DOI:10.32604/biocell.2022.018706

| BIOCELL DOI:10.32604/biocell.2022.018706 |  |
| Article |
Long non-coding RNA MIR22HG inhibits the adipogenesis of human bone marrow mesenchymal stem cells with the involvement of Wnt/β-catenin pathway
1Second Clinical Division, Peking University School and Hospital of Stomatology, Beijing, 100081, China
2Department of Orthodontics, Peking University School and Hospital of Stomatology, Beijing, 100081, China
3Central Laboratory, Peking University School and Hospital of Stomatology, Beijing, 100081, China
4National Center of Stomatology; National Clinical Research Center for Oral Diseases; National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing, 100081, China
*Address correspondence to: Yunfei Zheng, yunfei_zheng@bjmu.edu.cn; Lingfei Jia, jialingfei1984@sina.com
#Authors contributed equally
Received: 13 August 2021; Accepted: 18 October 2021
Abstract: Osteoporosis is a frequently occurring bone remodeling disorder worldwide with one characteristic being decreasing bone mineral density and a predisposition to bone fracture, which diminishes patients’ quality of life. Several studies showed that imbalance between the osteogenesis and adipogenesis of bone marrow mesenchymal stem cells (BMSCs) took part in the development of osteoporosis. In previous study, we found MIR22HG regulated the osteogenesis of human BMSCs positively. In this study, we found that MIR22HG was decreased during the adipogenesis of human BMSCs and exerted negative effects on adipogenesis with the involvement of Wnt/β-catenin signaling pathway both in vitro and in vivo. Nitazoxanide could inhibit Wnt signaling and relieve MIR22HG’s suppression on adipogenesis. These findings indicated that MIR22HG had great potential in clinical application for osteoporosis treatment and prevention.
Keywords: BMSCs; Osteoporosis; MIR22HG; Adipogenesis; Wnt/β-catenin pathway
Osteoporosis is a metabolic bone disease with one characteristic being increasing adipocyte tissue volume in bone marrow, which results in lower bone strength and fragility (Justesen et al., 2001; Meunier et al., 1971). It has been discussed for centuries and still remains a medical challenge worldwide (Rachner et al., 2011). The regulatory mechanism of osteoporosis is multifactorial, such as inflammation, angiogenesis, imbalance between osteoblasts and adipocytes (Lacativa and Farias, 2010; Rodríguez et al., 2009; Tong et al., 2019). In age-related osteoporosis, bone marrow mesenchymal stem cells (BMSCs) are inclined to differentiate into adipocytes rather than osteoblasts, generating an abnormal number of adipocytes and leading to osteoporosis. Wnt/β-catenin signaling is involved in osteoporosis as it takes an important part in regulating the balance between osteogenic and adipogenic differentiation of BMSCs (Huang et al., 2019; Takada et al., 2009). Activating the classical Wnt/β-catenin signaling pathway can stabilize β-catenin and promote osteogenesis (Canalis, 2013). Besides, Wnt signaling restrains adipogenesis via inhibiting the adipogenic transcription factors CCAAT enhancer-binding proteins (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) (Ross et al., 2000). Nitazoxanide (NTZ) is a clinically used antiparasitic drug and has been proved to efficiently inhibit Wnt signaling (Qu et al., 2018). Our work used NTZ as an inhibitor of Wnt signaling.
Long non-coding RNAs (lncRNAs) are non-protein-coding RNA molecules with an average length longer than 200 nucleotides, possessing multiple cellular functions such as chromatin organization, transcriptional regulation, and post-translational modification (West and Lagos, 2019). Many researchers have revealed lncRNAs’ important roles in adipogenesis (Chen et al., 2020; Pan et al., 2020; Shapira et al., 2017; Zhang et al., 2020). MIR22HG takes its name as it is the host gene of MiR-22 and has been validated to take effects in various cancers such as glioma, colorectal cancer, lung cancer, etc. Our group reported that MIR22HG was differentially expressed in the osteogenesis of human periodontal ligament stem cells (PDLSCs) and promoted the osteogenic differentiation of PDLSCs (Zheng et al., 2018). Moreover, MIR22HG was considerably decreased in BMSCs from osteoporotic mice compared with normal mice and promoted the osteogenesis of human BMSCs via PTEN/AKT pathway (Jin et al., 2020). However, the relationship between MIR22HG and adipogenesis of stem cells remains unknown. As a product of MIR22HG, MiR-22 promoted the osteogenesis and inhibited the adipogenesis of mesenchymal stem cells by targeting HDAC6 (Huang et al., 2012). To investigate whether MIR22HG itself directly influences the adipogenic differentiation of BMSCs, we decided to investigate the role of MIR22HG in the adipogenesis of BMSCs. Our research showed that MIR22HG could inhibit the adipogenesis of human BMSCs with the involvement of Wnt/β-catenin signaling. These findings suggest that MIR22HG might be a feasible target for osteoporosis treatment.
Human BMSCs were obtained from ScienCell Research Laboratories (San Diego, CA, USA) and cultured in growth medium made up of DMEM supplemented with 10% fetal bovine serum and 1% antibiotics. Cells were kept in a humidified incubator with 5% CO2 at 37°C. Adipogenesis was induced in standard growth medium supplemented with 50 nM insulin (Sigma-Aldrich, Saint Louis, MO, USA), 100 nM dexamethasone (Sigma-Aldrich), 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), and 200 μM indomethacin (Sigma-Aldrich) as described (Jin et al., 2017). The adipogenic medium was changed every 2 days, and cells were harvested at the indicated times.
Transfection of lentiviruses and small-interfering RNAs
The recombinant lentiviruses containing full-length MIR22HG and the scramble control were purchased from Cyagen Biosciences (Guangzhou, China) as described (Jin et al., 2020). Small interfering RNAs targeting MIR22HG and the scramble control were bought from Integrated Biotech Solutions Co. (Shanghai, China). The sequences are listed in Table 1.
RNA extraction and quantitative real-time PCR
Total RNA was extracted using TRIzol (Invitrogen) and reverse-transcribed into cDNA using a cDNA Reverse Transcription Kit (Takara). qRT-PCR was carried out using SYBR Green Master Mix on the ABI Prism 7500 Real-Time PCR System (Applied Biosystems). Relative expression was calculated by the 2−ΔΔCt method and normalized to the expression of GAPDH. Primers are shown in Table 1.
Protein extraction and Western blotting
The cells were lysed in Radio immunoprecipitation assay (RIPA) lysis buffer to extract total protein. Quantification of protein was performed using the BCA protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Thirty microgram proteins were resolved by 10% SDS–polyacrylamide gels and transferred to PVDF membranes (Millipore, Billerica, MA, USA). Primary antibodies against β-catenin and GAPDH (HuaxingBio Science, Beijing, China) were diluted 1:1000. The membranes were first incubated with primary antibodies overnight at 4°C, and then with corresponding secondary antibodies (1:10,000, Cell Signaling Technology). The band intensity was quantified by the Image J software (http://rsb.info.nih.gov/ij/) and normalized to the GAPDH band.
After 14 days adipogenic induction, cells were fixed with 4% formalin in PBS for 30 min, and then reacted with 60% saturated Oil Red O dye (Sigma-Aldrich) for 20 min. The Oil Red O-stained lipid droplets were photographed by LSM microscopy (Carl Zeiss, Oberkochen, Germany). To quantify the amount of lipid droplet formation, the stained cells were incubated with isopropanol to extract the dye from the lipid droplets, and the absorbance of reaction solution was then examined at 510 nM with a microplate reader (Bio-Rad).
Heterotopic bone formation assay in vivo
Human BMSCs were cultured in adipogenic medium for 1 week before the animal experiment. Then, cells were gathered, laden onto collagen sponge (8 mm × 8 mm × 2 mm) and then incubated at 37°C for 3 h, followed by centrifugation at 150×g for 5 min and implanted subcutaneously on the dorsal space of 5-week-old, BALB/c homozygous nude (nu/nu) mice (10 mice per group) as described previously (Huang et al., 2017; Jin et al., 2020). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Peking University Health Science Center (LA2014233) and were carried out in line with the Institutional Animal Guidelines.
All results are presented as mean ± SD. Statistical analysis was accomplished by Student’s t-test using IBM SPSS statistical software (version 21). Differences with P < 0.05 were treated statistically significant. All experiments were done for three repeats (N = 3).
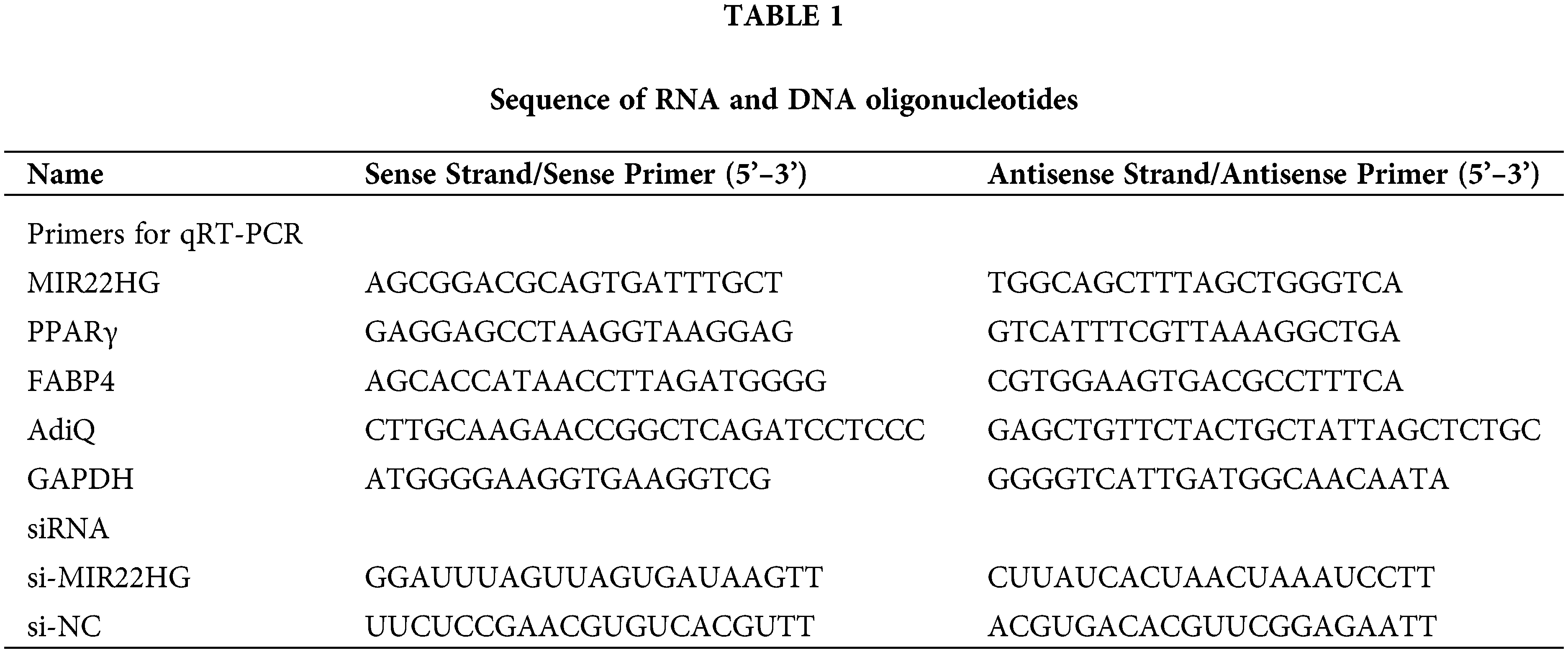
MIR22HG was decreased notably at the initial stage of the adipogenesis of BMSCs
We measured the expression of MIR22HG in the adipogenesis of human BMSCs to evaluate if MIR22HG is involved in this adipogenesis. As expected, the mRNA expressions of adipogenesis-associated genes fatty acid binding protein 4 (FABP4), adiponectin (AdiQ) and PPARγ were significantly increased (Figs. 1A–1C). qRT-PCR results showed that the expression of MIR22HG was greatly downregulated at the initial stage of the adipogenesis of BMSCs (Fig. 1D).
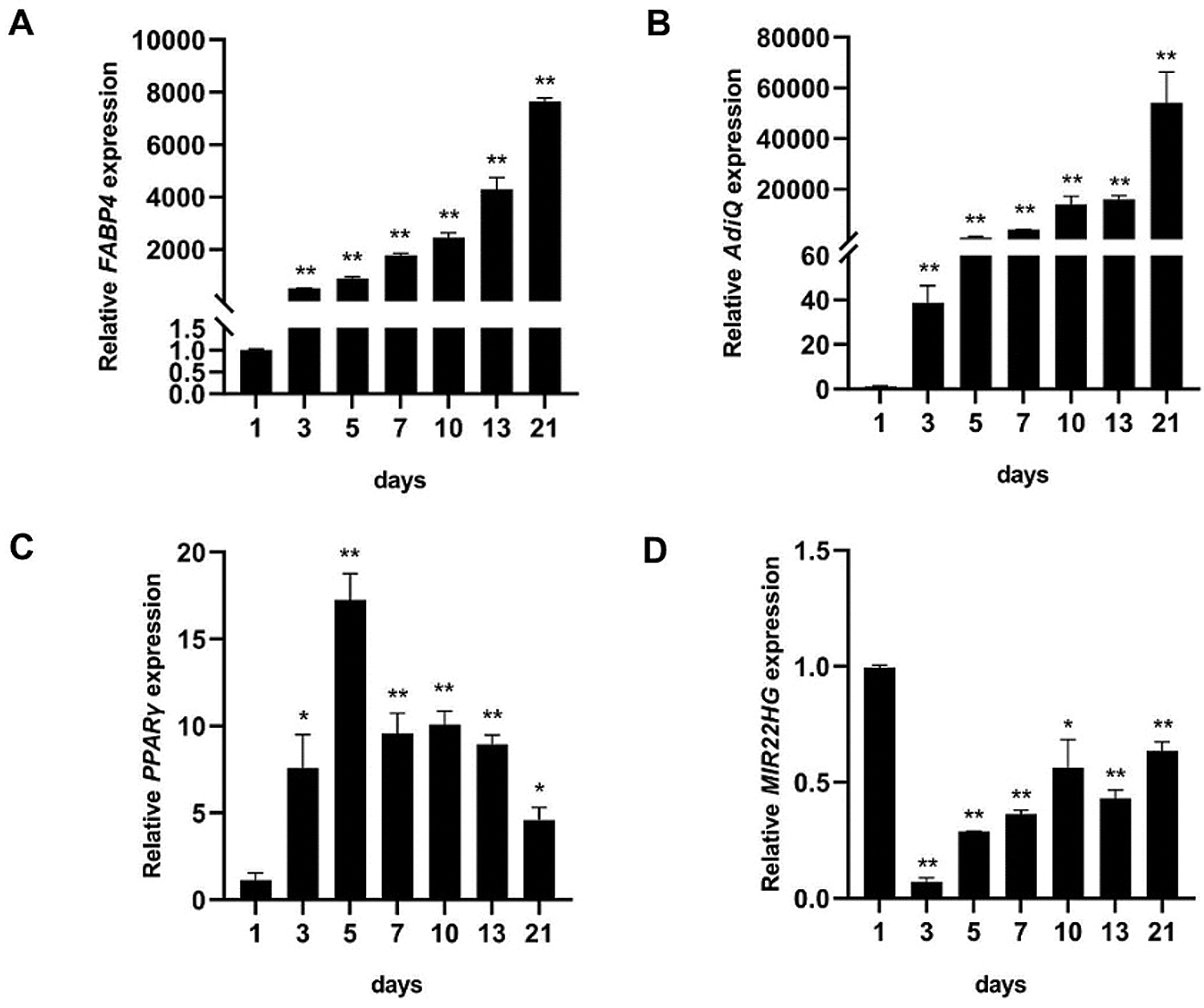
Figure 1: The expression pattern of adipogenic markers and MIR22HG in the adipogenesis of human BMSCs. (A–C) relative mRNA expression levels of FABP4, AdiQ, PPARγ were measured by qRT-PCR on days 1, 3, 5, 7, 10, 13, 21 of adipogenic induction. (D) qRT-PCR analysis of MIR22HG during the adipogenesis of human BMSCs. Results are presented as the mean ± SD, * P < 0.05, **P < 0.01, compared with day 1. Statistical analysis was accomplished by Student’s t-test, N = 3.
MIR22HG inhibited the adipogenic differentiation in vitro
To examine the role of MIR22HG in regulating the adipogenesis of human BMSCs, we transfected human BMSCs with recombinant lentiviruses containing full-length MIR22HG (MIR22HG group) and the scramble control (NC group), small interfering RNAs targeting MIR22HG (si-MIR22HG group) and the scramble control (si-NC group). The expression of MIR22HG was obviously decreased in si-MIR22HG transfected group compared to si-NC group and increased in MIR22HG group compared to NC group (Figs. 2A and 2D). The mRNA levels of adipogenic markers PPARγ and FABP4 were upregulated in si-MIR22HG group compared to si-NC group and downregulated in MIR22HG group compared to NC group both in proliferation medium (PM) and adipogenic medium (AM) (Figs. 2B, 2C, 2E and 2F). Oil Red O staining exposed a significant increase of lipid droplets in si- MIR22HG group compared to si-NC group and a decrease in MIR22HG group compared to NC group (Figs. 2G and 2H). All these results suggested MIR22HG took part in the adipogenesis of BMSCs.
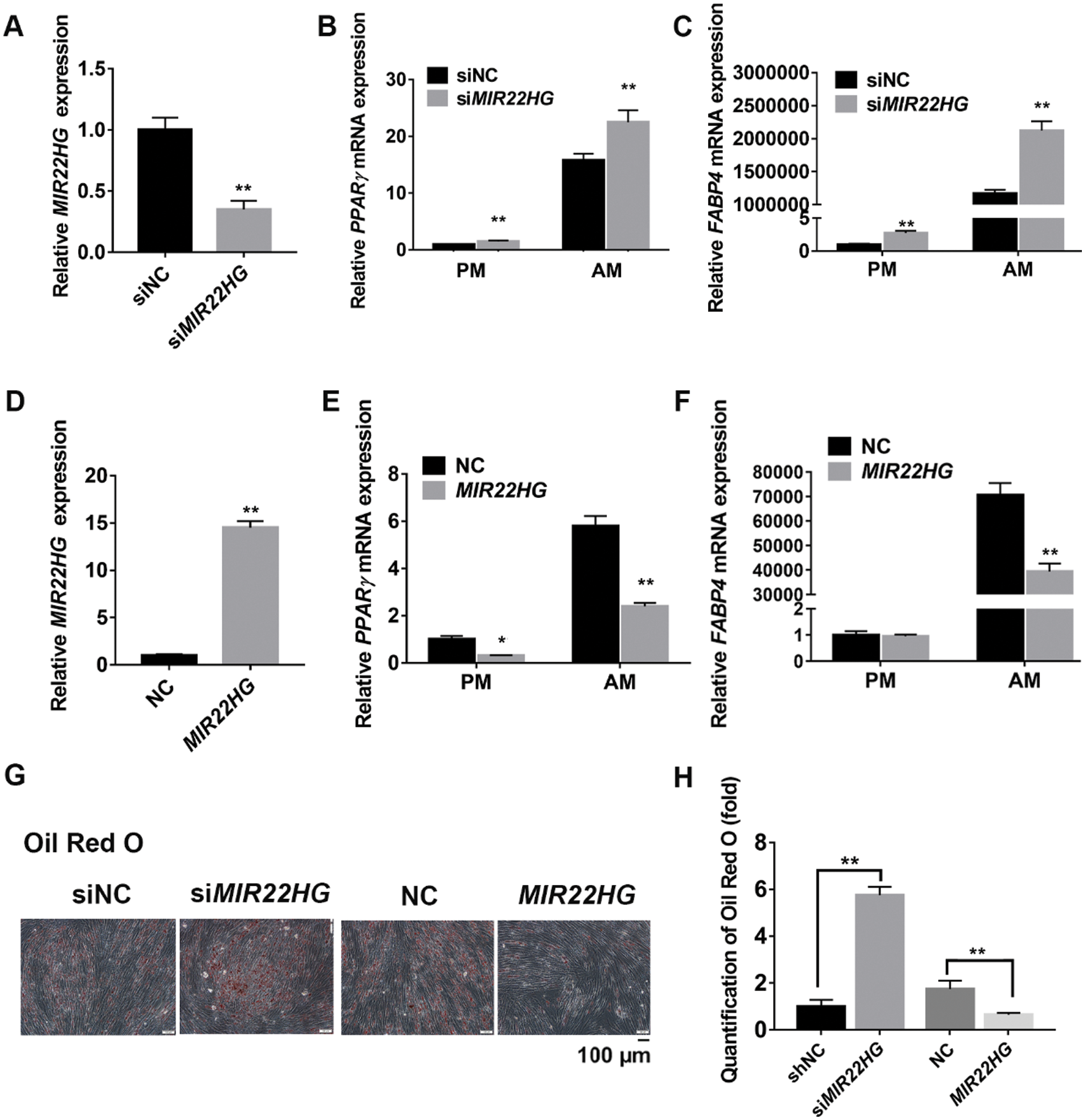
Figure 2: MIR22HG inhibited the adipogenesis of human BMSCs. The human BMSCs were transfected with small interfering RNA negative control (si-NC group), small interfering RNAs targeting MIR22HG (si-MIR22HG group), lentivirus overexpressing MIR22HG (MIR22HG group) or the scrambled vector (NC group). (A, D) the efficiency of transfection was measured by qRT-PCR. (B–C) and (E–F) cells were cultured in proliferation medium (PM) or adipogenic medium (AM) for 7 days. qRT-PCR analysis shows PPARγ and FABP4 mRNA expression. (G–H) images of Oil red O staining in the si-NC, si-MIR22HG, NC and MIR22HG groups (top row) after 14 days’ adipogenic induction. Histograms show quantification of Oil red O staining by spectrophotometry. Results are presented as the mean ± SD, *P < 0.05, **P < 0.01, compared with the si-NC and NC groups, relatively. Statistical analysis was accomplished by Student’s t-test, N = 3.
MIR22HG inhibited newly formed adipose tissue in vivo
Human BMSCs expressing si-MIR22HG, MIR22HG and the control were cultured in AM for 7 days. Then, cells were loaded onto Bio-Oss Collagen scaffolds and implanted in the dorsum of nude mice subcutaneously (Fig. 3A). After 8 weeks, the formation of adipose tissue was further characterized by Oil red O staining, showing intracellular lipid gathering (Fig. 3B). Quantification of the newly generated adipose tissue consistently showed a greater ratio of adipogenesis in the si-MIR22HG group (45.9%) compared to the si-NC group (12.6%) and a lower ratio in the MIR22HG group (4.1%) compared to the NC group (16.7%) (Fig. 3C). Those data in vivo provided further validation for our hypothesis.
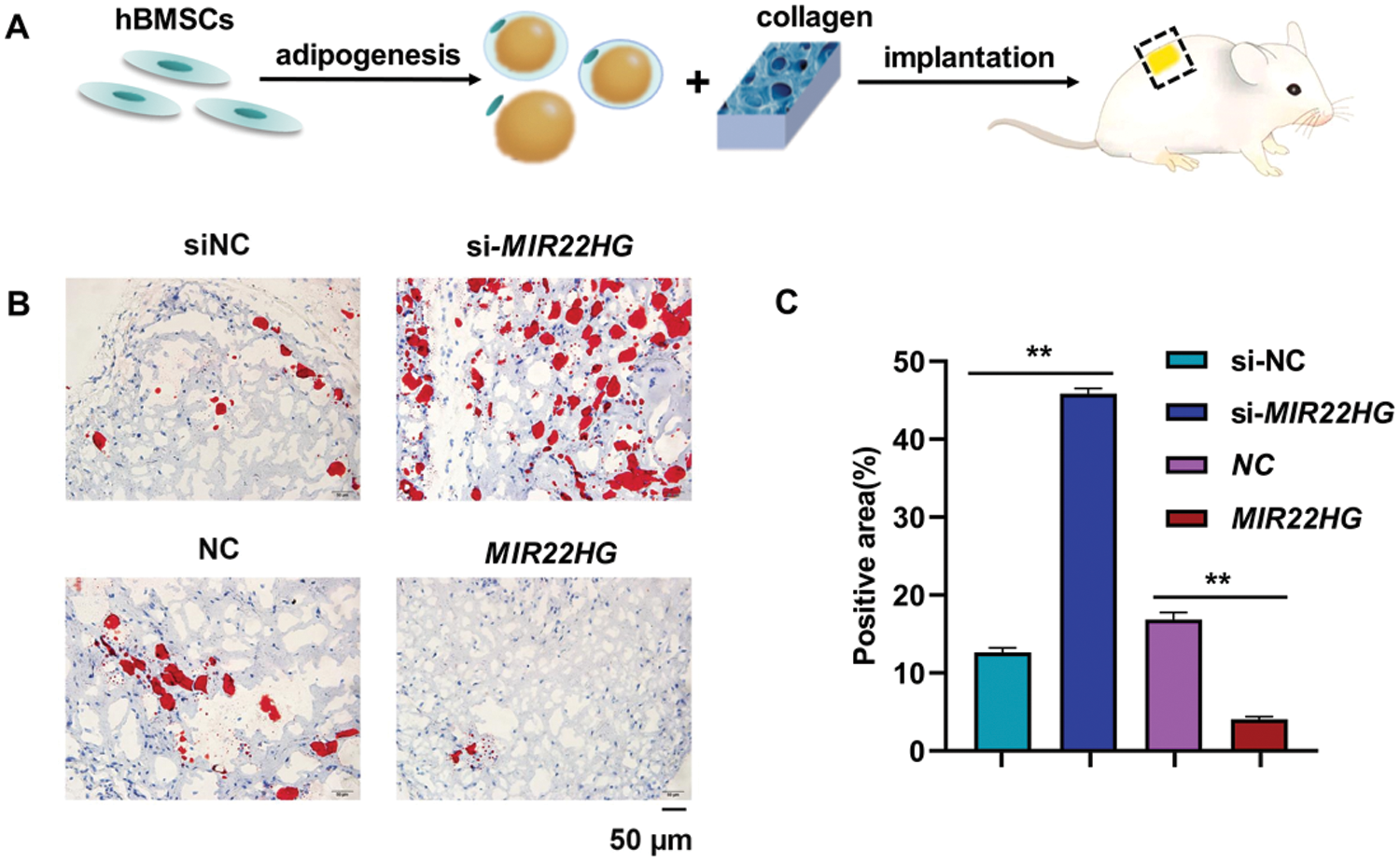
Figure 3: MIR22HG inhibited adipose tissue formation in vivo. (A) Schematic diagram illustrating the experimental setup. (B) Oil red O staining of the specimens implanted with adipogenic-induced human BMSC/collagen complex in si-NC group, si-MIR22HG group, NC group and MIR22HG overexpressed group. Scale bar, 50 μm. (C) quantitative analysis of the Oil red O-stained areas of newly generated tissue at 8 weeks after implantation. The areas were expressed as percentages of the total areas measured by Image J. Results are presented as the mean ± SD, *P < 0.05, **P < 0.01. Statistical analysis was accomplished by Student’s t-test, N = 3.
MIR22HG inhibited adipogenesis via β-catenin pathway
To figure out whether MIR22HG regulates adipogenesis via Wnt/β-catenin pathway in BMSCs, we performed western blot analysis and the results showed that MIR22HG overexpression promoted the protein level of β-catenin (Figs. 4A and 4B). NTZ was used as a β-catenin inhibitor dissolved in dimethyl sulfoxide (DMSO) and was added into PM/AM with the final concentration of 10 μM. With the treatment of NTZ, β-catenin was decreased, and the increase of β-catenin induced by MIR22HG was relieved compared to the control group (Figs. 4C and 4D). Furthermore, after 7 days of adipogenic induction under the treatment of NTZ in NC (NC+NTZ) and MIR22HG overexpression group (MIR22HG+NTZ), PPARγ and FABP4 were increased compared to NC group and MIR22HG group which were cultured without NTZ (Figs. 4E and 4F). Oil Red O staining disclosed that NTZ could relieve MIR22HG’s suppression on the adipogenesis (Figs. 4G and 4H). These data indicated that MIR22HG improved the expression of β-catenin and inhibited the adipogenesis, while NTZ showed an antagonistic effect.
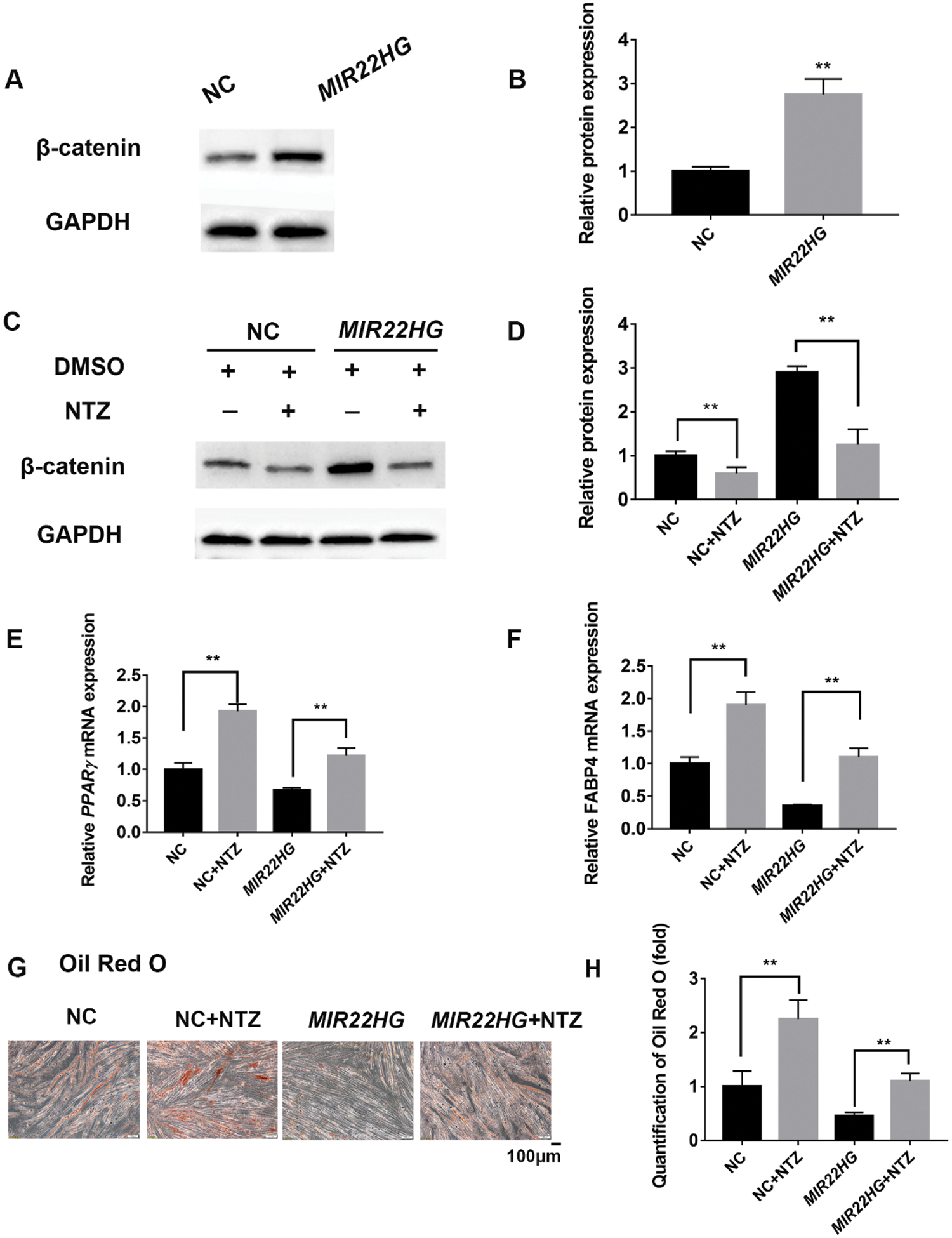
Figure 4: MIR22HG inhibited the adipogenesis of human BMSCs via Wnt/β-catenin pathway. (A–B) western blot analysis and quantification of protein expression of β-catenin and GAPDH in NC and MIR22HG groups. (C–D) NTZ and DMSO were added into PM respectively to treat cells for 96 h. Western blot analysis and quantification of protein expression of β-catenin and GAPDH in NC+DMSO, NC+NTZ, MIR22HG+DMSO and MIR22HG+NTZ groups. (E–F) relative mRNA expression levels of PPARγ and FABP4 were measured by qRT-PCR after 7 days adipogenic induction in NC and MIR22HG groups with or without NTZ treatment, relative to NC groups. (G–H), images and quantification of Oil Red O staining for lipid droplet in the NC, NC+NTZ, MIR22HG and MIR22HG+ NTZ groups. Results are presented as the mean ± SD, *P < 0.05, **P < 0.01. Statistical analysis was accomplished by Student’s t-test, N = 3.
In our previous study, osteoporosis mice models were set up successfully and our group found MIR22HG was considerably decreased in BMSCs from osteoporotic mice compared with normal mice. Because imbalance between the adipogenesis and osteogenesis of BMSCs is one factor of osteoporosis, we decided to explore the roles of MIR22HG in the osteogenic and adipogenic differentiation of BMSCs. Our previous study showed MIR22HG reinforced the osteogenesis of human BMSCs via lowering phosphatase and tensin homolog (PTEN) and activating AKT signaling (Jin et al., 2020). In this study, we found that MIR22HG was decreased during the adipogenesis of human BMSCs and regulated adipogenesis negatively both in vitro and in vivo with the involvement of Wnt/β-catenin signaling. Wnt/β-catenin pathway could not only inhibit adipogenesis but also stimulate the osteogenesis of BMSCs (Cawthorn et al., 2012; Si et al., 2006). AKT signaling was validated to involve in the adipogenesis of BMSCs (Song et al., 2017; Wang et al., 2017) and PTEN was a negative regulator of AKT/GSK3β/β-catenin signaling (Chen et al., 2019). Thus, our findings suggested that MIR22HG regulated the osteogenesis and adipogenesis through a complicated network with β-catenin being a core node.
Consistent to our study, MIR22HG was proved to be an inducer of the Wnt/β-catenin signaling pathway via its product, miR-22. MiR-22 targeted two inhibitors of β-catenin, SFRP2 and PCDH15 and exerted its effect on glioblastoma progression (Han et al., 2020). SFRP2 exerted inhibition through competing with Frizzled for binding Wnt (Kawano and Kypta, 2003). PCDH15 could decrease the activity of Wnt signaling. But the detailed mechanisms still remain unsolved (Han et al., 2020). Contrary to our findings, MIR22HG was reported to negatively regulate the Wnt/β-catenin pathway and inhibit cell proliferation and migration in cholangiocarcinoma (Hu et al., 2019). It seems that MIR22HG regulates Wnt/β-catenin pathway differently in special situations. In the present study, MIR22HG may also exert its function in the adipogenesis via miR22/SFRP2 and miR22/PCDH15 axes. Detailed mechanisms between MIR22HG and Wnt/β-catenin signaling need to be researched in the future. Additionally, NTZ was used as a single approach to inhibit Wnt/β-catenin pathway in our study. As a chemical compound, it was proved to influence autophagy differently in various cell lines (Shou et al., 2020; Sun et al., 2021; Wang et al., 2018). NTZ also significantly inhibited proliferation and promoted apoptosis in cardiomyocytes (Gong et al., 2021). Although effects of NTZ on BMSCs stay unidentified, it is possible that NTZ can influence many aspects of BMSCs such as proliferation, autophagy, and apoptosis. To better understand the mechanism, further study could focus on the effects of NTZ on BMSCs other than suppressing Wnt/β-catenin pathway.
In terms of clinical application, BMSCs are relatively easy to obtain and less likely to form tumor after implantation (Griffin et al., 2011). Advancement of BMSC-based treatments for the restoration of bone tissue is demonstrated by various human clinical studies (Gómez-Barrena et al., 2018; Gómez-Barrena et al., 2020; Rojewski et al., 2019). For instance, one Phase I clinical trial was completed in 2018. Osteoporosis patients were injected autologous fucosylated BMSCs collected 30 days before injection and BMSCs were proved to be feasible with no observed short-term adverse events (ClinicalTrials.gov identifier: NCT02566655) (Hu et al., 2019). However, BMSCs still have some shortcomings as potential seed cells for cell therapy in the treatment of osteoporosis such as inefficiency of osteogenic differentiation. Gene modification of BMSCs will make the BMSC-based treatments safer and more effective in the future. We suggested that MIR22HG might be a gene target to modify the ability of BMSCs to differentiate. Besides, Wnt/β-catenin signaling has been a target of several agents for osteoporosis. Romozumab is a newly developed anti-osteoporotic drug which binds a secreted protein sclerostin and reverses its inhibition on Wnt signaling. It was approved by Food and Drug Administration (FDA) in April 2019 (Mcclung et al., 2014). Our results showed that MIR22HG could regulate the expression of β-catenin and influence the adipogenesis. We hypothesized that MIR22HG might serve as a nucleic drug targeting Wnt signaling in the future.
Overall, this is the first study to recognize the negative effect of MIR22HG on the adipogenesis of human BMSCs with the involvement of Wnt/β-catenin signaling pathway. Our research suggested the potential of MIR22HG to become a therapeutic target of osteoporosis.
Availability of Data and Materials: The data that support the finding of this study are available from the corresponding author upon reasonable request.
Authors’ Contribution: The authors confirm contribution to the paper as follows: study conception and design: Yunfei Zheng, Lingfei Jia; data collection: Chanyuan Jin; analysis and interpretation of results: Chanyuan Jin, Ziyao Zhuang; draft manuscript preparation: Ziyao Zhuang. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: All animal experiments were approved by the Institutional Animal Care and Use Committee of the Peking University Health Science Center (LA2014233) and were carried out in line with the Institutional Animal Guidelines.
Funding Statement: This study was financially supported by grants from the National Natural Science Foundation of China (82071119, 82071142, 81700938, 81772876, 81800942).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Canalis E (2013). Wnt signalling in osteoporosis: Mechanisms and novel therapeutic approaches. Nature Reviews Endocrinology 9: 575–583. DOI 10.1038/nrendo.2013.154. [Google Scholar] [CrossRef]
Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati N, Martinez-Santibañez G, Macdougald OA (2012). Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 50: 477–489. DOI 10.1016/j.bone.2011.08.010. [Google Scholar] [CrossRef]
Chen Y, Li K, Zhang X, Chen J, Li M, Liu L (2020). The novel long noncoding RNA lncRNA-Adi regulates adipogenesis. Stem Cells Translational Medicine 9: 1053–1067. DOI 10.1002/sctm.19-0438. [Google Scholar] [CrossRef]
Chen YX, Zhu DY, Gao J, Xu ZL, Tao SC, Yin WJ, Zhang YL, Gao YS, Zhang CQ (2019). Diminished membrane recruitment of Akt is instrumental in alcohol-associated osteopenia via the PTEN/Akt/GSK-3β/β-catenin axis. The FEBS Journal 286: 1101–1119. DOI 10.1111/febs.14754. [Google Scholar] [CrossRef]
Gómez-Barrena E, Padilla-Eguiluz NG, Avendaño-Solá C, Payares-Herrera C, Velasco-Iglesias A, Torres F, Rosset P, Gebhard F, Baldini N, Rubio-Suarez JC (2018). A multicentric, open-label, randomized, comparative clinical trial of two different doses of expanded hBM-MSCs plus biomaterial versus iliac crest autograft, for bone healing in nonunions after long bone fractures: Study protocol. Stem Cells International 2018: 1–13. DOI 10.1155/2018/6025918. [Google Scholar] [CrossRef]
Gómez-Barrena E, Padilla-Eguiluz NG, García-Rey E, Hernández-Esteban P, Cordero-Ampuero J, Rubio-Suárez JC (2020). Validation of a long bone fracture non-union healing score after treatment with mesenchymal stromal cells combined to biomaterials. Injury—International Journal of the Care of the Injured 51: S55–S62. DOI 10.1016/j.injury.2020.02.030. [Google Scholar] [CrossRef]
Gong F, Shen T, Zhang J, Wang X, Fan G, Che X, Xu Z, Jia K, Huang Y, Li X (2021). Nitazoxanide induced myocardial injury in zebrafish embryos by activating oxidative stress response. Journal of Cellular and Molecular Medicine 25: 9740–9752. DOI 10.1111/jcmm.16922. [Google Scholar] [CrossRef]
Griffin M, Iqbal S, Bayat A (2011). Exploring the application of mesenchymal stem cells in bone repair and regeneration. The Bone & Joint Journal 93: 427–434. DOI 10.1302/0301-620X.93B4.25249. [Google Scholar] [CrossRef]
Han M, Wang S, Fritah S, Wang X, Zhou W, Yang N, Ni S, Huang B, Chen A, Li G (2020). Interfering with long non-coding RNA MIR22HG processing inhibits glioblastoma progression through suppression of Wnt/β-catenin signalling. Brain 143: 512–530. DOI 10.1093/brain/awz406. [Google Scholar] [CrossRef]
Hu X, Tan Z, Yang Y, Yang P (2019). Long non-coding RNA MIR22HG inhibits cell proliferation and migration in cholangiocarcinoma by negatively regulating the Wnt/β-catenin signaling pathway. The Journal of Gene Medicine 21: e3085. DOI 10.1002/jgm.3085. [Google Scholar] [CrossRef]
Huang S, Wang S, Bian C, Yang Z, Zhou H, Zeng Y, Li H, Han Q, Zhao RC (2012). Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells and Development 21: 2531–2540. DOI 10.1089/scd.2012.0014. [Google Scholar] [CrossRef]
Huang TB, Li YZ, Yu K, Yu Z, Wang Y, Jiang ZW, Wang HM, Yang GL (2019). Effect of the Wnt signal-RANKL/OPG axis on the enhanced osteogenic integration of a lithium incorporated surface. Biomaterials Science 7: 1101–1116. DOI 10.1039/C8BM01411F. [Google Scholar] [CrossRef]
Huang Y, Jin C, Zheng Y, Li X, Zhang S, Zhang Y, Jia L, Li W (2017). Knockdown of lncRNA MIR31HG inhibits adipocyte differentiation of human adipose-derived stem cells via histone modification of FABP4. Scientific Reports 7: 8080. DOI 10.1038/s41598-017-08131-6. [Google Scholar] [CrossRef]
Jin C, Jia L, Tang Z, Zheng Y (2020). Long non-coding RNA MIR22HG promotes osteogenic differentiation of bone marrow mesenchymal stem cells via PTEN/AKT pathway. Cell Death & Disease 11: 601. DOI 10.1038/s41419-020-02813-2. [Google Scholar] [CrossRef]
Jin C, Wang W, Liu Y, Zhou Y (2017). RAI3 knockdown promotes adipogenic differentiation of human adipose-derived stem cells by decreasing β-catenin levels. Biochemical and Biophysical Research Communications 493: 618–624. DOI 10.1016/j.bbrc.2017.08.142. [Google Scholar] [CrossRef]
Justesen J, Stenderup K, Ebbesen E, Mosekilde L, Steiniche T, Kassem M (2001). Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2: 165–171. DOI 10.1023/A:1011513223894. [Google Scholar] [CrossRef]
Kawano Y, Kypta R (2003). Secreted antagonists of the Wnt signalling pathway. Journal of Cell Science 116: 2627–2634. DOI 10.1242/jcs.00623. [Google Scholar] [CrossRef]
Lacativa PGS, Farias MLFD (2010). Osteoporosis and inflammation. Arquivos Brasileiros de Endocrinologia & Metabologia 54: 123–132. DOI 10.1590/S0004-27302010000200007. [Google Scholar] [CrossRef]
Mcclung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM (2014). Romosozumab in postmenopausal women with low bone mineral density. The New England Journal of Medicine 370: 412–420. DOI 10.1056/NEJMoa1305224. [Google Scholar] [CrossRef]
Meunier P, Aaron J, Edouard C, Vlgnon G (1971). Osteoporosis and the replacement of cell populations of the marrow by adipose tissue: A quantitative study of 84 iliac bone biopsies. Clinical Orthopaedics and Related Research 80: 147–154. DOI 10.1097/00003086-197110000-00021. [Google Scholar] [CrossRef]
Pan Y, Xie Z, Cen S, Li M, Liu W, Tang SA, Ye G, Li J, Zheng G, Li Z (2020). Long noncoding RNA repressor of adipogenesis negatively regulates the adipogenic differentiation of mesenchymal stem cells through the hnRNP A1-PTX3-ERK axis. Clinical and Translational Medicine 10: e227. DOI 10.1002/ctm2.227. [Google Scholar] [CrossRef]
Qu Y, Olsen JR, Yuan X, Cheng PF, Levesque MP, Brokstad KA, Hoffman PS, Oyan AM, Zhang W, Kalland KH (2018). Small molecule promotes β-catenin citrullination and inhibits Wnt signaling in cancer. Nature Chemical Biology 14: 94–101. DOI 10.1038/nchembio.2510. [Google Scholar] [CrossRef]
Rachner TD, Khosla S, Hofbauer LC (2011). Osteoporosis: Now and the future. The Lancet 377: 1276–1287. DOI 10.1016/S0140-6736(10)62349-5. [Google Scholar] [CrossRef]
Rodríguez JP, Astudillo P, Ríos S, Seitz G, Pino AM (2009). Adipogénesis y osteoporosis. Revista Médica de Chile 137: 827–836. DOI 10.4067/S0034-98872009000600015. [Google Scholar] [CrossRef]
Rojewski MT, Lotfi R, Gjerde C, Mustafa K, Veronesi E, Ahmed AB, Wiesneth M, Koerper S, Sensebé L, Layrolle P (2019). Translation of a standardized manufacturing protocol for mesenchymal stromal cells: A systematic comparison of validation and manufacturing data. Cytotherapy 21: 468–482. DOI 10.1016/j.jcyt.2019.03.001. [Google Scholar] [CrossRef]
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, Macdougald OA (2000). Inhibition of adipogenesis by Wnt signaling. Science 289: 950–953. DOI 10.1126/science.289.5481.950. [Google Scholar] [CrossRef]
Shapira SN, Lim HW, Rajakumari S, Sakers AP, Ishibashi J, Harms MJ, Won KJ, Seale P (2017). EBF2 transcriptionally regulates brown adipogenesis via the histone reader DPF3 and the BAF chromatin remodeling complex. Genes & Development 31: 660–673. DOI 10.1101/gad.294405.116. [Google Scholar] [CrossRef]
Shou J, Wang M, Cheng X, Wang X, Zhang L, Liu Y, Fei C, Wang C, Gu F, Xue F (2020). Tizoxanide induces autophagy by inhibiting PI3K/Akt/mTOR pathway in RAW264. 7 macrophage cells. Archives of Pharmacal Research 43: 257–270. DOI 10.1007/s12272-019-01202-4. [Google Scholar] [CrossRef]
Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H (2006). CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Molecular and Cellular Biology 26: 2955–2964. DOI 10.1128/MCB.26.8.2955-2964.2006. [Google Scholar] [CrossRef]
Song F, Jiang D, Wang T, Wang Y, Lou Y, Zhang Y, Ma H, Kang Y (2017). Mechanical stress regulates osteogenesis and adipogenesis of rat mesenchymal stem cells through PI3K/Akt/GSK-3β/β-catenin signaling pathway. BioMed Research International 2017: 1–10. DOI 10.1155/2017/6027402. [Google Scholar] [CrossRef]
Sun H, Ou T, Hu J, Yang Z, Lei Q, Li Y, Wang G, Li Y, Wu K, Wang S (2021). Nitazoxanide impairs mitophagy flux through ROS-mediated mitophagy initiation and lysosomal dysfunction in bladder cancer. Biochemical Pharmacology 190: 114588. DOI 10.1016/j.bcp.2021.114588. [Google Scholar] [CrossRef]
Takada I, Kouzmenko AP, Kato S (2009). Molecular switching of osteoblastogenesis versus adipogenesis: Implications for targeted therapies. Expert Opinion on Therapeutic Targets 13: 593–603. DOI 10.1517/14728220902915310. [Google Scholar] [CrossRef]
Tong X, Chen X, Zhang S, Huang M, Shen X, Xu J, Zou J (2019). The effect of exercise on the prevention of osteoporosis and bone angiogenesis. BioMed Research International 2019: 1–8. DOI 10.1155/2019/8171897. [Google Scholar] [CrossRef]
Wang X, Shen C, Liu Z, Peng F, Chen X, Yang G, Zhang D, Yin Z, Ma J, Zheng Z (2018). Nitazoxanide, an antiprotozoal drug, inhibits late-stage autophagy and promotes ING1-induced cell cycle arrest in glioblastoma. Cell Death & Disease 9: 1032. DOI 10.1038/s41419-018-1058-z. [Google Scholar] [CrossRef]
Wang Y, Liu Y, Fan Z, Liu D, Wang F, Zhou Y (2017). IGFBP2 enhances adipogenic differentiation potentials of mesenchymal stem cells from Wharton’s jelly of the umbilical cord via JNK and Akt signaling pathways. PLoS One 12: e0184182. DOI 10.1371/journal.pone.0184182. [Google Scholar] [CrossRef]
West KA, Lagos D (2019). Long non-coding RNA function in CD4+ T cells: What we know and what next? Non-coding RNA 5: 43. DOI 10.3390/ncrna5030043. [Google Scholar] [CrossRef]
Zhang T, Liu H, Mao R, Yang H, Zhang Y, Zhang Y, Guo P, Zhan D, Xiang B, Liu Y (2020). The lncRNA RP11-142A22. 4 promotes adipogenesis by sponging miR-587 to modulate Wnt5β expression. Cell Death & Disease 11: 475. DOI 10.1038/s41419-020-2550-9. [Google Scholar] [CrossRef]
Zheng Y, Li X, Huang Y, Jia L, Li W (2018). Time series clustering of mRNA and lncRNA expression during osteogenic differentiation of periodontal ligament stem cells. PeerJ 6: e5214. DOI 10.7717/peerj.5214. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |