DOI:10.32604/biocell.2022.019068

| BIOCELL DOI:10.32604/biocell.2022.019068 |  |
| Article |
Butyrate inhibits the bovine rumen epithelial cell proliferation via downregulation of positive regulators at G0/G1 phase checkpoint
Institute of Animal Culture Collection and Application, College of Animal Science and Technology, Yangzhou University, Yangzhou, 225009, China
*Address correspondence to: Guoqi Zhao, gqzhao@yzu.edu.cn
#These authors contributed equally to this work
Received: 01 September 2021; Accepted: 22 October 2021
Abstract: Short-chain fatty acids (SCFAs) butyrate promote the postnatal rumen epithelial development and maturation in ruminants. However, molecular mechanisms of effects of butyrate on the bovine rumen epithelial cells (BRECs) proliferation remain elusive. Therefore, purpose of this study was to investigate the effects of butyrate on the expression of genes and proteins at G0/G1 and S phase of BRECs cycle. Our results showed that BRECs treated with butyrate inhibited (P < 0.05) the proliferation of BRECs, relatively to control. Flow cytometric assays revealed that butyrate triggers the BRECs cycle arrest at the G0/G1 phase. qRT-PCR analyses of mRNA level of genes involved in the G0/G1 phase of cell cycle showed that butyrate significantly upregulated (P < 0.001) the expression of mRNA encoding p21Cip1 compared with control group, but it decreased (P < 0.05) the mRNA levels of cyclin D1 and CDK4 genes at G0/G1 phase checkpoint compared with control. Moreover, Western blot also revealed that butyrate downregulated the expression of cyclin D3, CDK6, p-Rb, and E2F1 proteins involved in the modulation of G0/G1 phase of cell cycle. In conclusion, our results demonstrated that butyrate inhibits the proliferation of BRECs via downregulation of positive regulators at G0/G1 phase checkpoint.
Keywords: Butyrate; Bovine rumen epithelial cells; Proliferation; G0/G1 phase
The high diet fiber is fermented into short-chain fatty acids (SCFAs) by rumen microorganisms in ruminants (Aschenbach et al., 2011). The SCFAs can provide the main energy requirement for ruminants. The total SCFAs mainly constitute acetate, propionate, and butyrate (Kristensen et al., 1998). The rumen papilla shows significant changes during calf rumen development before weaning (Lyford, 1988). The rumen shows the short papillae, and it accounts for less than 30% of the total stomach in newborn calves. However, the rumen accounts for 80% of the total stomach and has abundant and long papillae at 4 week-age. The rumen exhibits significant changes from birth to 4 weeks-age, which is probably involved in SCFAs. In particular, butyrate plays a vital role for rumen epithelial growth and development (Lane and Jesse, 1997).
Previous studies have demonstrated that addition of sodium butyrate in dietary ration can promote the ruminal papillae growth and maturation in young ruminants (Cavini et al., 2015; Gorka et al., 2009; Gorka et al., 2018). However, the culture medium containing butyrate disrupted the rumen epithelial cells proliferation in vitro (Gálfi et al., 1981) and other types of cells (Comalada et al., 2006; Fu et al., 2004; Liu et al., 2019), which is not consistent with results in vivo. The inhibitory effect of butyrate on the rumen epithelial cells proliferation in vitro has been speculated that the stimulatory effect of butyrate on the ruminal papillae growth and epithelial development is indirect signal pathway in vivo (Comalada et al., 2006; Fu et al., 2004).
The histone deacetylases (HDACs) can regulate the cellular proliferation, differentiation, and apoptosis (Abramova et al., 2006). Previous studies have shown that butyrate, a HDACs inhibitor, are able to inhibit the cell proliferation and stop the progression from G0/G1 and S phase of cell cycle (Khleif et al., 1996; Johnstone, 2002; Piekarz and Bates, 2004). The abnormal proliferation of transformed cells is commonly accompanied by the dysfunctions of negative regulators of the cell cycle, including the p16INK4A, p15INK4B, p21Cip1, p27Kip1 cyclin-dependent kinase inhibitors (CDKI), phosphorylation of Rb tumor suppressor and an elevation in the expression of positive regulators of the cell cycle, including the cyclin D/E, cyclin-dependent kinases (CDK), and E2F transcription factor (E2F), leading to bypass of G0/G1 and S phase of cell cycle (Khleif et al., 1996; Kiyono et al., 1998). In particularly, the CDK/E2F signal pathway play a central role in the control of cellular proliferation (Chong et al., 2009; Wu et al., 2001). In this study, we tested the possibility that butyrate affected the proliferation of bovine rumen epithelial cells (BRECs) by changing in key regulator involved in CDK/E2F signal pathway in vitro. Therefore, objective of the study was to determine mechanisms underlying the regulatory effects of butyrate on the BRECs proliferation in vitro.
Bovine rumen epithelial cells culture
All procedures involving dairy cows were complied with the guidelines of the Institutional Animal Care and Use Committee of Yang Zhou University (SYXK (Su) IACUC 2012-0029). Bovine rumen epithelial tissues from 6- to 7-month-old Holstein calves were obtained from the Experimental Farm of Yang Zhou University. Primary bovine rumen epithelial cells (BRECs) were immortalized by lentiviruses expressing SV40 large T antigen and validated by cytokeratin 18 (Zhan et al., 2019), and the immortal BRECs were collected at the Institute of Animal Culture Collection and Application (IACCA), Yangzhou University. These immortal BRECs used in this study were provided by IACCA, Yangzhou University (China). Cells were incubated in the DMEM/F12 medium containing 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, 1% non-essential amino-acids, 4 mm/L glutamine, 1× Insulin-Transferrin-Selenium (1× ITS; Invitrogen, Shanghai, China), 15 ng/mL EGF (Peprotech, Shanghai, China).
The BRECs were seeded at a density of 1 × 103 cells/well in 96-well plates. After overnight, the BRECs were treated in the absence (control group) or presence of 10, 20, 40 mM butyrate. Cells were then incubated for 1, 2, 3, 4, 5, or 6 days, respectively. Next, cell viability was detected using Cell Counting Kit-8 (CCK-8) Assay Kit (Dojindo, Shanghai, China) according to the manufacturer’s protocol. Briefly, 10 μL of CCK-8 solution was added to 200 μL of DMEM/F12 medium. After 2 h of incubation at 37°C, absorbance was measured using Multiskan Go microplate reader (Thermo Scientific, Shanghai, China) at 450 nm.
The BRECs were seeded at a density of 2 × 105 cells/well in 25-cm2 tissue culture plates and incubated in culture medium at 37°C under 5% CO2. Cells were isolated by trypsinization and washed by adding 40 mL PBS, and then centrifuged at 300 × g for 10 min to obtain the cell pellet. Cells were mixed with 5 mL cold 75% ethanol and stored at −20°C for 2 h. Thereafter, cells were washed in PBS and then incubated with stain buffer containing 5% horse serum. The supernatants were carefully discarded, and cells were treated by 100 µL PI/RNase Staining Buffer (BD Biosciences, Shanghai, China) for 15 min. DNA content were measured by flow cytometry (BD Biosciences, Shanghai, China), and characteristics of cell cycle phase were analyzed using ModFit LT software (Verity Software House, Topsham, USA).
RNA extraction and real-time quantitative PCR
Total RNA was extracted from BRECs using a TRIzol kit (Tiangen, Beijing, China) according to the manufacturer’s protocol. The 1 μg of total RNA reversely transcribed into cDNA using a 1× PrimeScript RT Master Mix (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. Quantitative RT-PCR (qRT-PCR) reactions were performed using SYBR® Premix Kit (Takara), and the qRT-PCR reaction was carried out on LightCycler96 System in a final volume of 20 μL using 96-well microwell plates. The qRT-PCR reactions were initially denatured at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. The primers were designed by Primer 6 software. The GAPDH gene was used as references. These primers used are listed in Table 1 and were synthesized by Suzhou Genewiz Biological Co. (Suzhou, China). The qRT-PCR analysis was calculated by the 2−ΔΔCT method.

Cells were extracted total protein using a radioimmunoprecipitation (RIPA) lysis buffer containing protease inhibitor buffer, and the cell lysates were incubated on ice for 30 min. Following centrifugation, cells were centrifuged at 20,000 × g for 10 min. Protein concentrations in the lysates were measured using a BCA protein assay kit (Thermo Scientific, Shanghai, China). Equal amounts of protein lysates were subjected to SDS-PAGE on a 10% polyacrylamide gel, following which polyacrylamide gels were transferred to nitrocellulose membranes (Pall, Shanghai, China). The membranes were blocked for 2 h with 5% skimmed milk in Tris-buffered saline with 0.1% Tween-20 (TBST) or 5% horse serum (for phosphorylation primary antibody). After blocking, membranes were incubated with the primary antibody at 4°C overnight by gentle shaking. The primary antibodies were obtained from Cell Signaling Technology (CST, Shanghai, China): GAPDH (1:1000; #2118), CDK2 (1:1000; #2546), CDK6 (1:1000; #3136), and cyclin D3 (1:1000; #2936). The primary antibodies were obtained from Abcam: phosphorylated (p)-Rb (1:1000; ab184796) and E2F1 (1:1000; ab179445). The following primary antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, Shanghai, China): E2F3 (1:200; SC-878). The membranes were incubated by a second incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000; CST, Shanghai, China). The chemiluminescence detection of HRP-conjugated secondary antibodies were performed using the Western Blotting Detection Reagent kit Super Signal West Femto Maximum Sensitivity Substrate (Thermo Scientific).
Values were expressed as mean ± standard error of the results. All results were evaluated by one-way analysis of variance (ANOVA) for post-hoc multiple comparisons using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered significant differences and P < 0.01 was considered extremely significant differences.
Butyrate inhibited bovine rumen epithelial cells proliferation
The immortalized BRECs have the ability to proliferate rapidly (Fig. 1). Compared with control, addition of 10, 20, and 40 mM butyrate to culture medium significantly inhibited the proliferation of BRECs (P < 0.05; Fig. 1). At 3 and 6 days of culture, there were significant differences in the proliferation of BRECs between control and butyrate treatment groups (P < 0.05; Fig. 1).
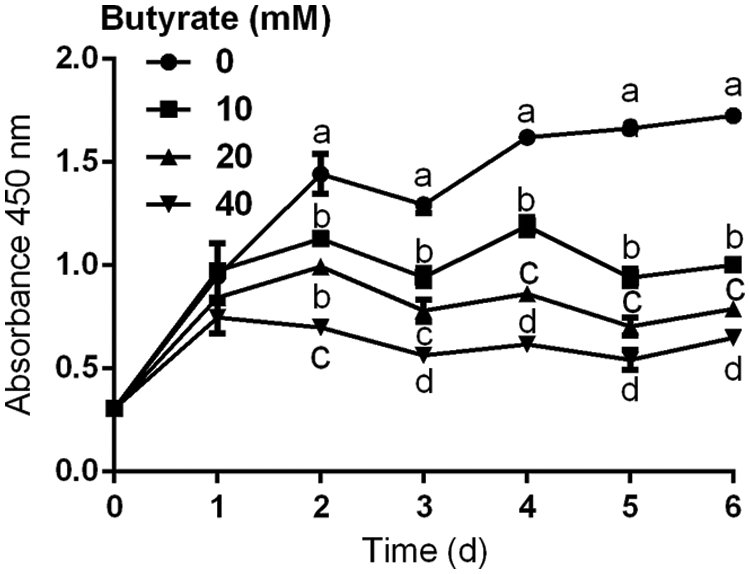
Figure 1: Effects of 10, 20, and 40 mM butyrate on the proliferation of BRECs. The BRECs were cultured in the absence (control group) or presence of 10, 20, and 40 mM butyrate. These cells were cultured for 1, 2, 3, 4, 5, or 6 days (N = 3 per group). Absorbance at 450 nm was measured in each well using an auto-microplate reader. Data are presented as means ± SEM (N = 3). Means with different letters (a–d) differed significantly according to time point.
Effects of butyrate on the bovine rumen epithelial cells cycle
As shown in Fig. 2, flow cytometric analysis also indicated that BRECs treated with 10, 20, and 40 mM butyrate included a higher proportion of cells in G0/G1 phase relatively to control group (P < 0.05; Figs. 2A and 2B). Consistent with this observation, the amount of BRECs treated by 10, 20, and 40 mM butyrate accounted for less cells at the S phase of cell cycle, relatively to control group (P < 0.05; Figs. 2A and 2B). These data suggest that butyrate inhibited the BRECs proliferation by disruption of progression from G0/G1 to S phase of the cell cycle.
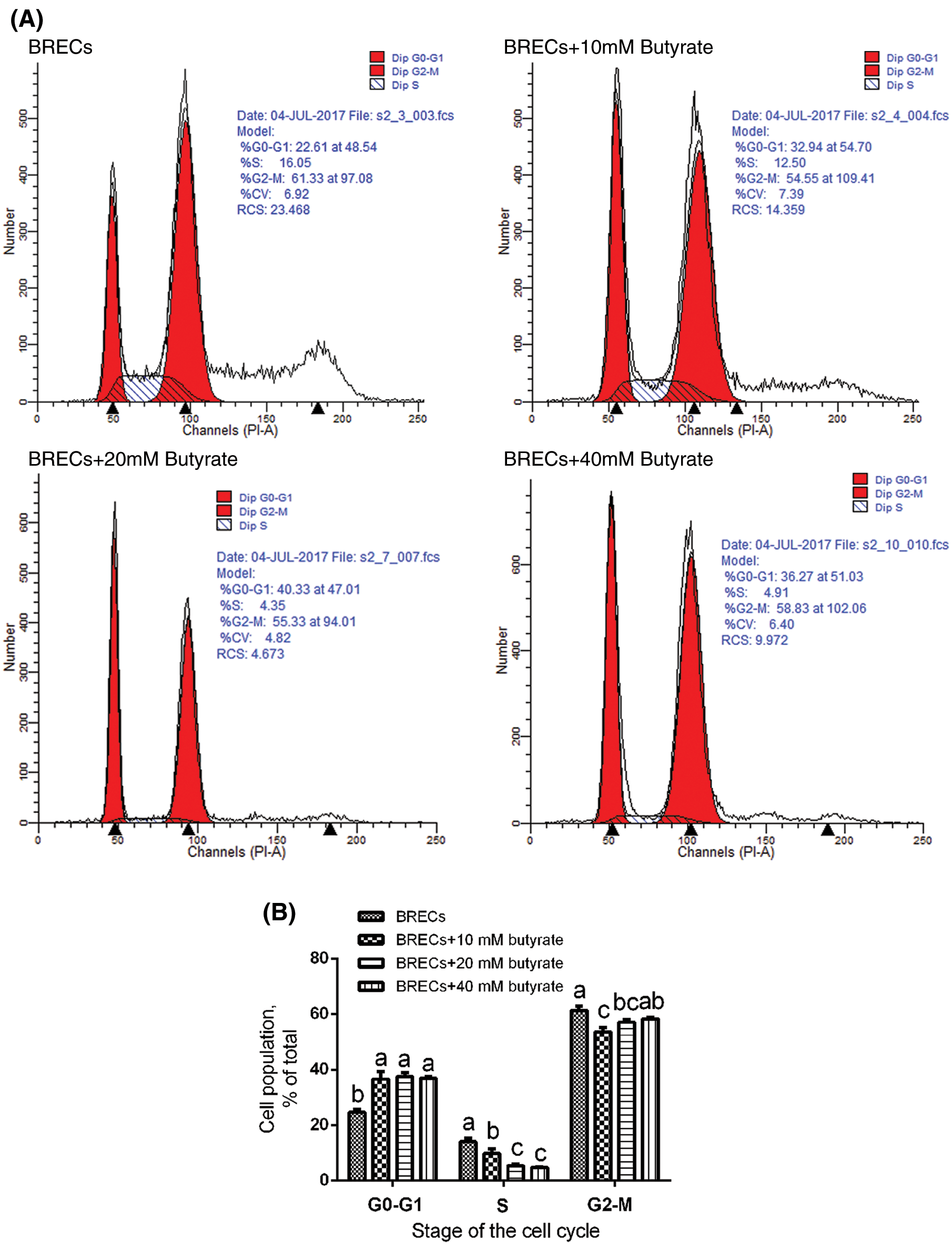
Figure 2: Effects of butyrate on cell cycle progression. The BRECs were cultured in the absence (control group) or presence of 10, 20, or 40 mM butyrate for 24 h. After incubation, cells were analyzed by flow cytometry. (A) A representative histogram of flow cytometric analyses. (B) Percentages of cells at different phases of the cell cycle. Data are presented as the means ± SEM (N = 3). Means with different letters (a–c) differed significantly according to treatment group.
Effect of butyrate on the mRNA expression of genes involved in the cellular proliferation regulator
As shown in Fig. 3, butyrate treatment increased the p21Cip1 expression compared with control (P < 0.001; Table 2), while no significant difference was shown in the mRNA level of p16INK4A and p27Kip1 between control and butyrate treatment (P > 0.05; Table 2). Surprisingly, the expression of p15INK4B was attenuated (P < 0.001) in BRECs treated by butyrate compares with control (Table 2). In addition, BRECs treated by butyrate significantly decreased (P < 0.05) the mRNA levels of CDK4 and cyclin D1 genes related to the modulation of G0/G1 phase of cell cycle compared with control, whereas there was no significant difference in the mRNA level of cyclin D2, cyclin E1, and cyclin E2 between control group and butyrate treatment (P > 0.05; Table 2). The levels of mRNA encoding HDAC1, HDAC7, and HDAC8 were attenuated (P < 0.05) in BRECs treated by butyrate in comparison with the control group (Table 2). The relationships between the mRNA expression of the cyclin-dependent kinase inhibitors p21Cip1 and CDK4, cyclin D1, HDACs mRNA expression was shown (Fig. 3). There is a negative correlation between p21Cip1 mRNA expression and CDK4, cyclin D1, HDAC1, HDAC7, and HDAC8 mRNA expression (Fig. 3; P < 0.001). These results indicated that p21Cip1 may play a vital role in the inhibitory role of BRECs proliferation.

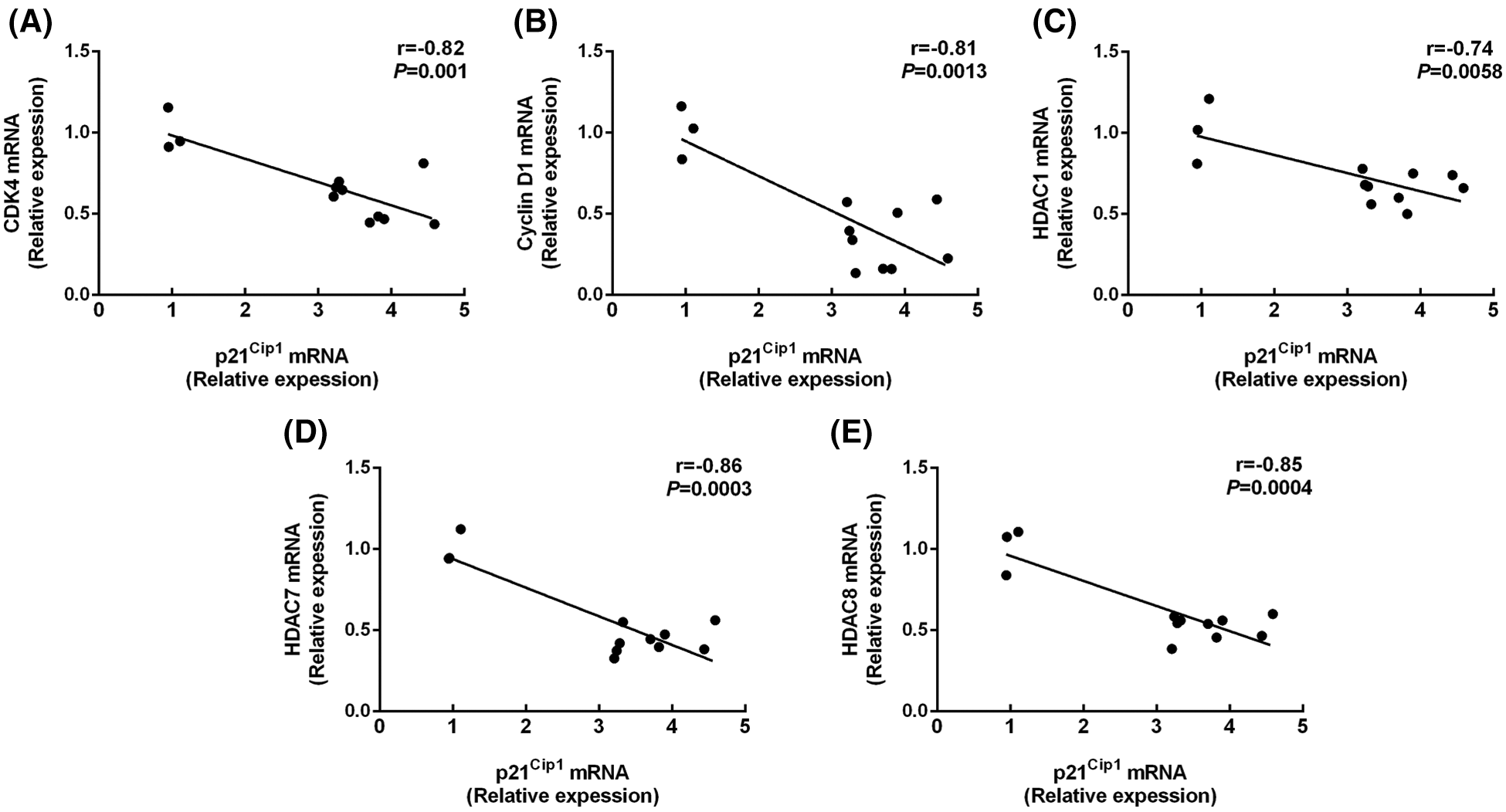
Figure 3: Correlations between the mRNA expression of the p21Cip1 and CDK4 (A), cyclin D1 (B), HDAC1 (C), HDAC7 (D), and HDAC8 (E) mRNA expression. The figure includes data were obtained from untreated BRECs or butyrate treated BRECs. Data are presented as the means ± SEM (N = 3).
Effect of butyrate on regulatory proteins at G0/G1 phase checkpoint
Although butyrate inhibited the proliferation of BRECs by upregulation of p21Cip1 and downregulation of CDK4 and cyclin D1, key protein expression of CDK/E2F involved in the cyclin-dependent kinase regulator downstream signal pathway should be investigated. To confirm that the effect of butyrate on the CDK/E2F signaling pathway key protein, the protein expression of genes related to the CDK/E2F pathway (i.e., CDK2, CDK6, cyclin D3, p-Rb, E2F1, and E2F3) was determined by Western blot analysis in control and butyrate treatment group (Fig. 4). The level of CDK6 protein involved in the modulation of G0/G1 phase of cell cycle was attenuated by addition of butyrate compared with control, whereas there was no significant difference in the expression of CDK2 protein related to the modulation of S phase of cell cycle between control and butyrate treatment (Fig. 4). In addition, butyrate treatment downregulated the level of cyclin D3 protein involved in the modulation of G0/G1 phase of cell cycle, relatively to control. Accordingly, the phosphorylation level of Rb was almost not expressed by addition of butyrate, compared to control (Fig. 4). There was no significant difference in the protein level of E2F3 between control and butyrate treatment. However, the protein expression of E2F1 activator was not found in BRECs treated by butyrate compared with control (Fig. 4). These findings suggested that butyrate decreased the expression of proteins (cyclin D3, CDK6, p-Rb, and E2F1) related to G1 progression phase of cell cycle, leading to an inhibition of BRECs proliferation.
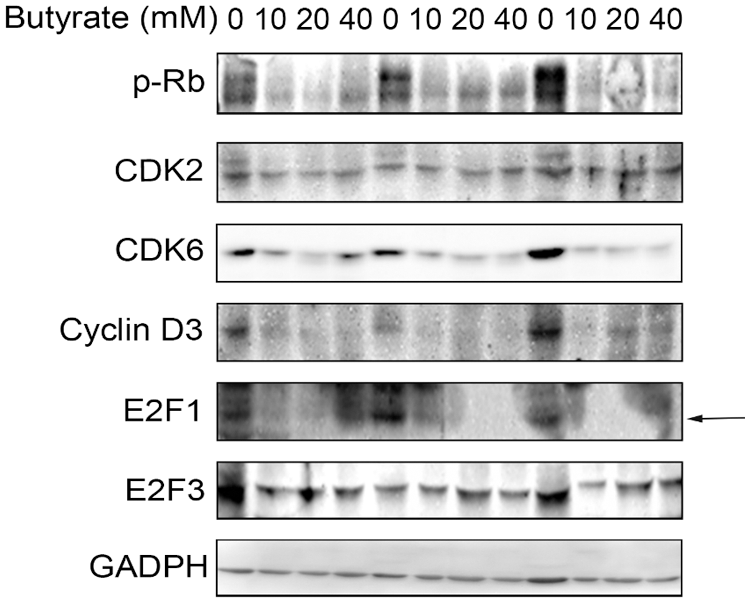
Figure 4: Effect of butyrate on regulatory proteins at G0/G1 phase checkpoint. The BRECs were cultured in the absence (control group) or presence of 10, 20, or 40 mM butyrate in vitro. After incubation, these cells were collected to extract total protein. Western blot analysis for CDK2, CDK6, cyclin D3, p-Rb, E2F1, and E2F3. Data are based on triplicate experiments.
The short-chain fatty acids (SCFAs), acetate, and propionate, and butyrate, which are produced by fermentation of high diet soluble fiber by the rumen microbiota, play a vital role in the rumen epithelial development and maturation in newborn ruminants. However, the mechanisms underlying the effects of butyrate on the BRECs growth remain largely unknown (Vi et al., 2003). Infusion of sodium butyrate promotes the growth and functional maturation of rumen papillae (Lane and Jesse, 1997; Liu et al., 2019). In contrast, the inhibitory effect of butyrate on the proliferation of colon epithelial cells, neutrophil, and intestinal epithelial cells in vitro has been reported (Aoyama et al., 2010; Comalada et al., 2006; Qiu et al., 2017). These reverse effects of butyrate on the cell proliferation between in vivo and in vitro was believed that rumen butyrate contributes to the development of rumen epithelium by other molecular pathways. Wang and Jiang (2010) reported that the BRECs treated by butyrate did not inhibit the proliferation of cells at the culture of 72 h, whereas the concentration of butyrate is only 1 mM. In fact, the different concentration butyrate should be used to investigate the inhibitory effect of butyrate on the proliferation of BRECs in vitro. In addition, the CDK/E2F signal pathway can control the cellular proliferation and the progression of the cell cycle (Chong et al., 2009; Wu et al., 2001). Therefore, we tested the possibility that butyrate affects the proliferation of BRECs by change in key regulator involved in CDK/E2F signal pathway in vitro. Our results demonstrated that butyrate significantly inhibited the proliferation of BRECs by downregulating the expression of mRNA and proteins related to G1 progression phase of cell cycle, including the cyclin D1, cyclin D3, CDK4, CDK6, p-Rb, and E2F1.
Our study has shown that butyrate inhibited the proliferation of BRECs by disruption of progression from G0/G1 to S phase of the cell cycle. The progression of the cell cycle from G0/G1 to S phase is regulated by cyclin, CDK, Rb, and E2F. The cyclin D1, D2, and D3 binds to the CDK4/6 and activates the CDK4/6, leading to the progression of the G0/G1 phase of the cell cycle (Sherr, 1994). However, the cyclin E1 and E2 interact with CDK2, triggering the progression of the S phase of the cell cycle (Lauper et al., 1998). The activity of cyclin/CDK complex are regulated by CDKI, such as p16INK4A, p15INK4B, p21Cip1, and p27Kip1 protein (Fridman and Tainsky, 2008). Previous study has shown that rumen fluid enhances the mRNA expression of p16INK4A and p21Cip1 to inhibit the progression of BRECs from the G1 phase to the S phase during the cell cycle (Wang and Jiang, 2010). However, the study did not investigate the expression level of CDK. In addition, rumen fluid did not change the cyclin D1, D2, D3, E1, and E2 expression levels (Wang and Jiang, 2010). Butyrate inhibited the progression of porcine intestinal epithelial cells from the G1 phase to the S phase of cell cycle by upregulating the p21Cip1 and p27Kip1 protein level and downregulating the CDK4 protein level (Qiu et al., 2017). Surprisingly, an increase in butyrate concentration from 0 to 10 mM decreased the CDK6 protein expression. Our study demonstrated that butyrate enhanced the mRNA expression of p21Cip1 in BRECs, and decreased the mRNA levels of CDK4, cyclin D1 and the protein expression of CDK6 and cyclin D3 of genes related to the modulation of G0/G1 phase of cell cycle, whereas butyrate did not change the mRNA levels of cyclin E1 and cyclin E2 and the protein expression of CDK2 of genes involved in the modulation of S phase of cell cycle. These results indicated that addition of butyrate inhibits the progression of BRECs at G0/G1 phase checkpoint during the cell cycle by upregulating the expression of p21Cip1 and downregulating the expression of cyclin D1, cyclin D3, CDK4, and CDK6.
The molecular mechanism of the inhibitory effect of butyrate on the positive regulators of cell cycle and proliferation of BRECs in vitro should be further investigated. The CDK/E2F pathway is believed to play a critical role in control of cellular proliferation (Gupta et al., 2015; Wu et al., 2001). Replicative senescence is commonly accompanied by an elevation in CDKI levels and downregulation of CDK and E2F activators (Khleif et al., 1996). In contrast, the activity of cyclin/CDK complex can trigger the phosphorylation of Rb, and this in turn causes the release of E2F activators that drive cell cycle progression for entering G1 into S-phase (Chen et al., 2009). Previous studies have shown that mice lacking E2F1 and E2F2 are able to survive and develop to adulthood. However, mice deficient for E2F1 and E2F3 or E2F2 and E2F3 activators cannot develop to adulthood during early embryonic development (Gupta et al., 2015; Wu et al., 2001). In addition, the mouse embryonic fibroblasts deficient for E2F1, E2F2, and E2F3 activators inhibited the expression of CDK and the phosphorylation of Rb, leading to a decrease in E2F target genes and a severe block in the proliferation of cell (Timmers et al., 2007; Wu et al., 2001). The butyrate downregulated the E2F1 expression and the activity of cyclin E/CDK2 complex, and enhanced the expression of p21Cip1, leading to the inhibitory effect of primary embryonic fibroblast proliferation (Abramova et al., 2006). Based on above studies, we investigated whether butyrate could mediate its inhibitory effect of cellular proliferation through modulation of the CDK/E2F signal pathway. In present study, our results showed that butyrate treatment caused the loss of p-Rb and E2F1 protein and decreased the cyclin D3 and CDK6 protein involved in the G0/G1 phase checkpoint in BRECs. The phosphorylation of Rb can disrupt the Rb/E2F complex to release of E2F activators during G0/G1 phase, leading to the proliferation of cell (Kiyono et al., 1998; Wu et al., 2001). Conversely, the activity of Rb/E2F complex induced by dephosphorylation of Rb or loss of E2F activators can inhibit the proliferation of cell (Kiyono et al., 1998; Wu et al., 2001). Our results demonstrated that butyrate can trigger the loss of E2F1 activators and a decrease in cyclin D3 and CDK6 protein levels at G0/G1 phase checkpoint, resulting in the block proliferation of BRECs. In conclusion, butyrate inhibits the progression of BRECs by downregulating the expression of genes and proteins involved in the G0/G1 phase checkpoint during the cell cycle.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Authors’ Contribution: Methodology, KZ and MCJ; writing—original draft preparation, KZ and MCJ; visualization, TYY and ZXH; funding acquisition, GQZ and KZ; writing—review and editing, GQZ and KZ; supervision, GQZ. All authors have read and agreed to the published version of the manuscript.
Ethics Approval: The experiments were approved by the Institutional Animal Care and Use Committee of Yang Zhou University (SYXK (Su) IACUC 2012-0029). The date of approval was 06 April 2016.
Funding Statement: This study was supported by the National Natural Science Foundation of China (No. 32002200), the Research Project of Natural Science Foundation of Jiangsu Province (BK20190898), and China Agriculture Research System of MOF and MARA.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abramova MV, Pospelova TV, Nikulenkov FP, Hollander CM, Fornace AJJr., Pospelov VA (2006). G1/S arrest induced by histone deacetylase inhibitor sodium butyrate in E1A + Ras-transformed cells is mediated through down-regulation of E2F activity and stabilization of beta-catenin. Journal of Biological Chemistry 281: 21040–21051. DOI 10.1074/jbc.M511059200. [Google Scholar] [CrossRef]
Aoyama M, Kotani J, Usami M (2010). Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26: 653–661. DOI 10.1016/j.nut.2009.07.006. [Google Scholar] [CrossRef]
Aschenbach JR, Penner GB, Stumpff F, Gabel G (2011). Ruminant Nutrition Symposium: Role of fermentation acid absorption in the regulation of ruminal pH. Journal of Animal Science 89: 1092–1107. DOI 10.2527/jas.2010-3301. [Google Scholar] [CrossRef]
Cavini S, Iraira S, Siurana A, Foskolos A, Ferret A, Calsamiglia S (2015). Effect of sodium butyrate administered in the concentrate on rumen development and productive performance of lambs in intensive production system during the suckling and the fattening periods. Small Ruminant Research 123: 212–217. DOI 10.1016/j.smallrumres.2014.11.009. [Google Scholar] [CrossRef]
Chen HZ, Tsai SY, Leone G (2009). Emerging roles of E2F in cancer: An exit from cell cycle control. Nature Reviews Cancer 9: 785–797. DOI 10.1038/nrc2696. [Google Scholar] [CrossRef]
Chong JL, Wenzel PL, Saenz-Robles MT, Nair V, Ferrey A et al. (2009). E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 462: 930–934. DOI 10.1038/nature08677. [Google Scholar] [CrossRef]
Comalada M, Bailon E, de Haro O, Lara-Villoslada F, Xaus J et al. (2006). The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. Journal of Cancer Research and Clinical Oncology 132: 487–497. DOI 10.1007/s00432-006-0092-x. [Google Scholar] [CrossRef]
Fridman AL, Tainsky MA (2008). Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene 27: 5975–5987. DOI 10.1038/onc.2008.213. [Google Scholar] [CrossRef]
Fu H, Shi YQ, Mo SJ (2004). Effect of short-chain fatty acids on the proliferation and differentiation of the human colonic adenocarcinoma cell line Caco-2. Chinese Journal of Digestive Diseases 5: 115–117. DOI 10.1111/j.1443-9573.2004.00167.x. [Google Scholar] [CrossRef]
Gálfi P, Veresegyházy T, Neogrády S, Kutas F (1981). Effect of sodium n-butyrate on primary ruminal epithelial cell culture. Zentralblatt für Veterinärmedizin Reihe A 28: 259–261. DOI 10.1111/j.1439-0442.1981.tb01189.x. [Google Scholar] [CrossRef]
Gorka P, Kowalski ZM, Pietrzak P, Kotunia A, Kiljanczyk R et al. (2009). Effect of sodium butyrate supplementation in milk replacer and starter diet on rumen development in calves. Journal of Physiology and Pharmacology 60: 47–53. [Google Scholar]
Gorka P, Kowalski ZM, Zabielski R, Guilloteau P (2018). Invited review: Use of butyrate to promote gastrointestinal tract development in calves. Journal of Dairy Science 101: 4785–4800. DOI 10.3168/jds.2017-14086. [Google Scholar] [CrossRef]
Gupta T, Saenz Robles MT, Pipas JM (2015). Cellular transformation of mouse embryo fibroblasts in the absence of activator E2F. Journal of Virology 89: 5124–5133. DOI 10.1128/JVI.03578-14. [Google Scholar] [CrossRef]
Johnstone RW (2002). Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nature Reviews Drug Discovery 1: 287–299. DOI 10.1038/nrd772. [Google Scholar] [CrossRef]
Khleif SN, DeGregor Z J, Yee CL, Otterson GA, Kaye FJ et al. (1996). Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proceedings of the National Academy of Sciences of the United States of America 93: 4350–4354. DOI 10.1073/pnas.93.9.4350. [Google Scholar] [CrossRef]
Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ (1998). Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396: 84–88. DOI 10.1038/23962. [Google Scholar] [CrossRef]
Kristensen NB, Danfaer A, Agergaard N (1998). Absorption and metabolism of short-chain fatty acids in ruminants. Archives of Animal Nutrition 51: 165–175. DOI 10.1080/17450399809381916. [Google Scholar] [CrossRef]
Lane MA, Jesse BW (1997). Effect of volatile fatty acid infusion on development of the rumen epithelium in neonatal sheep. Journal of Dairy Science 80: 740–746. DOI 10.3168/jds.S0022-0302(97)75993-9. [Google Scholar] [CrossRef]
Lauper N, Beck AR, Cariou S, Richman L, Hofmann K et al. (1998). Cyclin E2: A novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene 17: 2637–2643. DOI 10.1038/sj.onc.1202477. [Google Scholar] [CrossRef]
Liu L, Sun D, Mao S, Zhu W, Liu J (2019). Infusion of sodium butyrate promotes rumen papillae growth and enhances expression of genes related to rumen epithelial VFA uptake and metabolism in neonatal twin lambs. Journal of Animal Science 97: 909–921. DOI 10.1093/jas/sky459. [Google Scholar] [CrossRef]
Lyford SJ (1988). Growth and development of the ruminant digestive system. In: Church DC, Welch J (eds.The Ruminant Animal: Digestive Physiology and Nutrition, pp. 44–63. Chicago: Waveland Press, Prospect Heights. [Google Scholar]
Piekarz R, Bates S (2004). A review of depsipeptide and other histone deacetylase inhibitors in clinical trials. Current Pharmaceutical Design 10: 2289–2298. DOI 10.2174/1381612043383980. [Google Scholar] [CrossRef]
Qiu Y, Ma X, Yang X, Wang L, Jiang Z (2017). Effect of sodium butyrate on cell proliferation and cell cycle in porcine intestinal epithelial (IPEC-J2) cells. In Vitro Cellular & Developmental Biology-Animal 53: 304–311. DOI 10.1007/s11626-016-0119-9. [Google Scholar] [CrossRef]
Sherr CJ (1994). The ins and outs of RB: Coupling gene expression to the cell cycle clock. Trends in Cell Biology 4: 15–18. DOI 10.1016/0962-8924(94)90033-7. [Google Scholar] [CrossRef]
Totty ML, Morrell BC, Spicer LJ (2017). Fibroblast growth factor 9 (FGF9) regulation of cyclin D1 and cyclin-dependent kinase-4 in ovarian granulosa and theca cells of cattle. Molecular and Cellular Endocrinology 440: 25–33. DOI 10.1016/j.mce.2016.11.002. [Google Scholar] [CrossRef]
Timmers C, Sharma N, Opavsky R, Maiti B, Wu L, Wu J, Orringer D, Trikha P, Saavedra HI, Leone G (2007). E2f1, E2f2, and E2f3 control E2F target expression and cellular proliferation via a p53-dependent negative feedback loop. Molecular and Cellular Biology 27: 65–78. DOI 10.1128/MCB.02147-05. [Google Scholar] [CrossRef]
Vi RLB, Mcleod KR, Klotz JL, Heitmann RN (2003). Rumen development, intestinal growth and hepatic metabolism in the pre- and postweaning ruminant. Journal of Dairy Science 87: E55–E65. DOI 10.3168/jds.S0022-0302(04)70061-2. [Google Scholar] [CrossRef]
Wang A, Jiang H (2010). Rumen fluid inhibits proliferation and stimulates expression of cyclin-dependent kinase inhibitors 1A and 2A in bovine rumen epithelial cells. Journal of Animal Science 88: 3226–3232. DOI 10.2527/jas.2009-2769. [Google Scholar] [CrossRef]
Wang Z, Zhao T, Zhang P, Zhang S, Guan J, Ma X, Yin Y, Zhang J, Tang B, Li Z (2011). Histone deacetylase 1 down-regulation on developmental capability and histone acetylation in bovine oocytes and parthenogenetic embryos. Reproduction in Domestic Animals 46: 1022–1028. DOI 10.1111/j.1439-0531.2011.01778.x. [Google Scholar] [CrossRef]
Wu L, Timmers C, Maiti B, Saavedra HI, Sang L et al. (2001). The E2F1-3 transcription factors are essential for cellular proliferation. Nature 414: 457–462. DOI 10.1038/35106593. [Google Scholar] [CrossRef]
Zhan K, Gong XX, Jiang MC, Yang TY, Zhao GQ (2019). Short-chain fatty acids regulate the immune responses via G protein-coupled receptor 41 in bovine rumen epithelial cells. Frontiers in Immunology 10: 1399. DOI 10.3389/fimmu.2019.02042. [Google Scholar] [CrossRef]
Zhou Y, Akers RM, Jiang H (2008). Growth hormone can induce expression of four major milk protein genes in transfected MAC-T cells. Journal of Dairy Science 91: 100–108. DOI 10.3168/jds.2007-0509. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |