DOI:10.32604/biocell.2022.018845

| BIOCELL DOI:10.32604/biocell.2022.018845 |  |
| Article |
Biomonitoring of endosulfan toxicity in human
1Department of Biotechnology, Sardar Bhagwan Singh University, Former Sardar Bhagwan Singh Post Graduate Institute of Biomedical Science & Research, Dehradun, 248161, India
2Department of Biotechnology, National Institute of Technology, Raipur, 492010, India
*Address correspondence to: Awanish Kumar, awanik.bt@nitrr.ac.in
Received: 20 August 2021; Accepted: 12 November 2021
Abstract: Chemicals are comprehensively used worldwide to control herbs, weeds, pests, and other competing agents with various growing crops. The consumption of crops grown with these chemicals (even in small quantities) can upshot into accumulation in the human body. People can accidentally inhale these hazardous chemicals if they are in an area where they were applied. These chemicals can be ingested in a human with contaminated food and drinks. Ultimately it causes various adverse effects (chronic toxicity, teratogenic, mutagenic, carcinogenic effect, reproductive, and organ toxicity) on human health. Among the pool of these chemicals used as pesticides in the environment, exposure of endosulfan to humans has a potential health risk throughout the globe. The poisoning of endosulfan is reported frequently in society, therefore, this article highlights the toxicological and other harmful properties of endosulfan to humans and emphasized its essential biomonitoring. Its quick monitoring and effective hazard evaluation are possible with multi-omics technologies and some other analytical approaches. This review summarizes the introduction and mechanism of action of endosulfan along with some other toxic chemicals used in the agriculture work, the toxicity of endosulfan in human/environment, and importantly its biomonitoring (detection of chemicals and their metabolites in a biological sample) in human because biomonitoring is often considered as the most preferred method. Biomonitoring could be easily done in human samples (blood, hair, milk, and urine) with a multi-omics approach, which is a quick, reliable, and state-of-the-art technique.
Keywords: Endosulfan; Agricultural use; Toxicity; Biomonitoring in human
In the current scenario, chemical utilization processes are importantly exercised for the protection of crops. Various types of chemicals are available like herbicides, insecticides, fungicides, and other cidal agents. These chemicals show a negative impact on human health as well as animal health like causing diabetic conditions, endocrine disruption infertility, and cancer, etc. (Abdel Ghani and Abdallah, 2016; Al-Othman et al., 2015; Clementi et al., 2008; Mnif et al., 2011). Endosulfan is one of the most toxic pesticides and belongs the class I toxicity and it is used in agriculture extensively (Patočka et al., 2016). It is a chlorinated hydrocarbon available with different trade names like Afidan, Beosit, Cyclodan, Devisulfan, Endocide, Endocel, Endosol, FMC-5462, Hexasulfan, Hildan, Hoe-2671, Insectophene, Malix, Phaser, Thiodan, Thifor, Thionex, and Thimuletcinin different regions of the world. It is highly pesticide in various food crops including, coffee, fruits, tea, vegetables, and cereals, maize, sorghum, rice, etc. Endosulfan formulations include emulsifiable concentrate, wet powder, smoke tablets, and ultra-low liquid volume.
Endosulfan is a synthetic product having molecular formula (C9H6Cl6O3S) and its chemical structures are mentioned in Fig. 1.
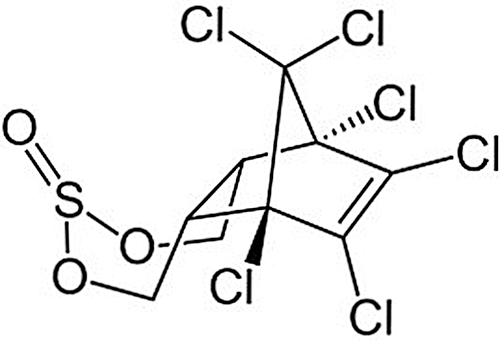
Figure 1: Showing the chemical structure of endosulfan (IPCS/CEC International Chemical Safety Cards, 1999).
IUPAC name of endosulfan is 6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepine-3–oxide and molecular weight is 406.93 g/mol (Cerrillo et al., 2005). Endosulfan is mainly brown in colour and solid and crystal from the structure. It has a slight sulfur dioxide odor. It is a mixture of two stereoisomers: alpha endosulfan, beta endosulfan, and these two isomers exist for a longer time under more acidic conditions. It is partially soluble in water (0.32 mg/L) and completely soluble in an organic solvent such as n-hexane, ethanol, toluene, ethyl acetate, and dichloromethane. The half-life of the alpha-isomer of endosulfan is 35 days and the beta-isomer is 150 days (Menezes et al., 2017). It decomposes on heating and produces toxic fumes of sulfur oxides and chlorine fumes. Consequently, these fumes react with bases and generate toxic sulfur dioxides fumes as a hazardous product (Ahlborg and Thunberg, 1980). Endosulfan is used as a insecticide and acaricide agent. It is used for the control of sucking, chewing, and boring insects and mites on a very wide range of crops, including fruit, vines, olives, vegetables, ornamentals, potatoes, cucurbits, cotton, tea, coffee, rice, cereals, maize, sorghum, oilseed crops, hops, hazels, sugar cane, tobacco, alfalfa, mushrooms, forestry, glasshouse crops, etc. Normally its effects on gastric action. It has a good capacity to adhere/adsorb to soils (Wauchope et al., 1992). Thus, endosulfan can enter into the human and animal body in various ways and create a negative effect on the human and animal health system. Endosulfan monitoring is another important factor as well as in the human body and animal body (Attaullah et al., 2018). It is a highly lethal pesticide and shows a relationship with oncogenic disease. Thus it has a negative effect on human health (Khwaja et al., 2013; Attaullah et al., 2019).
Various methods are present to endosulfan monitoring like circulatory fluid, hair in the human body but milk product is also another way to monitor the endosulfan in the animal body. All methods are used to define the toxicity level of endosulfan. It provides helps to define the appropriate level of toxicity of endosulfan. In this review, we discussed toxic agricultural chemicals like endosulfan, mechanism of action, side effects, and their biomonitoring in humans.
Endosulfan is a well-known chemical used for agricultural purposes. It has a specific mechanism of action to control the insect. However, it has shown a negative effect on the human body. Endosulfan mainly generates the adverse effect on nerve cells that are responsible for changing the various nerve cells events like altering the electrophysiological, enzymatic properties, and change in the kinetics of Na+ and K+ ion flow across the cell membrane. Consequently, endosulfan work as the destabilizing agent for calcium transport and ATPase activity in cells and it is also involved in phosphokinase activities (Richardson et al., 2019). Thus, endosulfan causes genotoxic effects and mutagenic effects in lymphocyte and liver hepatoblastoma cells (Pandey et al., 2006). These studies demonstrate that endosulfan work as an effective toxic molecule to human health. The ecological effect of endosulfan is highly poisonous to a living organism. It is highly toxic to birds, fish, and some other aquatic species and moderately toxic to some insects. Literature suggests that it is also toxic to humans and it enters the human body from agricultural products (Fig. 2).
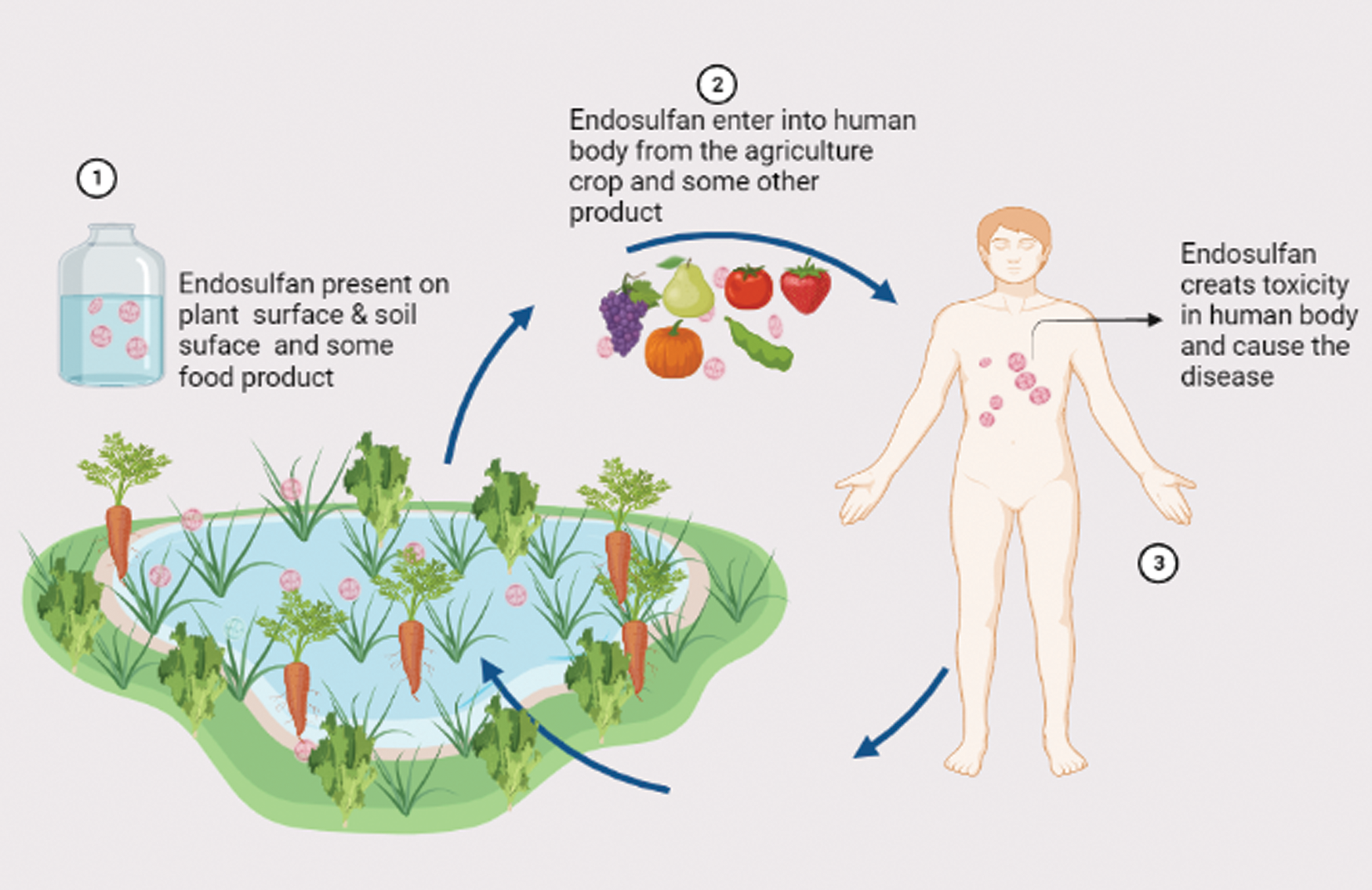
Figure 2: Entry of endosulfan in the human body from agricultural products that take a few days/week to excrete and cause many diseases.
It takes a few days to a few weeks to the excretion from the body and causes many diseases. Endosulfan is classified as a continuous organic pollutant and its human-related poisoning was reported from time to time (Kucuker et al., 2009). Therefore, biomonitoring of persistent endosulfan is very important for assessing human exposure to these chemicals because massive endosulfan biomonitoring programs can protect from health hazards. Growing research interest has been observed in the biomonitoring of harmful and toxic compounds over the last few years. Blood, urine, breast milk, hair, and meconium are the most used specimens for biomonitoring. The development of automated offline and online methods increases the demand for high-throughput analysis in biomonitoring programs (Rodríguez-Gonzalo et al., 2010). The scientific community is very much aware of the toxic effect of endosulfan, therefore, this article highlights the toxic effect of endosulfan in the next section that is used in agriculture frequently with special emphasis on its biomonitoring in humans which is very essential to protect farmers and other fauna from the hazardous effect of endosulfan.
As per the USA Environmental Protection Agency (USEPA), endosulfan is toxic, persistent chemical, and classified as extremely hazardous. Endosulfan is within the list of restricted-use chemicals. It is instructed that the labels for the product containing endosulfan should bear the words poison or danger because endosulfan shows the lethal effect in every phase of human life like the adult stage, adolescent, child stage.
Effect of endosulfan on adult human
A high risk of exposure to pesticides like endosulfan for humans is ascertained worldwide (Mnif et al., 2011). Due to the increasing level of endosulfan, it affects the female hormone estrogen which is capable of causing reproductive and developmental damage in both humans and animals (Patočka et al., 2016). The central nervous system is the main target of endosulfan toxicity and endosulfan exposure also causes liver tissue damage conjointly. Along with neurotoxin behavior, endosulfan conjointly acts as hematoxylin, genotoxin, and nephrotoxin. Laboratory studies also support its potential malignant neoplastic effects. Endosulfan has been connected to inherent physical disorders, mental disabilities, and deaths in farmworkers and communities across the world. Endosulfan poisoning has large symptoms including coma, convulsions, dizziness, headaches, upset, nausea, confusion, metabolic process depression, seizures, vomiting, and even death in severe cases. Therefore, it has been banned in many developed countries and it is gradually being phased out in others due to its persistent and poisonous nature, although its extensive uses continue in developing and under-developed countries. It is already illegal in Australia, Bangladesh, Brazil, Indonesia, Korea, Thailand, Taiwan, New Zealand, and Singapore, EU countries, UK, US but endosulfan is still in used India.
Effect of endosulfan on children
People have introduced varied dangerous compounds like organochlorine chemical pesticides like endosulfan, overwhelming metals, polychlorinated biphenyls (PCB), etc. The poisoning effects of endosulfan were discovered within the newborns (Silva and Carr, 2010). Underdeveloped growth and deformed limbs were noticed within the newborn calves due to endosulfan. Health disorders of terribly serious nature to the human population came into the limelight by the year 1990. Children were found to be the worst affected with innate anomalies, brain disease, epilepsy, abnormal condition, mental retardation, physical deformities, etc. Man and women were also suffering from varied chronic ailments that are irreversible and difficult to treat. The community living close to plantations had been complaining against the spraying of endosulfan from the middle of 1990 (Den Hond and Schoeters, 2006). A high concentration of endosulfan residues is found in those regions wherever intense agricultural activity is found. Residues are detected in an exceeding variety of media as well as air, sediments, surface water, aquatic vertebrates/invertebrates, terrestrial organisms, and humans additionally. α-endosulfan, β-endosulfan, and endosulfan-sulfate are detected in the food product. Endosulfan contents are typically higher in fruits and vegetables as compared to processed foods. As a result, dietary intake is anticipated to be the primary route of exposure to the adult population and children (Saxena et al., 1981).
Effect of endosulfan on the environment
From the literature, it is evident that endosulfan is a principal poisonous and toxic compound to living organisms. It is answerable for several deaths worldwide and causes many diseases in humans at has shown killing results on mammals and other animals. It has a detrimental effect on the environment by contaminating water, soils, and air (Sebastian and Raghavan, 2017). It is water-insoluble, however simply adheres to clay particles and persists in water/soil for many years. Endosulfan is quickly associated with soil particles or will be found readily linked to water bodies in surface water. Further, it dissolves in water, diminishes within the environment, and maybe reborn by the attack at the sulfite cluster via either hydrolysis or oxidation, to convert the poisonous Endosulfate (i.e., endosulfan sulfate), and the nontoxic endodiol (i.e., endosulfan diol), respectively. It enters into the soil, water, and air once sprayed as a pesticide to the crops. Some endosulfan could meet up with long separations because it arrives on harvests. The careless and random use of endosulfan to control the insects on a large variety of agricultural products has considerably raised the hazard risk for human health worldwide. To date, 62 countries have already voluntarily prohibited the use of endosulfan. If its use continues to be legal, it will kill many lives. Animals (fish, insects, invertebrates, shellfish) that live in endosulfan-contaminated soil or waters will increase endosulfan into their bodies and in this way, pesticide levels increase many times higher. Endosulfan is very poisonous to fish and aquatic invertebrates (Barr and Angerer, 2006). The Atlantic Salmon can tolerate endosulfan levels up to 500 μg/kg (Kapka-Skrzypczak et al., 2011). The National ban on the use of endosulfan under the Stockholm Convention ensured the endosulfan eradication from international use and provide a ray of hope to protect people and their environment (Cerrillo et al., 2005). Volatilization of pesticides within the sprayed environment like water, soil, and air leads to high levels of endosulfan. As per observation, it is found that in the majority region of the world, endosulfan exists within the vapor phase instead of the particulate phase. The relative stability of endosulfan within the atmosphere contributes long.
Wet deposition of endosulfan has been documented in areas of the Sierra-Nevada Mountain. Atmospheric transport of endosulfan to Arctic regions has been conjointly documented and its residues are discovered in numerous Arctic environments. It gradually causes neurotoxicity, genotoxicity, and endocrine gland toxicity (Sinha et al., 2001). The common method for human endosulfan exposure is through the consumption of contaminated food in those countries where endosulfan was not banned. Endosulfan has been found in several food products like oils, fruit, and vegetables (Silva and Carr, 2010). Exposure may also occur by smoking cigarettes (tobacco has endosulfan residues). These studies reflect that endosulfan chemicals generate some known adverse effects on humans and many side effects are still unknown. Endosulfan enters the human body through the respiratory route and creates lethal conditions in the human body (respiratory other disorders). Hence endosulfan requires more concern for comprehensive research further for human health concerns.
Biomonitoring of endosulfan in Human
Many available reports suggested that endosulfan is harmful and hazardous to humans. First, it is applied in agriculture and then it comes to the environment i.e., air, water, and food. Then using dermal contact, ingestion, and inhalation endosulfan is entered from these environmental sources to humans. Therefore, biomonitoring is required for this compound because biomonitoring is a very useful tool for assessing human exposure to these chemicals. Biomonitoring of endosulfan toxic effects can be done in a body fluid using omics markers identified by the multi-omics (state-of-the-art technique) (Fig. 3).
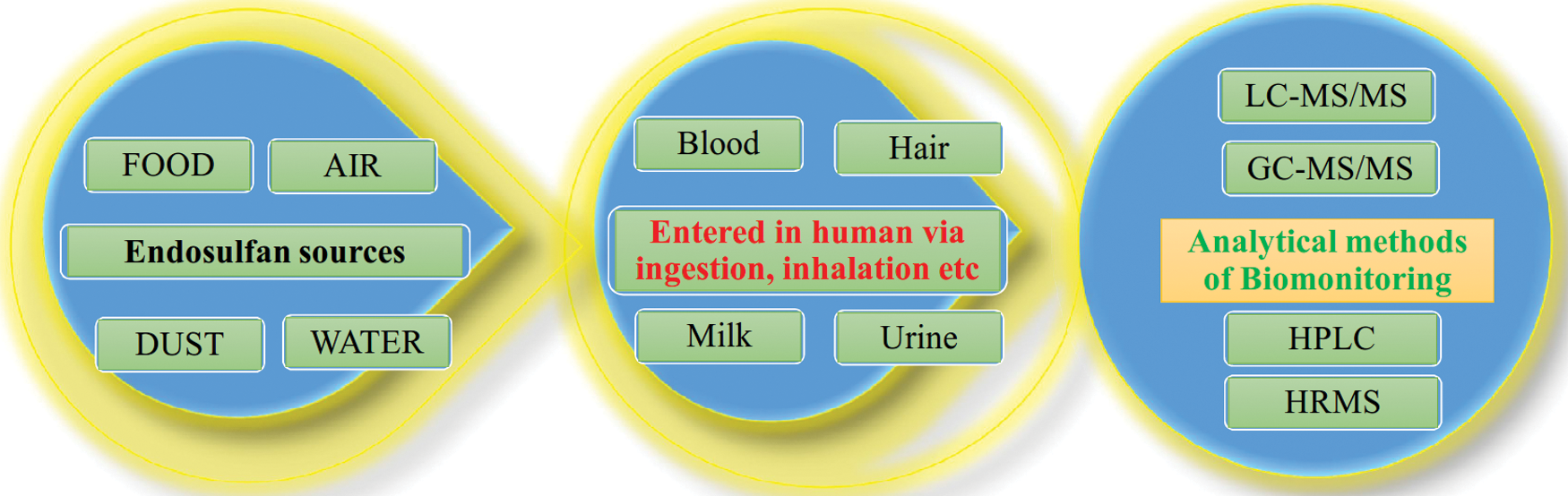
Figure 3: Flow of toxic endosulfan from the environment to humans and their biomonitoring using OMICS and other analytical methods.
Starting from sampling, quantification of endosulfan exposure, identification of biomarkers, its susceptibility could build the analytical exposure biology framework to unravel the human exposure. Further elucidation of endosulfan exposure in the individual requires the use of molecular analysis by various omics-approach (transcriptomics, proteomics, and metabolomics) because they are and state-of-the-art for the next-generation environmental toxicologists. A multi-omics study against the exposure to endosulfan in rats was done to unravel the developmental effects (Tarazona et al., 2019). But dedicated multi-omics studies are needed in humans for endosulfan biomonitoring in their body fluids and hairs.
Blood is a circulatory fluid and that is the easiest way to find out the level of toxicity in the human body. Endosulfan levels within the North Indian population were reported by Pathak et al. (2008). To achieve insight into this current chemical burden in newborns, this study examined the degree of organochlorine residues in the blood sample of healthy ladies with mature maternity. Hexachlorocyclohexane was the most abundant organochlorine present in blood samples, followed by endosulfan. Revealed information had shown a transfer rate of 60–70% of these pesticides from mothers to newborns. Because it might adversely influence the expansion and development of the newborn, therefore, this should be a great concern. Endosulfan poisoning in humans causes electrocardiogram alterations and high blood pressure (Moon and Chun, 2009). Over half of the patients developed internal organ toxicity, rhabdomyolysis, and hypotension (Karatas et al., 2006). The presence of endosulfan residues in cord blood, placenta, and breast milk suggests pre-and postnatal exposure. Risk levels is high for nearly all individuals. Endosulfan is accounted to suppress humoral immunity. It did not have any impact on free radical production. Endosulfan presentation (about 16 mg/kg) to rats diminished the level of superoxide dismutase and distended lipid peroxidation. Endosulfan is likewise able to exhaust glutathione (GSH) and instigate programmed cell death in human blood mononucleate cells in vitro (Ahmed et al., 2008).
Endosulfan instigated modifications in serum biochemical markers of oxidative stress in rabbits. It was suggested that vitamin C supplementation could also be helpful to keep the upcoming impacts of expanded aerobic pressure caused by endosulfan exposure (Ozdem et al., 2011). Endosulfan might repress cholinesterase movement in vitro and in vivo in Wistar rats and cross the placental boundary and influence the catalyst movement in male rodents (Silva and Carr, 2010). Regarding its neurotoxicological system of activity, endosulfan meddles with the capability of γ-aminobutyric corrosive (GABA: the primary neurotransmitter inhibitory). It restrains the authority of [35S]t-butylbicyclophosphorothionate (TBPS) to the picrotoxinin-authoritative site of the gamma-aminobutyric acid receptor in rodent brain synaptic films, interfering with chloride particle motion through the GABA-gated chloride channels (GABAA) in charge of diminishing neuronal volatility (Walse et al., 2003). Above discussed studies provide evidence that endosulfan toxicity is not only limited to one generation but also shows side effects in the next generation. Thus, endosulfan chemicals may be responsible for causing the genetic disorder. Hence endosulfan requires more study to find out the relation in the genetic disorder of humans.
Hair is another sample to find out the toxicity of endosulfan in the human body. Hair analysis also provides data regarding chronic exposure of endosulfan that is averaged over many months. The possibility of pesticides within the hair has been incontestable over the past few years. The unknown linkage between exposure of pesticides and the concentration in hair has restricted the popularity of this matrix as a pertinent tool for assessing human exposure. Controlled exposure to a mixture of pesticides composed of β-hexachlorocyclohexane, β-endosulfan, carbofuran, cyhalothrin, cypermethrin, diazinon, diflufenican, dieldrin, fipronil, p,p’-DDE, p,p’-DDT, pentachlorophenol, organophosphate, permethrin, propiconazole, oxadiazon, trifluralin, and propoxur was given to rat model. Results demonstrated the association between exposure intensity and ensuing pesticide concentration in hair. More obtained results additionally compare the findings from hair analysis to plasma and urine collected from similar rats. Hair, blood, and excrement were collected from rats and submitted to 90-day exposure to the same mixture of common pesticides at totally different levels. A linear relationship was found between the concentration of pesticides and exposure intensity within the hair of rats (Pearson 0.453–0.978, p < 0.01). A comparison of the result obtained from urine and plasma samples incontestable indicated the connection of hair analysis. For many chemicals, the superiority of hair was shown to differentiate animals from different groups. It reattributes animals to their correct group of exposure-based pesticide concentrations within the matrix. Therefore, this study powerfully supports hair analysis as a reliable tool to be used and throughout epidemiologic studies to be analyzed exposure related to adverse effects on health (Appenzeller et al., 2017). Rats were orally treated with blends of chlorinated pesticides and hair was collected to examine the pesticide accumulates overtime of up to a month. Quantitative and subjective investigation of the recuperated pesticides in hair was resolved to utilize gas chromatography. Results suggest that hair will be used as a biomarker for the checking of organochlorinated chemical deposits at low components per billion levels (Smith-Baker and Saleh, 2011). The middle and mean fixation values were prescribed for some chemical agents (for example use of lindane is recommended 4 µg/kg to 20 µg/kg, 3 times a week). The foremost noteworthy centralization of lindane in hair was watched in Greek provincial people, and the median concentrations of lindane were reported 70.2 pg/mg (Tsatsakis et al., 2008). Chlorinated pesticides were recognized in human hair, which indicates hair could be used for biomonitoring of chemicals.
Milk also works as a source to find out the level of toxicity. Endosulfan accumulates simply inside the fatty tissues of living organisms. Cerrillo et al. (2005) investigated the presence of α-endosulfan, β-endosulfan, and its metabolites in fatty tissues, non-fatty tissues, fluids from women of reproductive age, and children in Southern Spain. The highest concentration of commercial α-endosulfan and β-endosulfan was found in adipose tissue (mean value of 17.72 ng/g lipids) followed by human milk (mean value of 11.38 ng/ml milk). Endosulfan-ether (among endosulfan metabolites) was the foremost predominant compound in adipose tissues (68%) and bodily fluid (86%) samples (Cerrillo et al., 2005). A sample of milk containing endosulfan, endosulfan sulfate, or chlordane was conjointly ascertained. Milk and organs were monitored for endosulfan containing endosulfan sulfate, or chlordane was also observed by McCaskey and Liska (1967). Endosulfan buildup levels after death tests from a corpse were 1,270 ppm in the rumen content and 1.2, 1.1, and 0.6 ppm in the liver, kidney, and muscle tissue, respectively. Investigation of drain from cows that survived the harming uncovered a level more prominent than 1 ppm endosulfan quickly following the inebriation. This level diminished to 1 ppm toward the end of 33 days and was computed for endosulfan within the milk (McCaskey and Liska, 1967). Abusalama et al. (2014) performed a study within the Gezira space to know the organochlorine pesticide residues in raw cow’s milk. Five raw cow’s milk samples are collected from completely different places in Gezira space, which suffered from previous health issues caused by the unsafe use of pesticides. The samples were analyzed by applying completely different chromatographic methods for endosulfan and dichlorodiphenyltrichloroethane (DDT) residues. The findings showed that all investigated samples were found to contain endosulfan and DDT residues from 0.01 to 0.3 ppm for endosulfan and from 0.11 to 2.18 ppm, respectively. It is vital to say that the concentration of residues in some samples exceeded the quality levels were cited by FAO and WHO for acceptable daily intake as 0.006 ppm for endosulfan and 0.002 ppm for DDT. This study suggested the requirement to ban the use of endosulfan in agricultural activities in Gezira space (Sudan), promote health teaching programs among the farmers and encourage routine pesticides residues monitoring in food. Residues of pesticides were surveyed in 132 samples of cow milk collected from bulk transports (38 samples of raw milk) and market (94 samples of pasteurized milk) (Ciscato et al., 2002). These samples were analyzed by the multi-residue analytical methodology DFGS19 for pesticide contamination. More than 70 active substances were studied and the identification and quantification were done by gas chromatography. The results showed that 0.76% of samples were contaminated with hexachlorocyclohexane (HCH-α isomer) and 10.60% with endosulfan (α and β isomers). Each pesticide HCH and endosulfan found within the milk samples indicated their use in agriculture practices. Brazil does not permit the use of HCH since 1985 and endosulfan will be used solely for a few crops. These compounds should not be in milk because of their implications on human health. Milk and milk products are used in human life on a daily basis. If milk is contaminated by toxic chemicals and it enters into the human body through the mother feeding then it will surely cause toxicity in the new human body and is also responsible for causing various types of disease like cancer and some developmental disorders. Thus, endosulfan entered from one living body to another living body and spared the toxicity gradually. Endosulfan directly affects the human immune system. Hence, the human immune system is not capable to fight pathogens.
A study of endosulfan excretion in human urine was carried out by victimization associated with agriculture employees involved in spraying without gloves and masks. The urine samples were extracted with solid-phase extraction (SPE) cartridges and the compounds were separated/detected using Gas Chromatography-Tandem Mass qualitative analysis (GC-MS/MS) because of their high sensitivity in preventing most matrix interferences. The α- and β-isomers of endosulfan have been detected in concentrations ranging between 5368 and 2239 pg/ml. However, endosulfan-ether, endosulfan-lactone, and endosulfan-sulfate were not found on top of the detection limits. The results obtained were compared with the concentrations found for a non-occupational exposed man (Arrebola et al., 1999). Endosulfan metabolites are principally excreted in urine and excretory product. Urinary metabolites include β-endosulfan isomers and product like -sulfate, -lactone, -diol, and -ether (Martínez et al. 1998). The reproductive health events as well as abortion, sterility, intrauterine death (IUD), precocious time of life, and death were found considerably higher in the population when study compared to the control population. Above discussed studies demonstrate that urine is a good source to find the level of toxicity in the human body.
Biomonitoring of human body fluid has gained research interest and it would be an important/relevant matter for assessing the potential human health risks associated with exposure to the toxic pesticide endosulfan that has a negative impact on the environment as well as human health. Therefore, biomonitoring and the toxicological effect of endosulfan have been reviewed in this article. Selection of the biological specimen (blood, milk, hair, urine, etc.) for biomonitoring of endosulfan in humans is needed using various analytical methods (Fig. 3) for getting pharmacokinetics and pharmacodynamic information. This is easily possible for the involvement of high-throughput OMICS and other analytical methods for biomonitoring of pesticide endosulfan. These analytical methods measure either the parent pesticide or the biologically persistent metabolites. Defense against such toxic compounds is needful therefore review is focused on the biomonitoring and effect of endosulfan in the different tissue and body fluid for the broad readership of the field because as per best of our knowledge this issue is not reviewed extensively.
Acknowledgement: The authors sincerely acknowledge Sardar Bhagwan Singh Post Graduate Institute of Biomedical Science & Research), Balawala, Dehradun (UK), India, and National Institute of Technology, Raipur (CG), India for providing all kinds of support.
Authors’ Contribution: The authors confirm contribution to the paper as follows: study conception and design: Awanish Kumar, Santosh Kumar Karn; data collection: Santosh Kumar Karn; manuscript preparation: Santosh Kumar Karn, Aditya Upadhyay, Awanish Kumar. All authors reviewed and approved the final version of the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abdel Ghani SB, Abdallah OI (2016). Method validation and dissipation dynamics of chlorfenapyr in squash and okra. Food Chemistry 194: 516–521. DOI 10.1016/j.foodchem.2015.08.053. [Google Scholar] [CrossRef]
Abusalama EA, Elhassan AM, Errami M, Salghi R(2014). Pesticides Residues: Endosulfan and DDT in Cow’s milk in Gezira State. Sudan Moroccan Journal of Chemistry 2: 125–135. [Google Scholar]
Ahlborg UG, Thunberg TM (1980). Chlorinated phenols: Occurrence, toxicity, metabolism, and environmental impact. Critical Reviews in Toxicology 7: 1–35. DOI 10.3109/10408448009017934. [Google Scholar] [CrossRef]
Ahmed T, Tripathi AK, Ahmed RS, Das S, Suke SG, Pathak R, Chakraboti A, Banerjee BD (2008). Endosulfan-induced apoptosis and glutathione depletion in human peripheral blood mononuclear cells: Attenuation by N-acetylcysteine. Journal of Biochemical and Molecular Toxicology 22: 299–304. DOI 10.1002/jbt.20240. [Google Scholar] [CrossRef]
Al-Othman AA, Abd-Alrahman SH, Al-Daghri NM (2015). DDT and its metabolites are linked to increased risk of type 2 diabetes among Saudi adults: A cross-sectional study. Environmental Science and Pollution Research International 22: 379–386. DOI 10.1007/s11356-014-3371-0. [Google Scholar] [CrossRef]
Appenzeller BMR, Hardy EM, Grova N, Chata C, Faÿs F, Briand O, Schroeder H, Duca RC (2017). Hair analysis for the biomonitoring of pesticide exposure: Comparison with blood and urine in a rat model. Archives of Toxicology 91: 2813–2825. DOI 10.1007/s00204-016-1910-9. [Google Scholar] [CrossRef]
Arrebola FJ, Martínez Vidal JL, Fernández-Gutiérrez A (1999). Excretion study of endosulfan in urine of a pest control operator. Toxicology Letters 107: 15–20. DOI 10.1016/S0378-4274(99)00027-2. [Google Scholar] [CrossRef]
Attaullah M, Yousuf MJ, Amin M, Buneri ID, Rahim A, Anjum SI, Ilahi I (2019). Endosulfan concentrations in association with serum biochemical parameters and risk of cancer. Applied Ecology and Environmental Research 17: 5235–5244. DOI 10.15666/aeer/1702_52355244. [Google Scholar] [CrossRef]
Attaullah M, Yousuf MJ, Shaukat S, Anjum SI, Ansari MJ, Buneri ID, Tahir M, Amin M, Ahmad N, Khan SU (2018). Serum organochlorine pesticides residues and risk of cancer: A case-control study. Saudi Journal of Biological Sciences 25: 1284–1290. DOI 10.1016/j.sjbs.2017.10.023. [Google Scholar] [CrossRef]
Barr DB, Angerer J (2006). Potential uses of biomonitoring data: A case study using the organophosphorus pesticides chlorpyrifos and malathion. Environmental Health Perspectives 114: 1763–1769. DOI 10.1289/ehp.9062. [Google Scholar] [CrossRef]
Cerrillo I, Granada A, López-Espinosa MJ, Olmos B, Jiménez M, Caño A, Olea N, Fátima Olea-Serrano M (2005). Endosulfan and its metabolites in fertile women, placenta, cord blood, and human milk. Environmental Research 98: 233–239. DOI 10.1016/j.envres.2004.08.008. [Google Scholar] [CrossRef]
Ciscato CHP, Gebara AB, de Souza Spinosa H (2002). Pesticide residues in cow milk consumed in São Paulo City (Brazil). Journal of Environmental Science and Health, Part. B: Pesticides, Food Contaminants, and Agricultural Wastes 37: 323–330. DOI 10.1081/PFC-120004473. [Google Scholar] [CrossRef]
Clementi M, Tiboni GM, Causin R, La Rocca C, Maranghi F, Raffagnato F, Tenconi R (2008). Pesticides and fertility: An epidemiological study in Northeast Italy and review of the literature. Reproductive Toxicology 26: 13–18. DOI 10.1016/j.reprotox.2008.05.062. [Google Scholar] [CrossRef]
Den Hond E, Schoeters G (2006). Endocrine disrupters and human puberty. International Journal of Andrology 29: 264–290. DOI 10.1111/j.1365-2605.2005.00561.x. [Google Scholar] [CrossRef]
IPCS/CEC International Chemical Safety Cards (1999). Endosulfan (Mixed Isomers). https://inchem.org/documents/icsc/icsc/eics0742.htm. [Google Scholar]
Kapka-Skrzypczak L, Cyranka M, Skrzypczak M, Kruszewski M (2011). Biomonitoring and biomarkers of organophosphate pesticides exposure—state of the art. Annals of Agricultural and Environmental Medicine 18: 294–303. [Google Scholar]
Karatas AD, Aygun D, Baydin A (2006). Characteristics of endosulfan poisoning: A study of 23 cases. Singapore Medical Journal 47: 1030–1032. [Google Scholar]
Khwaja S, Mushtaq R, Mushtaq R, Yousuf M, Attaullah M, Tabbassum F, Faiz R (2013). Monitoring of biochemical effects of organochlorine pesticides on human health. Health 5: 1342–1350. DOI 10.4236/health.2013.58182. [Google Scholar] [CrossRef]
Kucuker H, Sahin O, Yavuz Y, Yürümez Y (2009). Fatal acute endosulfan toxicity: A case report. Basic & Clinical Pharmacology & Toxicology 104: 49–51. DOI 10.1111/j.1742-7843.2008.00216.x. [Google Scholar] [CrossRef]
Martínez Vidal JL, Arrebola FJ, Fernández-Gutiérrez A., Rams MA (1998). Determination of endosulfan and its metabolites in human urine using gas chromatography-tandem mass spectrometry. Journal of Chromatography. B, Biomedical Sciences and Applications 719: 71–78. [Google Scholar]
McCaskey TA, Liska BJ (1967). Effect of milk processing methods on endosulfan, endosulfan sulfate, and chlordane residues in milk. Journal of Dairy Science 50: 1991–1993. DOI 10.3168/jds.S0022-0302(67)87760-9. [Google Scholar] [CrossRef]
Menezes RG, Qadir TF, Moin A, Fatima H, Hussain SA, Madadin M, Pasha SB, Al Rubaish FA, Senthilkumaran S (2017). Endosulfan poisoning: An overview. Journal of Forensic and Legal Medicine 51: 27–33. DOI 10.1016/j.jflm.2017.07.008. [Google Scholar] [CrossRef]
Mnif W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B (2011). Effect of endocrine disruptor pesticides: A review. International Journal of Environmental Research and Public Health 8: 2265–2303. DOI 10.3390/ijerph8062265. [Google Scholar] [CrossRef]
Moon JM, Chun BJ (2009). Acute endosulfan poisoning: A retrospective study. Human & Experimental Toxicology 28: 309–316. DOI 10.1177/0960327109106488. [Google Scholar] [CrossRef]
Ozdem S, Nacitarhan C, Gulay MS, Hatipoglu FS, Ozdem SS (2011). The effect of ascorbic acid supplementation on endosulfan toxicity in rabbits. Toxicology and Industrial Health 27: 437–446. DOI 10.1177/0748233710388450. [Google Scholar] [CrossRef]
Pandey S, Nagpure NS, Kumar R, Sharma S, Srivastava SK, Verma MS (2006). Genotoxicity evaluation of acute doses of endosulfan to freshwater teleost Channa punctatus (Bloch) by alkaline single-cell gel electrophoresis. Ecotoxicology and Environmental Safety 65: 56–61. DOI 10.1016/j.ecoenv.2005.06.007. [Google Scholar] [CrossRef]
Pathak R, Suke SG, Ahmed RS, Tripathi AK, Guleria K, Sharma CS, Makhijani SD, Mishra M, Banerjee BD (2008). Endosulfan and other organochlorine pesticide residues in maternal and cord blood in North Indian population. Bulletin of Environmental Contamination and Toxicology 81: 216–219. DOI 10.1007/s00128-008-9459-9. [Google Scholar] [CrossRef]
Patočka J, Wu Q, França TCC, Ramalho TC, Pita R, Kuča K (2016). Clinical aspects of the poisoning by the pesticide endosulfan. Química Nova 39: 987–994. DOI 10.5935/0100-4042.20160102. [Google Scholar] [CrossRef]
Richardson JR, Fitsanakis V, Westerink RHS, Kanthasamy AG (2019). Neurotoxicity of pesticides. Acta Neuropathologica 138: 343–362. DOI 10.1007/s00401-019-02033-9. [Google Scholar] [CrossRef]
Rodríguez-Gonzalo E, García-Gómez D, Herrero-Hernández E, Carabias-Martínez R (2010). Automated sample treatment with the injection of large sample volumes for the determination of contaminants and metabolites in urine. Journal of Separation Science 33: 2240–2249. DOI 10.1002/jssc.201000202. [Google Scholar] [CrossRef]
Saxena MC, Siddiqui MK, Bhargava AK, Murti CR, Kutty D (1981). Placental transfer of pesticides in humans. Archives of Toxicology 48: 127–134. DOI 10.1007/BF00310482. [Google Scholar] [CrossRef]
Sebastian R, Raghavan SC (2017). Molecular mechanism of endosulfan action in mammals. Journal of Biosciences 42: 149–153. DOI 10.1007/s12038-016-9655-4. [Google Scholar] [CrossRef]
Silva MH, Carr WCJ (2010). Human health risk assessment of endosulfan: II. Dietary exposure assessment. Regulatory Toxicology and Pharmacology 56: 18–27. DOI 10.1016/j.yrtph.2009.08.015. [Google Scholar] [CrossRef]
Sinha N, Adhikari N, Saxena K(2001). Effect of endosulfan during fetal gonadal differentiation on spermatogenesis in rats. Environmental Toxicology and Pharmacology 10: 29–32. DOI 10.1016/S1382-6689(01)00066-7. [Google Scholar] [CrossRef]
Smith-Baker C, Saleh MA (2011). Hair as a marker for pesticides exposure. Journal of Environmental Science and Health. Part B, Pesticides, Food Contaminants, and Agricultural Wastes 46: 648–653. DOI 10.1080/03601234.2012.597701. [Google Scholar] [CrossRef]
Tarazona S, Bernabeu E, Carmona H, Gómez-Giménez B, García-Planells J, Leonards PEG, Jung S, Conesa A, Felipo V, Llansola M (2019). A multiomics study to unravel the effects of developmental exposure to endosulfan in rats: molecular explanation for sex-dependent effects. ACS Chemical Neuroscience 10: 4264–4279. DOI 10.1021/acschemneuro.9b00304. [Google Scholar] [CrossRef]
Tsatsakis AM, Tzatzarakis MN, Tutudaki M (2008). Pesticide levels in head hair samples of Cretan population as an indicator of present and past exposure. Forensic Science International 176: 67–71. DOI 10.1016/j.forsciint.2007.07.017. [Google Scholar] [CrossRef]
Walse SS, Scott GI, Ferry JL (2003). Stereoselective degradation of aqueous endosulfan in modular estuarine mesocosms: Formation of endosulfan γ-hydroxycarboxylate. Journal of Environmental Monitoring 5: 373–379. DOI 10.1039/B212165D. [Google Scholar] [CrossRef]
Wauchope RD, Buttler TM, Hornsby AG, Augustijn-Beckers PW, Burt JP (1992). The SCS/ARS/CES pesticide properties database for environmental decision-making. Reviews of Environmental Contamination and Toxicology 123: 1–155. DOI 10.1007/978-1-4612-2862-2. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |