaterium, B. stearothermophilus, B. cereus and B. mojavensis. Cellulases secreted by B. subtilis and B. pumilus break down 1,4-β-D-glycosidic linkages in cellulose products and chitinases released by PGPR not only targeted cell wall of phytopathogens but also degrade cuticle of major agricultural insects-pests. Another PGPR Paenibacillus ehimensis KWN38 showed disease suppressive activities against R. solani AG-1, F. oxysporum f. sp. lycopersici and Phytophthora capsici through secretion of hydrolases viz., chitinases, cellulases, glucanases and proteases (Naing et al., 2014). Lactobacillus bacteria also showed disease suppressive activities through production of lactic acids. However, in some PGPR, out of different hydrolytic enzymes produced such as chitinases, cellulases, proteases and pectinases, only protease enzymes secreted by Serratia plymuthica IC14 and Paenibacillus sp. B2 showed antagonistic activity against B. cinerea and Sclerotinia sclerotiorum (Kamensky et al., 2003; Wang et al., 2021).
Competition for space and nutrients
Rhizosphere region serves as an important interphase between plant roots and microorganisms, elucidated by different inorganic acids exudates by root surface, i.e., sugars, vitamins, amino acids, organic acids, nucleosides, phenolic compounds and phytosiderophores. These nutrients act as chemo-attractants for motile bacteria to migrate towards roots surface, providing niche to a diverse range of microorganisms, including pathogenic microbes (Vacheron et al., 2013). The ability of microorganisms to proliferate, efficiently colonize the root surface and persist at population density levels sufficient to generate plant beneficial effects determine their competitive rhizosphere colonization efficiency. Therefore, in the rhizospheric region, competition for nutrients and physical occupation sites is an indirect mechanism utilized by competitive PGPRs against pathogenic microbes that depend on external sources (Olanrewaju et al., 2019). These opportunistic PGPR microbes also compete with pathogenic microbe and overcome the toxins released by the pathogenic microbes and safeguard the rhizosphere by degradation of the same (de Souza et al., 2019). For example, inhabitation of certain bacterial strains, i.e., Pseudomonas fluorescens PJ0210 in the corn rhizosphere showed disease suppression by competing Pythium aphanidermatum and Bipolaris maydis for nutrients such as glucose, asparagine etc. (Wang et al., 2021). Hence it is necessary to understand the changes in the plant rhizosphere microbial composition and its microenvironment to control the spread of plant diseases (Chen et al., 2020).
Quorum sensing is an intercellular communication system among bacteria, which is governed by gene expression coupled with cell concentration and mediated by the diffusion of specific signal molecules such as N-acylhomoserine lactones (AHLs) (Hartmann et al., 2021). It regulates expression of several phenotypes contributing bacterial pathogenesis in Psuedomonas syringae, Pectobacterium atrosepticum, P. carotovorum, Dickeya solani, Erwinia amylovora, Ralstonia solanacearum, Agrobacterium tumefaciens, that aggravate virulence and infection potential of bacterial pathogens. In the rhizospheric region, certain PGPRs combat bacterial infections by adopting quorum interrupting strategies that interferes quorum sensing through enzymatic degradation of AHLs molecules, this mechanism is known as quorum quenching (QQ) and the PGPRs are known as QQ bacteria (Rosier et al., 2020). However, different types of enzymes responsible for AHLs degradation include lactonases, oxidoreductases and acylases, which attenuates virulence of bacterial pathogens, rather than inhibiting growth or killing bacterial pathogen. The first QQ enzyme was identified from gram positive Bacillus sp. strain 240B1, encoded by gene aiiA was known for the inactivation of AHL signal by hydrolysis of lactone ring (Dong et al., 2000). However, expression of aiiA gene significantly decreased AHLs release and suppressed soft rot disease by Pectobacterium carotovorum in potato, Brinjal, Chinese cabbage and celery. Introduction of aiiA gene cloned from PGPR Bacillus sp. into transgenic plants disrupted quorum sensing ability of the pathogen through degradation of autoinducers which in turn blocks the expression of virulence genes, thus alleviated diseases even after infection of the pathogen (Altaf et al., 2017). Biopriming of tomato seeds with Pseudomonas segetis strain P6 isolated from rhizosphere of Salicornia europaea protected tomato from P. syringae pv. Tomato by showing PGP activities and QQ activities through acylases-based enzymatic degradation of AHLs (Rodriguez et al., 2020).
Siderophores are low molecular weight (500–100 Da) iron scavengers, that chelate iron from surrounding environment and transport Fe3+ into microbial cell providing competitive advantage to PGPR microbes (Pahari et al., 2017). When siderophores released in a surrounding environment it solubilizes the iron and form an iron-siderophore complex which move through diffusion process and reached the cell membrane receptors of bacteria where active transport takes place after recognition (Suleman et al., 2018; Bradley et al., 2020). Bacterial siderophores are classified into four major classes which comprise phenol catecholates, carboxylate, pyoverdines and hydroxamates (Saha et al., 2016). Based on the chemical nature of their coordination sites with iron few siderophores have been classified as phenolates (e.g., Yersiniabactin) and others, in addition, as “mixed” e.g., pyoverdins, produced by Pseudomonas species and containing both hydroxamate and catecholate functional groups (Table 1) (Sah and Singh, 2015). The α-carboxylate kind of siderophores is produced by Zygomycetes (mucorales) group of fungi and a few bacteria, such as Rhizobium meliloti and coordinate iron through hydroxyl and carboxyl groups (Saha et al., 2016). Siderophores can be detected qualitatively and quantitatively by using Chrome Azurol S assay and CAS assay respectively. Siderophore-producing PGPRs belong to Pseudomonads, Bacillus, Rhizobium, Bradyrhizobium, Serratia and Streptomyces and numerous genes responsible for siderophore biosynthesis include pvdA, fpvI, fpvR, pvdF, pvdE, fpvA, pvdD, pvdJ, pvdI, pvcABCD (Pii et al., 2015).
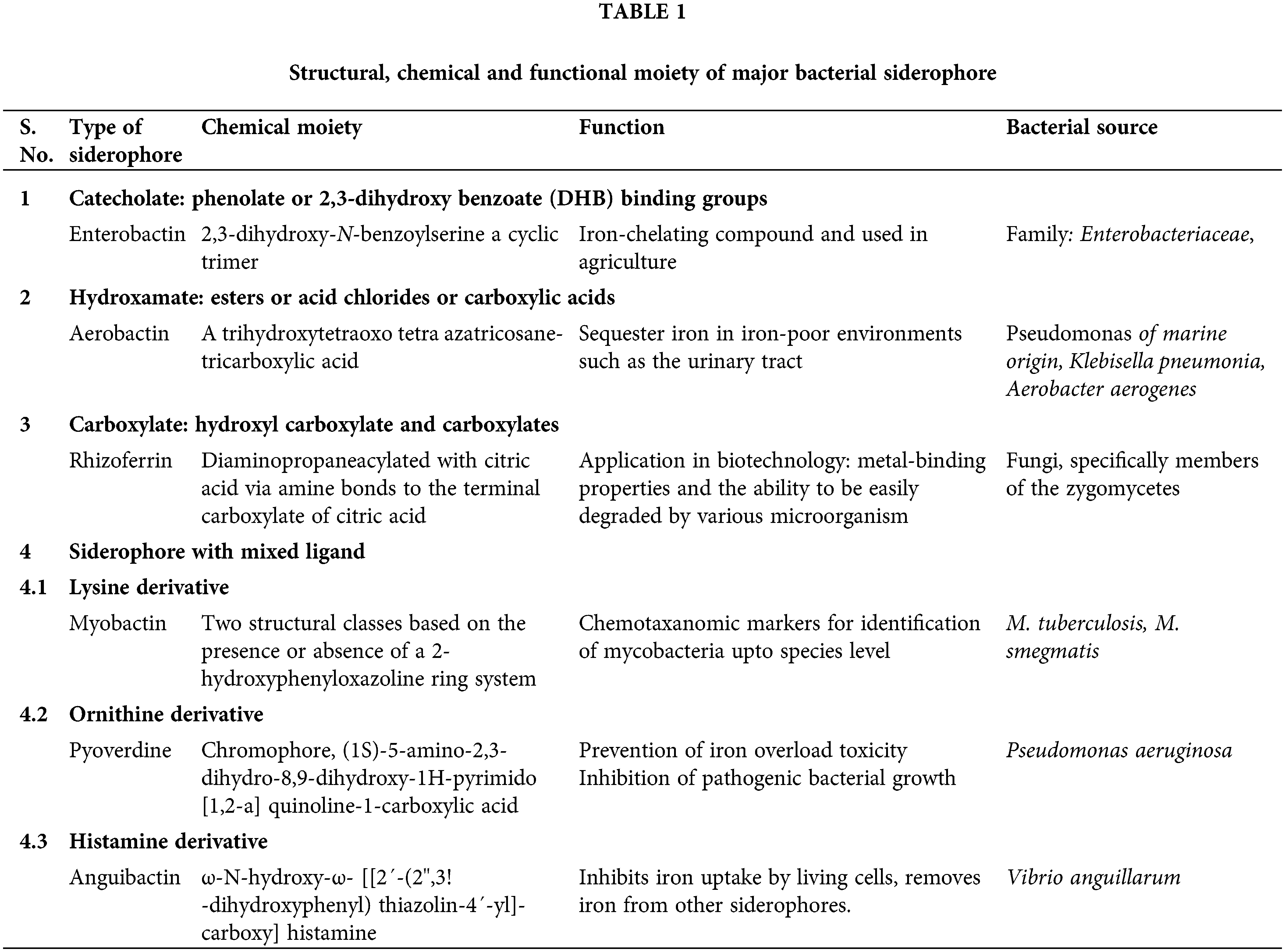
Seed biopriming with siderophore Pseudomonas strain GRP3 protected groundnut and mung bean crop from iron chlorosis by reduction in chlorotic symptom through enhanced chlorophyll content and reduced mobility of heavy metals in contaminated soils (Sayyed et al., 2013). These siderophores also used to treat radioactive wastes prior to storage or to decontaminate soils and water, yet, the understanding the chemical structures of different siderophores and the membrane receptors involved in Fe uptake has opened new areas for research (Syed et al., 2021). Crops, for instance oats assimilate iron using the microbial siderophores. Application of microbial siderophorogenic bioinoculants have been extensively studied and it was found that seed biopriming with siderophore producing bacteria especially with PGPR has protected groundnut crop from iron chlorosis. An improvement in overall growth and health of plants has been observed after treatment of seeds with siderophorogenic bioinoculants. Increased percentage of germination, root ramification, nodulation, height, foliage and chlorophyll content can be achieved only through seed bacterization with siderophoregenic Pseudomonas. It plays an important role to reduce phytopathogens proliferation by iron chelation and also promotes the plant growth by increased uptake of iron (Dey et al., 2020).
Iron acquisition through siderophore production is an important factor in deciding the competitive fitness of bacteria to grow in the plant roots vicinity and to compete with other microbes for iron in the rhizosphere (Lewis et al., 2019). It aids the plant to uptake iron in the presence of other metals such as Cadmium and Nickel. Siderophores produced by Pseudomonas generally have higher affinity with ferric ion. Pyoverdines are effective siderophore that can suppress the growth rate of non-iron chelating fungi and bacteria under in-vitro conditions and P. putida produces the pseudofactin siderophore that have the ability to get rid of the Fusarium oxysporum and Rhizoctonia solani from rhizosphere by lowering iron availability in soil (Kirienko et al., 2019). Soil bacterial isolates such as Azotobacter vinelandii MAC 259 and Bacillus cereus UW 85 siderophores can be used as efficient PGPR to increase yield. Siderophores of Bacillus megaterium from tea rhizosphere helps in the plant growth promotion and reduction of disease intensity. The provision of iron to plants through soil bacteria is more important when the plants are exposed to an environmental stress such as heavy metal pollution. In these cases, siderophores help to get rid of the stresses imposed on plants by high soil levels of heavy metals (Mandal and Kotasthane, 2014). The continuous use of fungicides leads to development of fungal resistant strains transforming fungicides ineffective. Microbial metabolites may help to control plant pathogens by enhancing the population of antagonistic microorganisms in the soil. Most of the Plant Growth-Promoting Bacteria can inhibit the harmful microorganisms by releasing siderophore, cyanide and antibiotics. Siderophores are themselves growth inhibitors of various plant pathogenic fungi, such as Phythium ultimum, Fusarium oxysporum veri dianthi and Sclerotinia sclerotiorum. Siderophore producing PGPRs viz., Rhizobium, Azotobacter, Azospirillum have been implicated in biocontrol aspect for several phytopathogens such as F. oxysporum, F. udum, F. solani, F. monoliforme, Colletotrichum gossypi, Ustulina zonata and Fomes lamnensis (Sayyed et al., 2013). In another study, siderophore rich culture of Alcaligenes faecalis exerted antifungal activity against Aspergillus niger NCIM 1025, A. flavus NCIM 650, F. oxysporum NCIM 1008 and Alternaria alternata IARI 715 (Sayyed and Chincholkar, 2009).
Release of root exudates & rhizomicrobiome regulation
Roots are able to secrete various chemical compounds into the soil and it is referred as root exudates. Roots are regulating soil microorganisms and change the physico-chemical properties of the soil and inhibit soil plant pathogens. Root exudates are released by plants in two different forms. One through passive (diffuse) and the other through active secretions. The exudates are rich in organic acids, amino acids, terpenoids, phenolic compounds, polyacteylenes, flavonoids, alkaloids, sugars, tannins, and secondary metabolites. Rhizosphere regions are highly populated with microbes including bacteria, actinomycetes, fungi and insects. Roots can secrete variety of proteins along with (Saeed et al., 2021) higher molecular weight compounds called as rhizodeposition that are released into the soil by plant roots that serve as nutritional source for rhizospheric microbes. Root exudates containing ions, water and oxygen are responsible for interaction of molecules between roots and rhizobacteria in the rhizospheric region, either as repellents against pathogens or few compounds as attractant towards beneficial microbes. Root exudates vary among the plant species, age, and it serve as a good nutritional source to rhizospheric microbes (Santoyo et al., 2021). Root excretions were divided into secretion with known function and secretion with unknown functions like lubrication, defence of plant roots, etc.
Root exudates are known for its high and low molecular weight compounds. The low molecular weight compounds are released by the plants through diffusion based on membrane permeability that includes organic acids, sugars, amino acids and phenolics that are released from root intact cells. These phenolic groups stimulate the plant growth. The mucilage (polysaccharides) and proteins secreted by epidermal and root cap cells constitute the high molecular weight compounds. These compounds facilitate soil and root interaction followed by the root movement in the soil (Galloway et al., 2020). Roots of soya bean released specific plant exudates called soya saponins (Tsuno et al., 2017). These soya saponins elicit the symbiotic relationship with soyabean and Bacillus diazoefficiens (Liu et al., 2015). Root exudates act as a messenger among the root and rhizobacteria in the rhizospheric soil (Walker et al., 2003). They protect the plants from harsh environment, help to store the ions, root soil interactions and so on. Young plants secrete about 30% of photosynthates and in that Jasmonic acids are involved in altering the rhizosphere microbes (Carvalhais et al., 2013), leading to richness of microbes including Lysinibacillus, Bacillales, Paenibacillus amylolyticus and Bacillus which participate in the defence mechanisms. Secondary metabolites in plant root exudates played an important role in symbiotic relationship among plants with fungi and bacteria (Pang et al., 2021). The secondary metabolite of root exudates has the function of antimicrobial, insecticidal, phytotoxic, anti-insecticides, antibiotics and hormonal properties (Bais et al., 2006). Exudates act as herbivore defence reaction in nearby plants, involved in root movement and reduction of metal toxicity (Hawes et al., 2016). These secondary metabolites attract or prevent the microbes and insects. The PGPR species include Azotobacter, Bacillus, Caulobacter, Erwinia, Micrococcous, Serratia, Cellulomonas, Flavigena, Agrobacterium, Pseudomonas, Arthrobacter, Azospirillum, Burkholderia, Chromobacterium (Duy et al., 2016; Hossain et al., 2016; Disi et al., 2019; Hassan et al., 2019). The nitrogen fixing Rhizobium includes Azorhizobium, Mesorhizobium, Allorhizobium, Bradyrhizobium that colonize and help legume plants directly or indirectly (Kumawat et al., 2019; Harman and Upho, 2019).
The rhizospheric bacteria facilitate optimal plant growth and development through bio-fertilization process, by undertaking two major activities such as nitrogen fixation and solubilization of phosphorus. Though nitrogen is present in ample amount in atmosphere, yet, it is non-utilizable by plants. Therefore, PGPRs involve intricate process of biological nitrogen fixation (BNF) mainly either by symbiotic association with plant or non-symbiotically in free living manner (Ahemad and Kibret, 2014). Symbiotic PGPRs residing within plant tissues directly exchange metabolites, for example, Bradyrhizobium, Mesorhizobium, Rhizobium, Sinorhizobium directly fix N2 in root nodules of leguminous plants, whereas, Frankia spp. fix N2 in roots of non-legumes (Laranjo et al., 2014). However, non-symbiotic PGPRs include Azotobacter, Azospirillum, Azoarcus, Burkholderia, Enterobacter, Gluconobacterium, Pseudomonas, Nostoc (Ahemad and Kibret, 2014). Nitrogenase enzyme, a metallo-enzyme complex comprising of two subunits, i.e., dinitrogenase with a metal cofactor i.e., iron (Fe), molybdenum (Mo), vanadium (V) and dinitrogenase reductase with Fe-protein, catalyse the conversion of atmospheric N2 to NH3, that can be easily assimilated by the plants (Hu and Ribbe, 2016).
Similarly, phosphorus, being the second most essential macronutrient, is involved in several metabolic pathways in plant system such as biosynthesis of macromolecules, cell signalling, photosynthesis and respiration (Khan et al., 2010). However, from insoluble organic (soil phytate, inositol phosphate, phosphomonomers and triesters) and inorganic (apatite, phosphate forms of aluminium and calcium) forms of phosphorus applied to the soil, 90%–95% are rendered unavailable and only 1 mg/Kg−1 are taken by plants (Pandey and Maheswari, 2007). Plants solubilize insoluble phosphates using different strategies, either through production of extracellular enzymes, i.e., phytases/phosphatases that hydrolyze phosphoric esters or through releasing mineral dissolving compounds and chelating agents such as hydroxyl ions, organic acid ions and CO2, that can be taken by plants in soluble forms i.e., monobasic (H2PO4−) and dibasic (HPO42−) ions (Sharma et al., 2013). PGPRs capable of solubilization of insoluble phosphates belong to bacterial genera viz., Azospirillum, Azotobacter, Burkholderia, Bacillus, Enterobacter, Pseudomnonas, Rhizobium, Serratia marcescens (Bhattacharya and Jha, 2012).
Phytohormones production/Phytostimulation
The rhizosphere and its surrounding soil have the ability to produce varieties of hormones, such as auxins, cytokinins, gibberelic acid, ethylene, known as phytohormones. These phytohormones are more essential things to stimulate plant growth in agriculture fields. These are also called as plant growth regulators or stimulators. Very low quantities of these stimulators are involved in plant growth regulations and these lesser quantities play various dimensions in plants growth and development (Egamberdieva et al., 2017). Among these hormones, indole 3-acetic acid (IAA) acts as notable signalling molecule in plant cell differentiation, cell division, cell expansion, apical dominance, root initiation of lateral and adventitious roots (Olanrewaju et al., 2017). PGPRs such as Mycobacterium, Rhizobium, Bradyrhizobium, Enterobacter, Klebsiella, Microbacterium, Dendrobium moschatum and P. fluorescens undertake indole-pyruvate and indole-acetaldehyde pathways for IAA biosynthesis using tryptophan in root exudates as precursor (Sayyed et al., 2019). These hormones influence alteration of plant auxin pool, increase root length and area causing greater absorption of nutrients and loosening of plant cell wall causing greater exudation by roots (Grobelak et al., 2015). The lesser auxin concentration in plants stimulates the plant growth and higher concentration inhibits the plant growth. Rhizospheric bacteria that produce these hormones, especially IAA can also act as signalling molecules to react on both plants as well as pathogens. Phytohormone production by Rhizobium was observed in the legume plants namely Vigna mungo, Sesbania sesbani and Crotalaria retusa.
Another phytohormone, ethylene is a highly active hormone involved in seed germination, leaf maturation, fruit ripening, senescence, root initiation and elongation at lower concentration. Ethylene concentration cause defoliation, leaf abscission, inhibits plant and root growth (Iqbal et al., 2017). In addition, many biotic and abiotic conditions such as pathogenic infections, drought, salinity, water logging, heavy metal toxicity stimulate higher levels of ethylene, thus, it is also referred as stress hormone (Ali et al., 2014; Devarajan et al., 2021). The 1-aminocyclopropane-1-carboxylate (ACC) is the precursor of ethylene which is formed while the plants undergo various stress namely drought conditions, floods and heavy metals. PGPRs including Acinetobacter, Azospirillum, Alcaligenes, Agrobacterium rhizogenes, Achromobacter, Bacillus, Burkholderia, Enterobacter, Pseudomonas, Serratia, Ralstonia and Rhizobium exhibit ACC deaminase activity that lowers ethylene levels, thereby, stimulating tolerance to abiotic and biotic stress in plants (Devarajan et al., 2021). ACC deaminase activity by PGPRs convert ethylene to α-ketobutyrate and ammonia, lowers ethylene production in response to pathogenic infection, that further stimulate root and shoot elongation, increased root nodulation and nutrient (N, P, K) uptake (Gupta and Pandey, 2019). Cytokinin is another important phytohormone involved in plant root development, boosting the cell division, improve the root hair formation, prevent the root elongation and initiate the shoot formation (Amara et al., 2015; Devi et al., 2020; Devarajan et al., 2021). It also plays vital role in leaf expansions, root growth, chlorophyll synthesis, nutritional signalling, branching and enhance seed germination. PGPRs capable of cytokinin production include Azotobacter, Pantoea agglomerans, Pseudomonas fluorescens, Paenibacillus polymyxa and Rhodospirillum rubrum (Dos Santos et al., 2020). These production and supply of phytohormones by the PGPR makes the plant to survive and overcome the stress during pathogen attack. This mechanism also helps the plant to address towards severe environmental conditions.
Induction of systemic resistance (ISR)
Induced resistance is defined as an enhancement of the plant’s defensive capacity against a broad spectrum of pathogens and pests that is acquired after appropriate stimulation. The elevated resistance produced by an inducing agent upon infection by a pathogen is called Induced Systemic Resistance (ISR) or Systemic Acquired Resistance (SAR). The induction of systemic resistance by rhizobacteria is referred as ISR, whereas by other agencies is called SAR. PGPRs activate ISR, a pathway involving jasmonate and ethylene signalling, conferring non-specific protection against pathogenic fungi, bacteria, viruses as well as against several insects and nematodes (Zamioudis and Pieterse, 2012). A large number of defense enzymes have been associated with ISR which include phenylalanine ammonia lyase (PAL), chitinase, β-1,3-glucanase, peroxidase (PO), polyphenol oxidase (PPO), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), lipoxygenase and proteinase inhibitors (Murali et al., 2021). Chitinases and β-1,3-glucanases show synergic antifungal activity that are related to the SAR mediated pathway which includes salicylic acid (SA) as signal molecule that is activated by necrotizing pathogens and chemical inducers. These enzymes also bring about liberation of molecules that elicit the first steps of induction of resistance, phytoalexins and phenolic compounds.
Bacterial traits of PGPRs including flagella, cell envelope, lipopolysaccharides (LPS), secondary metabolites viz., siderophores, antibiotics, lytic enzymes also operate as an elicitor of ISR, for example 2,3-butanediol in Bacillus subtilis GB03, dimethyl disulfide in B. cereus C1L, branched-chain alcohols in B. amyloliquefaciens IN937a, N-acylhomoserine lactones in Serratia liquefaciens MG1, S. plymuthica HRO-C48 and Pseudomonas putida IsoF (Pokhare et al., 2015; Kumar et al., 2015). Many strains of Bacillus confer broad spectrum of protection through significant reduction in the incidence or severity of diseases in wide range of hosts by elicitation of ISR which has been successfully demonstrated in field trials or greenhouse on crops including sugar beet, tomato, bell pepper, muskmelon, watermelon, tobacco, Arabidopsis, cucumber, potato, radish, carnation, bean, sugarcane, chilli, brinjal, rice, mango, finger millet etc. (Choudhary et al., 2015; Miljakovic et al., 2020). For example, bacterial consortium containing P. putida CRN-09 and B. subtilis CRN-16 conferred greater expression of ISR in mungbean against Macrophomina phaseolina by enhancing peroxidase, phenylalanine ammonia lyase, β-1,3-glucanase, polyphenol oxidase, chitinases activities (Sharma et al., 2018). Also, P. fluorescens exhibited a state of active defensive strategy against charcoal rot disease in chickpea through induction of systemic resistance. Induced resistance through accumulation of defence enzymes were also reported in rice and groundnut following combined application of Pseudomonas strains and Beauveria isolate (Karthiba et al., 2010; Hartmann et al., 2021).
Peroxidases have been found to play a major role in the regulation of plant cell elongation, phenol oxidation, polysaccharide cross-linking, IAA oxidation, cross linking of extension monomers, oxidation of hydroxyl–cinnamyl alcohols into free radical intermediates, wound healing, biosynthesis of lignin and other oxidative phenols. PO is associated with disease resistance in plants and enhanced levels of PO induced resistance in fluorescent pseudomonads were noticed in sugarcane in response to infection by Colletotrichum falcatum (Shair et al., 2021). Cucumber seedlings treated with PGPR Bacillus megaterium strain L8 induced resistance against seedling damping-off caused by Pythium aphanidermatum, through expression of several plant defense enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidase (PO), phenylalanine ammonia lyase (PAL) and polyphenol oxidase (PPO) activities in roots in a time course of 13 days (Liang et al., 2011). Yanti (2015) also observed that inoculation of PGPRs viz., Serratia marcescens strain N2.4 in shallot bulbs, increased PO enzymes activity up to 0.058 µm. mL−1 and 0.053 µm. mL−1 in roots and leaves, and further conferred induced resistance against bacterial leaf blight Xanthomonas axonopodis pv. allii. Inoculation of soft wheat seeds (Triticum aestivum L.) with Pseudomonas bacterial strains isolated from earthworm coprolites, showed significant antifungal and growth-promoting action through increased peroxidase activity in presence of Bipolaris sorokiniana as compared to non-bacterized plants (Minaeva et al., 2017; Shair et al., 2021). Priming of chilli seeds with beneficial rhizobacteria Bacillus sp. BSp.3/aM showed improved plant health of chilli, i.e., germination (98.00%), seedling vigor (1374±7.15 vigor index) and confer protection against seed-borne incidence caused by Colletotrichum capsici (Jayapala et al., 2019). The reduced anthracnose disease incidence up to 20.00% was attributed to induction of defense-related enzyme activities, i.e., PAL (95 units at 48 h post inoculation hpi), PO (6.49 units at 24 hpi), PPO (5.81 units at 24 hpi), lipoxygenase LOX (9.9 units at 24 hpi), phenolics (94.7 µg/g tissue at 120 hpi) and chitinase (94.7 µg/g tissue at 96 hpi), respectively.
PGPR treatment was found to enhance the expression of PO during plant pathogen interactions. Garcia-Seco et al. (2015) reported that three isoforms of PO were expressed on fruit peel tissue upon treatment with the P. fluorescens, FP7 amended with chitin. Two peroxidase isoforms have been induced in rice plants treated with fluorescent pseudomonads and challenged with R. solani. Chilli plants treated with mixtures of strains of PGPR viz., Pf1 + B. subtilis + Neem + chitin showed enhanced PO activity against CMV. Treatment with P. fluorescens Pf1 induced high level expression of PO in tomato plants against F. oxysporum f. sp. lycopersici and tea plants against blister blight disease (Wang et al., 2021).
PPO usually accumulates upon wounding in plants, which is achieved by octadecanoid defense signal pathway. In a study by Chen et al. (2000), cucumber roots treated with PGPRs Pseudomonas corrugatq 13 and P. aureofaciens 63–28 showed accumulation of antioxidant defense enzymes in root tissues viz., PAL, PO, PPO activity in 2–5 days lasting up to 16 days after bacterization. However, these enzyme activities increased upon challenge with root and crown rot pathogen Pythium aphanidermatum, peaked 4–6 days after inoculation of pathogen. Also, higher expression of PPO isoform was found evident in P. fluorescens Pf1 treated tomato plants in response to the infection of F. oxysporum f.sp. lycopersici. However, combined application of microbial consortia, i.e., B. subtilis, T. viride, P. fluorescens in sugarbeet and green gram plants expressed higher accumulation of PPO defense enzymes, upon inoculation with fungal pathogens viz., Sclerotium rolfsii and Macrophomina phaseolina (Narayanasamy, 2019).
Babu et al. (2015) reported PGPRs isolated from tomato rhizosphere exhibited protection against early blight disease of tomato through enhanced accumulation of antioxidant peroxidase (PO), and polyphenol peroxidase (PPO) enzymes. The result showed significant increase in seed germination, seedling vigour, growth and fruit weight of tomato, which was attributed to PGPRs ability to produce IAA, enhanced nutrient uptake and chlorophyll content in treated plants. Another PGPR, Bacillus spp. KPF-5, KPF-7, KPF-17, were also found to control blast disease of rice, Pyricularia oryzae by adopting defensive strategy manifested through induction of systemic resistance by elicitation of antioxidant enzymes, i.e., peroxidase (3.5–4.1-fold), polyphenol oxidase (3.0–3.8-fold), superoxide dismutase (1.7–1.9-fold) and phenylalanine ammonia lyase (3.9–4.4-fold) in rice leaves and roots (Rais et al., 2017). In addition, Bacillus spp. secreted multiple biocontrol determinants such as glucanase (1.0–1.3 U/mg of soil), protease (1.1-5.5 U/mg of soil), siderophores (6.5–42.8 µg/mL) in rhizosphere of rice varieties thus alleviating P. oryzae induced oxidative damage and suppressing blast disease incidence. Application of PGPR strains especially fluorescent Pseudomonads in horticultural crops such as tomato, chilli and banana expressed increased activity of PPO and its isoforms, when challenged by viruses such as Tomato spotted wilt virus, Cucumber mosaic virus and Banana bunchy top virus (Joni et al., 2020).
Phenylalanine ammonia lyase (PAL)
Phenylalanine ammonia lyase (PAL) is the first key enzyme involved in phenyl propanoid pathway and plays a key role in biosynthesis of phenolics and phytoalexins. PAL is also the key enzyme in inducing synthesis of salicylic acid (SA), which induces systemic resistance in many plants. An increase in the level of mRNAs encoding for PAL was recorded in the early stage of interaction between bean roots and various rhizobacteria. Induction of enzymes such as PAL and PO, leading to the accumulation of phenolics and lignin can occur in response to pathogen attack. Rhizobacterial treatment of rice, maize and tea seedlings with PGPRs viz., Pseudomonas, Bacillus, Staphylococcus, Ochrobactrum, Lysinibacillus, Micrococcus, Leifsonia, Exiguobacterium and Arthobacter triggered enzymatic (APX, CAT, Chitinase, PAL) and non-enzymatic (Proline, polyphenolics) antioxidant defense reactions, indicated its role in reduction of reactive oxygen species (ROS) burden and priming of plants towards stress mitigation (Bhattacharya et al., 2020). Rhizobacterial strains isolated from chilli rhizosphere viz., P. fluorescens PDS1, B. subtilis BDS1, B. cereus UK4, B. amyloliquefaciens UK2 and B. subtilis KA9 suppressed bacterial leaf blight of chilli, Ralstonia solanacearum (Kashyap et al., 2021). The antagonistic property is attributed to induce resistance in chilli leaf and root tissues (cv. Pusa) through enhancement of defensive enzymes such as PO (4.87-fold), PPO (9.30-fold), PAL (1.04-fold), SOD (9.49-fold) activities along with other PGP activities viz., IAA production (67.64%), phosphorus solubilization (79.41%), ammonia, HCN (58.82%) and siderophore production (55.88%).
Chitinases (PR3, PR4, PR8 and PR11) are PR-proteins which hydrolyze chitin, a major cell wall component of fungi, cuticle and peritrophic membrane in insects (Tetreau et al., 2015). Chitinase enzymes utilizes endolytic or exolytic mechanisms to cleave the bond between C1 and C4 of two consecutive N-acetyl glucosamine (GlcNAc). A large number of plants chitinases have been purified and characterized which are endochitinases with molecular weight ranging from 25 to 36 kDa (Aida et al., 2016). The chitinase production in plants was suggested as a part of defense mechanism in plants against fungal pathogens. Increased expression of chitinase activity and induction of more isoforms of chitinase was reported in many plant pathogen interaction studies (Backer et al., 2018).
Evidence of β-1,3-glucanases (PR-2) (EC 3.2.1.6) in disease resistance was first reported in dicots and β-1,3-glucanase genes constitute defense genes induced during pathogenesis (Su et al., 2016). Later, β-1,3-glucanases induction was demonstrated in monocot plants viz., barley, rice, wheat, and sorghum as a response mechanism against infection by necrotrophic pathogens. It has been reported rapid induction of two β-1,3-glucanases in the incompatible interaction between bean and C. lindemuthianum and it was also reported in other plant pathogen interactions (Chakraborty et al., 2019).
Strengthening of cell wall structure
The mechanism of inhibition includes cell wall strengthening by apposition caused by large amounts of callose and phenolic substances at the sites of attempted fungal invasion. In tomato plants, cell wall thickening was brought about by bacterization, deposition of phenolic compounds and formation of callose was observed and resulted in declined growth of F. oxysporum f.sp. radicis lycopersici in the epidermal layer and outer cortex of root system of treated plants (Amini and Jahanshir, 2009). The rapid strengthening of reaction sites of pathogen delays the infection process and allows sufficient time for the host to build up other defense reactions. In bean plants, seed treatment done with PGPR induces cell wall lignifications (Hilal et al., 2016). Agrobacterium rhizogenes Ri T-DNA transformed pea roots pre-inoculated with the endophytic bacterium, Bacillus pumilus SE34 were protected against the root rot pathogen F. oxysporum f.sp. pisi. Similar wall appositions and papillae were observed in pea roots treated with the P. fluorescens 63-28R upon challenge inoculation with either F. oxysporum f.sp. pisi or P. ultimum, indicating a general induction of physical defense barriers to pathogen ingress. Thickening of cell wall of cortical cells was induced in tomato was observed after colonization of roots by P. fluorescens WCS417. Bacillus pumilus strain SE 34 also induced strengthening of cell wall structure in tomato against F. oxysporum f.sp. radicis-lycopersici (Shivakrishnaprasad, 2019).
Exposure to pathogens or insects generates a cascade of events leading to the expression of phytohormones that causes the suppression of invading organisms. The term systemic acquired resistance (SAR) was first coined by Ross who described induced resistance in tobacco plants after infection with tobacco mosaic virus (TMV). During SAR, resistance reactions occur in the non-infected parts starting from the infection site. At the site of attack, the plants respond to pathogen infection through modifications of the cell wall, production of phytoalexins, production of pathogenesis related (PR) proteins and activation of programmed cell death or hypersensitive reaction (HR). Plants use a variety of cues, including the sense of touch (Mescher and Moraes, 2015), oviposition and salivary enzymes or oral secretions to detect herbivore invasion. Oviposition chemicals like benzyl cyanide deposited with eggs of the cabbage white butterfly (Pieris brassicae) act as an elicitor in inducing defense in brussels sprouts (Brassica oleracea) (Afenntoulis et al., 2021). The biochemical elicitors in insect oral secretions also plays an important role in eliciting systematic resistance in plants. Such biochemical oral elicitors include Caeliferins in grasshoppers (Schmelz, 2015), β-glucosidase in cabbage butterfly, volicitin in Spodoptera exigua, inceptin in maize fall armyworm.
The defensive response to herbivores usually begins at the plant cell plasma membrane where the perception of molecular patterns and defense effectors occurs. This in turn causes the elevation of cytosolic calcium that leads to depolarization of the plasma transmembrane potential followed by ion efflux/influx, mitogen-activated protein kinase (MAPK) activation (Zebelo and Maffei, 2015). These events lead to increase in production of phytohormones viz., auxins, cytokinins (CKs), gibberellins (GAs), salicylic acid (SA), jasmonic acid (JA), ethylene (ET), abscisic acid (ABA), and brassino steroids (BRs) or production of volatile organic compounds (VOC) (Mescher and Moraes, 2015). Induced resistance against pests is mediated by phenylpropanoid and octadecanoid pathways through the production of salicylic acid (SA) and jasmonic acid (JA), which affects insect growth and development of insects or through the release of volatiles for attraction of natural enemies. For example, the resistance in rice against the pathogen, leaf folder Cnaphalocrocis medinalis is mediated by ET and SA signalling pathways.
Rhizospheric engineering of PGPR to control plant diseases
Rhizosphere as defined as a narrow zone of interface between roots and soil environment harbours enormous reservoir of microbial community under the influence of organic materials, rhizodeposists, plant metabolites and plant debris. The two compartments of rhizosphere viz ecto and endorhizosphere extensively holds the association of plants with specific group of microbes interacting with one another as an individual, thus functioning as metaorganism or holobiont (Bordenstein and Theis, 2015). The population of selected microorganisms exerts numerous beneficial effects on plant and overall rhizosphere functioning such as enhancing plant growth by facilitating nutrient acquisition, tolerate abiotic stress as well as defence against phytopathogens. Such intricate relationship maintains rhizosphere in dynamic equilibrium and suggests its scope to engineer all its components viz. soil, plant and microbial population, etc., favouring plant growth and tolerance to various biotic and abiotic stresses. Moreover, owing to the drawbacks of conventional rhizosphere modification strategies in terms of maintenance of population densities that decline over time and distance from inoculation source, avenue of rhizosphere microbiome engineering emerged as potential alternative.
Engineering of rhizosphere microbiome
Rhizosphere engineering aimed to manipulate the components of rhizosphere microbiome by altering the rhizosphere through biological tools and approaches viz plant, microbiome and meta-organism approach to express a bias towards beneficial microorganisms enabling plants to evolve into better hosts (Quiza et al., 2015). It basically harnesses the variations in plant root exudate patterns or genetically alter it that influence microbial communities by either enhancing or inhibiting the growth of specific microorganisms (Quiza et al., 2015). The goal of rhizosphere engineering mainly governs direct plant-microbe interactions for enhanced beneficial outcomes such as nutrient cycling, mineralization, solubilisation, decomposition of organic matter, tolerance to abiotic stresses as well as disease resistance. It especially deals with plant defense machinery instrumental in engineering plant resistance to biotic stresses or microbial population engineering rather than single strain engineering.
Strategies for engineering rhizosphere microbiome
Engineering a “biased rhizosphere” is the novel procedure that involves expression of specific genes in transgenic plants to enable roots to produce specific nutritional compounds that are recognized by specific beneficial microorganisms (Sudheer et al., 2020). Plant root exudates play a pivotal role in attracting specific beneficial microorganisms, therefore altering root exudate compositions is determined as one of major approach to reshape rhizosphere microbiome (Olanrewaju et al., 2019). Also, understanding of root architecture, biochemical and molecular determinants around root or rhizosphere are also key determinant responsible for selective microbial enrichment (Kumar and Dubey, 2020). Several strategies responsible for rhizosphere modification includes manipulation of root border cells, engineering of inhibitors and enhancers as well as induction of microbial gene expression in host plant cell.
Microbe mediated rhizosphere engineering for plant disease control
Microbe mediated rhizosphere engineering usually deals with microbial community surrounding root system of the plant particularly PGPR, which is achieved through bio inoculation (Khan et al., 2019). Therefore, many rhizosphere-engineering strategies used for shaping microbiome requires an information database of PGPR as potential bio fertilizers, usually living in a symbiotic association with their host.
Additionally, the information on their functionality and persistence are also required for culturing of microbes to increase the cultivability of microbes into formulations. Some of these PGPRs includes rhizobia species viz. Rhizobium, Bradyrhizobium, Sinorhizobium, Mesorhizobium; diazotrophs viz. Azospirillum, Azotobacter, Acetobacter, PSBs viz. Bacillus, Streptomyces, Psuedomonas etc.
The mechanism underlying plant disease control via rhizosphere engineering of PGPR can be categorized into direct and indirect effects. Improved fertilization through solubilisation of phosphorus, iron and biological nitrogen fixation, plant growth modulation by inhibition of auxin or cytokinin as well as modulation of ethylene governs direct effects (Belimov et al., 2015). Whereas, indirect effects include niche occupancy leading to efficient colonization of roots, bio pesticide and bio control activities through production of antibacterial, antifungal, nematicide compounds as well as stimulation of plant defense mechanisms by systemic resistance induction and enhancing the pathogen triggered immunity (Huang and Zimmerli, 2014).
PGPR utilized as bacterial formulations (Newton and McLellan, 2015) plays an important role in fixing atmospheric nitrogen (e.g., diazotrophs viz. Acetobacter, Azospirrillum), redeem nitrogen from ammonia (NH4) and nitrate (NO3) as well as increase accessibility of diverse nutrients such as iron, phosphorus, zinc, copper and cadmium through different groups of phosphate solubilizing bacteria (PSB), siderophore producing bacteria and arbuscular mycorrhizal fungi (AMF) (Gomathy et al., 2018). Several rhizobacteria viz. Bacillus, Streptomyces, Psuedomonas have been recognized as potential biocontrol agents due to their ability of producing antibiotic compounds such as phenazine, DAPG, HCN, oligomycin, bacteriocins as well as antifungal compounds such as phoroglucinols, phenazines and pyoluteorin (Chaithanya, 2016). In addition to that, variety of phytohormones viz auxins or indole-3-acetic acid (IAA), gibberellins (GA), cytokinin are considered as a key constituent of plant-microbe interactions playing an essential role in plant growth and development (Gupta et al., 2018; Arun et al., 2020). Cross talk mediated by these chemicals viz. jasmonic acid, salicylic acid and ethylene signalling pathway plays an important role in activating systemic acquired resistance (SAR) and induced systemic resistance (ISR). Therefore, microbial inoculation of plant can induce broad term resistance in both above- or below ground plant parts, thus, priming plants against any cellular derivative determinants also known as microbe associated molecular patterns (MAMPs) viz. cell envelope elements, flagella, siderophore etc. (Malik et al., 2020).
Strategies to enhance the PGPR colonization
The colonization of plant rhizosphere by microorganisms from soil to seed is governed by several properties such as C-N availability, organic matter content, water availability, pH, geographical patterns including soil type and seasonality (Santoyo et al., 2021). Therefore, it is necessary to develop efficient strategies that can emphasize effective inoculation methods and modulate determinants for efficient colonization of plants by PGPRs as well as their consistent performance under field conditions (Lee et al., 2016). PGPR colonization of plants can be amended by biofilm formation as well as biochar application.
The plant-associated biofilms can establish on various plant parts such as leaves, roots, seeds and internal vascular structure (Backer et al., 2018). Among several advantages of biofilm formation on PGPR colonization, some are as follows:
• Ability of biofilm formation enhances bacterial survival in addition to enhancing plant growth through various PGPR-associated mechanisms.
• Biofilm formation confers higher resistance to antibiotics therefore leading to improved chance of survival in competitive soil environment.
• Biofilm also enhances plants growth indirectly through biocontrol of plant diseases via competitive colonization of rhizosphere as well as production of antimicrobial compounds.
• For e.g., single and dual-species biofilm produced by Psuedomonas, Trichoderma, Bradyrhizobium, Penicillium etc. showed PGPR activity such as greater ammonia production, IAA production, siderophore production as well as phosphate solubilization (Kumar et al., 2021).
• High PGPR activity have been reported in case of seed germination of cotton, root-shoot length of wheat, dry weight of soybean and nitrogen accumulation, seed germination and root length of maize post biofilm formation (Mohd and Ahmad, 2014).
• Exopolysaccharide and biofilm production by PGPR isolates (Bacillus tequilensis and Bacillus aryabhattai) found to be an important characteristic for salt tolerance in rice plants (Biochar is widely known as soil amendment due to its ability to improve soil fertility and increase crop yields. It has the dynamic ability to change soil fertility parameters such as pH, organic matter content, cation exchange capacity, nutrient retention, water retention, oxygen tension, bulk density, thus influencing microbial survival in soil and providing niche for microbes (Jenkins et al., 2017). In addition to that, use of biochar acts as a carrier material for microbial inoculants when applied as a seed coating, thus promote early colonization of rhizosphere with beneficial microorganisms (Deb et al., 2016).
Rhizosphere competence and compatibility with other microflora
The ability of PGPRs to colonize crop rhizosphere largely depends on their composition and amount of root exudates and most importantly competence that further lays foundation of structural development of microbial community. Rhizosphere competence governs the ecological fitness of PGPR as well as an associated risk with their colonization, competition as well as survival in the soil environment. Successful colonization of microbes depends on recognition, adherence, invasion, colonization, growth and interactions. Initially, crosstalk between plant roots and microbes are established by production of signals and PGPRs adhere to plant surface via pili, outer membrane proteins and flagella. Plant-microbe interactions further triggers signalling pathways producing secondary metabolites viz. phenolics, flavonoids, alkaloids, terpenoids etc. enhancing plant’s ability to resist pathogens. In addition, PGPR also promotes plant growth and development by producing plant growth hormones viz. auxin (IAA), cytokinin, gibberellins (Pang et al., 2021).
Recent research trends have highlighted the concept of development of multi-strain mixtures with the rationale to perform better in terms of nutrient acquisition, biotic and abiotic stress resistance with additive benefits in sustainable way (Vorholt et al., 2017). To which the issue of biological compatibility among multiple microbial strains on account of antagonistic interactions paves it way towards developing effective multi-strain mixtures to use as inoculants (Sarma et al., 2015). Microbial components in consortia are considered as compatible to each other, when they have no growth suppressive effect on each other during in vitro co-culture conditions either in contact or in close proximity or during plant rhizosphere colonization (Liu et al., 2018).
Inoculation of beneficial plant microbes such as PGPR viz. Bacillus, Psuedomonas, Trichoderma etc. in agricultural system has yielded beneficial outcomes in terms of increase crop growth as well as resistance against phytopathogens serve potential substitute for chemical pesticides. Several studies have reported gradual decline of bio-inoculant populations and their performance over distance and time of inoculation due to decrease in inoculant numbers, physiological status of inoculant cells, biotic interactions in soil as well as edaphic properties. Several other factors such as agronomic practices based on heavy use of agrochemicals, selection preferences of plants in selective association with introduced microbial community impact the efficacy of inoculants (Trivedi et al., 2017). The host selection pressure is mediated by host immune system, root exudates as well as indigenous endophytic microbes viz fungi, bacteria, micro-algae and viruses (Fister et al., 2016). In addition to their beneficial effects, several introduced microbes can also harbour or favour potential opportunistic pathogens that can harm root environment by disrupting ecological integrity as well as by inducing diseases.
The successful persistence of introduced microbes depends on their ability to cope with unfavorable conditions, to successfully compete with indigenous microorganisms, to overcome plant selection preferences and to establish, proliferate and to remain active. Therefore, increasing inoculation efficiency, performance as well persistence of effective bio-inoculants by subtracting its detrimental outcomes can be achieved by using indigenous microbes, genetic engineering tools as well as improved delivery methods.
Use of indigenous microbes, i.e., group of innate microbial communities inhabiting local soils, plant internal tissues and outer surfaces enhance persistence chance of PGPR due to their innate adaptability to plant environment that may increase the chance of inoculum survival (Banerjee et al., 2017). Indigenous microbes are harnessed by isolating microbes harbouring healthy plants with phenotype of interest that are used either alone or combined as a composite microbial consortium to improve overall crop fitness and performance of susceptible plant (Mueller and Sachs, 2015). Use of advanced genetic engineering tools such as RNAi and CRISPR/Cas9 can modify gene of interest in order to mine knowledge at genetic and transcriptional levels about their functions and expressions relevant to improved nutrient mobilisation as well as defense against plant pathogens.
Optimized delivery strategies represent fundamental aspect of bio-inoculation success as up to 90% of introduced microbes can be lost during field application (Vejan et al., 2016). Therefore, use of effective tools to improve formulations dispersal allowing controlled release of microbial inoculants can ensure feasibility, sustainability as well commercial success of microbe-mediated crop protection. Seed bio-priming, i.e., coating seeds with PGPR before sowing are effective in suppressing disease infection from germination to later stages of plant development (Junges et al., 2016). In addition, encapsulation technologies involving binding of seeds with microbial inoculants via liquid polymers, adhesives, gelatin, starch, methylcellulose, etc. showed improved germination, seedling vigour, fertilizer release rate and disease resistance (Jambhulkar and Sharma, 2014).
Genetic engineering of PGPR strains
Genetic engineering plays a pivotal role in identifying causes of variable strain performance offering a means to develop PGPR that are effective even at low inoculum doses under variety of environmental conditions. A successful strategy for strain development relies on the fact that the introduced PGPR must establish and maintain biologically active populations in competition with already-adapted resident microflora. In terms of strain colonization and performance, genetic engineering of individual fitness determinants targets particular gene involved in growth promotion either by modifying the timing or level of expression or transferring and expressing in alternate hosts with desirable attributes. Success of strain improvement strategy relies not only on plant growth enhancement but also on stable maintenance and expression of engineered trait, effects on fitness of modified strain as well as effect of modified strain on non-target organisms in environment.
Important consideration for the better use of PGPR
Development of new PGPR inocula with magnificent potential relies on efficient laboratory screening assays based on specific PGPR mechanisms viz. nitrogen fixation, auxin synthesis, ACC deaminase activity and calcium phosphate solubilization.
• PGPR formulations should be prepared with appropriate carrier material allowing efficient rhizosphere colonization under field conditions.
• Long term fumigation usually affects soil microbes and their interactions impeding nutrient acquisition and mobilization, also posing great challenge to rhizosphere colonization by PGPR inocula (Dangi et al., 2017).
• Designing microbial consortia addressing several problems such as bioremediation, plant growth promotion as well as disease resistance simultaneously would be a promising holistic management approach.
• Proper training of farmers and associated staffs for their efficient application is very important element in development and deployment of beneficial inocula (Itelima et al., 2018).
• Development of PGPR-based inoculants generally includes following steps:
a) Isolation of bacteria from roots or plant tissues.
b) Screening in laboratory under controlled growth environment.
c) Field screening for different crops, geographic locations, planting dates as well as soil types.
d) Evaluation of rhizosphere competence and bio-compatibility.
e) Standardization of delivery methods and management practices.
f) Bioassay confirming non-toxicological effects.
g) Product delivery formulation development.
h) Registration and regulatory approval.
i) Product availability in the market.
Future thrust area in PGPR/challenges
The rhizosphere microbiome studies tend to facilitate communication between the plant and surrounding soil environment contributing to create productivity metagenome leading to crop productivity. Studies concentrating comparative genomics as well as metabolomics to unveil synergistic and complementary mechanisms can be focused with the use of model plants grown under gnobiotic conditions. Microbial interactions and assembly possess direct relation with plant’s ability leading to selection of host-microbial association and “microbe-driven cropping system” is emerging as an approach to enhance plant fitness and productivity. Application of multiomics approach along with recent genetic engineering tools such as CRISPR can be the new talk of future research aiming for enhancing nutritional status, disease resistance and crop yield for achieving zero hunger goals for constantly increasing human population. Utilization of synthetic biology approaches exploiting positive microbiome interactions aiming to achieve food production and bioenergy under environmental stress conditions can be a major challenge. Additionally, the major scientific obstacle impeding further progress is the fundamental issues concerning microbial abundance and diversity, their functions as well as understanding on complex chemical and biological interactions occurring in the rhizosphere microbiome. To resolve these constraints, more stress should be given in encouraging development of eco-friendly alternatives, non-polluting amendments and novel natural biocontrol agents as well as genetically modified options.
The concept of achieving healthier crops with minimal inputs of fertilizers and agrochemicals without compromising its yield is a major challenge. Use of PGPR bio-inoculants as bio fertilizers as well as biocontrol agents simultaneously paves the way towards healthier and sustainable crop production. Knowledge about diverse PGPR microbiome in crop rhizosphere and their mechanism in plant growth promotion as well as protection against biotic stresses channelizes efficient microorganisms in beneficial way. Rhizosphere can be engineered through appropriate selection of crop species and varieties, by the introduction of microorganisms, soil amendments, by genetic modification as well as through microbial biological activities. Therefore, rhizosphere microbiome engineering is also emerging as dynamic technique for increasing bio-inoculant colonization, competence and persistence of beneficial microbiota in crop rhizosphere. In addition to that, it also provides opportunities to alter structures of microbial community increasing disease resistance in plants as well as uptake of nutrients. Therefore, designing and application of synthetically developed consortia from compatible multi strains should be given more emphasis as they show better results in terms of plant growth promotion as well as resistance to biotic stresses as compared to single strain.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contribution: PD and GM conceived the work and PD, GM and LD wrote the draft manuscript. PD and GM finalize manuscript. All authors reviewed the work, revised the final manuscript, and approved submission.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Adhya TK, Lal B, Mohapatra B, paul D, Das S (2018). Advances in Soil Microbiology: Recent Trends and Future Prospects. Singapore: Springer. [Google Scholar]
Afenntoulis DG, Cusumano A, Greenberg LO, Carrls L, Fatouros NE (2021). Attraction of Trichogramma Wasps to butterfly oviposition induced plant volatiles depends on Brassica species, Wasp strain and leaf necrosis. Frontiers in Ecology and Evolution 9: 703134. [Google Scholar]
Ahemad E, Kibret M (2014). Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. Journal of King Saudi University Science 26: 1–20. [Google Scholar]
Aida MF, Hanan M, Abd-Elnabey, Hassan AH, Ibrahim et al. (2016). Purification, characterization and antimicrobial activity of chitinase from marine-derived Aspergillus terreus. Egyptian Journal of Aquatic Research 42: 185–192. [Google Scholar]
Ali S, Charles TC, Glick BR (2014). Amelioration of high salinity stress damage by plant growth promoting bacterial endophytes that contain ACC deaminase. Plant Physiology and Biochemistry 80: 160–167. [Google Scholar]
Altaf M, Khan M, Abulreesh H, Ahmad I (2017). Quorum sensing in plant growth-promoting rhizobacteria and its impact on plant-microbe interaction. In: Singh DP, Singh HB, Prabha R (eds.Plant-Microbe Interactions in Agro-Ecological Perspectives, pp. 311–331. Springer Nature. [Google Scholar]
Amara U, Khalid R, Hayat R (2015). Soil bacteria and phytohormones for sustainable crop production. In: Maheshwari DK (eds.Bacterial metabolites in sustainable agroecosystem. Springer International, 87–103. [Google Scholar]
Amini, Jahanshir (2009). Induced resistance in tomato plants against Fusarium wilt invoked by nonpathogenic Fusarium, chitosan and bion. Plant Pathology Journal 25: 256–262. [Google Scholar]
Arun KD, Sabarinathan KG, Gomathy M, Kannan R, Balachandar D (2020). Mitigation of drought stress in rice crop with plant growth-promoting abiotic stress-tolerant rice phyllosphere bacteria. Journal of Basic Microbiology 60: 768–786. DOI 10.1002/jobm.202000011. [Google Scholar] [CrossRef]
Babu AN, Jogaiah S, Ito SI, Nagaraj AK, Tran LSP (2015). Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Science 231: 62–73. DOI 10.1016/j.plantsci.2014.11.006. [Google Scholar] [CrossRef]
Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D et al. (2018). Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Frontiers in Plant Science 9: 1473. DOI 10.3389/fpls.2018.01473. [Google Scholar] [CrossRef]
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology 57: 233–266. DOI 10.1146/annurev.arplant.57.032905.105159. [Google Scholar] [CrossRef]
Bakker P, Berendsen RL, Doornbos RL, Wintermans PCA, Pieterse CMJ (2013). The rhizosphere revisited: Root microbiomes. Frontiers in Plant Sciences 4: 165. DOI 10.3389/fpls.2013.00165. [Google Scholar] [CrossRef]
Banerjee A, Bareh DA, Joshi S (2017). Native microorganisms as potent bioinoculants for plant growth promotion in shifting agriculture (Jhum) systems. Journal of Soil Science and Plant Nursery 17: 127–140. DOI 10.4067/S0718-95162017005000010. [Google Scholar] [CrossRef]
Basu A, Prasad P, Das SN, Kalam S, Sayyed RZ et al. (2021). Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 13: 1140. DOI 10.3390/su13031140. [Google Scholar] [CrossRef]
Belimov A, Dodd IC, Safronova VI, Shaposhnikov AI, Azarova TS et al. (2015). Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deamine decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum). Annual Applied Biology 167: 11–25. DOI 10.1111/aab.12203. [Google Scholar] [CrossRef]
Bhattacharya PN, Jha DK (2012). Plant growth-promoting rhiuzobacteria (PGPREmergence in agriculture. World Journal of Microbiology and Biotechnology 28: 1327–1350. DOI 10.1038/s41598-020-72439-z. [Google Scholar] [CrossRef]
Bhattacharya C, Banerjee S, Acharya U, Mitra A, Mallick I et al. (2020). Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Scientific Reports 10: 15536. DOI 10.1038/s41598-020-72439-z. [Google Scholar] [CrossRef]
Bordenstein SR, Theis KR (2015). Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biology 13: e1002226. DOI 10.1371/journal.pbio.1002226. [Google Scholar] [CrossRef]
Bouffaud ML, Kyselkova M, Gouesnard B, Grundmann G, Muller D et al. (2012). Is diversitfication history of maize influencing selection of soil bacteria by roots? Molecular Ecology 21: 195–206. DOI 10.1111/j.1365-294X.2011.05359.x. [Google Scholar] [CrossRef]
Bradley JM, Svistunenko DA, Wilson MT, Hemmings AM, Moore GR et al. (2020). Bacterial iron detoxification at the molecular level. Journal of Biological Chemistry 295: 17602–17623. DOI 10.1074/jbc.REV120.007746. [Google Scholar] [CrossRef]
Bulgarelli D, Oter RG, Munch PC, Weiman A, Dröge J et al. (2015). Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host & Microbe 17: 392–403. DOI 10.1016/j.chom.2015.01.011. [Google Scholar] [CrossRef]
Cao Y, Pi H, Chandrangsu P, Li Y, Wang Y et al. (2018). Antagonism of two plant growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Scientific Reports 8: 4360. [Google Scholar]
Carvalhais LC, Dennis PG, Badri DV, Tyson GW, Vivanco JM et al. (2013). Activation of the jasmonic acid plant defence pathway alters the composition of rhizosphere bacterial communities. PLoS One 8: e56457. DOI 10.1371/journal.pone.0056457. [Google Scholar] [CrossRef]
Caulier S, Grills A, Colau G, Licciardi F, Liepin M et al. (2018). Versatile antagonistic activities of soil-borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and other potato pathogens. Frontiers of Microbiology 9: 143. [Google Scholar]
Chai YN, Schachtman DP (2022). Assessment of bacterial inoculant delivery methods for cereal crops. Frontiers of Microbiology. DOI 10.3389/fcimb.2022.791110. [Google Scholar] [CrossRef]
Chaithanya BH (2016). Biocontrol potentiality and plant growth promoting activity of P. fluorescens-A Review. International Journal of Agricultural Science Research 6: 169–176. [Google Scholar]
Chakraborty N, Mukherjee K, Sarkar A, Acharya K (2019). Interaction between bean and Colletotrichum gloeosporioides: Understanding through a biochemical approach. Plants 8: 345. [Google Scholar]
Chen C, Belanger RB, Benhamou N, Paulitz TC (2000). Defense enzymes induced in cucumber roots by treatment with plamnt growth-ptomoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiological and Molecular Plant Pathology 56: 13–23. [Google Scholar]
Chen Y, Wang W, Zhou D, Jing T, Li K et al. (2020). Biodegradation of lignocellulosic agricultural residues by a newly isolated Fictibacillus sp. YS-26 improving carbon metabolic properties and functional diversity of the rhizosphere microbial community. Bioresource Technology 310: 123381. [Google Scholar]
Choudhary DK, Kasotia A, Jain S, Vaishnav A, Kumari S et al. (2015). Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. Journal of Plant Growth Regulation 35: 276–300. DOI 10.1007/s00344-015-9521-x. [Google Scholar] [CrossRef]
Cordovez V, Rotoni C, Andreote F, Oyserman B, Carrion Víctor J et al. (2021). Successive plant growth amplifies genotype-specific assembly of the tomato rhizosphere microbiome. Science of the Total Environment 772: 144825. DOI 10.1016/j.scitotenv.2020.144825. [Google Scholar] [CrossRef]
Dangi S, Tirado-Corbala R, Gerik J, Hanson B (2017). Effect of long-term continuous fumigation on soil microbial communities. Agronomy 7: 37. DOI 10.3390/agronomy7020037. [Google Scholar] [CrossRef]
de Souza RSC, Armanhi JSL, Damasceno NB, Imperial J, Arruda P (2019). Genome sequences of a plant beneficial synthetic bacterial community reveal genetic features for successful plant colonization. Frontiers in Microbiology 10: 1779. DOI 10.3389/fmicb.2019.01779. [Google Scholar] [CrossRef]
Deb D, Kloft M, Lassig J, Walsh S (2016). Variable effects of biochar and P solubizing microbes on crop productivity in different in different soil conditions. Agroecology and Sustainable Food Systems 40: 145–168. DOI 10.1080/21683565.2015.1118001. [Google Scholar] [CrossRef]
Devarajan AK, Muthukrishanan G, Truu J, Truu M, Ostonen I et al. (2021). The foliar application of rice phyllosphere bacteria induces drought-stress tolerance in Oryza sativa (L.). Plants 10: 387. [Google Scholar]
Devi V, Gomathy M, Sabarinathan KG, Jeyshree M, Kalaiyarasi V (2020). Assessing the plant growth promoting activity of phylloplane associated plant bacteria of Rice. International Journal of Current Microbiology and Applied Sciences 9: 384–391. [Google Scholar]
Dey S, Regon P, Kar S, Panda SK (2020). Chelators of iron and their role in plant’s iron management. Physiology and Molecular Biology of Plants 26: 1541–1549. [Google Scholar]
Disi JO, Mohammad HK, Lawrence K, Kloepper J, Fadamiro H (2019). A soil bacterium can shape below ground interactions between maize, herbivores and entomopathogenic nematodes. Plant and Soil 437: 83–92. [Google Scholar]
Dong YH, Xu JL, Li XZ, Zhang LH (2000). AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. PNAS, 97: 3526–3531. [Google Scholar]
Dos Santos RM, Diaz PAE, Lobo LLB, Rigobelo EC (2020). Use of plant growth-promoting rhizobacteria in maize and sugarcane: Characteristics and applications. Frontiers in Sustainable Food Systems 4: 136. [Google Scholar]
Duy M, Hoi N, Ve N, Thuc L, Trang N (2016). Influence of Cellulomonas Flavigena, Azospirillum sp. and Psudomonas sp. on rice growth and yield grown in submerged soil amended in rice straw, Recent Trends in PGPR Research and Sustainable Crop Production, 238–242. [Google Scholar]
Egamberdieva D, Wirth SJ, Alqarawi AA, Abd_AEF, Hashem A (2017). Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Frontiers in Microbiology 8: 2104. [Google Scholar]
Fernando W, Nakkeeran S, Zhang Y, Savchuk S (2018). Biological control of Sclerotinia sclerotiorum (Lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop Protection 26: 100–107. [Google Scholar]
Fister S, Robben C, Witte AK, Schoder D, Wagner M et al. (2016). Influence of environmental factors on phage bacteria interactions and on the efficacy and infectivity of phage P100. Frontiers of Microbiology 7: 1152. [Google Scholar]
Galloway AF, Knox P, Krause K (2020). Sticky mucilages and exudates of plants: Putative microenvironmental design elements with biotechnological value. New Phyologist Foundation, 225(4), 1461–1469. [Google Scholar]
Garcia-Seco D, Zhang Y, Gutierrez-Mañero FJ, Martin C, Ramos-Solano B (2015). Application of Pseudomonas fluorescens to blackberry under field conditions improves fruit quality by modifying flavonoid metabolism. PLoS One 10: e0142639. [Google Scholar]
Glick BR (2012). Plant growth-promoting bacteria: mechanisms and applications. Scientifica: 963401. [Google Scholar]
Gomathy M, Sabarinathan KG, Sivasankari D, Pandiyarajan P (2018). Arbuscular mycorrhizal fungi and glomalin–Super glue. International Journal of Current Microbiological and Applied Sciences 7: 2853–2857. [Google Scholar]
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS et al. (2017). Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiological Research 27: 100210. [Google Scholar]
Grobelak A, Napora A, Kacprzak M (2015). Using plant growth promoting rhizobacteria (PGPR) to improve plant growth. Ecological Engineering 84: 22–28. [Google Scholar]
Gupta A, Sharma A, Pathak R, Kumar A, Sharma S (2018). Solid state fermentation of non-edible oil seed cakes for production of proteases and cellulases and degradation of anti-nutritional factors. Journal of Food Biotechnology Research 2: 3–8. [Google Scholar]
Gupta S, Pandey S (2019). ACC Deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French Bean (Phaseolus vulgaris) plants. Frontiers in Microbiology 10: 1506. [Google Scholar]
Hakim S, Naqqash T, Nawaz MS, Laraib I, Siddique MJ et al. (2021). Rhizosphere engineering with plant growth promoting microorganisms for agriculture and ecological sustainability. Frontiers in Sustainable Food Systems 5: 617157. [Google Scholar]
Harman GE, Upho N (2019). Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Scientifica 2019: 9106395. [Google Scholar]
Hartmann A, Klink S, Rothballer M (2021). Importance of N-Acyl-homoserine lactone-based quorum sensing and quorum quenching in pathogen control and plant growth promotion. Pathogens 10: 1561. [Google Scholar]
Hassan MK, McInroy JA, Jones J, Shantharaj D, Liles MR et al. (2019). Pectin-Rich amendment enhances soybean growth promotion and nodulation mediated by Bacillus velezensis strains. Plants 8: 120. [Google Scholar]
Hawes M, Allen C, Turgeon BG, Curlango-Rivera G, Minh Tran T et al. (2016). Root border cells and their role in plant defense. Annual Review of Phytopathology 54: 143–161. [Google Scholar]
Hilal A, Shafie Radwa, El-Sharkawy Hany (2016). Interaction between Bean yellow mosaic virus and Botrytis fabae on Faba Bean and the Possibility of their Control by Plant Growth Promoting rhizobacteria. Egyptian Journal of Phytopathology 44: 81–97. [Google Scholar]
Hiltner L (1904). Uber neuere Erfahrungen und Probleme auf dem Gebiete der Bodenbakteriologie unter besonderden berucksichtigung und Brache. Arb Dtsch Landwirtsch Gesellschaft 98: 59–78. [Google Scholar]
Hossain M, Ran C, Liu K, Ryu CM, Rasmussen-Ivey C et al. (2016). Deciphering the conserved genetic loci implicated in plant disease control through comparative genomics of Bacillus amyloliquefaciens subsp. Plantarum. Frontiers in Plant Science 6: 631. [Google Scholar]
Hu Y, Ribbe MW (2016). Biosynthesis of the metalloclusters of nitrogenases. Annual Review of Biochemistry 85: 455–483. DOI 10.1146/annurev-biochem-060614-034108. [Google Scholar] [CrossRef]
Huang PY, Zimmerli L (2014). Enhancing crop innate immunity: New promising trends. Frontiers of Plant Science 5: 624. DOI 10.3389/fpls.2014.00624. [Google Scholar] [CrossRef]
Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan MIR (2017). Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Frontiers in Plant Science 8: 475. DOI 10.3389/fpls.2017.00475. [Google Scholar] [CrossRef]
Itelima JU, Bang WJ, Onyimba IA, Oj E (2018). A review: Biofertilizers, a key player in enhancing soil fertility and crop productivity. Journal of Microbiology and Biotechnology Reprints 2: 22–28. [Google Scholar]
Jamali H, Sharma A (2020). Biocontrol potential of bacillus subtilis RH5 against sheath blight of rice caused by Rhizoctonia solani. Journal of Basic Microbiology 60: 268–280. [Google Scholar]
Jambhulkar P, Sharma P (2014). Development of bioformulation and delivery system of Pseudomonas fluorescens against bacterial leaf blight of rice (Xanthomonas oryzae pv. oryzae). Journal of Environmental Biology 35: 843. [Google Scholar]
Jayapala N, Mallikarjunaiah NH, Puttaswamy H, Gavirangappa H, Ramachandra NS (2019). Rhizobacteria Bacillus spp. induce resistance against anthracnose disease in chilli (Capsicum annum L.) through activating host defense response. Egyptian Journal of Biological Pest Control 29: 45. [Google Scholar]
Jenkins JR, Viger M, Arnold EC, Harris ZM, Ventura M et al. (2017). Biochar alters the soil microbiome and soil function: Results of next-generation amplicon sequencing across Europe. Global Change Biology Bioenergy 9: 591–612. [Google Scholar]
Joni FR, Hamid H, Yanti Y (2020). Effect of plant growth promoting rhizobacteria (PGPR) on increasing the activity of defense enzymes in tomato plants. International Journal of Environment, Agriculture Biotechnology 5: 1474–1479. [Google Scholar]
Junges E, Bralo, Muniz MF, Bastos BDO, Oruiski P (2016). Biopriming in bean seeds. Acta Agriculturae Scandinavica Section B 66: 207–214. [Google Scholar]
Kamensky M, Ovadis M, Chet I, Chernin L (2003). Soil-borne strain IC14 of Serratia plymuthica with multiple mechanisms of antifungal activity provides biocontrol of Botrytis cinerea and Sclerotinia sclerotiorum diseases. Soil Biology and Biochemistry 35: 323–331. [Google Scholar]
Karthiba L, Saveetha K, Suresh S, Raguchander T, Saravanakumar D et al. (2010). PGPR and entomopathogenic fungus bioformulation for the synchronous management of leaf folder pest and sheath blight disease of rice. Pest Management Science 66: 555–564. [Google Scholar]
Kashyap AS, Manzar N, Rajawat MVS, Kesharwani AK, Singh RP et al. (2021). Screening and biocontrol potential of rhizobacteria native to gangetic plains and hilly regions to induce systemic resistance and promote plant growth in chilli against bacterial wilt disease. Plants 10: 2125. [Google Scholar]
Kenawy A, Dallin DJ, Abo-Zaid, Malek RA, Ambehabati KK et al. (2019). Biosynthesis of antibiotics by PGPR and their role in biocontrol of plant diseases. In: Sayyed RZ (ed.Plant Growth Promoting Rhizobacteria for Sustainable Stress Management, vol. 13, pp. 1–35. DOI 10.1007/978-981-13-6986-5_1. [Google Scholar] [CrossRef]
Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA (2010). Plant growth promotion by phosphate solubilizing fungi-current perspective. Archives of Agronomy and Soil Science 56: 73–98. [Google Scholar]
Khan N, Bano A, Rahman MA, Guo J, Kang Z et al. (2019). Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Scientific Reports 9: 249. DOI 10.1038/s41598-019-38702-8. [Google Scholar] [CrossRef]
Kirienko DR, Kang D, Kirienko NV (2019). Novel Pyoverdine Inhibitors Mitigate Pseudomonas aeruginosa Pathogenesis. Frontiers in Microbiology 9: 3317. [Google Scholar]
Köhl J, Kolnaar R, Ravensberg WJ (2019). Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Frontiers in Plant Science 10: 845. [Google Scholar]
Kulimushi PZ, Basine GC, Nachihera GM, Thonart P, Ongena M (2018). Efficacy of Bacillus amyloliquifaciens as biocontrol agent to fungal diseases of maize under tropical climates: From lab to field assays in South Kivu. Environmental Science and Pollution Research 25: 29808–29821. [Google Scholar]
Kumar A, Dubey A (2020). Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. Journal of Advanced Research 24: 337–352. [Google Scholar]
Kumar A, Gond SK, Mishra A, Sharma VK, Verma SK et al. (2015). Salicyclic acid and its role in systemic resistance induced by Pseudomonas fluorescens to early blight disease of tomato. International Journal of Plant Research 51: 223–281. [Google Scholar]
Kumawat K, Sharma P, Sirari A, Singh I, Gill B et al. (2019). Synergism of Pseudomonas aeruginosa (LSE-2) nodule endophyte with Bradyrhizobium sp. (LSBR-3) for improving plant growth, nutrient acquisition and soil health in soybean. World Journal of Microbiology and Biotechnology 35: 47. [Google Scholar]
Kundan R, Pant G, Jado N, Agarwal PK (2015). Plant growth promoting rhizobacteria: Mechanism and current perspective. Journal of Fertilizers and Pesticides 6: 1000155. [Google Scholar]
Laranjo M, Alexandre A, Oliveita S (2014). Legume growth-promoting rhizobacteria: An overview on the Mesorhizobium genus. Microbiological Research 169: 2–17. [Google Scholar]
Lee SK, Lur HS, Lo KJ, Cheng KC, Chuang CC et al. (2016). Evaluation of the effects of different liquid inoculant formulations on the survival and plant-growth promoting efficiency of Rhodopsuedomonas palustris strain PS3. Applied Microbiology and Biotechnology 100: 7977–7987. [Google Scholar]
Lewis RW, Islam A, Opdahl L, Davenport JR, Sullivan TS (2019). Comparative genomics, siderophore production and iron scavenging potential of root zone soil bacteria isolated from ‘Concord’ grape vineyards. Microbial Ecology 78: 699–713. [Google Scholar]
Liang JG, Tao RX, Hao Z, Wang LP, Zhang X (2011). Induction of resistance in cucumber against seedling damping-off by PGPR Bacillus megaterium strain L8. African Journal of Biotechnology 10: 6920–6927. [Google Scholar]
Liu K, Mclnroy JA, Hu CH, Kloepper JW (2018). Mixtures of plant growth promoting rhizobacteria enhance biological control of multiple plant diseases and plant growth promotion in the presence of pathogens. Plant Disease 102: 67–72. [Google Scholar]
Liu Y, Guan D, Jiang X, Ma M, Li L et al. (2015). Proteins involved in nodulation competitiveness of two Bradyrhizobium diazoefficiens strains induced by soybean root exudates. Biology and Fertility of Soils 51: 251–260. [Google Scholar]
Lyu D, Zajonc J, Page A, Tanney CAS, Shah A et al. (2021). Plant Holobiont Theory: The phytomicrobiome plays a central role in evolution and success. Microorganisms 9: 675. [Google Scholar]
Mahmud K, Missaouli A, Lee K, Ghimire B, Presley HW et al. (2021). Rhizosphere microbiome manipulation for sustainable crop production. Current Plant Biology 27: 100210. [Google Scholar]
Maksimov ABCD, Abizgildina RR, Pusenkova LI (2011). Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens (review). Applied Biochemistry and Microbiology 47: 333–345. [Google Scholar]
Malik NAA, Kumar IS, Nadarajesh K (2020). Elicitor and receptor molecules: Orchestrators of plant defense and immunity. International Journal of Molecular Sciences 21: 963. [Google Scholar]
Mandal L, Kotasthane AS (2014). Isolation and assessment of plant growth promoting activity of siderophore producing Pseudomonad’s fluorescent in crops. International Journal of Agriculture Environment and Biotechnology 6: 103–105. [Google Scholar]
Martinez-Absalon S, Rojas-Solis D, Hernandez-Leon R, Prieto-Bajaras C, Orozco-Mosqueda MDC et al. (2014). Potential use and mode of action of the new strain Bacillus thuringiensis UM96 for the biological control of the grey mould phytopathogen Botrytis cinerea. Biocontrol Science and Technology 24: 1349–1362. [Google Scholar]
Mescher MC, Moraes CD (2015). Role of plant sensory perception in plant-animal interactions. Journal of Experimental Botany 66: 425–433. [Google Scholar]
Miljakovic D, Marinkovic J, Balesevic-Tubic S (2020). The significance of bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 8: 1037. [Google Scholar]
Minaeva OM, Akimova EE, Tereshchenko NN, Zyubanova TI, Apenysheva MV et al. (2017). Effect of Pseudomonas bacteria on peroxidase activity in wheat plants when infected with Bipolaris sorokiniana. Russian Journal of Plant Physiology 65: 717–725. [Google Scholar]
Mohd MA, Ahmad I (2014). A novel strain of P. fluorescens WS1 forms biofilm on root surface and enhances growth of wheat plant. International Conference in Agricultural and Horticultural Sciences, Hyderabad. [Google Scholar]
Mueller UG, Sachs JL (2015). Engineering microbiomes to improve plant and animal health. Trends in Microbiology 23: 606–617. [Google Scholar]
Murali M, Naziya B, Ansari MA, Alomary MN, Alyaha S et al. (2021). Biorespecting of rhizosphere-resident fungi: Their role and importance in sustainable agriculture. Journal of Fungi 7: 314. DOI 10.3390/jof7040314. [Google Scholar] [CrossRef]
Naing KW, Anees M, Kim SJ, Nam Y, Kim YC et al. (2014). Characterization of antifungal activity of Paenibacillus ehimensis KWN38 against soilborne phytopathogenic fungi belonging to various taxonomic groups. Annals of Microbiology 64: 55–63. [Google Scholar]
Nehra V (2011). Plant growth promoting rhizobacteria: A critical review. Life Science and Medicine Research 2011: 21–319. [Google Scholar]
Newton RJ, McLellan SL (2015). A unique assemblage of cosmopolitan freshwater bacteria and higher community diversity differentiate an urbanized estuary from oligotrophic lake Michigan. Frontiers in Microbiology 6: 1028. [Google Scholar]
Ni M, Wu Q, Wang J, Liu WC, Ren JH et al. (2018). Identification and comprehensive evaluation of a novel biocontrol agent Bacillus atrophaeus JZB120050. Journal of Environmental Science and Plant Health Part B 53: 777–785. [Google Scholar]
Olanrewaju OS, Ayangbenro AS, Glick BR, Babalola OO (2019). Plant health: Feedback effect of root exudates-rhizobiome interactions. Applied Microbiology and Biotechnology 103: 1155–1166. DOI 10.1007/s00253-018-9556-6. [Google Scholar] [CrossRef]
Olanrewaju OS, Glick BR, Babalola OO (2017). Mechanisms of action of plant growth promoting bacteria. World Journal of Microbial Biotechnology 33: 197. DOI 10.1007/s11274-017-2364-9. [Google Scholar] [CrossRef]
Pahari A, Pradhan A, Nayak S, Mishra, Bhusan B (2017). Bacterial siderophore as a plant growth promoter. In: Patra JK, Microbial Biotechnology, pp. 163–180. Springer Nature, 163–180. [Google Scholar]
Pandey P, Maheswari D (2007). Two-species microbial consortium for growth promotion of Cajanus cajan. Current Science 92: 1137–1142. [Google Scholar]
Pang Z, Chen J, Wang T, Gao C, Li Z et al. (2021). Linking plant secondary metabolites and plant microbiomes: A Review. Frontiers in Plant Sciences 12: 621276. DOI 10.3389/fpls.2021.621276. [Google Scholar] [CrossRef]
Peterson E, Kaur P (2018). Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Frontiers in Microbiology 9: 2928. DOI 10.3389/fmicb.2018.02928. [Google Scholar] [CrossRef]
Pii Y, Penn A, Terzano R, Crecchio C, Mimmo T et al. (2015). Plant-microorganism-soil interactions influence the Fe availability in the rhizosphere of cucumber plants. Plant Physiology and Biochemistry 87: 45–52. [Google Scholar]
Pokhare SS, Berliner T, Adak U, Kumar U, Mukherjee A (2015). Entomopathogenic nematodes: Insect biocontrol agents. Indian Farming, 65, 20–23. [Google Scholar]
Quiza L, St-Arnaud M, Yergeau E (2015). Harnessing phytomicrobiome signalling for rhizosphere microbiome engineering. Frontier in Plant Science 6: 1–11. [Google Scholar]
Rais A, Jabeen Z, Shair F, Hafeez FY, Hassan MN (2017). Bacillus spp., a biocontrol agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryza. PLoS One 21: e0187412. [Google Scholar]
Rodriguez M, Torres M, Blanco L, Bejar V, Sampedro I et al. (2020). Plant growth-promoting activity and quorum-quenching mediated biocontrol of bacterial phytopathogens by Pseudomonas segetis strain P6. Scientific Reports 10: 4121. [Google Scholar]
Rosier A, Beauregard PB, Bais HP (2020). Quorum quenching activity of the PGPR Bacillus subtilis UD1022 alters nodulation efficiency of Sinorhizobium meliloti on Medicago truncatula. Frontiers in Microbiology 11: 596299. [Google Scholar]
Saeed Q, Xiukang W, Haider FU, Kucerik J, Mumtaz MZ et al. (2021). Rhizosphere bacteria in plant growth promotion, biocontrol and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. International Journal of Molecular Sciences 22: 10529. [Google Scholar]
Sah S, Singh R (2015). Siderophore: Structural and functional characterization–A comprehensive review. Agriculture (Polnohospodarstvo) 61: 97–114. [Google Scholar]
Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharya S et al. (2016). Microbial siderophores and their potential applications: A review. Environmental Science and Pollution Research 23: 3984–3999. [Google Scholar]
Santoyo G, Urtis-Flores CA, Loeza-Lara PD, Carmen MD, Mosqueda O et al. (2021). Rhizosphere colonization determinants by plant growth promoting rhizobacteria (PGPR). Biology 10: 475. [Google Scholar]
Sarma BK, Yadav SK, Singh S, Singh HB (2015). Microbial consortia mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biology and Biochemistry 87: 25–33. [Google Scholar]
Sayyed RZ, Arora NK, Reddy MS (2019). Plant growth-promoting rhizobacteria for sustainable stress management. Microorganisms for Sustainability 1: 1–362. [Google Scholar]
Sayyed RZ, Chincholkar SB (2009). Siderophore producing A. fecalis: More biocontrol potential vis-a-vis chemical fungicide. Current Microbiology 58: 47–51. [Google Scholar]
Sayyed RZ, Chincholkar SB, Reddy MS, Gangurde N (2013). Siderophore producing PGPR for crop nutrition and phytopathogen suppression. In: Maheshwari DK, Bacteria in Agrobiology: Plant Disease Management. Springer. [Google Scholar]
Schmelz EA (2015). Impacts of insect oral secretions on defoliation-induced plant defense. Current Opinion in Insect Science 9: 7–15. [Google Scholar]
Shair F, Yasmin H, Hassan MN, Alzharani OM, Noureldeen A (2021). Pseudomonas spp. mediate defense response in sugarcane through differential exudation of root phenolics. Saudi Journal of Biological Sciences 28: 7528–7538. DOI 10.1016/j.sjbs.2021.09.030. [Google Scholar] [CrossRef]
Sharma CK, Vishnoi VK, Dubey RC, Maheshwari DK (2018). A twin rhizospheric bacterial consortium induces systemic resistance to a phytopathogen Macrophomina phaseolina in mung bean. Rhizosphere 5: 71–75. [Google Scholar]
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013). Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2: 587. [Google Scholar]
Sherathia D, Dey R, Thomas M, Dalsania T, Savsani K, Pal KK (2016). Biochemical and molecular characterization of DAPG-producing growth promoting rhizobacteria (PGPR) of groundnut (Arachis hypogea L.). Legume Research 39: 614–622. [Google Scholar]
Shivakrishnaprasad VS (2019). Symbiotic agriculture increasing knowledge on the mode of action of beneficial microorganisms. Agri food sciences, technologies and biotechnologies. Reggio, Italy. [Google Scholar]
Smith DL, Gravel V, Yergeau E (2017). Editorial: Singalling in the phytomicrobiome. Frontriers in Plant Science 8: 611. [Google Scholar]
Su Y, Wang Z, Liu F, Li Z, Peng Q et al. (2016). Isolation and characterization of ScGluD2, a new sugarcane beta-1,3-Glucanase D family gene induced by Sporisorium scitamineum, ABA, H2O2, NaCl, and CdCl2 Stresses. Frontiers in Plant Science 7: 1348. [Google Scholar]
Sudheer S, Bai RG, Usmani Z, Sharma M (2020). Insights on engineered microbes in sustainable agriculture: Biotechnological developmnets and future prospects. Genomics 21: 321–333. [Google Scholar]
Suleman M, Yasmin S, Rasul M, Yahya M, Atta BM et al. (2018). Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and effects on wheat. PLoS One. DOI 10.1371/journal.pone.0204408. [Google Scholar] [CrossRef]
Syed RBN, Shahzad R, Tayade R, Shahid M, Hussain A et al. (2021). Evaluation of potential of PGPR to protect tomato against Fusarium wilt and promote plant growth. PeerJ 16: e11194. [Google Scholar]
Tetreau G, Dittmer NT, Cao X, Agrawal S, Chen YR et al. (2015). Analysis of chitin-binding proteins from Manduca sexta provides new insights into evolution of peritrophin A-type chitin-binding domains in insects. Insect Biochemistry and Molecular Biology 62: 127–141. [Google Scholar]
Trivedi P, Schenck PM, Wallenstein MD, Singh BK (2017). Tiny microbes, big yields: Enhancing food crop production with biological solutions. Microbial Biotechnology 10: 999–1003. [Google Scholar]
Tsuno Y, Fujimatsu T, Endo K, Sugiyama A, Yazaki K (2017). Soyasaponins, a new class of root exudates in soybean (Glycine max). Plant and Cell Physiology 59: 366–375. [Google Scholar]
Uren NC (2007). Types, amounts and possible functions of compounds released into the rhizosphere by grown plants. In: Pinton R, Varanini Z, Nannipieri P (Eds.The Rhizosphere, Biochemistry and Organic Substances at the Soil Plant Interface, pp. 1–21. Boca Raton, FL: Taylor Francis group. [Google Scholar]
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moenne-Loccoz Y et al. (2013). Plant growth-promoting rhizobacteria and root system functioning. Frontiers of Plant Science 4: 356. [Google Scholar]
Van Loon LC (2007). Plant responses to plant growth-promoting rhizobacteria. European Journal of Plant Pathology 119: 243–254. DOI 10.1007/s10658-007-9165-1. [Google Scholar] [CrossRef]
Vandana UK, Rajkumari J, Singha LP, Satish L, Alavilli H et al. (2021). The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 10: 101. DOI 10.3390/biology10020101. [Google Scholar] [CrossRef]
Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A (2016). Role of plant growth promoting rhizobacteria in agricultural sustainability–A review. Molecules 21: 573. DOI 10.3390/molecules21050573. [Google Scholar] [CrossRef]
Vorholt JA, Vogel C, Carlstrom CI, Mueller DB (2017). Establishing causality: Opportunities of synthetic community for plant microbiome research. Cell Host and Microbe 22: 142–155. DOI 10.1016/j.chom.2017.07.004. [Google Scholar] [CrossRef]
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003). Root exudation and rhizosphere biology. Plant Physiology 132: 44–51. DOI 10.1104/pp.102.019661. [Google Scholar] [CrossRef]
Wang H, Liu R, You MP, Barbeti MJ, Chen Y (2021). Pathogen biocontrol using plant growth-promoting bacteria (PGPRRole of bacterial diversity. Microorganisms 9: 1988. [Google Scholar]
Yang D, Wang L, Wang T, Zhang Y, Zhang S et al. (2021). Plant growth promoting rhizobacteria HN6 induced the rhange and Reorganization of Fusarium Microflora in the rhizosphere of banana seedlings to construct a healthy banana microflora. Frontiers in Microbiology 12: 685408. [Google Scholar]
Yanti Y (2015). Peroxidase enzyme activity of rhizobacteria-induced shallots bulbs to induce resistance of shallot towards bacterial leaf blight (Xanthomonas axonopodis pv. allii). Procedia Chemistry 14: 501–507. [Google Scholar]
Zamioudis C, Pieterse CM (2012). Modulation of host immunity by beneficial microbes. Molecular Plant Microbe Interactions 25: 139–150. [Google Scholar]
Zebelo SA, Maffei ME (2015). Role of early signaling events in plant-insect interactions. Journal of Experimental Botany 66: 435–448. [Google Scholar]
Zhang R, Vivanco JM, Shen Q (2017). The unseen rhizosphere root-soil-microbe interactions for crop production. Current Opinion in Microbiology 37: 8–14. DOI 10.1016/j.mib.2017.03.008. [Google Scholar] [CrossRef]
Zhou Y, Hao L, Ji C, Zhou Q, Song X et al. (2021). The effect of salt-tolerant antagonistic bacteria CZ-6 on the rhizosphere microbial community of winter jujube (Ziziphus jujube Mill. Dongzao) in saline alkali land. BioMed Research International. DOI 10.1155/2021/5171086. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |