DOI:10.32604/biocell.2022.018651

| BIOCELL DOI:10.32604/biocell.2022.018651 |  |
| Article |
Calcitonin gene related peptide modified mesenchymal stem cells reduce restenosis after carotid balloon injury in rats
Department of Cardiology, Affiliated Hospital of Zunyi Medical University, Zunyi, 563003, China
*Address correspondence to: Panke Chen, cpkmail@163.com
Received: 08 August 2021; Accepted: 25 October 2021
Abstract: This work aimed to investigate the effects of calcitonin gene-related peptide (CGRP)-modified mesenchymal stem cells (MSCs) on vascular stenosis in carotid balloon-injured rats. The CGRP gene labeled with EGFP was transfected into bone marrow MSCs, and CGRP protein expression in MSCs was confirmed by immunofluorescence assays. A rat carotid balloon injury model was established using a surgical method. CGRP-modified MSCs were orthotopically transplanted into the injured area of the rats. At 28 days after cell transplantation, EGFP and CD31 expression was detected by immunofluorescence staining. Hematoxylin-eosin (H&E) staining was used to detect the intima/media area of the injured carotid artery stenosis site, and the expression of proliferating cell nuclear antigen (PCNA) was detected by immunohistochemistry. MSCs from rat bone marrow positively expressed CD29 and negatively expressed CD45. In vivo immunofluorescence staining showed that EGFP expression was significantly increased at the vascular injury site of rats transplanted with MSC_CGRP compared with the control group on the 28th day after cell transplantation and that the damaged vessels exhibited continuous CD31 expression. H&E staining showed that the vascular intimal proliferation area of rats transplanted with MSC_CGRP was significantly reduced compared with that of other groups. Furthermore, the immunohistochemistry results showed that PCNA expression in the endothelium of the MSC_CGRP group was lower than that of the other groups. Therefore, MSCs transfected with the CGRP gene can express the CGRP protein, and the implantation of CGRP-modified MSCs at the damaged site after carotid balloon-induced injury can reduce the neointimal area.
Keywords: Mesenchymal stem cells; Calcitonin gene-related peptide; Lentiviral transfection; Cell transplantation; Restenosis
Restenosis after percutaneous coronary intervention (PCI) is an important problem that has not been solved clinically (Zimmermann et al., 2019). Restenosis is mainly related to neointimal hyperplasia and vascular remodeling caused by endothelial injury and the proliferation of vascular smooth muscle cells (Spadaccio et al., 2020). Mesenchymal stem cells (MSCs) have the ability to self-renew and differentiate into vascular endothelial cells, vascular smooth muscle cells and other cell types, as well as promoting vascular regeneration and repair in ischaemic tissues through paracrine action; thus, these cells are more suitable than other stem cells for the treatment of clinical diseases (Andrzejewska et al., 2019; Brown et al., 2019). Our team previously showed that MSC transplantation can promote the repair of injured arterial endothelium, thereby reducing the degree of postoperative vascular stenosis, but its effect on vascular smooth muscle cells (VSMCs) is relatively weak (Shi et al., 1999, 2011). Therefore, the discovery of a novel cell capable of simultaneously repairing injured arterial endothelial cells and inhibiting the migration of VSMCs would be a good strategy to prevent and treat restenosis.
Calcitonin gene-related peptide (CGRP), which is widely distributed in the nervous and cardiovascular systems, is the strongest endogenous vasodilator peptide in the body (Moreno-Ajona et al., 2020); CGRP dilates blood vessels, inhibits the proliferation of VSMCs, protects endothelial cells and participates in angiogenesis (Russell et al., 2014). Moreover, this peptide can positively regulate MSC proliferation and migration. Therefore, this study aimed to investigate the effect of MSCs modified with CGRP on VSMCs, to study the intervention of vascular stenosis after carotid balloon injury in rats and to provide a new therapeutic strategy for vascular stenosis.
Culture and identification of MSCs
Bone marrow from male SD rats (license No. SCXK 2012-0005, Animal Experimental Center of Chongqing Third Military Medical University) was obtained by aseptic methods. The femur and tibia were completely separated bilaterally and the muscle tissue around the bone was removed. The bone marrow cavity was rinsed with a syringe into an EP tube containing DMEM, after which the mixture was centrifuged at 1000 r/s for 5 min. The centrifuged cells were then inoculated into DMEM culture flasks containing 10% FBS. When cells were grown to 80% confluence, they were digested with 0.25% trypsin and passaged. CD29-FITC and CD45-PE expression levels in MSCs were verified by flow cytometry prior to transfection experiments.
Lentivirus transfection into MSCs and immunocytochemistry to verify CGRP expression
P3 MSCs at a density of 3–5 × 104 cells/well were inoculated in 24-well plates and incubated at 37°C overnight; then, the medium was removed, and 500 µL of medium containing 1% FCS was added per well. MSCs from the MSC-EGFP group and the MSC-CGRP group were combined with lentivirus containing enhanced green fluorescent protein (Lv-EGFP) or EGFP and CGRP recombinant lentivirus containing enhanced green fluorescent protein (Lv-EGFP-CGRP) (in cooperation with Shanghai Yingwei Xin Co., Ltd., China) at a multiplicity of infection (MOI) of 30. After the cells were mixed, they were incubated overnight, and the culture medium was replaced with normal medium.
Passage 3 MSCs transfected with the control virus expressing EGFP only (Lv-EGFP) and the CGRP virus expressing EGFP and CGRP (Lv-EGFP-CGRP) were plated in 6-well plates at 1 × 104 cells/well. The slides were removed within 24–48 h for immunocytochemical detection; slides were fixed in 4% paraformaldehyde at room temperature for 30 min, incubated with 0.3% Triton-100 at room temperature for 30 min, and normal goat serum at room temperature for 20 min. Then, the normal goat serum was poured out but not washed away, and primary antibody (rabbit anti-rat CGRP polyclonal antibody, 1:200, Abcam) was added dropwise and incubated overnight at 4°C. Secondary antibody (donkey anti-rabbit IgG-TRITC, 1:100) was added and incubated at 37°C for 1 h, and after the samples were washed with PBS, the cell nuclei were re-stained with DAPI for 3–5 min. After 3 washes with PBS (5 min each), a fluorescence quenching agent was added, and CGRP-positive red cells were observed under a laser confocal microscope to identify whether the MSCs transfected with Lv-EGFP-CGRP expressed CGRP.
Establishment and grouping of carotid balloon injury models
Specific pathogen-free (SPF) adult male SD rats (Experimental Animal Center of Daping Hospital, license No. SCXK 2012-0005, weight: 300 ± 50 g) raised in an SPF-grade environment (certificate No. SYXK 2011-003) and handled according to the Animal Management Rules of the State Committee of Science and Technology of China (No. 2 on November 14, 1988, revised in 2011) were used in the experiments, which were approved by the Animal Experimental Ethics Committee of Zunyi Medical University (Nos. 201612A001, 20220120).
After the rats were anesthetized, a neck incision was made after disinfection, and the distal end of the external carotid artery was ligated. A 2F balloon catheter was inserted from the proximal end of the external carotid artery to the common carotid artery, which was approximately 2 cm, and 0.2 mL of saline was added to the balloon. After resistance was detected, the catheter was slowly pulled back three times after releasing water to exit the catheter. Then, 200 µL of 1 × 106 MSCs was injected into the injured artery and incubated for 30 min. The incision was sutured layer by layer, and the rats were returned to the animal room to continue feeding to the end of the experiment. Penicillin was injected intraperitoneally for 3 days after surgery (Fig. 1).
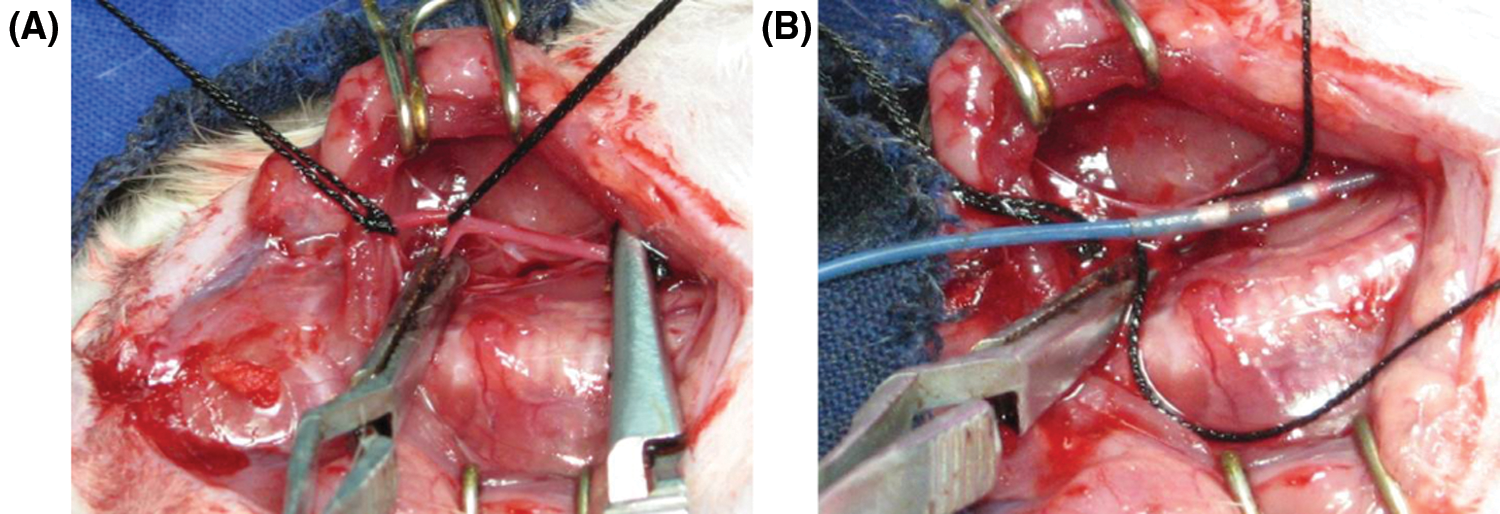
Figure 1: Establishment of the balloon injury model of the carotid artery. (A) Separation and visualization of the carotid artery. (B) Balloon injury to the carotid artery.
Control group (20 rats): After the carotid balloon injury, 300 µL of PBS was injected into the injured artery and locally incubated for 30 min.
MSC_EGFP group (20 rats): After the carotid balloon injury, 300 µL of Lv-EGFP-transfected MSCs (containing 1 × 106 cells) was injected into the injured artery and locally incubated for 30 min.
MSC-CGRP group (16 rats): After the carotid balloon injury, 300 µL of Lv-EGFP-CGRP-transfected MSCs (containing 1 × 106 cells) was injected into the injured artery and locally incubated for 30 min.
Collection and preservation of vascular tissue specimens
Rats were sacrificed on day 28, and the injured carotid arteries were fixed in 10% formalin, embedded in paraffin, and serially sectioned at 4 µm for histological examination. At the same time, some carotid tissues were collected, and a 6-μm frozen section was prepared, fixed in acetone for 5–10 min, and then frozen in a −80°C freezer for immunofluorescence staining.
The expression of EGFP and CD31 in the MSC-EGFP or MSC-CGRP groups was detected by immunolabeling to evaluate the homing and endothelialization of MSCs. The frozen sections were placed at room temperature for 30 min and then washed by PBS, incubated with 0.25% Triton for 10 min at room temperature and 3% H2O2 for 10 min at room temperature, washed with PBS, and incubated with normal goat serum for 45 min at room temperature. Then, the excess liquid was removed. Rabbit anti-rat CD31 (Abcam, 1:250) was added dropwise at 4°C overnight. Each section was incubated with donkey anti-rabbit TRITC-IgG (Abcam, 1:100) at 37°C for 45 min and washed with PBS in the dark. After anti-fluorescence quenching with a sealing tablet, the film was observed under a fluorescence microscope.
Hematoxylin-eosin (HE) staining and immunohistochemical staining
Hemorrhage HE staining was used to observe the histopathological morphology of balloon-damaged vessels, and Leica Qwin software was used to calculate changes in the angiogenic intima/medial area and stenosis rate. The steps were as follows: conventional xylene paraffin section dewaxing, gradient alcohol hydration, hematoxylin staining for 5–8 min, 1% hydrochloric acid alcohol differentiation for 10 s, washing with running water for 30 min, eosin staining for 2–3 min, gradient alcohol dehydration for 3 min, and treatment with xylene for transparentizing. After the sections were sealed with neutral resin, pathological changes were observed under a microscope.
The PCNA protein level was detected by immunohistochemical staining 28 days after cell transplantation. After dewaxing, antigen retrieval and blocking, the cells were incubated with the corresponding primary antibody at 4°C overnight, incubated with biotin secondary antibody for 1 h at room temperature, and then stained with SABC complex and DAB and counterstained with hematoxylin. The samples were observed for endocardial PCNA expression under a light microscope and imaged. Five fields were observed in each group, and the percentage of PCNA-positive cells in the total number of cells was calculated.
SPSS17.0 software was used for statistical analysis. All parameters are presented as the mean ± standard deviation (X ± S), and the differences between groups were detected by one-way ANOVA and t-tests. P < 0.05 was used to indicate statistical significance, while P < 0.05 was used to indicate a highly significant difference.
The formation of small colonies was noted on the 4th day of primary cell culture, and P3 MSCs were crosslinked in the form of a long fusiform, whirlpool-shaped configuration (Fig. 2A). Flow cytometry showed that the P3 MSCs expressed high levels of CD29 and low or no levels of CD45 (Fig. 2B), identifying them as MSCs.
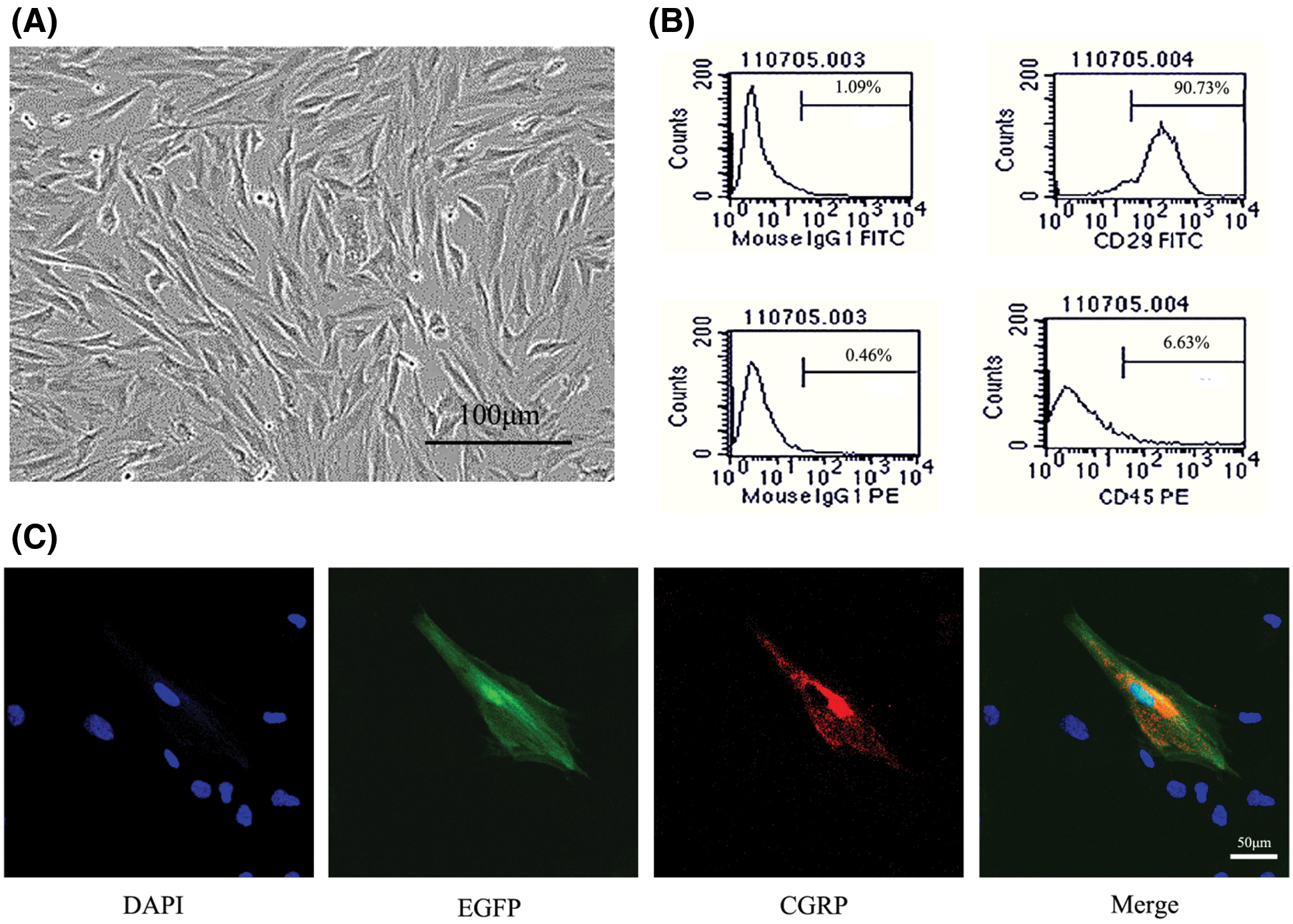
Figure 2: Identification of MSCs and expression of the CGRP protein in MSCs modified with CGRP. (A) The third generation of MSCs showed fusiform adherent growth. (B) MSCs showed positive expression of CD29 and negative expression of CD45. (C) CGRP-modified MSCs showed EGFP fluorescence (green) and expressed the CGRP protein (red).
CGRP protein expression in CGRP-modified MSCs
Immunofluorescence was used to detect the expression of the CGRP protein in CGRP-modified MSCs. The results of laser confocal microscopy showed that the CGRP protein could be expressed in the cytoplasm, and EGFP-positive expression was observed at the same site (Fig. 2C).
Local survival and differentiation of damaged blood vessels after cell transplantation
In the MSC_CGRP group on the 28th day after balloon injury, green fluorescence of EGFP expression and red fluorescence of CD31 could be observed at the site of balloon injury, suggesting that MSCs modified by the CGRP gene mediated by the lentivirus vector could be locally expressed and differentiated into endothelial cells after in vivo transplantation (Fig. 3).
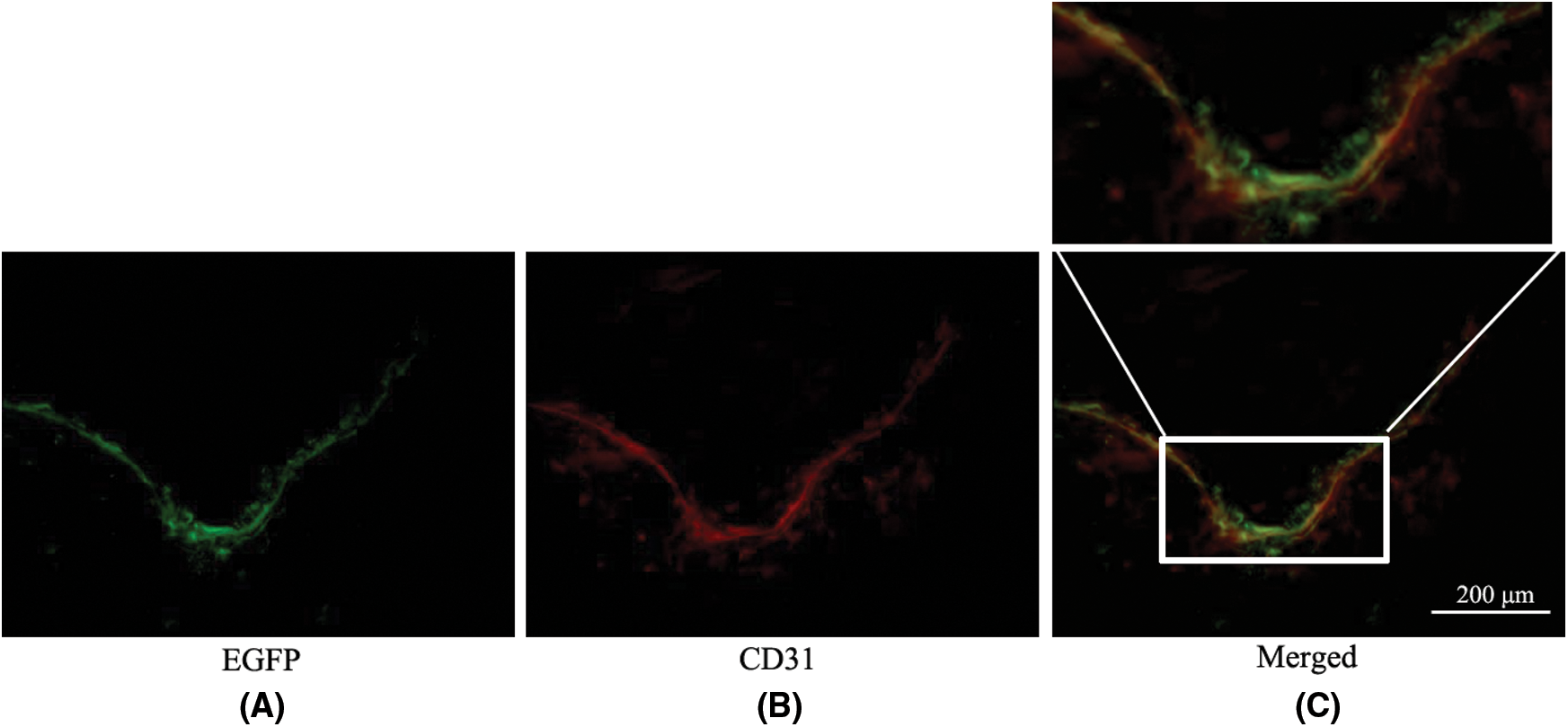
Figure 3: The colonization and differentiation of MSCs in the MSC_CGRP group were observed under a fluorescence microscope (×400). (A) EGFP expressed green fluorescence at the site of balloon injury. (B) CD31 is marked by TRITC and shows red fluorescence in the same field. (C) The merged image for green EGFP and red CD31 (the merged part of green and red showed yellow color).
Effect of cell transplantation on pathological changes in vascular tissues
H&E staining of vascular tissues from each group after 28 days of cell transplantation showed different degrees of intima hyperplasia and atherosclerotic plaque formation in each group (Fig. 4A). The ratio of intimal area/medial membrane area was calculated by Leica Qwin image analysis software, and the values of intimal area/medial membrane area in the model control group, MSC_EGFP group and MSC_CGRP group were (0.986 ± 0.06), (0.745 ± 0.04) and (0.484 ± 0.03), respectively, with significant differences among each group (Figs. 4A and 4B).
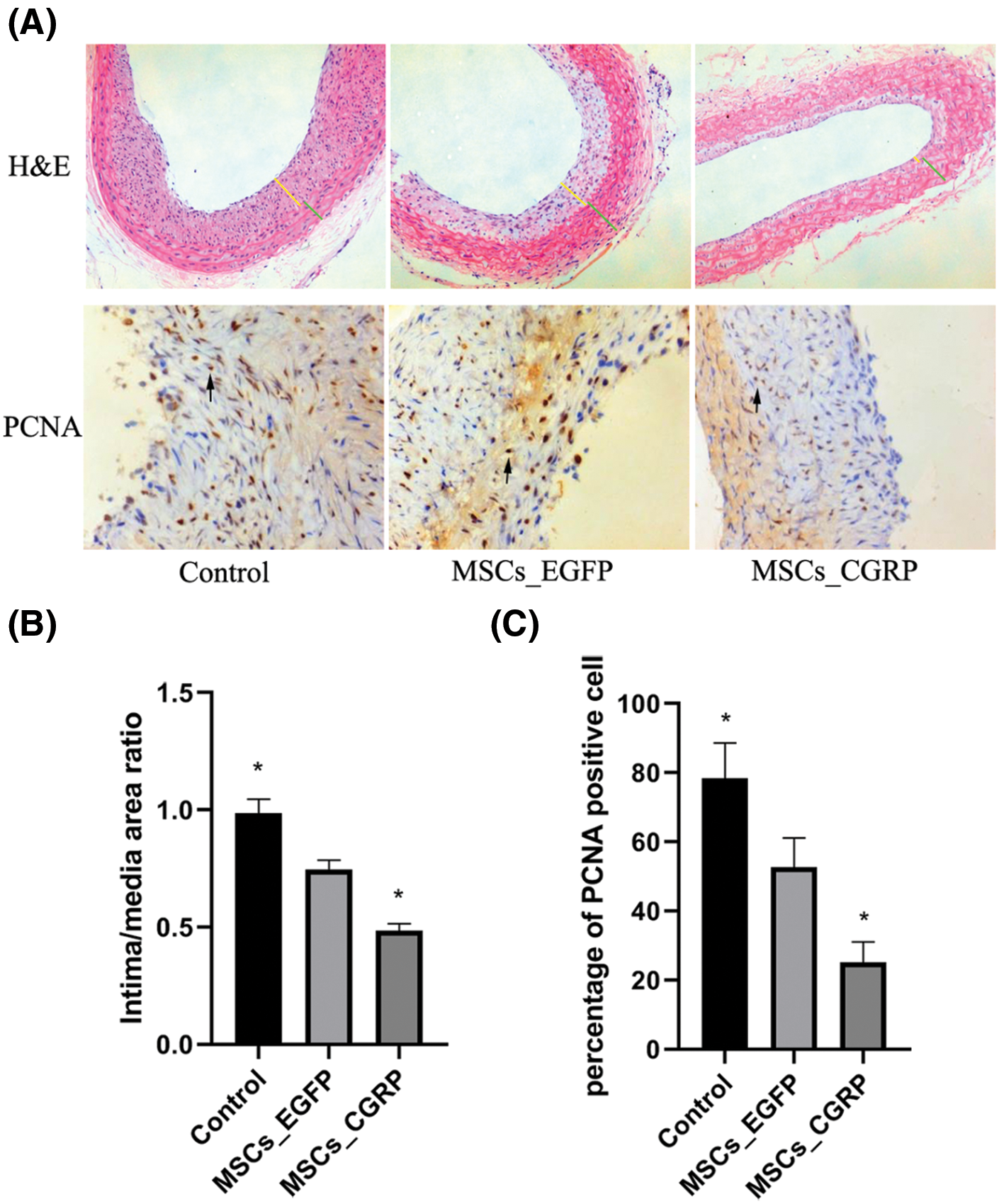
Figure 4: HE staining, immunohistochemical staining and semiquantitative analysis. (A) HE staining and PCNA immunohistochemical staining image of balloon injury vascular sites of MSCs, MSC_EGFP and MSC_CGRP group (yellow line: intima; green line: media; black narrow: PCNA-positive staining). (B) Semiquantitative analysis of the intimal/medial area ratio. (C) Semiquantitative analysis of the percentage of PCNA-positive cells.
Effect of cell transplantation on the proliferation of carotid artery VSMCs
The expression of PCNA-positive cells was found in the neointima of the three groups, with the highest expression in the model control group (78.37 ± 10.22%), followed by the MSC transplantation group (52.73 ± 8.42%), and the lowest expression was in the CGRP transplantation group (25.12 ± 5.96%). The differences between the groups were significant (P < 0.05) (Figs. 4A and 4C).
Abnormal intimal hyperplasia after vascular injury, such as atherosclerosis and in-stent restenosis (ISR), plays an important role in the development of occlusive vascular disease (Schwartz et al., 2004). ISR is a complex process involving multiple cell types and vascular wall tissues. Studies have found that VSMCs play an important role in the pathogenesis of vascular occlusion disease, and abnormal proliferation of VSMCs is believed to be the main cause of ISR (Kim et al., 2015). Promoting the rapid endothelialization of injured intima can inhibit the abnormal proliferation of VSMCs. MSCs can differentiate into endothelial cells, cardiac cells and VSMCs and are easily transfected with exogenous genes that are stably expressed; thus, they are the first type of cells to be used for genetic engineering at present (Xu et al., 2016). Studies have shown that MSCs can be differentiated into endothelial cells by transplantation into injured blood vessels, inhibiting neointimal hyperplasia and reducing the vascular stenosis rate (Ge et al., 2018; Qu et al., 2020; Saad et al., 2017). Studies have also shown that MSCs transplanted into myocardial infarction-related arteries can reduce inflammation and improve cardiac function, but the trial was terminated early due to aggravation of restenosis (Cho et al., 2019). In our study, we found that MSC transplantation in a rat model with myocardial infarction and carotid artery balloon injury improved cardiac function after infarction but only slightly reduced the balloon injury artery and had a relatively weak effect on inhibiting VSMC proliferation (Shi et al., 1999, 2011). Gene-modified MSC transplantation can optimize the therapeutic effect of MSC transplantation.
CGRP is a vasoactive peptide of the neuropeptide family that is widely distributed in the nervous and cardiovascular systems and has many physiological functions, such as powerful vasodilation, inhibition of VSMC proliferation, protection of endothelial cells and participation in angiogenesis (Ma et al., 2021; Xue et al., 2019). Multiple studies later confirmed that CGRP inhibited the proliferation of VSMCs (Liu et al., 2003; Schaeffer et al., 2003). Therefore, can CGRP inhibit the proliferation of VSMCs after transfection with MSCs without affecting the function of MSCs? Previous studies of our research group showed that MSCs transfected with CGRP did not affect the differentiation of the cells (Chen et al., 2015). In this study, lentivirus with CGRP was again used to transfect MSCS. Cell immunofluorescence detection showed that EGFP and CD31 fluorescent double staining was found in damaged blood vessels after the transplantation of MSCS modified with CGRP. Other studies have shown that in a mouse model of lower limb ischemia in which the CGRP gene was deleted, the angiogenesis of ischemic tissues was impaired (Zhu et al., 2021). Combined with the results of this study, these findings suggest that CGRP may enhance the repair effect of MSCs on the endothelium.
PCNA, as a cell cycle fission protein, is an important polymerase cofactor in DNA replication and division, which is necessary for cells to enter the cell cycle and proliferate in humans (Deshmukh et al., 2016; Genschel et al., 2017). PCNA expression in VSMCs is extremely low in the quiescent phase, but the expression is significantly increased in cells with active proliferation (Sun et al., 2017). Therefore, PCNA can be used as a marker for VSMC proliferation, as increased expression is associated with increased proliferation. In contrast, inhibiting the expression of PCNA can inhibit VSMC proliferation and migration. In vivo studies showed that compared with those of the control group and the lentivirus transfection group, PCNA expression in rats with carotid artery injury in the MSC group modified with CGRP was reduced.
In summary, MSCs gene-modified with CGRP can express CGRP protein. In vivo studies showed that MSCs modified with CGRP can reduce the formation of new intima after vascular injury, which provides a new strategy and experimental basis for the treatment of vascular proliferative diseases by genetically engineered cells.
Availability of Data and Materials: The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contribution: The authors confirm contribution to the paper as follows: study conception, design, and data collection: PC and SM; draft manuscript preparation: PC. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: This study and all experiments involved are under approval of the Zunyi Medical University Experiment Committee.
Funding Statement: This work was supported by the Technology Fund of Guizhou Province (Qian ke he LH zi [2014] 7576).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Andrzejewska A, Lukomska B, Janowski M (2019). Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells 37: 855–864. DOI 10.1002/stem.3016. [Google Scholar] [CrossRef]
Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR (2019). Mesenchymal stem cells: Cell therapy and regeneration potential. Journal of Tissue Engineering and Regenerative Medicine 13: 1738–1755. DOI 10.1002/term.2914. [Google Scholar] [CrossRef]
Chen P, Shi B, Xu G, Liu Z, Long X, Zhang W, Ma S (2015). Lentiviral vector mediated CGRP gene in vitro transfection and its effects on biological properties of MSC. Chongqing Medical Journal 14: 1873–1875. [Google Scholar]
Cho JW, Seo MS, Kang KK, Sung SE (2019). Effect of human thymus adipose tissue-derived mesenchymal stem cells on myocardial infarction in rat model. Regenerative Therapy 11: 192–198. DOI 10.1016/j.reth.2019.07.005. [Google Scholar] [CrossRef]
Deshmukh AL, Kumar C, Singh DK, Maurya P, Banerjee D (2016). Dynamics of replication proteins during lagging strand synthesis: A crossroads for genomic instability and cancer. DNA Repair 42: 72–81. DOI 10.1016/j.dnarep.2016.04.010. [Google Scholar] [CrossRef]
Ge Q, Zhang H, Hou J, Wan L, Cheng W et al. (2018). VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Molecular Medicine Reports 17: 1667–1675. [Google Scholar]
Genschel J, Kadyrova LY, Iyer RR, Dahal BK, Kadyrov FA, Modrich P (2017). Interaction of proliferating cell nuclear antigen with PMS2 is required for MutLα activation and function in mismatch repair. Proceedings of the National Academy of Sciences of the United States of America 114: 4930–4935. DOI 10.1073/pnas.1702561114. [Google Scholar] [CrossRef]
Kim JY, Kim KH, Lee WR, An HJ, Lee SJ et al. (2015). Apamin inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and migration through suppressions of activated Akt and Erk signaling pathway. Vascular Pharmacology 70: 8–14. DOI 10.1016/j.vph.2014.12.004. [Google Scholar] [CrossRef]
Liu N, Chen G, Wang X, Yao X, Su J, Li T, Wu X, Zhang Y, Tang J, Tang C (2003). Effects of certain vasoactive peptides on pathogenesis of vascular restenosis. Chinese Medical Sciences Journal 18: 1–8. [Google Scholar]
Ma WJ, Yin YC, Zhang BK, Li YJ, Li WQ (2021). Calcitonin gene-related peptide-mediated pharmacological effects in cardiovascular and gastrointestinal diseases (Review). Molecular Medicine Reports 23: 27. [Google Scholar]
Moreno-Ajona D, Pérez-Rodríguez A, Goadsby PJ (2020). Gepants, calcitonin-gene-related peptide receptor antagonists: What could be their role in migraine treatment? Current Opinion in Neurology 33: 309–315. DOI 10.1097/WCO.0000000000000806. [Google Scholar] [CrossRef]
Qu Q, Pang Y, Zhang C, Liu L, Bi Y (2020). Exosomes derived from human umbilical cord mesenchymal stem cells inhibit vein graft intimal hyperplasia and accelerate reendothelialization by enhancing endothelial function. Stem Cell Research & Therapy 11: 133. DOI 10.1186/s13287-020-01639-1. [Google Scholar] [CrossRef]
Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014). Calcitonin gene-related peptide: Physiology and pathophysiology. Physiological Reviews 94: 1099–1142. DOI 10.1152/physrev.00034.2013. [Google Scholar] [CrossRef]
Saad A, Dietz AB, Herrmann SMS, Hickson LJ, Glockner JF et al. (2017). Autologous mesenchymal stem cells increase cortical perfusion in renovascular disease. Journal of the American Society of Nephrology 28: 2777–2785. DOI 10.1681/ASN.2017020151. [Google Scholar] [CrossRef]
Schaeffer C, Vandroux D, Thomassin L, Athias P, Rochette L, Connat JL (2003). Calcitonin gene-related peptide partly protects cultured smooth muscle cells from apoptosis induced by an oxidative stress via activation of ERK1/2 MAPK. Biochimica et Biophysica Acta 1643: 65–73. DOI 10.1016/j.bbamcr.2003.09.005. [Google Scholar] [CrossRef]
Schwartz RS, Chronos NA, Virmani R (2004). Preclinical restenosis models and drug-eluting stents: Still important, still much to learn. Journal of the American College of Cardiology 44: 1373–1385. [Google Scholar]
Shi B, Guo Y, Wang Z, Wang D, Shen C (1999). Effects of transplantation of peripheral blood mesenchymal stem cells with hypoxia preconditioning on postangioplasty restenosis in rabbits. Chinese Journal of Pathophysiology 25: 1686–1691. [Google Scholar]
Shi B, Liu ZJ, Zhao RZ, Long XP, Wang DM, Wang ZL (2011). Effect of mesenchymal stem cells on cardiac function and restenosis of injured artery after myocardial infarction. Zhonghua Yi Xue Za Zhi 91: 2269–2273. [Google Scholar]
Spadaccio C, Antoniades C, Nenna A, Chung C, Will R, Chello M, Gaudino MFL (2020). Preventing treatment failures in coronary artery disease: What can we learn from the biology of in-stent restenosis, vein graft failure, and internal thoracic arteries? Cardiovascular Research 116: 505–519. DOI 10.1093/cvr/cvz214. [Google Scholar] [CrossRef]
Sun W, Huang Y, Yin T, Wang J, Du R, Qiu J, Zhang Y, Wang Y, Chen J, Wang G (2017). Effects of elemene on inhibiting proliferation of vascular smooth muscle cells and promoting reendothelialization at the stent implantation site. Biomaterials Science 5: 1144–1155. DOI 10.1039/C7BM00190H. [Google Scholar] [CrossRef]
Xu J, Wu D, Yang Y, Ji K, Gao P (2016). Endothelial-like cells differentiated from mesenchymal stem cells attenuate neointimal hyperplasia after vascular injury. Molecular Medicine Reports 14: 4830–4836. DOI 10.3892/mmr.2016.5799. [Google Scholar] [CrossRef]
Xue CD, Chen Y, Ren JL, Zhang LS, Liu X, Yu YR, Tang CS, Qi YF (2019). Endogenous intermedin protects against intimal hyperplasia by inhibiting endoplasmic reticulum stress. Peptides 121: 170131. DOI 10.1016/j.peptides.2019.170131. [Google Scholar] [CrossRef]
Zhu W, Sheng D, Shao Y, Zhang Q, Peng Y (2021). Neuronal calcitonin gene-related peptide promotes prostate tumor growth in the bone microenvironment. Peptides 135: 170423. DOI 10.1016/j.peptides.2020.170423. [Google Scholar] [CrossRef]
Zimmermann FM, Omerovic E, Fournier S, Kelbaek H, Johnson NP et al. (2019). Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: Meta-analysis of individual patient data. European Heart Journal 40: 180–186. DOI 10.1093/eurheartj/ehy812. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |